Abstract
Objective
Human microRNA-185 (miR-185) has been reported to act as a regulator of fibrosis and angiogenesis in cancer. However, miR-185 has not been investigated in patients with ST-segment elevation myocardial infarction (STEMI). We hypothesized that the changes in miR-185 levels in STEMI patients are related to the processes of myocardial healing and remodeling.
Methods
Between January 2011 and December 2013, 145 patients with STEMI (65.9±11.6 years old; 41 women) were enrolled. Initial and discharge serum samples collected from 20 patients with STEMI and mixed sera from 8 healthy controls were analyzed by a microarray. A quantitative reverse transcription polymerase chain reaction (RT-qPCR) analysis of miR-185 was performed in all 145 patients. The correlation between the miR-185 levels and the clinical, laboratory, angiographic, and echocardiographic parameters was analyzed.
Results
The microarray analysis revealed a biphasic pattern in miR-185 levels, with an initial decrease followed by an increase at discharge. The miR-185 levels at discharge were significantly correlated with the troponin-I, CK-MB, and area under the curve of CK-MB levels. There was a positive correlation between the transforming growth factor-β and miR-185 levels at discharge (ρ=0.242, p=0.026). A high wall motion score index and a low ejection fraction, as measured by echocardiography, and high B-type natriuretic peptide level at one month after STEMI were related to high miR-185 levels.
Conclusion
Our results showed that elevated miR-185 levels at the late stage of STEMI were related to a large amount of myocardial injury and adverse remodeling.
Keywords: human microRNA, miR-185, myocardial infarction
Introduction
microRNAs (miRNAs) are small (approximately 22 nucleotides in length) endogenous non-coding RNA molecules that function as post-transcriptional regulators of the gene expression (1). miRNAs have been detected in a wide range of body fluids, including serum and plasma, and the stability of circulating miRNAs has been demonstrated (1,2).
Because of their stability, many circulating miRNAs have been evaluated as potential biomarkers for the diagnosis and prediction of outcomes. In particular, numerous recent studies have investigated the utility of miRNAs as diagnostic and prognostic biomarkers in patients with acute myocardial infarction (AMI) (1,3-16). The healing process following AMI is complex, and many cell types, including inflammatory cells and fibroblasts, are recruited. In addition, the expression of numerous genes may be altered, and many miRNAs may be involved in these alterations. Therefore, the miRNAs involved in major myocardial healing may be crucial biomarkers for left ventricular (LV) remodeling and ensuing heart failure.
miR-185 has been reported to be involved in the migration and proliferation of cancer and inflammatory cells as well as in calcium homeostasis (17-21), which might be closely related to the cardiac healing process after AMI. However, to our knowledge, miR-185 levels have not been investigated in patients with AMI, specifically patients with ST-segment elevation myocardial infarction (STEMI).
The present study profiled the changes in miR-185 levels and investigated the relationship between these levels and LV remodeling parameters using echocardiography and known biomarkers of myocardial injury in patients with STEMI.
Materials and Methods
Patients
Between January 2011 and December 2013, 145 patients with STEMI were enrolled in the study. All patients were ≥18 years old and had been diagnosed with STEMI based on electrocardiogram findings and elevated levels of troponin-I. All individuals had had their first STEMI and achieved complete revascularization by primary percutaneous coronary intervention (PCI).
Blood samples were obtained before the primary PCI and at discharge (median duration: 4 days) and were stored at -80℃ until the analysis. The patients were treated according to the American College of Cardiology Foundation/American Heart Association (ACC/AHA) STEMI guidelines with statins, β-blockers, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and dual antiplatelet agents (22). In the initial screening for miRNAs that showed significant changes after STEMI, the miRNA expression profiles of patients with STEMI and healthy controls were compared. Of the 145 patients, 20 with STEMI who had no history of PCI, chronic kidney disease, diabetes, or ischemic stroke were selected, and their sera were analyzed by a microarray. For the control group (n=8; mean age, 57 years old; 4 women), the sera from patients with normal coronary arteries were analyzed by a microarray. The control subjects underwent conventional coronary angiography for atypical chest discomfort. Two patients in the control group had hypertension. None had a history of diabetes or any cardiovascular disease.
After selecting miR-185 as the focus of this study, quantitative reverse transcription polymerase chain reaction (RT-qPCR) assays were performed on samples from all enrolled patients with STEMI (n=145). All blood samples for this study were supplied by the Gyeongsang National University Biobank. Clinical, angiographic, and echocardiographic parameters were collected from the medical records of the study patients, and these parameters were analyzed for correlations with the miR-185 levels.
Institutional review board approval was obtained, and informed consent was waived for this study.
microRNA analyses
For the miRNA microarrays, we collected 3 pools of serum samples: 1 from the 20 selected patients with STEMI at admission, another from the same patients at discharge, and the last from the 8 controls. Each set of pooled 0.2 mL serum samples were evaluated with an Affymetrix miRNA 4.0 Array (GenoCheck, Ansan, South Korea). Serum miRNAs were isolated with the MicroRNA Extraction Kit (217184; QIAGEN, Hilden, Germany). Isolated miRNAs were reverse-transcribed using the TaqMan MicroRNA Reverse Transcription Kit (4366596; Applied Biosystem, Waltham, USA) according to the manufacturer's instructions. qPCR was performed using the TaqMan MicroRNA PCR Kit (4366596, 4440040; Applied Biosystem) and the miR-185 primer assay (002271; Applied Biosystem). The expression of the miRNAs was normalized to the level of the U6 small nuclear RNA (6,13,14).
Analyses of 20 selected patients were performed in triplicate to validate the qPCR findings. The levels of miR-185 and U6 did not vary significantly among individual samples. miRNAs were detected using the ABI 7500 Fast Real-Time PCR System (Applied Biosystems) by real-time monitoring of the increase in fluorescence of the TaqMan detector. miR-185 levels were calculated using the 2-ΔΔCT method (23).
Biomarker measurements
B-type natriuretic peptide (BNP) and C-reactive peptide levels were evaluated at admission. BNP levels were also evaluated at the one-month follow-up. We also estimated the amount of infarcted myocardium by measuring the creatine kinase-MB (CK-MB) and troponin-I levels. The peak value and area under the curve (AUC) of CK-MB were calculated. The AUC of the CK-MB levels was determined by the linear-trapezoidal method using the levels at baseline and at 6, 12, 18, 24, and 48 hours after admission (21). The transforming growth factor-β (TGF-β) levels in 84 randomly selected patients were analyzed using the same serum used for the miR-185 analysis. The TGF-β levels were determined using the TGF-β1 Quantikine ELISA Kit (R&D Systems, Minneapolis, USA).
Echocardiographic parameters
Echocardiographic parameters were assessed in the patients at admission and at follow-up one month after STEMI. The LV end diastolic volume (LVEDV), LVEDV index (LVEDVI; LVEDV/body surface area), LV end systolic volume (LVESV), LVESV index (LVESVI; LVESV/body surface area), stroke volume (LVEDV minus LVESV), LV ejection fraction (LVEF) by Simpson's method, and wall motion score index (WMSI) were also assessed in this study. The WMSI was calculated by dividing the sum of the scores by the number of segments visualized. Scores were assigned as follows: a normally contracting segment was assigned a score of 1; hypokinesis, 2; akinesis, 3; dyskinesis, 4; and aneurysmal, 5.
Statistical analyses
Statistical analyses were performed using the SPSS software program for Windows, version 21 (IBM Corporation, Chicago, USA). Continuous variables are presented as means ± standard deviations or median and interquartile range, and categorical variables are presented as absolute values and proportions (%). Correlations between the miRNA-185 levels and clinical, echocardiographic, and laboratory variables were analyzed using the Mann-Whitney U test. All p values refer to 2-tailed tests of significance, and values less than 0.05 were considered statistically significant.
Results
Temporal changes in miR-185 levels
To determine the temporal changes in the miRNAs as potential biomarkers, we analyzed samples collected at admission and discharge. Among the 2,578 serum miRNAs detected by an Affymetrix miRNA 4.0 Array, 27 showed a more than 2-fold difference between patients with STEMI and controls using any sample at admission and/or discharge (Table 1). The changes in the miRNA expression were grouped into three patterns. The first group, which included miR-3620-5p and miR-4487, had depressed values at both admission and discharge. The second group, which included miR-25-3p, miR-185-5p, and miR-1246, had initially depressed values that became elevated at discharge. The third group, which included all remaining miRNAs, had elevated values at the PCI stage that were maintained through discharge.
Table 1.
MicroRNAs with More than a Two-fold Change on the Microarray.
| At Admission | At Discharge | |
|---|---|---|
| Class 1 | Depressed | Depressed |
| miR-3620-5p | -1.674 | -2.195 |
| miR-4487 | -2.733 | -2.360 |
| Class 2 | Depressed | Elevated |
| miR-25-3p | -0.085 | 2.230 |
| miR-185-5p | 0.603 | 2.286 |
| miR-1246 | 0.097 | 3.656 |
| Class 3 | Elevated | Elevated |
| let-7b-5p | 3.399 | 5.545 |
| let-7c-5p | 1.973 | 3.847 |
| miR-16-5p | 2.273 | 5.380 |
| miR-92a-3p | 3.780 | 5.257 |
| miR-320a | 2.053 | 2.210 |
| miR-320b | 1.864 | 2.134 |
| miR-320c | 1.945 | 2.309 |
| miR-486-5p | 5.121 | 6.906 |
| miR-762 | 2.012 | 2.763 |
| miR-1237-5p | 2.290 | 2.568 |
| miR-3178 | 2.661 | 3.983 |
| miR-3613-3p | 2.817 | 2.872 |
| miR-3656 | 2.876 | 2.864 |
| miR-3665 | 4.508 | 3.300 |
| miR-3960 | 2.904 | 2.380 |
| miR-4466 | 3.744 | 3.440 |
| miR-4484 | 7.825 | 6.744 |
| miR-4668-5p | 2.448 | 2.580 |
| miR-6087 | 3.096 | 2.526 |
| miR-6089 | 3.553 | 2.871 |
| miR-6090 | 4.840 | 4.280 |
| miR-6126 | 2.746 | 2.441 |
Association of clinical and laboratory findings with the miR-185 levels at discharge
The average age of the patients was 65.9±11.6 years old. Almost all the patients were in Killip class I (85.5%), and multi-vessel disease was detected in 76 patients (52.4%). The 145 total patients were divided into 2 groups based on the miR-185 levels at discharge: the decreased miR-185 group (≤1.0) and the elevated miR-185 group (>1.0). The miR-185 levels were not significantly correlated with most clinical variables, including the medical history, coronary artery characteristics, and initial vital signs, although a correlation with a history of ischemic stroke was noted (Table 2).
Table 2.
Clinical Characteristics and Levels of miR-185 (n=145).
| Characteristics | Decreased miR-185 (n=38) |
Elevated miR-185 (n=107) |
p value |
|---|---|---|---|
| Age (mean±SD, years) | 65.0±9.5 | 66.4±12.2 | 0.530 |
| Females, n (%) | 9 (23.7%) | 32 (29.9%) | 0.534 |
| Body mass index (kg/m2) | 24.2±2.7 | 24.1±2.9 | 0.775 |
| Hypertension, n (%) | 15 (39.5%) | 56 (52.3%) | 0.191 |
| Diabetes mellitus, n (%) | 9 (23.7%) | 27 (25.2%) | 1.000 |
| Currently smoking, n (%) | 12 (31.6%) | 43 (40.2%) | 0.641 |
| Past medical history, n (%) | |||
| Ischemic heart disease | 2 (5.3%) | 13 (12.1%) | 0.355 |
| Heart failure | 0 (0%) | 0 (0%) | - |
| Revascularization | 2 (5.3%) | 9 (8.4%) | 0.728 |
| Ischemic stroke | 4 (10.5%) | 2 (1.9%) | 0.041 |
| Chronic kidney disease | 0 (0%) | 3 (2.8%) | 0.567 |
| SBP (mmHg) | 127.3±28.1 | 135.1±31.0 | 0.178 |
| DBP (mmHg) | 75.7±14.4 | 79.0±17.4 | 0.308 |
| Heart rate (beats/min) | 74.2±15.5 | 76.7±17.3 | 0.433 |
| Killip class, n (%) | 0.775 | ||
| class I | 34 (89.5%) | 90 (84.1%) | |
| class II | 1 (2.6%) | 7 (6.5%) | |
| class III | 1 (2.6%) | 3 (2.8%) | |
| class IV | 2 (5.3%) | 8 (7.5%) | |
| Infarct related artery, n (%) | 0.184 | ||
| Left main | 1 (2.6%) | 4 (3.7%) | |
| Left anterior descending | 18 (47.4%) | 54 (50.4%) | |
| Left circumflex | 2 (5.3%) | 16 (15.0%) | |
| Right coronary artery | 18 (47.4%) | 37 (34.6%) | |
| Multi-vessel disease, n (%) | 18 (47.4%) | 58 (54.2%) | 0.571 |
DBP: diastolic blood pressure, SBP: systolic blood pressure
In the initial echocardiographic findings, the group with elevated miR-185 levels showed a higher WMSI and lower ejection fraction than those with decreased miR-185 levels (WMSI: 1.4±0.3 vs. 1.2±0.2, p=0.004; ejection fraction: 50.2%±7.9% vs. 53.8%±8.4%, p=0.020, respectively). The relationships between the clinical parameters at one month after STEMI and miR-185 showed similar findings (Table 3). There was no significant correlation between the miR-185 levels and the LV volume or stroke volume. The interval changes in echocardiographic parameters did not differ markedly between the decreased and increased miR-185 groups (Table 3). Furthermore, there were no significant differences in the clinical outcomes, including cardiac death, hospitalization for heart failure, cardiac ischemic events, and all-cause death, between the groups (Table 4).
Table 3.
Echocardiographic Parameters and Levels of miR-185 (n=145).
| Parameters | Decreased miR-185 (n=38) |
Elevated miR-185 (n=107) |
p value |
|---|---|---|---|
| Initial echocardiographic exam | |||
| End-diastolic volume index, mL/m3 | 50.8±12.8 | 53.9±13.8 | 0.237 |
| End-systolic volume index, mL/m3 | 23.7±9.2 | 27.1±10.1 | 0.071 |
| Stroke volume, mL | 46.4±12.5 | 45.6±13.2 | 0.744 |
| Wall motion score index | 1.2±0.2 | 1.4±0.3 | 0.004 |
| Ejection fraction, % | 53.8±8.4 | 50.2±7.9 | 0.020 |
| 1 month follow-up exam | |||
| End-diastolic volume index, mL/m3 | 54.8±16.7 | 57.3±14.7 | 0.383 |
| End-systolic volume index, mL/m3 | 25.1±12.3 | 28.1±11.1 | 0.164 |
| Stroke volume, mL | 50.6±11.6 | 49.8±13.3 | 0.757 |
| Wall motion score index | 1.2±0.2 | 1.3±0.2 | 0.021 |
| Ejection fraction, % | 55.4±7.6 | 51.7±8.2 | 0.018 |
| Interval changes | |||
| LVEDV percent change, % | 4.3±19.4 | 3.8±21.3 | 0.869 |
| LVESV percent change, % | 7.2±28.3 | 9.3±40.4 | 0.780 |
| Stroke volume, mL | 4.2±11.8 | 4.2±11.6 | 0.983 |
| Ejection fraction, % | 1.6±5.7 | 1.5±6.2 | 0.946 |
Table 4.
Clinical Outcomes and Levels of miR-185 (n=145).
| Events, n (%) | Decreased miR-185 (n=38) |
Elevated miR-185 (n=107) |
p value |
|---|---|---|---|
| Cardiac death | 2 (5%) | 2 (2%) | 0.281 |
| Hospitalization for heart failure | 0 (0%) | 4 (4%) | 0.573 |
| Cardiac ischemic events | 6 (16%) | 14 (13%) | 0.785 |
| All major cardiovascular events | 6 (16%) | 18 (17%) | 1.000 |
| All cause of death | 4 (11%) | 4 (4%) | 0.207 |
Cardiac ischemic events include cardiac death, myocardial infarction, and coronary revascularization. All major cardiovascular events are cardiac ischemic events and ischemic stroke.
The correlation of biomarkers for myocardial injury and TGF-β with the miR-185 levels at discharge
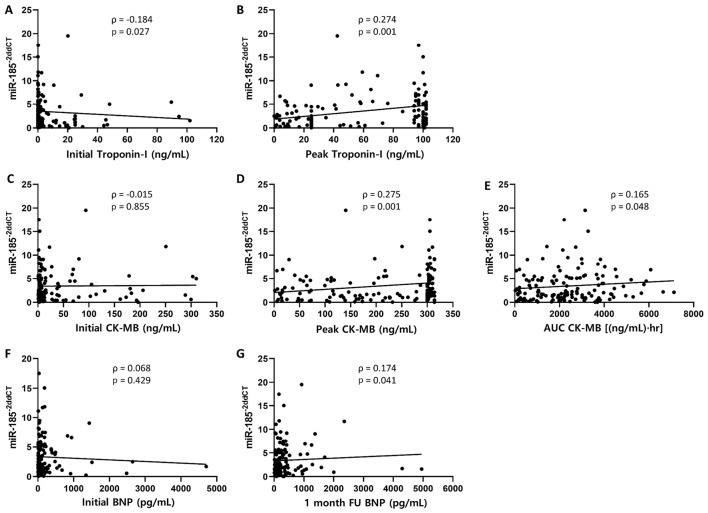
The miR-185 levels were significantly correlated with the levels of peak troponin-I, peak CK-MB, and total CK-MB (Spearman correlation ρ=0.274, p=0.001; ρ=0.275, p=0.001; and ρ=0.165, p=0.048, respectively; Fig. 1). The levels of BNP, a biomarker used for heart failure, were not correlated with the miR-185 levels at admission, but the BNP levels after 1 month showed a weak correlation with the miR-185 levels (ρ=0.174, p=0.041, Fig. 1). The analysis of the relationship between the elevated miR-185 group and the decreased miR-185 group showed a similar tendency (Table 5). The TGF-β levels of 84 randomly selected patients were analyzed using the same sera used to evaluate miR-185. The miR-185 levels at discharge were positively correlated with the TGF-β levels (ρ=0.242, p=0.026, Fig. 2).
Figure 1.
Biomarkers related to myocardial injury and miR-185 levels (n=145). Peak levels of troponin-I and CK-MB were positively correlated with the levels of miR-185 at discharge (Spearman’s correlation ρ=0.274, p=0.001 and ρ=0.275, p=0.001, respectively; B and D). The AUC of the CK-MB levels during hospitalization suggests that the total myocardial injury was weakly correlated with the miR-185 levels (ρ=0.165, p=0.048, E). The levels of BNP at 1-month follow-up (FU) were positively correlated with the miR-185 levels at discharge (ρ=0.174, p=0.041, G).
Table 5.
Biomarkers for Myocardial Injury and Levels of miR-185 (n=145).
| Parameters, (median, IQR) | Decreased miR-185 (n=38) |
Elevated miR-185 (n=107) |
p value |
|---|---|---|---|
| Troponin-I, initial, ng/mL | 0.7 (0.1, 5.9) | 0.2 (0.1, 3.1) | 0.076 |
| Troponin-I, peak, ng/mL | 25.0 (25.0, 59.0) | 62.1 (25.0, 97.0) | 0.017 |
| CK-MB, initial, ng/mL | 8.2 (3.0, 33.5) | 6.1 (3.1, 23.6) | 0.653 |
| CK-MB, peak, ng/mL | 165.9 (51.8, 289.7) | 293.3 (114.3, 303.0) | 0.003 |
| CK-MB, AUC, (ng/mL)·hr | 2,052.3 (689.4, 3,137.6) | 2,318.7 (1,195.3, 3,553.5) | 0.053 |
| BNP, initial, pg/mL | 40.0 (16.5, 140.0) | 51.5 (24.0, 184.3) | 0.218 |
| BNP, 1 month, pg/mL | 143.0 (108.0, 390.8) | 204.5 (108.0, 390.8) | 0.038 |
IQR: interquartile range, AUC: area under the curve, CK: creatine kinase, BNP: B-type natriuretic peptide
Figure 2.

Relationship between miR-185 and TGF-β (n=84). The miR-185 levels were positively correlated with the TGF-β levels (ρ=0.242, p=0.026).
Discussion
To our knowledge, this study is the first to report the changes in circulating miR-185 levels in patients with STEMI. The important findings of this investigation were as follows: (1) miR-185 was downregulated at the early stage after STEMI and upregulated at discharge (median four days after STEMI); (2) the miR-185 levels were significantly correlated with the peak troponin-I and both peak and total CK-MB, which are markers of the extent of myocardial injury; (3) a high WMSI, low ejection fraction, and high BNP levels after one month, which are known indicators of heart failure, were correlated with high levels of miR-185 at discharge; and (4) the miR-185 levels were significantly correlated with the levels of TGF-β, a key molecule in the beginning stage of infarcted myocardial healing.
Over the last decade, many studies have reported the involvement of miRNAs after AMI. The prognostic values of several miRNAs after AMI have been investigated, including miR-155 and miR-390 at discharge for 1-year cardiac death (10), miR-208b and miR-499-5p for heart failure and 30-day mortality (11), miR-150 for LV remodeling (12), and miR-208b and miR-34a for mortality or heart failure within 6 months (15). In previous studies of patients with AMI, blood was collected at different time points for the analysis of miRNAs. In most investigations, the blood samples were obtained just once, in a stable state, such as immediately before discharge, at discharge, or at five days after PCI. We hypothesized that the miRNA levels may possibly change dynamically during the healing stage, such as in the early inflammatory, proliferation, and maturation phases. Our microarray data showed that the miRNAs did indeed change over time and they were able to be grouped into three patterns. Among changed miRNAs, the levels of miR-25-3p, miR-185-5p, and miR-1246 decreased before PCI and significantly increased just before discharge, suggesting that these miRNAs are involved in the early inflammatory phase as well as the early proliferation phase of myocardial healing and post-AMI LV remodeling. Previous studies investigating miRNAs were conducted using sera or plasma to identify diagnostic biomarkers of AMI at the emergency department or at the time of PCI (5,9). miR-185, with its mildly reduced level in the acute phase of AMI, was not the focus of those studies, in contrast with the present study.
miR-185 has been implicated in various cancers (17-21). Many groups have reported that miR-185 was able to suppress the progression and migration of cells and alter the expression of genes related to angiogenesis (18,21). A recent report supported a direct negative correlation between miR-185 and stromal interaction molecule 1 (STIM1) in microvascular endothelial cells (24). STIM1, which is an endoplasmic reticulum calcium sensor, plays an important role in epithelial-to-mesenchymal transition, invasion, metastasis, and angiogenesis. Lei et al. (25) reported that in idiopathic pulmonary fibrosis, miR-185 blocked TGF-β-induced collagen V overexpression and alleviated TGF-β-induced epithelial to mesenchymal transition in alveolar epithelial cell lines. Kim et al. (26) showed that miR-185 exerted anti-hypertrophic effects in a transverse aortic construction model by modulating calcium signaling pathways, including nuclear factor of activated T (NFAT) cells and calcium/calmodulin-dependent protein kinase II delta (CaMKIIδ). Furthermore, another report showed that cardiomyocyte apoptosis due to prolonged endoplasmic reticulum stress was significantly reduced by miR-185 overexpression via direct targeting of Na+/H+ exchanger-1, a protein involved in endoplasmic reticulum stress (27). miR-185 is known to be expressed in cardiomyocytes, endothelial cells, and epithelial cells (25,28) and regulates angiogenesis, fibrosis, and apoptosis under various conditions (24-28). Although its direct targets and actions in AMI are unclear, especially in STEMI, miR-185 may be a modulator in the healing process accompanied by angiogenesis and fibrosis after AMI. Therefore, we focused on miR-185, and our data showed that the elevated miR-185 levels in the early stage of myocardial healing after STEMI responded to a large amount of injured myocardium, as demonstrated by cardiac enzyme levels and echocardiographic parameters. TGF-β is an important mediator of fibrosis and angiogenesis during myocardial healing after AMI (29). A previous study showed that miR-185 repressed the TGF-β expression (25). However, the present study showed that the TGF-β levels were positively correlated with miR-185 levels at discharge. The mechanistic relationship between miR-185 and TGF-β in infarcted myocardium remains unclear. We postulated that the TGF-β expression is influenced by a variety of biologic stimuli in the healing of infarcted myocardium, and elevated miR-185 levels might reflect a compensatory feedback mechanism for increased TGF-β (30).
Ho et al. (31) demonstrated that during hypoxia, the levels of miR-185 were decreased, whereas the levels of the miR-185 precursors were increased. In our study, the miR-185 levels were consistently decreased at PCI and increased at discharge, which might reflect the accumulation of precursors. Thus, the elevated levels may be related to the degree of initial hypoxic damage and the decreased LV systolic function. In contrast, Yu et al. (32) reported that the miR-185 levels were higher in patients with dilated cardiomyopathy than in controls. Among the patients with dilated cardiomyopathy, the group with high miR-185 levels showed a more favorable prognosis at the one-year follow-up than the group with low miR-185 levels. In contrast to the role of miR-185 in dilated cardiomyopathy characterized by chronic inflammation and progressive fibrosis, we suggest that the prognostic role of miR-185 may differ in myocardial infarction characterized by acute inflammation and intensive fibrosis. We also suggest that the prognostic role of the miR-185 level may differ based on the measurement timing, as our study results showed. However, we were unable to define the relationship between the miR-185 levels and clinical outcomes within one year or echocardiographic LV remodeling after one month. The prognostic role of high miR-185 levels after AMI might need to be determined in a larger prospective clinical study in the future.
Limitations
Several limitations associated with the present study warrant mention. First, we did not validate the miR-185 levels at admission. Because the timing of the symptom onset to hospital visit for AMI varied, we decided to evaluate the miR-185 levels at discharge, meaning that the patients in this study were stabilized. Second, this study used clinical data obtained from a single hospital center, so the number of patients and major clinical adverse outcomes were too small for statistical analyses. The small number of adverse outcomes in particular was attributed to enrolling patients after they had successfully undergone primary PCI. The predictive value of miR-185 for long-term outcomes was indeterminable, and the correlation between adverse remodeling parameters and the miR-185 levels was ultimately weak. Therefore, a large number of multi-center clinical studies will be needed to confirm these results. Finally, the Ct values of miR-185 were normalized relative to the amount of U6 in this study. U6 has been widely used for the normalization of microRNA levels in previous studies of myocardial infarction (9,13,14). However, it has also been reported that U6 is unsuitable for the normalization of serum microRNA levels under various disease conditions (33,34), as traditional reference genes, including U6, can be degraded in serum samples. In the present study, to exclude the influence of the degradation of U6 in serum samples, all qPCR procedures were performed in triplicate on different days.
Conclusion
Elevated circulating miR-185 levels in patients with late-stage STEMI were related to large amounts of myocardial injury and adverse remodeling parameters. miR-185 might be a useful biomarker for predicting LV remodeling after STEMI.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This work was supported by Kuhnil Pharmaceutical.
Acknowledgement
The biospecimens and clinical data used in this study were provided by Gyeongsang National University Hospital, a member of the Korea Biobank Network.
References
- 1.Romaine SP, Tomaszewski M, Condorelli G, Samani NJ. MicroRNAs in cardiovascular disease: an introduction for clinicians. Heart 101: 921-928, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 105: 10513-10518, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zile MR, Mehurg SM, Arroyo JE, Stroud RE, DeSantis SM, Spinale FG. Relationship between the temporal profile of plasma microRNA and left ventricular remodeling in patients after myocardial infarction. Circ Cardiovasc Genet 4: 614-619, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devaux Y, Vausort M, McCann GP, et al. A panel of 4 microRNAs facilitates the prediction of left ventricular contractility after acute myocardial infarction. PLoS One 8: e70644, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jakob P, Kacprowski T, Briand-Schumacher S, et al. Profiling and validation of circulating microRNAs for cardiovascular events in patients presenting with ST-segment elevation myocardial infarction. Eur Heart J 38: 511-515, 2017. [DOI] [PubMed] [Google Scholar]
- 6.Ai J, Zhang R, Li Y, et al. Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction. Biochem Biophys Res Commun 391: 73-77, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Wang GK, Zhu JQ, Zhang JT, et al. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J 31: 659-666, 2010. [DOI] [PubMed] [Google Scholar]
- 8.Devaux Y, Vausort M, Goretti E, et al. Use of circulating microRNAs to diagnose acute myocardial infarction. Clin Chem 58: 559-567, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Devaux Y, Mueller M, Haaf P, et al. Diagnostic and prognostic value of circulating microRNAs in patients with acute chest pain. J Intern Med 277: 260-271, 2015. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto S, Sakata Y, Nakatani D, et al. A subset of circulating microRNAs are predictive for cardiac death after discharge for acute myocardial infarction. Biochem Biophys Res Commun 427: 280-284, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Gidlöf O, Smith JG, Miyazu K, et al. Circulating cardio-enriched microRNAs are associated with long-term prognosis following myocardial infarction. BMC Cardiovasc Disord 13: 12, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devaux Y, Vausort M, McCann GP, et al. MicroRNA-150: a novel marker of left ventricular remodeling after acute myocardial infarction. Circ Cardiovasc Genet 6: 290-298, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Zile MR, Mehurg SM, Arroyo JE, Stroud RE, DeSantis SM, Spinale FG. Relationship between the temporal profile of plasma microRNA and left ventricular remodeling in patients after myocardial infarction. Circ Cardiovasc Genet 4: 614-619, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X, Dong Y, Chen S, et al. Circulating microRNA-146a and microRNA-21 predict left ventricular remodeling after ST-elevation myocardial infarction. Cardiology 132: 233-241, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Lv P, Zhou M, He J, et al. Circulating miR-208b and miR-34a are associated with left ventricular remodeling after acute myocardial infarction. Int J Mol Sci 15: 5774-5788, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong YM, Liu XX, Wei GQ, Da YN, Cha L, Ma CS. Prediction of long-term outcome after acute myocardial infarction using circulating miR-145. Scand J Clin Lab Invest 75: 85-91, 2015. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi Y, Forrest AR, Maeno E, Hashimoto T, Daub CO, Yasuda J. MiR-107 and miR-185 can induce cell cycle arrest in human non-small cell lung cancer cell lines. PLoS One 4: e6677, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qu F, Cui X, Hong Y, et al. MicroRNA-185 suppresses proliferation, invasion, migration, and tumorigenicity of human prostate cancer cells through targeting androgen receptor. Mol Cell Biochem 377: 121-130, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Tan Z, Jiang H, Wu Y, et al. miR-185 is an independent prognosis factor and suppresses tumor metastasis in gastric cancer. Mol Cell Biochem 386: 223-231, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, Liu X, Feng B, et al. STIM1, a direct target of microRNA-185, promotes tumor metastasis and is associated with poor prognosis in colorectal cancer. Oncogene 34: 4808-4820, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma X, Shen D, Li H, et al. MicroRNA-185 inhibits cell proliferation and induces cell apoptosis by targeting VEGFA directly in von Hippel-Lindau-inactivated clear cell renal cell carcinoma. Urol Oncol 33: 169.e1-e11, 2015. [DOI] [PubMed] [Google Scholar]
- 22. Wright RS, Anderson JL, Adams CD, et al. ; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American Academy of Family Physicians, Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons. J Am Coll Cardiol 57: e215-e367, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT Method. Methods 25: 402-408, 2011. [DOI] [PubMed] [Google Scholar]
- 24.Hou J, Liu L, Zhu Q, et al. MicroRNA-185 inhibits angiogenesis in human microvascular endothelial cells by targeting stromal interaction molecule 1. Cell Biol Int 40: 318-328, 2016. [DOI] [PubMed] [Google Scholar]
- 25.Lei GS, Kline HL, Lee CH, Wilkes DS, Zhang C. Regulation of collagen V expression and epithelial-mesenchymal transition by miR-185 and miR-186 during idiopathic pulmonary fibrosis. Am J Pathol 186: 2310-2316, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JO, Song DW, Kwon EJ, et al. miR-185 plays an anti-hypertrophic role in the heart via multiple targets in the calcium-signaling pathways. PLoS One 10: e0122509, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JO, Kwon EJ, Song DW, Lee JS, Kim DH. miR-185 inhibits endoplasmic reticulum stress-induced apoptosis by targeting Na+/H+ exchanger-1 in the heart. BMB Rep 49: 208-213, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li CC, Qiu XT, Sun Q, et al. Endogenous reduction of miR-185 accelerates cardiac function recovery in mice following myocardial infarction via targeting of cathepsin K. J Cell Mol Med 23: 1164-1173, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bujak M, Frangogiannis N. The role of TGF-β signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res 74: 184-195, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang S, Raghavachari S. Quantifying negative feedback regulation by micro-RNAs. Phys Biol 8: 055002, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho JJ, Metcalf JL, Yan MS, et al. Functional importance of Dicer protein in the adaptive cellular response to hypoxia. J Biol Chem 287: 29003-29020, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu M, Liang W, Xie Y, et al. Circulating miR-185 might be a novel biomarker for clinical outcome in patients with dilated cardiomyopathy. Sci Rep 6: 33580, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Felekkis K, Papaneophytou C. Challenges in using circulating micro-RNAs as biomarkers for cardiovascular diseases. Int J Mol Sci 21: 561, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benz F, Roderburg C, Cardenas DV, et al. U6 is unsuitable for normalization of serum miRNA levels in patients with sepsis or liver fibrosis. Exp Mol Med 20: e42, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]