Abstract
We herein report a case of pneumocystis pneumonia (PCP) in a 77-year-old woman with ovarian cancer who was receiving olaparib therapy. After the patient's second relapse of ovarian cancer, she was administered olaparib as maintenance therapy following successful completion of docetaxel and carboplatin therapy. On receiving olaparib, she showed symptoms of a fever and malaise. Based on laboratory and imaging findings, she was diagnosed with PCP. After treatment with corticosteroids and trimethoprim/sulfamethoxazole followed by atovaquone, the patient's general condition improved. The lymphocytopenia observed after olaparib administration may have been associated with the development of PCP.
Keywords: pneumocystis pneumonia, ovarian cancer, olaparib, lymphocytopenia
Introduction
Pneumocystis pneumonia (PCP) is a life-threatening opportunistic infection in immunocompromised hosts. With the improvement in anti-human immunodeficiency virus (HIV) treatments and increase in the number of patients undergoing immunosuppressive therapy, PCP has become a common complication in non-HIV-infection cases, such as cases with leukemia and solid malignancies, like lung cancer and breast cancer (1).
Recently, poly [adenosine diphosphate (ADP)-ribose] polymerase (PARP) inhibitors have begun to be used as a new therapeutic approach in the management of ovarian cancer (2). Olaparib is the first PARP inhibitor to be approved for maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer. However, thus far, no PCP cases related to olaparib administration have been reported.
We herein report a case of PCP in a patient receiving olaparib subsequent to cytotoxic chemotherapy for ovarian cancer.
Case Report
A 77-year-old woman was referred to Miyazaki Prefectural Miyazaki Hospital for treatment of an abdominal tumor. She complained of a bloated stomach and epigastric pain. An ascites puncture was performed, and cytology revealed adenocarcinoma. The patient had undergone total hysterectomy, bilateral appendectomy, and partial omentectomy. Under the diagnosis of stage IV ovarian cancer, she received seven cycles of paclitaxel and carboplatin (TC) therapy as adjuvant chemotherapy.
Her ovarian cancer reoccurred 18 months after the adjuvant chemotherapy, with the splenic hilum as the recurrence site. Thus, she received six cycles of docetaxel and carboplatin (DC) therapy. Immediately after the fourth chemotherapy session, she had bacterial pneumonia, which improved with ampicillin/sulbactam therapy. Six months after the DC therapy, the ovarian cancer relapsed at the pancreatic head, para-aortic lymph nodes, liver, and splenic hilum. Thereafter, DC therapy was readministered in six cycles and was judged to be effective, so olaparib administration was started as a maintenance therapy. Two months after the start of the olaparib therapy, the patient complained of a fever, fatigue, and appetite loss. She visited our emergency department and was admitted to the gynecology department. She was suspected as having pneumonia and was given ceftriaxone, but her symptoms did not improve. The gynecologist consulted with the department of respiratory medicine regarding her medical condition. The patient was then transferred to the department of respiratory medicine for treatment.
Her vital signs were as follows: temperature, 39.2°C; blood pressure, 107/64 mmHg; pulse, 86 beats/min; and respiratory rate, 26 cycles/min with an O2 saturation of 96% in room air. No anomalies were observed upon auscultation. Hemogram results showed a white blood cell count of 1,350 cells/mm3 (70.0% neutrophils, 26.0% lymphocytes, 6.0% monocytes, 2.0% eosinophils, and 1.0% basophils) and C-reactive protein level of 4.34 mg/dL. The total protein (5.7 g/dL) and albumin levels (3.2 g/dL) were decreased. The Krebs von den Lungen-6 and surfactant protein D levels were elevated (551 U/mL and 138 ng/mL, respectively). The β-D-glucan levels were measured with a kinetic turbidimetric assay by employing the WAKO™ β-Glucan Test (Wako Pure Chemical Industries, Tokyo, Japan). The serum β-D-glucan level was 8.6 pg/mL. In addition, the renal and liver function test results were normal, as well as the CA125 level (26.0 IU/L; Table).
Table.
Laboratory Findings on Admission.
| Urinalysis | Biochemistry | Bacteriology | ||||||
| Protein | (-) | TP | 5.7 | g/dL | β-D-glucan | 8.6 | pg/mL | |
| Sugar | (-) | Alb | 3.2 | g/dL | ||||
| Occult Blood | (-) | T-Bil | 0.6 | mg/dL | Selogogy | |||
| AST | 19 | U/L | HBsAg | (-) | ||||
| Hematology | ALT | 10 | U/L | HCVAb | (-) | |||
| WBC | 1,350 | /mm3 | LDH | 208 | U/L | HIVAb | (-) | |
| Band | 4.0 | % | BUN | 9.4 | mg/dL | |||
| Seg | 61.0 | % | Cr | 0.62 | mg/dL | Tumor marker | ||
| Eo | 2.0 | % | CRP | 4.34 | mg/dL | CA125 | 26.0 | IU/L |
| Ba | 1.0 | % | Glu | 100 | mg/dL | |||
| Mo | 6.0 | % | KL-6 | 551 | U/mL | |||
| Ly | 26.0 | % | SP-D | 138 | ng/mL | |||
| IgG | 734 | mg/dL | ||||||
| Hb | 7.5 | g/dL | ||||||
| Ht | 21.4 | % | ||||||
| Plt | 4.7×104 | /mm3 | ||||||
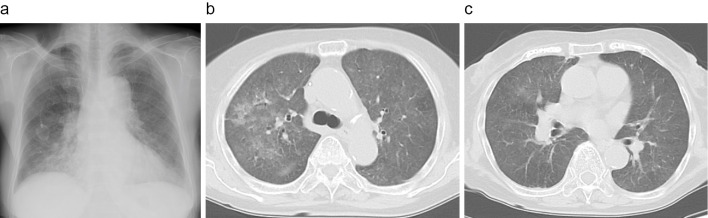
Chest radiography revealed ground-glass opacity (GGO) of the bilateral lung fields in addition to the known bronchiectasis of the mediastinal side of the lower right lung field. Chest computed tomography (CT) also revealed bilateral GGOs (Fig. 1). Based on these findings, we suspected PCP or drug-induced pneumonia. We therefore treated the patient with systemic corticosteroid therapy with oral prednisolone at 80 mg/day and trimethoprim/sulfamethoxazole (TMP/SMX; 15 mg/kg/day).
Figure 1.
Chest radiograph and computed tomography images on admission. a: Chest radiography findings showing bilateral diffuse ground-glass opacity. b, c: Chest computed tomography images showing bilateral diffuse ground-glass opacities with a subpleural-sparing pattern.
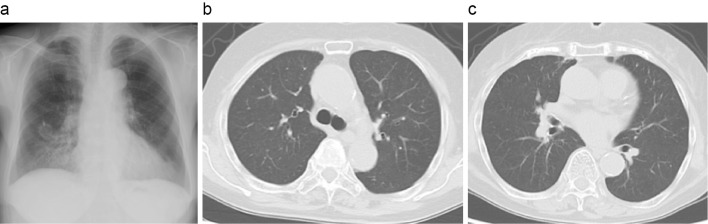
On the second day after the patient's transfer to our department, we performed bronchoalveolar lavage (BAL). An analysis of the BAL fluid (BALF) revealed a total cell count of 6.5×105/mL (histiocytes 53%, lymphocytes 38%, neutrophils 7%, and eosinophils 2%), with no malignant cells or pathogenic organisms detected. The treatments improved the patient's clinical condition. One week after bronchoscopy and BAL, polymerase chain reaction (PCR) of her BALF specimen was positive for Pneumocystis jirovecii DNA. Based on these results, she was diagnosed with PCP. We continued the administration of corticosteroids and TMP/SMX. The prednisolone dose was gradually tapered over three weeks. After seven days of administration, TMP/SMX was changed to atovaquone because of adverse events (appetite loss). The atovaquone therapy was continued for 14 days. After these treatments, the patient's general condition and chest X-ray photography and CT findings improved (Fig. 2), and the administration of PCP prophylaxis was also initiated with atovaquone. She did not wish to receive further treatment and was not re-administered olaparib.
Figure 2.
Chest radiograph and computed tomography images after treatment. a: Chest radiography. b, c: Chest computed tomography. Both images show improvement in bilateral diffuse ground-glass opacities.
Discussion
This is the first reported case of PCP in a patient receiving olaparib therapy. In the present case, the patient's chest radiograph presented with bilateral or diffuse GGO. In addition, high-resolution CT revealed diffuse GGO with a patchy distribution and subpleural sparing pattern. Although these imaging findings are relatively common in PCP (3), they are also found in interstitial pneumonitis, such as drug-induced lung injury. However, to our knowledge, there has been only one report of drug-induced lung injury due to olaparib. Thus, to exclude the possibility of drug-induced lung injury, we performed BAL for a definitive diagnosis of PCP.
The PCR assay of the patient's BALF specimen was positive for P. jirovecii DNA. Generally, microscopic detection of the organisms in respiratory specimens has been the golden standard for the diagnosis of PCP (4). However, non-HIV-infected patients with PCP have a lower burden of pneumocystis than HIV-positive patients, and the organisms are difficult to detect by a microscopic examination (4). In contrast, a PCR assay has 85-100% sensitivity and 79-96% specificity for the diagnosis of microscopically positive PCP (5,6). Owing to its high sensitivity, PCR assays are being used increasingly frequently for the microbiological diagnosis of PCP.
Although PCR testing has a high sensitivity, false positives are a critical problem. False positives for PCP-PCR have been reported in non-HIV immunocompromised patients (such as during steroid administration or immunosuppressive agents) and in patients with chronic respiratory diseases (7). However, in these reports, the subjects were clinically unsuspected of having PCP; therefore, bronchoscopy and PCR of BAL were performed to test for lung cancer, bacterial pneumonia, and other respiratory diseases. In addition, several reports have defined PCP-colonization (false positive) as PCP-PCR positivity in respiratory samples from patients without a clinical course of PCP or imaging findings (7,8). Based on these definitions, our case was considered at risk of developing PCP, had clinical features of PCP, and had characteristic imaging findings; therefore, we diagnosed our patient with PCP rather than as a false positive.
With respect to the present patient's β-D glucan levels, previous studies showed that the serum β-D glucan assay can be useful for the diagnosis of PCP (9). However, in non-HIV patients, pneumocystis burden levels have been reported to be very low. Regarding serum-β-D glucan, some studies have found that the β-D glucan value reflects the pneumocystis burden (10,11). Li et al. reported that the sensitivity of β-D glucan in non-HIV patients was relatively low compared with that in HIV-positive patients and, based on their meta-analysis, concluded that the decreased positivity rates might be explained by the lower burden of pneumocystis in non-HIV PCP individuals (6). In addition, Matsumura et al. reported that the sensitivity and specificity for discriminating probable PCP from colonization were 76.2% and 73.3%, respectively, at a cut-off of 6.0 pg/mL (12). Therefore, the low β-D glucan level in our case does not deny the possibility of PCP and is considered to reflect the low pneumocystis burden.
Recently, PCP has emerged as a threat to non-HIV-infected, immunocompromised patients, such as those receiving chemotherapy for hematological malignancies or solid tumors, and patients receiving immunosuppressive agents for organ transplantation or connective tissue diseases. Among patients with solid tumors, those with primary or metastatic brain tumors, lung cancer, and breast cancer are at a high risk for PCP (13). However, the occurrence of PCP in patients with ovarian cancer is extremely rare (14). In contrast, the administration of corticosteroids is considered a risk factor for developing PCP in non-HIV-infected patients (15). However, corticosteroids were not administered to the present patient.
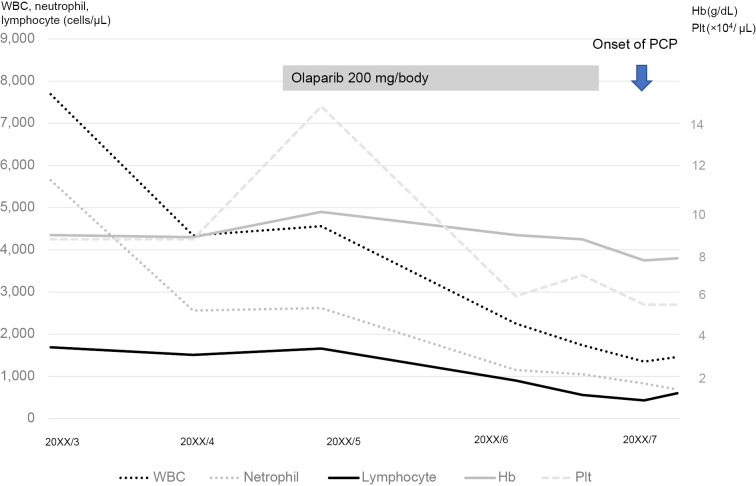
It is necessary to discuss the relationship between the intensity of chemotherapy in solid tumors and the risk of developing PCP. Dose-dense chemotherapy has also been reported to increase the risk of developing PCP in patients with breast cancer (16,17). According to these reports, after multiple chemotherapies, lymphopenia can lead to an increased risk for developing PCP. Although the present patient did not undergo intensive chemotherapy, lymphocytopenia was observed after olaparib administration, which may have been associated with the development of PCP (Fig. 3). Olaparib is an oral PARP inhibitor that has shown clinical effectiveness for ovarian (18) and breast cancers (19). Class-specific adverse effects include anemia, fatigue, and nausea, which are commonly associated with all available PARP inhibitors (2). As serious adverse effects, myelosuppression (anemia, neutropenia, leukopenia, thrombocytopenia, and lymphopenia) and interstitial lung disease have been reported. The frequency of olaparib-induced lymphopenia of G3 and higher has been reported to range from 0.5% to 4.3% (20,21). The incidence of lymphopenia due to olaparib is low, but careful monitoring is required.
Figure 3.
Clinical course of the patient. On initiation of olaparib administration, the number of lymphocytes began to gradually decrease.
Conclusions
This is the first reported case of PCP in a patient receiving olaparib therapy to treat ovarian cancer. Lymphocytopenia occurring after olaparib administration may be associated with the development of PCP. The incidence of lymphopenia due to olaparib is low, but careful monitoring is required.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Fillatre P, Decaux O, Jouneau S, et al. Incidence of Pneumocystis jiroveci pneumonia among groups at risk in HIV-negative patients. Am J Med 127: 1242.e11-1242.e17, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee S, Gonzalez-Martin A, Harter P, et al. First-line PARP inhibitors in ovarian cancer: summary of an ESMO Open - Cancer Horizons round-table discussion. ESMO Open 5: 1-10, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salzer HJF, Schäfer G, Hoenigl M, et al. Clinical, diagnostic, and treatment disparities between HIV-infected and non-HIV-infected immunocompromised patients with Pneumocystis jirovecii pneumonia. Respiration 96: 52-65, 2018. [DOI] [PubMed] [Google Scholar]
- 4.Azoulay É, Bergeron A, Chevret S, Bele N, Schlemmer B, Menotti J. Polymerase chain reaction for diagnosing pneumocystis pneumonia in non-HIV immunocompromised patients with pulmonary infiltrates. Chest 135: 655-661, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Tasaka S. Recent advances in the diagnosis and management of Pneumocystis pneumonia. Tuberc Respir Dis (Seoul) 83: 132-140, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li WJ, Guo YL, Liu TJ, Wang K, Kong JL. Diagnosis of pneumocystis pneumonia using serum (1-3)-β-D-Glucan: a bivariate meta-analysis and systematic review. J Thorac Dis 7: 2214-2225, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris A, Norris KA. Colonization by Pneumocystis jirovecii and its role in disease. Clin Microbiol Rev 25: 297-317, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calderón EJ. Pneumocystis infection: seeing beyond the tip of the iceberg. Clin Infect Dis 50: 354-356, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Tasaka S, Kobayashi S, Yagi K, et al. Serum (1→3) β-D-glucan assay for discrimination between Pneumocystis jirovecii pneumonia and colonization. J Infect Chemother 20: 678-681, 2014. [DOI] [PubMed] [Google Scholar]
- 10.Tokuda H, Sakai F, Yamada H, et al. Clinical and radiological features of Pneumocystis pneumonia in patients with rheumatoid arthritis, in comparison with methotrexate pneumonitis and Pneumocystis pneumonia in acquired immunodeficiency syndrome: a multicenter study. Intern Med 47: 915-923, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura H, Tateyama M, Tasato D, et al. Clinical utility of serum β-D-glucan and KL-6 levels in Pneumocystis jirovecii pneumonia. Intern Med 48: 195-202, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Matsumura Y, Ito Y, Iinuma Y, et al. Quantitative real-time PCR and the (1→3)-β-D-glucan assay for differentiation between Pneumocystis jirovecii pneumonia and colonization. Clin Microbiol Infect 18: 591-597, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Sepkowitz KA, Brown AE, Telzak EE, Gottlieb S, Armstrong D. Pneumocystis carinii pneumonia among patients without AIDS at a cancer hospital. JAMA J Am Med Assoc 267: 832-837, 1992. [PubMed] [Google Scholar]
- 14.Watanabe M, Aoki Y, Kurata H, Tanaka K. Pneumocystis carinii pneumonia in a patient with stage IV ovarian cancer. Gynecol. Oncol. 87: 225-227, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Vananuvat P, Suwannalai P, Sungkanuparph S, et al. Primary prophylaxis for Pneumocystis jirovecii pneumonia in patients with connective tissue diseases. Semin Arthritis Rheum 41: 497-502, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Tolaney SM, Najita J, Winer EP, Burstein HJ. Lymphopenia associated with adjuvant anthracycline/taxane regimens. Clin Breast Cancer 8: 352-356, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Waks AG, Tolaney SM, Galar A, et al. Pneumocystis jiroveci pneumonia (PCP) in patients receiving neoadjuvant and adjuvant anthracycline-based chemotherapy for breast cancer: incidence and risk factors. Breast Cancer Res Treat 154: 359-367, 2015. [DOI] [PubMed] [Google Scholar]
- 18.Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Obstet Gynecol Surv 69: 594-596, 2014. [DOI] [PubMed] [Google Scholar]
- 19.Shen M, Pan H, Chen Y, et al. A review of current progress in triple-negative breast cancer therapy. Open Med 15: 1143-1149, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 18: 1274-1284, 2017. [DOI] [PubMed] [Google Scholar]
- 21.Ray-Coquard I, Pautier P, Pignata S, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med 381: 2416-2428, 2019. [DOI] [PubMed] [Google Scholar]