Abstract
The core function of hematopoietic stem and progenitor cells (HSPCs) is to provide lifelong production of all lineages of the blood and immune cells. The mechanisms that modulate HSPC homeostasis and lineage biasing are not fully understood. Growing evidence implicates the aryl hydrocarbon receptor (AHR), an environment-sensing transcription factor, as a regulator of hematopoiesis. AHR ligands modulate the frequency of mature hematopoietic cells in the bone marrow and periphery, while HSPCs from mice lacking AHR (AHR KO) have increased proliferation. Yet, whether AHR modulates HSPC lineage potential and directs differentiation toward specific lineage-biased progenitors is not well understood. This study revealed that AHR KO mice have an increased proportion of myeloid-biased HSCs and myeloid-biased multipotent progenitor (MPP3) cells. Utilizing inducible AHR knockout mice (iAHR KO), it was discovered that acute deletion of AHR doubled the number of MPP3 cells and altered the composition of downstream lineage-committed progenitors, such as increased frequency of pregranulocyte/premonocyte committed progenitors. Furthermore, in vivo antagonism of the AHR led to a 2.5-fold increase in the number of MPP3 cells and promoted myeloid-biased differentiation. Using hematopoietic-specific conditional AHR knockout mice (AHRVav1) revealed that increased frequency of myeloid-biased HSCs and myeloid-biased progenitors is driven by AHR signaling that is intrinsic to the hematopoietic compartment. These findings demonstrate that the AHR plays a pivotal role in regulating steady-state hematopoiesis, influencing HSPC homeostasis and lineage potential. In addition, the data presented provide potential insight into how deliberate modulation of AHR signaling could help with the treatment of a broad range of diseases that require the hematopoietic compartment.
Keywords: HSC, AHR, lineage biasing, differentiation, hematopoiesis
Introduction
Hematopoiesis is the tightly regulated process by which cells of the blood, including circulating immune cells, arise from hematopoietic stem and progenitor cells (HSPCs). Maintaining HSPCs is essential for continuously producing and replenishing blood and immune cells over the entire lifespan. HSPCs consist of hematopoietic stem cells (HSCs) and multipotent progenitor (MPP) cells. HSCs are rare multipotent cells that self-renew and also generate an entirely new hematopoietic system [1]. HSCs are generally maintained in a quiescent state, but quickly proliferate and differentiate into MPP cells in response to signals from the environment [2,3]. MPP cells are a heterogenous population. MPP1 cells can support the generation of all mature lineages with limited self-renewal capacity, and are only able to support hematopoiesis for a short period of time [4]. MPP2 cells are biased toward megakaryocyte/erythrocyte progenitor cells, MPP3 cells are myeloid biased, and MPP4 cells are lymphoid biased [4,5].
The balance between HSCs and MPP subpopulations and their lineage potential is crucial in maintaining proper quantities of different types of blood cells. Disruption in the potential of HSPCs to give rise to a particular lineage can result in commitment of progenitors to alternative lineages, which can contribute to diseases such as acute myeloid leukemia and acute myelodysplastic syndrome [6,7]. Understanding factors that regulate HSPC lineage specification provides insight into blood-based diseases as well as ways to generate progenitor cells to treat hematopoietic diseases and disorders.
HSPCs are regulated by a complex network of soluble mediators that influence the expression and function of transcription factors [8]. While numerous transcription factors have been discovered to regulate hematopoiesis, the specific roles of many transcription factors in modulating HSPCs are not fully understood. Recent studies implicate the aryl hydrocarbon receptor (AHR) as an important regulator of hematopoiesis, but the precise nature of the AHR's role in modulating HSPCs is unclear.
The AHR is a ligand-regulated environment-sensing transcription factor that binds a wide variety of synthetic and naturally derived small molecules [9,10]. Evidence that the AHR is an important regulator of hematopoiesis includes that AHR-binding small molecules significantly affect the hematopoietic compartment [11–13]. For example, exposure to exogenous chemicals that activate the AHR, such as dioxins and polychlorinated biphenyls, correlates with increased incidence of hematopoietic cancers in several human population-based studies [14–16], and AHR antagonism increased proliferation of human HSCs in vitro [17]. In addition, numerous reports using animal models demonstrate that treatment with AHR ligands influences hematopoietic cells in the bone marrow and periphery, although the direction and nature of changes observed depend on experimental context [18–20]. Moreover, mice that lack the AHR (AHR KO mice) exhibit abnormal frequencies of HSPCs [21–23]. Taken together, these studies highlight that AHR signaling has an important role in regulation of the hematopoietic compartment.
Despite evidence that treatment with AHR ligands influences aspects of hematopoiesis, the endogenous function of the AHR in regulation of HSPC homeostasis is less clear. Prior studies suggest that the AHR regulates hematopoiesis by directing differentiation toward specific lineage-committed progenitor cells [13,24]. Yet, it is unclear whether AHR signaling modulates hematopoietic differentiation even earlier by directing HSCs toward distinct lineage-biased MPP subsets. To address this, we used different transgenic AHR KO mouse models and an AHR antagonist to determine whether absence or attenuation of AHR signaling altered homeostatic regulation of HSPC lineage specification. These studies show that even in the absence of exogenous stressors, AHR signaling modulates homeostatic HSPC differentiation. Altered lineage potential of HSPCs is associated with not only aging but also an increased incidence of disease such as autoimmunity and carcinogenesis. Understanding not only the environmental signals but also the molecules that regulate lineage-biasing of HSPCs is an important area of research that will provide key insight into new approaches to treat multiple human diseases.
Materials and Methods
Animals and treatments
C57Bl/6 mice (age 5–6 weeks) were purchased from the Jackson Laboratory (Bar Harbor, ME). Initial breeding stocks for B6.AhRtm1Bra (AHR KO) and AHRfx/fx mice were provided by Dr. Christopher Bradfield (University of Wisconsin), and colonies were continually maintained at the University of Rochester. Initial breeding stock for B6.Cg-TgA2Kio/J (Vav1cre) mice and B6.129-Gt(ROSA)26Sortm1(cre/ERT2)Tyj/J (CreERT2) mice was obtained from the Jackson Laboratories. Female AHRfx/fx mice were crossed with male CreERT2 mice to generate Ahrfx/fxCreERT2 mice (AHRCreERT2). Excision of the Ahr in AHRCreERT2 mice was induced by treatment with tamoxifen (i.p., 25 mg/kg body weight; Sigma, St. Louis, MO), administered every 24 h for 3 days. After treatment with tamoxifen, AHRCreERT2 mice were referred to as inducible AHR KO mice (iAHR KO). Assessment of bone marrow cells was initiated 14 days after last administration of tamoxifen. Female AHRfx/fx mice were crossed with male Vav1cre transgenic mice to create a colony of Vav1creAhrfx/fx (AHRVav1) mice. The genotype of all mice was determined using PCR [20,25–28]. A complete list of all primers used is provided in Supplementary Table S1. AHR was antagonized by injecting mice (i.p.) with 100 μg of CH223191 (Tocris, United Kingdom) suspended in corn oil. As a control, another group of mice was administered corn oil (i.p.). All data presented were generated using female mice, and all experiments were initiated when mice were between 6 and 8 weeks of age. All mice were housed in microisolator cages in a specific pathogen-free facility at the University of Rochester, with a 12-h light/12-h dark cycle and an ambient temperature between 20°C and 22°C, and were provided food and water ad libitum. All animal treatments followed all regulations and guidelines and were conducted with prior approval of the Institutional Animal Care and Use Committee of the University of Rochester.
Isolation of hematopoietic cells
Briefly, bones were crushed with mortar and pestle to release bone marrow cells, which were suspended in Iscove's modified Dulbecco's medium (IMDM) (12440053; Gibco) supplemented with 2.5% fetal bovine serum (1677714; HyClone). The cells were then passed through a 40 μm nylon filter to remove stromal aggregates and debris [25]. Erythrocytes were removed by hypotonic lysis, and the number of bone marrow cells was determined using a TC10 Cell Counter (Nexelcom Bioscience) with cell viability assessed using trypan blue exclusion.
Flow cytometry
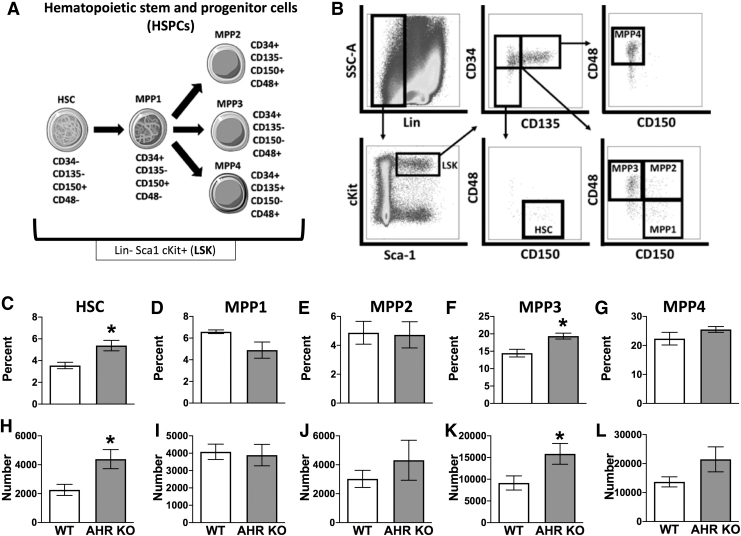
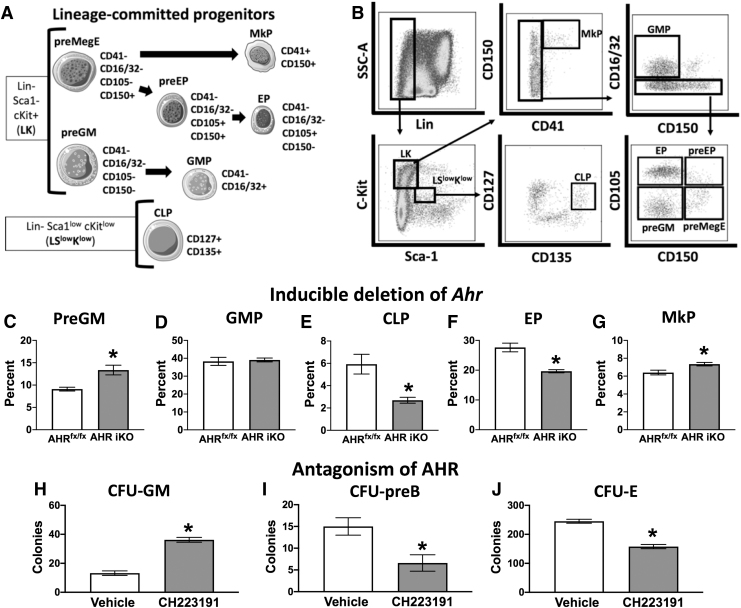
Mature hematopoietic cells were excluded using a cocktail containing monoclonal antibodies directed at the following cell lineage markers: CD3 (Pac Blue; Clone: 17A2), CD45R (Pac Blue; Clone: RA3-6B2), CD11b (Pac Blue; Clone: M1/70), TER-119 (Pac Blue; Clone: TER-119), and Ly-6G/C (Pac Blue; Clone: RB6-8C5). To identify HSCs, MPPs, and common lymphoid progenitors (CLPs), bone marrow cells were preincubated with rat Ig and anti-mouse CD16/32 (clone 93) before incubation with previously determined optimal concentrations of the following fluorochrome-conjugated monoclonal antibodies: lineage cocktail, Sca1 (PE-CF594; Clone: D7), cKit (BV650; Clone: 2B8), CD34 (FITC; Clone: RAM34), CD135 (PE; Clone: A2F10.1), CD48 (APC-cy7; Clone: HM48-1), CD150 (PE-cy7; Clone: TC15-12F112.2), and CD127 (APC; Clone: A7R34). For identification of lineage-committed progenitors, bone marrow cells were preincubated with rat Ig before incubation with the following fluorochrome-conjugated monoclonal antibodies: lineage cocktail, Sca1 (AF647; Clone: D7), cKit (BV650; Clone: 2B8), CD41 (FITC; Clone: MWReg30), CD16/32 (APC-cy7; Clone: 93), CD150 (PE-cy7; Clone: TC15-12F112.2), and CD105 (PE; Clone: MJ7/18). All antibodies were purchased from either BD (San Jose, CA) or BioLegend (San Diego, CA), and were titrated before using in experiments. Cell viability was >95%. Fluorescence minus one control was used to assess nonspecific fluorescence and define all gating parameters. Two to three million bone marrow cells were stained, and 1 million events were collected using an LSRII flow cytometer (BD Biosciences, San Jose, CA). Data were analyzed using the FlowJo software program (TreeStar, Ashland, OR). The specific combinations of molecular markers used to identify different HSPC and lineage-committed progenitor cell populations are detailed in Figs. 1A and 4A. The gating strategies used are outlined in Figs. 1B and 4B.
FIG. 1.
AHR KO mice have increased proportions of HSCs and myeloid-biased MPP3 cells. Bone marrow cells were isolated from female C57Bl/6 (WT) and global AHR knockout (KO) mice (8–12 weeks of age; 4–5 mice per group). (A) Graphic depicts the specific combinations of cell surface markers that were used to define the indicated subsets of HSPCs. Lineage-negative (Lin−) cells were defined as cells that do not express CD3, CD11b, CD45R, TER-119, or Ly-6G/C. (B) Gating strategy used to identify HSCs and MPP subsets. (C–G) Mean percentage of LSK (Lin− Sca1+ cKit+) cells that were (C) HSCs, (D) MPP1, (E) MPP2, (F) MPP3, and (G) MPP4 cells from the bone marrow of WT and AHR KO mice. (H–L) Number of (H) HSC (I) MPP1, (J) MPP2, (K) MPP3, and (L) MPP4 cells in the bone marrow of WT and AHR KO mice. Error bars represent SEM. Asterisks denote P < 0.05 (Student's t-test). AHR, aryl hydrocarbon receptor; HSPCs, hematopoietic stem and progenitor cells; MPP, multipotent progenitors; SEM, standard error of the mean; WT, wild type.
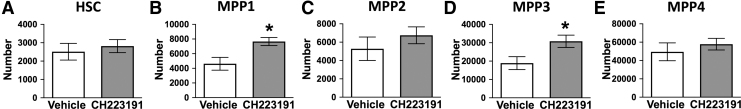
FIG. 4.
Antagonism of AHR increased the number of MPP1 and MPP3 cells. Female C57BL/6J mice (8 weeks of age; 6 mice per group) were given CH223191 (100 μg) or control (corn oil) by i.p. injection 48 h before isolation of bone marrow cells. Cells were labeled as in Fig. 1 and analyzed by flow cytometry. Graphs show the number of (A) HSC (B) MPP1, (C) MPP2, (D) MPP3, and (E) MPP4 cells in the bone marrow of mice treated with vehicle or CH223191. Error bars represent SEM. Asterisks denote P < 0.05 (Student's t-test).
Hematopoietic colony-forming unit assays
Colony-forming unit-erythroid (CFU-E), CFU-granulocyte macrophage (CFU-GM), and CFU-pre-B lymphocyte (CFU-Pre-B) assays were performed using Methocult media, following the manufacturer's instructions (https://www.stemcell.com/mouse-colony-forming-unit-cfu-assays-using-methocult.html; Stem Cell Technologies, Vancouver, Canada). Specifically, M3434 Methocult medium was used to enumerate CFU-GMs, CFU-Es were quantified using M3334 Methocult medium, and CFU-pre-B cells were measured using M3630 Methocult medium. Briefly, bone marrow cells from individual mice were resuspended in IMDM at 2 × 105 cells/mL for CFU-GM assays, and 1 × 106 cells/mL for CFU-E and CFU-preB assays. For all CFU assays, 300 μL of inoculated IMDM was added to 3 mL of Methocult medium. Cells were dispensed into six-well plates and incubated at 37°C in 5% CO2. The number of CFU-E was determined after 2 days of culture, and the number of CFU-GM and CFU-preB cells was determined after 7–12 days in culture. The plates were scored for CFU colonies using an inverted microscope [29].
Statistical analysis
All statistical analyses were performed using JMP software (Version 14; SAS, Cary, NC). A two-way ANOVA, followed by a Tukey's honest significant difference post hoc test, was used to compare differences between multiple independent variables (eg, multiple genotypes and different treatment groups). Differences between the mean values of two groups at a single point in time were assessed utilizing a two-tailed Student's t test. Differences in mean values were considered statistically significant when P values were ≤0.05. Error bars on all graphs indicate the standard error of the mean.
Results
AHR KO mice have increased proportions of myeloid-biased HSCs and MPPs
To delineate how absence of the AHR affects the composition of HSPCs, which include HSCs and phenotypically distinct MPP subpopulations, bone marrow cells from AHR KO and wild-type (WT) mice were analyzed using flow cytometry. Figure 1A illustrates the specific cell surface markers, and Fig. 1B depicts the overall gating strategy used to identify HSCs and MPP1, MPP2, MPP3, and MPP4 cells [4]. Bone marrow cells from AHR KO mice had 35% greater percentage (Fig. 1C) and twice the number of HSCs (Fig. 1H) compared to WT mice. There were no statistically significant differences in the percent (Fig. 1D) or number (Fig. 1I) of MPP1s in AHR KO mice compared to WT mice. However, comparison of lineage-biased MPP cells from AHR KO and WT mice revealed a statistically significant increase in the percent (Fig. 1F) and number (Fig. 1K) of myeloid-biased MPP3 cells. In contrast, neither the percentage nor number of MPP2 (Fig. 1E, J) and MPP4 cells (Fig. 1G, L) were different in WT and AHR KO mice.
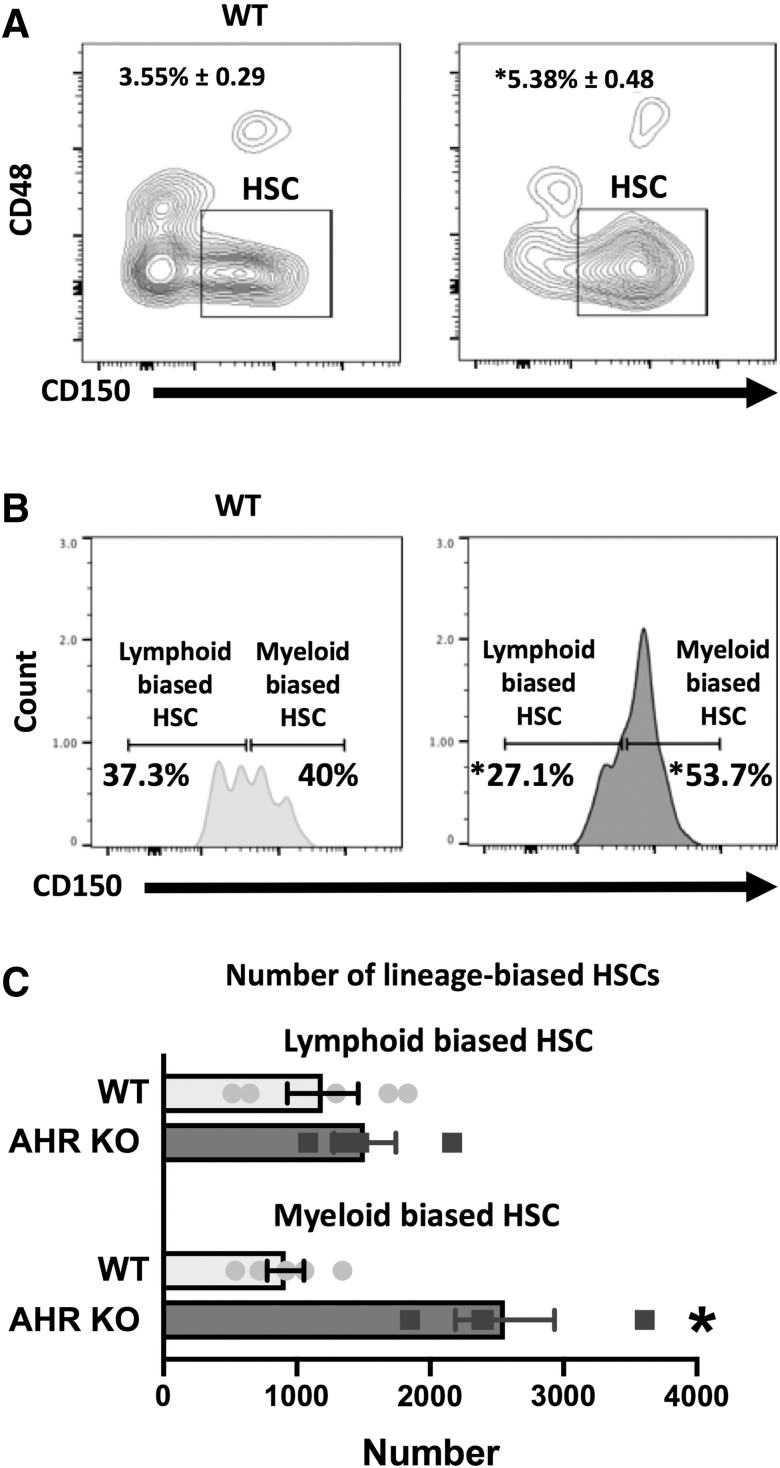
HSCs are not a homogeneous population and can be further subdivided, with HSCs that express lower levels of CD150 tending to be more lymphoid biased, whereas those that express higher levels of CD150 being myeloid biased [30]. Since there was an observed increase in the number of HSCs in AHR KO mice, we set out to determine if this was due to altered proportions of lineage-biased HSCs, by analyzing the ratio of HSCs that were CD150hi (myeloid-biased HSC) or CD150lo (lymphoid-biased HSC). The percentage of bone marrow cells that were HSCs was higher in AHR KO mice compared to WT mice (Fig. 2A). However, within this population of cells, the percent of lymphoid-biased HSCs was lower, and percent of myeloid-biased HSCs was higher in AHR KO mice (Fig. 2B). In addition, there was a 2.5-fold increase in the number of myeloid-biased HSCs in AHR KO mice compared to WT mice (Fig. 2C). These findings suggest that AHR may influence lineage commitment events very early on during hematopoiesis, as AHR KO mice exhibited increased proportions of myeloid-biased HSCs and MPP3 cells.
FIG. 2.
AHR KO mice have increased proportions of myeloid-biased HSCs. C57Bl/6 (WT) and AHR KO mice (8–12 weeks of age; 4–5 mice per group). (A) Mean percentage of LSK cells that were HSCs. (B) Mean percentage of HSCs that were CD150Low (lymphoid-biased HSC) or CD150High (myeloid-biased HSC). (C) Number of lymphoid-biased HSCs or myeloid-biased HSCs in the bone marrow of AHR KO and WT mice. Error bars represent SEM. Asterisks denote P < 0.05 (Student's t-test).
Acute attenuation of AHR altered MPP homeostasis and elevated the number of myeloid-biased MPP3 cells
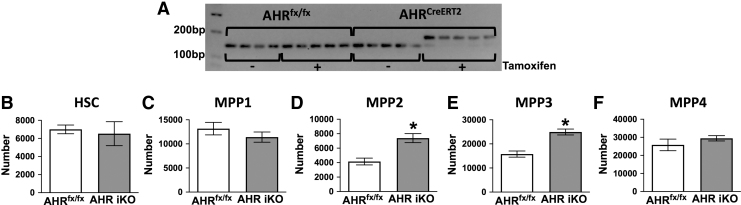
A limitation of studies using global AHR KO mice is that they do not express AHR at any point in time during their lifespan. Therefore, the increased proportion of HSCs and myeloid-biased MPP3s observed in AHR KO mice may be caused by the chronic lack of AHR. To address this, we utilized inducible AHR KO mice (AHR iKO). Tamoxifen treatment induced excision of the Ahr (Fig. 3A). There was no difference in the number of HSCs in AHR iKO mice compared to tamoxifen-treated AHRfx/fx mice (Fig. 3B). There was also no difference in the number of MPP1 cells in AHR iKO mice compared to tamoxifen-treated AHRfx/fx mice (Fig. 3C). However, there was a 2.5-fold increase in the number of MPP2 (Fig. 3D) and MPP3 (Fig. 3E) cells in AHR iKO mice (Fig. 3E). In contrast to MPP2 and MPP3 cells, there was no significant difference in the number of MPP4 cells (Fig. 3F).
FIG. 3.
Inducible deletion of AHR increased the proportion of MPP3 cell. Female AHRfx/fx or Ahrfx/fxCreERT2 (AHR iKO) mice were administered tamoxifen (i.p., 25 mg/kg BW) every 24 h for 3 days (8 weeks of age; 5 mice per group). Bone marrow cells were isolated 2 weeks after the final dose of tamoxifen. (A) Ahr gene excision in bone marrow cells was confirmed using PCR followed by agarose gel electrophoresis. The image depicts PCR products: the unexcised Ahr gene yields a 140 bp PCR product, whereas when excised, the PCR product is 180 bp. (B–F) Isolated bone marrow cells were labeled as in Fig. 1 and analyzed using flow cytometry. Graphs show the mean number of (B) HSC (C) MPP1, (D) MPP2, (E) MPP3, and (F) MPP4 cells in the bone marrow of tamoxifen-treated AHRfx/fx and AHR iKO mice. Error bars represent SEM. Asterisks denote P < 0.05 (Student's t-test).
To further test the idea that attenuating AHR induces acute changes to the proportion of HSPC subpopulations, we used the pharmacological AHR antagonist CH223191 [31]. CH223191 treatment significantly increased the number of MPP1 (Fig. 4B) and MPP3 cells (Fig. 4D), but did not alter the number of HSCs (Fig. 4A), MPP2s (Fig. 4C), or MPP4 cells (Fig. 4E). When integrated with findings from AHR iKO mice, these results indicate that increased proportion of MPP3 cells is an acute response to the loss of AHR, whereas increased HSC frequency appears to require chronic lack of AHR.
Acute attenuation of AHR altered the composition of lineage-committed hematopoietic progenitors
Given that acute deletion of AHR increased the frequency of myeloid-biased MPP3s, we next determined whether this altered the composition of downstream hematopoietic lineage-committed progenitors in the bone marrow. Figure 5A depicts the cell surface markers used to define lineage-committed progenitors [4], and Fig. 5B outlines the gating scheme used. AHR iKO mice had an increased percentage of pregranulocyte/premonocyte precursors (preGM) (Fig. 5C), with no alteration to the frequency of granulocyte/monocyte precursors (GMP) (Fig. 5D) compared to tamoxifen-treated AHRfx/fx mice. There was a twofold decrease in the percentage of CLPs in AHR iKO mice compared to tamoxifen-treated AHRfx/fx mice (Fig. 5E). There was a 29% decrease in the percentage of erythroid-committed precursor (EP) cells in AHR iKO mice compared to tamoxifen-treated AHRfx/fx mice (Fig. 5F). In addition, iAHR KO mice had an increased frequency of megakaryocyte-committed progenitors (MkP) compared to tamoxifen-treated AHRfx/fx mice (Fig. 5G). Consistent with these observations, functional evaluation of HSPC differentiation, assessed by utilizing CFU assays, revealed that treatment of mice with CH223191 increased the number of CFU-GM cells (Fig. 5H), and decreased the number of CFU-preB (Fig. 5I) and CFU-E (Fig. 5J). Overall, these results indicate that acute attenuation of AHR not only affects the distribution of lineage-biased MPPs but also the composition and function of downstream lineage-committed progenitors.
FIG. 5.
Inducible deletion of AHR and AHR antagonism altered downstream lineage-committed progenitors. AHRfx/fx or Ahrfx/fxCreERT2 (AHR iKO) mice (8–12 weeks of age; 4–5 per group) were administered tamoxifen (i.p., 25 mg/kg BW) every 24 h for 3 days. Bone marrow cells were isolated 2 weeks after the final dose of tamoxifen. (A) The cartoon outlines the combination of cell surface markers that were used to define indicted lineage-committed progenitors. (B) Gating strategy used to identify lineage-committed progenitors. (C, D) Graphs present the mean percentage of Lin− Sca1− cKit+ (LK) cells that were myeloid-committed precursors. (C) preGM cells and (D) GMP cells. (E) The graph shows the mean percentage of CLPs, presented as percentage of lineage−Sca1lowcKitlow cells. Graphs show mean percentage of LK cells that were (F) EP, portrayed as the percentage of LK cells that were EPs, and (G) MkP, portrayed as the percentage of LK cells that were MkPs. (H–J) Female C57BL/6J mice (8 weeks of age; 6 per group) were given CH223191 (100 μg) or control (corn oil) by i.p. injection twice, 2 days apart, and sacrificed 48 h after the final dose. (H) Isolated bone marrow cells from individual mice (2 × 104 cells/mouse) were cultured in media that drive myeloid differentiation, and the number of CFU-GM was determined. (I) Bone marrow cells (0.5 × 105) were cultured in media that drive lymphoid differentiation, and the number of CFU-preB was determined. (J) Bone marrow cells (1 × 105) were cultured in media that drive erythroid differentiation, and CFU-E were enumerated. Error bars represent SEM. Asterisks denote P < 0.05 (Student's t-test). CFU-E, erythroid progenitor colonies; CFU-GM, granulocyte/monocyte progenitor colony-forming units; CFU-preB, pre-B lymphoid progenitor colonies; CLPs, common lymphoid progenitors; EP, erythroid-committed precursors; GMP, granulocyte/monocyte precursors; MkP, megakaryocyte-committed progenitors; preGM, pregranulocyte/premonocyte precursors.
The AHR-meditated increase in myeloid lineage specification is driven by factors intrinsic to the hematopoietic compartment
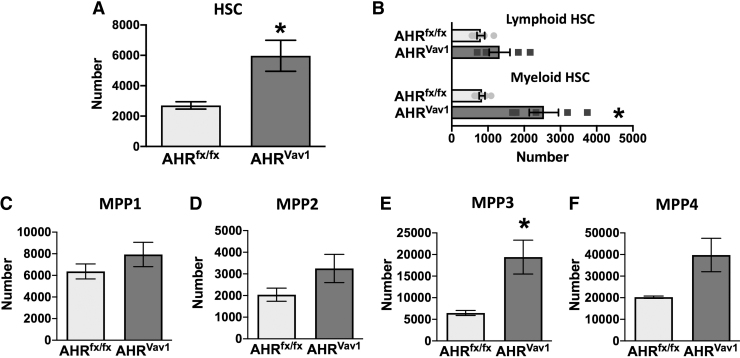
The AHR is expressed in both hematopoietic and nonhematopoietic cells [32,33]. To determine if intrinsic AHR signaling within the hematopoietic compartment drives alteration in the proportion of HSCs and lineage-biased MPPs, we utilized conditional AHR knockout mice (AHRVav1 mice) [20,25]. AHRVav1 mice had double the number of HSCs (Fig. 6A) and a 2.5-fold increase in the number of myeloid-biased HSCs (Fig. 6B) compared to AHRfx/fx mice. In addition, AHRVav1 mice exhibited differences in the proportions of MPP subpopulations (Fig. 6C–F) that were similar to those observed in AHR KO (Fig. 3C–F); particularly, there was a statistically significant increase in the number of MPP3 cells (Fig. 6E). These results indicate that the lack of AHR in the hematopoietic compartment is sufficient to the drive alterations in HSCs and lineage-biased MPPs.
FIG. 6.
Conditional deletion of AHR induces expanded numbers of myeloid-biased HSCs and MPP3 cells. Bone marrow cells were isolated from female AHRfx/fx or AHRVav1 mice (8 weeks of age; 5 per group) and stained for analysis using flow cytometry. The cell surface markers and gating strategy used were identical to the approach outlined in Fig. 1A and B. (A) Number of HSCs in AHRfx/fx and AHRVav1 mice. (B) Number of HSCs that were CD150High (Myeloid-biased HSC) or CD150Low (lymphoid-biased HSC) in AHRfx/fx and AHRVav1 mice. (C–F) Number of (C) MPP1, (D) MPP2, (E) MPP3, and (F) MPP4 cells in the bone marrow. Error bars represent SEM. Asterisks denote P < 0.05 (Student's t-test).
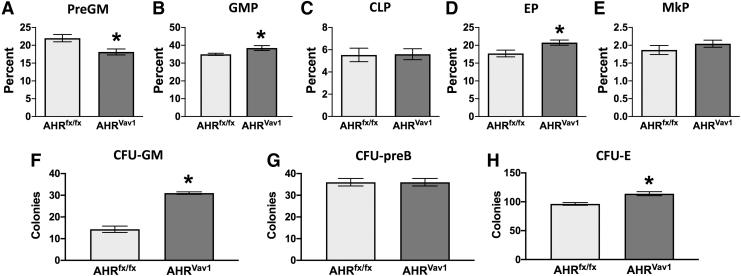
Given that hematopoietic-intrinsic AHR deletion increased the proportion of myeloid-biased HSCs and MPPs, we next ascertained if there were alterations to the proportion of downstream, lineage-committed progenitor cells. Analysis of myeloid lineage-committed progenitors revealed a 10% decrease in preGM precursors (Fig. 7A), and a 5% increase in the frequency of GMPs in AHRVav1 mice compared to AHRfx/fx mice (Fig. 7B). Deletion of the AHR from the hematopoietic compartment also increased the frequency of EP (Fig. 7D). In contrast, there were no significant differences in the percentage of CLPs (Fig. 7C) or MkP (Fig. 7E) in AHRVav1 mice and AHRfx/fx mice. In addition, compared to AHRfx/fx mice, bone marrow cells from AHRVav1 mice generated two times more CFU-GMs (Fig. 7F). There was also a slight, but statistically significant, increase in the number of CFU-Es in AHRVav1 mice compared to AHRfx/fx mice (Fig. 7H). However, there was no difference in CFU-preB numbers in AHRVav1 mice compared to AHRfx/fx (Fig. 7G). These data indicate that AHR regulates myeloid and erythroid lineages intrinsically in the hematopoietic compartment, but modulation of lymphoid and megakaryocyte lineage progenitors is also influenced by AHR signals from nonhematopoietic tissues.
FIG. 7.
Absence of AHR from the hematopoietic compartment is sufficient to alter the proportion of lineage-committed progenitors. (A–E) Bone marrow cells were isolated from female AHRfx/fx or AHRVav1 mice (8 weeks of age; 4–5 per group). Cells were labeled with antibodies against the cell surface markers indicated in Fig. 5A and analyzed using the gating strategy depicted in Fig. 5B. (A, B) Graphs present the percentage of (A) preGM cells and (B) GMP cells [denoted as the percentage of Lin−Sca1− cKit+ (LK) cells]. (C) The graph shows the mean percentage of CLPs, depicted as percentage of lineage−Sca1lowcKitlow cells. (D) Graph shows the frequency of EP (as the percentage of LK cells). (E) Graph shows the frequency of MkP as a percentage of LK cells. (F–H) Bone marrow cells were isolated from female AHRfx/fx or AHRVav1 mice (8 weeks of age; 3 per group). Bone marrow cells from individual mice were cultured in media that support lineage-specific differentiation, measured as CFU. (F) Bone marrow cells (2 × 104) were cultured in media that drive myeloid differentiation, and CFU-GMs were enumerated. (G) Cells (1 × 105) were cultured in media that drive lymphoid differentiation, and the mean number of CFU-preB was determined. (H) Cells (1 × 105 per mouse) were cultured in media that drive erythroid differentiation, measured as CFU. Graph depicts the mean number of CFU-E. Error bars represent SEM. Asterisks denote P < 0.05 (Student's t-test).
Discussion
Maintaining a pool of HSPCs that resupplies all the cells of the blood and immune system across the lifespan is central to continued health. There is growing evidence that the AHR integrates the responses of the hematopoietic compartment to environmental cues, thereby influencing hematopoiesis [34]. This includes compelling evidence that treatment with AHR ligands modulates aspects of hematopoiesis [35–37]. Indeed, it has been shown that AHR ligands impact differentiation and functions of specific lineages of hematopoietic cells, particularly in the context of a stressor [18,34,38,39]. However, the contribution of AHR to maintaining HSPC homeostasis is less clear. Also uncertain is whether AHR signaling impinges on the lineage differentiation potential of HSPCs. The findings reported herein demonstrate that the AHR regulates HSPCs at primitive stages of lineage commitment. Consistent with prior studies [21,40], global AHR KO mice exhibited skewing of hematopoietic progenitor cells. Utilizing novel inducible AHR knockout mice, hematopoietic-specific AHR knockout mice, and a pharmacological AHR antagonist, this study reveals that acute and intrinsic regulation of AHR signaling in the hematopoietic compartment modulate myeloid-biased lineage specification in HSPCs. Overall, these new findings further highlight AHR as an important regulator in the homeostatic maintenance of HSPCs.
A key finding of this study was that even during steady-state hematopoiesis, the absence or attenuation of AHR signaling affected not only lineage-biased progenitor cells but also lineage-biased HSCs. In particular, global AHR KO mice and mice in which AHR was conditional ablated from the hematopoietic system exhibited higher frequencies of myeloid-biased HSCs and myeloid-biased progenitor cells, compared to WT mice. This is consistent with previous research, in which serial transplantation experiments demonstrated that HSCs from AHR KO mice had increased self-renewal capacity, with enhanced granulocyte and monocyte production after tertiary transplantation [21,22]. Results from this work extend this and indicate that AHR may influence lineage-biased cues in primitive HSCs. Increased myeloid biasing in hematopoietic stem cells and progenitors is a hallmark of an aged hematopoietic system [41–43]. Increasing evidence suggests enhanced myeloid biasing is due, in part, to age-related HSC deterioration [41,44–46]. Also, many of the genes and pathways that are altered in leukemic transformation of HSCs also play a role in aging [41]. Leukemic stem cells may have mechanisms that repress AHR signaling [47]. Furthermore, absence of the AHR increased development of myeloproliferative disorders, which supports that AHR signaling sustains hematopoietic progenitor cell function [48]. It has also been reported that the downregulation of AHR drives a myeloproliferative phenotype in chronic myeloid leukemia [49]. These findings collectively support that modulation of AHR signaling in HSPCs may serve a critical role in both aging and disease progression of the hematopoietic system, in part, by impacting HSPC homeostasis.
Another finding of this work is that in vivo antagonism of AHR increased proportions of myeloid-biased progenitors. AHR antagonism has been shown to expand human HSCs in vitro [17], and recent clinical transplantation trials utilizing an AHR antagonist to expand human cord blood HSCs showed significant expansion in the number of HSCs, with increased engraftment [50]. In addition, patients transplanted with human cord blood HSCs expanded utilizing an AHR antagonist had enhanced recovery of neutrophils [50,51]. Although these clinical studies of transplantation did not examine progenitor cells, the greater recovery of neutrophils aligns with increased myeloid progenitors observed in mice treated with an AHR antagonist and in AHR KO mice. Furthermore, there are reports of AHR antagonism promoting the production of human DCs from ex vivo cultured human hematopoietic progenitors [52]. Thus, modulation of AHR has potential clinical relevance not only for increasing the expansion of HSC expansion to improve yield for transplantation but also may provide a targetable molecule for the ex vivo expansion of specific lineage-committed precursors.
The data presented further support that AHR signaling both inside and outside of the hematopoietic cells is important for AHR-mediated regulation of lineage specification of HSPCs. Interestingly, the study reveals the important in vivo interplay of AHR signaling between hematopoietic and nonhematopoietic cells in the regulation of the early erythroid-megakaryocyte axis. This finding is consistent with studies that suggest AHR influences the erythroid-megakaryocyte axis, although the impact may depend on the type of ligand [13,53] For instance, in vitro antagonism of AHR promoted megakaryopoiesis [53], whereas in vitro AHR activation promoted erythropoiesis in human hematopoietic progenitor cells [13]. However, in this in vivo study, the directionality of changes to the frequency of erythroid and megakaryocyte progenitors was dependent on the presence or absence of AHR signaling in nonhematopoietic cells. These changes further highlight the role of AHR signaling in the bone marrow microenvironment, playing a significant role in regulating HSPCs. In the bone marrow microenvironment, there are multiple complex interactions between HSPCs and other cells types such as mesenchymal stem cells, endothelial cells, and immune cells [54]. Lack of AHR has been found to alter the bone marrow stroma, with increased numbers of mesenchymal stem cells [22]. The evidence further supports the need to research the interaction of AHR signaling in both hematopoietic and nonhematopoietic tissue and how this signaling can impact the early stages of hematopoietic lineages.
Conclusion
Overall, the research reported herein demonstrates that the AHR modulates primitive hematopoiesis at steady state, that is, even in the absence of exogenous stressors to the bone marrow compartment. Regulating the balance between HSCs and MPPs, and influencing lineage commitment among MPPs, are essential to maintaining the optimal number of blood and immune cells. When this finely tuned system is imbalanced, diseases and disorders that range from cytopenia to hematological malignancies can arise [6,7,55]. These new findings add to the mounting evidence that AHR is an important governor of this tightly regulated system. More broadly, this new information supports the idea that the AHR not only shapes HSPC programming in an enduring manner but also affects HSPC lineage specification transiently. Moreover, AHR-regulated mechanisms are likely different among HSPC subpopulations. Given that multipotent and lineage-committed progenitors need to be nimble and poised to respond appropriately to external cues, and the growing interest in HSPC immune-modulating potential [56], the AHR is an important regulator of their responses and can potentially be harnessed in ways that can help reduce or treat a broad range of communicable and noncommunicable diseases.
Supplementary Material
Acknowledgments
The authors are grateful to Dr. Timothy Bushnell and Mr. Matt Cochran at the URMC Flow Cytometry Core, and to Catherine Donegan for maintaining the mouse colonies used in this research. Figures 1A and 5A were modified from Servier Medical Art licensed under Creative Common Attribution 3.0 Unported License (http://smart.servier.com/).
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by grants from the following grants from the US National Institutes of Environmental Health Science, National Institutes of Health: P30ES01247, T32ES07026, R01ES04862, and R01ES030300.
Supplementary Material
References
- 1. Orkin SH and Zon LI. (2008). Hematopoiesis: an evolving paradigm for stem cell biology. Cell 132:631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA and Trumpp A. (2009). IFNalpha activates dormant haematopoietic stem cells in vivo. Nature 458:904–908. [DOI] [PubMed] [Google Scholar]
- 3. King KY and Goodell MA. (2011). Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat Rev Immunol 11:685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cabezas-Wallscheid N, Klimmeck D, Hansson J, Lipka DB, Reyes A, Wang Q, Weichenhan D, Lier A, von Paleske L, et al. (2014). Identification of regulatory networks in HSCs and their immediate progeny via integrated proteome, transcriptome, and DNA methylome analysis. Cell Stem Cell 15:507–522. [DOI] [PubMed] [Google Scholar]
- 5. Pietras EM, Reynaud D, Kang YA, Carlin D, Calero-Nieto FJ, Leavitt AD, Stuart JM, Gottgens B and Passegue E. (2015). Functionally distinct subsets of lineage-biased multipotent progenitors control blood production in normal and regenerative conditions. Cell Stem Cell 17:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ye M, Zhang H, Yang H, Koche R, Staber PB, Cusan M, Levantini E, Welner RS, Bach CS, et al. (2015). Hematopoietic differentiation is required for initiation of acute myeloid leukemia. Cell Stem Cell 17:611–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shastri A, Will B, Steidl U and Verma A. (2017). Stem and progenitor cell alterations in myelodysplastic syndromes. Blood 129:1586–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wilkinson AC and Gottgens B. (2013). Transcriptional regulation of haematopoietic stem cells. Adv Exp Med Biol 786:187–212. [DOI] [PubMed] [Google Scholar]
- 9. Esser C and Rannug A. (2015). The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol Rev 67:259–279. [DOI] [PubMed] [Google Scholar]
- 10. Soshilov AA and Denison MS. (2014). Ligand promiscuity of aryl hydrocarbon receptor agonists and antagonists revealed by site-directed mutagenesis. Mol Cell Biol 34:1707–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoon BI, Hirabayashi Y, Kawasaki Y, Kodama Y, Kaneko T, Kanno J, Kim DY, Fujii-Kuriyama Y and Inoue T. (2002). Aryl hydrocarbon receptor mediates benzene-induced hematotoxicity. Toxicol Sci 70:150–156. [DOI] [PubMed] [Google Scholar]
- 12. Garrett RW and Gasiewicz TA. (2006). The aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin alters the circadian rhythms, quiescence, and expression of clock genes in murine hematopoietic stem and progenitor cells. Mol Pharmacol 69:2076–2083. [DOI] [PubMed] [Google Scholar]
- 13. Smith BW, Rozelle SS, Leung A, Ubellacker J, Parks A, Nah SK, French D, Gadue P, Monti S, et al. (2013). The aryl hydrocarbon receptor directs hematopoietic progenitor cell expansion and differentiation. Blood 122:376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. (1997). IARC Working Group on the Evaluation of Carcinogenic Risks to Humans: Polychlorinated Dibenzo-Para-Dioxins and Polychlorinated Dibenzofurans. Lyon, France, 4–11 February 1997. IARC Monogr Eval Carcinog Risks Hum 69:1–631. [PMC free article] [PubMed] [Google Scholar]
- 15. Bertazzi PA, Consonni D, Bachetti S, Rubagotti M, Baccarelli A, Zocchetti C and Pesatori AC. (2001). Health effects of dioxin exposure: a 20-year mortality study. Am J Epidemiol 153:1031–1044. [DOI] [PubMed] [Google Scholar]
- 16. Kramer S, Hikel SM, Adams K, Hinds D and Moon K. (2012). Current status of the epidemiologic evidence linking polychlorinated biphenyls and non-hodgkin lymphoma, and the role of immune dysregulation. Environ Health Perspect 120:1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boitano AE, Wang J, Romeo R, Bouchez LC, Parker AE, Sutton SE, Walker JR, Flaveny CA, Perdew GH, et al. (2010). Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science 329:1345–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thurmond TS, Staples JE, Silverstone AE and Gasiewicz TA. (2000). The aryl hydrocarbon receptor has a role in the in vivo maturation of murine bone marrow B lymphocytes and their response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol 165:227–236. [DOI] [PubMed] [Google Scholar]
- 19. Bankoti J, Burnett A, Navarro S, Miller AK, Rase B and Shepherd DM. (2010). Effects of TCDD on the fate of naive dendritic cells. Toxicol Sci 115:422–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boule LA, Burke CG, Jin GB and Lawrence BP. (2018). Aryl hydrocarbon receptor signaling modulates antiviral immune responses: ligand metabolism rather than chemical source is the stronger predictor of outcome. Sci Rep 8:1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Singh KP, Garrett RW, Casado FL and Gasiewicz TA. (2011). Aryl hydrocarbon receptor-null allele mice have hematopoietic stem/progenitor cells with abnormal characteristics and functions. Stem Cells Dev 20:769–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Unnisa Z, Singh KP, Henry EC, Donegan CL, Bennett JA and Gasiewicz TA. (2016). Aryl hydrocarbon receptor deficiency in an Exon 3 deletion mouse model promotes hematopoietic stem cell proliferation and impacts endosteal niche cells. Stem Cells Int 2016:4536187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gasiewicz TA, Singh KP and Bennett JA. (2014). The Ah receptor in stem cell cycling, regulation, and quiescence. Ann N Y Acad Sci 1310:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lindsey S and Papoutsakis ET. (2012). The evolving role of the aryl hydrocarbon receptor (AHR) in the normophysiology of hematopoiesis. Stem Cell Rev Rep 8:1223–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bennett JA, Singh KP, Welle SL, Boule LA, Lawrence BP and Gasiewicz TA. (2018). Conditional deletion of Ahr alters gene expression profiles in hematopoietic stem cells. PLoS One 13:e0206407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schmidt JV, Su GH, Reddy JK, Simon MC and Bradfield CA. (1996). Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U S A 93:6731–6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Walisser JA, Glover E, Pande K, Liss AL and Bradfield CA. (2005). Aryl hydrocarbon receptor-dependent liver development and hepatotoxicity are mediated by different cell types. Proc Natl Acad Sci U S A 102:17858–17863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feil R, Brocard J, Mascrez B, LeMeur M, Metzger D and Chambon P. (1996). Ligand-activated site-specific recombination in mice. Proc Natl Acad Sci U S A 93:10887–10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vannini N, Campos V, Girotra M, Trachsel V, Rojas-Sutterlin S, Tratwal J, Ragusa S, Stefanidis E, Ryu D, et al. (2019). The NAD-booster nicotinamide riboside potently stimulates hematopoiesis through increased mitochondrial clearance. Cell Stem Cell 24:405–418 e7. [DOI] [PubMed] [Google Scholar]
- 30. Beerman I, Bhattacharya D, Zandi S, Sigvardsson M, Weissman IL, Bryder D and Rossi DJ. (2010). Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc Natl Acad Sci U S A 107:5465–5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao B, Degroot DE, Hayashi A, He G and Denison MS. (2010). CH223191 is a ligand-selective antagonist of the Ah (Dioxin) receptor. Toxicol Sci 117:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Frericks M, Meissner M and Esser C. (2007). Microarray analysis of the AHR system: tissue-specific flexibility in signal and target genes. Toxicol Appl Pharmacol 220:320–332. [DOI] [PubMed] [Google Scholar]
- 33. Wheeler JL, Martin KC, Resseguie E and Lawrence BP. (2014). Differential consequences of two distinct AhR ligands on innate and adaptive immune responses to influenza A virus. Toxicol Sci 137:324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Negishi T, Kato Y, Ooneda O, Mimura J, Takada T, Mochizuki H, Yamamoto M, Fujii-Kuriyama Y and Furusako S. (2005). Effects of aryl hydrocarbon receptor signaling on the modulation of TH1/TH2 balance. J Immunol 175:7348–7356. [DOI] [PubMed] [Google Scholar]
- 35. Casado FL, Singh KP and Gasiewicz TA. (2011). Aryl hydrocarbon receptor activation in hematopoietic stem/progenitor cells alters cell function and pathway-specific gene modulation reflecting changes in cellular trafficking and migration. Mol Pharmacol 80:673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Singh KP, Wyman A, Casado FL, Garrett RW and Gasiewicz TA. (2009). Treatment of mice with the Ah receptor agonist and human carcinogen dioxin results in altered numbers and function of hematopoietic stem cells. Carcinogenesis 30:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sakai R, Kajiume T, Inoue H, Kanno R, Miyazaki M, Ninomiya Y and Kanno M. (2003). TCDD treatment eliminates the long-term reconstitution activity of hematopoietic stem cells. Toxicol Sci 72:84–91. [DOI] [PubMed] [Google Scholar]
- 38. Funatake CJ, Marshall NB and Kerkvliet NI. (2008). 2,3,7,8-Tetrachlorodibenzo-p-dioxin alters the differentiation of alloreactive CD8+ T cells toward a regulatory T cell phenotype by a mechanism that is dependent on aryl hydrocarbon receptor in CD4+ T cells. J Immunotoxicol 5:81–91. [DOI] [PubMed] [Google Scholar]
- 39. Vorderstrasse BA and Kerkvliet NI. (2001). 2,3,7,8-Tetrachlorodibenzo-p-dioxin affects the number and function of murine splenic dendritic cells and their expression of accessory molecules. Toxicol Appl Pharmacol 171:117–125. [DOI] [PubMed] [Google Scholar]
- 40. Singh KP, Casado FL, Opanashuk LA and Gasiewicz TA. (2009). The aryl hydrocarbon receptor has a normal function in the regulation of hematopoietic and other stem/progenitor cell populations. Biochem Pharmacol 77:577–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ and Weissman IL. (2005). Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A 102:9194–9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sudo K, Ema H, Morita Y and Nakauchi H. (2000). Age-associated characteristics of murine hematopoietic stem cells. J Exp Med 192:1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liang Y, Van Zant G and Szilvassy SJ. (2005). Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood 106:1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee J, Yoon SR, Choi I and Jung H. (2019). Causes and mechanisms of hematopoietic stem cell aging. Int J Mol Sci 20:1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Geiger H, de Haan G and Florian MC. (2013). The ageing haematopoietic stem cell compartment. Nat Rev Immunol 13:376–389. [DOI] [PubMed] [Google Scholar]
- 46. Akunuru S and Geiger H. (2016). Aging, clonality, and rejuvenation of hematopoietic stem cells. Trends Mol Med 22:701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ly M, Rentas S, Vujovic A, Wong N, Moreira S, Xu J, Holzapfel N, Bhatia S, Tran D, et al. (2019). Diminished AHR signaling drives human acute myeloid leukemia stem cell maintenance. Cancer Res 79:5799–5811. [DOI] [PubMed] [Google Scholar]
- 48. Singh KP, Bennett JA, Casado FL, Walrath JL, Welle SL and Gasiewicz TA. (2014). Loss of aryl hydrocarbon receptor promotes gene changes associated with premature hematopoietic stem cell exhaustion and development of a myeloproliferative disorder in aging mice. Stem Cells Dev 23:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gentil M, Hugues P, Desterke C, Telliam G, Sloma I, Souza LEB, Baykal S, Artus J, Griscelli F, et al. (2018). Aryl hydrocarbon receptor (AHR) is a novel druggable pathway controlling malignant progenitor proliferation in chronic myeloid leukemia (CML). PLoS One 13:e0200923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wagner JE Jr., Brunstein CG, Boitano AE, DeFor TE, McKenna D, Sumstad D, Blazar BR, Tolar J, Le C, et al. (2016). Phase I/II Trial of StemRegenin-1 expanded umbilical cord blood hematopoietic stem cells supports testing as a stand-alone graft. Cell Stem Cell 18:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stefanski H, Brunstein CG, McKenna DH, Sumstad D, Miller JS, Blazar BR, DeFor TE, Boitano AE, Cooke MP, et al. (2019). Mgta-456, an aryl hydrocarbon receptor (AHR) antagonist based expansion of CD34+ hematopoietic stem cells (HSC), permits selection of better HLA matched cord blood units (CBUs) and promotes faster neutrophil recovery and uniform engraftment with potentially less acute graft-vs-host disease (GVHD). Blood 134:804. [Google Scholar]
- 52. Thordardottir S, Hangalapura BN, Hutten T, Cossu M, Spanholtz J, Schaap N, Radstake TR, van der Voort R and Dolstra H. (2014). The aryl hydrocarbon receptor antagonist StemRegenin 1 promotes human plasmacytoid and myeloid dendritic cell development from CD34+ hematopoietic progenitor cells. Stem Cells Dev 23:955–967. [DOI] [PubMed] [Google Scholar]
- 53. Strassel C, Brouard N, Mallo L, Receveur N, Mangin P, Eckly A, Bieche I, Tarte K, Gachet C and Lanza F. (2016). Aryl hydrocarbon receptor-dependent enrichment of a megakaryocytic precursor with a high potential to produce proplatelets. Blood 127:2231–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Boulais PE and Frenette PS. (2015). Making sense of hematopoietic stem cell niches. Blood 125:2621–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Giles AJ, Reid CM, Evans JD, Murgai M, Vicioso Y, Highfill SL, Kasai M, Vahdat L, Mackall CL, et al. (2016). Activation of hematopoietic stem/progenitor cells promotes immunosuppression within the pre-metastatic niche. Cancer Res 76:1335–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wildes TJ, Flores CT and Mitchell DA. (2019). Concise review: modulating cancer immunity with hematopoietic stem and progenitor cells. Stem Cells 37:166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.