Abstract
The skin is a complex and multifunctional organ, in which the static versus dynamic balance is responsible for its constant adaptation to variations in the external environment that is continuously exposed. One of the most important functions of the skin is its ability to act as a protective barrier, against the entry of foreign substances and against the excessive loss of endogenous material. Human skin imposes physical, chemical and biological limitations on all types of permeating agents that can cross the epithelial barrier. For a molecule to be passively permeated through the skin, it must have properties, such as dimensions, molecular weight, pKa and hydrophilic-lipophilic gradient, appropriate to the anatomy and physiology of the skin. These requirements have limited the number of commercially available products for dermal and transdermal administration of drugs. To understand the mechanisms involved in the drug permeation process through the skin, the approach should be multidisciplinary in order to overcome biological and pharmacotechnical barriers. The study of the mechanisms involved in the permeation process, and the ways to control it, can make this route of drug administration cease to be a constant promise and become a reality. In this work, we address the physicochemical and biopharmaceutical aspects encountered in the pathway of drugs through the skin, and the potential added value of using solid lipid nanoparticles (SLN) and nanostructured lipid vectors (NLC) to drug permeation/penetration through this route. The technology and architecture for obtaining lipid nanoparticles are described in detail, namely the composition, production methods and the ability to release pharmacologically active substances, as well as the application of these systems in the vectorization of various pharmacologically active substances for dermal and transdermal applications. The characteristics of these systems in terms of dermal application are addressed, such as biocompatibility, occlusion, hydration, emollience and the penetration of pharmacologically active substances. The advantages of using these systems over conventional formulations are described and explored from a pharmaceutical point of view.
Keywords: Dermal application, Solid lipid nanoparticles, Nanostructured lipid carriers, Bioavailability, Skin permeation
Dermal application; Solid lipid nanoparticles; Nanostructured lipid carriers; Bioavailability, Skin permeation.
1. Introduction
In recent decades, the skin has been explored as an alternative route for topical, dermal and transdermal administration of drugs. However, it was only after the 1960s that the processes involved in skin permeation began to disclosed, however, advances are still modest. In fact, the number of articles published does not reflect the number of products available on the market.
The first studies reporting the importance of knowing the drug solubility (in vehicles) in the rate of skin permeation date back to the early 1940s [1]. In the 1950s, it was documented that molecules with log P between 1 and 3 permeated skin more easily [2, 3, 4].
Despite the numerous advantages that the skin presents as administration route for a range of drugs, for systemic effects, the number of molecules developed with physicochemical characteristics suitable for the transdermal route remains very limited. Nicotine and nitroglycerin, currently considered as drug models of skin permeation, were properly characterized in the 1950s [5]. To overcome this difficulty, alternatives have been proposed, such as nano and microencapsulation. On the other hand, the skin permeation and penetration of the drug do not only depend on the physicochemical properties drug and its delivery system [6, 7, 8], but also the anatomy, physiology and biochemical skin structure. The knowledge of biopharmaceutical aspects is instrumental to identify the skin's barrier properties, so that they can be overcome to ensure transcutaneous permeation.
The topical administration of drugs is typically associated to a higher patient's compliance because it a non-invasive route. The effect may be non-systemic (if the drug remains in within the skin layers) or systemic (if the drug reaches the blood circulation and, in this way, we have a transdermal administration). Another advantage of using the skin for drug administration refers to the avoidance of first pass metabolism through the liver and also the gastrointestinal tract (in comparison to the oral route), both phenomena responsible for the reduced drug bioavailability. However, the presence of the stratum corneum as the outermost layer of epidermis is responsible for limiting the type and amount of drug that can be administered through this route. To increase drug permeation/penetration through the skin, several techniques have been proposed. Examples of “active” methods include the iontophoresis (which uses the electricity to "force" the entry of molecules through the skin [9, 10, 11, 12]), electroporation (by applying a high-voltage pulse to the skin [13, 14]) and sonophoresis (by application of ultrasound [15, 16]). Regarding more “passive” methods to increase the permeation of molecules, the use of supersaturated solutions [17, 18, 19], absorption promoters [20, 21], andlipid nanoparticles [22, 23, 24, 25] have been proposed.
In this review, we provide a systematic analysis of the anatomical, physiological and biochemical characteristics of the skin that may influence the permeation/penetration of drugs, their physicochemical properties and the potential advantages of using lipid nanoparticles as carriers to increase skin permeation of loaded molecules.
2. General aspects of skin tissue
The epidermis and the dermis are two main strata that structurally perform specific functions. The epidermis is the epithelial layer of ectodermal origin that lines the superficial part of the skin, and the dermis is the connective layer of mesodermal origin that protects and supports the body and its organs. The epithelial tissue is made up of cells arranged in continuous laminae, densely grouped and held tightly together by numerous cellular junctions. The apical surface of the epithelial cells is directed towards the outermost surface of the skin and the basal surface adheres to the adjacent connective tissue.
The skin presents desmosomes, hemidesmosomes and cell-cell junctions called “tight junction”. Desmosomes are complex cytoplasmic expansions, consisting of protein chains of desmoplaquin and placoglobin. Desmosomes are linked by transmembrane glycoproteins, which extend through the spaces between adjacent cell membranes, fixing one cell to another through electrostatic bonds. Hemidesmosomes, structurally similar to half a desmosome, connect cells to extracellular material. The tight junction connects neighbouring cells (cell-cell connection) of the tissues that cover the surface of the organs and body cavities, making it difficult for substances to pass through the cells and separating the apical part from the basolateral part of the cells. The fixation between the basal surface of the epidermis and the connective tissue occurs through a thin extracellular layer, called the basal membrane, which consists of two laminae, namely, basal lamina and reticular lamina. The basal lamina contains collagen fibers and other proteins, while the reticular lamina contains structural proteins, such as reticular fibers and fibronectin [26].
The epidermis is a stratified and keratinized squamous epithelium. The cells present in the superficial layers are flattened, while those present in the deeper layers have variable shapes. Basal cells are deeper, and are forced to target the apical surface as they grow by cell division. Over time, this type of cells dehydrates and stiffens. The dermis consists of connective tissue and comprises a true gel, rich in mucopolysaccharides and fibrillar material of three types (i.e., elastic, collagen and reticular fibers), in addition to a few other cells such as fibroblasts, macrophages and some adipocytes. In the dermis there are also blood and lymphatic vessels and structures derived from the epidermis, such as hair follicles, sebaceous glands and sweat glands, which may also offer a pathway for drugs to enter. Areolar tissue and the adipose tissue are below the dermis. Since the skin is the largest organ in the body, accounting for about 16% of body weight, it is a dynamic, complex system integrated by an array of cells, tissues and matrix elements. The skin is responsible for the physical integrity and for maintaining the biochemical activities (body temperature, sensory information). The homeostasis of the skin essentially depends on the stable organization and the cohesion between epidermis and dermis. Both are interconnected by the dermo-epidermal junctions, which comprise basal keratinocytes, basal dermo-epidermal membrane and the papillary dermis [27].
2.1. Epidermis
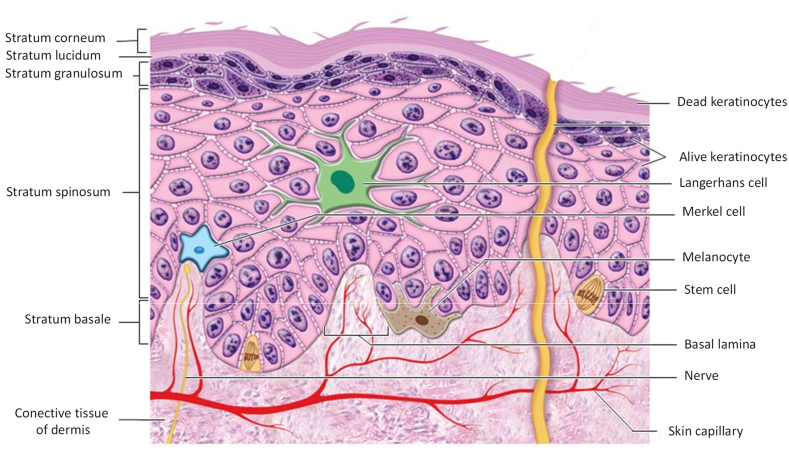
The epidermis is the superficial layer of the skin with a thickness varying between 1.3 mm (palms and feet) and 0.6 mm (face) has no vascular network of its own. The epidermis contains four main types of cells, arranged in characteristic layers or strata; namely keratinocytes, melanocytes, Langerhans cells and Merkel cells (Figure 1).
Figure 1.
Cell types and different layers of epidermis [own drawing].
The strata of the epidermis are divided into stratum corneum (10–20 μm) and viable epidermis (90–100 μm). The viable epidermis is divided into three distinct strata (basal, prickly and granular). The cells of these strata undergo continuous differentiation to give rise to the outermost layer of the skin, the stratum corneum. In places on the body where exposure to friction is high, such as on the fingertips, on the palm and on the sole of the foot, the epidermis has an additional layer, called the lucid layer, in addition to a thicker corneal layer [28, 29].
2.2. Cells of epidermis
Keratinocytes make up most of the epidermis. These cells are responsible for the production of keratin, a resistant and fibrous protein whose function is to protect the skin and underlying tissues from external agents, such as heat, the invasion of microorganisms and the penetration of chemical agents. Melanocytes represent approximately 8% of the cells of the epidermis. These produce melanin, a pigment responsible for skin tone that absorbs ultraviolet light and reduces its harmful effects on the skin. Langerhans cells are formed from the bone marrow and migrate to the epidermis. These cells represent the small portion of epidermal cells and still participate in the immune response. Merkel cells are the fewest cells, located in the basal layer, joining the keratinocytes by means of desmosomes and acting as mechanical receptors for tactile function. Due to continuous renewal, basal cells are responsible for maintaining the epidermis. Melanocytes, Langerhans and Merkel cells are dispersed among the basal stratum keratinocytes [26].
2.3. Strata of the epidermis
The basal stratum is the deepest of all the constituents of the epidermis. This consists of a single row of cuboidal or columnar keratinocytes, which separates the epidermis from the dermis. The keratinocyte nuclei in the basal layer are large, and the cytoplasm contains many ribosomes, a small Golgi complex, few mitochondria, and some rough endoplasmic reticulum. The cytoskeleton, within the cells of the basal layer, includes intermediate filaments composed of keratin. The spiny or malpighian layer, located more externally in relation to the basal layer, has 8 to 10 layers of polyhedral keratinocytes, which have the same organelles as the cells in the basal layer. The spinous layer contains keratin filaments that bind to neighbouring cells through desmosomes. Keratin filaments and desmosomes are responsible for maintaining the cohesion between cells of the epidermis and for resistance against friction. Lamellar granules, which are considered the first sign of keratinization, appear for the first time in the spinous layer. These granules contain lipids (e.g., ceramides, cholesterol and fatty acids), and enzymes (e.g., proteases, acid phosphatase, lipases and glycosidases). Lamellar granules migrate to the surface and expel their contents by exocytosis. The released lipids thus coat the surface promoting its lipid barrier properties [30]. This lipid coating may pose some advantages to the use of lipid nanoparticles for drug delivery [31].
The granular layer is composed of 3–5 rows of flattened polygonal cells, with a cytoplasm full of basophilic granules, keratohyaline compounds, and lamellar granules. These cells have dark granules of the keratohyaline protein, which is a differentiation phenomenon compared to other cells. These granules contain profilagrin, the precursor to philagrin, a protein that intersects with keratin filaments, responsible for the structure and together with the stratum corneum for the mechanical resistance of the skin [32, 33].
In the thick skin of plantar regions, the lucid layer is more evident and consists of a thin layer of flat, eosinophilic and translucent cells. This layer has numerous keratin filaments in the cytoplasm and desmosomes can still be found between the cells [34].
The stratum corneum is the most superficial layer of the epidermis and one of the most important barriers against the loss of physiologically essential substances and the penetration of xenobiotics from the external environment into the body, including drugs and drug delivery systems. Although it is a thinner layer about 10–20 μm, it ensures the protection from mechanical aggressions and is responsible for the primary defence against ultraviolet light exposure [35].
The stratum corneum has the formidable ability to adjust its thickness according to the aggressions of the surrounding environment, thus individuals in inhospitable environments or those more exposed to ultraviolet radiation have thicker skin. This property causes a great variation between and within individuals that can be reflected in a failure in percutaneous absorption. In in-vitro and ex-vivo studies with synthetic or natural membranes, and even in human studies, this aspect is seldomly observed, and in scientific research it invariably leads to errors in interpretation of results [36].
2.4. Dermis
The dermis is mainly composed of connective tissue and comprises a thickness between 1,000 to 1,200 μm. Connective tissue consists of two basic elements, namely, cells and matrix. The matrix is composed of fibers and a fundamental substance, the component of the connective tissue that occupies the spaces between cells and fibers. Unlike epithelia, connective tissues are very vascularized [34, 37]. The dermis can be divided into a superficial papillary region and a deep reticular region. The papillary region consists of areolar connective tissue, containing thin elastic fibers. The areolar connective tissue contains several types of cells, including fibroblasts, macrophages, plasma cells and adipocytes, as well as three types of fibers (collagen, elastic and reticular) and the fundamental substance. The areolar connective tissue combined with the adipose tissue forms the subcutaneous layer below the dermis (hypodermis), this layer of tissue ensures the connection of the skin to the underlying tissues and the organs.
The elastic fibers consist of protein molecules, called elastin, surrounded by glycoprotein, called fibrillin, an essential component for the stability of the elastic fiber. Reticular fibers are formed by collagen with a glycoprotein layer and participate in the formation of the basement membrane.
The reticular region consists of irregular dense connective tissue, whose collagen fibers have an irregular disposition. The connective tissue comprises a true gel, rich in mucopolysaccharides (fundamental substance) and fibrillar material, in addition to the few cells present such as fibroblasts, macrophages and some adipocytes [38].
In the dermis, blood and lymphatic vessels and structures derived from the epidermis are also found, such as hair follicles, sebaceous glands and sweat glands. The dermis serves as a support for the epidermis and joins the skin to the subcutaneous cell tissue or hypodermis. The papillary layer is thin and is composed of loose connective tissue that forms the dermal papillae. In this layer, Meissener's corpuscles (responsible for tactile sensitivity), and Ruffini and Paccini's corpuscles (sensitive terminal organs responsible for heat and vibration, respectively), are found [28].
The hypodermis, also called the subcutaneous layer, is composed of adipose and loose connective tissue that joins the dermis to underlying organs. Depending on the region and the degree of nutrition of the organism, it may have a variable layer of adipose tissue. The hypodermis is anchored to the dermis through fibers and functions as a fat deposit [39].
2.5. Intercellular adhesion elements
In addition to the main cells, there are intercellular adhesion elements in the epidermis represented by cytokeratins, tonofibrils and desmosomes. Cytokeratins are proteins synthesized by keratinocytes that gradually accumulate inside cells to the corneal layer. Cytokeratins are essential in the formation of the cytoskeleton and desmosomes. As the keratinocyte develops, other types of cytokeratins are formed, e.g., in the granular layer. These cytokeratins are called 1, 10, 6 and 16 and in the spinous layer the cytokeratins are called 5, 14 and 6b. Tonofibrils are intermediate keratin filaments that bind to desmosome plaques, increasing the level of cell adhesion. The desmosome plate consists of several protein chains (desmoplaquin and placoglobin) in which the tonofilaments and transmembrane bonds formed by desmogleins are inserted. Desmosomes connect the keratinocytes of the granular layer with the corneocytes of the stratum corneum [26].
2.6. Dermoepidermal adhesion elements
The dermoepidermal junction is the zone of the basement membrane that forms the interface between the epidermis and the dermis. Its main function is to resist against external mechanical forces, sustain the epidermis, determine the polarity of its growth, organize the cytoskeleton of the basal cells, produce signals during development and function as a semipermeable barrier. The thickness of the dermoepidermal junction varies from 60 to 140 nm, presenting a tangled structure of collagens, types IV and VII, with longitudinal fibers [40].
In addition to its role in cellular cohesion, the dermoepidermal junction also represents a zone of exchange between keratinocytes present in the epidermis and dermal connective tissue. The dermoepidermal junction can be divided into three compartments: anchoring filaments (hemidesmosomes) [41], which bind to the basal keratinocytes; dense lamina, formed mainly by type IV collagen; dense sub-layer formed by anchoring fibrils (type VII collagen). The dermoepidermal junction also includes tenascin proteins, types I and III, collagens and fibrillin-1.
2.7. Epidermal metabolism
The study of epidermal metabolism is very important with regard to the transdermal release of drugs, as well as in the safety and efficient local treatment in case of topically applied drugs. Lytic enzymes participate in the process of differentiating the anatomical and physiological barrier and are responsible for the physiological degradation of cellular components (such as proteins and lipids) and also act in the epidermal metabolism of xenobiotics [39].
Over the past ten years, many studies have demonstrated significant metabolic processes in the skin due to the effects of enzymes located mainly on the epidermis. Enzymatic activities detected in the skin and its location in the various strata of the skin tissue have a marked effect on the permeation of foreign compounds. Thus, many pharmacological or toxic effects of drugs and the components of the formulation or its metabolites, may be associated with the nature and magnitude of the skin metabolism of drugs.
The impact of the metabolism that occurs on the skin, the effects of the topically applied drug and the factors capable of biotransformations by biochemical modulators are thus important factors of skin protection [42].
The main difficulties encountered in studies of skin metabolism of drugs derive from the relatively complex structure of this organ and from the different levels of enzymatic activities. However, aspects related to the action of numerous enzymes have been ruled out during the planning, development and evaluation of new formulations [39].
The extent to which skin metabolism may be involved in skin permeation and the fate of topically applied xenobiotics has been assessed through cell culture, structurally intact and metabolically active. One of the first studies using [14C] benzo-(a)-pyrene demonstrated that all classes of metabolites of this compound were found in the culture medium [43].
Although the skin is considered an important site of extrahepatic metabolism, the information found on the role of skin metabolism in the fate of topically applied drugs is superficial. Although the extent of cutaneous metabolism is modest when compared to metabolism in the liver, it is important to consider the effect of the metabolic function inherent in the local and systemic transdermal distribution. However, there is still a need to investigate to what extent skin metabolism is involved with the bioavailability of drugs administered by the transdermal route. However, it is already evident in recent publications that enzymes active in skin tissue have the ability to biotransform topically applied compounds with a consequent change in the pharmacological effect [42].
Studies carried out to locate the enzymatic activity in the skin revealed that sebaceous cells are the most metabolically active cell type, followed by differentiated keratinocytes and basal cells [44, 45, 46]. Other studies showed that the sebaceous glands exhibit significant amounts of 5α-reductase, responsible, for example, for the conversion of testosterone to 5α-dihydrotestosterone. Applying immunohistochemical methods, it was observed that the epidermis, sebaceous glands and hair follicle present high enzymatic activity, mainly cytochrome p-450 isoenzymes [44, 47, 48]. Although hundreds of enzymes are present in the skin, studies with polycyclic hydrocarbons and steroids have revealed the presence of oxidative reactions (aryl hydrocarbon hydrolase, 7-ethoxycoumarin O-deethylase, epoxide hydrolase, 17β hydroxysteroid and monooxygenase-dependent cytochrome P-450) and hydrolytics (esterase, leucine-aminopeptidase and aminopeptidase), both distributed in the epidermis, hair follicle, basal lamina, capillary endothelium, sweat gland and superficial dermis [42, 44, 49].
3. Anatomical and physiological barriers to percutaneous absorption of drugs
The transdermal route has attracted the interest of the scientific community, due to the associated biomedical advantages. In modern pharmaceutical practice, drugs are applied to the skin for local (epithelial surface), dermatological (skin cutaneous) or for transdermal (systemic) effect. A drug in contact with the skin surface can penetrate the skin tissue through two potential routes: directly through the stratum corneum or through the adnexal orifices (hair follicle, sebaceous glands and sweat glands) [50].
The permanent discussion of the relative importance of the adnexal orifices and pores in relation to transport through the stratum corneum, has been open for some time, due to the lack of an adequate experimental model that allows to separate each of the permeation pathways [50, 51] and simultaneously evaluate biotransformation through multiple enzyme activity [44]. Since each pathway cannot be fully discussed separately, it would be more relevant to focus on globally discussing the barriers imposed by the cutaneous tissue to drug permeation [30, 52]. On the other hand, the adnexal orifices comprise an area for permeation of approximately 0.1% of the total area of the skin, and their distribution is irregular throughout the entire organ, which makes the discussion even more palliative.
As research progresses, new delivery systems for unconventional administration routes have been designed. During the last decade, the transcutaneous route has received increasing interest for the systemic administration of drugs. A considerable number of modern technologies, including the concept of targeted drug delivery, with the aim of promoting the release in the site of action and improve its bioavailability [53, 54].
Overcome the impermeable nature of the skin barrier, different pharmaceutical approaches are being continuously proposed to improve the systemic delivery of drugs through the skin, e.g., pro-drugs or hydrophobic analogues, permeation enhancers, saturated semi-solid formulations, micro- and nanoparticles [55]. Many of these innovative products end up being discarded because they are not able to effectively overcome epithelial barriers.
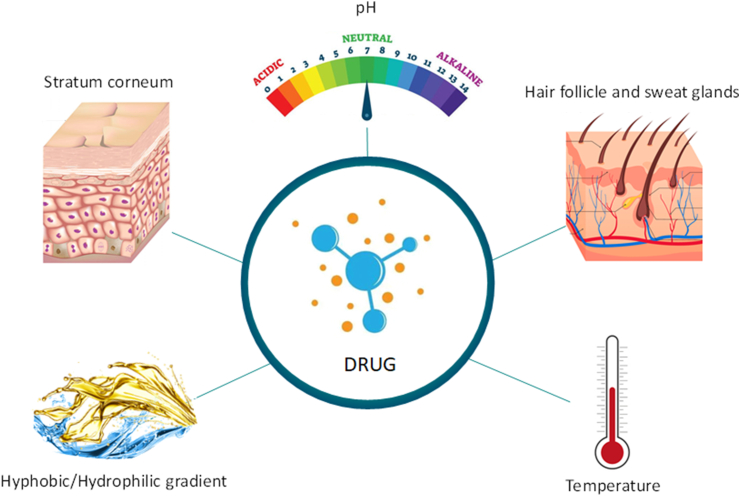
Cutaneous microvasculature is considered a secondary skin barrier. This acts as a dissipator of molecules that diffuse through the primary skin barrier, ensuring maximum diffusion into the capillaries, which negatively affect the transdermal delivery of drugs and other chemical compounds [56]. Thus, the permeability of drugs through the epidermis is governed by several anatomical and physiological barriers, as schematically represented in Figure 2.
Figure 2.
Main anatomical and physiological barriers that govern the percutaneous absorption of drugs [own drawing].
3.1. Stratum corneum
The stratum corneum together with the stratum granulosa adapt the skin to external signals, with modulations appropriate to their defence functions. As in vitro or ex vivo models often ignore the complexity of skin barriers, the correlation with in vivo behavior is always controversial [57].
Due to the exceptional composition of the stratum corneum, with long chain ceramides, free fatty acids and cholesterol as the main classes of lipids, the lipid organization in this layer is different from other biological membranes. In the stratum corneum, two lamellar phases are present with repetition distances of approximately 6.0 and 13.0 nm. In fact, most of the defensive functions of the epidermis are present in the stratum corneum [36, 58].
The epidermal barrier is multifunctional attributed to the interaction between macrostructures (cross-sectional organization of corneocytes) and microstructures (organization of lipid intercorneocytes), bi and three-dimensional supramolecular organization of the lipid matrix and other cellular structures and elements and enzymes that make up the stratum corneum [59, 60].
The corneocytes are organized into clusters (a cluster of about 12 cells). These clusters are separated from each other by layers with low permeation resistance, and are responsible for delimiting the intercluster penetration route. The intercorneocyte lipids are organized in parallel and as repeated bilayers, head-to-head and tail-to-tail. Intercellular lipids can be classified into four categories: cholesterol and its derivatives, ceramides, free fatty acids and triglycerides. About 80% of these lipids are nonpolar, and the small hydrophilicity of their molecules is due to the presence of hydrophilic groups, which are capable of forming hydrogen bonds with lipids, water and some chemical compounds [29].
The tail-tail region of intercorneocytes is known as the intercellular, nonpolar, transepidermal route [58] or percutaneous absorption lipid route. It has been suggested that the skin may contain aqueous pores, thus being a possible hydrophilic route for percutaneous absorption [61, 62], however, the penetration of water and polar molecules is very low [63]. Nevertheless, the different types of aqueous pores, whose opening dimension on the surface ranges between 0.4 and 2.8 nm, have been investigated as a cutaneous penetration pathway for nanomaterials [51, 58, 64].
The stratum corneum comprises the outermost part of the cutaneous tissue, about 5–20 μm and is composed of corneocytes surrounded by lipid regions and is the main barrier for dermal and transdermal administration routes of drugs, either in their free form or loaded in delivery systems or nanoparticles. As most of the drugs applied to the skin permeate throughout the lipid domains, the organization of the lipids is considered to be very important for the barrier function of the skin [52].
The concept of the stratum corneum, as a limited permeability barrier, has been schematically and mathematically represented as a two-compartment model. This model is generally represented as an impermeable matrix of cells, filled with keratin embedded in a lipid matrix and is compared to a wall, where the bricks represent the cells and the mortar is represented by the intercellular lipids. This arrangement forms a tortuous path for the diffusion of molecules through the stratum corneum, hindering the drug penetration and absorption [65].
The chemical nature and disposition of intercellular lipids are also uncommon, and provide greater resistance against drug diffusion. This structural organization defines the location of certain barrier functions, both in the extracellular compartment and in the cytoplasm. In the extracellular compartment, important protective functions are found, such as a barrier against microorganisms, elimination of toxic chemical agents and allergens, and selective absorption. In the intracellular compartment (cytoplasmic) there are barriers against ultraviolet rays, activation of cytokines and thermoregulation. The hydration and impermeability of the barrier are controlled by the intra and extracellular compartments.
Lamellar bodies contain not only lipids (sphingolipids, phospholipids and cholesterol), but also lipid hydrolases, proteolytic enzymes, structural proteins, enzyme inhibitors, and antimicrobial peptides. Lipid hydrolases, in addition to their biochemical functions, are responsible for protection against xenobiotics from fatty substances. The performance of lipid nanoparticles as drug delivery systems is also impaired by this skin defence mechanism. Thus, the lamellar secretory system determines that certain sets of protective functions remain within the interstices. These protective functions are generally driven by stimuli that increase the production and secretion of lamellar bodies, which inactivate the action of transport systems. On the other hand, a spectrum of structural proteins and non-secreted enzymes remain within the corneocytes to ensure a different set of protective functions for the skin [59].
3.2. Hydrophobic-hydrophilic gradient and pH
The chemical composition of the stratum corneum, considering all structural sub-elements, contains water (5–20%), lipids (10–12%), proteins (60–70%) and other unidentified compounds (5–8%). However, chemically, the stratum corneum is referred to as a hydrophobic stratum, regardless of its high protein content. Among these proteins, 10–15% is soluble in water [51, 66]. The greater amount of water in the cells of the viable epidermis contributes to the hydrophilicity of the stratum corneum. Consequently, an epidermal hydrophilic-hydrophobic balance (HLB) can be expected towards the granular stratum [60, 67].
From a skin protection perspective, HLB is an additional defence strategy against the entry of hydrophobic compounds into the skin. In fact, it is widely reported that intermediate oil/water distribution coefficient values are an important parameter in percutaneous absorption [67]. The higher the HLB, the greater the drug bioavailability. Drugs, whose oil/water distribution gradient favours solubility in a hydrophobic environment (log D ≥ 3) [68], would have greater difficulty permeating the viable epidermis [69]. This same principle can be applicable to drugs-loaded lipid nanoparticles.
The composition and location of the chemical components that make up the stratum corneum is also responsible for a non-linear pH gradient between the lower and upper parts of the layer [70, 71]. The pH ranges between 4.5 and 5.5 on the surface of the stratum corneum of mammals, reaches pH values between 6.8 and 7.0 at the interface of the granular and horny strata [60, 72]. Thus, acid salts with pKa above 5.0 facilitate cutaneous penetration through the intracellular route to the surface of the stratum corneum, however their diffusion would be limited at the interface of the granular and horny strata. In the first barrier (pH 4.5–5.5), the acid salt molecule would be in the non-ionized form and, in the granular layer (pH 6.8–7.0), the molecule would be in the ionized form in greater proportion.
Much evidence suggests that the pH gradient of the stratum corneum is involved in antimicrobial defence, in the homeostasis of the permeation barrier, in the integrity and cohesion of the stratum corneum, and in the flaking processes [36, 70, 71].
The formation of the epidermal barrier involves several pH-dependent enzymes, especially those related to the hydrophobic components and their destruction by flaking. Such hydrolases include glucocerebrosidase, sphingomyelinase acid, acid lipases, phosphatases and phospholipases. Glucocerebrosidase with an optimum pH of 5.6 is involved in the synthesis of important ceramides [71].
At the interface between the granular layer and the compact layer, there is a release of acid hydrolases into the extracellular space by differentiated keratinocytes. Acid hydrolases are an important biochemical factor in defending the skin against the entry of drugs and other chemical compounds with acidic characteristics, which would, in principle, be a threat to cutaneous homeostasis [70]. The formation of an acid mantle in the most superficial part of the skin and a pH gradient in a non-polar hydrophobic environment is regulated by different mechanisms, such as the excretion of lactic acid from sweat glands, excretion of triglycerides that are transformed into acids free fatty acids by normal microflora, presence of free fatty acids in intercorneocytes and presence of membranes involved in sodium exchange (Na+) [36]. Another important mechanism of acidification is through the non-oxidative deamination of histidine by histidase to form trans-urochanic acid. This defence mechanism comprising the acid mantle and the pH gradient negatively influences the penetration of electrostatically charged compounds, as well as transporters whose zeta potential deviates from zero [36, 71]. Trans-urochanic acid also has immunosuppressive and carcinogenic effects [73].
In short, the penetration of drugs and other foreign compounds in the stratum corneum, as well as their progression through the viable epidermis is limited by nanoporosity, HLB and pH. Consequently, structural dimensions, oil/water distribution coefficient and surface properties are among the most important physicochemical parameters to be considered when developing new dosage forms.
3.3. Hair follicle and sweat glands
Drugs aimed for skin permeation should be relatively small molecules (<500Da), lipophilic and devoid of electrical charges [74]. When penetrating agents are nanometric, the analysis of the structure and nanoporosity of the stratum corneum suggests, firstly, that in healthy individuals these agents have to be smaller than 5.0 or 7.0 nm in order to have an opportunity to diffuse intact through the entire liquid part of the lipid bilayers [66]. Although not proven, nanostructures must be less than 36 nm in size to potentially be able to harness aqueous pores for permeation through the skin [51, 63, 64].
In general, the composition and physicochemical properties of nanometric agents, or their components, can improve or limit penetration and diffusion through the skin. These same properties can also be responsible for maintaining the physical integrity of nanoparticles when these agents come into contact with the components of the skin. Therefore, it is reasonable to estimate that lipid nanoparticles can interact at different intensities with cutaneous lipids, with which they can melt [58]. The obtained lipid film (i.e., from the melting of lipid nanoparticles with the lipids of skin) may promote skin hydration. Although in this case nanoparticles do not penetrate the skin nor through its appendages, an hydrated skin is more permeable than a dried skin to the drugs that are released from lipid matrices being melted creating a sustained-release depot [24].
Despite the compact structure of the stratum corneum, the sweat glands and hair follicles open space on the skin surface, thus providing entry doors that, in principle, can be used for skin permeation of drugs. These openings thus form the transfolicular penetration route.
The sweat glands extend from the stratum corneum to the deepest layers of the skin are about 3.0–5.0 mm long and are involved in thermoregulation, acid excretion and drainage of impurities. The serous fluid excreted by the sweat glands is a diluted aqueous mixture of organic acids, carbohydrates, amino acids, nitrogenous substances, vitamins and electrolytes [75, 76].
Hair follicles are pilosebaceous units with excretory properties, and serve as a route for the elimination of fatty substances (sebum). The composition of sebum is a mixture of fatty acids (15–28%), waxes (25–26%), triglycerides (57-30%), cholesterol (1–1.5%) and squalene (10–12%). The pilosebaceous units extend about 4 mm into the skin, with this invagination followed by the stratum corneum by approximately 0.1 mm [77]. Consequently, it is possible to anticipate that a drug or its carrier system, of appropriate dimensions and composition, could reach the viable epidermis or the systemic circulation, depending on the depth of penetration.
Although some studies consider the transfolicular route to be an important route for percutaneous absorption, this opinion is not consensual [78, 79]. In addition to the small area that these holes provide at the skin surface, it is commonly argued that the excretion of sweat and sebum would impair the penetration of xenobiotics.
Another aspect that has attracted scientific research in skin permeation is the phenomenon of opening and closing the hair follicle. Several studies support the idea that a substance (lipophilic or hydrophilic) applied topically penetrates the skin through the hair follicle, during a mass flow from the inside to the skin, i.e., the growth of the hair or the production of sebum [78, 80, 81, 82]. To explain this phenomenon, it was assumed that there is a layer that closes some follicles and that can be removed only by hair growth, during the excretion of sebum [78], or by using an exfoliating agent [81].
The study by Otberg et al. [81] showed that, during the application of a formulation on the skin, only 74% of the hair follicles of the forearm were open, while a pre-treatment of the skin with mechanical peeling was able to open all the hair follicles for the penetration of the topically applied substance. Other studies however demonstrated the importance of hair follicles in the absorption of molecules and large particles, small hydrophilic molecules and in the reservoir function [83, 84]. Vogt et al. [82] showed that, after peeling, the penetration of fluorescent particles with dimensions between 40 and 1,500 nm increased. Additionally, they found that the 40 nm particles not only penetrated deeply into the follicular duct, but also penetrated through the hair follicle epithelium. These data suggest that delivery systems can be used for depositing drugs in the hair follicle duct, and that small size nanoparticles can be used as targeted-delivery of drugs. However, no data on stability and/or dispersibility in sweat or sebum are reported in the articles cited here and the intense enzymatic activity seems to be overlooked.
Since the processes that shape follicular drug penetration are yet poorly understood, there is an emerging need for methodologies that can quantify follicular penetration and deposition of drugs and/or their delivery systems applied to the skin.
3.4. Temperature
Although little mentioned in skin permeation studies, it seems certain to state that in any mechanistic study it would be instructive to determine how the rate of transcutaneous permeation could be influenced by temperature, as well as by hydration, lipophilia, presence of enzymes, cutaneous microvasculature or by macrophages. The influence of temperature on the rate of skin permeation could be better understood through the thermodynamic evaluation of the process and the determination of activation energies. In fact, by increasing infrared irradiation (exposure to the sun), fever, or dilation of vascularization by increasing blood flow or the number of capillaries, the skin permeability increases significantly. In summary, this is due to fluidization of the intercorneocyte lipid tails and/or an increase in dermal clearance [60, 85].
4. Factors affecting the drug absorption through the skin
The description of the physical barriers of the stratum corneum highlights that the larger openings and more permeable discontinuous areas define nanoporous nature of this outermost layer of epidermis. Thus, by the analysis of the skin anatomy it can be anticipated that in healthy individuals the penetration of nanoparticles with dimensions between 5-7 nm or 36 nm may happen through the intercellular route or through the aqueous pores, respectively. Similarly, larger particles (10,000–210,000 nm) could penetrate the skin through the transfolicular route. Drug delivery systems with nanometric dimensions could simultaneously use different routes of penetration into the skin, increasing the potential for drug penetration. In addition, the intercluster region may contain greater penetration pathways and should be studied more carefully [58].
All research on transdermal delivery of drugs and transcutaneous permeation reveals that there are many other factors to be considered and that skin permeation is not necessarily the final step to increase drug bioavailability or even local action. Even the penetration of nanometric particles can be affected, simply, by the opening or closing of the sweat and sebaceous glands.
4.1. Skin physiological conditions and administration sites
Age, skin type and sex hormones influence the permeability of the skin due to a slightly different chemical composition in the stratum corneum structure. Thus, depending on the region of the body, cutaneous absorption through the transepidermal and transfolicular routes may be favoured or impaired.
Percutaneous absorption of drugs is governed by the integrity and regional variations of the skin, the dimensions and density of the aqueous and hydrophobic pores and the path of the lipid fluids. As stated above, a commonly highlighted fact is the variation in the thickness of the stratum corneum in regions of the body, such as the face and the palms of the hands and feet. The pilosebaceous units are also unevenly distributed in the body, where their density, the diameter of the hairy holes and the volume of the follicular infundibulum vary in each region [47, 86].
The integrity of the stratum corneum may be compromised by dermatological diseases and other pathological conditions, in addition to trauma, extensive use of detergents, prolonged exposure to air conditioning and to a dry environment. These circumstances may be responsible for different changes in the skin due to the extraction and modification of intercorneocyte lipids or by dehydration of the skin [87].
Formulations to be applied to unhealthy skins are generally studied on intact skin, without considering the differences in structure and chemical composition between healthy and sick individuals and between different species [29]. Thus, the comparison of experimental data should consider the location of the body in in-vivo studies and the conditions of the biopsy sources in ex-vivo studies [44, 88].
Dehydration of the stratum corneum in healthy individuals is currently one of the risk factors that most affect skin permeation. In fact, excessive use of detergents and air conditioning can have profound effects on the skin, such as exfoliation and cracks [86, 89].
The hydration of the stratum corneum is responsible for a barrier reduction. In fact, a water content in the order of 20–50% promotes the swelling of the corneocytes with a consequent reduction in cell compaction and resistance to diffusion. Under these conditions, hydrophobic drugs would find an unfavorable HLB. The increase in transcutaneous permeation by hydration depends on the type of skin and the physicochemical properties of the penetrating agent [90].
Another relevant aspect is the skin temperature. The environmental conditions (latitude, temperature, humidity) in which individuals are exposed when coming into contact with the formulations must be taken into account. Therefore, it is necessary to be careful with the data obtained in-vitro, before evaluating whether a particular drug or nanoparticle is or is not absorbed through the skin [59].
4.2. Physicochemical characteristics of penetrating molecules
More than 70 years of research on percutaneous absorption of drugs reveal that the solubility of the drug in the carrier system plays a fundamental role in the amount of drug permeating the skin. Since permeation is a thermodynamically driven event, saturated formulations ensure maximum transcutaneous penetration [90].
Since only the non-ionized fraction of the molecule will be transported to the viable epidermis, the pH of the carrier system and the pKa of the penetrating molecule must be carefully chosen. The oil/water partition coefficient should favour diffusion in both water and lipids, since very lipophilic molecules would diffuse more easily in the stratum corneum, but would have difficulty leaving it and migrating to the deeper layers of the skin. On the other hand, a hydrophilic substance will also find it difficult to melt with the lipids composing the sebum, compromising its absorption [91].
To permeate significantly through the skin, the molar mass of the permeating agents should be less than 500 Da [74]. However, this property does not exclude that larger molecules can penetrate the stratum corneum and disorganize the supramolecular lipid structure, favouring the intentional or accidental entry of other molecules and/or nanoparticles [58, 92].
The potential for the skin components to establish chemical interaction with the drug and the local enzymatic metabolism cannot be overlooked. Bonding with normal skin components will prevent further penetration, while metabolism can reduce the amount of drug molecules that can permeate it effectively [44]. Skin metabolism normally exists for the conversion of endogenous substances (for example, hormones, steroids, inflammatory mediators, lipids) and for the removal of xenobiotics [47, 48, 93].
Another important parameter is the diffusion coefficient of the penetrating molecule, between the delivery system and the skin. In homogeneously diluted solutions, the diffusion coefficient can be calculated using the Stokes-Einstein equation provided that the limiting factor for absorption is not the dimension of the penetrating molecule. The diffusion coefficient undergoes few changes for molecules with a molar mass between 100 and 1000 Da. For smaller molecules the diffusion coefficient is affected by changes in polarity [94, 95].
4.3. Physicochemical properties of the formulation
When a formulation is applied to the skin, all its constituents undergo the same absorption process, albeit different extents. As most products are not applied occlusively, formulations tend to change their composition due to the presence of water or volatile compounds that evaporate. When the skin perspires and/or the fatty components of the skin are excreted, the sebum can be mixed with the applied formulation. Consequently, different synergistic combinations or interactions between the delivery system, the drug and the skin components will be established. Thus, the physicochemical properties of the drug delivery system are very important and crucial in skin permeation [96] and the non-volatile constituents will be absorbed to different degrees.
More viscous formulations reduce the diffusion coefficient of the molecule in the vehicle, delaying or avoiding partitioning with the skin and consequently absorption. Extremely lipophilic formulations will compete with the lipophilic stratum corneum, making it difficult to partition [97]. On the other hand, occlusive formulations can moderately increase absorption. Besides, the presence of solvent molecules, surfactants, absorption enhancers, and other compounds in the formulation can alter or damage the stratum corneum by different processes, causing a significant increase in the absorption of all components of the formulation applied to the skin [98].
The efficiency and safety of formulations developed for topical, dermal and transdermal drug delivery can generally be distinguished when dispersed in water, based on organic solvents or in ointments, creams, gels, or other semi-solids. It is certain that each of these formulations will affect the barriers of the skin and the permeation pathways differently.
4.4. Physicochemical properties of nanoparticles
Several parameters govern the entry of nanoparticles through the skin, namely, (i) the size of the particles, which will influence the ability to penetrate the skin, (ii) the penetration pathway, and (iii) the diffusion coefficient between the formulation and the skin [99, 100]. Together with the dimension, shape and surface properties of the nanoparticles (charges and polarity), the selection of the penetration pathway, the depth of penetration through that pathway, and the diffusion coefficient in the skin will influence the amount of drug entering the skin [100].
Regarding the shape, despite their characteristic solid core, lipid nanoparticles are not always rigid. The deformable shape and their dimension versus orientation must also be considered. Thus, the fact that a mathematical model is used to describe the diffusion of non-spherical and deformable nanostructures will not be entirely possible. Depending on the spatial orientation of the drug, permeation will vary continuously. It is also obvious that rigid or deformable shapes and their orientations can influence the passage of delivery systems through a given structure with defined porosity, as well as their aggregation in penetration through a narrow path [101].
With respect to the surface electrical charge, this property influences both the risk of nanoparticles’ aggregation and the skin diffusion. It is the coating of nanoparticles that interacts first with the skin components, and may influence positively or negatively the nanoparticles diffusion [100, 101]. The surface charges can be associated with the ionizable residues of the materials, influencing the pKa as a whole, from which it could be anticipated that charged particles would not penetrate through the skin. However, this would be a simplistic answer, because it does not contemplate that the amount of charge can vary when nanoparticles come into contact with the slightly acidic characteristics of the skin [58]. In addition, positively or negatively charged surfaces can interact differently with various skin components and follow different penetration pathways [102].
Particles’ physicochemical stability is another important parameter as it can modify their surface properties and influence the ratio and penetration pathways through the skin [80]. At the level of lipid nanoparticles, lipid stability may also be related to their ability to form micelles after contact with specific structures or components of the skin [103]. This may happen in case phospholipids are used as surfactants in lipid nanoparticles composition.
To sum up, besides the effect of the drug solubility and oil/water partition coefficient, the nanoparticles size influences the general stability, the surface properties, the deformability and, therefore, the diffusion coefficient of the drug in the nanoparticles and through the skin. Thus, the potential for drug-loaded nanoparticles to be absorbed through the skin, without considering these variables would be rather inaccurate.
5. Lipid nanoparticles
Lipid nanoparticles emerged in the early 90's and over the last decades have shown to be promising delivery systems for a range of chemically different drugs. These particles have a mean diameter between 50 and 400 nm, are made up of lipids and stabilized by surfactants. There are mainly two types of lipid nanoparticles, which are essentially distinguished by their composition, i.e., when they are composed only of solid lipids, they are called solid lipid nanoparticles (SLN). When their composition includes, in addition to solid lipids, a portion of a liquid lipid (commonly an oil), they are called nanostructured lipid vectors (NLC). To be considered nanoparticles, i.e., based on a solid lipid matrix, they have to melt above the body and room temperatures (i.e., in general, above 40 °C).
SLN and NLC received special attention due to the countless applications and advantages e.g. (i) the low toxicity and high biocompatibility due to the composition in physiological lipids, already existing in the organism, (ii) the capacity of a controlled/sustained release of the loaded drug, which occurs by erosion of the lipid matrix or by degradation by enzymes, such as lipases, (iii) protection of labile and sensitive drugs, (iv) high physicochemical stability, (vi) improvement of the bioavailability of the loaded drug, which automatically reduces the amount of drug needed to exhibit therapeutic effect and (v) the possibility of site-specific delivery of drugs. All of these advantages make SLN and NLC potential delivery systems to overcome biological barriers encountered by conventional dosage forms. SLN and NLC may however pose some challenges in formulation development due to (i) low loading capacity especially for hydrophilic or more polar drugs, (ii) risk of drug expulsion from the lipid matrix due to polymorphic changes over storage time, and (iii) the relatively high amount of water that is needed to produce the lipid nanoparticles dispersion, which may increase the risk of microbiological contaminations and need to add preservatives that induce side reactions.
Since NLC incorporate a mixture of solid lipid and liquid lipid in their composition, a more amorphous structure results in comparison to a more organized structure of SLN. This results in NLC of higher loading capacity for lipophilic drugs than SLN. The greater the matrix crystallinity, the lower the loading capacity. Over storage time, lipids show the trend to reorganize in more stable polymorphic forms, and these changes result in increased risk of drug expulsion from the matrices [104]. Soon after production, matrices specially produced from triglycerides exhibit a polymorphic form ɑ, which is the least stable and most amorphous form, and incorporates the greatest amount of drug. During the storage period, these matrices turn into a more stable form, the β form which, because it is more crystalline, will expel previously loaded drug molecules from the lipid matrix.
5.1. Excipients for lipid nanoparticles
The main excipients used in lipid nanoparticles production are essentially lipids, surfactants and water. The choice of lipid will directly affect the physicochemical characteristics of the obtained nanoparticles, i.e., their morphology, size, polydispersity and their zeta potential value. In addition, the composition of the lipid will dictate its crystallinity and as such the ability for the incorporation of drug molecules. In addition, the organization of the lipid molecules at the matrix level can adopt three distinct polymorphic forms, namely the amorphic form ɑ, the orthorhombic perpendicular form β′ and the parallel triclinic form β. The crystallinity increases from ɑ, to β′ and β.
The choice of the appropriate lipid lies mainly in the ability to solubilize/disperse the drug, i.e., prior to production, a study of the lipid profile should be carried out, which consists in reaching the highest drug solubility in the lipid material [105]. For this purpose, several solid lipids should be selected in the case of SLN production, or an ideal mixture of solid lipid with liquid lipid in the case of NLC production. The lipids most commonly applied in these systems for topical application are ceramides, triglycerides, fatty acids and fatty alcohols. Usually, between 5 to 30% of lipid is used for the production of SLN/NLC [104, 106]. As discussed in the previous sections, several of these lipids composing SLN and NLC are natural compounds of the skin and of sebum, which altogether ensure that these lipid nanoparticles are biocompatible, biotolerable and biodegradable.
5.1.1. Lipids
The solid lipids commonly used for the production of lipid nanoparticles include mixtures of mono-, di- and triglycerides, such as Precirol®ATO5 (glyceryl distearate) [107, 108, 109], Compritol®888ATO (glyceryl dibehenate) [38, 110], Dynasan®114 (glyceryl trimyristate, C14) [111,112], Dynasan®116 (glyceryl tripalmitate, C16) [113,114], Dynasan®118 (glyceryl tristearate, C18) [115], Imwitor®900K (mixture of mono and diacylglycerols based on hydrogenated fatty acids containing 40–55% glyceryl monostearate) [110], and Softisan®100 (mixture of triglycerides composed of fatty acids with chains from C10 to C18) [116,117].
Among commonly used fatty acids is stearic acid [118, 119], which is a fatty acid saturated with an 18-carbon chain, C18. Its application in SLN and NLC promote flexibility to the lipid matrix, thus offering the opportunity to accommodate a high amount of drug molecules. Another class of lipids derived from fatty acids are fatty alcohols, which are also of high safety and tolerability. Examples are cetyl alcohol [120] and stearyl alcohol [120, 121]. These types of lipids can be used with absorption promoters due to the ability to cause disturbances in the lipid packaging of the skin [120].
Ceramides are another class of highly applied solid lipids that are mainly composed of esters of fatty acids and fatty alcohols. Other examples are cocoa butter [113, 122], carnauba wax [123], beeswax [123, 124] and cetyl palmitate [112]. Regarding liquid lipids, some oils rich in fatty acids can be listed, such as almond oil [125], olive oil [125], oleic acid [126, 127], squalene (shark liver oil) [128], ricin or castor oil [127, 129].
The most used medium chain triglycerides are miglyol®810 [130] and miglyol®812 [131,132] (esters of caprylic acid), Labrafac® (medium chain triglycerides derived from caprylic acid) [133], Labrafil® (glycerides associated with polyethylene glycol esters (PEG)) [134, 135, 136], Maisine® (glyceryl monolinolate) [137], Lauroglycol® (propylene glycol monolaurate) [138] and Capryol® (propylene glycol monocaprilate) [139].
5.1.2. Surfactants
Surfactants are an essential component for the production of nanoparticles, so that surface tension is reduced and particle distribution facilitated. Surfactants are usually amphiphilic molecules whose hydrophilic group is directed towards the aqueous phase and the lipophilic group towards the oily phase of the nanoparticles. The knowledge of the hydrophilic-lipophilic balance (HLB) of surfactants, which is directly related to their solubility, is instrumental to select the best for the stability of a SLN/NLC composition [118]. The choice of the ideal surfactant should respond to two principles, i.e., quantitative and qualitative compatibility. Regarding quantitative compatibility, surfactants should be used in low concentrations (0.5–5.0%) in the formulation due to their ability to irritate the skin and often form other nanometric structures in the formulation, such as micelles. The qualitative compatibility refers to its chemical structure and the ability of its chains to interact and adjust with the lipid chains on the surface of the nanoparticles to stabilize two immiscible phases. These types of interactions are usually electrostatic, since the lipophilic part of the surfactant is attracted by the lipid molecules. A physical adsorption of these to the surface of the molecule can be extended by the lipid chains composing the lipid matrix. The hydrophilic groups of the surfactants are responsible for the repulsive forces between particles, which depend on the volume of the dispersion and chemical nature of the surfactant [140].
For the production of SLN and NLC, surfactants can be chosen according to their HLB value and to the nature and structure of their hydrophilic groups. Depending on the purpose, non-ionic, anionic and cationic can be used [141, 142]. For safety reasons, the most commonly used surfactants are non-ionic, which promote stereochemical stabilization of nanoparticles. Charged surfactants such as anionic (negative charge) and cationic (positive charge) promote the stability of nanoparticles by electrochemical stabilization resulting in repulsion between them and, consequently, reducing the risk of particles sedimentation or aggregation. For topical application, to the use of cationic surfactants may be an interesting option considering the anionic character of the skin [143], to promote an electrostatic interaction between the particles and the skin. Cationic nanoparticles are therefore not expected to penetrate into the skin but will increase bioadhesion, the particles are retained longer onto the skin, which may lead to improved absorption of the drug that is released from the particles through the skin. Examples of cationic or positively charged molecules that are described to stabilize lipid nanoparticles include stearylamine [144, 145, 146], quaternary ammonium salts [110, 147], and Esterquat 1 [146]. As for anionic or negatively charged surfactants, these are usually applied as co-surfactants, at very low concentration (<1%wt), and include bile salts, such as cholate and sodium taurocholate.
The most widely used non-ionic surfactants in the production of SLN and NLC are polysorbates (Tween® 20, 40, 60, 80) [117], sorbitans (Span®20, 40, 60, 80), sorbitol esters (Mirj® 45, 52, 53, 59), tyloxapol and triblocks of polyoxypropylene copolymers (poloxamers) [148]. Esters of stearic, lauric, oleic and palmitic acids are also frequently employed. Because of their non-irritation and non-toxic profile, non-ionic surfactants are preferentially used, especially for dermal and transdermal formulation as, unlike surfactants with ionic charge, non-ionically charged molecules do not cause disruption of the lipids that make up the skin [149].
Lecithins or phosphatidylcholines derived from soy or egg, are also frequently used as surfactants, usually added to the inner lipid phase of SLN/NLC dispersions as co-surfactants. Generally, soy lecithin contains more saturated fatty acid chains than egg lecithin [106]. Besides their role as co-surfactant in reducing the mean particle size due to their amphiphilic character [150, 151], lecithins may act as absorption enhancers [152].
5.2. Production of lipid nanoparticles
The choice of the method to produce SLN and NLC will determine the quality of the nanoparticles dispersion obtained, in particular, its mean particle size, polydispersity, encapsulation efficiency and loading capacity for a certain drug, and also how the drug is accommodated in the lipid matrices. Methods that promote the drug dispersion within the lipid matrix will contribute to develop SLN/NLC with modified-release profile, whereas methods that place the drug onto the surface of the particles will promote an immediate release of drug which may only be useful for a local/topical effect and less for dermal and transdermal drug release. The factors that most influence the choice of method are the characteristics of the drug to be loaded [105, 153], i.e. (i) solubility, (ii) molecular weight, (iii) thermolability, (iv) susceptibility to oxidation and (v) the chemical structure [24, 154]. Prior to any choice of method, a solid lipid must be chosen, in the case of SLN production and in the case of NLC a mixture of liquid and solid lipid that can solubilize or incorporate the largest amount of drug to achieve the highest loading capacity (LC) and encapsulation efficiency (EE) as possible. These parameters (LC and EE) are used as quality indicators in the development of a certain SLN/NLC formulation and are determined as follows:
where Wloaded drug refers to the mass of drug (g) that was quantified inside the particles, the Wlipids to the total mass of lipids (g) used in the production, and the Wdrug taken in production refers to the mass of drug initially weighted and taken in the production process.
The literature describes several production methods to develop SLN and NLC, which may or may not require the use of organic solvents [155, 156, 157, 158]. The most commonly applied method is the high-pressure homogenization (HPH) which can be run either at cold or hot temperature. In both processes, the drug is solubilized or dispersed inpreviously melted lipids. In the case of hot HPH, a hot aqueous surfactant solution is added to the lipid phase heated at the same temperature, and this mixture is homogenized by high shear or by means of ultrasounds to produce a pre-emulsion. This pre-emulsion is then processed by the high-pressure homogenizer for 3 to 5 homogenization cycles at 500 bar. After reducing the particle size by cavitation forces, the obtained nanoemulsion will be cooled so that the nanoparticles can be formed by recrystallization of the lipid now composing the core of SLN/NLC. Cold HPH undergoes a slight modification compared to hot HPH, which happens immediately after solubilizing the drug in melted lipid phase, when this mixture is cooled by liquid nitrogen or dry ice. This mixture is subsequently ground in a mortar, producing microparticles that are resuspended in an aqueous surfactant solution at room temperature. The reduction of micro to nanoparticles occurs in the high-pressure homogenizer at room temperature or below. This process is suitable for thermolabile drugs. The cold HPH approach was developed to overcome some flaws related to hot HPH, which include the risk of drug distribution in the aqueous phase during the homogenization process and problems related to lipid crystallization, being able to avoid “supercooled melts”. In the hot HPH and upon cooling the nanoemulsion, if the drug is too hydrophilic, the lipid will recrystallize first creating the matrices onto which the drug will be precipitated. For more lipophilic drugs and for the cold HPH, the drug will be preferentially located inside the lipid matrices contributing to achieve a modified release profile [159, 160].
The microemulsion method was firstly developed by Gasco et al. [161] and has been adapted by several research groups [119, 145, 162, 163]. In this method, and as happens with the hot HPH, the drug is added to the melted lipids and an aqueous solution of surfactant is added at the same temperature. This mixture is homogenized under high shear to produce a micro emulsion and then diluted in cold water. This dilution causes a thermal shock that breaks the microemulsion into a nanoemulsion [164].
The double emulsion method emerged as an alternative to load hydrophilic drugs, firstly described by Garcia-Fuentes et al. [165], and improved by Fangueiro et al. [117]. In addition to SFAs with hydrophilic characteristics, the method can be applied for the incorporation of peptides and proteins or labile API, where these will be incorporated in an internal aqueous phase surrounded by a lipid matrix. These aqueous droplets containing the API allow them to be protected from chemical and/or enzymatic degradation. First, the hydrophilic drug is solubilized in an internal aqueous phase and added to the lipid phase to form a primary w/o (water-in-oil) emulsion through high sheer homogenization or sonication. Secondarily, this w/o emulsion will be dispersed in an external aqueous surfactant solution to form an w/o/w (water-in-oil-in-water) emulsion [117]. In this method, at least two surfactants are necessarily applied. The surfactant with a low HLB value will be added to the lipid phase and is used to stabilize the first interface (w/o). The second surfactant usually has a high HLB value and is added to the external aqueous phase to stabilize the second interface (o/w) (oil-in-water) [148]. This method is commonly referred as suitable for the loading hydrophilic drug molecules, including peptides and proteins, which are added to the inner aqueous phase of the first w/o emulsion, prior to the production of the double emulsion. These inner aqueous droplets protect the drug from chemical and/or enzymatic degradation and usually result in higher EE and LC, when compared to methods that require the use of high temperatures.
The solvent emulsification-evaporation method is usually applied when the lipophilic drug is not soluble in lipids [166]. In this method, an organic solvent, not miscible in water (cyclohexane, chloroform) but which solubilizes the lipid, is added to an aqueous solution of surfactant to form an o/w emulsion. Then the organic solvent is removed by evaporation. Prior to the production of o/w, the drug to be loaded is added to the inner organic solution in which the lipid has been dissolved.
In the solvent displacement method, also called nanoprecipitation method, a water-miscible organic solvent (e.g. ethanol, methanol, acetone) is used to dissolve the selected lipid compounds to produce the inner organic solution in which the drug is also added. It was initially described for the production of polymeric nanoparticles [167], and later adapted for SLN and NLC [168, 169, 170]. The lipid phase is injected into an aqueous surfactant solution of under magnetic stirring. SLN/NLC are formed after complete removal of the solvent by diffusion or distillation, with precipitation of the particles [159].
In the solvent emulsification-diffusion method, a solution of a semi-polar organic solvent capable of solubilizing the previously saturated lipid is used to ensure thermodynamic balance. This solution is added to an aqueous surfactant solution to obtain an o/w emulsion. The saturated solution prevents the diffusion of the solvent from the droplets into the aqueous phase. The formation of SLN and NLC is obtained by adding an excess of water to the emulsion in order to facilitate the diffusion of the droplet solvent [164, 171].
A phase inversion method has also been described to produce lipid nanoparticles [172]. This method involves two steps. In the first stage, all components are melted and magnetically stirred using a temperature program that goes from 25 to 85 °C and then decreased to 60 °C. These three temperature cycles are applied so that the phase inversion process defined by that temperature range occurs. In the second stage, an irreversible shock is caused by the addition of ice water. This process leads to the formation of nanoparticles.
Another method adapted from the production of polymeric nanoparticles has been described [173]. This method produces particles by coacervation in a controlled manner, through the use of alkaline salts of fatty acids. The basis of the method lies in the interaction of a micellar solution of these salts (i.e. sodium stearate, sodium palmitate, sodium myristate or sodium behenate) and an acid solution (coacervation solution) in the presence of an amphiphilic stabilizing agent of a polymeric nature [173, 174]. SLN or NLC can be obtained by lowering the pH of the medium. It is a simple, economically accessible and thermosensitive method that allows the incorporation of various types of drugs and can be easily scaled-up [175].
5.3. Incorporation of SLN and NLC and mechanisms of drug release
The literature describes three models for each type of lipid nanoparticles, i.e., SLN and NLC [160]. The SLN models are described according to the place of drug in the matrices, whereas NLC models are described according to the type of lipid mixtures used to nanostructure their cores. In SLN type I, the amount of drug and lipid is equivalent, which will cause the precipitation of lipid and drug together resulting in the homogeneous distribution of the drug in the lipid matrix. In SLN type II, there is a greater amount of lipid compared to the drug. In this case, the lipid will precipitate faster, creating solid lipid cores onto which the drug will precipitate. This type of nanoparticles provides the location of API preferentially on the surface of the particles. For hydrophilic drugs, this type of SLN is also observed as the drug molecules that escaped into the aqueous phase will be placed onto the recently formed solid cores with the cooling down of the dispersion. In SLN type III there is a greater amount of drug relative to the lipid, then the precipitation of drug occurs first and the lipid precipitates covering the drug cores. This type of nanoparticles provides the location of the drug preferentially in the nanoparticles core, being surrounded by lipids.
The location of the drug in the lipid matrix governs the release profile. SLN type I will provide a prolonged release. SLN type II will offer a fast/burst release, whereas SLN type III will provide a sustained release.
With respect to the morphology of NLC, type I, or the imperfect crystal model, is obtained when mixing solid lipids with sufficient amounts of oils which hinder the capacity of the former in recrystalize in a highly ordered form. The NLC type II or the amorphous model is obtained when using special lipids (e.g., dibutyl adipate, hydroxy octacosanyl hydroxystearate, isopropyl myristate) that do not recrystalize upon cooling the nanoemulsion. The NLC type III, or the multiple model, is obtained when mixing solid lipids with medium/long chain triglycerides (MCT, LCT) in a ratio that the solubility of MCT or LCT in the selected solid lipids is exceeded creating nanocompartments of oil entrapped in the solid matrix. Amongst the three types, this multiple model promotes a burst release followed by a prolonged release of drug [160].
5.4. Lipid nanoparticles for topical, dermal and transdermal application
The added value of lipid nanoparticles for skin administration can be linked to properties, such as improved skin hydration, adhesiveness, occlusion, lubrication and skin emollience, besides their use in delivering the drug for local/topical action, or to exhibit a deeper action in the dermis or systemically. The first barrier to overcome is the stratum corneum. The lipids that make up this layer, namely ceramides, fatty acids and cholesterol, form a continuous structure that hinders the passage of certain substances [176]. On the other hand, lipid nanoparticles are composed of lipids that resemble those existing both in the sebum and in the skin itself. This may either promote dissolution of SLN/NLC in the sebum followed by the release of the drug to have a topical or dermal effect, or nanoparticles can enter the skin through different pathways and appendages (as discussed above) reaching the systemic circulation and have a transdermal effect.
Adhesiveness of nanoparticles to the skin is desirable for the purpose of skin hydration and to increase the time of contact of the drug with the skin, allowing to increase its cutaneous absorption. The smaller the size the greater the surface of the particles available for skin adhesion [177].
Conventional topical formulations alone do not ensure long-term skin hydration, particularly when the stratum corneum is excessively dry. An ideal formulation to be applied on this type of skin wound be one with the capacity to form a dense lipid film and create oclusiveness. The strong adhesion of SLN onto human skin was confirmed by a stripping test followed by electron microscopy analysis to detect the presence of the lipid film, which explain the occlusion and hydration phenomena promoted by SLN [178].
Regarding the occlusion caused by these systems, it is also mainly due to their nanometric size, which in turn gives a huge surface area to the particles when in contact with the skin [179]. Lipid nanoparticles reduce the transepidermal water loss by evaporation with enhanced occlusion. This phenomenon favors the penetration of the drug into the skin [180]. The occlusion factor depends strongly on the crystallinity of the nanoparticles [181], i.e., the higher the crystallinity of nanoparticles, the higher the occlusion factor.
The occlusion factor of SLN and NLC has already been extensively reported [181]. It is determined in vitro using the Vringer and Ronde test [182]. The test is based on the placement of the formulation onto a nitrocellulose filter paper on top of vial previously filled with water. Evaporation of water is recorded at skin temperature (≈32 °C) over a period of time. As a reference, a vial with water and covered with filter paper only is used.
Other features that nanoparticles offer to the skin include lubrication, hydration and emollience. The emollience is mainly due to the spherical structure that these particles usually present. This structure gives them viscoelastic behaviour, which is commonly evaluated by applying oscillation tests, obtaining information on the inter and intra-particle forces. With these tests, it is possible to distinguish whether the formulation has an elastic or viscous character [183]. Lipid nanoparticles also offer a mechanical barrier to the skin, protecting it against harsh environments, skin irritation and allergic reactions [183].
Lipid nanoparticle formulations have a white color, and can be dispersed into creams or gels to obtain semi-solid consistency and appealing organoleptic properties. SLN and NLC are able to retain flavors and promote an extended release of the fragrance over time when topically applied [183]. To ensure no skin irritation, SLN and NLC dispersions should be compatible with the physiological pH of the skin, which is slightly acidic (4.2–5.6) [184]. However, there are studies in which pH strongly affects the physical and chemical stability of nanoparticles [185, 186].
Some authors have investigated the permeation of NLC containing tripterin, drug for the treatment of melanoma [187]. The studies revealed that the positive charges of the particles contributed to the increase in percutaneous absorption of tripterin.
Sanna et al. [120] studied skin permeation of SLN and the results showed that the permeation of econazole nitrate is dependent on the structure of the lipid, namely fatty alcohols. In addition, they proved that the permeation is increased due to the interaction of the lipids present in the nanoparticles and the lipids that make up the skin structure. Another research group analyzed the permeation of idebenone-loaded SLN, a potent anti-oxidant drug from ubiquinone A [188]. The authors proved that none of the tested SLN managed to permeate the deepest layers of the skin, with the drug being more retained in the epidermis. Thus, the aim is not always the systemic absorption of drug; in cases of sunscreens and other cosmetic ingredients, the ideal is to be located on the surface of the stratum corneum and epidermis, as its absorption into the systemic circulation can lead to harmful effects.
Table 1 lists several examples of drugs that have been formulated in SLN and/or NLC for topical, dermal and transdermal administration.
Table 1.
Examples of drugs loaded in SLN and/or NLC for topical, dermal and transdermal administration.
| Drug | Therapeutic properties | Type of nanoparticle | References |
|---|---|---|---|
| Aceclofenac | Anti-inflammatory Anti-rheumatoid |
SLN | [189] |
| NLC | [126] | ||
| Alpha-pinene | Anti-inflammatory Anti-cancer |
NLC | [190, 191] |
| Betamethasone valerate | Atopic eczema | SLN | [108] |
| Citral | Anti-cancer | NLC | [192] |
| Clotrimazole | Anti-fungal | SLN and NLC | [104, 132] |
| Econazole nitrate | Anti-fungal | SLN | [133] |
| Fluticasone propionate | Anti-inflammatory corticosteroid | NLC | [109] |
| Ketoprofen | Anti-inflammatory | NLC | [133] |
| Lidocain | Local anesthetic | SLN and NLC | [130] |
| Limonene | Anti-cancer | SLN | [23] |
| Prednicarbate | Atopic eczema | SLN | [108] |
| Psoralen | Psoriasis | SLN and NLC | [128] |
| Resveratrol | Anti-oxidant | SLN and NLC | [131] |
| Retinyl retinoate | Anti-aging | NLC | [136] |
| Sildenafil citrate | Erectile dysfunction | SLN and NLC | [137] |
| Vitamin A | Anti-oxidant Anti-tumoral |
SLN | [180] |
6. Conclusions
The skin is a complex and multifunctional organ, and many of its characteristics ensure its barrier function, allowing the skin to constantly adapt to variations in the external environment. One of the most important functions of the skin is its ability to act as a protective barrier, thus preventing foreign substances from entering the body and excessive loss of endogenous material. Human skin imposes physical, chemical and biological limitations on all types of permeating agents that can cross the epithelial barrier. The drug to be released passively through the skin requires properties such as lipophilia, size, shape, molar mass, pKa and HLB. These requirements have limited the number of products commercially available for dermal and transdermal delivery. As a result, several strategies have emerged in recent years to improve the permeation of drugs through the skin. These strategies can be classified into active and passive methods. The passive approach to increase the permeability of the skin implies the optimization of the molecule, the formulation or the use of nanoparticles with adequate dimensions, shapes and composition. However, for drugs with a molar mass greater than 500 Da, the result is, in most cases, insignificant. Active methods that normally involve physical or mechanical actions to increase release have shown interesting results. These methods have been successful for drugs of different lipophilicity and molecular weight, such as proteins, peptides and oligonucleotides. Among the methods, electrical (iontophoresis, electroporation), mechanical (abrasion, ablation and perforation) and other energy related techniques such as ultrasound, radio frequency, laser and temperature stand out. For these release methods to compete with approaches already on the market, the main issues are related to effectiveness, safety, reproducibility, cost-effectiveness and scale-up facilities. The mechanisms that support the penetration and diffusion of nanoparticles in the skin are not perfectly explained or even critically addressed. The size of the nanoparticle is considered the most important prerequisite for absorption. However, other properties, such as the effect of surface charges, pKa and composition of the nanoparticles, can modulate their entry into the skin. There is still a difficulty in defining certain terms such as nanomaterials, nanoparticles, carriers, drug delivery systems and vectors, which are used without care to clarify the reader about their differences. When making comparisons on penetration, stability and bioavailability, authors generally compare different systems and results obtained by different methodologies. In-vitro or ex-vivo permeation studies often lead the reader to believe that the results obtained can be extrapolated to in-vivo evaluations. However, many aspects related to the skin barrier system, in particular enzymatic metabolism are not considered. A starting point for understanding the mechanisms involved in the skin permeation process must be a multidisciplinary approach to biological and pharmacotechnical problems. The understanding of the mechanisms encountered in the permeation process, and the ways to control, can make this administration route of drugs stop being a promise and become a reality. Evidence exists that lipid nanoparticles allow controlling and delivering various drugs for skin permeation. Besides, their composition makes these systems safe for dermatological and cosmetic use. However, for a lipid nanoparticle formulation to be a successful product, aspects related to the right choice of lipid for a certain drug, the surfactant, the production method and then the appropriate choice of the final semi-solid (cream, gel) have to be considered.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by the Portuguese Science and Technology Foundation (FCT) from the Ministry of Science and Technology (MCTES) (UIDB/04469/2020) (CEB strategic fund) and UIDB/04033/2020 (CITAB), and European Funds (PRODER/COMPETE) and FEDER, under the Partnership Agreement PT2020.
Data availability statement
No data was used for the research described in the article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Footnotes
This article is a part of the Lipid-Based Nanoparticles Special issue.
References
- 1.Rothman S. The principles of percutaneous permeation. J. Lab. Clin. Med. 1943;28:1305–1321. [Google Scholar]
- 2.Vallette G. Percutaneous absorption. Pharm. J. 1953;20:461–462. [Google Scholar]
- 3.Hadgraft J.W., Somers G.F. Percutaneous absorption. J. Pharm. Pharmacol. 1956;8:625–634. doi: 10.1111/j.2042-7158.1956.tb12194.x. [DOI] [PubMed] [Google Scholar]
- 4.Davies R.F.M. Penetration of dermatological vehicles. Pharm. J. 1950;1:74–76. [Google Scholar]
- 5.Hadgraft J., Lane M.E. Skin permeation: the years of enlightenment. Int. J. Pharm. 2005;305:2–12. doi: 10.1016/j.ijpharm.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Hossain M.A., Ahmed S.U., Plakogiannis F.M. Effect of vehicle systems, pH and enhancers on the permeation of highly lipophilic aripiprazole from Carbopol 971P gel systems across human cadaver skin. Drug Dev. Ind. Pharm. 2012;38:323–330. doi: 10.3109/03639045.2011.602978. [DOI] [PubMed] [Google Scholar]
- 7.Kogan A., Garti N. Microemulsions as transdermal drug delivery vehicles. Adv. Colloid Interf. Sci. 2006;123–126:369–385. doi: 10.1016/j.cis.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Xiang T.X., Anderson B.D. The relationship between permeant size and permeability in lipid bilayer membranes. J. Membr. Biol. 1994;140:111–122. doi: 10.1007/BF00232899. [DOI] [PubMed] [Google Scholar]
- 9.Balaguer-Fernandez C., Femenia-Font A., Muedra V., Merino V., Lopez-Castellano A. Combined strategies for enhancing the transdermal absorption of midazolam through human skin. J. Pharm. Pharmacol. 2010;62:1096–1102. doi: 10.1111/j.2042-7158.2010.01142.x. [DOI] [PubMed] [Google Scholar]
- 10.Kolli C.S., Chadha G., Xiao J., Parsons D.L., Babu R.J. Transdermal iontophoretic delivery of selegiline hydrochloride, in vitro. J. Drug Target. 2010;18:657–664. doi: 10.3109/10611860903494237. [DOI] [PubMed] [Google Scholar]
- 11.Krishnan G., Roberts M.S., Grice J., Anissimov Y.G., Benson H.A. Enhanced transdermal delivery of 5-aminolevulinic acid and a dipeptide by iontophoresis. Biopolymers. 2011;96:166–171. doi: 10.1002/bip.21520. [DOI] [PubMed] [Google Scholar]
- 12.Singh N.D., Banga A.K. Controlled delivery of ropinirole hydrochloride through skin using modulated iontophoresis and microneedles. J. Drug Target. 2013 doi: 10.3109/1061186X.2012.757768. [DOI] [PubMed] [Google Scholar]
- 13.Charoo N.A., Rahman Z., Repka M.A., Murthy S.N. Electroporation: an avenue for transdermal drug delivery. Curr. Drug Deliv. 2010;7:125–136. doi: 10.2174/156720110791011765. [DOI] [PubMed] [Google Scholar]
- 14.Escobar-Chavez J.J., Bonilla-Martinez D., Villegas-Gonzalez M.A., Revilla-Vazquez A.L. Electroporation as an efficient physical enhancer for skin drug delivery. J. Clin. Pharmacol. 2009;49:1262–1283. doi: 10.1177/0091270009344984. [DOI] [PubMed] [Google Scholar]
- 15.Escobar-Chavez J.J., Bonilla-Martinez D., Villegas-Gonzalez M.A., Rodriguez-Cruz I.M., Dominguez-Delgado C.L. The use of sonophoresis in the administration of drugs throughout the skin. J. Pharm. Pharmaceut. Sci. 2009;12:88–115. doi: 10.18433/j3c30d. [DOI] [PubMed] [Google Scholar]
- 16.Herwadkar A., Sachdeva V., Taylor L.F., Silver H., Banga A.K. Low frequency sonophoresis mediated transdermal and intradermal delivery of ketoprofen. Int. J. Pharm. 2012;423:289–296. doi: 10.1016/j.ijpharm.2011.11.041. [DOI] [PubMed] [Google Scholar]
- 17.Iervolino M., Cappello B., Raghavan S.L., Hadgraft J. Penetration enhancement of ibuprofen from supersaturated solutions through human skin. Int. J. Pharm. 2001;212:131–141. doi: 10.1016/s0378-5173(00)00603-7. [DOI] [PubMed] [Google Scholar]
- 18.Moser K., Kriwet K., Kalia Y.N., Guy R.H. Enhanced skin permeation of a lipophilic drug using supersaturated formulations. J. Contr. Release. 2001;73:245–253. doi: 10.1016/s0168-3659(01)00290-5. [DOI] [PubMed] [Google Scholar]
- 19.Leveque N., Raghavan S.L., Lane M.E., Hadgraft J. Use of a molecular form technique for the penetration of supersaturated solutions of salicylic acid across silicone membranes and human skin in vitro. Int. J. Pharm. 2006;318:49–54. doi: 10.1016/j.ijpharm.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 20.Shetty P.K., Suthar N.A., Menon J., Deshpande P.B., Avadhani K., Kulkarni R.V., Mutalik S. Transdermal delivery of lercanidipine hydrochloride: effect of chemical enhancers and ultrasound. Curr. Drug Deliv. 2013 doi: 10.2174/1567201811310040007. [DOI] [PubMed] [Google Scholar]
- 21.Shokri J., Azarmi S., Fasihi Z., Hallaj-Nezhadi S., Nokhodchi A., Javadzadeh Y. Effects of various penetration enhancers on percutaneous absorption of piroxicam from emulgels. Res. Pharm. Sci. 2012;7:225–234. [PMC free article] [PubMed] [Google Scholar]
- 22.Cimino C., Maurel O.M., Musumeci T., Bonaccorso A., Drago F., Souto E.M.B., Pignatello R., Carbone C. Essential oils: pharmaceutical applications and encapsulation strategies into lipid-based delivery systems. Pharmaceutics. 2021;13 doi: 10.3390/pharmaceutics13030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Souto E.B., Zielinska A., Souto S.B., Durazzo A., Lucarini M., Santini A., Silva A.M., Atanasov A.G., Marques C., Andrade L.N., Severino P. (+)-Limonene 1,2-epoxide-loaded SLNs: evaluation of drug release, antioxidant activity, and cytotoxicity in an HaCaT cell line. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21041449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Souto E.B., Baldim I., Oliveira W.P., Rao R., Yadav N., Gama F.M., Mahant S. SLN and NLC for topical, dermal, and transdermal drug delivery. Expet Opin. Drug Deliv. 2020;17:357–377. doi: 10.1080/17425247.2020.1727883. [DOI] [PubMed] [Google Scholar]
- 25.Carbone C., Fuochi V., Zielinska A., Musumeci T., Souto E.B., Bonaccorso A., Puglia C., Petronio Petronio G., Furneri P.M. Dual-drugs delivery in solid lipid nanoparticles for the treatment of Candida albicans mycosis. Colloids Surf. B Biointerfaces. 2020;186:110705. doi: 10.1016/j.colsurfb.2019.110705. [DOI] [PubMed] [Google Scholar]
- 26.Proksch E., Brandner J.M., Jensen J.-M. The skin: an indispensable barrier. Exp. Dermatol. 2008;17:1063–1072. doi: 10.1111/j.1600-0625.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu H., Ishiko A., Masunaga T., Kurihara Y., Sato M., Bruckner-Tuderman L., Nishikawa T. Most anchoring fibrils in human skin originate and terminate in the lamina densa. Lab. Invest. 1997;76:753–763. [PubMed] [Google Scholar]
- 28.Madison K.C. Barrier Function of the Skin: ‘‘La Raison d’EOE tre’’ of the Epidermis. J. Invest. Dermatol. 2003;121:231–241. doi: 10.1046/j.1523-1747.2003.12359.x. [DOI] [PubMed] [Google Scholar]
- 29.Bouwstra J.A., Ponec M. The skin barrier in healthy and diseased state. Biochim. Biophys. Acta. 2006;1758:2080–2095. doi: 10.1016/j.bbamem.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 30.Menon G.K. New insights into skin structure: scratching the surface. Adv. Drug Deliv. Rev. 2002;54:S123–S130. doi: 10.1016/s0169-409x(02)00121-7. [DOI] [PubMed] [Google Scholar]
- 31.Souto E.B., Doktorovova S. Chapter 6 - solid lipid nanoparticle formulations pharmacokinetic and biopharmaceutical aspects in drug delivery. Methods Enzymol. 2009;464:105–129. doi: 10.1016/S0076-6879(09)64006-4. [DOI] [PubMed] [Google Scholar]
- 32.Eckert R.L., Rorke E.A. Molecular biology of keratinocyte differentiation. Environ. Health Perspect. 1989;80:109–116. doi: 10.1289/ehp.8980109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elias P.M. Therapeutic implications of a barrier-based pathogenesis of atopic dermatitis. Ann. Dermatol. 2010;22:245–254. doi: 10.5021/ad.2010.22.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leveque J.L., Corcuff P., de Rigal J., Agache P. In vivo studies of the evolution of physical properties of the human skin with age. Int. J. Dermatol. 1984;23:322–329. doi: 10.1111/j.1365-4362.1984.tb04061.x. [DOI] [PubMed] [Google Scholar]
- 35.Marquez-Lago T.T., Allen D.M., Thewalt J. A novel approach to modelling water transport and drug diffusion through the stratum corneum. Theor. Biol. Med. Model. 2010;7:33. doi: 10.1186/1742-4682-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elias P.M. Stratum corneum defensive functions: an integrated view. J. Invest. Dermatol. 2005;125:183–200. doi: 10.1111/j.0022-202X.2005.23668.x. [DOI] [PubMed] [Google Scholar]
- 37.Waller J.M., Maibach H.I. Age and skin structure and function, a quantitative approach (I): blood flow, pH, thickness, and ultrasound echogenicity. Skin Res. Technol. 2005;11:221–235. doi: 10.1111/j.0909-725X.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- 38.Jain S., Mistry M.A., Swarnakar N.K. Enhanced dermal delivery of acyclovir using solid lipid nanoparticles. Drug Deliv. Transl. Res. 2011;1:395–406. doi: 10.1007/s13346-011-0036-0. [DOI] [PubMed] [Google Scholar]
- 39.Schaefer H., Filaquier C. Skin metabolism. Pathol. Biol. 1992;40:196–204. [PubMed] [Google Scholar]
- 40.Roig-Rosello E., Rousselle P. The human epidermal basement membrane: a shaped and cell instructive platform that aging slowly alters. Biomolecules. 2020;10:1607. doi: 10.3390/biom10121607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walko G., Castañón M.J., Wiche G. Molecular architecture and function of the hemidesmosome. Cell Tissue Res. 2015;360:363–378. doi: 10.1007/s00441-014-2061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Q., Grice J.E., Wang G., Roberts M.S. Cutaneous metabolism in transdermal drug delivery. Curr. Drug Metabol. 2009;10:227–235. doi: 10.2174/138920009787846350. [DOI] [PubMed] [Google Scholar]
- 43.Kao J., Hall J., Shugart L.R., Holland J.M. An in vitro approach to studying cutaneous metabolism and disposition of topically applied xenobiotics. Toxicol. Appl. Pharmacol. 1984;75:289–298. doi: 10.1016/0041-008x(84)90211-4. [DOI] [PubMed] [Google Scholar]
- 44.Steinsträsser I., Merkle H.P. Dermal metabolism of topically applied drugs: pathways and models reconsidered. Pharm. Acta Helv. 1995;70:3–24. doi: 10.1016/0031-6865(94)00047-y. [DOI] [PubMed] [Google Scholar]
- 45.Chytil F. Retinoic acid: biochemistry and metabolism. J. Am. Acad. Dermatol. 1986;15:741–747. doi: 10.1016/s0190-9622(86)70229-6. [DOI] [PubMed] [Google Scholar]
- 46.Puche C., José M., Cabero A., Meseguer A. Expression and enzymatic activity of the P450c17 gene in human adipose tissue. Eur. J. Endocrinol. 2002;146:223–229. doi: 10.1530/eje.0.1460223. [DOI] [PubMed] [Google Scholar]
- 47.Coomes M.W., Norling A.H., Pohl R.J., Müller D., Fouts J.R. Foreign compound metabolism by isolated skin cells from the hairless mouse. J. Pharmacol. Exp. Therapeut. 1983;225:770–777. [PubMed] [Google Scholar]
- 48.Pohl R.J., Coomes M.W., Sparks R.W., Fouts J.R. 7-ethoxycoumarin O-deethylation activity in viable basal and differentiated keratinocytes isolated from the skin of the hairless mouse. Drug Metab. Dispos. 1984;12:25–34. [PubMed] [Google Scholar]
- 49.Finnen M.J., Shuster S. Phase 1 and Phase 2 drug metabolism in isolated epidermal cells from adult hairless mice and in whole human hair follicles. Biochem. Pharmacol. 1985;34 doi: 10.1016/0006-2952(85)90735-x. 3571-3355. [DOI] [PubMed] [Google Scholar]
- 50.Cevc G., Vierl U. Nanotechnology and the transdermal route: a state of the art review and critical appraisal. J. Contr. Release. 2010;141:277–299. doi: 10.1016/j.jconrel.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 51.Cevc G. Lipid vesicles and other colloids as drug carriers on the skin. Adv. Drug Deliv. Rev. 2004;56:675–711. doi: 10.1016/j.addr.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 52.Wertz P.W., van den Bergh B. The physical, chemical and functional properties of lipids in the skin and other biological barriers. Chem. Phys. Lipids. 1998;91:85–96. doi: 10.1016/s0009-3084(97)00108-4. [DOI] [PubMed] [Google Scholar]
- 53.Proksch E., Fölster-Holst R., Bräutigam M., Sepehrmanesh M., Pfeiffer S., Jensen J.M. Role of the epidermal barrier in atopic dermatitis. J. Dtsch. Dermatol. Ges. 2009;7:899–910. doi: 10.1111/j.1610-0387.2009.07157.x. [DOI] [PubMed] [Google Scholar]
- 54.Dhote V., Bhatnagar P.K., Mishra P.K., Mahajan S.C., Mishra D.K., Ionthophoresis A potential emergence of a transdermal drug delivery system. Sci. Pharm. 2012;80:1–28. doi: 10.3797/scipharm.1108-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tiwary A.K., Sapra B., Jain S. Innovations in transdermal drug delivery: formulations and techniques. Recent Pat. Drug Deliv. Formulation. 2007;1:23–36. doi: 10.2174/187221107779814087. [DOI] [PubMed] [Google Scholar]
- 56.Cevc G., Vierl U. Spatial distribution of cutaneous microvasculature and local drug clearance after drug application on the skin. J. Contr. Release. 2007;118:18–26. doi: 10.1016/j.jconrel.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 57.Fuchs E. Scratching the surface of skin development. Nature. 2007;445:834–842. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baroli B. Penetration of nanoparticles and nanomaterials in the skin: fiction or reality? J. Pharm. Sci. 2010;99:21–50. doi: 10.1002/jps.21817. [DOI] [PubMed] [Google Scholar]
- 59.Elias P.M., Chol E.H. Interactions among stratum corneum defensive functions. Exp. Dermatol. 2005;14:719–726. doi: 10.1111/j.1600-0625.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- 60.Lee S.H., Jeong S.K., Ahn S.K. An update of the defensive barrier function of skin. Yonsei Med. J. 2006;30:293–306. doi: 10.3349/ymj.2006.47.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Loftsson T., Vogensen S.B., Brewster M.E., Konrádsdóttir F. Effects of cyclodextrins on drug delivery through biological membranes. J. Pharm. Sci. 2007;96:2532–2546. doi: 10.1002/jps.20992. [DOI] [PubMed] [Google Scholar]
- 62.Weiss S.C. Conventional topical delivery systems. Dermatol. Ther. 2011;24:471–476. doi: 10.1111/j.1529-8019.2012.01458.x. [DOI] [PubMed] [Google Scholar]
- 63.Potts R.O., Francoeur M.L. The influence of stratum corneum morphology on water permeability. J. Invest. Dermatol. 1991;96:495–499. doi: 10.1111/1523-1747.ep12470197. [DOI] [PubMed] [Google Scholar]
- 64.Tang H., Mitragotri S., Blankschtein D., Langer R. Theoretical description of transdermal transport of hydrophilic permeants: application to low-frequency sonophoresis. J. Pharm. Sci. 2001;90:545–568. doi: 10.1002/1520-6017(200105)90:5<545::aid-jps1012>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 65.Guy R.H., Hadgraft J. Physicochemical aspects of percutaneous penetration and its enhancement. Pharm. Res. (N. Y.) 1988;5:753–758. doi: 10.1023/a:1015980516564. [DOI] [PubMed] [Google Scholar]
- 66.Johnson M.E., Blankschtein D., Langer R. Evaluation of solute permeation through the stratum corneum: lateral bilayer diffusion as the primary transport mechanism. J. Pharm. Sci. 1997;86:1162–1172. doi: 10.1021/js960198e. [DOI] [PubMed] [Google Scholar]
- 67.Moss G.P., Dearden J.C., Patel H., Cronin M.T.D. Quantitative structure-permeability relationships (QSPRs) for percutaneous absorption. Toxicol in Vitro. 2002;16:299–317. doi: 10.1016/s0887-2333(02)00003-6. [DOI] [PubMed] [Google Scholar]
- 68.Lapins M., Arvidsson S., Lampa S., Berg A., Schaal W., Alvarsson J., Spjuth O. A confidence predictor for logD using conformal regression and a support-vector machine. J. Cheminf. 2018;10:17. doi: 10.1186/s13321-018-0271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mitragotri S. Modeling skin permeability to hydrophilic and hydrophobic solutes based on four permeation pathways. J. Contr. Release. 2003;86:69–92. doi: 10.1016/s0168-3659(02)00321-8. [DOI] [PubMed] [Google Scholar]
- 70.Wagner H., Kostka K.-H., Lehr C.-M., Schaefer U.F. pH profiles in human skin: influence of two in vitro test systems for drug delivery testing. Eur. J. Pharm. Biopharm. 2003;55:57–65. doi: 10.1016/s0939-6411(02)00125-x. [DOI] [PubMed] [Google Scholar]
- 71.Schmid-Wendtner M.H., Korting H.C. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol. Physiol. 2006;19:296–302. doi: 10.1159/000094670. [DOI] [PubMed] [Google Scholar]
- 72.Blank H.I. Measurement of pH of the skin surface. J. Invest. Dermatol. 1939;2:67–79. [Google Scholar]
- 73.Kullavanijaya P., Lim H.W. Photoprotection. J. Am. Acad. Dermatol. 2005;52:937–958. doi: 10.1016/j.jaad.2004.07.063. [DOI] [PubMed] [Google Scholar]
- 74.Brown M.B., Martin G.P., Jones S.A., Akomeah F.K. Dermal and transdermal drug delivery systems: current and future prospects. Drug Deliv. 2006;13:175–187. doi: 10.1080/10717540500455975. [DOI] [PubMed] [Google Scholar]
- 75.Patterson M.J., Galloway S.D., Nimmo M.A. Variations in regional sweat composition in normal human males. Exp. Physiol. 2000;85:869–875. doi: 10.1111/j.1469-445x.2000.02058.x. [DOI] [PubMed] [Google Scholar]
- 76.Smallegange R.C., Verhulst N.O., Takken W. Sweaty skin: an invitation to bite? Trends Parasitol. 2011;27:143–148. doi: 10.1016/j.pt.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 77.Meidan V.M. Methods for quantifying intrafollicular drug delivery: a critical appraisal. Expet Opin. Drug Deliv. 2010;7:1095–1108. doi: 10.1517/17425247.2010.503954. [DOI] [PubMed] [Google Scholar]
- 78.Lademann J., Knorr F., Richter H., Blume-Peytavi U., Vogt A., Antoniou C., Sterry W., Patzelt A. Hair follicles--an efficient storage and penetration pathway for topically applied substances. Skin Pharmacol. Physiol. 2008;21:150–155. doi: 10.1159/000131079. [DOI] [PubMed] [Google Scholar]
- 79.Genina E.A., Altshuler G.B., Tuchin V.V., Bashkatov A.N., Sinichkin Y.P., Kochubey V.I., Lakodina N.A. In vitro and in vivo study of dye diffusion into the human skin and hair follicles. J. Biomed. Opt. 2002;7:471–477. doi: 10.1117/1.1486247. [DOI] [PubMed] [Google Scholar]
- 80.Viola J.R., El-Andaloussi S., Oprea I.I., Smith C.E. Non-viral nanovectors for gene delivery: factors that govern successful therapeutics. Expet Opin. Drug Deliv. 2010;7:721–735. doi: 10.1517/17425241003716810. [DOI] [PubMed] [Google Scholar]
- 81.Otberg N., Richter H., Knuttel A., Schaefer H., Sterry W., Lademann J. Laser spectroscopic methods for the characterization of open and closed follicles. Laser Phys. Lett. 2004;1:46–49. [Google Scholar]
- 82.Vogt A., Combadiere B., Hadam S., Stieler K.M., Lademann J., Schaefer H., Autran B., Sterry W., Blume-Peytavi U. 40 nm, but not 750 or 1,500 nm, nanoparticles enter epidermal CD1a+ cells after transcutaneous application on human skin. J. Invest. Dermatol. 2006;126:1316–1322. doi: 10.1038/sj.jid.5700226. [DOI] [PubMed] [Google Scholar]
- 83.Essa E.A., Bonner M.C., Barry B.W. Human skin sandwich for assessing shunt route penetration during passive and iontophoretic drug and liposome delivery. J. Pharm. Pharmacol. 2001;54:1481–1490. doi: 10.1211/002235702135. [DOI] [PubMed] [Google Scholar]
- 84.Dokka S., Cooper S.R., Kelly S., Hardee G.E., Karras J.G. Dermal delivery of topically applied oligonucleotides via follicular transport in mouse skin. J. Invest. Dermatol. 2005;124:971–975. doi: 10.1111/j.0022-202X.2005.23672.x. [DOI] [PubMed] [Google Scholar]
- 85.Mitragotri S. Temperature dependence of skin permeability to hydrophilic and hydrophobic solutes. J. Pharm. Sci. 2007;96:1832–1839. doi: 10.1002/jps.20793. [DOI] [PubMed] [Google Scholar]
- 86.Tagami H. Location-related differences in structure and function of the stratum corneum with special emphasis on those of the facial skin. Int. J. Cosmet. Sci. 2008;30:413–434. doi: 10.1111/j.1468-2494.2008.00459.x. [DOI] [PubMed] [Google Scholar]
- 87.Marjukka Suhonen T., Bouwstra J.A., Urtti A. Chemical enhancement of percutaneous absorption in relation to stratum corneum structural alterations. J. Contr. Release. 1999;59:149–161. doi: 10.1016/s0168-3659(98)00187-4. [DOI] [PubMed] [Google Scholar]
- 88.Reed J.T., Ghadially R., Elias P.M. Skin type, but neither race nor gender, influence epidermal permeability barrier function. Arch. Dermatol. 1995;131:1134–1138. [PubMed] [Google Scholar]
- 89.Zhai H., Maibach H.I. Effects of skin occlusion on percutaneous absorption: an overview. Skin Pharmacol. Appl. Skin Physiol. 2001;14:1–10. doi: 10.1159/000056328. [DOI] [PubMed] [Google Scholar]
- 90.Moser K., Kriwet K., Froehlich C., Kalia Y.N., Guy R.H. Supersaturation: enhancement of skin penetration and permeation of a lipophilic drug. Pharm. Res. (N. Y.) 2001;18:1006–1011. doi: 10.1023/a:1010948630296. [DOI] [PubMed] [Google Scholar]
- 91.Swarbrick J., Lee G., Brom J., Gensmantel N.P. Drug permeation through human skin II: permeability of ionizable compounds. J. Pharm. Sci. 1984;73:1352–1355. doi: 10.1002/jps.2600731006. [DOI] [PubMed] [Google Scholar]
- 92.Abdul Rasool B.K., Gareeb R.H., Fahmy S.A., Abdul Rasool A.A. Meloxicam β-cyclodextrin transdermal gel: physicochemical characterization and in vitro dissolution and diffusion studies. Curr. Drug Deliv. 2011;8:381–391. doi: 10.2174/156720111795767942. [DOI] [PubMed] [Google Scholar]
- 93.Oesch F., Fabian E., Oesch-Bartlomowicz B., Werner C., Landsiedel R. Drug-metabolizing enzymes in the skin of man, rat, and pig. Drug Metab. Rev. 2007;39:659–698. doi: 10.1080/03602530701690366. [DOI] [PubMed] [Google Scholar]
- 94.Khan G.M., Hussain A., Hanif R.M. Preparation and evaluation of 5, 9-dimethyl-2-cyclopropyl-2-decanol as a penetration enhancer for drugs through rat skin. Pak. J. Pharm. Sci. 2011;24:451–457. [PubMed] [Google Scholar]
- 95.Sareen R., Kumar S., Gupta G.D. Meloxicam carbopol-based gels: characterization and evaluation. Curr. Drug Deliv. 2011;8:407–415. doi: 10.2174/156720111795768013. [DOI] [PubMed] [Google Scholar]
- 96.Aboelwafa A., El-Setouhy D., Elmeshad A. Comparative study on the effects of some polyoxyethylene alkyl ether and sorbitan fatty acid ester surfactants on the performance of transdermal carvedilol proniosomal gel using experimental design. AAPS PharmSciTech. 2010;11:1591–1602. doi: 10.1208/s12249-010-9539-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jain D., Pathak K. Design, characterization, and evaluation of meloxicam gel prepared by suspension and solution polymerization using solubility parameter as the basis for development. AAPS PharmSciTech. 2010;11:133–142. doi: 10.1208/s12249-009-9369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sankar V., Ruckmani K., Durga S., Jailani S. Proniosomes as drug carriers. Pak. J. Pharm. Sci. 2010;23:103–107. [PubMed] [Google Scholar]
- 99.Alves M.P., Scarrone A.L., Santos M., Pohlmann A.R., Guterres S.S. Human skin penetration and distribution of nimesulide from hydrophilic gels containing nanocarriers. Int. J. Pharm. 2007;341:215–220. doi: 10.1016/j.ijpharm.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 100.Letchford K., Burt H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: micelles, nanospheres, nanocapsules and polymersomes. Eur. J. Pharm. Biopharm. 2007;65:259–269. doi: 10.1016/j.ejpb.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 101.Neely B.J., Madihally S.V., Robinson R.L., Gasem K.A.M. Nonlinear quantitative structure-property relationship modeling of skin permeation coefficient. J. Pharm. Sci. 2009;98:4069–4084. doi: 10.1002/jps.21678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kataoka K., Itaka K., Nishiyama N., Yamasaki Y., Oishi M., Nagasaki Y. Smart polymeric micelles as nanocarriers for oligonucleotides and siRNA delivery. Nucleic Acids Symp. Ser. 2005;49:17–18. doi: 10.1093/nass/49.1.17. [DOI] [PubMed] [Google Scholar]
- 103.Sarker D.K. Engineering of nanoemulsions for drug delivery. Curr. Drug Deliv. 2005;2:297–310. doi: 10.2174/156720105774370267. [DOI] [PubMed] [Google Scholar]
- 104.Souto E.B., Muller R.H. Investigation of the factors influencing the incorporation of clotrimazole in SLN and NLC prepared by hot high-pressure homogenization. J. Microencapsul. 2006;23:377–388. doi: 10.1080/02652040500435295. [DOI] [PubMed] [Google Scholar]
- 105.Doktorovova S., Souto E.B., Silva A.M. Hansen solubility parameters (HSP) for prescreening formulation of solid lipid nanoparticles (SLN): in vitro testing of curcumin-loaded SLN in MCF-7 and BT-474 cell lines. Pharmaceut. Dev. Technol. 2018;23:96–105. doi: 10.1080/10837450.2017.1384491. [DOI] [PubMed] [Google Scholar]
- 106.Souto E.B., Doktorovova S., Boonme P. Lipid-based colloidal systems (nanoparticles, microemulsions) for drug delivery to the skin: materials and end-product formulations. J. Drug Deliv. Sci. Technol. 2011;21:43–54. [Google Scholar]
- 107.Das S., Ng W.K., Kanaujia P., Kim S., Tan R.B.H. Formulation design, preparation and physicochemical characterizations of solid lipid nanoparticles containing a hydrophobic drug: effects of process variables. Colloids Surf. B Biointerfaces. 2011;88:483–489. doi: 10.1016/j.colsurfb.2011.07.036. [DOI] [PubMed] [Google Scholar]
- 108.Sivaramakrishnan R., Nakamura C., Mehnert W., Korting H.C., Kramer K.D., Schäfer-Korting M. Glucocorticoid entrapment into lipid carriers — characterisation by parelectric spectroscopy and influence on dermal uptake. J. Contr. Release. 2004;97:493–502. doi: 10.1016/j.jconrel.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 109.Doktorovova S., Araujo J., Garcia M.L., Rakovsky E., Souto E.B. Formulating fluticasone propionate in novel PEG-containing nanostructured lipid carriers (PEG-NLC) Colloids Surf. B Biointerfaces. 2010;75:538–542. doi: 10.1016/j.colsurfb.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 110.Doktorovova S., Shegokar R., Rakovsky E., Gonzalez-Mira E., Lopes C.M., Silva A.M., Martins-Lopes P., Muller R.H., Souto E.B. Cationic solid lipid nanoparticles (cSLN): structure, stability and DNA binding capacity correlation studies. Int. J. Pharm. 2011;420:341–349. doi: 10.1016/j.ijpharm.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 111.Aditya N.P., Patankar S., Madhusudhan B., Murthy R.S.R., Souto E.B. Arthemeter-loaded lipid nanoparticles produced by modified thin-film hydration: pharmacokinetics, toxicological and in vivo anti-malarial activity. Eur. J. Pharmaceut. Sci. 2010;40:448–455. doi: 10.1016/j.ejps.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 112.Martins S., Tho I., Souto E., Ferreira D., Brandl M. Multivariate design for the evaluation of lipid and surfactant composition effect for optimisation of lipid nanoparticles. Eur. J. Pharmaceut. Sci. 2012 doi: 10.1016/j.ejps.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 113.Kuo Y.-C., Chung C.-Y. Solid lipid nanoparticles comprising internal Compritol 888 ATO, tripalmitin and cacao butter for encapsulating and releasing stavudine, delavirdine and saquinavir. Colloids Surf., B. 2011;88:682–690. doi: 10.1016/j.colsurfb.2011.07.060. [DOI] [PubMed] [Google Scholar]
- 114.Kuo Y.-C., Lin C.-W. Effect of electromagnetic field and surface modification on the electrical behavior of novel solid lipid nanoparticles covered with l-arginine. Colloids Surf., B. 2009;71:45–51. doi: 10.1016/j.colsurfb.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 115.Noack A., Hause G., Mäder K. Physicochemical characterization of curcuminoid-loaded solid lipid nanoparticles. Int. J. Pharm. 2011 doi: 10.1016/j.ijpharm.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 116.Zhang S.-h., Shen S.-c., Chen Z., Yun J.-x., Yao K.-j., Chen B.-b., Chen J.-z. Preparation of solid lipid nanoparticles in co-flowing microchannels. Chem. Eng. J. 2008;144:324–328. [Google Scholar]
- 117.Fangueiro J.F., Gonzalez-Mira E., Martins-Lopes P., Egea M.A., Garcia M.L., Souto S.B., Souto E.B. A novel lipid nanocarrier for insulin delivery: production, characterization and toxicity testing. Pharmaceut. Dev. Technol. 2013;18:545–549. doi: 10.3109/10837450.2011.591804. [DOI] [PubMed] [Google Scholar]
- 118.Severino P., Pinho S.C., Souto E.B., Santana M.H.A. Polymorphism, crystallinity and hydrophilic–lipophilic balance of stearic acid and stearic acid–capric/caprylic triglyceride matrices for production of stable nanoparticles. Colloids Surf. B Biointerfaces. 2011;86:125–130. doi: 10.1016/j.colsurfb.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 119.Ghadiri M., Fatemi S., Vatanara A., Doroud D., Najafabadi A.R., Darabi M., Rahimi A.A. Loading hydrophilic drug in solid lipid media as nanoparticles: statistical modeling of entrapment efficiency and particle size. Int. J. Pharm. 2012;424:128–137. doi: 10.1016/j.ijpharm.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 120.Sanna V., Caria G., Mariani A. Effect of lipid nanoparticles containing fatty alcohols having different chain length on the ex vivo skin permeability of Econazole nitrate. Powder Technol. 2010;201:32–36. [Google Scholar]
- 121.Souto E.B., Wissing S.A., Barbosa C.M., Muller R.H. Comparative study between the viscoelastic behaviors of different lipid nanoparticle formulations. J. Cosmet. Sci. 2004;55:463–471. [PubMed] [Google Scholar]
- 122.Kim B.-D., Na K., Choi H.-K. Preparation and characterization of solid lipid nanoparticles (SLN) made of cacao butter and curdlan. Eur. J. Pharmaceut. Sci. 2005;24:199–205. doi: 10.1016/j.ejps.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 123.Kheradmandnia S., Vasheghani-Farahani E., Nosrati M., Atyabi F. Preparation and characterization of ketoprofen-loaded solid lipid nanoparticles made from beeswax and carnauba wax. Nanomed. Nanotechnol. Biol. Med. 2010;6:753–759. doi: 10.1016/j.nano.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 124.Attama A.A., Müller-Goymann C.C. Effect of beeswax modification on the lipid matrix and solid lipid nanoparticle crystallinity. Colloids Surf., A. 2008;315:189–195. [Google Scholar]
- 125.Pardeike J., Weber S., Haber T., Wagner J., Zarfl H.P., Plank H., Zimmer A. Development of an itraconazole-loaded nanostructured lipid carrier (NLC) formulation for pulmonary application. Int. J. Pharm. 2011;419:329–338. doi: 10.1016/j.ijpharm.2011.07.040. [DOI] [PubMed] [Google Scholar]
- 126.Patel D., Dasgupta S., Dey S., Ramani Y.R., Ray S., Mazumder B. Nanostructured lipid carriers (NLC)-Based gel for the topical delivery of aceclofenac: preparation, characterization, and in vivo evaluation. Sci. Pharm. 2012;80:749–764. doi: 10.3797/scipharm.1202-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Muchow M., Maincent P., Muller R.H., Keck C.M. Production and characterization of testosterone undecanoate-loaded NLC for oral bioavailability enhancement. Drug Dev. Ind. Pharm. 2011;37:8–14. doi: 10.3109/03639045.2010.489559. [DOI] [PubMed] [Google Scholar]
- 128.Fang J.Y., Fang C.L., Liu C.H., Su Y.H. Lipid nanoparticles as vehicles for topical psoralen delivery: solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC) Eur. J. Pharm. Biopharm. 2008;70:633–640. doi: 10.1016/j.ejpb.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 129.González-Mira E., Nikolić S., García M.L., Egea M.A., Souto E.B., Calpena A.C. Potential use of nanostructured lipid carriers for topical delivery of flurbiprofen. J. Pharm. Sci. 2010;100:242–251. doi: 10.1002/jps.22271. [DOI] [PubMed] [Google Scholar]
- 130.Pathak P., Nagarsenker M. Formulation and evaluation of lidocaine lipid nanosystems for dermal delivery. AAPS PharmSciTech. 2009;10:985–992. doi: 10.1208/s12249-009-9287-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gokce E.H., Korkmaz E., Dellera E., Sandri G., Bonferoni M.C., Ozer O. Resveratrol-loaded solid lipid nanoparticles versus nanostructured lipid carriers: evaluation of antioxidant potential for dermal applications. Int. J. Nanomed. 2012;7:1841–1850. doi: 10.2147/IJN.S29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Souto E.B., Wissing S.A., Barbosa C.M., Mueller R.H. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int. J. Pharm. 2004;278:71–77. doi: 10.1016/j.ijpharm.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 133.Cirri M., Bragagni M., Mennini N., Mura P. Development of a new delivery system consisting in drug -“ in cyclodextrin “- in nanostructured lipid carriers for ketoprofen topical delivery. Eur. J. Pharm. Biopharm. 2012;80:46–53. doi: 10.1016/j.ejpb.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 134.Chen Y., Yuan L., Zhou L., Zhang Z.H., Cao W., Wu Q. Effect of cell-penetrating peptide-coated nanostructured lipid carriers on the oral absorption of tripterine. Int. J. Nanomed. 2012;7:4581–4591. doi: 10.2147/IJN.S34991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Teeranachaideekul V., Müller R.H., Junyaprasert V.B. Encapsulation of ascorbyl palmitate in nanostructured lipid carriers (NLC)—Effects of formulation parameters on physicochemical stability. Int. J. Pharm. 2007;340:198–206. doi: 10.1016/j.ijpharm.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 136.Lee S., Jeong J., Kim S., Lee K., Ahn B., Kang M., Choi Y. Topical formulation of retinyl retinoate employing nanostructured lipid carriers. J. Pharm. Invest. 2012;42:243–250. [Google Scholar]
- 137.Elnaggar Y.S., El-Massik M.A., Abdallah O.Y. Fabrication, appraisal, and transdermal permeation of sildenafil citrate-loaded nanostructured lipid carriers versus solid lipid nanoparticles. Int. J. Nanomed. 2011;6:3195–3205. doi: 10.2147/IJN.S25825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kumbhar D.D., Pokharkar V.B. Engineering of a nanostructured lipid carrier for the poorly water-soluble drug, bicalutamide: physicochemical investigations. Colloids Surf., A. 2013;416:32–42. [Google Scholar]
- 139.Alam M.I., Baboota S., Ahuja A., Ali M., Ali J., Sahni J.K. Intranasal administration of nanostructured lipid carriers containing CNS acting drug: pharmacodynamic studies and estimation in blood and brain. J. Psychiatr. Res. 2012;46:1133–1138. doi: 10.1016/j.jpsychires.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 140.Rosen M.J. third ed. ed. John Wiley & Sons, Inc; New Jersey, USA: 2004. Surfactants and Interfacial Phenomena. [Google Scholar]
- 141.Doktorovova S., Kovacevic A.B., Garcia M.L., Souto E.B. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: current evidence from in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2016;108:235–252. doi: 10.1016/j.ejpb.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 142.Doktorovova S., Shegokar R., Martins-Lopes P., Silva A.M., Lopes C.M., Muller R.H., Souto E.B. Modified Rose Bengal assay for surface hydrophobicity evaluation of cationic solid lipid nanoparticles (cSLN) Eur. J. Pharmaceut. Sci. 2012;45:606–612. doi: 10.1016/j.ejps.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 143.Curdy C., Kalia Y.N., Guy R.H. Post-iontophoresis recovery of human skin impedance in vivo. Eur. J. Pharm. Biopharm. 2002;53:15–21. doi: 10.1016/s0939-6411(01)00216-8. [DOI] [PubMed] [Google Scholar]
- 144.Kuo Y.-C., Chen H.-H. Entrapment and release of saquinavir using novel cationic solid lipid nanoparticles. Int. J. Pharm. 2009;365:206–213. doi: 10.1016/j.ijpharm.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 145.Pedersen N., Hansen S., Heydenreich A.V., Kristensen H.G., Poulsen H.S. Solid lipid nanoparticles can effectively bind DNA, streptavidin and biotinylated ligands. Eur. J. Pharm. Biopharm. 2006;62:155–162. doi: 10.1016/j.ejpb.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 146.Vighi E., Ruozi B., Montanari M., Battini R., Leo E. pDNA condensation capacity and in vitro gene delivery properties of cationic solid lipid nanoparticles. Int. J. Pharm. 2010;389:254–261. doi: 10.1016/j.ijpharm.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 147.Tabatt K., Kneuer C., Sameti M., Olbrich C., Müller R.H., Lehr C.-M., Bakowsky U. Transfection with different colloidal systems: comparison of solid lipid nanoparticles and liposomes. J. Contr. Release. 2004;97:321–332. doi: 10.1016/j.jconrel.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 148.Fangueiro J.F., Andreani T., Egea M.A., Garcia M.L., Souto S.B., Souto E.B. Experimental factorial design applied to mucoadhesive lipid nanoparticles via multiple emulsion process. Colloids Surf. B Biointerfaces. 2012;100:84–89. doi: 10.1016/j.colsurfb.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 149.Som I., Bhatia K., Yasir M. Status of surfactants as penetration enhancers in transdermal drug delivery. J. Pharm. BioAllied Sci. 2012;4:2–9. doi: 10.4103/0975-7406.92724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schubert M.A., Harms M., Müller-Goymann C.C. Structural investigations on lipid nanoparticles containing high amounts of lecithin. Eur. J. Pharmaceut. Sci. 2006;27:226–236. doi: 10.1016/j.ejps.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 151.Schubert M.A., Muller-Goymann C.C. Characterisation of surface-modified solid lipid nanoparticles (SLN): influence of lecithin and nonionic emulsifier. Eur. J. Pharm. Biopharm. 2005;61:77–86. doi: 10.1016/j.ejpb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 152.Dreher F., Walde P., Walther P., Wehrli E. Interaction of a lecithin microemulsion gel with human stratum corneum and its effect on transdermal transport. J. Contr. Release. 1997;45:131–140. [Google Scholar]
- 153.Campos J.R., Fernandes A.R., Sousa R., Fangueiro J.F., Boonme P., Garcia M.L., Silva A.M., Naveros B.C., Souto E.B. Optimization of nimesulide-loaded solid lipid nanoparticles (SLN) by factorial design, release profile and cytotoxicity in human Colon adenocarcinoma cell line. Pharmaceut. Dev. Technol. 2019;24:616–622. doi: 10.1080/10837450.2018.1549075. [DOI] [PubMed] [Google Scholar]
- 154.Mahant S., Rao R., Souto E.B., Nanda S. Analytical tools and evaluation strategies for nanostructured lipid carrier-based topical delivery systems. Expet Opin. Drug Deliv. 2020;17:963–992. doi: 10.1080/17425247.2020.1772750. [DOI] [PubMed] [Google Scholar]
- 155.Shimojo A.A.M., Fernandes A.R.V., Ferreira N.R.E., Sanchez-Lopez E., Santana M.H.A., Souto E.B. Evaluation of the influence of process parameters on the properties of resveratrol-loaded NLC using 2(2) full factorial design. Antioxidants. 2019:8. doi: 10.3390/antiox8080272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Souto E.B., Doktorovova S., Zielinska A., Silva A.M. Key production parameters for the development of solid lipid nanoparticles by high shear homogenization. Pharmaceut. Dev. Technol. 2019;24:1181–1185. doi: 10.1080/10837450.2019.1647235. [DOI] [PubMed] [Google Scholar]
- 157.Fernandes A.R., Ferreira N.R., Fangueiro J.F., Santos A.C., Veiga F.J., Cabral C., Silva A.M., Souto E.B. Ibuprofen nanocrystals developed by 2(2) factorial design experiment: a new approach for poorly water-soluble drugs. Saudi Pharmaceut. J. 2017;25:1117–1124. doi: 10.1016/j.jsps.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Severino P., Santana M.H., Souto E.B. Optimizing SLN and NLC by 2(2) full factorial design: effect of homogenization technique. Mater. Sci. Eng. C Mater. Biol. Appl. 2012;32:1375–1379. doi: 10.1016/j.msec.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 159.E.B. Souto, R.H. Müller, Lipid nanoparticles (SLN and NLC) for drug delivery, in: J. Domb, Y. Tabata, M.N.V.R. Kumar, S. Farber (Eds.) Nanoparticles for Pharmaceutical Applications, American Scientific Publishers2007, pp. 103-122.
- 160.Souto E.B., Almeida A.J., Müller R.H. Lipid nanoparticles (SLN®, NLC®) for cutaneous drug delivery:structure, protection and skin effects. J. Biomed. Nanotechnol. 2007;3:317–331. [Google Scholar]
- 161.Gasco M.R. Solid lipid nanospheres from warm microemulsion. Pharmaceut. Technol. Eur. 1997;9:52–58. [Google Scholar]
- 162.Fontana G., Maniscalco L., SchilIaci D., Cavallaro G., Giammona G. Preparation, characterization and in vitro antitumoral activity of solid lipid nanoparticles (SLN) containing tamoxifen. Drug Deliv. 2005;12:385–392. doi: 10.1080/10717540590968855. [DOI] [PubMed] [Google Scholar]
- 163.Patel P.A., Patravale V.B. AmbiOnp: solid lipid nanoparticles of amphotericin B for oral administration. J. Biomed. Nanotechnol. 2011;7:632–639. doi: 10.1166/jbn.2011.1332. [DOI] [PubMed] [Google Scholar]
- 164.Sinha V.R., Srivastava S., Goel H., Jindal V. Solid lipid nanoparticles (SLN´s) - trends and implications in drug targeting. Int. J. Adv. Pharmaceut. Sci. 2011;1:212–238. [Google Scholar]
- 165.Garcia-Fuentes M., Torres M.R., Alonso J.M. Design of lipid nanoparticles for the oral delivery of hydrophilic macromolecules. Colloids Surf., B. 2002;27:159–168. [Google Scholar]
- 166.Sjöström B., Bergenstahl B. Preparation of submicron drug particles in lecithin-stabilized o/w emulsions I. Model studies of the precipitation of cholestreryl acetate. Int. J. Pharm. 1992;88:53–62. [Google Scholar]
- 167.Quintanar-Guerrero D., Allémann E., Fessi H., Doelker E. Pseudolatex preparation using a novel emulsion–diffusion process involving direct displacement of partially water-miscible solvents by distillation. Int. J. Pharm. 1999;188:155–164. doi: 10.1016/s0378-5173(99)00216-1. [DOI] [PubMed] [Google Scholar]
- 168.Dong Y., Ng W.K., Shen S., Kim S., Tan R.B.H. Solid lipid nanoparticles: continuous and potential large-scale nanoprecipitation production in static mixers. Colloids Surf. B Biointerfaces. 2012;94:68–72. doi: 10.1016/j.colsurfb.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 169.Hu F.Q., Yuan H., Zhang H.H., Fang M. Preparation of solid lipid nanoparticles with clobetasol propionate by a novel solvent diffusion method in aqueous system and physicochemical characterization. Int. J. Pharm. 2002;239:121–128. doi: 10.1016/s0378-5173(02)00081-9. [DOI] [PubMed] [Google Scholar]
- 170.Videira M.A., Botelho M.F., Santos A.C., Gouveia L.F., de Lima J.J., Almeida A.J. Lymphatic uptake of pulmonary delivered radiolabelled solid lipid nanoparticles. J. Drug Target. 2002;10:607–613. doi: 10.1080/1061186021000054933. [DOI] [PubMed] [Google Scholar]
- 171.Wissing S.A., Kayser O., Muller R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004;56:1257–1272. doi: 10.1016/j.addr.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 172.Heurtault B., Saulnier P., Pech B., Proust J.E., Benoit J.P. A novel phase inversion-based process for the preparation of lipid nanocarriers. Pharm. Res. (N. Y.) 2002;19:875–880. doi: 10.1023/a:1016121319668. [DOI] [PubMed] [Google Scholar]
- 173.Battaglia L., Gallarate M., Cavalli R., Trotta M. Solid lipid nanoparticles produced through a coacervation method. J. Microencapsul. 2010;27:78–85. doi: 10.3109/02652040903031279. [DOI] [PubMed] [Google Scholar]
- 174.Corrias F., Lai F. New methods for lipid nanoparticles preparation. Recent Pat. Drug Deliv. Formulation. 2011;5:212–213. doi: 10.2174/187221111797200597. [DOI] [PubMed] [Google Scholar]
- 175.Battaglia L., Gallarate M. Expert Opin Drug Deliv; 2012. Lipid Nanoparticles: State of the Art, New Preparation Methods and Challenges in Drug Delivery; pp. 497–508. [DOI] [PubMed] [Google Scholar]
- 176.Bouwstra J.A., Honeywell-Nguyen P.L., Gooris G.S., Ponec M. Structure of the skin barrier and its modulation by vesicular formulations. Prog. Lipid Res. 2003;42:1–36. doi: 10.1016/s0163-7827(02)00028-0. [DOI] [PubMed] [Google Scholar]
- 177.Wissing S.A., Müller R.H. Cosmetic applications for solid lipid nanoparticles (SLN) Int. J. Pharm. 2003;254:65–68. doi: 10.1016/s0378-5173(02)00684-1. [DOI] [PubMed] [Google Scholar]
- 178.Wissing S.A., Müller R.H. Solid lipid nanoparticles (SLN)-a novel carrier for UV blockers. Pharmazie. 2001;56:783–786. [PubMed] [Google Scholar]
- 179.Müller R.H., Radtke M., Wissing S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002;54(Supplement):S131–S155. doi: 10.1016/s0169-409x(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 180.Jenning V., Gysler A., Schaefer-Korting M., Gohla S.H. Vitamin A loaded solid lipid nanoparticles for topical use: occlusive properties and drug targeting to the upper skin. Eur. J. Pharm. Biopharm. 2000;49:211–218. doi: 10.1016/s0939-6411(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 181.Wissing S.A., Müller R.H. The influence of the crystallinity of lipid nanoparticles on their occlusive properties. Int. J. Pharm. 2002;242:377–379. doi: 10.1016/s0378-5173(02)00220-x. [DOI] [PubMed] [Google Scholar]
- 182.de Vringer T., de Ronde H.A. Preparation and structure of a water-in-oil cream containing lipid nanoparticles. J. Pharm. Sci. 1995;84:466–472. doi: 10.1002/jps.2600840415. [DOI] [PubMed] [Google Scholar]
- 183.Souto E.B., Muller R.H. Cosmetic features and applications of lipid nanoparticles (SLN, NLC) Int. J. Cosmet. Sci. 2008;30:157–165. doi: 10.1111/j.1468-2494.2008.00433.x. [DOI] [PubMed] [Google Scholar]
- 184.Prow T.W., Grice J.E., Lin L.L., Faye R., Butler M., Becker W., Wurm E.M., Yoong C., Robertson T.A., Soyer H.P., Roberts M.S. Nanoparticles and microparticles for skin drug delivery. Adv. Drug Deliv. Rev. 2011;63:470–491. doi: 10.1016/j.addr.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 185.Freitas C., Muller R.H. Stability determination of solid lipid nanoparticles (SLN) in aqueous dispersion after addition of electrolyte. J. Microencapsul. 1999;16:59–71. doi: 10.1080/026520499289310. [DOI] [PubMed] [Google Scholar]
- 186.Wei C.C., Ge Z.Q. Influence of electrolyte and poloxamer 188 on the aggregation kinetics of solid lipid nanoparticles (SLNs) Drug Dev. Ind. Pharm. 2012;38:1084–1089. doi: 10.3109/03639045.2011.640331. [DOI] [PubMed] [Google Scholar]
- 187.Chen Y., Zhou L., Yuan L., Zhang Z.H., Liu X., Wu Q. Formulation, characterization, and evaluation of in vitro skin permeation and in vivo pharmacodynamics of surface-charged tripterine-loaded nanostructured lipid carriers. Int. J. Nanomed. 2012;7:3023–3032. doi: 10.2147/IJN.S32476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Montenegro L., Sinico C., Castangia I., Carbone C., Puglisi G. Idebenone-loaded solid lipid nanoparticles for drug delivery to the skin: in vitro evaluation. Int. J. Pharm. 2012;434:169–174. doi: 10.1016/j.ijpharm.2012.05.046. [DOI] [PubMed] [Google Scholar]
- 189.Dasgupta S., Mazumder B., Ghosh S.K., Kaurav S.S. Solid lipid nanoparticles (SLNs) for topical delivery of aceclofenac by using xanthan gum: ex vivo and in vivo evaluation. Curr. Drug Deliv. 2012 [PubMed] [Google Scholar]
- 190.Zielinska A., Ferreira N.R., Durazzo A., Lucarini M., Cicero N., Mamouni S.E., Silva A.M., Nowak I., Santini A., Souto E.B. Development and optimization of alpha-pinene-loaded solid lipid nanoparticles (SLN) using experimental factorial design and dispersion analysis. Molecules. 2019:24. doi: 10.3390/molecules24152683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zielinska A., Ferreira N.R., Feliczak-Guzik A., Nowak I., Souto E.B. Loading, release profile and accelerated stability assessment of monoterpenes-loaded solid lipid nanoparticles (SLN) Pharmaceut. Dev. Technol. 2020;25:832–844. doi: 10.1080/10837450.2020.1744008. [DOI] [PubMed] [Google Scholar]
- 192.Zielinska A., Martins-Gomes C., Ferreira N.R., Silva A.M., Nowak I., Souto E.B. Anti-inflammatory and anti-cancer activity of citral: optimization of citral-loaded solid lipid nanoparticles (SLN) using experimental factorial design and LUMiSizer(R) Int. J. Pharm. 2018;553:428–440. doi: 10.1016/j.ijpharm.2018.10.065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.