Abstract
Cardiovascular disease, including myocardial infarction (MI), is the leading cause of death in the western world. Following MI, a large number of cardiomyocytes are lost and inflammatory cells such as monocytes and macrophages migrate into the damaged region to remove dead cells and tissue. These inflammatory cells secrete growth factors to induce degradation of the extracellular matrix in the myocardium and recruit cardiac fibroblasts. However, the contribution of specific macrophage subsets on cardiac cell function and survival in the steady state as well as in the diseased state is not well known. There is an increasing demand for in vitro cardiac disease models to bridge the critical missing link in the existing experimental methods. In this review, studies using in vitro models to examine the interaction between macrophages and cardiac cells, including cardiomyocytes, endothelial cells, and fibroblasts, are summarized to better understand the complex inflammatory cascade post-MI. The current challenges and the future directions of in vitro cardiac models are also discussed. Detailed and more mechanistic insights into macrophages and cardiac cell interactions during the multiphase repair process could potentially revolutionize the development of treatments and diagnostic alternatives.
Impact statement
The inflammatory cascade postmyocardial infarction (MI) is very complex. In vitro cardiac disease model studies bridge the critical missing link in the existing experimental methods and provide insights, including multicellular interaction post-MI. Detailed and more mechanistic insights into macrophages and cardiac cell interactions during the multiphase repair process could potentially revolutionize in developing treatments and diagnostic alternatives.
Keywords: cardiac inflammation, cardiac disease models, cardiac cell interaction, macrophages
Introduction
The postmyocardial infarction (MI) microenvironment is very complex. Lack of oxygen and nutrients causes hypoxia and massive amounts of cell death, igniting both an innate and adaptive downstream immune response. Despite the knowledge that inflammation plays a crucial role in the pathogenesis of MI injury,1–3 little is known about cellular crosstalk between resident myocardial and inflammatory cells and the key factors that dictate either constructive or maladaptive healing.
With the dynamic nature of the heart, it is difficult to directly assess interaction of various cells and their true healing potential in vivo. Standard cell culture techniques do not provide a realistic environment in which functional outcomes of a given cell therapy can be evaluated. Animal models often involve such a complex combination of factors that it is difficult to interpret the outcomes. To bridge this critical missing link in the available experimental methods, which typically leap from cell cultures in a Petri dish to experimental animal models to clinical trials, in vitro models are required. In this review, we will provide an overview of studies using in vitro models to understand the complex inflammatory cascade post-MI, including the interaction between macrophages and cardiac cells post-MI.
Post-MI Inflammation
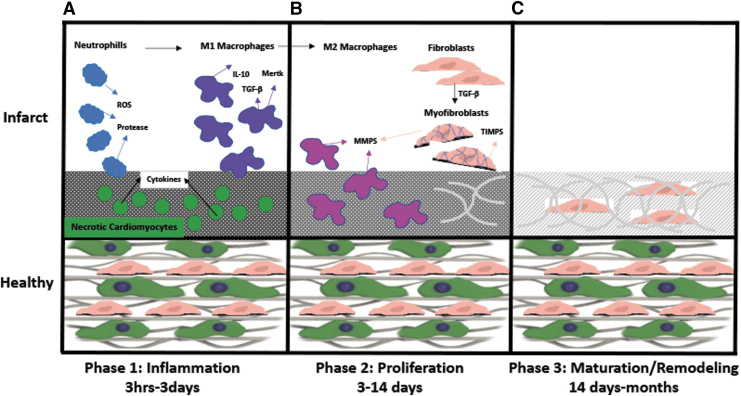
Following MI, there are multiple overlapping stages of repair which include inflammation, proliferation, and remodeling. Initially, cell death and debris as well as proinflammatory cytokines and chemokines, including interleukins from both necrotic and apoptotic cells initiates neutrophil infiltration (Fig. 1A).1 Neutrophils are the first to arrive and are short lived. They contribute to the infarct injury by secreting proteases and generating reactive oxygen species (ROS) before turning apoptotic (Fig. 1A).4 ROS is involved in proangiogenic or profibrotic roles although excess release of ROS is shown to be detrimental post-MI.5,6 Neutrophils initiate monocyte recruitment and eventual macrophage differentiation at the site of injury (Fig. 1A),1 replacing embryonic-derived tissue resident macrophages7 and taking on distinct phenotypes.7,8
FIG. 1.
An overview of the post-MI multiphase reparative process, including initial inflammatory phase (A), followed by proliferative phase (B), leading to remodeling/maturation phase (C). IL, interleukin; MI, myocardial infarction; MMP, matrix metalloproteinases; ROS, reactive oxygen species; TGF-β, transforming growth factor beta; TIMP, tissue inhibitor of metalloproteinase.
Proinflammatory or classically activated M1 phenotype macrophages are initially responsible for removing necrotic cardiomyocytes and fibroblasts along with apoptotic neutrophils that have responded before them.9 Phagocytic function of macrophages is known as a regulator of inflammation through clearing of the numerous apoptotic cells such as neutrophils and promoting secretion of M2-like anti-inflammatory mediators such as interleukin (IL)-10 and transforming growth factor beta (TGF-β).10 Specifically, efferocytosis, or the clearing of apoptotic cells, is one of the most important functions of proinflammatory macrophages post-MI. It has been found that macrophage myeloid-epithelial-reproductive tyrosine kinase (Mertk) is expressed by proinflammatory macrophages and it is necessary and sufficient for proper engulfment and efferocytosis in mice.11 In fact, depleted Mertk expression corresponded to apoptotic cardiomyocyte accumulation, increased infarct size and adverse remodeling suggesting Mertk is an essential receptor for proper apoptotic cardiomyocyte/macrophage interaction. Similar results were also found in primary human adult cardiomyocytes and macrophages.12
As healing continues, repair moves into the proliferative phase where macrophage polarization shifts toward the anti-inflammatory or alternatively activated M2 phenotype, promoting myofibroblast and vascular cell infiltration (Fig. 1B).13 In the diseased state, fibroblasts and macrophages secrete high levels of matrix metalloproteinases (MMPs), such as MMP214 and MMP9,15 which cause extensive matrix breakdown, altering the mechanical properties of the tissues. This shift in mechanical properties causes changes in fibroblast secretion profile to increased expression of TGF-α and TGF-β.16 Additionally, the loss of IL-1β and IL-10 expression during proliferative phase allows fibroblasts to transdifferentiate into myofibroblasts.17 Myofibroblasts express α-SMA, which allows them to form adhesion bridges with extracellular matrix (ECM) fibronectin, initiating a contractile force that is maintained until collagen fiber deposition can reinforce the infarct as it matures (Fig. 1B).16 Inflammatory and reparative cells eventually become apoptotic and collagenous scar tissue is formed (Fig. 1C).18 An intricate and complex inflammatory cascade regulates the resulting tissue remodeling, leading to structural and functional alterations, which can cause an irreversible decrease in cardiac pump performance.
A recent study demonstrated that the inhibition of the innate immune response through treatment with a potent antioxidant called 3-bromo-4,5-dihydroxybenzaldehyde (BDB) resulted in a noticeable improvement in survival and cardiac function of mice due to inhibition of macrophage infiltration and proinflammatory cytokine production.19 Similarly, a study by Tokutome et al. used nanoparticles (NPs) loaded with an anti-inflammatory drug named Pioglitazone to target monocytes and macrophages. NPs inhibited monocyte recruitment and inflammatory gene expression in macrophages while promoting a more M2 type macrophage phenotype. These NPs lessened ischemia/reperfusion injury and significantly reduced mouse mortality.20 These recent studies further support the need for a more in-depth understanding of the cells governing the inflammatory cascade post-MI to develop successful therapies for post-MI regeneration.
Even though macrophages are among the key cells activated during this initial phase of the host response and mediate inflammation post-MI,1,21 it remains unknown what mechanisms underlie the macrophage polarization shift. In addition, while the classification of macrophage phenotypes into M1–M2 paradigm is widely accepted and used, there has been an increasing body of evidence recently demonstrating a wide phenotypic variation of macrophages in vivo post-MI.22 Further investigation accounting for the heterogeneity of macrophages will be critical to the prevention of detrimental remodeling as the prolonging of inflammation is associated with dysfunctional matrix deposition, scar formation, and heightened cardiomyocyte apoptosis.1
Interaction of Macrophages with Cardiomyocytes
Despite its abundancy in both healthy and injured heart, the role of macrophages in the heart has been underappreciated in the past.1 Macrophages have long been thought to only possess immune-related function, such as phagocytosis. However, it has recently been demonstrated that resident cardiac macrophages play a role during healthy and steady condition as well.23,24 Resident cardiac macrophages not only express the gap junction protein connexin43 (Cx43) and directly couple with conducting cells in the atrioventricular (AV) node, but also depolarize along with them.23,24 In fact, macrophage coupling changes the resting membrane potential of cardiomyocytes, which may accelerate their repolarization. Additionally, delayed AV conduction is seen after both macrophage ablation and deletion of macrophage Cx43 suggesting that macrophages play a crucial role in healthy, steady-state cardiac conduction.23,24
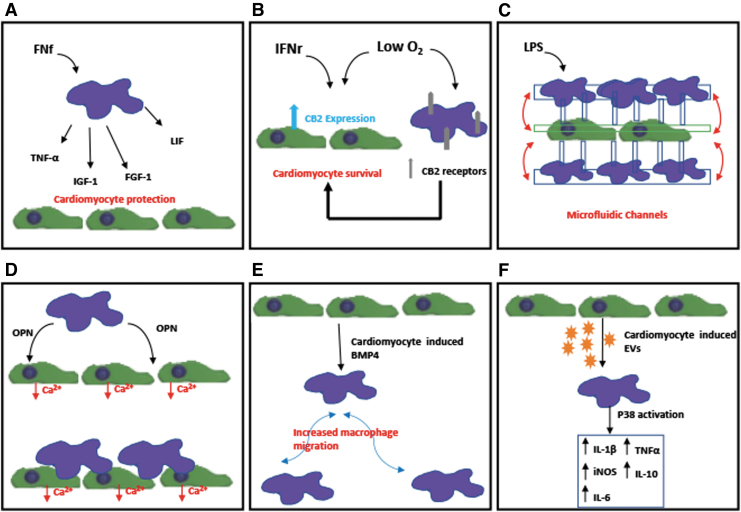
In addition, the effects of macrophages on survival and apoptosis of murine embryonic and adult cardiomyocytes have been demonstrated. A recent study by Trial et al. examined the effect of rat splenic macrophages on the survival of primary rat cardiomyocytes up to 24 h.25 They demonstrated that macrophages activated using 110 ug per 106 cells of fibronectin fragments (FNf) protect cardiomyocytes from apoptosis through the secretion of tumor necrosis factor-α (TNF-α), fibroblast growth factor-1 (FGF-1), insulin-like growth factor 1 (IGF-1), and leukemia-inhibiting factor (LIF) (Fig. 2A). In vitro stimulation of human monocytes with FNf also induced overexpression of TNF-α, FGF-1, IGF-1, and LIF suggesting that tissue degradation signals such as FNf may stimulate cardiomyocyte-protective macrophage phenotype.
FIG. 2.
Schematics of in vitro studies demonstrating the interaction between cardiomyocytes and macrophages: (A) Demonstration of macrophages activated by FNf-protecting cardiomyocytes from apoptosis through the secretion of TNF-α, FGF-1, IGF-1, and LIF.25 (B) Demonstration of both hypoxia and IFNγ treatment inducing the expression of CB2 receptor in cardiomyocytes and macrophages and that CB2 receptor could play a critical role in the survival of cardiomyocytes post-MI.26 (C) Potential use of microfluidic coculture device for real-time monitoring of interaction of macrophages and cardiac cells.27 (D) Demonstration of both macrophage-derived factors, specifically matricellular protein OPN and direct coupling with macrophages affect calcium-handling function of cardiomyocytes.28 (E) Demonstration of cardiomyocyte-conditioned media causing increased migration of proinflammatory macrophage through BMP4.29 (F) EVs derived from cardiomyocytes promoted a proinflammatory phenotype in macrophages with increased expression of iNOS, IL-1β, IL-6, TNF-α, and IL-10 through activation of the map kinase pathway p38.30 CB2, cannabinoid receptor 2; EVs, extracellular vesicles; FGF-1, fibroblast growth factor-1; FNf, fibronectin fragments; IFNγ, interferon-gamma; IGF-1, insulin-like growth factor 1; iNOS, inducible nitric oxide synthase; LIF, leukemia inhibiting factor; LPS, lipopolysaccharides; OPN, osteopontin; TNF-α, tumor necrosis factor-α.
Heinemann et al. examined embryonic and adult murine cardiomyocytes under normoxic and hypoxic conditions for up to 24 h to explore the role of cannabinoid receptor 2 (CB2) during inflammation phase post-MI (Fig. 2B).26 It has been shown that CB2 is associated with myocardial adaptation and inflammatory modulation. Both hypoxia and interferon-gamma treatment induced the expression of CB2 receptor in cardiomyocytes. They have also demonstrated that proinflammatory primary murine macrophages express higher levels of CB2 receptor compared with anti-inflammatory macrophages and their expression of CB2 receptor was further increased when cultured under hypoxia. During coculture, when CB2 receptor expression was knocked down in pro-inflammatory macrophages, higher cardiomyocyte apoptosis was demonstrated, suggesting that CB2 receptor could play a critical role in the survival of cardiomyocytes post-MI.
Moreover, a recent study by Ai et al. utilized microfluidic channels to coculture RAW264.7 cell line with H9c2 myoblasts for up to 3 days (Fig. 2C).27 Their microfluidic device allowed spatial separation of heterogeneous cells while maintaining soluble factor communication. They have shown that macrophages polarized using lipopolysaccharides (LPS) into a proinflammatory phenotype promoted myocyte apoptosis through mitochondrial damage. This study demonstrates the potential of the microfluidic coculture device for real-time monitoring of inflammatory response for myocardial disease and for anti-inflammatory drug screening.
Finally, the most recent study by our group examined the effects of direct and indirect interaction with polarized macrophage subsets on the function of mouse embryonic stem cell-derived cardiomyocytes (mES-CM) in vitro (Fig. 2D).28 This study demonstrated that both macrophage-derived factors and direct coupling with macrophages affect calcium-handling function of mES-CM. Specifically, it is shown that macrophage-derived matricellular protein osteopontin plays a role in affecting mES-CM store-operated calcium entry, a calcium-handling mechanism known to be upregulated post-MI.
Interestingly, until recently, how surviving cardiomyocytes might influence macrophages post-MI was largely unexplored. Pallotta et al. found that human stem cell-derived cardiomyocyte-conditioned media caused increased migration of proinflammatory macrophage through BMP4 by using a three-dimensional (3D) inverted invasion assay (Fig. 2E).29 However, as the differentiated cardiomyocytes were ∼76% pure, contributions by other cell types, including fibroblast and endothelial-like cells were not fully assessed. Another study by Almeida et al. explored the role of extracellular vesicles in crosstalk between cardiomyocytes and macrophages.30 Cardiomyoblast cell line (H9c2) and neonatal cardiomyocytes were cultured under both normal and ischemic conditions and EVs were collected from their media. H9c2-derived EVs promoted a proinflammatory phenotype in RAW264.7 macrophages with increased expression of inducible nitric oxide synthase (iNOS), IL-1β, and IL-6 through activation of the map kinase pathway p38. This was similar to the phenotype seen in tissue-resident cardiac macrophages.31 Similar, but not identical, p38 activation was seen in macrophages cultured in neonatal CM-derived EVs, which caused upregulation of iNOS, IL-1β, IL-6, IL-10, and TNFα (Fig. 2F). Interestingly, ischemia induced increased adhesion of macrophages to ECM component fibronectin, but reduced their phagocytic behavior and direct interaction or adhesion to cardiomyocytes.30 Additionally, EVs isolated from human serum of both healthy and MI patients induced changes in macrophage phenotype, however, contamination of EV samples with other plasma components prevented further exploration of human EV-specific effects. This study underlines the importance of understanding how macrophage phenotype is affected by cardiomyocytes post-MI and how it mediates cardiac repair.
Interaction of Macrophages with Endothelial Cells
The vascular network, composed of endothelial cells (ECs), is estimated to be within 2–3 μm of any cardiomyocytes in the heart,32,33 with ECs being the most abundant nonmyocyte cell type in the myocardium.34 Complex paracrine interactions between ECs and cardiomyocytes are important in both the healthy and diseased tissue.32,33 They play a crucial role in inflammation and are the first cell type to recognize and respond to injury and infection in many cases.35 Post-MI, ECs become activated by binding damage-associated molecular patterns (DAMPs) that are released from necrotic cardiomyocytes through toll-like receptors (TLRs).17,36 Activated ECs respond to injury by producing ROS and proinflammatory cytokines, such as monocyte chemoattractant-1 (MCP-1), which initiate monocyte and macrophage infiltration to the infarct.37 Additionally, activated ECs begin to express numerous adhesion proteins such as p-selectins, which help inflammatory cells like leukocytes bind to the vascular walls, and enter the tissue as the tight junctions between ECs loosen and the endothelium permeability changes in response to inflammation.17,35
Once the initial proinflammatory monocytes and macrophages have infiltrated the tissue, they begin to secrete proteases and cytokines, such as MMP9 and TNF-α, while removing dead cells and debris.38,39 Macrophages regulate angiogenesis by breaking down the ECM and basement membrane to allow proliferating ECs to form sprouts. After a few days, when the phenotype of macrophage shifts from proinflammatory to anti-inflammatory, prohealing phenotype, macrophages begin to express and secrete proangiogenic factors, such as vascular endothelial growth factor (VEGF) and tissue inhibitor of metalloproteinase 1 (TIMP-1), which inhibit MMP action and promote tissue remodeling and scar formation.37,39
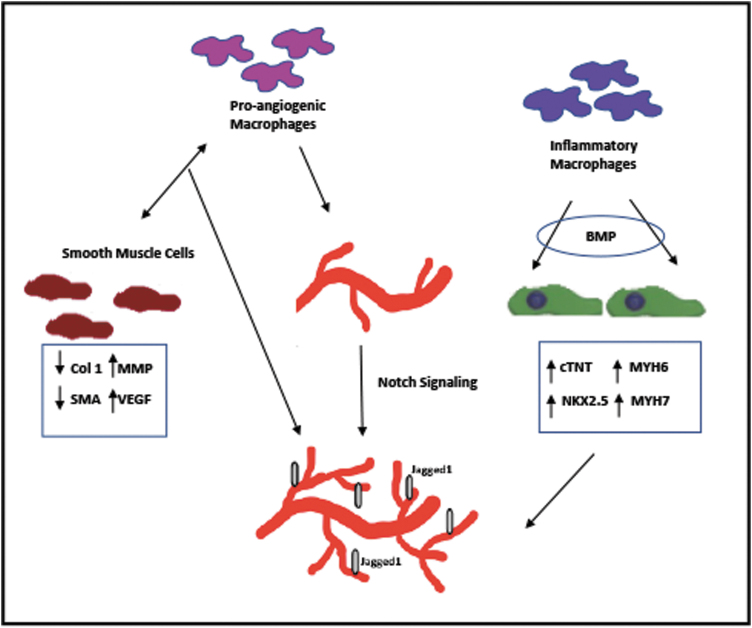
Since it is difficult to fully understand interaction of macrophages and ECs from complex in vivo studies, several groups have utilized in vitro models to more closely investigate the effect of inflammation on angiogenesis (Fig. 3). Tattersall et al. found that bone marrow-derived macrophages are proangiogenic by using a bead capillary sprouting assay.40 The presence of macrophages increased the number of human umbilical vein endothelial cells (HUVECs) as well as the length of HUVEC sprouts when in coculture for up to 6 days. Polarization of macrophages into a proinflammatory phenotype enhanced these “angiotrophic” effects through Notch signaling. Notch signaling is responsible for a myriad of physiological processes and allows neighboring cells to communicate with one another. Specifically, Jagged1, a cell surface ligand that interacts with numerous Notch receptors, was essential for EC sprouting. Additionally, the addition of pericytes enhanced EC sprouting through Notch1 signaling suggesting that support cells also play a significant role in wound healing. Further studies are necessary to explore the effects of inflammation on the supporting cells.
FIG. 3.
A schematic summary of studies demonstrating the crosstalk between macrophages and other cardiac cells. The presence of macrophages enhanced proliferation and sprouting of endothelial cells through Notch1 signaling.40 Coculture of macrophages and SMCs depressed SMC expression of collagen type I, whereas upregulating VEGF and MMP expression, suggesting the role of macrophage-SMC crosstalk in regulation of angiogenesis.41 Inflammatory macrophage-derived BMPs enhanced the expression of cardiac markers in cardiomyocytes and BMP retention in the tissue fostered endothelial cell sprouting and tube formation.42 SMC, smooth muscle cell; VEGF, vascular endothelial growth factor.
Butoi et al. used transwell culture setup to explore how macrophages affect not only ECs, but also smooth muscle cells (SMCs), which serve as support cells for vascular networks and angiogenesis.41 Coculture of macrophages and SMCs depressed SMC expression of ECM proteins such as collagen type I. Inversely, the expression of VEGF and MMP was significantly increased in both cell types when in coculture for up to 3 days. Furthermore, conditioned media from the coculture induced EC sprouting and tube formation when cultured in Matrigel, demonstrating a proangiogenic milieu of factors. This study suggests that macrophage-SMC crosstalk may play a crucial role in the regulation of angiogenesis and ECM deposition post-MI.
Extended from their previous study demonstrating the effects of inflammatory macrophage-derived BMPs on stem cell-derived cardiomyocytes two dimensionally (2D),29 Pallotta et al. recently explored the effects of BMPs on cardiomyocytes using a 3D in vitro model.42 Enhanced expression of cardiac markers by human embryonic stem cell (ESC)-derived cardiomyocytes was examined when cultured in a 3D collagen tissue preconditioned with BMPs. Additionally, BMP retention in the tissue fostered HUVEC sprouting and tube formation, suggesting that BMP-loaded scaffolds or therapies may not only enhance cardiac differentiation of stem cell sources but also enhance in vitro angiogenesis post-MI. Future studies may include a combination of cardiomyocytes, ECs, and support cells, such as pericytes and SMCs, which would mimic more closely the complex in vivo tissue composition.
Interaction of Macrophages with Fibroblasts and Myofibroblasts
Cardiac fibroblasts are key to the physiological structure and function of the heart as they form a 3D network between cells and tissue layers and are involved in electrical signaling. While cardiac fibroblasts have long been considered to be the most abundant cell type in the myocardium, recent findings suggest that fibroblasts make up only about 15% of nonmyocytes in the heart.34 After the initial inflammation phase post-MI, cardiac fibroblasts become activated into cardiac myofibroblasts, which rapidly proliferate and migrate into the necrotic myocardium to synthesize the ECM and initiate the remodeling process.43–45
It has been demonstrated that fibroblasts are activated by DAMPs released by necrotic cardiomyocytes post-MI.46,47 Additionally, fibroblasts express proinflammatory cytokines, MMPs, and break down the ECM.17,18 Nakaya et al. recently demonstrated that cardiac myofibroblasts phagocytize apoptotic cardiomyocytes through milk fat globule/epidermal growth factor 8 (MGF-E8). Following MGF-E8-mediated engulfment of apoptotic cells, myofibroblast transition from a pro- to an anti-inflammatory phenotype48 contributing to collagen synthesis, healing, and scar formation.17 This transition mirrors the shift of macrophages from proinflammatory to anti-inflammatory phenotype, suggesting that continuous and intricate communication between fibroblasts and macrophages exist in the post-MI microenvironment.
One of the key factors implicated in the crosstalk between fibroblasts and macrophages is TGF-β. TGF-β is a multifunctional protein known to have three isoforms present mostly in the latent form. It can be activated by a number of agonists, including ROS, MMPs, and other cytokines and is also involved in numerous mitogen-activated protein kinase pathways. Macrophages and fibroblasts are both the main sources and effectors of TGF-β post-MI.1,18,49,50 TGF-β is known to have both pro- and anti-inflammatory effects on macrophages by contributing to monocyte recruitment and activation, as well as suppression of proinflammatory macrophage phenotype. TGF-β is also known to promote myofibroblast differentiation, ECM deposition, and inhibition of MMPs through induction of TIMP expression.1,49–51 With its complex role in post-MI, it is difficult to study TGF-β signaling in vivo due to knockout fatality.50
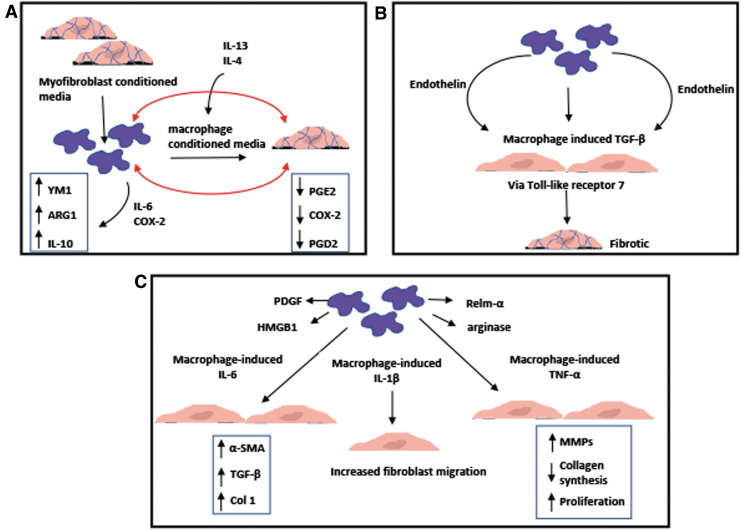
A recent in vitro study examined myofibroblast/macrophage crosstalk through conditioned media experiments.52 Murine dermal myofibroblast-conditioned media enhanced the expression of markers, such as ARG1 and YM1, as well as secretion of IL-10 in anti-inflammatory bone marrow-derived macrophages, through IL-6 and COX-2 (Fig. 4A). Myofibroblast-derived IL-6 and PGD2 increased ARG1 activity and expression, whereas LPS induced IL-10 production. Conditioned media from macrophages, which were cultured in myofibroblast conditioned media with IL-4 and IL-13, resulted in reduced fibroblast migration and COX-2, PGE2, and PGD2 expression.52 This study demonstrates that the feedback communication between fibroblasts and macrophages can directly promote wound healing and tissue repair.
FIG. 4.
Schematics of in vitro studies demonstrating the interaction between macrophages and cardiac fibroblasts/myofibroblasts: (A) Bidirectional crosstalk through IL-6, PGE2, and PGD2 between myofibroblasts and macrophages enhanced anti-inflammatory phenotype in both cells.52 (B) Macrophages activated by immune complexes induced cardiac fibroblasts to secrete TGF-β in an endothelin-1-dependent manner through TLR7 leading to a fibrosing phenotype.56 (C) Macrophage-secreted IL-1β induced significant increase in migration of cardiac fibroblasts.56 Macrophages increased expression of α-SMA and collagen type I and production of TGF-β in cardiac fibroblasts through IL-6 signaling.57 Macrophage-induced TNF-α enhances proliferation of cardiac fibroblasts, collagen degradation, MMP activity, and inflammatory cytokine production.58 PDGF, platelet-derived growth factor; TLR7, toll-like receptor 7.
Recent studies have also implicated the expression of TGF-β with the development of fibrosis. Alvarez et al. explored the relationship between macrophages and fibroblasts in association with congenital heart block. It was demonstrated that conditioned media from human macrophages activated by immune complexes induced human fetal cardiac fibroblasts to secrete TGF-β in an endothelin-1-dependent manner through toll-like receptor 7 (TLR7) leading to a fibrosing phenotype (Fig. 4B).53 This study supports the role of endothelin-1 in profibrotic responses in linking TLR7 inflammatory signaling. In another study, Ma et al. explored the role of TGF-β in hypertensive cardiac fibrosis. They demonstrated that in vitro coculture with primary peritoneal mouse macrophages increased the expression of α-SMA and collagen type I and production of TGF-β in neonatal mouse cardiac fibroblasts through IL-6 signaling (Fig. 4C).54 In the skin injury model, myeloid cell-specific STAT3 signaling has been found to be associated with TGF-β expression in macrophages and fibroblasts.55 Specifically, IL-10 decreased both macrophage and fibroblast-derived TGF-β expression in a STAT3-dependent manner. This study highlights the protective role of STAT3 signaling in immune cell-mediated skin fibrosis demonstrating a link between STAT3 signaling and TGF-β. However, whether similar relationship between STAT3 signaling and TGF-β expression is found in post-MI microenvironment awaits further investigations.
IL-1β, a protein known to be associated with TGF-β, is secreted by macrophages. It inhibits the conversion of fibroblasts to myofibroblasts until the proinflammatory microenvironment is cleared and new ECM can be deposited.16,18 Mitchell et al. used a modified Boyden chamber to examine the role of proinflammatory cytokines on migration of neonatal rat cardiac fibroblasts in vitro.56 They demonstrated that macrophage-secreted IL-1β induced significant increase in the migration of cardiac fibroblasts (Fig. 4C). Similar to IL-1β, TNF-α is also secreted by inflammatory macrophages and is involved in the migratory phenotype and function of fibroblasts postinjury. This study suggests that further elucidation of mechanisms that control cardiac fibroblast migration can potentially aid in developing therapies for physiological remodeling.
Siwik et al. demonstrated that TNF-α reduces collagen synthesis and increases MMP production in both neonatal and adult rat cardiac fibroblasts in vitro (Fig. 4C).57 In addition, Sun et al. utilized a knockout mouse model to demonstrate that TNF-α induced proliferation of cardiac fibroblasts, collagen degradation, MMP activity, and inflammatory cytokine production.58 Excessive TNF-α was also associated with left ventricular dysfunction and rupture, further demonstrating the importance of a better understanding of the detailed and complex interactions between macrophages and fibroblasts.
Other proteins secreted by macrophages after myocardial injury include platelet-derived growth factor (PDGF) and high mobility box 1 protein (HMGB1) (Fig. 4C). PDGF is a chemoattractant for fibroblasts59 and HMGB1-induced chemokine releases in cardiac fibroblasts through the receptor RAGE, which regulates wound healing and scar formation.60 Anti-inflammatory macrophages secrete arginase, which ultimately metabolizes to proline, a major component of collagen synthesis by fibroblasts and myofibroblasts.61 Relm-α, which controls LH-2-mediated fibrotic collagen crosslinking is also secreted by anti-inflammatory macrophages.62 Macrophages are also shown to promote apoptosis of fibroblasts and myofibroblasts, however, details regarding that relationship are not fully understood.63,64 A more recent in vitro study demonstrated the importance of understanding how the temporal shift in macrophage phenotype affects fibroblast phenotype; with proinflammatory macrophage-conditioned media promoting proinflammatory gene expression in fibroblasts and anti-inflammatory macrophage-conditioned media-induced fibroblast proliferation. Additionally, fibroblasts first cultured with proinflammatory macrophages and then anti-inflammatory macrophages demonstrated decreased expression of inflammatory markers and increased collagen secretion,65 demonstrating plasticity of fibroblast phenotype. This further reveals the complexity of interactions between macrophages and fibroblasts and further studies are needed to fully understand the inflammatory microenvironment post-MI.
Sources of Macrophages
Various sources of macrophages, including immortalized cell lines, primary cells and pluripotent stem cell-derived cells have been considered and utilized thus far (Table 1). While each cell source exhibits its own list of benefits and limitations, all sources have been successfully used in macrophage biology. One of the most commonly used immortalized cell line is RAW264.7, a monocyte/macrophage-like cell line derived from Abelson leukemia-infected BALB/c mice.66–68 These cells are relatively easy to culture and have a highly stable proliferative capacity. RAW264.7 cells can also be polarized into different macrophage phenotypes by using cytokines, such as LPS or IL-4.66 Similarly, THP-1, a human monocytic leukemia cell line69–71 and U937, a human promonocytic myeloid leukemia cell line, have also been widely used.72,73 THP-1 cells are easily cultured, adherent cells that can be easily differentiated into macrophages through phorbol-12-myristate-13-acetate (PMA) and further polarized into different phenotypes. U937 cells possess a theoretically unlimited proliferative capacity and can be differentiated into macrophages. One of the benefits of these cell lines is less variability among batches as they have relatively homogenous genetic backgrounds. However, they are immortalized cells derived from malignant background and they still need to be fully characterized to determine whether they possess all the native features of primary cells.72
Table 1.
Summary of Various Sources of Macrophages Commonly Used
| Cell type | References | |
|---|---|---|
| Cell line | RAW264.7 (mouse) | 66–68 |
| THP-1 (human) | 69–71 | |
| U937 (human) | 72,73 | |
| Primary | Peripheral blood | 74–76 |
| Bone marrow derived | 77,78 | |
| Pluripotent stem cell derived | mESC | 81–83 |
| hESC | 84,85 | |
| hiPSC | 86–88 |
hESC, human embryonic stem cell; hiPSC, human induced pluripotent stem cell; mESC, mouse embryonic stem cell.
Primary cells, including peripheral blood-derived macrophages, are also widely used in the field of inflammation research. While these cells are relatively easy to isolate, it is difficult to obtain consistent and high yields from patients. In addition, tissue-specific phenotype is not well represented in peripheral blood macrophages.74–76 Alternatively, bone marrow-derived macrophages are another commonly used primary cell source, although it requires more complex isolation technique.77,78 They exhibit more expansive proliferative capacity in vitro, however, there is some debate regarding the reproducibility of the expansion technique as it has been found that cell density may not only affect cell surface marker expression but also functional aspects.77,79,80
Most recently, studies have demonstrated the use of pluripotent stem cells in deriving monocytes and macrophages. Several groups have demonstrated successful differentiation of macrophages from ESC.81,82 Dreymueller et al. showed that murine ESC-derived macrophages exhibited a more anti-inflammatory or M2 phenotype compared with bone marrow-derived macrophages. Despite their anti-inflammatory phenotype, these cells delayed the healing of deep skin wound.83 Similarly, Haideri et al. derived murine ESC macrophages and found their phenotype to be different from bone marrow-derived macrophages through reduced phagocytic function as well as reduced response to LPS and enhanced response to IL-4. However, when used in a liver fibrosis model, ESC macrophages significantly reduced hepatic fibrosis by downregulating myofibroblasts and activated liver progenitors, showcasing their potential therapeutic capacity.84 Anderson et al. demonstrated that human ESC-derived CD34 hematopoietic progenitor cells can be differentiated into macrophages following colony formation and treatment with GM-CSF and M-CSF.85 The resulting cells expressed characteristic macrophage cell surface markers, exhibited phagocytic function and cytokine secretion.85
Most recently, Cao et al. developed an efficient protocol to obtain induced pluripotent stem cell (iPSC)-derived macrophages.86 They performed a thorough characterization demonstrating that iPSC-derived macrophages exhibit similar gene expression profiles to peripheral blood macrophages, including CD68, and similar cell surface marker expression, including CD11b, CD18, and CD45. Additionally, iPSC-derived macrophages were successfully polarized into both M1 and M2 phenotype, similar to peripheral blood-derived macrophages, expressing classical pro and anti-inflammatory markers, respectively. These cells also expressed typical endocytic, phagocytic, and efferocytotic function.86 Mucci et al. described a robust protocol for the generation of murine iPSC-derived macrophages and explored an option for using these macrophages as a tool for disease modeling of hereditary pulmonary alveolar proteinosis.87 Additionally, iPSC-derived macrophages can be used to more easily obtain quiescent macrophages on a large scale, yet consistent as is needed for the study of certain pathogens such as Salmonella, which infect macrophages.88 As described by these studies, iPSC-derived macrophages offer a unique advantage in disease-specific modeling, providing a platform for developing advanced therapeutic approaches. Moreover, iPSC-derived macrophages with high proliferative capacity to allow for an abundant supply for experimentation would be beneficial. Further identification and analysis of specific macrophage populations by taking into account for heterogeneity and variation in macrophage phenotype will facilitate our understanding of the phenotype regulations and novel signaling pathways for therapeutic applications.
Conclusion and Future Directions
Despite recent progress in efforts to better understand the post-MI microenvironment, there remains much unknown details surrounding cardiac cell crosstalk and their ultimate effects on cardiac healing and function. To date, only a limited number of studies examined any phenotypic or functional changes in cardiomyocytes in evaluating the effect of coculture with macrophages or macrophage-derived factors. Further investigation examining the changes in cardiomyocyte function in response to subsets of polarized macrophage would provide useful information. Additionally, more studies using human primary or stem cell-derived cell types instead of murine cell type to investigate the effect of direct interaction between cells would be critical for clinical translation. While previous studies focused more on the initial proinflammatory response using proinflammatory macrophages, further studies are needed using macrophages of other phenotypes, as there are more emerging evidence suggesting the wide phenotypic spectrum of macrophages.22 The results from these studies can provide insights into the optimal timing of clinical interventions and therapies.
With the dynamic nature of the heart, it is difficult to directly assess the interaction of cardiomyocytes with neighboring or infiltrating cell types and their true healing potential in vivo. Standard cell culture techniques do not provide a realistic environment in which functional outcomes of a given intervention can be evaluated. Animal models often involve such a complex combination of factors that it is difficult to interpret the outcomes. To bridge this critical missing link in the available experimental methods, which typically leap from cell cultures in a Petri dish to experimental animal models of heart disease to clinical trials, in vitro models are required.
However, an alternative to existing models that focus almost exclusively on mimicking the healthy cardiac microenvironment with the goal of providing a living surgical replacement, there is a growing need for in vitro diseased experimental models to study heart failure. While there are multiple ways to generate disease models ranging from simple 2D cultures to organ-on-chip models, organoids, and complex 3D cocultures, an appropriate model should be selected based on the purpose of the study and the questions being asked. Advanced 3D tissue models allowing independent control of cell and extracellular components can serve as a powerful tool to simulate cell interactions in 3D tissues to directly evaluate function and interaction of cell and tissue during reparative process post-MI. Moreover, in vitro diseased tissue model mimicking the multiphase post-MI condition with multiple cell types rather than cocultures can better recapitulate tissue-level pathophysiology. Detailed and mechanistic studies on the interaction of various cell types and the microenvironment during multiple phases of post-MI repair will lead to a better understanding of important factors needed to develop therapies for cardiovascular diseases. A 3D diseased tissue model or organoids can also serve as a platform for efficiently screening candidate therapeutic strategies provided, and they reproduce key aspects of natural cardiac tissue function. Taken together, in vitro models and studies provide a reliable platform to gain insights into otherwise complex interactions and these findings can be validated in in vivo animal models for complete understanding of macrophage and cardiac cell signaling in the post-MI environment.
Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the National Institutes of Health [NIH R15 HL145726]; and the National Science Foundation [NSF CAREER 1653464].
References
- 1. Frangogiannis, N.G. Regulation of the inflammatory response in cardiac repair. Circ Res 110, 159, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marchant, D.J., Boyd, J.H., Lin, D.C., Granville, D.J., Garmaroudi, F.S., and McManus, B.M.. Inflammation in myocardial diseases. Circ Res 110, 126, 2012. [DOI] [PubMed] [Google Scholar]
- 3. Swirski, F.K., and Nahrendorf, M.. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 339, 161, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ma, Y., Yabluchanskiy, A., and Lindsey, M.L.. Neutrophil roles in left ventricular remodeling following myocardial infarction. Fibrogenesis Tissue Repair 6, 11, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qin, F., Simeone, M., and Patel, R.. Inhibition of NADPH oxidase reduces myocardial oxidative stress and apoptosis and improves cardiac function in heart failure after myocardial infarction. Free Radic Biol Med 43, 271, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Zhao, W., Zhao, T., Chen, Y., Ahokas, R.A., and Sun, Y.. Reactive oxygen species promote angiogenesis in the infarcted rat heart. Int J Exp Pathol 90, 621, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lavine, K.J., Epelman, S., Uchida, K., et al. . Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci U S A 111, 16029, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nahrendorf, M., Swirski, F.K., Aikawa, E., et al. . The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204, 3037, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nathan, C., and Ding, A.. Nonresolving inflammation. Cell 140, 871, 2010. [DOI] [PubMed] [Google Scholar]
- 10. Huynh, M.L., Fadok, V.A., and Henson, P.M.. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest 109, 41, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wan, E., Yeap, X.Y., Dehn, S., et al. . Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ Res 113, 1004, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang, S., Yeap, X.Y., Grigoryeva, L., et al. . Cardiomyocytes induce macrophage receptor shedding to suppress phagocytosis. J Mol Cell Cardiol 87, 171, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Troidl, C., Mollmann, H., Nef, H., et al. . Classically and alternatively activated macrophages contribute to tissue remodelling after myocardial infarction. J Cell Mol Med 13, 3485, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O'Rourke, S.A., Dunne, A., and Monaghan, M.G.. The role of macrophages in the infarcted myocardium: orchestrators of ECM Remodeling. Front Cardiovasc Med 6, 101, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Halade, G.V., Jin, Y.F., and Lindsey, M.L.. Matrix metalloproteinase (MMP)-9: a proximal biomarker for cardiac remodeling and a distal biomarker for inflammation. Pharmacol Ther 139, 32, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baum, J., and Duffy, H.S.. Fibroblasts and myofibroblasts: what are we talking about? J Cardiovasc Pharmacol 57, 376, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ong, S.-B., Hernández-Reséndiz, S., Crespo-Avilan, G.E., et al. . Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther 186, 73, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Frangogiannis, N.G. The inflammatory response in myocardial injury, repair and remodeling. Nat Rev Cardiol 11, 255, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ji, N., Lou, H., Gong, X., Fu, T., and Ni, S.. Treatment with 3-bromo-4,5-dihydroxybenzaldehyde improves cardiac function by inhibiting macrophage infiltration in mice. Korean Circ J 48, 933, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tokutome, M., Matoba, T., Nakano, Y., et al. . Peroxisome proliferator-activated receptor-gamma targeting nanomedicine promotes cardiac healing after acute myocardial infarction by skewing monocyte/macrophage polarization in preclinical animal models. Cardiovasc Res 115, 419, 2019. [DOI] [PubMed] [Google Scholar]
- 21. Frangogiannis, N.G. Emerging roles for macrophages in cardiac injury: cytoprotection, repair, and regeneration. J Clin Invest 125, 2927, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peet, C., Ivetic, A., Bromage, D.I., and Shah, A.M.. Cardiac monocytes and macrophages after myocardial infarction. Cardiovasc Res 116, 1101, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hulsmans, M., Clauss, S., Xiao, L., et al. . Macrophages facilitate electrical conduction in the heart. Cell 169, 510, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yap, J., Cabrera-Fuentes, H.A., Irei, J., Hausenloy, D.J., and Boisvert, W.A.. Role of macrophages in cardioprotection. Int J Mol Sci 20, 2474, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Trial, J., Rossen, R.D., Rubio, J., and Knowlton, A.A.. Inflammation and ischemia: macrophages activated by fibronectin fragments enhance the survival of injured cardiac myocytes. Exp Biol Med 229, 538, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heinemann, J.C., Duerr, G.D., Keppel, K., et al. . CB2 receptor-mediated effects of pro-inflammatory macrophages influence survival of cardiomyocytes. Life Sci 138, 18, 2015. [DOI] [PubMed] [Google Scholar]
- 27. Ai, X., Lu, W., Zeng, K., Li, C., Jiang, Y., and Tu, P.. Microfluidic coculture device for monitoring of inflammation-induced myocardial injury dynamics. Anal Chem 90, 4485, 2018. [DOI] [PubMed] [Google Scholar]
- 28. Hitscherich, P.G., Xie, L.-H., Del Re, D., and Lee, E.J.. The effects of macrophages on cardiomyocyte calcium-handling function using in vitro culture models. Physiol Rep 7, e14137, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pallotta, I., Sun, B., Wrona, E.A., and Freytes, D.O.. BMP protein-mediated crosstalk between inflammatory cells and human pluripotent stem cell-derived cardiomyocytes. J Tissue Eng Regen Med 11, 1466, 2017. [DOI] [PubMed] [Google Scholar]
- 30. Almeida Paiva, R., Martins-Marques, T., Jesus, K., et al. . Ischaemia alters the effects of cardiomyocyte-derived extracellular vesicles on macrophage activation. J Cell Mol Med 23, 1137, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pinto, A.R., Paolicelli, R., Salimova, E., et al. . An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLoS One 7, e36814, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brutsaert, D.L. Cardiac endothelial-myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol Rev 83, 59, 2003. [DOI] [PubMed] [Google Scholar]
- 33. Shah, A.M., Grocott-Mason, R.M., Pepper, C.B., et al. . The cardiac endothelium: cardioactive mediators. Prog Cardiovasc Dis 39, 263, 1996. [DOI] [PubMed] [Google Scholar]
- 34. Pinto, A.R., Ilinykh, A., Ivey, M.J., et al. . Revisiting cardiac cellular composition. Circ Res 118, 400, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mai, J., Virtue, A., Shen, J., Wang, H., and Yang, X.-F.. An evolving new paradigm: endothelial cells—conditional innate immune cells. J Hematol Oncol 6, 61, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu, J., Wang, H., and Li, J.. Inflammation and inflammatory cells in myocardial infarction and reperfusion injury: a double-edged sword. Clin Med Insights Cardiol 10, 79, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kumar, A.G., Ballantyne, C.M., Michael, L.H., et al. . Induction of monocyte chemoattractant protein-1 in the small veins of the ischemic and reperfused canine myocardium. Circulation 95, 693, 1997. [DOI] [PubMed] [Google Scholar]
- 38. Nahrendorf, M., and Swirski, F.K.. Monocyte and macrophage heterogeneity in the heart. Circ Res 112, 1624, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lambert, J.M., Lopez, E.F., and Lindsey, M.L.. Macrophage roles following myocardial infarction. Int J Cardiol 130, 147, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tattersall, I.W., Du, J., Cong, Z., et al. . In vitro modeling of endothelial interaction with macrophages and pericytes demonstrates Notch signaling function in the vascular microenvironment. Angiogenesis 19, 201, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Butoi, E., Gan, A.M., Tucureanu, M.M., et al. . Cross-talk between macrophages and smooth muscle cells impairs collagen and metalloprotease synthesis and promotes angiogenesis. Biochim Biophys Acta 1863, 1568, 2016. [DOI] [PubMed] [Google Scholar]
- 42. Pallotta, I., Sun, B., Lallos, G., Terrenoire, C., and Freytes, D.O.. Contributions of bone morphogenetic proteins in cardiac repair cells in three-dimensional in vitro models and angiogenesis. J Tissue Eng Regen Med 12, 349, 2018. [DOI] [PubMed] [Google Scholar]
- 43. Weber, K.T., Sun, Y., Bhattacharya, S.K., Ahokas, R.A., and Gerling, I.C.. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol 10, 15, 2013. [DOI] [PubMed] [Google Scholar]
- 44. Brown, R.D., Ambler, S.K., Mitchell, M.D., and Long, C.S.. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol 45, 657, 2005. [DOI] [PubMed] [Google Scholar]
- 45. Fan, D., Takawale, A., Lee, J., and Kassiri, Z.. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair 5, 15, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang, W., Lavine, K.J., Epelman, S., et al. . Necrotic myocardial cells release damage-associated molecular patterns that provoke fibroblast activation in vitro and trigger myocardial inflammation and fibrosis in vivo. J Am Heart Assoc 4, e001993, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Turner, N.A. Inflammatory and fibrotic responses of cardiac fibroblasts to myocardial damage associated molecular patterns (DAMPs). J Mol Cell Cardiol 94, 189, 2016. [DOI] [PubMed] [Google Scholar]
- 48. Nakaya, M., Watari, K., Tajima, M., et al. . Cardiac myofibroblast engulfment of dead cells facilitates recovery after myocardial infarction. J Clin Invest 127, 383, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Peters, T., Sindrilaru, A., Hinz, B., et al. . Wound-healing defect of CD18(-/-) mice due to a decrease in TGF-beta1 and myofibroblast differentiation. EMBO J 24, 3400, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bujak, M., and Frangogiannis, N.G.. The role of TGF-β signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res 74, 184, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jivraj, N., Phinikaridou, A., Shah, A.M., and Botnar, R.M.. Molecular imaging of myocardial infarction. Basic Res Cardiol 109, 397, 2014. [DOI] [PubMed] [Google Scholar]
- 52. Fernando, M.R., Giembycz, M.A., and McKay, D.M.. Bidirectional crosstalk via IL-6, PGE2 and PGD2 between murine myofibroblasts and alternatively activated macrophages enhances anti-inflammatory phenotype in both cells. Br J Pharmacol 173, 899, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Alvarez, D., Briassouli, P., Clancy, R.M., et al. . A novel role of endothelin-1 in linking Toll-like receptor 7-mediated inflammation to fibrosis in congenital heart block. J Biol Chem 286, 30444, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ma, F., Li, Y., Jia, L., et al. . Macrophage-stimulated cardiac fibroblast production of IL-6 is essential for TGF beta/Smad activation and cardiac fibrosis induced by angiotensin II. PLoS One 7, e35144, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Do, N.-N., Willenborg, S., Eckes, B., et al. . Myeloid cell–restricted STAT3 signaling controls a cell-autonomous antifibrotic repair program. J Immunol 201, 663, 2018. [DOI] [PubMed] [Google Scholar]
- 56. Mitchell, M.D., Laird, R.E., Brown, R.D., and Long, C.S.. IL-1beta stimulates rat cardiac fibroblast migration via MAP kinase pathways. Am J Physiol Heart Circ Physiol 292, H1139, 2007. [DOI] [PubMed] [Google Scholar]
- 57. Siwik, D.A., Chang, D.L., and Colucci, W.S.. Interleukin-1beta and tumor necrosis factor-alpha decrease collagen synthesis and increase matrix metalloproteinase activity in cardiac fibroblasts in vitro. Circ Res 86, 1259, 2000. [DOI] [PubMed] [Google Scholar]
- 58. Sun, M., Dawood, F., Wen, W.H., et al. . Excessive tumor necrosis factor activation after infarction contributes to susceptibility of myocardial rupture and left ventricular dysfunction. Circulation 110, 3221, 2004. [DOI] [PubMed] [Google Scholar]
- 59. Li, J., Kim, Y.N., and Bertics, P.J.. Platelet-derived growth factor-stimulated migration of murine fibroblasts is associated with epidermal growth factor receptor expression and tyrosine phosphorylation. J Biol Chem 275, 2951, 2000. [DOI] [PubMed] [Google Scholar]
- 60. Rossini, A., Zacheo, A., Mocini, D., et al. . HMGB1-stimulated human primary cardiac fibroblasts exert a paracrine action on human and murine cardiac stem cells. J Mol Cell Cardiol 44, 683, 2008. [DOI] [PubMed] [Google Scholar]
- 61. Albina, J.E., Mills, C.D., Henry, W.L.Jr., and Caldwell, M.D.. Temporal expression of different pathways of 1-arginine metabolism in healing wounds. J Immunol 144, 3877, 1990. [PubMed] [Google Scholar]
- 62. Knipper, J.A., Willenborg, S., Brinckmann, J., et al. . Interleukin-4 receptor alpha signaling in myeloid cells controls collagen fibril assembly in skin repair. Immunity 43, 803, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Desmouliere, A., Redard, M., Darby, I., and Gabbiani, G.. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol 146, 56, 1995. [PMC free article] [PubMed] [Google Scholar]
- 64. Diez-Roux, G., and Lang, R.A.. Macrophages induce apoptosis in normal cells in vivo. Development 124, 3633, 1997. [DOI] [PubMed] [Google Scholar]
- 65. Ploeger, D.T.A., Hosper, N.A., Schipper, M., Koerts, J.A., de Rond, S., and Bank, R.A.. Cell plasticity in wound healing: paracrine factors of M1/M2 polarized macrophages influence the phenotypical state of dermal fibroblasts. Cell Commun Signal 11, 29, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Taciak, B., Bialasek, M., Braniewska, A., et al. . Evaluation of phenotypic and functional stability of RAW 264.7 cell line through serial passages. PLoS One 13, e0198943, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Elisia, I., Pae, H.B., Lam, V., Cederberg, R., Hofs, E., and Krystal, G.. Comparison of RAW264.7, human whole blood and PBMC assays to screen for immunomodulators. J Immunol Methods 452, 26, 2018. [DOI] [PubMed] [Google Scholar]
- 68. Wu, Q., Qi, Y., Wu, N., et al. . Expression and anti-inflammatory role of activin receptor-interacting protein 2 in lipopolysaccharide-activated macrophages. Sci Rep 7, 10306, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Singla, R.D., Wang, J., and Singla, D.K.. Regulation of Notch 1 signaling in THP-1 cells enhances M2 macrophage differentiation. Am J Physiol Heart Circ Physiol 307, H1634, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Qin, Z. The use of THP-1 cells as a model for mimicking the function and regulation of monocytes and macrophages in the vasculature. Atherosclerosis 221, 2, 2012. [DOI] [PubMed] [Google Scholar]
- 71. Chanput, W., Mes, J.J., and Wichers, H.J.. THP-1 cell line: an in vitro cell model for immune modulation approach. Int Immunopharmacol 23, 37, 2014. [DOI] [PubMed] [Google Scholar]
- 72. Chanput, W., Peters, V., and Wichers, H.. THP-1 and U937 cells. In: Verhoeckx, K., Cotter, P., Lopez-Exposito, I., et al., eds. The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models. Springer International Publishing: Cham (CH), 2015, p. 147. [PubMed] [Google Scholar]
- 73. McDade, J.K., Brennan-Pierce, E.P., Ariganello, M.B., Labow, R.S., and Michael Lee, J.. Interactions of U937 macrophage-like cells with decellularized pericardial matrix materials: influence of crosslinking treatment. Acta Biomater 9, 7191, 2013. [DOI] [PubMed] [Google Scholar]
- 74. Rozner, A.E., Dambaeva, S.V., Drenzek, J.G., Durning, M., and Golos, T.G.. Generation of macrophages from peripheral blood monocytes in the rhesus monkey. J Immunol Methods 351, 36, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ginhoux, F., and Jung, S.. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol 14, 392, 2014. [DOI] [PubMed] [Google Scholar]
- 76. Frodermann, V., and Nahrendorf, M.. Macrophages and cardiovascular health. Physiol Rev 98, 2523, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Manzanero, S. Generation of mouse bone marrow-derived macrophages. Methods Mol Biol 844, 177, 2012. [DOI] [PubMed] [Google Scholar]
- 78. Davis, B.K. Isolation, culture, and functional evaluation of bone marrow-derived macrophages. Methods Mol Biol 1031, 27, 2013. [DOI] [PubMed] [Google Scholar]
- 79. Lee, C.M., and Hu, J.. Cell density during differentiation can alter the phenotype of bone marrow-derived macrophages. Cell Biosci 3, 30, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. West, M.E.D., Sefton, E.J.B., and Sefton, M.V.. Bone marrow-derived macrophages enhance vessel stability in modular engineered tissues. Tissue Eng Part A 25, 911, 2019. [DOI] [PubMed] [Google Scholar]
- 81. Subramanian, A., Guo, B., Marsden, M.D., et al. . Macrophage differentiation from embryoid bodies derived from human embryonic stem cells. J Stem Cells 4, 29, 2009. [PMC free article] [PubMed] [Google Scholar]
- 82. Karlsson, K.R., Cowley, S., Martinez, F.O., Shaw, M., Minger, S.L., and James, W.. Homogeneous monocytes and macrophages from human embryonic stem cells following coculture-free differentiation in M-CSF and IL-3. Exp Hematol 36, 1167, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dreymueller, D., Denecke, B., Ludwig, A., and Jahnen-Dechent, W.. Embryonic stem cell-derived M2-like macrophages delay cutaneous wound healing. Wound Repair Regen 21, 44, 2013. [DOI] [PubMed] [Google Scholar]
- 84. Haideri, S.S., McKinnon, A.C., Taylor, A.H., et al. . Injection of embryonic stem cell derived macrophages ameliorates fibrosis in a murine model of liver injury. NPJ Regen Med 2, 14, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Anderson, J.S., Bandi, S., Kaufman, D.S., and Akkina, R.. Derivation of normal macrophages from human embryonic stem (hES) cells for applications in HIV gene therapy. Retrovirology 3, 24, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cao, X., Yakala, G.K., van den Hil, F.E., Cochrane, A., Mummery, C.L., and Orlova, V.V.. Differentiation and functional comparison of monocytes and macrophages from hiPSCs with peripheral blood derivatives. Stem Cell Reports 12, 1282, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mucci, A., Kunkiel, J., Suzuki, T., et al. . Murine iPSC-derived macrophages as a tool for disease modeling of hereditary pulmonary alveolar proteinosis due to Csf2rb deficiency. Stem Cell Reports 7, 292, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hale, C., Yeung, A., Goulding, D., et al. . Induced pluripotent stem cell derived macrophages as a cellular system to study salmonella and other pathogens. PLoS One 10, e0124307, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]