Abstract
Disruption of circadian rhythms occurs in rotating shift-work, jetlag, and in individuals with irregular sleep schedules. Circadian disruption is known to alter inflammatory responses and impair immune function. However, there is limited understanding of how circadian disruption modulates cancer-induced inflammation. Inflammation is a hallmark of cancer and is linked to worse prognosis and impaired brain function in cancer patients. Here, we investigated the effect of circadian disruption on cancer-induced inflammation in an orthotopic breast cancer model. Using a validated chronic jetlag protocol that advances the light-cycle by 8 h every 2 days to disrupt circadian rhythms, we found that circadian disruption alters cancer-induced inflammation in a tissue-specific manner, increasing inflammation in the body and brain while decreasing inflammation within the tumor tissue. Circadian disruption did not affect inflammation in mice without tumors, suggesting that the impact of circadian disruption may be particularly detrimental in the context of underlying inflammatory conditions, such as cancer. Importantly, circadian disruption did not affect tumor burden, suggesting that increased inflammation was not a result of increased cancer progression. Overall, these findings identify the importance of healthy circadian rhythms for limiting cancer-induced inflammation.
Keywords: Chronic jetlag, 4T1 breast Cancer, Cytokines, Neuroinflammation, Metastasis, Clock genes, Circadian rhythms
Highlights
-
•
Circadian disruption enhances cancer-induced inflammation in the body and brain.
-
•
The profile of inflammatory cytokines altered by circadian disruption is tissue specific.
-
•
Changes in inflammatory profiles by circadian disruption are not due to enhanced tumor burden.
1. Introduction
Disruption of circadian rhythms affects the immune system and alters inflammatory responses (Cuesta et al., 2016). Circadian rhythms are endogenously generated cycles that regulate many physiological processes, including metabolism, cellular proliferation, immune function, and neurocognitive function (Baxter and Ray, 2020). In mammals, the suprachiasmatic nucleus of the hypothalamus is the body's master circadian clock, which synchronizes and aligns circadian rhythms throughout the body in response to environmental light/dark patterns (Weaver, 1998). Disruption to light/dark patterns can cause disruption of circadian rhythms (Vetter, 2020). Shift-work, trans-meridian flights (jetlag), and pervasive exposure to artificial light at night are all known to disrupt circadian rhythms (Cain et al., 2020; Pilorz et al., 2018).
The importance of robust circadian rhythms for health and well-being is now accepted (Allada and Bass, 2021), and disruption of circadian clocks is associated with many chronic health issues including cancer. Epidemiological studies have demonstrated increased cancer rates in populations susceptible to circadian disruption like shift workers (Fagundo-Rivera et al., 2020; Pariollaud and Lamia, 2020), and circadian rhythm disruption has been shown to influence efficacy and toxicity of cancer treatments like chemotherapy in patients (Hrushesky, 1985; Lévi, 1996). Preclinical cancer models support these findings with evidence that circadian disruption enhances cancer progression (Aiello et al., 2020), impairs anti-cancer immunity (Hadadi et al., 2020), and promotes tumor resistance to chemotherapy (Xiang et al., 2015, 2019). One key mechanism linking the circadian system and cancer is inflammation (Sulli et al., 2019), which is likely bidirectional. Inflammation has been demonstrated to disrupt circadian rhythms and blunt clock gene expression (Guo et al., 2015; Xiong et al., 2019). On the other hand, disruption of circadian rhythms affects the immune system and alters inflammatory responses (Cuesta et al., 2016). Moreover, cancer itself has been shown to flatten circadian rhythms in Ly6cHi (proinflammatory) monocytes in a mouse model of breast cancer (Sullivan et al., 2019).
Inflammation is a hallmark of cancer (Hanahan and Weinberg, 2011) that promotes cancer progression (Deshmukh et al., 2019), and is associated with poor prognosis (Diakos et al., 2014), reduced treatment efficacy (Slaviero et al., 2003), and cancer-related morbidities including fatigue and cognitive and affective dysfunction (Lacourt et al., 2018; Pyter et al., 2009; Walker et al., 2018). Inflammatory cytokines can be produced by tumor cells (Walker et al., 2018) or tumor-associated stromal cells (Solinas et al., 2009), and once in circulation can have detrimental systemic effects (Diakos et al., 2014). Cancer-induced inflammation can also promote the synthesis of pro-inflammatory mediators in organs distant to the tumor site, including the brain (Santos et al., 2019). Treatment with the anti-inflammatory drug aspirin reduces long-term mortality from cancer (Rothwell et al., 2011) and reduces distant metastasis (Rothwell et al., 2012), demonstrating that inflammation can directly affect long-term outcomes in clinical populations. While it has been shown in non-cancer settings that circadian disruption dysregulates inflammatory responses (Baxter and Ray, 2020) and is linked to cognitive and affective dysfunction (Karatsoreos et al., 2011; Otsuka et al., 2020), it is not known whether circadian disruption modulates cancer-induced inflammation.
Here, we implanted mammary carcinoma cells into mice with established circadian disruption to determine how circadian disruption affects cancer-induced inflammation. Using a validated chronic jetlag protocol to disrupt circadian rhythms we investigated changes in cancer-associated inflammatory gene expression in distinct sites of the body and brain - the hypothalamus which houses the master clock (suprachiasmatic nucleus), the liver which is anatomically and functionally distinct from the orthotopic tumor site (mammary fatpad) and known to be sensitive to circadian disruption (Ferrell and Chiang, 2015), and the tumor itself. We also explored whether altered inflammation was associated with changes in primary tumor growth, metastasis, memory impairment, and depression-like behavior. We chose to explore the impact of circadian disruption using 4T1.2 mammary tumor-induced inflammation because the link between breast cancer and circadian rhythms is well established in the clinic and in preclinical studies (Fagundo-Rivera et al., 2020; Sullivan et al., 2019), and we have previously characterized the effect of mammary tumors on memory and sickness behavior using this model (Walker et al., 2018). The findings demonstrate that circadian disruption enhanced the inflammatory profile induced by cancer, which may have important implications for survival outcomes and quality of life for cancer patients exposed to environmental factors that disrupt circadian rhythms.
2. Methods
2.1. Animals and ethics
80 BALB/c female mice (8–12 weeks old) (Monash University, Australia) were used in this study and housed individually in standard shoebox cages in a temperature and humidity-controlled environment. Food and water were available ad libitum. All procedures involving mice were carried out under protocols approved by the Monash University Animal Ethics Committee (protocol number MIPS.2017.46) and in accordance with National Health and Medical Research Council guidelines. Animals were monitored daily to ensure they were not experiencing distress.
2.2. Experimental design
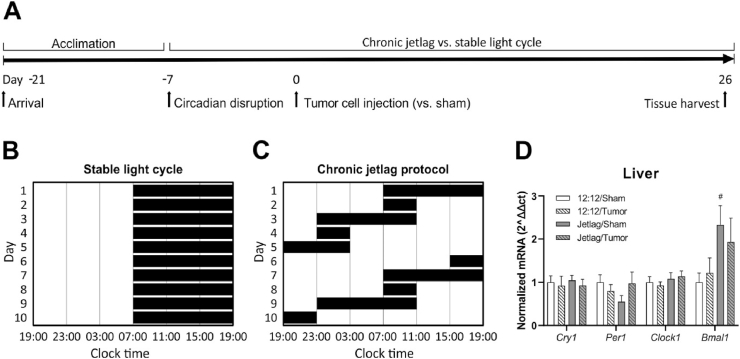
Mice were randomly allocated to experimental groups of 10 per group (12:12 light-dark cycle ± tumors; jetlag ± tumors) across 2 replications. Briefly, one week prior to tumor cell or vehicle injection, mice remained on either a 12:12 h light:dark schedule or began exposure to the jetlag protocol. Tumor growth, metastasis, body weights and behavior were assessed across the course of the experiment until day 26 when the light schedules of mice aligned and tissue was collected for examination. The experimental timeline is outlined in Fig. 1A.
Fig. 1.
Experimental design and effect of chronic jetlag on clock gene expression. A. Experimental design used to evaluate the effects of chronic jetlag on cancer-induced inflammation. B, C. Graphical representations of the stable and rotating light schedules used to induce chronic jetlag. D. Expression of clock genes in liver tissue as determined using qRT-PCR (n = 3–5 per group). Error bars represent SEM. # indicates significant effect of chronic jetlag exposure at the p < 0.05 level.
2.3. 4T1.2 mammary adenocarcinoma cell line
4T1.2 tumor cells, syngenic to BALB/c mice, were used in this study (Lelekakis et al., 1999; Sloan et al., 2004). Tumor cells were transduced to express codon-optimized luciferase and mCherry under control of the ubiquitin C promoter (Kaminskas et al., 2013). Cell line identity was confirmed by short tandem repeat profiling (Cellbank, Australia). Cells were determined to be mycoplasma free before injection using a MycoAlert™ Mycoplasma Detection Kit (Lonza, Australia) or using primers as previously described (Uphoff and Drexler, 2002).
2.4. Breast cancer model
For this study, the 4T1.2 mammary carcinoma cell line was chosen as 4T1 derived tumors have been shown to induce higher concentrations of proinflammatory cytokines in the brain, liver and serum compared to other mammary tumor cell lines (Walker et al., 2017). Furthermore, we have previously characterized the cytokines and chemokines released by these cells in vitro (Walker et al., 2018). 1 × 105 4T1.2 luciferase tagged tumor cells were injected in 20 μL PBS (Invitrogen, USA) into the left 4th mammary gland of anesthetized (3% isoflurane) mice. Control (sham) non-tumor bearing mice received injections of 20 μL PBS into the left 4th mammary fat pad under anesthesia. Primary tumor growth was monitored by bioluminescence imaging under 3% isoflurane anesthesia until tumors became palpable and signal saturation occurred (after day 10 of growth). Tumors were then monitored by digital calliper every 2–3 days with primary tumor volume calculated using the formula: (length × width2)/2. Distant metastasis was monitored twice weekly by bioluminescence imaging under 3% isoflurane anesthesia. Mice were injected with d-luciferin (150 mg/kg, Promega) via tail vein, and primary tumor burden and metastasis was quantified using an IVIS Lumina II (PerkinElmer) (Kim-Fuchs et al., 2014; Sloan et al., 2010). Non-tumor bearing mice that received PBS underwent identical procedures as tumor bearing mice including anesthesia.
2.5. Circadian rhythm disruption
To determine the impact of circadian disruption on cancer-induced inflammation, the light schedule of mice in the experimental group was advanced manually by setting an analogue light switch timer forward by 8 h every two days. For example, lights may turn on at 0700 and off at 1900 but turn back on at 1100 allowing only 4 h of darkness that day, whereas control mice were kept on a stable 12:12 h light:dark cycle (Fig. 1C). This chronic jetlag protocol disrupts circadian rhythms in rodents (Filipski et al., 2004), without impacting total sleep quantity (Gao et al., 2020). Mice in the control condition were maintained on a stable 12:12 h light:dark schedule (Fig. 1B). To avoid exposing control mice to light during scheduled dark periods, which enhances tumor progression (Agbaria et al., 2019), all testing was conducted during the light phase, within 4 h of lights-on relative to each light cycle condition. Upon arrival at our institute, mice were stabilized on a consistent 12:12 h light:dark schedule for a minimum of two weeks, after which half of the mice began the chronic jetlag protocol. Mice were maintained on either the stable light/dark schedule or the chronic jetlag schedule starting one week prior to tumor cell injection and continuing until the end of the experiment. During light phases, light levels were a maximum of 170 lux in the housing room, using overhead fluorescent white light. During dark phases, mice were kept in very low light levels (<0.3 lux). The timing of euthanasia and tissue collection for molecular and biochemical analyses occurred on a day when the timing of the light schedule coincided for both the control and chronic jetlag groups.
2.6. Behavioral testing
Memory and depression-like behavior were used to examine changes in mood and neurocognitive function in response to circadian disruption and mammary adenocarcinoma. Experimenters were unaware of the treatment conditions of mice during behavioral testing. Memory changes were assessed using the novel object/novel place recognition test and depression-like behavior using the forced swim and sucrose preference tests. All mice underwent testing in the novel object/novel place recognition test and forced swim test (n = 20 per group). Half of the mice in each group were tested on the sucrose preference test (n = 10 per group).
Memory assessment. The novel object/novel place recognition test was conducted in a separate testing room under low lighting (<1.3 lux, at level of test arena), achieved with a low wattage lamp facing into a corner of the testing room. Mice were habituated and tested within a 40 min window. Mice were tested one week after initiation of the chronic jetlag protocol (i.e. 24 h prior to tumor cell injection) and one week after. We have established previously that decreased performance on the novel object/novel place recognition test is observable within a week of tumor inoculation (Walker et al., 2018) and thus, an examination past this time point, as cancer burden becomes greater, was deemed unlikely to show possible synergistic effects between circadian disruption and tumor development on cognition.
To acclimate, mice were exposed to the arena 2–3 times for 5 min and then underwent habituation to the novel object/novel place recognition task 3–4 times prior to the start of the experiment to ensure mice reached 65% or greater novel object recognition. This was necessary to ensure mice had adequate exposure to the arena in order to reduce aversiveness given testing was conducted during their light phase when mice are less active. The novel object/novel place recognition test was performed and analyzed with Viewer III software (Biobserve GmbH, Bonn, Germany), as described previously (Walker et al., 2018). Novel object recognition was calculated by determining the percentage of time spent with the novel object divided by the total time exploring all the objects in the arena. Seventeen mice were excluded because they spent less than 20 s exploring the familiar objects in the learning phase.
Assessment of depression-like behavior. Sucrose preference was assessed by providing mice with access to two water bottles throughout the experiment. One bottle contained drinking water and the other bottle contained 1% (w/v) sucrose in drinking water. The percentage of sucrose solution over the total liquid consumed was calculated. The amount of plain water and sucrose solution consumed was weighed and changed every 2–3 days or whenever mice were removed from their cages for testing. Following tumor cell injection, sucrose preference for each mouse was averaged across three time periods: early (2–10 days post-injection), mid (10–17 days post-injection), and late (17–26 days post-injection) stages of cancer progression.
The forced swim test was recorded in complete darkness under infrared lighting 9 days after tumor cell injection as described previously (Walker et al., 2013). Briefly, mice were placed individually into a plastic cylinder (20 cm diameter) filled to a depth of 15 cm of water maintained at 24 ± 1 °C. The same water and bucket were used for a maximum of two mice before being changed. FST lasted for 6 min and mice were immediately returned to their home cage. The total time spent immobile was determined by an experimenter unaware of treatment conditions.
2.7. Tissue collection
Mice were euthanized using CO2 at 26 days after tumor cell injection. Culling and tissue collection occurred at a consistent time-point, between 2 and 4 h after lights on, with collection counterbalanced between groups. Following euthanasia, mice were transcardially perfused with PBS to clear blood from tissue which may confound gene expression analysis. Then, brains were removed and the hypothalamus dissected out and frozen immediately on dry ice. Livers, spleens and primary tumors were dissected, weighed, and frozen immediately on dry ice. All tissues were stored at −80 °C until processing.
2.8. Gene expression analysis
Quantitative real-time PCR was used to assess tissue-specific changes in the expression of genes related to inflammation, circadian rhythm regulation, and cellular senescence, caused by tumors and chronic jetlag. Tissue from mice that underwent all behavioral procedures was selected for gene expression analysis. Following extraction and quality control, final group sizes used for gene expression analysis for each tissue are: Hypothalamus: 12:12/Sham (n = 7), 12:12/Tumor (n = 6), Jetlag/sham (n = 4), Jetlag/Tumor (n = 5); Liver: 12:12/Sham (n = 4), 12:12/Tumor (n = 3), Jetlag/sham (n = 5), Jetlag/Tumor (n = 4); and Tumor: 12:12/Tumor (n = 8), Jetlag/Tumor (n = 8). RNA was extracted from the hypothalamus, liver and primary tumor using the Trizol method (Invitrogen, Carlsbad, CA, USA) following the manufacturer's protocol (Life Technologies). Assessment of RNA quality and concentration was carried out with the Agilent Technologies 2100 Bioanalyzer and Nanodrop ND-1000 spectrophotometer. Complementary DNA (cDNA) was synthesized from 1 μg total RNA per sample using SuperScript® First-Strand Synthesis Kit IV and random hexamers following the manufacturer's protocol (Life Technologies). We measured expression of the genes Per1, Cry1, Clock and Bmal1/Arntl for their role in the regulation of circadian rhythms. We measured the cytokine-related genes Il6, Il1b, Il10, Tnf, and Ifngr2 for their role in inflammatory processes, and Cox2 and Stat3 for their regulatory roles in cytokine signaling. The mRNA expression of 15 genes was measured in a 7900HT Fast Real-Time PCR System (Applied Biosystems) using pre-designed Taqman Gene Expression Assays for: 1) inflammation genes: Il6 (Mm00446190_m1), Il1b (Mm00434228_m1), Il10 (Mm01288386_m1), Tnf (Mm00443258_m1), Ifngr2 (Mm00492626_m1), Cox2 (Mm03294838_g1), and Stat3 (Mm01219775_m1); 2) circadian clock genes: Per1 (Mm00501813_m1), Cry1 (Mm00514392_m1), Clock (Mm00455950_m1), Bmal1/Arntl (Mm00500223_m1); and 3) housekeeper genes: Tbp (Mm00446973_m1) and Ubc (Mm01201237_m1). No reverse transcriptase controls and no template controls were included to rule out genomic DNA contamination and reagent contamination, respectively. Normalized relative quantities (2−ΔΔCt) of each mRNA were calculated for each gene and normalized against the geometric mean of Tbp and Ubc for each mouse, that did not differ between groups.
2.9. Statistical analysis
Two-way ANOVAs (light cycle, tumor, light cycle × tumor) were used to assess the effects of 4T1.2 tumors and circadian disruption on: expression of each gene in liver or hypothalamus; behavior in the sucrose preference test, forced swim test or novel object/novel place recognition test; and spleen weight. There were two between-subjects factors – light cycle (two levels: control vs. chronic jetlag) and tumor (two levels: 4T1.2 vs. PBS vehicle control). Mixed-design repeated-measures ANOVAs were used to assess the effects of 4T1.2 tumor cells and light cycle disruption on sucrose preference following tumor cell injection, bodyweight and primary and metastatic tumor growth with ‘time’, defined as days post-tumor injection as the within-subjects factor. A Greenhouse-Geisser correction epsilon (ε) was used to correct for violations of sphericity. Where significant main or interaction effects were found, post-hoc Fisher's least significant difference tests were used to test for pairwise differences between groups. One-way ANOVAs were used to examine the effects of the light cycle (control vs. chronic jetlag) on the expression of each gene in primary tumors. Independent samples t-tests were used to assess differences in final tumor mass, sucrose preference, and bodyweight before 4T1.2 tumor cell injection. Significance was set a priori at p < 0.05 (two-tailed).
3. Results
3.1. Chronic jetlag alters clock gene expression in the liver
To confirm that chronic jetlag exposure disrupted circadian rhythms, we evaluated changes in expression of clock genes, Clock1, Bmal1, Cry1, and Per1 in liver and brain. Chronic jetlag exposure increased Bmal1 expression in the liver of non-tumor bearing mice (jetlag main effect; F(1, 12) = 4.052, p = 0.038); Fig. 1D). Bmal1 is a key component of the mammalian molecular clock, where the Clock1 and Bmal1 proteins form dimers that inhibit Bmal1 and Clock1 transcription, creating a transcriptional/translational feedback loop with a ∼24 h cycle (Hernandez-Rosas et al., 2020). No other statistically significant differences in circadian gene expression in the liver or hypothalamus were found. These findings are consistent with evidence demonstrating that the chronic jetlag protocol causes phase-shifting in clock gene expression profiles (Iwamoto et al., 2014).
3.2. Tumors induce inflammation in the liver and hypothalamus which is exacerbated by chronic jetlag
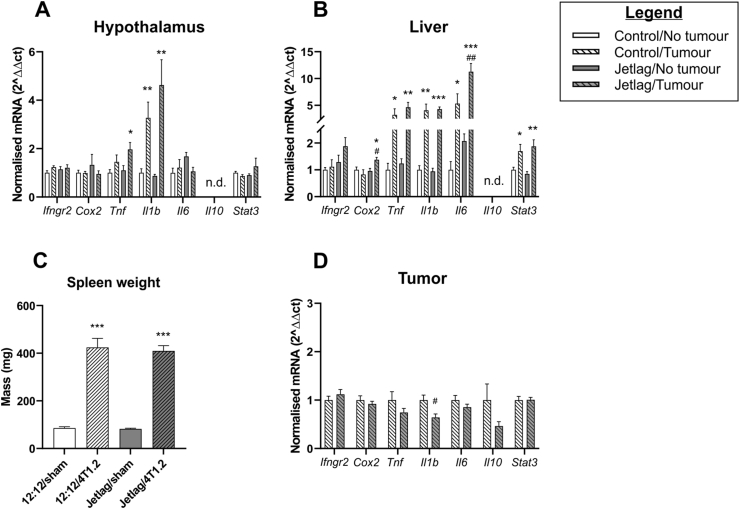
To determine how chronic jetlag impacts cancer-induced neuroinflammation, we assessed cytokine and Stat3 gene expression within the hypothalamus. The hypothalamus was selected because it is known to be sensitive to changes in peripheral inflammation (Radler et al., 2014) and because it houses the suprachiasmatic nucleus, the master circadian clock (Weaver, 1998). Tumors increased expression of hypothalamic Tnf (tumor main effect: F(1, 16) = 7.600, p = 0.014) and Il1b (tumor main effect: F(1, 16) = 26.142, p < 0.001). Post-hoc analysis revealed that only tumor bearing mice exposed to chronic jetlag demonstrated increased hypothalamic Tnf expression, suggesting that chronic jetlag affects cancer-induced inflammation in this organ (Fig. 2A).
Fig. 2.
Effect of jetlag on cancer-induced inflammation. Expression of inflammation-related genes as determined using qRT-PCR in: A. hypothalamus; B. liver. C. Splenomegaly induced by tumors. D. Expression of inflammation-related genes as determined using qRT-PCR in tumors. Error bars represent SEM. ∗, ∗∗, ∗∗∗ indicates statistically significant differences between tumor bearing mice and non-tumor bearing mice exposed to the same light-cycle condition at the p < 0.05, 0.01 and 0.001 levels, respectively, as determined using post-hoc analysis. #, ## indicates statistically significant differences between control and jetlag exposed tumor-bearing mice at the p < 0.05 and 0.01 levels, respectively, as determined using post-hoc analysis.
To determine how chronic jetlag impacts cancer-induced inflammation in the body, we assessed transcription of cytokines in the liver, and Stat3, a key transcriptional regulator of inflammatory cytokines (Yu et al., 2009). The liver was chosen because liver function is tightly controlled by circadian rhythms and circadian disruption alters gene expression in this organ (Ferrell and Chiang, 2015). While the presence of a tumor on its own had no effect on hepatic Cox2 expression, chronic jetlag induced hepatic Cox2 expression (chronic jetlag × tumor interaction: F(1, 12) = 6.547, p = 0.025; chronic jetlag main effect; F(1, 12) = 4.782, p = 0.049), and post-hoc analysis showed this interaction in tumor bearing mice only (Fig. 2B). While the presence of a tumor increased Il6 expression in the liver, chronic jetlag nearly doubled cancer-induced hepatic Il6 expression (chronic jetlag main effect: F(1, 10) = 8.467, p = 0.016; tumor main effect: F(1, 10) = 31.560, p < 0.001; Fig. 2B). Tumors increased expression of hepatic Tnf (tumor main effect: F(1, 12) = 20.073, p = 0.001), Il1b (tumor main effect: F(1, 12) = 45.016, p < 0.001), and Stat3 (tumor main effect; F(1, 12) = 25.311, p < 0.001), but these factors were unaffected by chronic jetlag (Fig. 2B).
To determine if changes in brain and liver inflammation were associated with other markers of peripheral inflammation we assessed spleen weight: a measure of myeloid cell expansion and inflammation in tumor bearing mice (Bronte and Pittet, 2013; Steenbrugge et al., 2019; Walker et al., 2018; Walker et al., 2017). Spleen mass increased in tumor bearing mice compared to non-tumor bearing mice, as previously reported (Walker et al., 2018, Fig. 2C), while exposure to chronic jetlag had no effect on splenomegaly caused by tumors (tumor main effect: F(1, 72) = 236.854, p < 0.001) (Fig. 2C). These findings show that chronic jetlag exerted organ-specific effects on expression of inflammatory cytokines in cancer-bearing mice, but not in tumor-free mice.
3.3. Chronic jetlag exposure decreased expression of Il1b in tumors
Inflammatory signaling within the tumor microenvironment affects tumor progression and metastasis (Shalapour and Karin, 2015). To determine how chronic jetlag impacts inflammation within the tumor microenvironment, we assessed cytokine and Stat3 gene expression in tumor tissue. Chronic jetlag decreased Il1b expression in tumors (chronic jetlag main effect: F(1, 13) = 8.438, p = 0.012), but did not modulate expression of other cytokines or Stat3 (Fig. 2D).
3.4. Changes in inflammation caused by chronic jetlag are not the result of increased primary tumor size or metastasis
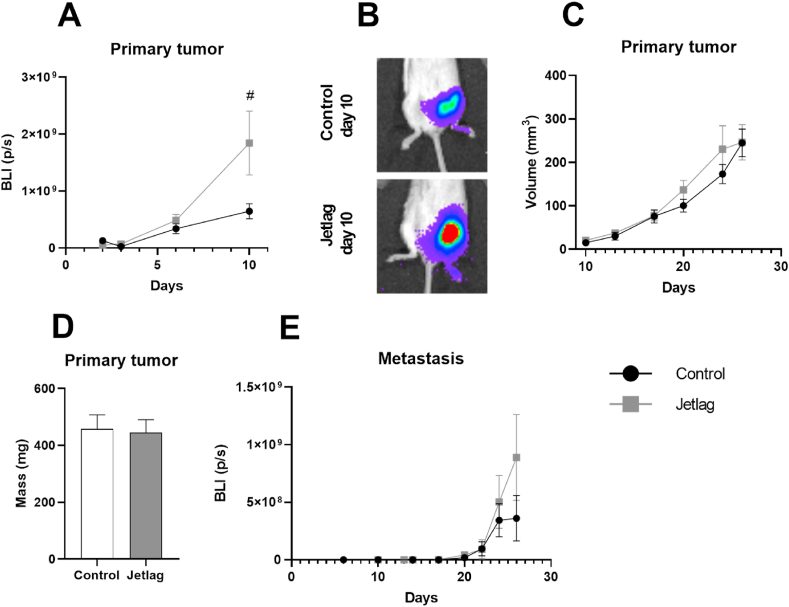
To determine whether the effects of chronic jetlag on cancer-induced inflammation were a consequence of changes in cancer burden, we assessed in vivo changes in primary tumor growth and metastasis using bioluminescence imaging. Chronic jetlag increased primary tumor growth 10 days after tumor cell injection as measured by bioluminescence imaging (chronic jetlag main effect: F(1, 38) = 4.797, p = 0.035; time main effect: F(1.078, 40.979) = 12.805, p = 0.001, ε = 0.539) (Fig. 3A and B). However, these differences were not reflected in calliper measurements following day 10 (chronic jetlag main effect: F(1, 32) = 0.025, p = 0.876; time main effect: F(2.076, 66.427) = 27.477, p < 0.001, ε = 0.692; Fig. 3C), nor in the final tumor mass weighed ex vivo (t(36) = 0.87, p = 0.853; Fig. 3D). Chronic jetlag did not significantly affect metastasis (chronic jetlag main effect: F(1, 34) = 1.294, p = 0.263; time main effect: F(1.728, 58.767) = 7.125, p = 0.003, ε = 0.576) (Fig. 3E). In summary, there was no significant difference in cancer burden at the end of the experiment, when assessment of inflammatory gene expression was conducted.
Fig. 3.
Effects of chronic jetlag on tumor growth and metastasis. A. Bioluminescent imaging of early tumor growth. B. Representative images of primary tumor size at day 10 for control and jetlag-treated tumor bearing mice. C. Primary tumor growth from day 10–26 as measured with callipers. D. Primary tumor mass measured ex-vivo. E. Metastasis progression measured by bioluminescent imaging. Days are measured post-4T1.2 tumor cell injection. Error bars represent SEM. # indicates significant effect of chronic jetlag exposure at the p < 0.05 level. BLI (p/s): bioluminescence intensity (photons/second).
3.5. Impact of chronic jetlag on behavior and cognition
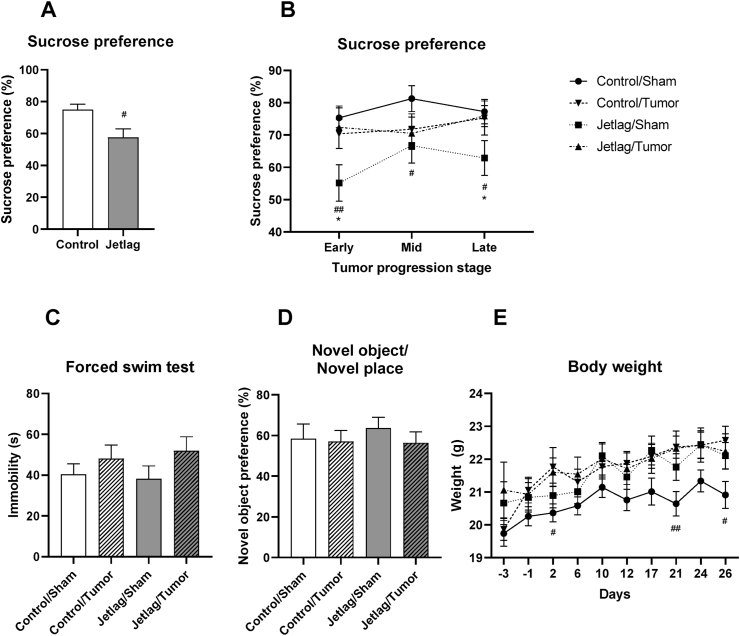
Because inflammation is causally implicated in cancer-induced depression and memory impairment (Marquie et al., 2015; Otsuka et al., 2020; Pyter et al., 2009; Walker et al., 2018), we assessed the impact of chronic jetlag on cancer-induced depression-like behavior and memory impairment. To assess changes in mood-related outcomes, performance in the sucrose preference test and forced swim test were evaluated. Chronic jetlag exposure promoted anhedonia in non-tumor bearing mice, decreasing sucrose preference compared to control mice maintained in stable light conditions (t(18) = 2.765, p = 0.013) (Fig. 4A). Chronic jetlag exposure decreased sucrose preference in non-tumor bearing mice only across early (2–10 days post-injection), mid (10–17 days post-injection), and late (17–26 days post-injection) stages of cancer progression (Fig. 4B; chronic jetlag × tumor interaction: F(1, 35) = 5.452, p = 0.025; chronic jetlag main effect: F(1, 35) = 4.862, p = 0.034). The absence of jetlag-induced anhedonia in tumor bearing mice may reflect changes in metabolism caused by cancer (Schwartsburd, 2019) rather than a change in anhedonia per se. Tumor bearing mice showed a trend towards increased immobility in the forced swim test (tumor main effect: F(1, 73) = 3.124, p = 0.081), but this was not affected by chronic jetlag (F(1, 73) = 0.278, p = 0.6; Fig. 4C).
Fig. 4.
Effects of chronic jetlag exposure and tumors on depression-like behavior, memory and sickness response. A. Anhedonia assessed by sucrose preference test prior to 4T1.2 tumor cell injection in jetlag exposed mice. B. Anhedonia assessed by sucrose preference test in sham and 4T1.2 tumor bearing mice exposed to jetlag. C. Changes in depression-related behavior in the forced swim test. D. Memory performance assessed in the novel object/novel place recognition test. E. Bodyweight change throughout the experiment (tumor cell injected on day 0). Error bars represent SEM. #, ## indicates significant effect of jetlag exposure at the p < 0.05 and 0.01 levels, respectively. ∗ indicates significant effect of 4T1.2 tumor cell injection at the p < 0.05 level.
The novel object/novel place recognition test is a functional tool to assess cognitive deficits in cancer models (Walker et al., 2018). Neither chronic jetlag nor tumors affected novel object recognition at 7 days post 4T1.2 tumor cell injection (chronic jetlag main effect: F(1, 58) = 0.154, p = 0.696; tumor main effect: F(1, 58) = 0.535, p = 0.468; Fig. 4D).
To determine if behavioral findings were confounded by sickness, which occurs in the late stages (>26 days) of 4T1.2 cancer progression (Walker et al., 2018), we measured bodyweight. All mice gained weight from the start of the experiment (time main effect: F(3.108, 229.976) = 19.625, p < 0.001, ε = 0.444; Fig. 4E) suggesting that tumor bearing mice were not overtly sick by the end of the experiment. Exposure to chronic jetlag increased body weight among non-tumor bearing mice, but not tumor bearing mice following tumor cell or sham injection (day 1 – day 26) (time × chronic jetlag interaction; F(3.108, 229.976) = 3.566, p = 0.014, ε = 0.444; Fig. 4E). This is consistent with evidence demonstrating that circadian disruption is linked to metabolic dysfunction (for review: (Casiraghi et al., 2016; Shetty et al., 2018). Exposure to chronic jetlag did not affect bodyweight prior to 4T1.2 tumor cell injection (day −3 – day 0) (t(78) = 1.089, p = 0.279; Fig. 4E). These findings showed that exposure to chronic jetlag reduced sucrose preference and increased weight-gain in non-tumor bearing mice only.
4. Discussion
This study demonstrates for the first time that disruption of circadian rhythms in tumor bearing mice exacerbates cancer-induced inflammation, independently of changes in primary tumor growth or metastasis. Circadian disruption increased inflammation in the presence of cancer only, suggesting that circadian disruption may be particularly detrimental for cancer patients. These effects were tissue and cytokine specific, which may have implications for optimising treatment using systemic cytokine targeting therapies. Because the impact of inflammation on cancer-related processes, such as metastasis, can be mediated by tumor cell-type (Greten and Grivennikov, 2019), understanding how circadian disruption impacts inflammatory processes throughout the body, and in different types of cancer, will be critical to improving outcomes in cancer patients with circadian disruption. Together with previous evidence demonstrating a role for inflammation in causing cancer-related cognitive dysfunction and poor prognosis, these findings highlight the importance of healthy circadian rhythms in managing cancer-induced inflammation.
Circadian disruption enhanced cancer-induced inflammation in the brain and the body, but the inflammatory profile was tissue-dependent. Specifically, cancer-induced Tnf was potentiated in the hypothalamus by chronic jetlag whereas cancer-induced transcription of Il6 was sensitive to circadian disruption in the liver. These findings suggest that inflammation-mediated processes may be more vulnerable to disruption in cancer patients exposed to circadian disruption. For example, TNF signaling in the hypothalamus potentiates HPA-axis activity (Silverman et al., 2005), and the impact of chronic inflammation on HPA-axis function is linked to fatigue and depression in cancer patients (Ahmad et al., 2021; Schmidt et al., 2016). Meanwhile, hepatic Il-6 is an important regulator of the acute phase response (Ehlting et al., 2021) and is involved in metastatic spread in some types of breast cancer (Ara and Declerck, 2010; Asgeirsson et al., 1998; Chen et al., 2018), processes that may be further impacted in cancer patients experiencing circadian disruption. The impact of these organ-specific changes on the efficacy of systemic cytokine targeting therapies (e.g. anti-Il-6 antibodies) or associated side effects in cancer patients with circadian disruption is unknown, and may be an important consideration for optimising treatment with these therapies.
In contrast to increased inflammation in the hypothalamus and liver, circadian disruption reduced Il1b expression within the tumor. Within the tumor microenvironment, interleukin-1 is primarily produced by immune cells (Zhang et al., 2020), suggesting that decreased Il1b expression in tumors found here may reflect changes in the function or number of tumor-infiltrating immune cells (Wu et al., 2018). Consistent with that, circadian disruption has been linked to immunosuppression within the tumor microenvironment in a mouse model of spontaneous metastatic breast cancer (Aiello et al., 2020). However, Il1b is associated with both pro- and anti-tumor effects (Bent et al., 2018) and further work is needed to determine the clinical significance of these changes. Taken together, these findings demonstrate that circadian disruption exerts cytokine- and tissue-specific effects on cancer-induced inflammation. Because inflammatory microenvironments are also important regulators of pre-metastatic niche formation (Peinado et al., 2017) and organotropic metastasis (Chen et al., 2018), understanding how circadian disruption impacts inflammatory processes throughout the body, and in different types of cancer, will be critical to improving outcomes in cancer patients with circadian disruption.
Circadian disruption exerted limited effects on growth of the primary tumor or metastasis, which may be due to the tumor model or the type and timing of circadian disruption. Exposure to artificial light at night and disruption of the light/dark cycle are two common environmental methods used to dysregulate circadian rhythms in rodents. Artificial light at night suppresses melatonin synthesis, enhancing tumor progression in mice via melatonin-dependent mechanisms (Agbaria et al., 2019; Zubidat et al., 2018). Methodological heterogeneity, including different rodent strains, tumor types, and relative timing of circadian disruption to tumor initiation/induction and length of study, means the mechanisms linking chronic jetlag to enhanced tumorigenesis are less clear. Three of four prior studies using a comparable chronic jetlag protocol to this study, in similarly aggressive tumors models, found that circadian rhythm disruption accelerates growth of heterotopically transplanted osteosarcoma and pancreatic adenocarcinoma (Filipski et al., 2004, 2006), and Lewis lung carcinoma (Wu et al., 2012). Notably, the fourth study, using a cre-inducible lung tumor model, reported that circadian rhythm disruption after tumor initiation increased tumor growth but circadian rhythm disruption prior to tumor initiation did not (Papagiannakopoulos et al., 2016), which is consistent with our findings. This suggests that the specific timing of transition to circadian disruption may exert an important effect on tumor growth. While in vivo assessment of metastasis raised the possibility that circadian rhythms may modulate metastatic progression (Fig. 3E), different experimental designs are needed to determine this effect. For example, surgical resection of the primary tumor following the onset of metastasis would enable extended assessment of metastatic progression without the ethical constraints associated with large primary tumor size. Nevertheless, more research is required to determine how cancer progression is impacted by the susceptibility of different tumor types to circadian rhythm disruption, and how timing of the transition into circadian disruption impacts cancer progression. This may have important implications for human shift-workers, many of whom regularly transition between periods of recovery from, and exposure to, circadian disruption.
Here, we investigated if the increased inflammation caused by circadian rhythm disruption in tumor bearing mice exacerbated memory impairment and depression-like behavior. Disruption of circadian rhythms promoted anhedonia, but neither chronic jetlag nor tumor status affected memory or depression-like behavior in the forced-swim test. We and others have demonstrated that cancer impairs cognition and increases depression-like behaviors in rodents (Pyter et al., 2009; Walker et al., 2018); however, this effect is not always observed (Walker et al., 2017). In addition, chronic jetlag has been shown to impair memory and increase immobility in the forced swim test in rats (Horsey et al., 2019). In the present study, it is possible that testing during the light (inactive) phase of the light/dark cycle masked the effects of cancer and chronic jetlag on performance in the novel object/novel place recognition test and forced swim test. Indeed, control mice without tumors showed a 10% reduction in novel object preference compared to our previous findings in mice tested in the dark (active) phase of the light/dark cycle using the same tumor model, same sex, and same strain of mice in the same testing facility (Walker et al., 2018). Given circadian rhythm disruption exacerbated cancer-induced inflammation, and inflammation is strongly implicated in neuropsychiatric disorders (Bauer and Teixeira, 2019), further research is warranted to determine how elevated cancer-induced inflammation resulting from circadian rhythm disruption impacts cognition and affect-related behaviors.
While the findings of this study improve our understanding of how circadian disruption interacts with cancer-induced inflammation, some limitations should be considered when interpreting these findings. The timeframe of these experiments was constrained by ethical guidelines for metastatic disease. It is possible that changes in metastasis caused by circadian disruption would have been observable over a longer timeframe, as has been reported in a spontaneous metastatic breast cancer model (Hadadi et al., 2020), which progresses more slowly than the transplanted 4T1.2 model used here. Further research is needed to determine how circadian disruption affects metastasis in 4T1.2 mammary adenocarcinoma models. The use of cytokine gene-expression was selected as it allowed us to investigate tissue-specific inflammation without being confounded by the infiltration of systemic cytokines into tissue. It will be important for future work to determine how these changes in gene expression impact cytokine signaling function within different organs of the body and regions of the brain. Finally, future work should aim to determine how chronic circadian disruption impacts the temporal profile of cancer-induced inflammation, which may lead to optimized chronotherapeutic interventions in cancer patients with circadian disruption (Borniger et al., 2017).
5. Conclusion
Our findings demonstrate that circadian disruption exacerbates cancer-induced inflammation in the liver and hypothalamus and demonstrates that the profile of cancer-induced inflammation is organ specific. The findings indicate that inflammation beginning in the body is not always directly reflected in the brain and demonstrates that differences in inflammatory signatures between the body and brain can be further magnified by circadian disruption. Cancer-induced inflammation is mediated by cancer type, stage, treatment, and location, suggesting that the impact of circadian disruption inflammation may also be mediated by these factors. In cancer patients, inflammation is associated with decreased survival and treatment efficacy (Diakos et al., 2014), and elevated risk of cancer-associated side effects including pain, cognitive impairment, and depression (Diakos et al., 2014; Patel et al., 2015; Santos and Pyter, 2018). Therefore, understanding how circadian rhythm disruption impacts the inflammatory profile induced by different types of cancer will help tailor treatments for cancer patients, improving patient outcomes and quality of life.
Funding
This work was supported by the National Breast Cancer Foundation, Australia [PF-15-014]; the National Health and Medical Research Council, Australia [1147498]; Monash University Interdisciplinary Research Scheme Competitive, Australia; the Schizophrenia Research Institute, Australia and Neuroscience Research Australia (NeuRA), Australia. EKS is supported by the National Breast Cancer Foundation, Australia [IIRS-20-025] and Cancer Council Victoria Grants-in-Aid, Australia.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
EKS is a member of the SAB for Cygnal Therapeutics.
References
- Agbaria S., Haim A., Fares F., Zubidat A.E. Epigenetic modification in 4T1 mouse breast cancer model by artificial light at night and melatonin - the role of DNA-methyltransferase. Chronobiol. Int. 2019;36(5):629–643. doi: 10.1080/07420528.2019.1574265. [DOI] [PubMed] [Google Scholar]
- Ahmad M.H., Rizvi M.A., Fatima M., Mondal A.C. Pathophysiological implications of neuroinflammation mediated HPA axis dysregulation in the prognosis of cancer and depression. Mol. Cell. Endocrinol. 2021;520:111093. doi: 10.1016/j.mce.2020.111093. [DOI] [PubMed] [Google Scholar]
- Aiello I., Fedele M.L.M., Roman F., Marpegan L., Caldart C., Chiesa J.J., Golombek D.A., Finkielstein C.V., Paladino N. Circadian disruption promotes tumor-immune microenvironment remodeling favoring tumor cell proliferation. Sci. Adv. 2020;6(42) doi: 10.1126/sciadv.aaz4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allada R., Bass J. Circadian mechanisms in medicine. N. Engl. J. Med. 2021;384(6):550–561. doi: 10.1056/NEJMra1802337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ara T., Declerck Y.A. Interleukin-6 in bone metastasis and cancer progression. Eur. J. Cancer. 2010;46(7):1223–1231. doi: 10.1016/j.ejca.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgeirsson K.S., Olafsdottir K., Jonasson J.G., Ogmundsdottir H.M. The effects of IL-6 on cell adhesion and e-cadherin expression in breast cancer. Cytokine. 1998;10(9):720–728. doi: 10.1006/cyto.1998.0349. [DOI] [PubMed] [Google Scholar]
- Bauer M.E., Teixeira A.L. Inflammation in psychiatric disorders: what comes first? Ann. N. Y. Acad. Sci. 2019;1437(1):57–67. doi: 10.1111/nyas.13712. [DOI] [PubMed] [Google Scholar]
- Baxter M., Ray D.W. Circadian rhythms in innate immunity and stress responses. Immunology. 2020;161(4):261–267. doi: 10.1111/imm.13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bent R., Moll L., Grabbe S., Bros M. Interleukin-1 beta-A friend or foe in malignancies? Int. J. Mol. Sci. 2018;19(8) doi: 10.3390/ijms19082155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borniger J.C., Walker W.H., II, Gaudier-Diaz M.M., Stegman C.J., Zhang N., Hollyfield J.L., Nelson R.J., DeVries A.C. Time-of-Day dictates transcriptional inflammatory responses to cytotoxic chemotherapy. Sci. Rep. 2017;7:41220. doi: 10.1038/srep41220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte V., Pittet M.J. The spleen in local and systemic regulation of immunity. Immunity. 2013;39(5):806–818. doi: 10.1016/j.immuni.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain S.W., McGlashan E.M., Vidafar P., Mustafovska J., Curran S.P., Wang X., Mohamed A., Kalavally V., Phillips A.J.K. Evening home lighting adversely impacts the circadian system and sleep. Sci. Rep. 2020;10(1):1–10. doi: 10.1038/s41598-020-75622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casiraghi L.P., Alzamendi A., Giovambattista A., Chiesa J.J., Golombek D.A. Effects of chronic forced circadian desynchronization on body weight and metabolism in male mice. Phys. Rep. 2016;4(8) doi: 10.14814/phy2.12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Hoffmann A.D., Liu H., Liu X. Organotropism: new insights into molecular mechanisms of breast cancer metastasis. NPJ Precis Oncol. 2018;2(1):4. doi: 10.1038/s41698-018-0047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuesta M., Boudreau P., Dubeau-Laramee G., Cermakian N., Boivin D.B. Simulated night shift disrupts circadian rhythms of immune functions in humans. J. Immunol. 2016;196(6):2466–2475. doi: 10.4049/jimmunol.1502422. [DOI] [PubMed] [Google Scholar]
- Deshmukh S.K., Srivastava S.K., Poosarla T., Dyess D.L., Holliday N.P., Singh A.P., Singh S. Inflammation, immunosuppressive microenvironment and breast cancer: opportunities for cancer prevention and therapy. Ann. Transl. Med. 2019;7(20):593. doi: 10.21037/atm.2019.09.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diakos C.I., Charles K.A., McMillan D.C., Clarke S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–503. doi: 10.1016/S1470-2045(14)70263-3. 3. [DOI] [PubMed] [Google Scholar]
- Ehlting C., Wolf S.D., Bode J.G. Acute-phase protein synthesis: a key feature of innate immune functions of the liver. Biol. Chem. 2021;402(9):1129–1145. doi: 10.1515/hsz-2021-0209. [DOI] [PubMed] [Google Scholar]
- Ferrell J.M., Chiang J.Y. Circadian rhythms in liver metabolism and disease. Acta Pharm. Sin. B. 2015;5(2):113–122. doi: 10.1016/j.apsb.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundo-Rivera J., Gómez-Salgado J., García-Iglesias J.J., Gómez-Salgado C., Camacho-Martín S., Ruiz-Frutos C. Relationship between night shifts and risk of breast cancer among nurses: a systematic review. Medicina (Kaunas) 2020;56(12) doi: 10.3390/medicina56120680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipski E., Delaunay F., King V.M., Wu M.W., Claustrat B., Grechez-Cassiau A., Guettier C., Hastings M.H., Francis L. Effects of chronic jet lag on tumor progression in mice. Cancer Res. 2004;64(21):7879–7885. doi: 10.1158/0008-5472.CAN-04-0674. [DOI] [PubMed] [Google Scholar]
- Filipski E., Li X.M., Levi F. Disruption of circadian coordination and malignant growth. Cancer Causes Control. 2006;17(4):509–514. doi: 10.1007/s10552-005-9007-4. [DOI] [PubMed] [Google Scholar]
- Gao Q., Khan S., Zhang L. Brain activity and transcriptional profiling in mice under chronic jet lag. Sci. Data. 2020;7(1):361. doi: 10.1038/s41597-020-00709-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten F.R., Grivennikov S.I. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B., Yang N., Borysiewicz E., Dudek M., Williams J.L., Li J., Maywood E.S., Adamson A., Hastings M.H., Bateman J.F., White M.R.H., Boot-Handford R.P., Meng Q.J. Catabolic cytokines disrupt the circadian clock and the expression of clock-controlled genes in cartilage via an NFкB-dependent pathway. Osteoarthritis Cartilage. 2015;23(11):1981–1988. doi: 10.1016/j.joca.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadadi E., Taylor W., Li X.M., Aslan Y., Villote M., Riviere J., Duvallet G., Auria C., Dulong S., Raymond-Letron I., Provot S., Bennaceur-Griscelli A., Acloque H. Chronic circadian disruption modulates breast cancer stemness and immune microenvironment to drive metastasis in mice. Nat. Commun. 2020;11(1):3193. doi: 10.1038/s41467-020-16890-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hernandez-Rosas F., Lopez-Rosas C.A., Saavedra-Velez M.V. Disruption of the molecular circadian clock and cancer: an epigenetic link. Biochem. Genet. 2020;58(1):189–209. doi: 10.1007/s10528-019-09938-w. [DOI] [PubMed] [Google Scholar]
- Horsey E.A., Maletta T., Turner H., Cole C., Lehmann H., Fournier N.M. Chronic jet lag simulation decreases hippocampal neurogenesis and enhances depressive behaviors and cognitive deficits in adult male rats. Front. Behav. Neurosci. 2019;13:272. doi: 10.3389/fnbeh.2019.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrushesky W.J. Circadian timing of cancer chemotherapy. Science. 1985;228(4695):73–75. doi: 10.1126/science.3883493. [DOI] [PubMed] [Google Scholar]
- Iwamoto A., Kawai M., Furuse M., Yasuo S. Effects of chronic jet lag on the central and peripheral circadian clocks in CBA/N mice. Chronobiol. Int. 2014;31(2):189–198. doi: 10.3109/07420528.2013.837478. [DOI] [PubMed] [Google Scholar]
- Kaminskas L.M., Ascher D.B., McLeod V.M., Herold M.J., Le C.P., Sloan E.K., Porter C.J. PEGylation of interferon alpha2 improves lymphatic exposure after subcutaneous and intravenous administration and improves antitumor efficacy against lymphatic breast cancer metastases. J. Contr. Release. 2013;168(2):200–208. doi: 10.1016/j.jconrel.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatsoreos I.N., Bhagat S., Bloss E.B., Morrison J.H., McEwen B.S. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc. Natl. Acad. Sci. U. S. A. 2011;108(4):1657–1662. doi: 10.1073/pnas.1018375108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Fuchs C., Le C.P., Pimentel M.A., Shackleford D., Ferrari D., Angst E., Hollande F., Sloan E.K. Chronic stress accelerates pancreatic cancer growth and invasion: a critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain Behav. Immun. 2014;40:40–47. doi: 10.1016/j.bbi.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacourt T.E., Vichaya E.G., Chiu G.S., Dantzer R., Heijnen C.J. The high costs of low-grade inflammation: persistent fatigue as a consequence of reduced cellular-energy availability and non-adaptive energy expenditure. Front. Behav. Neurosci. 2018;12:78. doi: 10.3389/fnbeh.2018.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévi F. Chronopharmacology and chronotherapy of cancers. Pathol. Biol. 1996;44(7):631–644. [PubMed] [Google Scholar]
- Lelekakis M., Moseley J.M., Martin T.J., Hards D., Williams E., Ho P., Lowen D., Javni J., Miller F.R., Slavin J., Anderson R.L. A novel orthotopic model of breast cancer metastasis to bone. Clin. Exp. Metastasis. 1999;17(2):163–170. doi: 10.1023/a:1006689719505. [DOI] [PubMed] [Google Scholar]
- Marquie J.C., Tucker P., Folkard S., Gentil C., Ansiau D. Chronic effects of shift work on cognition: findings from the VISAT longitudinal study. Occup. Environ. Med. 2015;72(4):258–264. doi: 10.1136/oemed-2013-101993. [DOI] [PubMed] [Google Scholar]
- Otsuka T., Thi Le H., Kohsaka A., Sato F., Ihara H., Nakao T., Maeda M. Adverse effects of circadian disorganization on mood and molecular rhythms in the prefrontal cortex of mice. Neuroscience. 2020;432:44–54. doi: 10.1016/j.neuroscience.2020.02.013. [DOI] [PubMed] [Google Scholar]
- Papagiannakopoulos T., Bauer M.R., Davidson S.M., Heimann M., Subbaraj L., Bhutkar A., Bartlebaugh J., Vander Heiden M.G., Jacks T. Circadian rhythm disruption promotes lung tumorigenesis. Cell Metabol. 2016;24(2):324–331. doi: 10.1016/j.cmet.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariollaud M., Lamia K.A. Cancer in the fourth dimension: what is the impact of circadian disruption? Cancer Discov. 2020;10(10):1455. doi: 10.1158/2159-8290.CD-20-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S.K., Wong A.L., Wong F.L., Breen E.C., Hurria A., Smith M., Kinjo C., Paz I.B., Kruper L., Somlo G., Mortimer J.E., Palomares M.R., Irwin M.R., Bhatia S. Inflammatory biomarkers, comorbidity, and neurocognition in women with newly diagnosed breast cancer. J Natl Cancer Inst. 2015;107(8) doi: 10.1093/jnci/djv131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H., Zhang H., Matei I.R., Costa-Silva B., Hoshino A., Rodrigues G., Psaila B., Kaplan R.N., Bromberg J., Kang Y., Bissell M.J., Cox T.R., Giaccia A.J., Erler J.T., Hiratsuka S., Ghajar C.M., Lyden D. Pre-metastatic niches: organ-specific homes for metastases. Nat. Rev. Cancer. 2017;17(5):302–317. doi: 10.1038/nrc.2017.6. [DOI] [PubMed] [Google Scholar]
- Pilorz V., Helfrich-Forster C., Oster H. The role of the circadian clock system in physiology. Pflügers Archiv. 2018;470(2):227–239. doi: 10.1007/s00424-017-2103-y. [DOI] [PubMed] [Google Scholar]
- Pyter L.M., Pineros V., Galang J.A., McClintock M.K., Prendergast B.J. Peripheral tumors induce depressive-like behaviors and cytokine production and alter hypothalamic-pituitary-adrenal axis regulation. Proc. Natl. Acad. Sci. U. S. A. 2009;106(22):9069–9074. doi: 10.1073/pnas.0811949106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radler M.E., Hale M.W., Kent S. Calorie restriction attenuates lipopolysaccharide (LPS)-induced microglial activation in discrete regions of the hypothalamus and the subfornical organ. Brain Behav. Immun. 2014;38:13–24. doi: 10.1016/j.bbi.2013.11.014. [DOI] [PubMed] [Google Scholar]
- Rothwell P.M., Fowkes F.G., Belch J.F., Ogawa H., Warlow C.P., Meade T.W. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377(9759):31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- Rothwell P.M., Wilson M., Price J.F., Belch J.F., Meade T.W., Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379(9826):1591–1601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- Santos J.C., Pyter L.M. Neuroimmunology of behavioral comorbidities associated with cancer and cancer treatments. Front. Immunol. 2018;9:1195. doi: 10.3389/fimmu.2018.01195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos J.C., Bever S.R., Sullivan K.A., Pyter L.M. Cancer and cancer survival modulates brain and behavior in a time-of-day-dependent manner in mice. Sci. Rep. 2019;9(1):6497. doi: 10.1038/s41598-019-42880-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M.E., Semik J., Habermann N., Wiskemann J., Ulrich C.M., Steindorf K. Cancer-related fatigue shows a stable association with diurnal cortisol dysregulation in breast cancer patients. Brain Behav. Immun. 2016;52:98–105. doi: 10.1016/j.bbi.2015.10.005. [DOI] [PubMed] [Google Scholar]
- Schwartsburd P. Cancer-induced reprogramming of host glucose metabolism: "vicious cycle" supporting cancer progression. Front. Oncol. 2019;9:218. doi: 10.3389/fonc.2019.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalapour S., Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J. Clin. Invest. 2015;125(9):3347–3355. doi: 10.1172/JCI80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty A., Hsu J.W., Manka P.P., Syn W.K. Role of the circadian clock in the metabolic syndrome and nonalcoholic fatty liver disease. Dig. Dis. Sci. 2018;63(12):3187–3206. doi: 10.1007/s10620-018-5242-x. [DOI] [PubMed] [Google Scholar]
- Silverman M.N., Pearce B.D., Biron C.A., Miller A.H. Immune modulation of the hypothalamic-pituitary-adrenal (HPA) axis during viral infection. Viral Immunol. 2005;18(1):41–78. doi: 10.1089/vim.2005.18.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaviero K.A., Clarke S.J., Rivory L.P. Inflammatory response: an unrecognised source of variability in the pharmacokinetics and pharmacodynamics of cancer chemotherapy. Lancet Oncol. 2003;4(4):224–232. doi: 10.1016/s1470-2045(03)01034-9. [DOI] [PubMed] [Google Scholar]
- Sloan E.K., Priceman S.J., Cox B.F., Yu S., Pimentel M.A., Tangkanangnukul V., Arevalo J.M.G., Morizono K., Karanikolas B.D.W., Wu L., Sood A.K., Cole S.W. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70(18):7042–7052. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan E.K., Stanley K.L., Anderson R.L. Caveolin-1 inhibits breast cancer growth and metastasis. Oncogene. 2004;23(47):7893–7897. doi: 10.1038/sj.onc.1208062. [DOI] [PubMed] [Google Scholar]
- Solinas G., Germano G., Mantovani A., Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J. Leukoc. Biol. 2009;86(5):1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- Steenbrugge J., Vander Elst N., Demeyere K., De Wever O., Sanders N.N., Van Den Broeck W., Dirix L., Laere S.V., Meyer E. Comparative profiling of metastatic 4T1- vs. Non-metastatic py230-based mammary tumors in an intraductal model for triple-negative breast cancer. Front. Immunol. 2019;10:2928. doi: 10.3389/fimmu.2019.02928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulli G., Lam M.T.Y., Panda S. Interplay between circadian clock and cancer: new frontiers for cancer treatment. Trends in cancer. 2019;5(8):475–494. doi: 10.1016/j.trecan.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K.A., Bever S.R., McKim D.B., Godbout J.P., Sheridan J.F., Obrietan K., Pyter L.M. Mammary tumors compromise time-of-day differences in hypothalamic gene expression and circadian behavior and physiology in mice. Brain Behav. Immun. 2019;80:805–817. doi: 10.1016/j.bbi.2019.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uphoff C.C., Drexler H.G. Comparative PCR analysis for detection of mycoplasma infections in continuous cell lines. In Vitro Cell. Dev. Biol. Anim. 2002;38(2):79–85. doi: 10.1290/1071-2690(2002)038<0079:CPAFDO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Vetter C. Circadian disruption: what do we actually mean? Eur. J. Neurosci. 2020;51(1):531–550. doi: 10.1111/ejn.14255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A.K., Budac D.P., Bisulco S., Lee A.W., Smith R.A., Beenders B., Kelley K.W., Dantzer R. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology. 2013;38(9):1609–1616. doi: 10.1038/npp.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A.K., Chang A., Ziegler A.I., Dhillon H.M., Vardy J.L., Sloan E.K. Low dose aspirin blocks breast cancer-induced cognitive impairment in mice. PLoS One. 2018;13(12) doi: 10.1371/journal.pone.0208593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker W.H., II, Borniger J.C., Surbhi Zalenski A.A., Muscarella S.L., Fitzgerald J.A., Zhang N., Gaudier-Diaz M.M., DeVries A.C. Mammary tumors induce central pro-inflammatory cytokine expression, but not behavioral deficits in balb/C mice. Sci. Rep. 2017;7(1):8152. doi: 10.1038/s41598-017-07596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver D.R. The suprachiasmatic nucleus: a 25-year retrospective. J. Biol. Rhythm. 1998;13(2):100–112. doi: 10.1177/074873098128999952. [DOI] [PubMed] [Google Scholar]
- Wu M., Zeng J., Chen Y., Zeng Z., Zhang J., Cai Y., Ye Y., Fu L., Xian L., Chen Z. Experimental chronic jet lag promotes growth and lung metastasis of Lewis lung carcinoma in C57BL/6 mice. Oncol. Rep. 2012;27(5):1417–1428. doi: 10.3892/or.2012.1688. [DOI] [PubMed] [Google Scholar]
- Wu T.C., Xu K., Martinek J., Young R.R., Banchereau R., George J., Turner J., Kim K.I., Zurawski S., Wang X., Blankenship D., Brookes H.M., Marches F., Obermoser G., Lavecchio E., Levin M.K., Bae S., Chung C.H., Smith J.L., Cepika A.M., Oxley K.L., Snipes G.J., Banchereau J., Pascual V., O'Shaughnessy J., Palucka A.K. IL1 receptor antagonist controls transcriptional signature of inflammation in patients with metastatic breast cancer. Cancer Res. 2018;78(18):5243–5258. doi: 10.1158/0008-5472.CAN-18-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang S., Dauchy R.T., Hauch A., Mao L., Yuan L., Wren M.A., Belancio V.P., Frasch T., Blask D.E., Hill S.M. Doxorubicin resistance in breast cancer is driven by light at night-induced disruption of the circadian melatonin signal. J. Pineal Res. 2015;59(1):60–69. doi: 10.1111/jpi.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang S., Dauchy R.T., Hoffman A.E., Pointer D., Frasch T., Blask D.E., Hill S.M. Epigenetic inhibition of the tumor suppressor ARHI by light at night-induced circadian melatonin disruption mediates STAT3-driven paclitaxel resistance in breast cancer. J. Pineal Res. 2019;67(2) doi: 10.1111/jpi.12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X.-Y., Liang J., Xu Y.-Q., Liu Y. The Tilapia collagen peptide mixture TY001 protects against LPS-induced inflammation, disruption of glucose metabolism, and aberrant expression of circadian clock genes in mice. Chronobiol. Int. 2019;36(7):1013–1023. doi: 10.1080/07420528.2019.1606821. [DOI] [PubMed] [Google Scholar]
- Yu H., Pardoll D., Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer. 2009;9(11):798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Borcherding N., Kolb R. IL-1 signaling in tumor microenvironment. Adv. Exp. Med. Biol. 2020;1240:1–23. doi: 10.1007/978-3-030-38315-2_1. [DOI] [PubMed] [Google Scholar]
- Zubidat A.E., Fares B., Fares F., Haim A. Artificial light at night of different spectral compositions differentially affects tumor growth in mice: interaction with melatonin and epigenetic pathways. Cancer Control. 2018;25(1) doi: 10.1177/1073274818812908. 1073274818812908. [DOI] [PMC free article] [PubMed] [Google Scholar]