Abstract
Itaconic acid (IA) is a biologically based unsaturated dicarboxylic acid secreted by mammalian cells. While IA has potential for use in multiple applications, information regarding the influence of IA on animal production remains scarce. This study investigated the effects of dietary IA supplementation on the growth performance, nutrient digestibility, slaughter variables, blood parameters, and intestinal morphology of broiler chickens. A total of 360 one-day-old Arbor Acre broiler chicks were allotted to 6 groups, with 10 chicks per cage and 6 replicates per group in a randomized complete block design. Broiler chicks were fed a basal diet with 0 (control), 0.2, 0.4, 0.6, 0.8, or 1.0% IA. The experimental period lasted from 1 to 42 d of age. Dietary IA supplementation did not affect average daily gain (ADG) and feed/gain ratio (F/G) but quadratically increased average daily feed intake (ADFI) and linearly increased crude protein (CP) digestibility during the grower period (d 22–42). A higher breast and thigh muscle yield and a lower abdominal fat yield were observed in a linear and quadratic manner with the IA supplementation. Adding IA to the diet had significant effects on superoxide dismutase (SOD), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and catalase (CAT) levels in serum at d 21 and on total antioxidation capacity (T-AOC) at d 42. There were linear and quadratic increases in villus height and the villus height/crypt depth ratio (V/C) of the duodenum and villus height of the jejunum with the supplementation of IA. Regression analyses for ADFI, dressed yield, breast and thigh muscle yield, abdominal fat yield, serum ALT, CAT, and SOD levels, villus length of the duodenum and jejunum, and V/C of the duodenum indicated that the optimal dietary IA supplementation would be from 0.4 to 0.7%. From an economic perspective, a level of 0.4% IA in the broiler diet is recommended for improving the nutrient digestibility, slaughter performance, antioxidant ability, and intestinal morphology of broiler chickens.
Key words: alternatives to antibiotics, organic acid, intestinal health, itaconate, antioxidant function
INTRODUCTION
Antibiotic growth promoters (AGPs) have been widely used in animal feeds to promote rapid growth and reduce mortality, especially in the poultry and pig industries (Gromwell, 2002; Miles et al., 2006). Continual and unregulated use of AGPs in food animals has been demonstrated to lead to antibiotic resistance, a phenomenon that is considered a hazard to human health and the environment (Nataliya et al., 2019). As a result, the use of AGPs in animal feeds has been banned in the European Union and China. For this reason, researchers have sought to find suitable alternatives to AGPs to maintain the production performance and feed efficiency of farm animals, especially in the intensive and scaled-up broiler industry (Liu et al., 2017; Yang et al., 2019). Several alternatives to antibiotic growth promoters have been suggested, including organic acids, probiotics, herbs and herbal extracts, enzymes, and essential oils (Khan et al., 2012a,b; Abudabos et al., 2017; Omonijo et al., 2018; Ding et al., 2021; Fikry et al., 2021; Kuralkar and Kuralkar, 2021; Melaku et al., 2021; Rao et al., 2021). Among these, organic acids are one of the most studied alternatives, and they have been utilized for more than 5 decades to preserve feed and as additives to improve the performance of broilers (Khosravinia et al., 2015). Organic acids have been demonstrated to promote growth and exert antimicrobial as well as immune- and/or gut health-regulating activities in broilers (Emami et al., 2013; Sabour et al., 2019; Emili et al., 2021).
Itaconic acid (IA) is a derivate of the tricarboxylic acid cycle in the mitochondrial matrix derived from the decarboxylation of cis-aconitate by immunoresponsive gene 1 (Michelucci et al., 2013; Cordes et al., 2016). As a renewable organic acid, IA is rated by the US Department of Energy as one of the top 12 value-added products according to biomass, and it has gained increasing attention from researchers (Cruz et al, 2018; Zhao et al., 2018). Although IA has been used for industrial purposes, predominantly in polymer synthesis, many recent studies have demonstrated that it can restrict bacterial growth by inhibiting the bacterial enzyme isocitrate lyase and inhibiting proinflammatory cytokines by activating nuclear-factor-E2-related factor 2 (Nrf2) signaling, thus protecting the host against pathogen invasion, oxidative injury, and other stresses (Michelucci et al., 2013; Mills et al., 2018). In rats, an IA-enriched diet led to reduced visceral fat accumulation, suggesting that IA plays a role in energy metabolism or nutrition modulation (Booth et al., 1952). However, knowledge regarding the influence of dietary supplementation of IA in livestock and poultry remains scarce. Therefore, this study aimed to investigate the effects of dietary IA supplementation on growth and slaughter performance, nutrient digestion, blood biochemical parameters, and the intestinal morphology of broiler chickens.
MATERIALS AND METHODS
Birds and Experimental Design
All experimental procedures used were approved by the Animal Care and Use Committee of Shenyang Agricultural University as a protocol number 202006047. A total of 360 one-day-old Arbor Acre broiler chicks (male and female in half) were randomly assigned to 6 treatment groups with 6 replicates (10 birds per replicate). The supplemental levels of IA were 0 (control group), 0.2, 0.4, 0.6, 0.8, and 1.0%. The IA product used in the current study was provided in powder by YouQiYi Medicine Trading Co. (Fujian, China), which contained 99.6% IA.
Diet and Management
The experimental period lasted from 1 to 42 d of age. All birds were housed in wire cages (length 0.8 × width 0.65 × height 0.4 m), and feed and water were provided ad libitum throughout the experiment. The temperature in the house was maintained at 35°C for the first 3 d post hatching and then gradually decreased by 0.5°C per day until reaching a final temperature of 24°C. All birds received continuous light for the first 24 h and were then exposed to 23 h of light until the end of the study. Corn-soybean meal-based diets were formulated to meet or exceed the feeding standard of China for broiler chickens (NY/T33-2004, Ministry of Agriculture of the People's Republic of China, 2004). The diet did not contain any supplemented antibiotics. The composition of the diet and nutrient levels are presented in Table 1.
Table 1.
Ingredient composition and nutrient levels of basal diets (air dry basis).
| Items | Content (%) |
||||
|---|---|---|---|---|---|
| 1–21 d | 22–42 d | 1–21 d | 22–42 d | ||
| Ingredients | Analyzed values, % | ||||
| Corn | 61.92 | 60.00 | Crude protein | 21.19 | 20.49 |
| Soybean meal | 28.00 | 27.60 | Crude fat | 3.24 | 6.13 |
| Corn gluten meal | 2.82 | 3.50 | Calcium | 0.99 | 0.75 |
| Soybean oil | 0.80 | 3.50 | Total phosphorus | 0.89 | 0.64 |
| Monosodium glutamate residue | 1.50 | 1.50 | Calculated composition, % | ||
| Limestone | 1.50 | 1.00 | Metabolizable energy (MJ/kg) | 12.15 | 12.98 |
| Calcium hydrophosphate | 1.40 | 1.00 | Available phosphorus | 0.37 | 0.30 |
| Sodium chloride | 0.30 | 0.25 | Lysine | 1.34 | 1.28 |
| Choline chloride | 0.10 | 0.10 | Methionine | 0.50 | 0.51 |
| L-lysine | 0.65 | 0.65 | Threonine | 1.00 | 0.83 |
| DL-methionine | 0.18 | 0.20 | Tryptophan | 0.28 | 0.21 |
| L-threonine | 0.23 | 0.10 | |||
| Premix1 | 0.60 | 0.60 | |||
| Total | 100.00 | 100.00 | |||
The premix provided per kg of diets: Cu (as copper sulfate), 16 mg; Fe (as ferrous sulfate), 180 mg; Mn (as manganese sulfate), 87 mg; Zn (as zinc sulfate) 100 mg, I (as potassium iodide) 0.70 mg, Se (as sodium selenite) 0.40 mg; vitamin A, 10,000 IU; vitamin D, 3,000 IU; vitamin K3, 3 mg; thiamine, 5 mg; riboflavin, 10 mg; pyridoxine, 13 mg; cobalamin, 0.01; niacin, 50 mg; pantothenic acid, 17 mg; folic acid, 0.3 mg; biotin, 0.2 mg.
Samples Collection
Before the experiment began, the experimental diets were sampled once and stored at −20°C for chemical analysis. Feces were collected on d 19 to 21 and d 40 to 42 from each cage, supplemented with 10% hydrochloric acid to fix excreta nitrogen after collection, and dried in forced air (65°C) for 72 h. Samples of feed and feces were ground through a 0.45-mm screen prior to being analyzed for dry matter (DM), crude protein (CP), ether extract (EE), gross energy (GE), calcium (Ca), and total phosphorus (TP). At 21 and 42 d, the birds that were sampled were feed deprived for 12 h. In detail, one bird with an average body weight (BW) from each cage (3 male and 3 female each treatment) was selected to obtain blood samples via the wing vein. Blood was collected into vacuum tubes, centrifuged at 3,000 × g for 15 min to obtain serum, and stored at −20°C for further analysis. Then, the selected birds were euthanized and the abdomen was opened to remove the small intestinal segments immediately, and samples from middle sections (1 cm) of the duodenum, jejunum, and ileum were collected and stored in 8% formalin solution for later microscopic measurement of intestinal morphology.
Growth Performance and Mortality
Mortality was recorded daily for each replicate cage. Feed intake (FI) and BW were measured on d 21 and 42 in the early morning after a 12-h period of feed deprivation. The feed/gain ratio (F/G) was calculated as follows: F/G = (Total FI / (Total BW end period – total BW start period + total BW of dead birds)).
Nutrient Digestibility Determination
Acid insoluble ash (AIA) was used as a digestibility indicator to determine the apparent total tract digestibility. AIA, DM, CP, EE, Ca, and TP in feed and feces were measured by the AOAC method (2000). The GE of feed and fecal samples was determined using adiabatic oxygen bomb calorimetry (C2000, IKA, Germany). The digestibility was calculated by the following formula: digestibility (%) = (100 – A1/A2 × F2/F1 × 100), in which A1 represents the AIA content of the feed, A2 represents the AIA content of the feces, F1 represents the nutrient content of the feed, and F2 represents the nutrient content of the feces.
Slaughter Yields Determination
To evaluate the slaughter performance, at the end of the experiment (42 d of age), one bird with an average BW from each cage (3 male and 3 female each treatment) was selected, individually weighed, and sacrificed after 4 h of feed deprivation. The birds were manually dissected to determine carcass, breast muscle, thigh muscle, and abdominal fat weight and yield. All yields were calculated as follows: dressed weight = BW – (blood + feather) weights; half-eviscerated weight = dressed weight – (trachea + esophagus + crop + intestine + spleen + pancreas + gallbladder + reproductive organ + contents and membrane of gizzard) weights; eviscerated weight = half-eviscerated weight – (heart + liver + proventriculus + gizzard + fat around abdomen and gizzard + head + neck + claw) weights; dressed yield (%) = 100 × dressed weigh/BW; half-eviscerated yield (%) = 100 × half-eviscerated weight/BW; eviscerated yield (%) = 100 × eviscerated weight/BW; breast muscle yield (%) = 100 × breast muscle weight/eviscerated weight; thigh muscle yield (%) = 100 × thigh muscle weight/eviscerated weight; abdominal fat yield (%) = 100 × fat around abdomen and gizzard/eviscerated weight.
Serum Biochemical Parameters Analysis
The concentrations of blood serum superoxide dismutase (SOD), catalase (CAT), total antioxidation capacity (T-AOC), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were detected by commercial kits (Jiangsu BaoLai Biotechnology Co., Yancheng, China) according to the manufacturer's instructions. Each parameter was determined in triplicate simultaneously on the same plate. Additionally, the differences in the coefficient of variation among parallels had to be less than 10% to guarantee the reproducibility of repeated measurements.
Intestinal Histomorphological Measurement
After fixing in formalin for 48 h, tissues from the duodenum, jejunum, and ileum were dehydrated and embedded in paraffin wax, and then the samples were stained with hematoxylin and eosin. The measurements were performed with an Olympus optical microscope using Olympus SC180 software (BX46, Tokyo, Japan).
Statistical Analysis
All data were analyzed by one-way ANOVA procedure using SPSS software (version 23, SPSS Inc., Chicago, IL) and checked normality and homogeneity of variance before statistical analysis. Replicate was defined as an experimental unit for the trial. Statistical differences between treatment groups were compared using Tukey's multiple comparison test. Polynomial contrasts were used to test the linear and quadratic response to the increasing levels of IA in diets. Quadratic regressions (Y = aX2 + bX + c) were fitted to the responses of the dependent variables to dietary IA supplemented levels. The extremum response for IA was defined as IA =–b/(2 × a). The results are presented as the means and standard error of the mean (SEM), and differences were considered significant at P < 0.05.
RESULTS
Growth Performance
As shown in Table 2, no differences (P > 0.05) in BW, ADG, or F/G were observed among groups during the starter (d 1–21), grower (d 22–42), and whole periods (d 1–42). However, dietary supplementation with IA had a quadratic effect on ADFI during the grower (P = 0.005) and whole periods (P = 0.007), and the 0.6% IA group had the highest ADFI and the 1.0% IA group had the lowest ADFI. The factors that showed a quadratic response were selected for further analysis by quadratic regression related to the dietary IA levels (Table 3). The maximum responses for ADFI at d 22 to 42 and d 1 to 42 were 0.50 and 0.48%, respectively.
Table 2.
Effects of dietary IA supplementation on growth performance of broiler chickens.
| Item | Added IA, % |
SEM |
P-value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.2 | 0.4 | 0.6 | 0.8 | 1.0 | A | L | Q | ||
| BW day 1 (g) | 43.0 | 42.5 | 43.0 | 42.5 | 42.5 | 43.0 | 0.07 | 0.252 | 0.708 | 0.407 |
| BW d 21 (g) | 859.2 | 872.5 | 874.2 | 872.5 | 855.0 | 845.0 | 4.68 | 0.386 | 0.200 | 0.074 |
| BW d 42 (g) | 2,487.4 | 2,490.2 | 2,563.9 | 2,544.4 | 2,501.4 | 2,472.3 | 15.02 | 0.462 | 0.844 | 0.064 |
| Starter (1–21 d) | ||||||||||
| ADG (g) | 38.9 | 39.5 | 39.6 | 39.5 | 38.7 | 38.2 | 0.22 | 0.379 | 0.202 | 0.070 |
| ADFI (g) | 56.7 | 56.6 | 56.6 | 56.6 | 56.3 | 55.9 | 0.23 | 0.918 | 0.302 | 0.601 |
| F/G (g/g) | 1.46 | 1.43 | 1.43 | 1.43 | 1.45 | 1.46 | 0.01 | 0.667 | 0.638 | 0.118 |
| Grower (22–42 d) | ||||||||||
| ADG (g) | 77.5 | 77.6 | 80.9 | 79.3 | 78.4 | 77.5 | 0.67 | 0.676 | 0.963 | 0.174 |
| ADFI (g) | 162.9abc | 161.7bc | 164.6abc | 168.1a | 166.6ab | 159.2c | 0.83 | 0.013 | 0.983 | 0.005 |
| F/G (g/g) | 2.11 | 2.09 | 2.04 | 2.12 | 2.13 | 2.06 | 0.02 | 0.739 | 0.980 | 0.959 |
| Whole period (1–42 d) | ||||||||||
| ADG (g) | 58.2 | 58.6 | 60.2 | 59.4 | 58.5 | 57.8 | 0.35 | 0.425 | 0.718 | 0.061 |
| ADFI (g) | 109.8abc | 109.2bc | 110.6ab | 112.3a | 111.4ab | 107.5c | 0.45 | 0.022 | 0.736 | 0.007 |
| F/G (g/g) | 1.89 | 1.87 | 1.84 | 1.89 | 1.90 | 1.86 | 0.01 | 0.736 | 0.896 | 0.800 |
Broiler chickens receiving a basal diet (0) or diets supplemented with 0.2, 0.4% 0.6, 0.8, or 1.0% IA; A, P value of one-way ANOVA; L, P value of linear analysis; Q, P value of quadratic analysis.
Abbreviations: ADG, average daily gain; ADFI, average daily feed intake; BW, body weight; F/G, feed/gain ratio.
Mean values within a row with different superscript letters were significantly different (P < 0.05).
Table 3.
Estimation of the extremum response for dietary IA levels based on quadratic regressions in broiler chickens.
| Dependent variables | Regression equation | R2 | P | Extremum response to IA levels (%) |
|---|---|---|---|---|
| ADFI (22–42 d), g/d | Y=-21.68X2+21.64X+160.99 | 0.195 | 0.005 | 0.50 |
| ADFI (1–42 d), g/d | Y=-11.48X2+11.09X+108.81 | 0.188 | 0.007 | 0.48 |
| Dressed yield, % | Y=-5.74X2+5.59X+91.32 | 0.135 | 0.025 | 0.49 |
| Breast muscle yield, % | Y=-2.46X2+2.88X+27.04 | 0.262 | 0.006 | 0.58 |
| Thigh muscle yield, % | Y=-0.98X2+1.27X+20.65 | 0.301 | 0.015 | 0.65 |
| Abdominal fat yield, % | Y=0.07X2-0.10X+1.46 | 0.303 | 0.031 | 0.69 |
| ALT (d 21), mg/L | Y=-34.41X2+42.13X+101.33 | 0.413 | <0.01 | 0.61 |
| CAT (d 21), ng/L | Y=-10.46X2+11.60X+57.30 | 0.245 | 0.001 | 0.56 |
| SOD (d 42), pg/mL | Y=-11.52X2+13.59X+31.80 | 0.158 | 0.045 | 0.59 |
| Villus length of the duodenum (d 42), μm | Y=-217.39X2+294.74X+814.64 | 0.422 | <0.01 | 0.68 |
| V/C of the duodenum (d 42) | Y=-1.98X2+2.79X+8.38 | 0.187 | 0.033 | 0.70 |
| Villus length of the jejunum (d 42), μm | Y=-190.66X2+254.67X+730.36 | 0.214 | 0.019 | 0.67 |
Extremum was the maximum or minimum response to dietary IA levels according to each regression equation (%); R2, determination coefficient; P, P value of quadratic effect; Y was the dependent viable; X was the dietary IA level (%).
Abbreviations: ADFI, average daily feed intake; ALT, alanine aminotransferase; CAT, catalase; SOD, superoxide dismutase; V/C, villus length/crypt depth.
Nutrient Digestibility
When the effect of dietary IA supplementation on nutrient digestibility was analyzed (Table 4), during the starter period the dietary IA supplementation had no effects on the digestibility of DM, GE, CP, EE, Ca, or TP. Notably, supplementation with IA in the diet linearly increased CP (P = 0.002) and GE (P = 0.035) digestibility during the grower period.
Table 4.
Effects of dietary IA supplementation on nutrient digestibility (%) of broiler chickens.
| Item | Added IA, % |
SEM |
P-value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.2 | 0.4 | 0.6 | 0.8 | 1.0 | A | L | Q | ||
| Starter (1–21 d) | ||||||||||
| DM | 80.3 | 80.6 | 80.6 | 81.0 | 80.7 | 80.6 | 0.13 | 0.825 | 0.425 | 0.345 |
| CP | 64.0 | 64.1 | 64.3 | 64.2 | 64.1 | 63.8 | 0.19 | 0.990 | 0.743 | 0.538 |
| EE | 75.7 | 76.3 | 76.9 | 76.6 | 76.0 | 76.2 | 0.58 | 0.995 | 0.939 | 0.633 |
| Ca | 58.6 | 58.5 | 58.7 | 59.2 | 59.0 | 58.5 | 0.26 | 0.964 | 0.731 | 0.565 |
| TP | 47.9 | 48.8 | 48.9 | 48.9 | 48.7 | 47.8 | 0.27 | 0.748 | 0.904 | 0.122 |
| GE | 76.5 | 76.6 | 76.6 | 76.7 | 76.7 | 76.6 | 0.05 | 0.866 | 0.402 | 0.310 |
| Grower (22–42 d) | ||||||||||
| DM | 80.3 | 80.6 | 80.8 | 80.8 | 80.4 | 80.2 | 0.10 | 0.278 | 0.526 | 0.024 |
| CP | 62.1b | 63.2ab | 64.0ab | 65.0a | 64.7a | 64.6a | 0.29 | 0.029 | 0.002 | 0.093 |
| EE | 79.9 | 81.4 | 81.4 | 81.4 | 81.1 | 81.2 | 0.28 | 0.618 | 0.340 | 0.194 |
| Ca | 58.7 | 58.7 | 58.7 | 58.7 | 58.7 | 58.6 | 0.02 | 0.598 | 0.205 | 0.174 |
| TP | 47.6 | 48.4 | 48.5 | 48.5 | 48.3 | 47.8 | 0.18 | 0.577 | 0.833 | 0.066 |
| GE | 76.8 | 76.9 | 77.4 | 77.7 | 77.6 | 77.3 | 0.12 | 0.169 | 0.035 | 0.143 |
Broiler chickens receiving a basal diet (0) or diets supplemented with 0.2, 0.4, 0.6, 0.8, or 1.0% IA; A, P value of one-way ANOVA; L, P value of linear analysis; Q, P value of quadratic analysis.
Abbreviations: Ca, calcium; CP, crude protein; DM, dry matter; EE, ether extract; GE, gross energy; TP, total phosphorus.
Mean values within a row with different superscript letters were significantly different (P < 0.05).
Carcass and Parts Yield
At 42 d of age, IA supplementation had no (P > 0.05) effects on dressed yield or the all- or half-eviscerated yield but increased breast muscle yield (P = 0.032) and thigh muscle yield (P = 0.020) and decreased abdominal fat yield (P = 0.033) (Table 5). Notably, both dressed yield (P = 0.025) and breast muscle yield (P = 0.006) displayed quadratic responses to the IA supplementation level, and the 0.6% and 0.4% IA groups showed respective maximum values. In addition, there were linear (P = 0.012) and quadratic (P = 0.015) increases in the thigh muscle yield with the supplementation of IA, with the maximum responses in the 0.4% and 0.6% IA groups. Increasing levels of IA decreased abdominal fat yield in a linear (P = 0.008) and quadratic (P = 0.031) manner, with the lower values occurring in all IA groups. According to a quadratic regression analysis, the minimum response for abdominal fat yield was observed at 0.69%, while the optimal IA levels that maximized dressed yield, breast muscle yield, and thigh muscle yield were 0.49, 0.58, and 0.65%, respectively (Table 3).
Table 5.
Effects of dietary IA supplementation on slaughter performance of broiler chickens.
| Item | Added IA, % |
SEM |
P-value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.2 | 0.4 | 0.6 | 0.8 | 1.0 | A | L | Q | ||
| Dressed yield (%) | 94.2 | 95.4 | 95.7 | 95.8 | 94.4 | 94.2 | 0.27 | 0.237 | 0.580 | 0.025 |
| Half-eviscerated yield (%) | 88.2 | 89.2 | 88.7 | 88.7 | 87.5 | 87.6 | 0.28 | 0.503 | 0.186 | 0.299 |
| All-eviscerated yield (%) | 76.3 | 76.3 | 76.3 | 76.4 | 75.4 | 75.3 | 0.40 | 0.935 | 0.395 | 0.593 |
| Breast muscle yield (%) | 26.9b | 27.7a | 27.9a | 27.8a | 27.6a | 27.6a | 0.10 | 0.032 | 0.100 | 0.006 |
| Thigh muscle yield (%) | 20.7c | 20.8bc | 21.1a | 21.1a | 21.0ab | 21.0ab | 0.04 | 0.020 | 0.012 | 0.015 |
| Abdominal fat yield (%) | 1.5a | 1.4b | 1.4b | 1.4b | 1.4b | 1.4b | 0.00 | 0.033 | 0.008 | 0.031 |
Broiler chickens receiving a basal diet (0) or diets supplemented with 0.2%, 0.4%, 0.6%, 0.8%, or 1.0% IA; A, P value of one-way ANOVA; L, P value of linear analysis; Q, P value of quadratic analysis.
Mean values within a row with different superscript letters were significantly different (P < 0.05).
Serum Biochemical Parameters
Diets supplemented with IA had (P < 0.05) effects on SOD, ALT, AST, and VAT levels in serum at d 21 and on T-AOC at d 42 (Table 6). There were linear increases in SOD (P < 0.001) and AST (P = 0.001) with the supplementation of IA at d 21 and quadratic increases occurred in ALT (P < 0.01) and CAT (P = 0.001), and the 0.6 and 0.4% IA groups had the respective maximum values. Serum levels of ALT (P = 0.010) and T-AOC (P = 0.001) were linearly increased by the addition of IA at d 42, and the 1.0% IA group had the maximum. Serum SOD level at d 42 was increased in a quadratic manner (P = 0.045), and the highest value was observed in the 0.6% IA group. As shown in Table 3, for serum ALT and CAT concentrations at 21 d of age, the optimal IA levels were 0.6 and 0.56%, respectively, while for SOD at 42 d of age the optimum was 0.59%.
Table 6.
Effects of dietary IA supplementation on serum biochemical parameters of broiler chickens
| Item | Added IA, % |
SEM |
P-value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.2 | 0.4 | 0.6 | 0.8 | 1.0 | A | L | Q | ||
| Starter (1–21 d) | ||||||||||
| SOD (pg/ml) | 32.5c | 34.1c | 33.7c | 35.9b | 38.0a | 37.5ab | 0.40 | <0.01 | <0.001 | 0.920 |
| ALT (ng/L) | 102.6c | 106.6bc | 109.9b | 120.4a | 109.8b | 109.4b | 1.14 | <0.01 | 0.001 | <0.01 |
| AST (ng/L) | 126.0b | 127.3b | 127.7b | 128.2b | 130.0ab | 134.7a | 0.81 | 0.023 | 0.001 | 0.172 |
| CAT (ng/L) | 57.6c | 57.9c | 61.7a | 61.1ab | 58.3c | 59.1bc | 0.38 | 0.001 | 0.197 | 0.001 |
| T-AOC (U/ml) | 5.9 | 5.7 | 5.8 | 6.0 | 5.8 | 5.6 | 0.08 | 0.765 | 0.403 | 0.522 |
| Grower (22–42 d) | ||||||||||
| SOD (pg/ml) | 32.6 | 32.5 | 35.9 | 36.2 | 35.6 | 33.5 | 0.57 | 0.193 | 0.209 | 0.045 |
| ALT (ng/L) | 107.7 | 108.4 | 114.7 | 114.7 | 116.2 | 122.9 | 1.86 | 0.173 | 0.010 | 0.787 |
| AST (ng/L) | 135.6 | 139.5 | 139.9 | 140.6 | 140.6 | 140.6 | 2.00 | 0.982 | 0.512 | 0.666 |
| CAT (ng/L) | 55.1 | 58.4 | 60.0 | 55.0 | 55.8 | 55.6 | 0.76 | 0.289 | 0.519 | 0.217 |
| T-AOC (U/mL) | 5.5b | 5.9ab | 6.0a | 6.2a | 6.3a | 6.4a | 0.09 | 0.021 | 0.001 | 0.287 |
Broiler chickens receiving a basal diet (0) or diets supplemented with 0.2, 0.4, 0.6, 0.8, or 1.0% IA; SEM, standard error of the mean; A, P value of one-way ANOVA; L, P value of linear analysis; Q, P value of quadratic analysis.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CAT, catalase; SOD, superoxide dismutase; T-AOC, total antioxidation capacity.
Mean values within a row with different superscript letters were significantly different (P < 0.05).
Intestinal Morphology
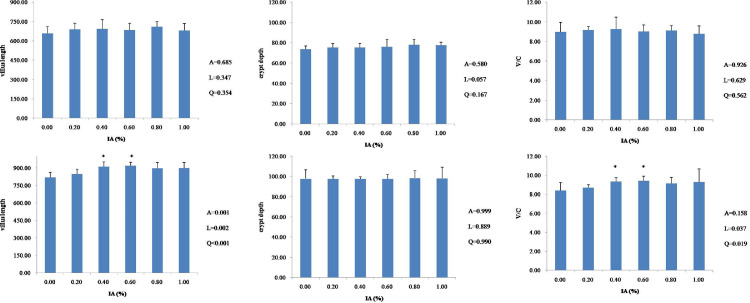
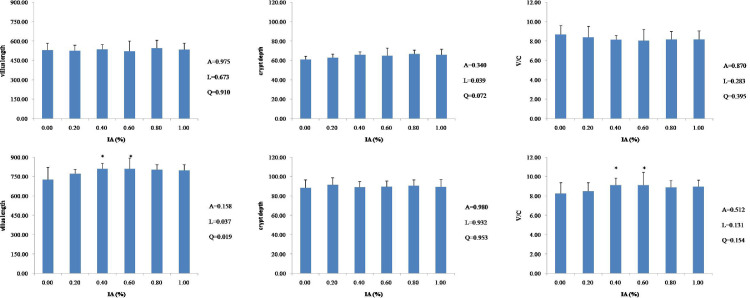
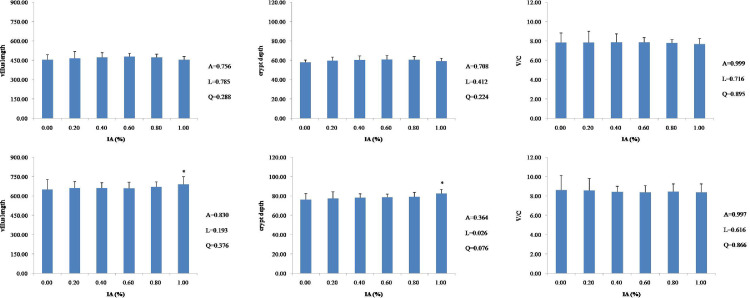
Compared to the control, dietary supplementation with IA did not affect (P > 0.05) villus length, crypt depth, or V/C of the duodenum (Figure 1), jejunum (Figure 2), or ileum (Figure 3) at 21 d of age. At 42 d of age, there were linear (P < 0.05) and quadratic (P < 0.05) increases in villus length and V/C of the duodenum with the supplementation of IA, and both 0.4% and 0.6% IA groups had higher values than other groups (P < 0.05). The villus length of the jejunum was linearly (P = 0.037) and quadratically (P = 0.019) increased in response to the increased level of IA supplementation, and both 0.4 and 0.6% IA groups showed higher values of villus length and V/C than other groups. There were no significant differences (P > 0.05) in villus length, crypt depth, or V/C of the ileum after dietary supplementation with IA, except that dietary 1.0% IA supplementation increased (P < 0.05) villus length and crypt depth. The results from a quadratic regression analysis showed that the optimal IA levels that maximized villus length and V/C of the duodenum and villus length of the jejunum were 0.68, 0.70, and 0.67%, respectively (Table 3).
Figure. 1.
Effects of dietary IA supplementation on duodenal villus length, crypt depth, and V/C ratio at 21 d (top row) and 42 d (bottom row) in broiler chickens. Broiler chickens receiving a basal diet (0) or diets supplemented with 0.2, 0.4, 0.6, 0.8, or 1.0% IA; intestines from each experimental group (n = 6) were sectioned and stained with HE. Columns with * indicate a significant difference (P < 0.05). Abbreviations: HE, hematoxylin-eosin stain; V/C, villus length/crypt depth; A, P value of one-way ANOVA; L, P value of linear analysis; Q, P value of quadratic analysis.
Figure. 2.
Effects of dietary IA supplementation on jejunum villus length, crypt depth, and V/C ratio at 21 d (top row) and 42 d (bottom row) in broiler chickens. Broiler chickens receiving a basal diet (0) or diets supplemented with 0.2, 0.4, 0.6, 0.8, or 1.0% IA; intestines from each experimental group (n = 6) were sectioned and stained with HE. Columns with * indicate a significant difference (P < 0.05). Abbreviations: HE, hematoxylin-eosin stain; V/C, villus length/crypt depth; A, P value of one-way ANOVA; L, P value of linear analysis; Q, P value of quadratic analysis.
Figure 3.
Effects of dietary IA supplementation on ilem villus length, crypt depth, and V/C ratio at 21 d (top row) and 42 d (bottom row) in broiler chickens. Broiler chickens receiving a basal diet (0) or diets supplemented with 0.2, 0.4, 0.6, 0.8, or 1.0% IA; intestines from each experimental group (n = 6) were sectioned and stained with HE. Columns with * indicate a significant difference (P < 0.05). Abbreviations: HE, hematoxylin-eosin stain; V/C, villus length/crypt depth; A, P value of one-way ANOVA; L, P value of linear analysis; Q, P value of quadratic analysis.
DISCUSSION
With the coming of the antibiotic-free feeding era, it is extremely urgent to seek effective and pollution-free alternatives as to antibiotics in animal production (Thacker, 2013; Mehdi et al., 2018; Lopez-Galvez et al., 2021). Organic acids have been commonly applied to animal feed due to their beneficial effects, such as antimicrobial activity, enhancement of nutrient digestibility, and improvement of intestinal morphology (Suiryanrayna and Ramana, 2015; Adewole et al., 2021). As a renewable organic acid, IA has received increasing attention from researchers due to its multiple biological functions (Hooftman and O'Neill, 2019; Zhu, 2020). This study showed that dietary supplementation with IA did not affect BW, ADG, or F/G but increased ADFI during the grower period (d 22–42). For the growth performance of broiler chickens, previous studies showed that dietary supplementation with 3% citric acid or 0.1% organic acid blends (combinations of citric acid, lactic acid, formic acid, acetic acid, propionic acid, sodium butyrate, and phosphoric acid) increased the ADG and ADFI of broilers and reduced the F/G ratio (Khosravinia et al., 2015; Sabour et al., 2019; Emili et al., 2021); however, this was not always the case. Several studies also revealed that the addition of 0.03% citric acid blends (combinations of citric acid, fumaric acid, sorbic acid, and malic acid) to diets did not affect weight gain, FI, or F/G (Yang et al., 2019; Adewole et al., 2021). Differences in results among these studies may be related to the source, dose, and type of organic acids used in the broiler diets. ADG and feed conversion efficiency are of utmost importance for the profitability of the broiler industry. Although there were no significant differences in BW, ADG, or F/G among the groups, broiler chicks fed 0.4% IA showed a higher BW, ADG, and a lower F/G than those in the control group from d 1 to d 42, indicating that dietary supplementation with IA did not compromise the growth performance of broiler chickens.
Interestingly, dietary IA supplementation increased CP digestibility and the percentages of breast muscle and thigh meat, and decreased the abdominal fat percentage while producing no difference in ADG. This implied that IA may play an important role in energy metabolism or protein deposition. Improved digestion for CP was also found in previous studies, and this improvement may be attributed to the enhancement of antimicrobial and/or anti-inflammatory activity and the promotion of nutrient absorption by increasing the intestinal villus height in broilers (Yang et al., 2019; Emili et al., 2021; Fikry et al., 2021; Zhu et al., 2021). Similar to our study, research by Abdel-Fattah et al. (2008) and Yang et al. (2019) found that dietary supplementation with organic acids decreased abdominal fat deposition. They speculated that the addition of citric acid or its blends to the diet may inhibit glycolysis, stimulate glycogenesis, and consequently decrease abdominal fat deposition. IA was previously demonstrated to inhibit phosphofructokinase 2, an enzyme that catalyzes the phosphorylation of fructose-6-phosphate to fructose-2,6-biphosphate. Fructose-2,6-biphosphate in turn allosterically activates phosphofructokinase 1, which catalyzes the phosphorylation of fructose-6-phosphate to fructose-1,6-bisphosphate in the glycolytic pathway (Yalcin et al., 2009; O'Neill, 2015). It has been shown that IA can suppress the synthesis of fatty acids from glucose due to its inhibitory effect on the glycolytic pathway. The results from Sakai et al. (2004) showed that the addition of IA to the diet led to reduced visceral fat accumulation in rats. All of these results indicated that IA has potential as a key regulator of nutrient repartition and could thereby contribute to reduced fat accumulation or increased protein deposition in broilers.
The pursuit of a rapid growth rate and intensive feeding in modern broilers make them particularly vulnerable to oxidative stress arising from various sources such as diseases, high temperatures, and exposure to toxic substances from water and feed (Chen et al., 2021a). The oxidative stress results in inferior growth, disruption of cellular antioxidant defenses, compromised health status, and lower product quality in broiler chickens (Abdel-Monerm et al., 2021; Chen et al., 2021b). The enzymes SOD and CAT are important antioxidant defenses of the host (Chen et al., 2021c). In this study, dietary IA supplementation significantly increased SOD and CAT contents in the serum at d 1 to 21 and T-AOC at d 22 to 42, indicating that IA can help reduce oxidative stress. Aminotransferases, including ALT and AST, are well-known markers for liver injury (Jia et al, 2021). A growing body of evidence strongly suggests that higher serum ALT or AST levels are substantially correlated with increased liver fat accumulation and excessive inflammation (Feng et al., 2020). Surprisingly, the addition of higher amounts of IA to the diet tended to increase ALT and AST levels at d 1 to 21, but whether IA compromised liver function should be further explored.
The intestine is not only the host's largest digestion and absorption site but also the host's largest immune organ. Intestinal morphology is an important indicator of the health of the digestive tract and the response of the intestine to various feed substances (Liu et al., 2017). As villus length and crypt depth or the V/C ratio are important indicators of the maturity and functional capacity of intestine, the longer the villus length and the shallower the crypt depth or the higher the V/C ratio in the small intestine, the greater the ability to absorb nutrients (Du and Guo, 2021). A decreased V/C ratio indicates damage to the mucosa and reduced digestion and absorption and is often accompanied by diarrhea and growth retardation (Lan et al., 2020). Many previous studies have reported beneficial effects of organic acids on the intestinal development of broiler chickens, for example, dietary administration of butyric acid, fumaric acid, or citric acid considerably increased the duodenal, jejunal, or ileal villus height of broilers (Adil et al., 2010; Khosravinia et al., 2015; Sabour et al., 2019). Similarly, our findings also showed an increase in the villus length and V/C of the duodenum and jejunum in broiler chickens after dietary IA supplementation, indicating that IA can contribute to improving enteric development of broilers.
In conclusion, this study has provided evidence that adding IA to the diet can increase nutrient digestibility and slaughter performance, enhance antioxidant ability, and modulate intestinal morphology of broiler chickens. According to the quadratic regression analysis, dietary supplementation with 0.4 to 0.7% IA would be optimal not only for maximizing ADFI, dressed yield, breast and thigh muscle yield, serum ALT, CAT, and SOD levels, villus length of the duodenum and jejunum, and the V/C ratio of the duodenum, but also for minimizing abdominal fat yield in broilers. From the standpoint of economic costs and benefits, 0.4% IA in broiler diets would be recommended.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (31772618).
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2022.101732.
Appendix. Supplementary materials
REFERENCES
- Abdel-Fattah S., EI-Sanhoury M., EI-Mednay N., Abdel-Azeem F. Thyroid activity, some blood constituents, organs morphology and performance of broiler chicks fed supplemental organic acids. Int. J. Poult. Sci. 2008;7:215–222. [Google Scholar]
- Abdel-Monerm A.M.E., Shehata A.M., Khidr R.E., Paswan V.K., Ibrahim N.S., EI-Ghoul A.A., Aldhumri S.A., Gabr S.A., Mesalam N.M., Elbaz A.M., Elsayed M.A., Wakwak M.M., Ebeid T.A. Nutritional manipulation to combat heat stress in poultry-a comprehensive review. J. Therm. Biol. 2021;98 doi: 10.1016/j.jtherbio.2021.102915. [DOI] [PubMed] [Google Scholar]
- Abudabos A.M., Alyemni A.H., Dafalla Y.M., Khan R.U. Effect of organic acid blend and Bacillus subtilis alone or in combination on growth traits, blood biochemical and antioxidant status in broilers exposed Salmonella typhimurium challenge during the starter phase. J. Appl. Anim. Res. 2017;45:538–542. [Google Scholar]
- Adewole D.I., Oladokun S., Santin E. Effects of organic acids-essential oils blend and oat fiber combination on broiler chicken growth performance, blood parameters, and intestinal health. Anim. Nutr. 2021;7:1039–1051. doi: 10.1016/j.aninu.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adil S., Banday T., Bhat G.A., Mir M.S., Rehman M. Effect of dietary supplementation of organic acids on performance, intestinal histomorphology, and serum biochemistry of broiler chickens. Vet. Med. Int. 2010;2010 doi: 10.4061/2010/479485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC . Association of Official Analytical Chemist; Washington, DC: 2000. Official Methods of Analysis. [Google Scholar]
- Booth A.N., Taylor J., Wilson R.H., Deeds F. The inhibitory effects of itaconic acid in vitro and in vivo. J. Biol. Chem. 1952;195:697–702. [PubMed] [Google Scholar]
- Chen S., Yong Y., Ju X. Effect of heat stress on growth and production performance of livestock and poultry: mechanism to prevention. J. Therm. Biol. 2021;99 doi: 10.1016/j.jtherbio.2021.103019. [DOI] [PubMed] [Google Scholar]
- Chen Y.P., Gu Y.F., Zhao H.R., Zhou Y.M. Dietary squalene supplementation alleviates diquat-induced oxidative stress and liver damage of broiler chickens. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Xing T., Li J., Zhang L., Jiang Y., Gao F. Hydrogen peroxide-induced oxidative stress impairs redox status and damages aerobic metabolism of breast muscle in broilers. Poult. Sci. 2021;100:918–925. doi: 10.1016/j.psj.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes T., Wallace M., Michelucci A., Divakaruni A.S., Sapcariu S.C., Sousa C., Koseki H., Cabrales P., Murphy A.N., Hiller K., Metallo C.M. Immunoresponsive gene 1 and itaconate inhibit succinate dehydrogenase to modulate intracellular succinate levels. J. Biol. Chem. 2016;291:14274–14284. doi: 10.1074/jbc.M115.685792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz J.C., Castro A.M., Servulo E.F.C. World market and biotechnological production of itaconic acid. 3 Biotech. 2018;8:138. doi: 10.1007/s13205-018-1151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S., Yan W., Ma Y., Fang J. The impact of probiotics on gut health via alternation of immune status of monogastric animals. Anim. Nutr. 2021;7:24–30. doi: 10.1016/j.aninu.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du E., Guo Y. Dietary supplementation of essential oils and lysozyme reduces mortality and improves intestinal integrity of broiler chickens with necrotic enteritis. Anim. Sci. J. 2021;92:e13499. doi: 10.1111/asj.13499. [DOI] [PubMed] [Google Scholar]
- Emami N.K., Naeini S.Z., Ruiz-Feria C.A. Growth performance, digestibility, immune response and intestinal morphology of male broilers fed phosphorus deficient diets supplemented with microbial phytase and organic acids. Livest. Sci. 2013;127:506–513. [Google Scholar]
- Emili V.R., Balakrishnan U., Yasir B., Chandrasekar S. Effect of dietary supplementation of acidifiers and essential oils on growth performance and intestinal health of broiler. J. Appl. Poult. Res. 2021;5 [Google Scholar]
- Feng X., Wen Y., Peng F.F., Wang N., Zhan X., Wu X. Association between aminotransferase/alanine aminotransferase ratio and cardiovascular disease mortality in patients on peritoneal dialysis: a multi-center retrospective study. BMC Nephrol. 2020;21:209. doi: 10.1186/s12882-020-01840-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikry A.M., Attia A.I., Lsmail I.E., Alagawany M., Reda F.M. Dietary citric acid enhances growth performance, nutrient digestibility, intestinal microbiota, antioxidant status, and immunity of Japanese quails. Poult. Sci. 2021;6 doi: 10.1016/j.psj.2021.101326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromwell G.L. Why and how antibiotics are used in swine production. Anim. Biotechnol. 2002;13:7–27. doi: 10.1081/ABIO-120005767. [DOI] [PubMed] [Google Scholar]
- Hooftman A., O'Neill L. The immunomodulatory potential of the metabolite itaconate. Trends Immunol. 2019;40:687–698. doi: 10.1016/j.it.2019.05.007. [DOI] [PubMed] [Google Scholar]
- Jia J., Yang Y., Liu F., Zhang M., Xu Q., Guo T., Wang L., Peng Z., He Y., Wang Y., Zhang Y., Zhang H., Shen H., Zhang Y., Yan D., Ma X., Zhang P. The association between serum alanine aminotransferase and hypertension: a national based cross-sectional analysis among over 21 million Chinese adults. BMC Cordiovasc. Disord. 2021;21:145. doi: 10.1186/s12872-021-01948-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan R.U., Naz S., Javdani M., Nikousefat Z., Selvaggi M., Tufarelli V., Laudadio V. The use of turmeric (Curcuma longa) in poultry feed. Worlds Poult. Sci. J. 2012;68:97–103. [Google Scholar]
- Khan R.U., Naz S., Nikousefat Z., Tufarelli V., Laudadio V. Thymus vulgaris: alternative to antibiotics in poultry feed. Worlds Poult. Sci. J. 2012;68:401–408. [Google Scholar]
- Khosravinia H., Nourmohammadi R., Afzali N. Productive performance, gut morphometry, and nutrient digestibility of broiler chicken in response to low and high dietary levels of citric acid. J. Appl. Poult. Res. 2015;24:470–480. [Google Scholar]
- Kuralkar P., Kuralkar S. Role of herbal products in animal production-an updated review. J. Ethnopharmacol. 2021;278 doi: 10.1016/j.jep.2021.114246. [DOI] [PubMed] [Google Scholar]
- Lan R., Li Y., Chang Q., Zhao Z. Dietary chitosan oligosaccharides alleviate heat-stress-induced intestinal oxidative stress and inflammatory response in yellow-feather broilers. Poult. Sci. 2020;99:6745–6752. doi: 10.1016/j.psj.2020.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yang X., Xin H., Chen S., Yang C., Duan Y., Yang X. Effects of a protected inclusion of organic acids and essential oils as antibiotic growth promoter alternative on growth performance, intestinal morphology and gut microflora in broilers. Anim. Sci. J. 2017;88:1414–1424. doi: 10.1111/asj.12782. [DOI] [PubMed] [Google Scholar]
- Lopez-Galvez G., Lopez-Alonso M., Pechova A., Mayo B., Dierick N., Gropp J. Alternatives to antibiotics and trace elements (copper and zinc) to improve gut health and zootechnical parameters in piglets: a review. Anim. Feed. Sci. Tech. 2021;271 [Google Scholar]
- Mehdi Y., Letourneau-Montminy M.P., Gaucher M.L., Chorfi Y., Suresh G., Rouissi T., Brar S.K., Cote C., Ramirez A.A., Godbout S. Use of antibiotics in broiler production: global impacts and alternatives. Anim. Nutr. 2018;4:170–178. doi: 10.1016/j.aninu.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melaku M., Zhong R., Han H., Wan F., Yi B., Zhang H. Butyric and citric acids and their salts in poultry nutrition: effects on gut health and intestinal microbiota. Int. J. Mot. Sci. 2021;22:10392. doi: 10.3390/ijms221910392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelucci A., Cordes T., Ghelfi J., Pailot A., Reiling N., Goldmann O., Binz T., Wegner A., Tallam A., Rausell A., Buttini M., Linster C.L., Medina E., Balling R., Hiller K. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc. Nat. Acad. Sci. U. S. A. 2013;110:7820–7825. doi: 10.1073/pnas.1218599110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R.D., Butcher G.D., Henry P.R., Littell R.C. Effect of antibiotic growth promoters on broiler performance, intestinal growth parameters, and quantitative morphology. Poult. Sci. 2006;85:476–485. doi: 10.1093/ps/85.3.476. [DOI] [PubMed] [Google Scholar]
- Mills E.L., Ryan D.G., Prag H.A., Dikovskaya D., Menon D., Zaslona Z., Jedrychowski M.P., Costa A.S.H., Higgins M., Hams E., Szpyt J., Runtsch M.C., King M.S., McGouran J.F., Fischer R., Kessler B.M., McGettick A.F., Hughes M.M., Carroll R.G., Booty L.M., Knatko E.V., Meakin P.J., Ashford M.L.J., Modis L.K., Brunori G., Sevin D.C., Fallon P.G., Caldwell S.T, Kunji E.R.S., Chouchani E.T., Frezza C., Dinkova-Kostova A.T., Hartley R.C., Murphy M.P., O'Neill L.A. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. 2018;556:113–117. doi: 10.1038/nature25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataliya R., Annemarie K., Sigrid M., Ulrike Z., Charles H., Konrad D. The application of antibiotics in broiler production and resulting antibiotic resistance in Escherichia coli: a global overview. Poult. Sci. 2019;98:1791–1804. doi: 10.3382/ps/pey539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omonijo F.A., Ni L., Gong J., Wang Q., Lahaye L., Yang C. Essential oils as alternatives to antibiotics in swine production. Anim. Nutr. 2018;4:126–136. doi: 10.1016/j.aninu.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill L. A broken Krebs cycle in macrophages. Immunity. 2015;42:393–394. doi: 10.1016/j.immuni.2015.02.017. [DOI] [PubMed] [Google Scholar]
- Rao Rama S.V., Raju M.V.L.N., Nagalakshmi D., Prakash B., Paul S.S. Effect of supplementation of graded concentrations of xylanase and a-amylase on performance, slaughter variables, and energy digestibility in broiler chickens fed corn-soybean meal-based diet. J. Appl. Poult. Res. 2021;30 [Google Scholar]
- Sabour S., Tabeidian S., Sadeghi G. Dietary organic acid and fiber sources affect performance, intestinal morphology, immune responses and gut microflora in broilers. Anim. Nutr. 2019;5:156–162. doi: 10.1016/j.aninu.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai A., Kusumota A., Kiso Y., Furuya E. Itaconate reduces visceral fat by inhibiting fructose 2,6-bisphosphate synthesis in rat liver. Nutrition. 2004;11-12:997–1002. doi: 10.1016/j.nut.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Suiryanrayna M., Ramana J. A review of the effects of dietary organic acids fed to swine. J. Anim. Sci. Biotechnol. 2015;6:45. doi: 10.1186/s40104-015-0042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker P. Alternatives to antibiotics as growth promoters for use in swine production: a review. J. Anim. Sci. Biotechnol. 2013;4:35. doi: 10.1186/2049-1891-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin A., Telang S., Clem B., Chesney J. Regulation of glucose metabolism by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases in cancer. Exp. Mol. Pathol. 2009;86:174–179. doi: 10.1016/j.yexmp.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Yang X., Liu Y., Yan F., Yang C., Yang X. Effects of encapsulated organic acids and essential oils on intestinal barrier, microbial count, and bacterial metabolites in broiler chickens. Poult. Sci. 2019;98:2858–2865. doi: 10.3382/ps/pez031. [DOI] [PubMed] [Google Scholar]
- Zhao M., Lu X., Zong H., Li J., Zhuge B. Itaconic acid production in microorganism. Biotechnol. Lett. 2018;40:455–464. doi: 10.1007/s10529-017-2500-5. [DOI] [PubMed] [Google Scholar]
- Zhu X. Recent progress in biological functions of itaconic acid. Chin. J. Anim. Nutr. 2020;32:998–1002. [Google Scholar]
- Zhu X., Guo Y., Liu Z., Yang J., Tang H., Wang Y. Itaconic acid exerts anti-inflammatory and antibacterial effects via promoting pentose phosphate pathway to produce ROS. Sci. Rep. 2021;11:18173. doi: 10.1038/s41598-021-97352-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.