Abstract
The Editors of Tissue Engineering Part A are officially retracting the article entitled, Endothelial/Mesenchymal Stem Cell Crosstalk Within Bioprinted Cocultures, by Santoro M, Awosika TO, Snodderly KL, Hurley-Novatny AC, Lerman MJ, Fisher JP. Tissue Eng Part A 2020;26(5-6):339–349. DOI: 10.1089/ten.tea.2019.0175.
After publication of the article, one of the coauthors of the paper discovered a potential flaw in the analysis that was thought to be dispatched prior to the submission of the paper to Tissue Engineering Part A but was not.
One of the co-editors of Tissue Engineering, John P. Fisher, PhD, is named as the senior author on the paper. Dr. Fisher has expressed deep regret about this error, and in the best interest of the scientific community and the readership of Tissue Engineering, has displayed rectitude in requesting the official retraction of his own article. Additionally, Dr. Fisher and his laboratory have strengthened the internal review processes of data collection and analysis so as to ensure that a similar error does not occur again in the future.
Tissue Engineering remains committed to preserving the integrity of the scientific literature and the community it serves.
Introduction
Tissue damage and organ function loss is a common outcome of several pathologies and traumas. Organ transplantation remains the standard treatment for these conditions, as the human body is unable to regenerate large tissues, such as long bones. Unfortunately, the demand for donated organs exceeds the growing demand, limiting the number of patients that can gain benefit from organ transplantation. Tissue engineering (TE) seeks to address this shortcoming by developing viable implantable scaffolds that can initially serve to replace tissue and subsequently foster tissue regeneration.1,2
A ubiquitous requirement for any engineered tissue is the presence of a vascular network that provides nutrients and sustains local tissue homeostasis.2,3 Conventional TE strategies rely on the regenerative ability of the body to form such vascular network throughout the scaffold.4 Yet, ingrowth of preexisting blood vessels is slow and results in limited cell viability within large scaffolds.5 Hence, scaffold vascularization has been recognized as one of the key challenges in TE and the major obstacle toward the regeneration of viable tissues and organs of clinically relevant size for human use.1,6
Blood vessel formation is a complex process that yet can be summarized into two main steps1: the sprouting and assembly of endothelial cells (ECs) into primitive capillaries (angiogenesis) and2 their stabilization and maturation through recruitment and differentiation of mesenchymal stem cells (MSCs) into mural cells (arteriogenesis).3 Accordingly, several studies have investigated the coculturing of ECs and MSCs to form perfusable blood vessels.7–11 Chen et al. showed that ECs generate an extensive capillary network when cocultured with bone marrow-derived MSCs in gelatin methacrylate (GelMA) scaffolds. In addition, the system formed functional anastomoses with the existing vasculature upon in vivo implantation.8 Jeon et al. mirrored these data and showed how MSCs undergo a phenotypic transition toward a mural cell lineage and colocalize with ECs, consistent with the process of arteriogenesis.9
However, MSCs were found to exert an antiangiogenic action in other instances. Otsu et al. observed an MSC dose-dependent production of reactive oxygen species that induced apoptosis of capillary network.10 Similarly, Menge et al. registered decreased EC proliferation and vascular network formation in vivo when ECs and MSCs are in direct cell contact.11 Given these contrasting findings, there is little agreement on how ECs and MSCs drive the formation of new vasculature and how these findings can be leveraged to engineer vascularized scaffolds.
Toward this end, we sought to investigate the cross talk between ECs and MSCs occurring within bioprinted scaffolds hosting different EC:MSC ratios or EC:MSC coculture modalities (direct cell–cell contact vs. paracrine signaling). We hypothesize that regulation of angiogenic and arteriogenic pathways is both time- and MSC dose dependent.
Both cells were loaded in GelMA, a hydrogel system successfully used in different TE strategies8,12,13 and, subsequently, processed through extrusion-based bioprinting. Regulation of signaling pathways involved in angiogenesis and/or arteriogenesis was assayed both using gene and protein expression. Bioprinting has been leveraged to replicate vessel morphology and to deposit specific cells in a spatial pattern similar to that of native vasculature.13–15 Understanding the mechanisms governing angiogenesis/arteriogenesis within bioprinted EC:MSC cocultures allows obtaining information that can be used as designate criteria to engineer bioprinted scaffolds hosting a vascular template in future studies.
Materials and Methods
Cell culture
Human umbilical vein endothelial cells (HUVECs; Lonza) were cultured in complete endothelial growth medium (EGM-Plus BulletKit; Lonza) according to manufacturer's guidelines. Rat mesenchymal stem cells (rMSCs; EMD Millipore) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% (vol/vol) fetal bovine serum and 1% (vol/vol) antibiotic/antimycotic (Anti-Anti) (Thermo Fisher Scientific, Waltham, MA). Human mesenchymal stem cells (hMSCs; RoosterBio) were cultured in complete growth media (RoosterNourish™-MSC; RoosterBio) according to manufacturer's instructions. All cell types were subpassaged at 70–80% confluency and used for experiment at passage 4.
GelMA synthesis
GelMA was synthesized as in previous studies.16–18 In brief, type A porcine skin gelatin (Sigma-Aldrich) was dissolved in phosphate buffered saline (PBS; Thermo Fisher Scientific) at a 10% (w/v) concentration for 20 min at 50°C. Methacrylic anhydride (MA; Sigma-Aldrich) was then added at 0.6 g of MA/1 g of gelatin under vigorous stirring for 1 h. The reacting solution was then diluted 1:1 with PBS, centrifuged, and the pellet was discarded. The supernatant was dialyzed against deionized water at 50°C using dialysis cassettes (10 kDa molecular weight cutoff; Thermo Fisher Scientific) for 4 days. The deionized water was changed thrice a day. Finally, the dialyzed GelMA was lyophilized for 2 days and stored at −80°C until further use.
Bioink preparation and scaffold bioprinting
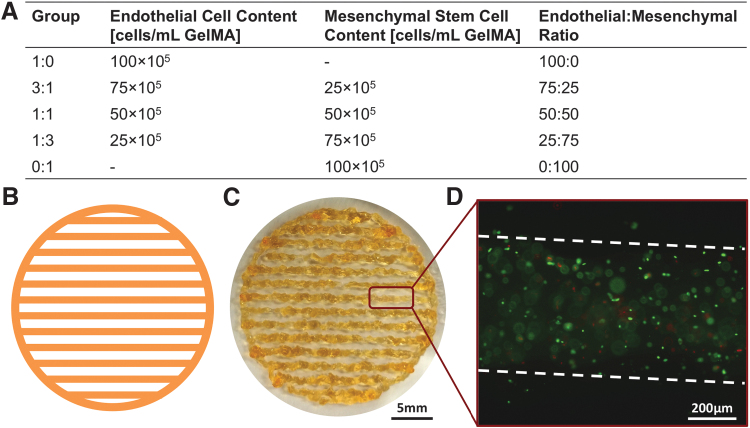
The lyophilized GelMA powder was dissolved at 7.5% (w/v) in PBS at 50°C for 20 min and vortexed for 10 s every 5 min. Photoinitiator 2-hydroxy-1-(4-(hydroxyethoxy)phenyl)-2-methyl-1-propanone (Irgacure 2959; BASF) was then added a concentration of 0.2% (w/v) at 50°C for 15 min under mixing. Cells were lifted by incubating with trypsin/EDTA 0.25% (Thermo Fisher Scientific) for 3 min, counted with a hemocytometer, and centrifuged to form a cell pellet with endothelial:stem cell ratios according to the experimental design shown in Figure 1A. The GelMA bioink was cooled to 37°C and used to resuspend the cell pellet. The resulting cell-laden bioink was then transferred into a syringe barrel and allowed to equilibrate for 1 h at 15°C.
FIG. 1.
Experimental layout and scaffold fabrication. (A) EC and MSC were loaded in GelMA according to five different ratios, starting from a solution of PBS containing 7.5% w/v GelMA and an overall cell concentration of 106 cells/mL GelMA. (B) Scaffolds were first CAD designed and (C) subsequently fabricated using extrusion-based bioprinting. (D) Upon bioprinting, scaffolds were analyzed using Live/Dead staining to ascertain that the printing conditions did not negatively affect cell viability. In (D), live and dead cells are stained in green and red, respectively, while the broken white line indicates the fiber contours. EC, endothelial cell; GelMA, gelatin methacrylate; MSC, mesenchymal stem cell; PBS, phosphate buffered saline.
All scaffolds in this study were fabricated using a commercially available extrusion-based bioprinter (3D-Bioplotter; EnvisionTEC). Scaffolds were designed as disks (25 mm in diameter, 400 μm thickness) composed of fibers 400 μm in diameter and 1 mm center-to-center fiber spacing (Fig. 1B, C). Each scaffold was extruded directly into a 35 mm diameter petri dish placed over a cooling platform set at 8°C and subsequently photocrosslinked with UV light (0.09 mW/cm2) for 90 s. Finally, 2 mL of complete endothelial growth medium (EGM-Plus BulletKit; Lonza) was added to each petri dish, and samples were maintained in a water-jacketed incubator at 37°C and 5% CO2 (HeraCell 150i; Thermo Fisher Scientific) up to 14 days. Media was replaced every 3 days for the whole duration of the experiment.
Experimental design
In the first experiment, HUVECs and rMSCs were lifted from tissue culture flasks and mixed according to five different HUVEC:rMSC ratios (1:0, 3:1, 1:1, 1:3, and 0:1, respectively) in the GelMA bioink. The overall cell concentration was kept constant among groups at 1 × 106 cells/mL GelMA bioink (Fig. 1A). Samples were harvested after 1, 7, and 14 days and analyzed for DNA quantification and gene expression.
In a second study, the same experimental design was used; however, hMSCs were substituted for rMSCs. Samples were harvested after 1, 7, and 14 days, assayed for gene expression and protein expression, and imaged using immunofluorescence microscopy. In both studies the ratio of HUVECs to rMSCs (or hMSCs) is referred to as EC:MSC.
Finally, HUVECs and hMSCs were mixed in a 3:1 ratio and either homogeneously encapsulated in the GelMA bioink (Direct coculture) or singularly encapsulated in GelMA bioink and bioprinted adjacently (Indirect coculture). To avoid confounding factors (e.g., oxygen gradients), all hydrogels contain 106 cells/mL, and the size/volume of the two hydrogels containing solely HUVECs or hMSCs in the Indirect coculture group matches that of the scaffold in the Direct coculture group. In this additional study, aimed at elucidating the relative effect of direct cell–cell contact and paracrine signaling, samples were analyzed for gene and protein expression.
DNA quantification
Double-stranded DNA content was quantified using Quant-iT PicoGreen (Thermo Fisher Scientific) following the manufacturer's protocol. Scaffolds were dissolved in TRIzol (Thermo Fisher Scientific) for 30 min and mechanically mixed using a pipette every 5 min. The scaffold/TRIzol mixture was then processed with a DNeasy Blood & Tissue Kit (Qiagen) to isolate the total DNA. Isolated DNA was mixed with the diluted dye in technical triplicate and compared to a standard DNA ladder (n = 4). Fluorescence measurements were read on a M5 SpectraMax plate reader (Molecular Devices) with an excitation wavelength of 490 nm and emission read at 538 nm.
Gene expression
Scaffolds were dissolved in TRIzol (Thermo Fisher Scientific) for 30 min and mechanically mixed using a pipette every 5 min. The scaffold/TRIzol mixture was then processed with an RNeasy Plus Mini Kit (Qiagen) to isolate the total RNA, which was then reverse transcribed to complementary DNA (cDNA) using a High Capacity cDNA Archive Kit (Thermo Fisher Scientific). Next, quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was performed by combining the cDNA solution with Universal Master Mix, oligonucleotide primers, and TaqMan probes, all provided by Thermo Fisher Scientific (n = 4).
Samples were tested against the following human and/or rat targets: vascular endothelial growth factor (VEGF)-A, platelet-derived growth factor, (PDGF) subunit B, transforming growth factor-β1 (TGF-β1), and TEK receptor tyrosine kinase (TIE-2). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as endogenous gene control. Further information on the probe can be found in Supplementary Table S1.
qRT-PCR was performed using a 7900HT real-time PCR System (Applied Biosystems) at thermal conditions of 2 min at 50°C, 10 min at 95°C, 40 cycles of 15 s at 95°C, and 1 min at 60°C. The relative gene expression level of each target gene was normalized to the mean of GAPDH in each group, and then the fold change was determined relative to homotypic EC cultures (i.e., group 1:0) at day 1. Fold change was calculated using the ΔΔCT relative comparative method as described previously.17,18
Protein expression
Conditioned medium from each sample (n = 4 per group) was harvested after 1, 7, and 14 days of culture and stored at −80°C until testing using Enzyme-Linked Immunosorbent Assay (ELISA) to determine secretion levels of ligands VEGF (BioGems), angiopoietin-1 (ANG-1; RayBiotech), PDGF (RayBiotech), and TGF-β1 (BioGems). Samples were thawed to room temperature and analyzed according to the manufacturer's protocol. A microplate reader (M5 SpectraMax, Molecular Devices) was used to measure the optical density in each sample.
Immunofluorescence microscopy
Scaffolds were tested through live/dead assay to verify that the bioprinting process did not have any detrimental effect on cell viability. Toward this end, 2 h after bioprinting scaffolds were rinsed in PBS for 5 min to remove extra media and then incubated in the dark for 30 min with a PBS-based solution containing 2 μM ethidium homodimer and 4 μM calcein AM (Life Technologies). Afterward, scaffolds were rinsed thrice in PBS and imaged on a fluorescent microscope (Nikon).
Another set of scaffolds was cultured for 7 days, rinsed in PBS, fixed in 4% paraformaldehyde, fixed in 1% sucrose in PBS for 4 h, and then rinsed thrice. Scaffolds were permeabilized using 0.05% Triton X-100 for 5 min, rinsed with PBS, and blocked with 5% goat serum and 1% bovine serum albumin in PBS. Scaffolds were then incubated with mouse primary antibodies against either α-smooth muscle actin (α-SMA) or smooth muscle myosin-heavy chain 1 (SMMHC1), rinsed, and incubated with a goat anti-mouse secondary antibody conjugated with Alexa Fluor 647 (Thermo Fisher Scientific).
Next, scaffolds stained for α-SMA were incubated with rabbit anti-vascular endothelial cadherin (VE-Cad), and scaffolds stained for SMMHC1 were incubated with rabbit anti-CD34 antibody. After rinsing, scaffolds were incubated with goat anti-rabbit antibody conjugated with Alexa Fluor 488 (Thermo Fisher Scientific). All primary antibodies were purchased from Abcam. Cell nuclei were counter stained with 4′,6-diamidino-2-phenylindole (DAPI), and the scaffolds were rinsed thrice with PBS before imaging on a fluorescent microscope (Nikon). Imaging was performed with a Nikon Ti2 microscope (Nikon) mounted with Nikon DS Ri2-Qi2 camera.
Statistical analysis
Where applicable, data are expressed as mean ± standard deviation. Statistical analysis was performed in Minitab software using a one-factor analysis of variance test followed by Tukey's honestly significant difference post hoc test. Differences were considered significant for p < 0.05.
Results
In the present work, ECs and MSCs were loaded in GelMA coculture according to five different ratios while keeping the same overall cell density (Fig. 1A). Regardless of the EC:MSC ratio used and the cell source (either human or rat), the vast majority of cells were viable (calcein AM staining, green), with only a negligible fraction of cells positive for marker of cell death ethidium homodimer-1 (red, Fig. 1D). Once we ascertained that the bioprinting process did not have a detrimental effect on cell viability we proceeded by investigating how EC:MSC ratio impacts the expression of factors involved in angiogenesis and arteriogenesis.
Mesenchymal stem cells have a time- and dose-dependent effect on the expression of angiogenic factors in bioprinted human/rat cocultures
We initially evaluated the effect of different EC:MSC ratios on cell growth and pathway regulation in hybrid bioprinted cocultures containing HUVECs and rMSCs.
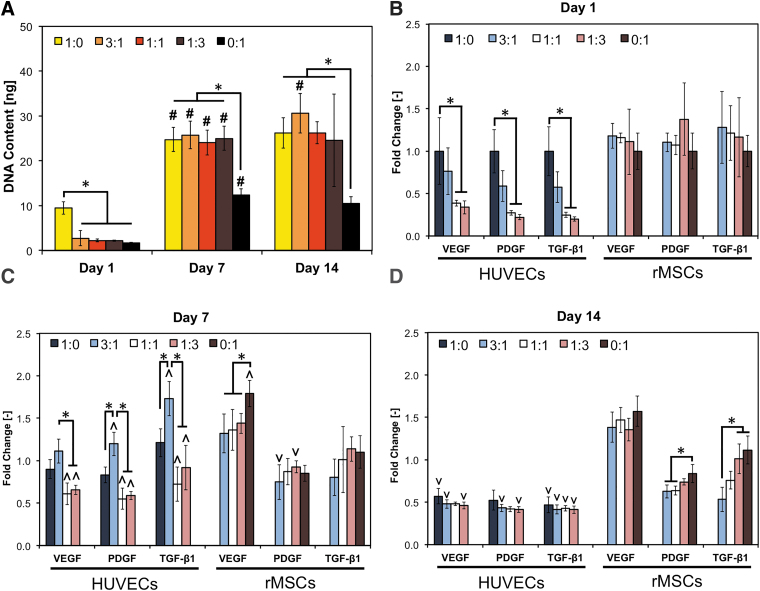
All experimental groups displayed an increase in cell content over the course of 14 days, as measured using DNA quantification (Fig. 2A). All groups showed a significant increase in cell content only between day 1 and 7 except for coculture group EC:MSC 3:1, where a further increase in DNA content occurred at day 14. DNA content in homotypic HUVEC cultures (i.e., group EC:MSC 1:0) was found to be significantly higher than in any MSC-containing groups at day 1. No significant differences in DNA content was observed at either day 7 or 14 among all experimental groups except for homotypic rMSC cultures (i.e., group EC:MSC 0:1), which consistently displayed a lower cell content than other groups.
FIG. 2.
Regulation of angiogenic/arteriogenic pathways in bioprinted hybrid cocultures. HUVECs and rMSCs were cocultured in five different ratios (1:0, 3:1, 1:1, 1:3, and 0:1) and analyzed after 1, 7, and 14 days for cell growth (A) and for the expression of key angiogenic/arteriogenic markers using RT-PCR (B–D, respectively). In panel A, data are reported as mean ± standard deviation (n = 6); “*” indicates statistical significance among different coculture ratios within each time point, while “#” indicates difference from the previous time point within each coculture ratio (p < 0.05). In (B–D), data are reported as mean ± standard deviation (n = 4), and “*” indicates statistical significance among different coculture ratios within each gene/time point tested (p < 0.05). Within each gene/group, a significant increase or decrease compared to previous time point is indicated by ∧ and ∨, respectively. HUVEC, human umbilical vein endothelial cell; rMSC, rat MSC; RT-PCR, reverse transcriptase–polymerase chain reaction.
To determine how the EC:MSC ratio affects the expression of markers associated with angiogenesis and/or arteriogenesis, we then profiled the cells using qRT-PCR for genes involved in angiogenesis (VEGF, PDGF) and arteriogenesis (PDGF, TGF-β1), as shown in Figure 2B–D. The use of hybrid human/rat cocultures allowed to perform species-specific gene expression and to evaluate independently the regulation of human genes in the HUVECs or of rat genes in the rMSCs. Toward this end, we conducted preliminary tests to ascertain the absence of cross-reactivity between human primers with rMSCs and rat primers with HUVECs (data not shown), as indicated by the lack of human gene and rat gene data for EC:MSC cocultures 0:1 and 1:0, respectively (Fig. 2B–D).
After 1 day of coculture, we observed an rMSC dose-dependent downregulation of human genes in the HUVECs, while gene expression in rMSCs was unaffected by the presence of HUVECs (Fig. 2B). Conversely, at day 7 HUVECs in group EC:MSC 3:1 had significantly higher human gene expression than any other group (Fig. 2C).
All coculture groups registered higher expression of human genes at day 7 compared to day 1 (as shown by the ∧ symbol), a phenomenon not observed for homotypic HUVEC cultures. In addition, the presence of HUVECs negatively impacted expression of VEGF in rMSCs, but had negligible effect on regulation of PDGF and TGF-β1 genes at day 7.
At day 14, expression of angiogenic human genes (i.e., in HUVECs) is downregulated compared to day 7 regardless of the EC:MSC ratio tested (∨ symbol, Fig. 1D). Expression of murine genes (i.e., in rMSCs) at day 14 is comparable to day 7, and an rMSC dose-dependent upregulation of rat genes PDGF and TGF-β1 was observed.
The inhibitory-to-stimulatory effect of MSCs on the regulation of angiogenic factors is mirrored in bioprinted human cocultures
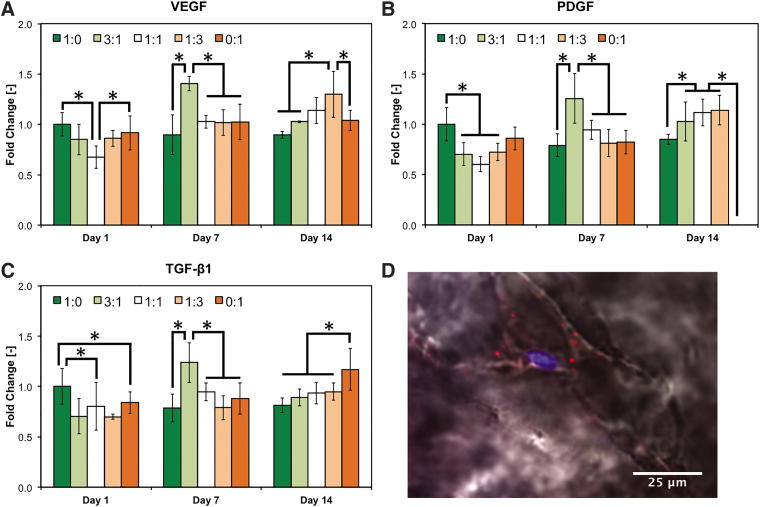
To validate these data in a fully human coculture system, which is more relevant from a translational standpoint, we repeated the previous experiment using HUVECs and hMSCs. EC:MSC group 1:0 showed higher gene expression than any other hMSC-containing group after 1 day of culture (Fig. 3). Strikingly, both homotypic culture groups (i.e., EC:MSC 1:0 and 0:1) exhibited higher VEGF expression than coculture group EC:MSC 1:1, indicating the detrimental effect of coculturing at early stage (Fig. 3A).
FIG. 3.
Regulation of angiogenic/arteriogenic pathways in bioprinted human cocultures. HUVECs and hMSCs were cocultured in five different ratios (1:0, 3:1, 1:1, 1:3, and 0:1) and analyzed after 1, 7, and 14 days for the expression of key angiogenic/arteriogenic markers using RT-PCR (A–C, respectively). In (A–D), data are reported as mean ± standard deviation (n = 4), and “*” indicates statistical significance among different coculture ratios within each gene/time point tested (p < 0.05). (D) Representative staining of EC:MSC 3:1 coculture group showing a cell positive for SMMHC1 (red), which is indicative of arteriogenic differentiation into perivascular cells. Cells were counter stained for nuclei (blue), and bright-field imaging (black/white) highlights the cell body within the GelMA hydrogel. hMSC, human MSC; SMMHC1, smooth muscle myosin-heavy chain 1.
Similar to the previous experiment, expression of all angiogenic/arteriogenic genes was maximized in coculture group EC:MSC 3:1 after day 7. By day 14 this peak shifted toward groups containing a higher fraction of hMSCs, with the greatest expression of VEGF and TGF-β1 in culture groups EC:MSC 1:3 and 0:1, respectively (Fig. 3A–C). Expression of PDGF was similar in all coculture groups and significantly higher that either homotypic culture groups (i.e., EC:MSC 1:0 and 0:1, Fig. 3B). Gene expression of TIE-2 receptor did not vary with the EC:MSC ratio selected nor temporally (Supplementary Fig. S1).
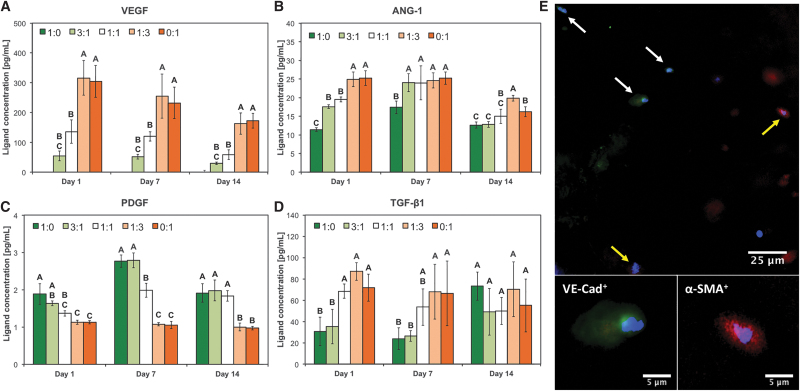
The same angiogenic/arteriogenic factors were assayed also for protein expression using ELISA, as shown in Figure 4. We observed an hMSC-dose dependent upregulation of VEGF, ANG-1, and TGF-β1 at all time points. Conversely, expression of PDGF ligand was downregulated in a MSC-dose dependent manner (Fig. 4C).
FIG. 4.
Expression of angiogenic/arteriogenic factors in bioprinted human cocultures. HUVECs and hMSCs were cocultured in five different ratios (1:0, 3:1, 1:1, 1:3, and 0:1) and analyzed after 1, 7, and 14 days for the expression of key angiogenic/arteriogenic factors using ELISA (A–D, respectively). In (A–D), data are reported as mean ± standard deviation (n = 4), and groups not connected by the same letter within each factor/time point tested are statistically different (p < 0.05). (E) Representative staining of bioprinted scaffold cultured at EC:MSC 3:1 ratio. Cells were stained for nuclei (blue), VE-Cad (green), and α-SMA (red). White and yellow arrows indicate HUVECs and hMSCs, respectively. High magnification insets show positive staining for VE-Cad in HUVECs (bottom left) and for α-SMA in hMSCs (bottom right). α-SMA, α-smooth muscle actin; ELISA, enzyme-linked immunosorbent assay; VE-Cad, vascular endothelial cadherin.
Immunofluorescent staining was also performed to evaluate cell phenotype and eventual differentiation. The majority of HUVECs maintained their endothelial phenotype, as witnessed by the expression of VE-Cad, while hMSCs remained positive for α-SMA, indicating that both cells did not dedifferentiate in our system (Fig. 4E). Furthermore, hMSCs adapted a perivascular cell-like phenotype when cocultured with ECs, as indicated by positive staining SMMHC1 (Fig. 3D) not present in homotypic hMSC cultures (i.e., group EC:MSC 0:1, not shown).
Heterotypic cell–cell contact promotes expression of angiogenic factors, while paracrine signaling supports expression of arteriogenic factors
As gene analyses showed that EC:MSC 3:1 group seemed to outperform the other groups (Figs. 2, 3), we conducted an additional experiment to determine which EC:MSC cross talk mechanism (direct cell–cell contact vs. paracrine signaling) might be responsible for the results previously shown.
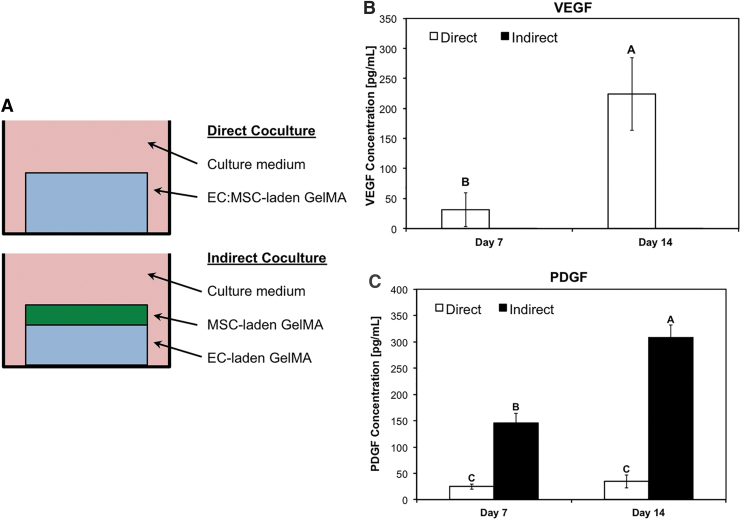
HUVECs and hMSCs were either loaded together in GelMA, as in the rest of this study (Direct coculture, Fig. 5A), or separately loaded in GelMA and bioprinted as two adjacent scaffolds, preventing heterotypic cell–cell contact but only diffusion of paracrine factors between the two cell types (Indirect coculture, Fig. 5A).
FIG. 5.
Different expression of angiogenic/arteriogenic factors in direct versus indirect bioprinted cocultures. (A) Schematic showing direct and indirect cocultures of EC:MSC bioprinted scaffolds. In the direct coculture, HUVECs and hMSCs were loaded at a 3:1 ratio within GelMA and bioprinted in a multiwell plate (blue box). In the indirect coculture, a hydrogel containing only HUVECs is bioprinted (blue box), followed by another hydrogel (green) loaded only with hMSCs. In this latter case, both hydrogels are loaded at a concentration of 106 cells/mL while maintaining an overall EC:MSC 3:1 ratio. In addition, the sum of the two hydrogels equals the volume of the bioprinted hydrogels used in the direct coculture group. Scaffolds were analyzed after 7 and 14 days using ELISA for the expression of VEGF (B) and PDGF (C). In (B, C), data are reported as mean ± standard deviation (n = 4), and groups not connected by the same letter within each factor/time point tested are statistically different (p < 0.05). PDGF, platelet-derived growth factor; VEGF, vascular endothelial growth factor.
Over the course of 14 days, no significant differences were found with gene expression except for upregulation at day 7 in the direct coculture group (Supplementary Fig. S2A–D). On a protein level, ELISA testing revealed a dramatic upregulation of angiogenic growth factor VEGF in the Direct coculture group at both day 7 and 14 (Fig. 5B). Conversely, PDGF was upregulated in the Indirect coculture group at both time points (Fig. 5C). ANG-1 and TGF-β1 levels did not vary significantly between Direct and Indirect coculture (Supplementary Fig. S2E, F).
Discussion
The development of tissue-engineered surrogates to address the ever-growing shortage of organ donors is hampered by the inability to fabricate viable constructs of relevant size for use in human patients.1,6 Aside from isolated instances where the tissue is rather avascular (e.g., cartilage) or where the high surface area-to-volume ratio allows for nutrient exchange through diffusion (e.g., skin, cornea), the presence of vasculature is indispensable for providing cells with nutrients and for maintaining tissue homeostasis.1
The scientific community has investigated different strategies to address this shortcoming. One of the most popular approaches involves the use of the two key cells involved in blood vessel formation, which are the ECs that initially assemble into capillaries (angiogenesis) and the MSCs responsible for their latter stabilization (arteriogenesis).3,19
Bioprinting is an advantageous technique in vascular TE applications, as it allows to pattern simultaneously the EC:MSC vasculogenic population, as well as the cells/factors responsible for the regeneration of the target tissue. By doing so, bioprinting allows the mimicry of native tissue architecture while assuring a distributed and interconnected pattern of the vasculogenic bioink.20,21
Along this rationale, we designed a scaffold where the radius of the cell-laden fibers is comparable to the characteristic length for oxygen diffusion in tissues (∼200 μm),22,23 which allows us to ignore hypoxia-mediated mechanisms and focus on heterotypic EC:MSC cross talk (Fig. 1).24 As the EC:MSC ratio in the vascular niche evolves temporally and spatially,2,3 we explored five different EC:MSC ratios to determine the ideal ratio that results in the strongest upregulation of angiogenic factors and serve as a design parameter for future bioprinting applications focused on the formation of vascular structures within the scaffolds (Fig. 1).
A controversy emerged in literature where MSCs contribute to vascular network formation in some cases while exerting an inhibitory effect on EC proliferation in others.7–11 One of the controversial aspects involves cell proliferation, which was found to be promoted, as well as impaired, in EC:MSC cocultures compared to homotypic EC cultures. Menge et al. reported that MSCs stabilize ECs and prevent their proliferation in as little as 24 h.11 This effect appeared to require direct EC:MSC contact and was associated with enhanced colocalization of VE-Cad and β-catenin at the cell membrane. Our data are consistent with these findings, as evidenced by the detrimental effect of MSCs on cell proliferation within 1 day of coculturing and the presence of VE-Cad+ ECs (Figs. 2A–4E).
In contrast, multiple investigators have repeatedly demonstrated that mutual paracrine signaling between ECs and MSCs promotes cell proliferation within 2–3 days.25–27 Our study corroborates these data as well, as witnessed by the enhanced proliferation occurring within 1 week in all cocultures compared to homotypic EC or MSC cultures (Fig. 2A and Supplementary S3).
As shown by Granja and coworkers,27 the enhanced proliferation is not dependent on the EC:MSC ratio selected. Therefore the contrasting findings present in literature could be reconciled by considering the different timescales between juxtacrine and paracrine signaling. The almost instantaneous formation of cell–cell communications prevents cell proliferation and appears to be the controlling mechanism until a “critical” concentration of paracrine factors (usually within 2–3 days) is produced by the cells, at which point proliferation of both cells is strongly promoted.
Another frequently overlooked aspect involves the relative contribution of ECs and MSCs on the regulation of key angiogenic and arteriogenic pathways. Multiple investigators demonstrated a more robust angiogenic response in EC:MSC cocultures compared to EC homotypic cultures but do not elaborate as to which cell type is responsible for the upregulation of angiogenic markers.27,28 The use of a hybrid human/rat coculture system was specifically pursued to address this issue. Interestingly, MSCs exhibited a dose-dependent stimulatory effect on EC expression of angiogenic factors (Fig. 2), which is consistent with the VEGF signaling cascade driving early vasculogenesis described in literature.2 A possible explanation for this disruption of VEGF signaling is offered by Lee et al., which showed that MSC-derived exosomes downregulate VEGF expression in a dose-dependent (i.e., MSC fraction dependent) manner.29
Accessory cells like MSCs become essential during the later stages of angiogenesis, distinguished by a significant upregulation of angiogenic and arteriogenic pathways. Our system seems to mimic this trend, as evidenced by the enhanced EC:MSC cross talk after 1 week of coculture. As the coculture progresses, the MSC secretome drives VEGF expression (Fig. 2C and Fig. 4) and PDGF and TGF-β1 regulation (Fig. 2D and Fig. 4), thus supporting the standard notion of MSC-mediated upregulation of angiogenic markers present in literature. This inhibitory-to-stimulatory switch in MSC action occurs in a manner that mimics the normal events surrounding blood vessel formation,2,3 although the timeline observed in vitro (14 days) might not necessarily overlap with the one occurring in vivo.30
The use of a hybrid coculture exposed the above-mentioned phenomena but heterotypic human–rat interactions might have affected pathways involved not only in angiogenesis/arteriogenesis but also in immune response, such as the TGF-β1 signaling cascade.31 To avoid these complications we repeated the same analyses in a fully human coculture and observed that the gene expression data (Fig. 3) are congruent with our hybrid testing.
While the relative contributions of ECs and MSCs could not be ascertained in human/human cocultures, the highest gene expression shifts over time toward groups with a low EC:MSC ratio, confirming that the primary drivers of angiogenesis are primarily ECs and secondarily MSCs. In addition, the correlation between VEGF expression and EC:MSC ratio (Fig. 3A) is exceptionally analogous to the one reported by Cui and colleagues, who used a similar system (HUVECs and umbilical cord-derived MSCs in GelMA) but for skin TE applications.28
Strikingly, ECs were the primary source of arteriogenic factor PDGF, which is associated with the recruitment of mural cells in vivo by ECs.32 At the same time, MSCs secreted high levels of ANG-1 and TGF-β1, which in vivo drive vessel stabilization by maximizing heterotypic EC:MSC interactions.3 Furthermore, MSCs in coculture positively stained for perivascular markers SMMHC1 and α-SMA (Figs. 3, 4). These data suggest a phenotypic transition of MSCs into mural-like cells consistent with the process of arteriogenesis. Yet, it is worth reiterating that the current work focused solely on the cellular cross talk governing EC:MSC interactions within bioprinted cocultures and did not investigate the formation of functional capillary structures.
Among the different mechanisms regulating angiogenesis in EC:MSC coculture, paracrine and juxtacrine signaling seem to have opposite effects on pathway regulation. To further elucidate how these two mechanisms operate, we performed an experiment where cell–cell contact between EC and MSCs was present or not (Direct and Indirect coculture, respectively).
Besides a mild upregulation of gene expression observed in the Direct coculture groups (Supplementary Fig. S2), VEGF levels were remarkably higher in Direct coculture conditions, while PDGF had greater expression in the Indirect coculture samples (Fig. 5). Kirkpatrick and colleagues found similar results in a 2D coculture of ECs with primary osteoblasts, while Augusti and colleagues demonstrated that PDGF expression was strongly downregulated in spheroidal cocultures of ECs and smooth muscle cells.33,34
Together, these data suggest that juxtacrine signaling between ECs and mesodermal cells, whether or not committed toward a bone lineage, favors regulation of angiogenic pathways and is consistent with the notion that vasculogenesis and osteogenesis evolve simultaneously in the native bone niche.20,21
In evaluating how EC:MSC cross talk occurs within bioprinted cocultures, this study solidifies the beneficial effect of MSCs on the overall regulation of angiogenic factors, as measured by upregulation of VEGF and expression of VE-Cad, as well as on arteriogenic factors, as witnessed by the adoption of a mural-like phenotype by MSCs. Our data suggest that an EC:MSC ratio of 3:1 results in the strongest upregulation of key signaling pathways. Given the diametrically opposed effect of Direct versus Indirect coculture modalities, further bioprinting designs may consider the presence of both EC:MSC-laden bioinks, as well as the adjacent deposition of bioinks containing solely MSCs or ECs.
We contend that the further incorporation of angiogenic drivers, such as shear stress gradients,35 would result in an even more robust promotion of angiogenic/arteriogenic markers. Future works will elucidate how the EC:MSC cross talk presented in this study translates into the actual formation of vascular structures, therefore reconciling the expression of angiogenic genes/proteins with the macroscopic process of angiogenesis and arteriogenesis.
Conclusions
In this study we sought to elucidate the cross talk occurring between ECs and MSCs when cocultured in different ratios or modalities. Using hybrid human/rat cocultures we pinpointed the role of each cell type on the overall regulation of genes/proteins involved in angiogenesis, showing how MSCs inhibited expression of angiogenesis factors in ECs early on, likely due to juxtacrine signaling-mediated inhibition of cell proliferation. The inhibitory action of MSCs reverts to a stimulatory one in a time-dependent manner, as corroborated in fully human cocultures.
Interestingly, the balance between juxtacrine and paracrine signaling (i.e., Direct vs. Indirect coculture) dictates expression of angiogenic or arteriogenic growth factors. Altogether, these findings can inform the design of vascularized constructs. In preparation for future testing of the constructs, the EC:MSC ratio and/or in vitro maturation time can be tuned to maximize expression of angiogenic signaling pathways and to elicit the formation of any vascular structure in vivo.
Supplementary Material
Acknowledgments
Dr. Santoro acknowledges support from the MSCRF Postdoctoral Fellowship Program. Dr. Lerman acknowledges support from the National Institute of Standards and Technology Measurement Science Fellowship Program (2014-NIST-MSE-01).
Disclosure Statement
No competing financial interests exist.
Funding Information
This research was supported by the Maryland Stem Cell Research Fund (MSCRF) Research Grant (No. 4300811) and the National Institute of Biomedical Imaging and Bioengineering/National Institutes of Health (NIBIB/NIH) Center for Engineering Complex Tissues (P41 EB023833).
Supplementary Material
References
- 1. Novosel, E.C., Kleinhans, C., and Kluger, P.J.. Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev 63, 300, 2011. [DOI] [PubMed] [Google Scholar]
- 2. Bae, H., Puranik, A.S., Gauvin, R., et al. . Building vascular networks. Sci Transl Med 4, 160ps23, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nat Med 6, 389, 2000. [DOI] [PubMed] [Google Scholar]
- 4. Muschler, G.F., Nakamoto, C., and Griffith, L.G.. Engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg Am 86, 1541, 2004. [DOI] [PubMed] [Google Scholar]
- 5. Rouwkema, J., and Khademhosseini, A.. Vascularization and angiogenesis in tissue engineering: beyond creating static networks. Trends Biotechnol 34, 733, 2016. [DOI] [PubMed] [Google Scholar]
- 6. Auger, F.A., Gibot, L., and Lacroix, D.. The pivotal role of vascularization in tissue engineering. Annu Rev Biomed Eng 15, 177, 2013. [DOI] [PubMed] [Google Scholar]
- 7. Nassiri, S.M., and Rahbarghazi, R.. Interactions of mesenchymal stem cells with endothelial cells. Stem Cells Dev 23, 319, 2014. [DOI] [PubMed] [Google Scholar]
- 8. Chen, Y.C., Lin, R.Z., Qi, H., et al. . Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels. Adv Funct Mater 22, 2027, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jeon, J.S., Bersini, S., Whisler, J.A., et al. . Generation of 3D functional microvascular networks with human mesenchymal stem cells in microfluidic systems. Integr Biol 6, 555, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Otsu, K., Das, S., Houser, S.D., Quadri, S.K., Bhattacharya, S., and Bhattacharya, J.. Concentration-dependent inhibition of angiogenesis by mesenchymal stem cells. Blood 113, 4197, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Menge, T., Gerber, M., Wataha, K., et al. . Human mesenchymal stem cells inhibit endothelial proliferation and angiogenesis via cell–cell contact through modulation of the VE-cadherin/β-catenin signaling pathway. Stem Cells Dev 22, 148, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gauvin, R., Chen, Y.C., Lee, J.W., et al. . Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials 33, 3824, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jia, W., Gungor-Ozkerim, P.S., Zhang, Y.S., et al. . Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 106, 58, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kang, H.-W., Lee, S.J., Ko, I.K., Kengla, C., Yoo, J.J., and Atala, A.. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol 34, 312, 2016. [DOI] [PubMed] [Google Scholar]
- 15. Kolesky, D.B., Homan, K.A., Skylar-Scott, M.A., and Lewis, J.A.. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci U S A 113, 3179, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuo, C.-Y., Wilson, E., Fuson, A., et al. . Repair of tympanic membrane perforations with customized, bioprinted ear grafts using chinchilla models. Tissue Eng Part A 24, 527, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kuo, C.-Y., Shevchuk, M., Opfermann, J., et al. . Trophoblast–endothelium signaling involves angiogenesis and apoptosis in a dynamic bioprinted placenta model. Biotechnol Bioeng 116, 181, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arumugasaamy, N., Ettehadieh, L.E., Kuo, C.Y., et al. . Biomimetic placenta-fetus model demonstrating maternal–fetal transmission and fetal neural toxicity of zika virus. Ann Biomed Eng 46, 1963, 2018. [DOI] [PubMed] [Google Scholar]
- 19. Carmeliet, P., and Jain, R.K.. Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Byambaa, B., Annabi, N., Yue, K., et al. . Bioprinted osteogenic and vasculogenic patterns for engineering 3D bone tissue. Adv Healthc Mater 6, 1700015, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cui, H., Zhu, W., Nowicki, M., Zhou, X., Khademhosseini, A., and Zhang, L.G.. Hierarchical fabrication of engineered vascularized bone biphasic constructs via dual 3D bioprinting: integrating regional bioactive factors into architectural design. Adv Healthc Mater 5, 2174, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grimes, D.R., Kelly, C., Bloch, K., and Partridge, M.. A method for estimating the oxygen consumption rate in multicellular tumour spheroids. J R Soc Interface 11, 20131124, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Olive, P.L., Vikse, C., and Trotter, M.J.. Measurement of oxygen diffusion distance in tumor cubes using a fluorescent hypoxia probe. Int J Radiat Oncol Biol Phys 22, 397, 1992. [DOI] [PubMed] [Google Scholar]
- 24. Krock, B.L., Skuli, N., and Simon, M.C.. Hypoxia-induced angiogenesis: good and evil. Genes Cancer 2, 1117, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saleh, F.A., Whyte, M., Ashton, P., and Genever, P.G.. Regulation of mesenchymal stem cell activity by endothelial cells. Stem Cells Dev 20, 391, 2011. [DOI] [PubMed] [Google Scholar]
- 26. Rahbarghazi, R., Nassiri, S.M., Khazraiinia, P., et al. . Juxtacrine and paracrine interactions of rat marrow-derived mesenchymal stem cells, muscle-derived satellite cells, and neonatal cardiomyocytes with endothelial cells in angiogenesis dynamics. Stem Cells Dev 22, 855, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bidarra, S.J., Barrias, C.C., Barbosa, M.A., Soares, R., Amédée, J., and Granja, P.L.. Phenotypic and proliferative modulation of human mesenchymal stem cells via crosstalk with endothelial cells. Stem Cell Res 7, 186, 2011. [DOI] [PubMed] [Google Scholar]
- 28. Zhang, X., Li, J., Ye, P., Gao, G., Hubbell, K., and Cui, X.. Coculture of mesenchymal stem cells and endothelial cells enhances host tissue integration and epidermis maturation through AKT activation in gelatin methacryloyl hydrogel-based skin model. Acta Biomater 59, 317, 2017. [DOI] [PubMed] [Google Scholar]
- 29. Lee, J.K., Park, S.R., Jung, B.K., et al. . Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One 8, e84256, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hathout, E., Chan, N.K., Tan, A., et al. . In vivo imaging demonstrates a time-line for new vessel formation in islet transplantation. Pediatr Transplant 13, 892, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Travis, M.A., and Sheppard, D.. TGF-β activation and function in immunity. Annu Rev Immunol 32, 51, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hellström, M., Gerhardt, H., Kalén, M., et al. . Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol 153, 543, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Herzog, D.P.E., Dohle, E., Bischoff, I., and Kirkpatrick, C.J.. Cell communication in a coculture system consisting of outgrowth endothelial cells and primary osteoblasts. Biomed Res Int 2014, 320123, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Korff, T., Kimmina, S., Martiny-Baron, G., and Augusti, H.G.. Blood vessel maturation in a 3-dimensional spheroidal coculture model: direct contact with smooth muscle cells regulates endothelial cell quiescence and abrogates VEGF responsiveness. FASEB J 15, 447, 2002. [DOI] [PubMed] [Google Scholar]
- 35. Galie, P.A., Nguyen, D.-H.T., Choi, C.K., Cohen, D.M., Janmey, P.A., and Chen, C.S.. Fluid shear stress threshold regulates angiogenic sprouting. Proc Natl Acad Sci U S A 111, 7968, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.