Abstract
Trypanosoma cruzi-associated megaesophagus was diagnosed in a domestic Louisiana-born llama with no significant travel history. The llama resided in the same rural area of greater New Orleans, Louisiana, where the first human autochthonous case of Chagas disease was identified in the state. Venous blood from the llama tested positive for T. cruzi kinetoplastid DNA by conventional PCR. The cardiac evaluation was unremarkable, while thoracic radiographs revealed generalized megaesophagus. The llama received supportive care, but was ultimately humanely euthanized. The esophagus was severely distended throughout its length on necropsy, and histologic evaluation showed no microscopic changes in esophageal tissue and minimal to mild lymphoplasmacytic inflammation in cardiac tissue. T. cruzi DNA was detected by conventional PCR in the esophagus, small intestine, and blood despite no protozoan organisms being observed in multiple tissue sections examined. This report contributes to the growing body of evidence of local transmission of T. cruzi in the southern United States, and Chagas disease should be considered a differential diagnosis when evaluating llamas and other large animal species for esophageal dysfunction. There is little research describing megaesophagus or Chagas disease in llamas, and this report aims to increase awareness about this zoonotic disease that is becoming more frequently reported in the southern United States.
Keywords: Chagas disease, Trypanosoma cruzi, megaesophagus, esophageal dilatation, llama, autochthonous Chagas case
Introduction
Trypanosoma cruzi, the zoonotic parasite that causes Chagas disease, is a major public health concern in several countries spanning from Argentina to the southern United States (USA) (Bern et al. 2011). There is increasing evidence of local transmission of Chagas disease in the southern USA (Bern et al. 2011, Lynn et al. 2020). The first autochthonous human case in Louisiana was identified in June 2006 in a rural area of greater New Orleans (Dorn et al. 2007), and ∼10% of all locally acquired cases in the USA since 2000 have originated from Louisiana (Dorn et al. 2007, LOPH 2018, Lynn et al. 2020). Human infection may occur secondary to spillover from zoonotic transmission cycles, and understanding the relationships between mammalian hosts, insect vector species, and the environment is critical to assess the risk of disease transmission in human and veterinary medicine.
Parasite transmission primarily occurs after feces of an infected, blood-sucking triatomine insect contaminate bite wounds or mucous membranes (Bern et al. 2011). There are more than 130 triatomine species in the Americas and while 11 have been reported in the USA, Triatoma sanguisuga is the main vector of T. cruzi in Louisiana. Initial surveys around the site of the first autochthonous human Chagas disease case reported positive infections in 56–66% of the T. sanguisuga insects collected (Dorn et al. 2007, Cesa et al. 2011, Herrera et al. 2015). The high prevalence of T. cruzi among this vector population is likely perpetuating local disease transmission among domestic and wild mammals in Louisiana's rural areas (Elmayan et al. 2019, Majeau et al. 2020).
T. cruzi has a complex life cycle that involves multiple vertebrate hosts in Louisiana and throughout the USA (Bern et al. 2011, Waleckx et al. 2014). In Louisiana, there is evidence of local transmission to wild mammals, including rodents (76%) (Herrera et al. 2015, Pronovost et al. 2018), opossums (37–60%) (Barr et al. 1991a, Houk et al. 2010), raccoons (23–43%) (Majeau et al. 2020), and armadillos (1–37%) (Yaeger 1988, Barr et al. 1991a). Additionally, the reported prevalence among domestic dogs in Louisiana has ranged from 1% to 16% over the past 40 years (Elmayan et al. 2019), and the overall prevalence among nonhuman primates born and raised at the Tulane National Primate Research Center in Covington, Louisiana, was 1.6% (Dorn et al. 2012, Herrera et al. 2019).
While it is well known that triatomine vectors are nonspecific feeders and will feed on livestock and other large animals (Kjos et al. 2013, Dumonteil et al. 2018, 2020), there are no reports of Chagas disease in llamas or other camelids, and the clinical features of the disease in these species remain unclear. In humans, T. cruzi manifests in acute or chronic forms of the disease (WHO 2015). Chronic Chagas disease is characterized by T. cruzi amastigotes becoming localized in particular tissues and organs. Cardiac and digestive tissues are most commonly targeted, which may lead to arrhythmias and/or heart failure and megaesophagus/megacolon, respectively (WHO 2015). Specific clinical features in animals have been documented in mice (Okumura and Correa Neto 1961), dogs (Okumura and Correa Neto 1961, Barr et al. 1991b), and nonhuman primates (Marsden et al. 1976, Mubiru et al. 2014), and cardiac and gastrointestinal tissues were similarly affected. Presented here is the first documented case of possible T. cruzi-associated megaesophagus in a domestic llama (Lama glama) located in the same geographic area as the first human autochthonous Chagas case (Dorn et al. 2007), as well as specific clinical features of the disease and diagnostic methods used.
Materials and Methods
Clinical evaluation for Chagas disease
In 2017, we received the case of a 4-year-old, male castrated llama; born near Covington, Louisiana, USA; raised in the greater New Orleans area; and with no travel history. The llama was experiencing significant weight loss and choking episodes from the time of his castration in March 2016. Episodes were characterized by a rolling motion in the midsection of his neck with a grunting noise, potentially indicating an inability to appropriately regurgitate food. Intermittent, mild upper respiratory infections were also reported. To manage the weight loss and suspected megaesophagus, the llama was transitioned to elevated feeding, soaking of feed, monthly ivermectin deworming, fenbendazole deworming every 3–6 months, and daily administration of Red Cell vitamin–iron–mineral supplement. The llama lived in an area where zoonotic transmission of T. cruzi had historically been documented (Dorn et al. 2007), and based on the similar clinical presentation to chronic human Chagas disease, a jugular venous blood sample was collected and evaluated for T. cruzi infection.
The llama was evaluated at the Louisiana State University Veterinary Teaching Hospital (LSU VTH) and a comprehensive physical examination, oral examination, complete blood count (CBC), serum biochemical assay, and McMaster's fecal flotation were performed. After continued decline in quality of life, the llama returned to the LSU VTH for an echocardiogram, electrocardiogram (ECG), radiographs, and humane euthanasia with postmortem examination.
Pathological and histological evaluation
Following euthanasia, a complete necropsy was performed, and all tissues were evaluated macroscopically. Routine tissue collection was performed according to standard procedures. Tissue samples were fixed in 10% buffered formalin solution, trimmed, and embedded in paraffin blocks. Five-micrometer sections were prepared and stained with hematoxylin and eosin (H&E) for routine histopathological evaluation. Additional tissues from the heart (atria, ventricles, septum, and apex), pericardial adipose, esophagus, small intestine (pooled duodenum, jejunum, and ileum), descending colon, liver, kidney, spleen, mesenteric lymph node, and skeletal muscle (right and left thighs) were collected for DNA extraction.
Molecular identification of T. cruzi
Blood samples, collected in 2017 and 2019, and necropsy tissues, including the esophagus, heart, and small intestine, were analyzed for the presence of T. cruzi DNA. Blood samples were mixed with an equal volume of 6 M guanidine-HCL and 0.2 M EDTA (pH 8) and stored at room temperature (Elmayan et al. 2019), and tissues were stored at 4°C. DNA from blood and tissue samples was promptly extracted using the DNeasy extraction kit (QIAGEN, Valencia, CA) according to the manufacturer's instructions and stored at 4°C. Remaining blood-guanidine samples were kept at room temperature and tissues were kept at −20°C for long-term storage. Conventional PCR was performed on all samples using two different molecular markers for T. cruzi. TCZ1 (5′CGAGCTCTTGCCCACACGGGTGCT-3′) and TCZ2 (5′-CCTCCAAGCAGCGGATAGTTCAGG-3′) primers were used to target satellite DNA (satDNA) (Moser et al. 1989) that produce a band of 188 bp specific for T. cruzi, and the minicircle variable region of the kinetoplast DNA (kDNA) was targeted using the primers, 121 (5′-AAATAATGTACGGG(T/G)GAGATGCATGA-3′) and 122 (5′-GGTTCGATTGG GGTTGGTGTAATATA-3′), amplifying a band of 330 bp specific for T. cruzi kDNA (Wincker et al. 1994). DNA from a T. cruzi reference strain WB1 was used as the positive control. Molecular grade water was also used as the negative control in the PCR. The PCR products were separated on a 2% agarose gel and visualized using a Bio-Rad Gel Doc system (Bio-Rad Laboratories, Hercules, CA).
Results
Clinical evaluation
The llama had a poor body condition with a weight of 63.6 kg (previously recorded 72.5 kg in 2016), continued difficulty regurgitating food, and frequent sneezing of C1 compartment contents from nasal passages. CBC and serum chemistry values are provided in Table 1. The echocardiogram revealed normal structure, motion, and position of the cardiac chambers. No evidence of valvular incompetence or congenital malformations was noted. Limited information is available regarding normal cardiac dimensions of new-world camelids, but patient dimensions were consistently lower than published measurements of cardiac dimensions for adult Huacaya alpacas (72 ± 6 kg) (Margiocco et al. 2009). The electrocardiogram revealed a normal sinus rhythm.
Table 1.
Serum Biochemical Analysis and Complete Blood Cell Count
| Parametera | Resultb | Rangec | Units |
|---|---|---|---|
| Chemistry panel | |||
| Glucose | 138 | 71–149 | mg/dL |
| AST | 120 | 36–76 | U/L |
| GGT | 39 | 6–29 | U/L |
| Alk phosphatase | 71 | 0–10 | U/L |
| CK | 37 | 14–238 | U/L |
| TBIL | 0.1 | 0.0–0.2 | mg/dL |
| Total protein | 5.9 | 4.7–7.3 | g/dL |
| Albumin | 3.7 | 3.6–6.1 | g/dL |
| Globulin | 2.2 | g/dL | |
| BUN | 14 | 9–34 | mg/dL |
| Creatinine | 2.76 | 1.1–3.2 | mg/dL |
| Ca | 8.7 | 7.9–10.9 | mg/dL |
| Phos | 3.1 | 2.6–10.7 | mg/dL |
| Na | 148 | 148–158 | mM |
| K | 4.5 | 3.7–6.2 | mM |
| Cl | 118 | 103–125 | mM |
| Bicarbonate | 21.0 | mM | |
| AGAP | 13.5 | 13–27 | mM |
| MG | 2.1 | mg/dL | |
| Calculated OSMO | 296 | mM | |
| CBC | |||
| RBC | 11.58 | 10.3–15.2 | 106/μL |
| HGB | 13.4 | 10.6–16.5 | g/dL |
| Platelet count | 282 | 299–775 | 103/μL |
| MPV | 7.1 | 4.0–6.5 | fL |
| PCT | 0.2 | % | |
| PLT comment | With clumps | ||
| Plasma appearance | Normal | ||
| Protein | 6.4 | 5.3–7.0 | g/dL |
| PCV | 30 | % | |
| HPP | 300 | mg/dL | |
| Tech: | 513 | ||
| WBC | 8.8 | 7.89–20.27 | 103/μL |
| Neutrophils | 5.5 (62) | 3.67–10.96 | 103/μL (%) |
| Lymphocytes | 1.58 (18) | 2.13–6.41 | 103/μL (%) |
| Monocytes | 0.1 (1.0) | 0.08–0.71 | 103/μL (%) |
| Eosinophils | 1.7 (19.0) | 0.16–3.29 | 103/μL (%) |
Values outside the reference range are listed in bold letters.
Reference values provided by UC Davis School of Veterinary Medicine, Davis, CA.
Values provided by LSU VTH&C Clinical Pathology Laboratory, Baton Rouge, LA.
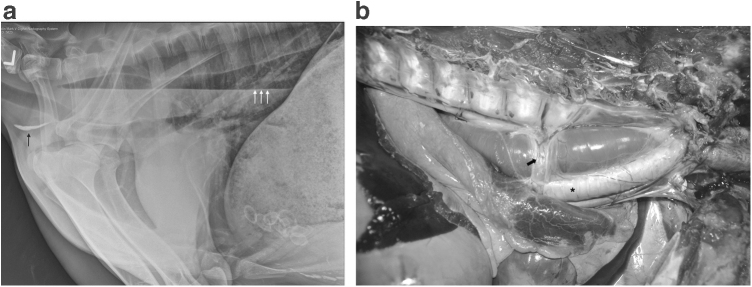
Thoracic radiographs showed generalized megaesophagus, which was most severe in the thoracic esophagus (Fig. 1a). Gas was present in the cranial cervical esophagus, and there was gas and fluid in the caudal cervical and thoracic esophagus, creating a fluid line, with a small amount of gravity-dependent mineral debris. Mild alveolar opacity was noted in the right ventral lung, which was thought to be due to either artifacts from superimposed thoracic limbs or potential aspiration pneumonia. Normal ingesta and mineralized material were noted in C1 and C3 of the stomach; the remainder of the examination was normal.
FIG. 1.
Megaesophagus. (a) Left lateral thoracic radiograph showing diffuse distention of the thoracic esophagus. The esophagus contains fluid and gas that creates a gas–liquid interface (white arrows) as well as gravity-dependent mineral debris (black arrow). (b) Gross necropsy image of the esophagus, located between the vertebral column dorsally (top of photo) and the trachea ventrally (asterisk), enlarged, and focally narrowed by the overlying azygos vein (arrow) where it courses over the esophagus.
Macroscopic findings
Necropsy identified severe megaesophagus as the primary gross finding (Fig. 1b). The esophagus was distended throughout its length, with narrowing in a focal area of the thoracic segment associated with the overlying azygos vein where it coursed over the esophagus. The esophageal measurements were as follows: cervical/neck portion, 2.5 cm diameter; middle neck portion, 1.2 cm diameter; thoracic inlet portion, 2.5 cm diameter; intrathoracic portion (cranial to the azygos vein), 5.5 cm diameter; caudal to the azygos vein, 6.5 cm diameter; and at the esophageal hiatus, 5 cm diameter (reference range: 2.54 cm cranial cervical; 3.89 cm caudal thoracic (Sukon et al. 2009)). No significant lesions and discoloration of the heart were noted. The right ventricular wall (RV) was 0.5 cm thick; the left ventricular wall (LV) and interventricular septum measured 1.7 cm for an RV:LV ratio = 1: 3.4 (reference range: RV:LV = 1: 2–3). Rare individual saccules within the C1 stomach compartment contained concretions and were considered to be within normal limits. No other significant lesions were noted.
Histologic findings
Histologic examination of the heart showed minimal to mild alterations, including occasional rowing of myocardial nuclei and subjectively increased numbers of sarcolemmal nuclei and interstitial cells. The interventricular septum showed similar alterations as well as a focal area degenerative cardiomyocytes characterized by shrunken and hypereosinophilic cytoplasm and pyknotic nuclei surrounded by a few mononuclear cells. The left heart showed a single aggregate of lymphocytes and plasma cells within the interstitium (Fig. 2). Sections of skeletal muscle from both right and left thighs showed similar minimal to mild alterations, including subjectively increased numbers and occasional rowing of sarcolemmal nuclei and mildly swollen sarcoplasm within individual myofibers.
FIG. 2.
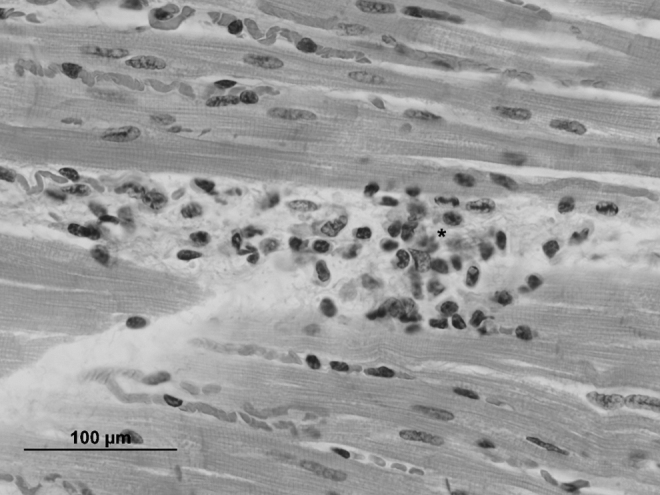
Photomicrograph of the heart with a small area of lymphocytic to plasmacytic inflammation (asterisk) in the interstitium, H&E stain (scale bar = 100 μm).
There were no histologic abnormalities identified in six sections of the esophagus. The bronchioles and alveoli contained scattered protozoan organisms, but were considered incidental findings (terminal aspiration). The stomach contained cross sections of nematode larvae, which were considered to be insignificant in this llama. Parasitological testing yielded Trichostrongylus-type ova and Eimeria spp., which were also not considered significant. The mucosal capillaries of the small and large intestines were congested, and lymphoid follicles of the mesenteric lymph node were hyperplastic. No significant abnormalities were seen within the examined sections of the spleen, liver, kidney, tongue, and urinary bladder. No protozoan organisms compatible with T. cruzi were observed in any tissues.
Molecular identification of T. cruzi
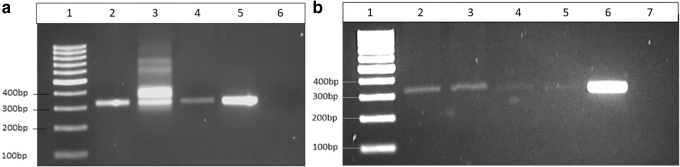
The presence of T. cruzi DNA was successfully identified in blood collected in 2017 (Fig. 3a), blood collected in 2019 (Fig. 3b), and in tissues from the esophagus and small intestine using kDNA as the molecular target (Fig. 3b). All other tissues, including cardiac tissues, showed no T. cruzi DNA amplification targeting kDNA. There was no T. cruzi DNA amplification using the satDNA as the molecular target, and positive and negative controls performed as expected for both reactions.
FIG. 3.
DNA gel electrophoresis of PCR amplicons from llama blood and tissue samples from different collection years. (a) Blood samples collected in the year 2017: Lane 1: 1-kb ladder; Lane 2: blood sample No. 1 (9/11/17); Lane 3: blood sample No. 2 (9/27/2017); Lane 4: blood sample No. 3 (10/2/2017); Lane 5: Trypanosoma cruzi positive control (strain WB1); and Lane 6: negative control. (b) Blood and tissue samples collected in the year 2019: Lane 1: 1-kb ladder; Lane 2: blood sample No. 1 (9/13/2019); Lane 3: blood sample No. 2 (10/28/2019); Lane 4: esophagus tissue; Lane 5: small intestine tissue; Lane 6: T. cruzi positive control (strain WB1); and Lane 7: negative control.
Discussion
The clinical evaluation and molecular diagnostics observed in the present case are consistent with T. cruzi-associated megaesophagus. To the best of our knowledge, this is possibly the first case of a chagasic mega syndrome documented in a llama or any other large animal species, as it has previously only been described in nonhuman primates (Marsden et al. 1976) and experimental dogs and mice (Okumura and Correa Neto 1961). Although megaesophagus is not uncommon in camelids (Watrous et al. 1995), there are very few cases documenting such findings. Esophageal dysfunction secondary to congenital vascular ring abnormalities has been reported in crias (McKenzie et al. 2010), and among llamas, idiopathic cases of acquired megaesophagus were most frequently reported (Watrous et al. 1995). There have been no previous reports of acquired megaesophagus associated with infectious diseases in llamas, although myasthenia gravis and generalized inflammatory myopathies, including those resulting from infectious disease, are common neuromuscular disorders that cause acquired megaesophagus in small animals (Evans et al. 2004, Mace et al. 2012). Tetanus produces esophageal dysfunction in humans and dogs (Dieringer and Wolf 1991) and Toxoplasma gondii, Neospora caninum, Borrelia burgdorferi, Ehrlichia canis, and Rickettsia rickettsii have also been implicated in megaesophagus (Mace et al. 2012). However, the history, symptoms, detection of T. cruzi DNA, and mild histologic changes are consistent with a clinical diagnosis of T. cruzi and potentially associated megaesophagus. Additionally, T. cruzi DNA was also found in small intestine tissue, showing a possible case of digestive Chagas disease (WHO 2015).
These findings are consistent with those described in humans and some veterinary species. In humans, dilation and thickening of the esophagus result from the presence of inflammatory lesions within layers of smooth muscle cells and subsequent parasympathetic denervation (Teixeira et al. 2006), which can occur throughout the digestive tract, but are generally more common in the esophagus and colon (Teixeira et al. 2006). Nonetheless, it has been reported that inflammatory lesions were present in only half of human T. cruzi megaesophagus cases despite positive identification of parasite DNA by PCR in 77% of esophageal tissues and 90% of blood samples (Lages-Silva et al. 2001). This tissue tropism of the parasite was reflected in the current case as DNA was positively identified in the esophagus and small intestine and neither organ showed histologic changes or signs of inflammatory infiltrates. T. cruzi lesions are commonly unevenly distributed across affected tissues and organs (Teixeira et al. 2011), which possibly contributed to the apparent absence of histologic lesions in this llama's esophagus. This, in addition to blood samples that tested positive for T. cruzi DNA, supports the diagnosis of Chagas disease and associated megaesophagus.
A small population of lymphocytes and plasma cells was identified in the left heart, consistent with very common findings of lymphoplasmacytic myocarditis in humans (Higuchi Mde et al. 1993, Teixeira et al. 2006), as well as chagasic dogs (Barr et al. 1991b), coyotes (Curtis-Robles et al. 2016, Hodo et al. 2020), raccoons (Curtis-Robles et al. 2016), and cats (Zecca et al. 2020). Enlargement of the heart is a common feature of Chagas disease in humans (Teixeira et al. 2006), and it is unclear if the increased RV:LV ratio is associated with T. cruzi infection. Alterations in tissue architecture and cardiac function due to Chagas disease result from the presence of inflammatory infiltrates within the heart tissue and subsequent damage to cardiomyocytes and the cardiac conduction system (Teixeira et al. 2006). The severity of tissue damage correlates with the degree of inflammatory infiltration, and T. cruzi parasites are rarely found microscopically (Higuchi Mde et al. 1993). Similar alterations may occur in striated skeletal muscle, and all observed changes within this llama's heart and skeletal muscle were minimal to mild and scattered. These changes could be suggestive of prior cardiac or muscular damage with regenerative change, although such damage could be nonspecific for Chagas disease. Even so, the mild histologic findings, the limited presence of degenerative cardiomyocytes and lymphocytic to plasmacytic inflammation, and the absence of parasitic organisms adequately correspond to the normal echocardiogram and ECG findings observed upon examination.
There is limited information available detailing how Chagas disease manifests in large mammals and what clinical presentations are more common. One report on experimental pigs showed no macroscopic alterations from T. cruzi, yet demonstrated histologic perivasculitis in the heart, meninges, and kidney, as well as lymphoid hyperplasia with necrosis in the spleen (Yauri et al. 2016). An additional report described symptoms and pathologic lesions observed in a chagasic horse that were primarily neurologic, and no histologic lesions were apparent in the heart or other organs (Bryan et al. 2016). The horse and the llama presented here received advanced diagnostics as companion animals, but in contrast, most food animal species do not live long enough for chronic signs of Chagas disease to develop or are sent to slaughter if signs of acute illness are shown. These practices and the likelihood that advanced diagnostics may be cost-prohibitive for large animals kept as companion animals may contribute to the lack of awareness and underreporting of this parasite in large animal species.
Human infections with T. cruzi are treated with the antitrypanosomal medications, benznidazole and nifurtimox (Rodriques Coura and de Castro 2002, Bern et al. 2007). Recently, both drugs were approved by the U.S. Food and Drug Administration (FDA) for the treatment of Chagas disease in pediatric patients under 18 years of age. (Traynor 2017, Thakare et al. 2021). Effective treatment has been recorded in acute phases and recent chronic infections, and it is recommended that patients receive treatment for late chronic infections without clinical manifestations or if clinical changes are mild (Rodriques Coura and de Castro 2002). These drugs have been rarely used in canine Chagas cases as supportive care is more common (Guedes et al. 2002, Bern et al. 2011). Similar to humans, these medications are more likely to be effective in earlier phases of the disease in the absence of more severe disease pathology (Guedes et al. 2002). It is unclear if these drugs would be effective against T. cruzi in camelids, but treatment would have likely been unrewarding in this case due to the severity of megaesophagus.
The prognosis for T. cruzi-associated megaesophagus in ruminants and camelids is likely poor, even with aggressive medical management. The ability of these animals to adequately digest and obtain nutrients from feed and forage high in cellulose is possible through a combination of physiologic factors. Salivation, chewing, swallowing, regurgitation, and rechewing are essential for adequate nutrient digestion in all ruminants and camelids, including llamas (Esteban and Thompson 1988, Church 1993). C1 and C2 of the camelid stomach, equivalent to the rumen and reticulum of a ruminant (Esteban and Thompson 1988), house a population of microorganisms that digest and break down plant material and produce volatile fatty acids, which are essential for energy production (Church 1993). The esophagus plays a critical role in ensuring the bidirectional movement of food for chewing and rechewing (Church 1993) and the presence of megaesophagus can hinder this process. This was observed in the case reported here and what ultimately contributed to the llama's decline in health and eventual euthanasia.
This case provides a detailed clinical evaluation of locally transmitted Chagas disease in a new mammalian host and also contributes to the growing body of evidence that T. cruzi is actively circulating in humans and animals within the USA. It is critical to fully describe all T. cruzi infections in any chagasic mammalian hosts to understand what transmission cycles may exist among triatomines, humans, domestic species, and local wildlife. These data will help bridge knowledge gaps regarding the eco-epidemiology of the parasite, which is essential for estimating the subsequent risk of disease in veterinary medicine and the potential for spillover into human medicine (Pronovost et al. 2018). It is feasible that disease transmission has persisted among humans and animals throughout the previous decade in this rural area of greater New Orleans as this llama, born in the USA, had a limited travel history and resided in the same geographic area where T. cruzi has historically been detected over several years. As such, it is probable that the prevalence of disease in human and veterinary medicine is underreported and there is an increased need for proactive surveillance.
Conclusion
This report describes the first documented case of locally transmitted Chagas disease and associated megaesophagus in a domestic llama. There is little information available detailing Chagas disease or megaesophagus in llamas, and this report increases awareness for owners and veterinarians about this zoonotic parasite and provides veterinarians new diagnostic information for the clinical setting. This information collectively contributes to characterization of T. cruzi in the southern USA, yet there are still many features of local disease transmission that remain unclear.
Ethics Statement
All procedures were performed during the standard clinical care of the animal, with consent from the owner. The animal was treated humanely and received a high standard of veterinary care at all times.
Acknowledgment
The authors would like to thank Weihong Tu for her assistance with molecular diagnostics.
Authors' Contributions
C.P.H. and E.D. conceived the study and designed the study protocol. C.A.H., C.M.S., and R.E.B. collected blood samples and clinically evaluated the case. R.J. interpreted diagnostic imaging. A.M.C. performed the cardiac evaluation. E.S., R.W.B., J.M.T., and C.A.H. performed necropsies and provided tissue samples. E.S. and R.W.B. performed and interpreted histopathologic examination of tissues. A.M., H.P., and J.M.T. carried out the molecular analysis. C.P.H., E.D., and J.M.T. carried out interpretation of the data. All authors drafted the manuscript and critically revised it for intellectual content. All authors read and approved the final manuscript. C.P.H. and E.D. are guarantors of the article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was funded, in part, by the Tulane ByWater Institute Faculty Fellowships in Interdisciplinary Collaboration 2020 to C.P.H., the Louisiana Board of Regents through the Board of Regents Support Fund [contract number LESASF (2018–21)-RD-A-19] to E.D., and the Ruth L. Kirschstein National Research Service Award [grant number 5T32OD11124–15 (MPI)] to J.M.T.
References
- Barr SC, Brown CC, Dennis VA, Klei TR. The lesions and prevalence of Trypanosoma cruzi in opossums and armadillos from southern Louisiana. J Parasitol 1991a; 77:624–627. [PubMed] [Google Scholar]
- Barr SC, Brown CC, Dennis VA, Klei TR. Pathologic features of dogs inoculated with North American Trypanosoma cruzi isolates. Am J Vet Res 1991b; 52:2033–2039. [PubMed] [Google Scholar]
- Bern C, Montgomery SP, Herwaldt BL, Rassi Jr A, et al. Evaluation and treatment of Chagas disease in the United States: A systematic review. JAMA 2007; 298:2171–2181. [DOI] [PubMed] [Google Scholar]
- Bern C, Kjos S, Yabsley MJ, Montgomery SP. Trypanosoma cruzi and Chagas' disease in the United States. Clin Microbiol Rev 2011; 24:655–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan LK, Hamer SA, Shaw S, Curtis-Robles R, et al. Chagas disease in a Texan horse with neurologic deficits. Vet Parasitol 2016; 216:13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesa K, Caillouet KA, Dorn PL, Wesson DM. High Trypanosoma cruzi (Kinetoplastida: Trypanosomatidae) prevalence in Triatoma sanguisuga (Hemiptera: Redviidae) in southeastern Louisiana. J Med Entomol 2011; 48:1091–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church DC. The Ruminant Animal Digestive Physiology and Nutrition. Prospect Heights, IL: Waveland Press, Inc., 1993. [Google Scholar]
- Curtis-Robles R, Lewis BC, Hamer SA. High Trypanosoma cruzi infection prevalence associated with minimal cardiac pathology among wild carnivores in central Texas. Int J Parasitol Parasites Wildl 2016; 5:117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieringer TM, Wolf AM. Esophageal hiatal hernia and megaesophagus complicating tetanus in two dogs. J Am Vet Med Assoc 1991; 199:87–89. [PubMed] [Google Scholar]
- Dorn PL, Perniciaro L, Yabsley MJ, Roellig DM, et al. Autochthonous transmission of Trypanosoma cruzi, Louisiana. Emerg Infect Dis 2007; 13:605–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn PL, Daigle ME, Combe CL, Tate AH, et al. Low prevalence of Chagas parasite infection in a nonhuman primate colony in Louisiana. J Am Assoc Lab Anim Sci 2012; 51:443–447. [PMC free article] [PubMed] [Google Scholar]
- Dumonteil E, Ramirez-Sierra MJ, Perez-Carrillo S, Teh-Poot C, et al. Detailed ecological associations of triatomines revealed by metabarcoding and next-generation sequencing: Implications for triatomine behavior and Trypanosoma cruzi transmission cycles. Sci Rep 2018, 8:4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumonteil E, Pronovost H, Bierman EF, Sanford A, et al. Interactions among Triatoma sanguisuga blood feeding sources, gut microbiota and Trypanosoma cruzi diversity in southern Louisiana. Mol Ecol 2020; 29:3747–3761. [DOI] [PubMed] [Google Scholar]
- Elmayan A, Tu W, Duhon B, Marx P, et al. High prevalence of Trypanosoma cruzi infection in shelter dogs from southern Louisiana, USA. Parasit Vectors 2019; 12:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban LR, Thompson JR. The Digestive System of New World Camelids - Common Digestive Disorders of Llamas. Ames, Iowa: Iowa State University Veterinarian, 1988; 50:9. [Google Scholar]
- Evans J, Levesque D, Shelton GD. Canine inflammatory myopathies: A clinicopathologic review of 200 cases. J Vet Intern Med 2004; 18:679–691. [DOI] [PubMed] [Google Scholar]
- Guedes PM, Veloso VM, Tafuri WL, Galvao LM, et al. The dog as model for chemotherapy of the Chagas' disease. Acta Trop 2002, 84:9–17. [DOI] [PubMed] [Google Scholar]
- Herrera CP, Licon MH, Nation CS, Jameson SB, et al. Genotype diversity of Trypanosoma cruzi in small rodents and Triatoma sanguisuga from a rural area in New Orleans, Louisiana. Parasit Vectors 2015; 8:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera C, Majeau A, Didier P, Falkenstein KP, et al. Trypanosoma cruzi diversity in naturally infected nonhuman primates in Louisiana assessed by deep sequencing of the mini-exon gene. Trans R Soc Trop Med Hyg 2019; 113:281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi Mde L, De Brito T, Martins Reis M, Barbosa A, et al. Correlation between Trypanosoma cruzi parasitism and myocardial inflammatory infiltrate in human chronic chagasic myocarditis: Light microscopy and immunohistochemical findings. Cardiovasc Pathol 1993; 2:101–106. [DOI] [PubMed] [Google Scholar]
- Hodo CL, Banuelos RM, Edwards EE, Wozniak EJ, et al. Pathology and Discrete Typing Unit Associations of Trypanosoma Cruzi Infection in Coyotes (Canis latrans) and Raccoons (Procyon lotor) of Texas, USA. J Wildl Dis 2020; 56:134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk AE, Goodwin DG, Zajac AM, Barr SC, et al. Prevalence of antibodies to Trypanosoma cruzi, Toxoplasma gondii, Encephalitozoon cuniculi, Sarcocystis neurona, Besnoitia darlingi, and Neospora caninum in North American opossums, Didelphis virginiana, from southern Louisiana. J Parasitol 2010; 96:1119–1122. [DOI] [PubMed] [Google Scholar]
- Kjos SA, Marcet PL, Yabsley MJ, Kitron U, et al. Identification of bloodmeal sources and Trypanosoma cruzi infection in triatomine bugs (Hemiptera: Reduviidae) from residential settings in Texas, the United States. J Med Entomol 2013; 50:1126–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lages-Silva E, Crema E, Ramirez LE, Macedo AM, et al. Relationship between Trypanosoma cruzi and human chagasic megaesophagus: Blood and tissue parasitism. Am J Trop Med Hyg 2001; 65:435–441. [DOI] [PubMed] [Google Scholar]
- LOPH. Chagas Disease- American Trypanosomiasis Annual Report. Baton Rouge, LA: Louisiana Office of Public Health Infectious Disease Epidemiology Section, 2018. [Google Scholar]
- Lynn MK, Bossak BH, Sandifer PA, Watson A, et al. Contemporary autochthonous human Chagas disease in the USA. Acta Trop 2020; 205:105361. [DOI] [PubMed] [Google Scholar]
- Mace S, Shelton GD, Eddlestone S. Megaesophagus. Compend Contin Educ Vet 2012; 34:E1. [PubMed] [Google Scholar]
- Majeau A, Pronovost H, Sanford A, Cloherty E, et al. Raccoons As an Important Reservoir for Trypanosoma cruzi: A Prevalence Study from Two Metropolitan Areas in Louisiana. Vector Borne Zoonotic Dis 2020; 20:535–540. [DOI] [PubMed] [Google Scholar]
- Margiocco ML, Scansen BA, Bonagura JD. Camelid cardiology. Vet Clin North Am Food Anim Pract 2009; 25:423–454. [DOI] [PubMed] [Google Scholar]
- Marsden PD, Seah SK, Draper CC, Pettitt LD, et al. Experimental Trypanosoma cruzi infections in rhesus monkeys. II. The early chronic phase. Trans R Soc Trop Med Hyg 1976; 70:247–251. [DOI] [PubMed] [Google Scholar]
- McKenzie EC, Seguin B, Cebra CK, Margiocco ML, et al. Esophageal dysfunction in four alpaca crias and a llama cria with vascular ring anomalies. J Am Vet Med Assoc 2010, 237:311–316. [DOI] [PubMed] [Google Scholar]
- Moser DR, Kirchhoff LV, Donelson JE. Detection of Trypanosoma cruzi by DNA amplification using the polymerase chain reaction. J Clin Microbiol 1989; 27:1477–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mubiru JN, Yang A, Dick Jr. EJ, Owston M, et al. Correlation between presence of Trypanosoma cruzi DNA in heart tissue of baboons and cynomolgus monkeys, and lymphocytic myocarditis. Am J Trop Med Hyg 2014; 90:627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura M, Correa Neto A. Experimental production of “megas” in animals inoculated with Trypanosoma cruzi. Rev Hosp Clin Fac Med Sao Paulo 1961; 16:338–341. [PubMed] [Google Scholar]
- Pronovost H, Peterson AC, Chavez BG, Blum MJ, et al. Deep sequencing reveals multiclonality and new discrete typing units of Trypanosoma cruzi in rodents from the southern United States. J Microbiol Immunol Infect 2018; 53:622–633. [DOI] [PubMed] [Google Scholar]
- Rodriques Coura J, de Castro SL. A critical review on Chagas disease chemotherapy. Mem Inst Oswaldo Cruz 2002; 97:3–24. [DOI] [PubMed] [Google Scholar]
- Sukon P, Timm KI, Valentine BA. Esophageal anatomy of the Llama (Llama glama). Int J Morphol 2009; 27:811–817. [Google Scholar]
- Teixeira AR, Nitz N, Guimaro MC, Gomes C, et al. Chagas disease. Postgrad Med J 2006; 82:788–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira AR, Hecht MM, Guimaro MC, Sousa AO, et al. Pathogenesis of Chagas' disease: Parasite persistence and autoimmunity. Clin Microbiol Rev 2011; 24:592–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakare R, Dasgupta A, Chopra S. Update on nifurtimox for treatment of Chagas disease. Drugs Today (Barc) 2021; 57:251–263. [DOI] [PubMed] [Google Scholar]
- Traynor, K. Benznidazole approved for Chagas disease in children. Am J Health Syst Pharm 2017; 74:1519. [DOI] [PubMed] [Google Scholar]
- Waleckx E, Suarez J, Richards B, Dorn PL. Triatoma sanguisuga blood meals and potential for Chagas disease, Louisiana, USA. Emerg Infect Dis 2014; 20:2141–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watrous BJ, Pearson EG, Smith BB, Snyder SP, et al. Megaesophagus in 15 llamas: A retrospective study (1985–1993). J Vet Intern Med 1995; 9:92–99. [DOI] [PubMed] [Google Scholar]
- WHO. Chagas disease in Latin America: An epidemiological update based on 2010 estimates. Wkly Epidemiol Rec 2015; 90:33–43. [PubMed] [Google Scholar]
- Wincker P, Britto C, Pereira JB, Cardoso MA, et al. Use of a simplified polymerase chain reaction procedure to detect Trypanosoma cruzi in blood samples from chronic chagasic patients in a rural endemic area. Am J Trop Med Hyg 1994; 51:771–777. [DOI] [PubMed] [Google Scholar]
- Yaeger RG. The prevalence of Trypanosoma cruzi infection in armadillos collected at a site near New Orleans, Louisiana. Am J Trop Med Hyg 1988; 38:323–326. [DOI] [PubMed] [Google Scholar]
- Yauri V, Castro-Sesquen YE, Verastegui M, Angulo N, et al. Domestic Pig (Sus scrofa) as an Animal Model for Experimental Trypanosoma cruzi Infection. Am J Trop Med Hyg 2016; 94:1020–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca IB, Hodo CL, Slack S, Auckland L, et al. Prevalence of Trypanosoma cruzi infection and associated histologic findings in domestic cats (Felis catus). Vet Parasitol 2020; 278:109014. [DOI] [PubMed] [Google Scholar]