Abstract
We herein report a case of acute exacerbation of idiopathic pulmonary fibrosis (AE-IPF) triggered by COVID-19. An 87-year-old woman tested positive for COVID-19 on a polymerase chain reaction test, and computed tomography revealed ground-glass opacity (GGO) superimposed on a background pattern consistent with usual interstitial pneumonia. Considering these data, we diagnosed her with AE-IPF. She experienced worsening of dyspnea and expansion of the GGO. Therefore, we introduced high-dose steroids (methylprednisolone 250 mg/day for 3 days). After the treatment, the pulmonary infiltrates improved. She was discharged from our hospital without severe disability. High-dose steroids can be a viable treatment option for AE-IPF triggered by COVID-19.
Keywords: COVID-19, acute exacerbation, idiopathic pulmonary fibrosis, high-dose steroid, case report
Introduction
Coronavirus disease 2019 (COVID-19) pneumonia is an acute respiratory disease caused by severe acute respiratory syndrome coronavirus 2 infection. Several drugs, including steroids and remdesivir, have been shown to be effective in recent randomized controlled trials for patients with severe COVID-19 (1,2). However, despite the development of therapeutic strategies, patients with COVID-19 still exhibit a high mortality rate, especially among the elderly and those with comorbidities, including diabetes, renal failure, and respiratory diseases (3,4). In particular, respiratory diseases, such as chronic obstructive pulmonary disease and interstitial lung disease (ILD), are also strongly associated with a high mortality (3,5).
Idiopathic pulmonary fibrosis (IPF) is an irreversible and progressive ILD with an unknown etiology. Some patients with IPF experience acute exacerbation (AE) of the disease, with episodes of the sudden acceleration of the disease process (6,7). AE-IPF is a severe and life-threatening complication of IPF that occurs without any identifiable causes or triggers, such as surgical intervention or infection (8).
We herein report a patient with AE-IPF triggered by COVID-19 who was successfully treated with a high-dose steroid.
Case Report
An 87-year-old asymptomatic woman was diagnosed with COVID-19 by transcription polymerase chain reaction (PCR). Three days later, she was admitted to our hospital to quarantine her from her family. She had a cough and slightly worsening dyspnea without hypoxia (oxygen saturation of 98% on ambient air). There were no extra-thoracic manifestations suggesting the presence of an underlying connective tissue disease (CTD). Routine blood tests showed a white blood cell count of 2,880/mm3, C-reactive protein of 0.09 mg/dL, lactate dehydrogenase 204 U/mL, D-dimer 1.8 μg/mL, KL-6 370 U/mL, ferritin 106.7 ng/mL, and positivity for anti-SSA(Ro) antibody.
Chest X-ray and computed tomography (CT) showed bilateral ground-glass opacity (GGO) superimposed on a background reticular shadow and honeycomb in the bilateral lower lobes, suggesting the usual interstitial pneumonia pattern (Fig. 1A, 2A-B, 3A). Given these findings, we diagnosed her with AE-IPF triggered by COVID-19 and placed her on favipiravir from the first day of admission.
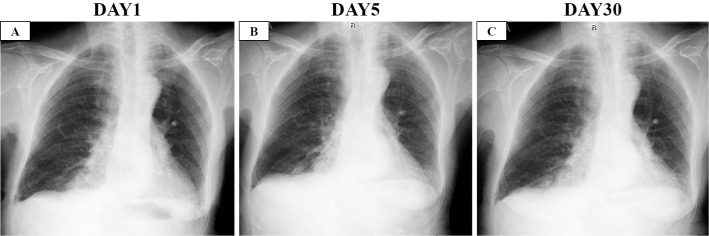
Figure 1.
Chest X-ray images. A: On the day of admission to our hospital. B: On the day of the initiation of steroid therapy (five days after admission). C: Two weeks after the initiation of steroids.
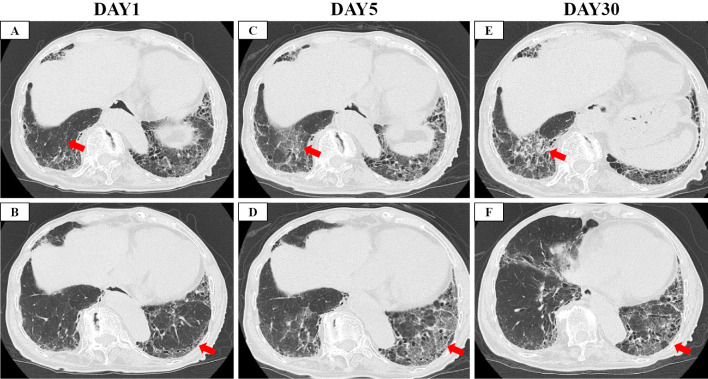
Figure 2.
Computed tomography images. A, B: On the day of admission to our hospital. C, D: On the day of the initiation of steroid therapy (five days after admission). E, F: Two weeks after the initiation of steroids. Red arrows show newly observed ground-glass opacity (GGO), and red arrow heads show honeycomb.
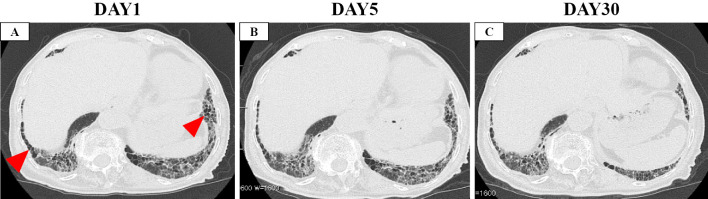
Figure 3.
Computed tomography images. A: On the day of admission to our hospital. B: On the day of the initiation of steroid therapy (five days after admission). C: Two weeks after the initiation of steroids. Red arrows show newly observed ground-glass opacity (GGO), and red arrow heads show honeycomb.
Five days later, she experienced hemoptysis, worsening of dyspnea (without hypoxia), elevated D-dimer 4.3 μg/mL, and expansion of GGO on CT (Fig. 1B, 2C-D, 3B). Therefore, we decided to initiate intravenous high-dose steroids (methylprednisolone 250 mg/day for 3 days followed by methylprednisolone 1.0 mg/kg/day, total 40 mg/day). In addition, we switched her anti-viral therapy from favipiravir to remdesivir. With this treatment, the dyspnea and pulmonary infiltrate of the left lower lobe gradually improved (Fig. 1C, 2E-F, 3C). The systemic steroid was tapered from day 8 of steroid therapy initiation and discontinued entirely on day 32. Finally, she was discharged from our hospital on day 34 without severe disability.
Discussion
We successfully treated a patient with AE-IPF triggered by COVID-19 with high-dose steroids. The recent guidelines concerning the management of patients with COVID-19 recommend the short-term use of low dose steroids (e.g. dexamethasone 6.0 mg for 10 days) based on the recovery study (1,9,10). However, the optimum dose and duration of steroid therapy, especially in high-risk groups, including those with IPF, remain unclear.
A recent study reported that high-dose steroids (methylprednisone 250 mg/day for 3 days) was a viable treatment option for severe COVID-19 (11). Kitayama et al. also reported a case of COVID-19 pneumonia resembling an AE-ILD rescued by steroid pulse therapy (12). Both patients with COVID-19 and IPF are exposed to hyperinflammatory states with elevated levels of cytokines, including interleukin 6 (IL-6) (13-15). Furthermore, high-dose steroids, including pulse therapy, are often used to manage AE-IPF (16,17). These data imply that AE-IPF triggered by COVID-19 may need higher doses of steroids to suppress the disease activity than those with idiopathic and other triggers. Although the optimal duration of maintenance steroid therapy in AE-IPF is unclear, we terminated steroid therapy on day 32. This is because our patient was elderly, so we were concerned about adverse events with long-term maintenance steroid therapy. In addition, most studies have applied a short-term protocol of steroid therapy (3-10 days) for severe COVID-19 patients (1,2,11). In contrast, the Japanese guideline for the treatment of IPF suggests long-term maintenance of steroid therapy after an AE event (17). Further evidence is needed to answer clinical questions concerning maintenance steroid therapy in AE-IPF triggered by COVID-19.
The present patient met the diagnostic criteria for AE-IPF, and an AE event was triggered by COVID-19 (8). Previous studies have suggested that some cases of AE-IPF are induced by viral infections (18,19). Song et al. noted no marked differences in the prognosis between patients with “idiopathic” AE and those whose condition was triggered by infection (20). In contrast, Kondoh et al. reported that AE-ILD triggered by COVID-19 had a worse prognosis than that triggered by other causes (21). Furthermore, Drake et al. reported that patients with COVID-19 with preexisting ILD had a significantly higher mortality than those without ILD (5). These data indicate that AE-IPF triggered by COVID-19 results in greater disease severity than idiopathic and other infection-triggered AE-IPF. Although the 90-day mortality rate of AE-ILD triggered by COVID-19 is especially high (75%), our patient recovered without severe disability with steroid pulse therapy from the early stage of the disease onset (21). These findings suggest that the early initiation of intensive treatment, including pulse steroid therapy, may be beneficial for patients with AE-IPF triggered by COVID-19.
In summary, we reported a patient with AE-IPF triggered by COVID-19 successfully treated with high-dose steroids. Both patients with COVID-19 and IPF are exposed to hyperinflammatory states. Therefore, high-dose steroids can be a useful treatment option for AE-IPF triggered by COVID-19 to control disease activity.
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images
The authors state that they have no Conflict of Interest (COI).
References
- 1. Horby P, Lim WS, Emberson JR, et al. ; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med 384: 693-704, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sterne JAC, Murthy S, Diaz JV, et al. ; The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between administration of systemic corticosteroids and mortality among critically Ill patients with COVID-19: a meta-analysis. JAMA 324: 1330-1341, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med 180: 1345-1355, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ioannou GN, Locke E, Green P, et al. Risk factors for hospitalization, mechanical ventilation, or death among 10131 US veterans with SARS-CoV-2 infection. JAMA Netw Open 3: e2022310, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drake TM, Docherty AB, Harrison EM, et al. Outcome of hospitalization for COVID-19 in patients with interstitial lung disease. An international multicenter study. Am J Respir Crit Care Med 202: 1656-1665, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collard HR, Moore BB, Flaherty KR, Brown KK, Kaner RJ, King TE Jr, et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 176: 636-643, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kondoh Y, Taniguchi H, Kawabata Y, Yokoi T, Suzuki K, Takagi K. Acute exacerbation in idiopathic pulmonary fibrosis. Analysis of clinical and pathologic findings in three cases. Chest 103: 1808-1812, 1993. [DOI] [PubMed] [Google Scholar]
- 8.Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An international working group report. Am J Respir Crit Care Med 194: 265-275, 2016. [DOI] [PubMed] [Google Scholar]
- 9. National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guideline [Internet]. [cited 2020 Feb 18]. Available from: https://www.covid19treatmentguidelines.nih.gov/. [PubMed]
- 10.Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edalatifard M, Akhtari M, Salehi M, et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID19 patients results from a randomised controlled clinical trial. Eur Respir J 56: 2002808, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitayama T, Kitamura H, Hagiwara E, et al. COVID-19 pneumonia resembling an acute exacerbation of interstitial pneumonia. Intern Med 59: 3207-3211, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webb BJ, Peltan ID, Jensen P, et al. Clinical criteria for COVID-19-associated hyperinflammatory syndrome: a cohort study. Lancet Rheumatol 2: e754-e763, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JH, Jang JH, Park JH, et al. The role of interleukin-6 as a prognostic biomarker for predicting acute exacerbation in interstitial lung diseases. PLoS One 16: e0255365, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arai TMH, Hirose M, Kida H, et al. Prognostic significance of serum cytokines during acute exacerbation of idiopathic interstitial pneumonias treated with thrombomodulin. BMJ Open Respir Res 8: e000889, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim DS, Park JH, Park BK, Lee JS, Nicholson AG, Colby T. Acute exacerbation of idiopathic pulmonary fibrosis: frequency and clinical features. Eur Respir J 27: 143-150, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Homma S, Bando M, Azuma A, et al. Japanese guideline for the treatment of idiopathic pulmonary fibrosis. Respir Investig 56: 268-291, 2018. [DOI] [PubMed] [Google Scholar]
- 18.Wootton SC, Kim DS, Kondoh Y, et al. Viral infection in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 183: 1698-1702, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.On R, Matsumoto T, Kushima H, Hirano R, Fujita M. Prevalence of viral infection in acute exacerbation of interstitial lung diseases in Japan. Respir Investig 58: 473-478, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song JW, Hong SB, Lim CM, Koh Y, Kim DS. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J 37: 356-363, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Kondoh Y, Kataoka K, Ando M, et al. COVID-19 and acute exacerbation of interstitial lung disease. Respir Investig 59: 675-678, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]