Version Changes
Revised. Amendments from Version 1
Further clarification of the study design: An analytic case-control followed by an interventional one arm clinical trial design (quasi experiment) were performed to fulfill the preset objectives. The case-control study included 89 prediabetic patients with peripheral neuropathy (Group A) and 89 prediabetics without peripheral neuropathy (Group B). Patients of group A were invited to participate in a quasi-experiment by administration of therapeutic dose of vitamin D. None of the 89 patients participating in the case-control study refused to be enrolled in the interventional study
Laboratory studies included: ionized calcium, phosphorous, PTH, and 25(OH)vitamin D, FBG, 2h-PPBG, glycated hemoglobin and total lipid profile (total cholesterol, low density lipoprotein (LDL) and triglycerides)
During the study, we found few patients with ionized calcium 0.8 mmol/L. they were 11 out of 178 prediabetic patients. They were asymptomatic (mostly due to chronic hypocalcemia) and once they were recognized to be hypocalcemic, they were given the proper management.
Table 1 is updated (removed TSH/ creatinine and FBG added)
Table 2 is updated (p=0.095 NS)
Vitamin D level showed a negative but statistically insignificant correlation with the severity of peripheral neuropathy (assessed by SF-MPQ) (r= 0.032, p= 0.766) and pain score (assessed by DN4 questionnaire) (r= 0.052, p= 0.629). Figure 1a/1b removed and discussion and conclusion are updated to new findings.
Table 4 changed ( MNB=McNemar Bowker test between patients with peripheral neuropathy before and after vitamin D injection. Paired t-test in both Short McGill and DN4 <0.001)
Underlying data DOI updated
References have been modified accordingly
Abstract
Background: Vitamin D deficiency is seen more frequently in diabetic patients with distal symmetrical polyneuropathy. Unfortunately, there is a shortage of data concerning prediabetic individuals with peripheral neuropathy (PN). Therefore, we aimed to study the association of vitamin D deficiency with PN severity and to determine the effect of vitamin D supplementation on PN in prediabetics.
Methods: A case-control study was conducted consisting of 89 prediabetic individuals with PN and a control group of prediabetics without PN, recruited from the outpatient department of the National Institute of Diabetes and Endocrinology, Cairo, Egypt. All patients were screened for PN using clinical examination and Douleur Neuropathique 4 diagnostic questionnaire (DN4). Group A (with PN) was assessed for neuropathic severity using the Short-Form McGill Pain Questionnaire (SF-MPQ). In addition, 25-hydroxyvitamin D, ionized calcium, phosphorus, parathyroid hormone (PTH), glycated hemoglobin (HbA1c), fasting blood glucose (FBG), 2-hour post 75g glucose (2h-PPBG) and lipid profile were measured for both groups. Prediabetic patients with PN were given vitamin D3 200.000 IU IM monthly for three months. After three months, clinical assessment, DN4, SF-MPQ and all laboratory measures were repeated.
Results: Vitamin D was not associated with the severity of PN patients. However, supplementation of vitamin D resulted in a highly significant improvement in glycemic parameters , p≤0.001. Interestingly, neuropathy score and severity before vitamin D supplementation were (6.4±1.6 and 28.3±7.2) and after became (2.5±0.9 and 17±6.3, p≤0.001).
Conclusion: Correction of vitamin D deficiency in prediabetics with PN as well as hypovitaminosis D, improves glycemic parameters, PN score and severity.
Keywords: prediabetes, vitamin D, peripheral neuropathy, neuropathic score
Editorial note:
After being made aware of issues with this article, the F1000Research Editorial Team worked closely with the authors’ institution, who completed a rigorous investigation of the concerns raised. Investigation was carried out by a multidisciplinary internal auditing committee formed of endocrinology and public health individuals across the Faculty of Medicine - Ain Shams University. The committee reviewed the study datasets and analyses and their findings identified various inaccuracies that require correction. In line with our article policies around permanency of content, in order to maintain the integrity and completeness of the scholarly record, F1000Research allows authors to publish revised versions, and any errors that become apparent during peer review or later can be corrected through the publication of new versions. We have worked with the article authors towards correction of the manuscript and publication of a new version. Corrections and changes relative to the previous version are clearly summarized in the ‘Amendments’ section at the start of the new version.
Introduction
Diabetes mellitus (DM), a significant world health problem, is a metabolic disease, which occurs due to a defect in insulin release and or insulin resistance 1 . Globally, the prevalence of type 2 diabetes (T2DM) is high and rising across all regions 2 .
There is a higher frequency of idiopathic polyneuropathy, small fiber neuropathy and painful sensory neuropathy among prediabetics. These findings suggest an involvement of the small unmyelinated nerve fibers that carry pain, temperature, and regulate autonomic function during prediabetes, before the development of diabetes 3 .
Vitamin D, which is a fat-soluble hormone, has multiple physiological roles, which extends far beyond calcium metabolism 4 . Vitamin D deficiency is a worldwide health problem, patients with prediabetes, T2DM, gestational diabetes and obesity represent a high-risk group 5 .
Recently, a lot of studies have been done to assess the association between vitamin D level and the diabetic peripheral neuropathy in patients with diabetes mellitus and to study the effect of vitamin D on painful neuropathy, but there is a lack of data concerning prediabetic individuals 1 .
The aim of this work was to determine the association of vitamin D deficiency with peripheral neuropathy severity and evaluate the effect of vitamin D supplementation on peripheral neuropathy in prediabetics with hypovitaminosis D.
Methods
An analytic case-control followed by an interventional one arm clinical trial design (quasi experiment) were performed to fulfill the preset objectives. The case-control study included 89 prediabetic patients with peripheral neuropathy (Group A) and 89 prediabetics without peripheral neuropathy (Group B).
Study Participants and Case Definition: Prediabetic individuals were diagnosed, according to the American Diabetes Association 2019, with impaired fasting (100–125 mg/dl) and/or impaired glucose tolerance (140–199 mg/dl), and/or glycated haemoglobin (5.7–6.4%) 6 (they were all ages between 18–60 years old). Participants were recruited from the National Institute of Diabetes and Endocrinology (NIDE), Cairo, Egypt, in the period from September 2018 to March 2019 after proven informed written consent.
Ethical Considerations: A written informed consent was obtained from all patients. All data were made confidential and an ethical approval on study conduction was obtained from the Local Research Ethical Committee (REC) of the Faculty of Medicine, Ain Shams University. FWA 000017858.
All participants were subjected to full medical history including smoking habits, alcohol consumption, drug history, thorough clinical examination including blood pressure, weight, height and BMI.
Screening for peripheral neuropathy
All participants were screened for peripheral neuropathy by 10 g monofilament for assessing the loss of protective sensation, tuning fork (vibration sense testing using a 128-Hz tuning fork), ankle reflex, pinprick (for perception of pain) and Douleur Neuropathic 4 diagnostic questionnaire (DN4) 7 that assesses symptoms reflecting pain in the form of burning, painful, cold, electric shocks, tingling, pins and needles. If the patients score is ≥4 the patient likely suffers from neuropathic pain. Patients found to have peripheral neuropathy were given the Short-Form McGill Pain Questionnaire (SF-MPQ) 8 that assesses the severity of pain; an increase in the score indicates increasing severity.
Laboratory studies
Laboratory studies included: ionized calcium, phosphorous, PTH, and 25(OH)vitamin D, FBG, 2h-PPBG, glycated hemoglobin and total lipid profile (total cholesterol, low density lipoprotein (LDL) and triglycerides)
Participants were first instructed to fast for eight hours (overnight fasting), 10 ml of venous blood were then collected by venipuncture without tourniquet. 2 ml of the collected blood were taken in an EDTA containing tube for the assay of the glycated hemoglobin and it was stored at 4°C to be carried out within one week. 2 ml were taken in a fluoride containing tube and then separated by centrifugation and the sample was used for measurement of FBG, serum Ca, phosphorus, PTH and 25(OH)vitamin D. 2 ml sample were collected two hours after 75 g oral glucose load for the measurement of the 2h-PPBG. On a separate day, 2 ml of venous blood were collected by venipuncture (after an overnight 12 hour fast), the sample was collected in a fluoride containing tube and then separated by centrifugation and used for measurement of total lipid profile (total cholesterol, low density lipoprotein (LDL), triglycerides (TG)) by enzyme colorimetric assay. Total cholesterol level was measured by Quantitative Enzymatic-Colorimetric assay (Catalogue Number: 1010/ manufacturer: Stanbio-Laboratory, Inc., USA/ Boerne, Texas/ 1/2018). Triglyceride level was measured by Quantitative Enzymatic-Colorimetric assay (Stanbio LiquiColor Triglycerides/ Catalog Number: 2100/ manufacturer: Stanbio-Laboratory, Inc., USA/ Boerne, Texas, USA/ 03/2018). LDL cholesterol can be determined as the difference between total cholesterol and the cholesterol content of the supernatant (HDL and VLDL) after precipitation of LDL fraction by polyvinyl sulphate in the presence of polyethylene-glycol monomethyl ether. Calculation LDL= Cholesterol- (HDL+ Triglyceride/5). HDL level was measured by Quantitative Enzymatic-Colorimetric assay (Stanbio HDL cholesterol/ Catalog Number: 0599/ manufacturer: Stanbio-Laboratory, Inc., USA/ Boerne, Texas, USA/ 02/2018). Serum 25-hydroxyvitamin D level was measured by an ELISA kit, which is a solid phase enzyme-linked immunosorbent assay (ELISA, Catalogue Number: 10501, Chemux Bioscience, Inc., Hayward, CA/ 10/2018). Parathormone level was measured by an ELISA kit with a normal range of 10–55 pg/ml (ELISA, Catalogue Number: KAP1481, DIAsource ImmunoAssays S.A, Nivelle, Belgium/ 2/2018). Glycated hemoglobin was measured by quantitative colorimetric determination of glycated haemoglobin in whole blood (Catalog Number: 0350/ manufacturer: Stanbio-Laboratory, Inc., Boerne, Texas, USA/ 06/2018). Fasting blood glucose, 2h-PPBG were measured by Stanbio Glucose LiquiColor (Oxidase) (Catalog Number: 1070, manufacturer: Stanbio-Laboratory, Inc., USA, Boerne, Texas, USA/ 04/2018). Vitamin D status was assessed according to Hovsepian et al., and classified as Sufficient if higher than 30ng/ml, Insufficient if between 20–29 ng/ml and Deficient if less than 20 ng/ml 9 .
Exclusion criteria
Patients with renal impairment, hypo or hyperthyroidism, patients on vitamin D supplementation or antiepileptic or any medication affecting calcium and vitamin D level, pregnant or breast-feeding females were excluded from the study.
Based on the results of previous studies, the majority of patients with and without peripheral neuropathy were found to be either insufficient or deficient as regard their level of vitamin D. The current analytical study has also demonstrated that all prediabetics with and without peripheral neuropathy were also either insufficient or deficient as regard their level of vitamin D.
Interventional Study : Patients of group A were invited to participate in a quasi-experiment by administration of therapeutic dose of vitamin D. None of the 89 patients participating in the case-control study refused to be enrolled in the interventional study. 200.000 IU of Vitamin D (cholecalciferol) were intramuscularly administered every month for three successive months to all patient with peripheral neuropathy.
Clinical assessments were repeated in the last visit after three months to assess the improvement in peripheral neuropathy in those patients. Retesting is advised after three months, as suppression of parathyroid hormone after supplementation with cholecalciferol takes at least three months and the response differs between individuals. So, most guidelines recommend repeat testing after three months 10 .
All laboratory tests were conducted at the beginning of the study and after three months of vitamin D supplementation.
Statistical analysis
Collected data were analyzed using SPSS (version 17, 2012, IBM Corporation, USA) (An open-access alternative that can perform an equivalent function is the R Stats package). The continuous quantitative variables included Age (Years), BMI (kg/m2), Systolic BP (mmHg), Diastolic BP (mmHg), HbA1c (%), 2h-PPBG (mg/dl), T. cholesterol (mg/dl), LDL (mg/dl), HDL (mg/dl), TG (mg/dl), 25 (OH) Vit D (ng/ml), Ionized Ca (mg/dl), Phosphorus (mg/dl), PTH (pg/ml) and they were described as mean and standard deviation. Student’s T-test was used to compare two independent groups (group A and group B) for quantitative data. Continuous variables before and after vitamin D intake were assessed using paired t-test. Regarding categorical/qualitative data, we measured vitamin D status (Sufficient, Insufficient, Deficient) and they were presented as numbers and percentages and the Chi-Square test was used to compare two independent groups (group A and group B) with qualitative data. Additional qualitative data that were measured were clinical examination for peripheral neuropathy using ankle reflex, tuning fork (vibration) and 10 g monofilament and they were presented as numbers and percentages and McNemar and McNemar Bowker tests were used to compared group A before and after vitamin D supplementation.
Results
Comparison between the two studied groups regarding clinical and laboratory characteristics is shown in Table 1. 20.2% of patients in group A (With PN) reported vitamin D insufficiency (N=18), while deficiency was evident in 79.8% (N=71) and none reported normal level of vitamin D. While group B (Without PN): 9 (10.1%) were insufficient, 80 (89.9%) were deficient and none were sufficient ( Table 2); with a non-significant difference in the mean level of vitamin D level between the two groups (13.957 ± 6.3603 ng/ml (group A) vs 14.594 ± 3.9318 ng/mL (group B) (P>0.05) ( Table 1).
Table 1. Comparison between the two studied prediabetic patient with (Group A) and without (Group B) peripheral Neuropathy.
| Group(A)
(N=89) |
Group (B)
(N=89) |
Sig | Student t test | |
|---|---|---|---|---|
| Mean±SD | Mean±SD | P-value | T-test | |
|
Age
(Years) |
50.416±7.9841 | 42.876±10.4183 | ≤0.001 | -5.419 |
|
BMI
(kg/m2) |
30.416±2.9573 | 30.236±3.2193 | 0.699 | -0.388 |
|
Systolic BP
(mmHg) |
131.52±10.068 | 129.82±10.784 | 0.28 | -1.08 |
|
Diastolic BP
(mmHg) |
80.28±9.925 | 74.38±8.147 | ≤0.001 | -4.33 |
|
HbA1c
(%) |
5.94±0.20 | 5.91±0.22 | 0.353 | -0.931 |
|
FBG
(mg/dl) |
101.92±15.28 | 92.02±12.00 | ≤0.001 | -4.806 |
|
2h-PPBG
(mg/dl) |
150.04±26.18 | 121.75±29.92 | ≤0.001 | -6.713 |
|
T.cholesterol
(mg/dl) |
197.730±25.55 | 191.32±30.04 | 0.127 | -1.532 |
|
LDL
(mg/dl) |
135.00±19.76 | 112.48±29.37 | ≤0.001 | -6.000 |
|
HDL
(mg/dl) |
43.70±12.70 | 55.70±15.10 | ≤0.001 | 5.7 |
|
TG
(mg/dl) |
112.58±27.88 | 115.12±21.22 | 0.495 | 0.684 |
|
25 (OH) Vit D
(ng/ml) |
13.95±6.36 | 14.59±3.93 | 0.423 | 0.804 |
|
Ionized Ca
(mg/dl) |
4.591±0.4220 | 4.244±0.5114 | ≤ 0.001 | -4.940 |
|
Phosphorus
(mg/dl) |
3.434±0.1167 | 3.582±0.2203 | ≤ 0.001 | 5.612 |
|
PTH
(pg/ml) |
80.22±18.07 | 72.34±16.67 | ≤ 0.05 | -3.021 |
BMI= body mass index, BP= blood pressure, HbA1c = glycated haemoglobin, 2h-PPBG = 2 hour post 75g glucose, T.cholesterol = total cholesterol, LDL = low density lipoprotein, , HDL = high density lipoprotein, TG = serum triglycerides, 25 (OH) Vit D= 25 hydroxy Vitamin D, Ionized Ca= ionized calcium, PTH = serum parathyroid hormone.
Table 2. Vitamin D status among the studied groups.
| Vitamin D status | Total | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sufficient | Insufficient | Deficient | |||||||
| Number | % | Number | % | Number | % | Number | % | ||
| Group (A) | 0 | 0% | 18 | 20.2 | 71 | 79.8 | 89 | 100 | 0.095 NS |
| Group (B) | 0 | 0% | 9 | 10.1 | 80 | 89.9 | 89 | 100 | |
| Total | 0 | 0% | 27 | 15 | 151 | 85 | 178 | 100 | |
*Group A= Prediabetic patients with peripheral neuropathy
Group B= Prediabetic patients without peripheral neuropathy
Vitamin D level showed a negative but statistically insignificant correlation with the severity of peripheral neuropathy (assessed by SF-MPQ) (r= 0.032, p= 0.766) and pain score (assessed by DN4 questionnaire) (r= 0.052, p= 0.629)
After vitamin D supplementation
There was a highly significant improvement in vitamin D level in group (A) after intramuscular injection of vitamin D, from which 42 (47%) prediabetic patients became sufficient, 38 (42.7%) became insufficient and only 9 (10.1%) remained deficient (P≤0.001). There was a highly significant improvement of glycemic profile as shown in ( Table 3).
Table 3. Comparison of prediabetic patients with PN before and after Vitamin D Supplementation (Group A).
| Group (A) before
vitamin D (N=89) |
Group (A) after
vitamin D (N=89) |
Sig. | Paired
t test |
|
|---|---|---|---|---|
| Mean±SD | Mean±SD | P-value | t-test | |
|
HbA1C
(%) |
5.94±0.20 | 5.707±0.33 | ≤ 0.001 | 7.45 |
|
FBG
(mg/dl) |
101.921±15.2831 | 92.258±15.2722 | ≤ 0.001 | 6.203 |
|
2h-75g glucose
(mg/dl) |
150.045±26.1816 | 102.000±16.7359 | ≤ 0.001 | 17.309 |
*HbA1c = glycated haemoglobin, FBG= fasting blood glucose, 2h-75g glucose=2-hour post 75g glucose
Neuropathic pain score and its severity
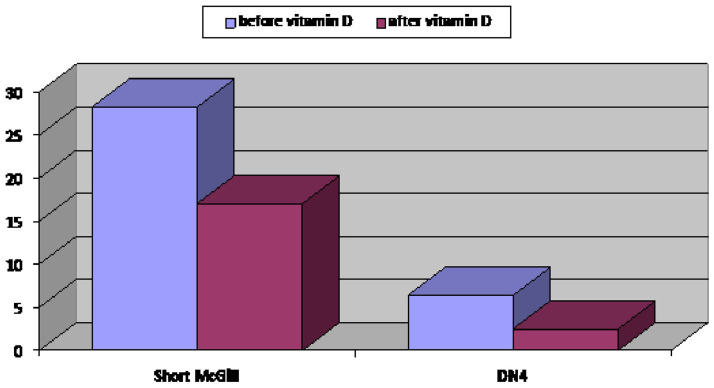
A statistically significant improvement in neuropathic pain severity was observed by the SF-MPQ (28.3±7.2 before and 17±6.3 after vitamin D supplementation, P≤0.001). There was also a statistically significant reduction in the DN4 questionnaire score from 6.39 ±1.64 to 2.5 ± 0.9, ≤0.001 (≥4 denote neuropathic pain) with an improvement of neuropathic pain in 82% of patients (73 out of 89) (P≤0.001) ( Figure 1).
Figure 1. Neuropathic pain score assessed by Douleur Neuropathic 4 score and severity of peripheral neuropathy assessed by the Short-Form McGill Pain Questionnaire score of group A (prediabetic patients with peripheral neuropathy) before and after vitamin D supplementation.
Clinical examination for peripheral neuropathy
We found a highly significant improvement in vibration sense by tuning fork and protective sense measured by the 10 g monofilament test (P≤0.001), while there was no improvement regarding ankle reflex (P>0.05) ( Table 4).
Table 4. Peripheral neuropathy indicators (ankle reflex, tuning fork vibration and 10 g monofilament) in group A (Prediabetics with PN) before and after vitamin D injection.
| Before | After | MNB | |||
|---|---|---|---|---|---|
| Ankle reflex | P value | ||||
| Ankle reflex |
Absent
N (%) |
Present
N (%) |
NS | ||
| Absent | 1 (100.0) | 0 (0.0) | |||
| Present | 0 (0.0) | 88 (100.0) | |||
| Vibration | |||||
| Vibration |
Absent
N (%) |
Reduced
N (%) |
Present
N (%) |
47.0
<0.001 |
|
| Absent | 2 (100.0) | 29 (85.3) | 12 (22.6) | ||
| Reduced | 0 (0.0) | 5 (14.7) | 6 (11.3) | ||
| Present | 0 (0.0) | 0 (0.0) | 35 (66.0) | ||
| Monofilament After | |||||
| Monofilament |
Absent
N (%) |
Reduced
N (%) |
Present
N (%) |
68.0
<0.001 |
|
| Absent | 6 (100.0) | 35 (92.1) | 27 (60.0) | ||
| Normal | 0 (0.0) | 0 (0.0) | 12 (26.7) | ||
| Reduced | 0 (0.0) | 3 (7.9) | 6 (13.3) | ||
MNB=McNemar Bowker test between patients with peripheral neuropathy before and after vitamin D injection
Paired t-test in both Short McGill and DN4 <0.001
Discussion
Diabetic peripheral neuropathy in recently diagnosed diabetic patients may reach about 8% and more than 50% in patients with long-standing diabetes 11 . Recently, the American Diabetes Association stated that there is no strong evidence that supports the lifestyle management or efficacy of glycemic control in the treatment of neuropathic pain, which means that pharmaceutical interventions such as pregabalin, duloxetine, or tapentadol are the only way of treatment 12 . Accordingly, we aimed to demonstrate the association of vitamin D status with peripheral neuropathy and determine the effect of vitamin D supplementation on painful neuropathy in prediabetics.
Even with our sunny country, none of our patients (0%) had sufficient vitamin D level.
Kuchay et al., 2015 in their study found that prediabetes patients were 54.3% vitamin D deficient, 21.3% were insufficient and only 24.4% were sufficient despite abundant sunshine in India 13 .
Kuchay et al., (2015) demonstrated an association between vitamin D status and prevalence of diabetes, with low prevalence in people with high vitamin D status and a belief that a serum 25(OH) vitamin D level of 15 ng/mL or less may be a threshold at which vitamin D deficiency confers negative effect on insulin sensitivity 13 . This was confirmed when nearly 50% of patients with prediabetes had serum 25(OH) vitamin D levels below 15 ng/mL 13 . On the contrary, Rolim et al., (2016) found the association between HbA1c and 25(OH) vitamin D controversial and glycemic control was not associated with vitamin D level 14 . Luo et al., (2009) stated that there was no impact of hypovitaminosis D on metabolic syndrome status and HbA1c 15 .
The association between vitamin D status and prevalence of diabetes can be explained through the effect of vitamin D on pancreatic β‐cell function and plasma calcium. Vitamin D deficiency decreases serum calcium, which regulates insulin synthesis and release 16 .
On the other hand, administration of vitamin D causes increase in serum calcium, decrease in circulating free fatty acid levels, increase in insulin release and improvement in glucose levels 17 .
Shehab et al., (2012) study on 210 diabetic patients, from which 87 had peripheral neuropathy, first found that vitamin D deficiency was significantly associated with diabetic peripheral neuropathy 18 . In agreement with Shillo et al., (2019) who reported that serum vitamin D levels were lower in patients with painful DPN than in those with painless DPN, and pain scores were negatively correlated with serum vitamin D levels 19 .
On the contrary, Basit et al., (2016) acknowledged that there was no significant correlation between 25 (OH) vitamin D status with either total McGill pain location, McGill pain score, DN4 or positive symptoms 20 and this was inconsistent with the present study. Studies by Usluogullari et al., (2015) also found no difference in the prevalence of vitamin D deficiency between diabetic peripheral neuropathy patients and controls 21 .
However, Shehab et al., (2012) study confirmed that vitamin D was the only independent risk factor for diabetic peripheral neuropathy 18 . While in China, He et al., (2017) declared that deficiency of vitamin D is an independent risk factor for diabetic peripheral neuropathy and can be considered a potential biomarker for peripheral neuropathy in diabetic Chinese patients 22 .
On the other hand, Alkhatatbeh et al., (2019) showed that the only significant predictor for neuropathic pain was female gender, while vitamin D level, BMI, age, FBG, duration of T2DM, DBP and SBP were not 23 . The divergence in the results of previous studies may be due to the use of different methods to assess neuropathy and because the studies were directed on different populations.
Injection of vitamin D 200.000 IU intramuscular every month for three successive months is in accordance with the guidelines for vitamin D supplementation and treatment of deficiency in Central Europe individuals with proved vitamin D deficiency which require higher doses of vitamin D than doses recommended for the general population. The therapeutic dose in severe deficiency should be 1.000–10.000 IU/day (~50.000 IU/week), depending on the patient’s body weight and age. The duration of the treatment varies from 1–3 months, depending on the degree of vitamin D deficiency 24 . Our patients showed significant improvement and reduction in neuropathy severity score and also showed clinical improvement by monofilament and tuning fork. This is in line with Bell (2012) who found great improvement in neuropathic symptoms after supplementation with 50.000 IU of vitamin D 2 every week in a case report of a patient suffering from diabetic peripheral neuropathy. The patient had been refractory to different types of treatment like tricyclic's, gabapentin, oxycodone and pregabalin 25 . As well, Shehab et al., (2015) in their study applied vitamin D replacement therapy as a single intramuscular vitamin D dose of 300.000 IU and this application significantly enhanced the DN4 questionnaire scores of the patients with diabetic neuropathy 26 . Correspondingly, Lee and Chen (2008) showed that oral cholecalciferol resulted in an approximate 50% reduction in painful neuropathic symptoms and a significant reduction in SF-MPQ score from 32.1 to 19.4; however, this study had neither a placebo group nor was randomized, leaving it open to considerable bias 27 .
Possible explanation of previous studies was demonstrated in vitro by Fukuoka et al., (2001) and in vivo by Riaz et al., (1999) who considered vitamin D as a neurotrophic substance, which modulates neuronal growth and differentiation, and neuromuscular functions 28, 29 . Its exact role in diabetic neuropathic pain is uncertain; insufficiency of vitamin D may increase damage of diabetic nerve and may affect the function of nociceptors leading to pain at a higher threshold of serum 25 (OH) vitamin D concentration higher than that in the non-diabetic individuals 27 .
Therefore, the results of previous studies corroborate our findings that vitamin D supplementation improves peripheral neuropathy and can be used as a safe treatment for peripheral neuropathy in prediabetic patients with hypovitaminosis D.
Opposing previous results, a study by Alam et al., (2016) reported no significant decrease in neuropathic pain scores after vitamin D administration 30 . This study was based on all or none values instead of assessing the quantity of pain score, which may have led to a failure of observing a reduction in pain scoring.
Glycemic parameters of our patients showed significant improvement after the administration of 200.000 IU of vitamin D every four weeks for 12 weeks, which was the same result found by Kuchay et al., (2015) who revealed that correcting vitamin D deficiency in people with prediabetes significantly reduces FBG, two hours plasma glucose and A1C levels in 12 months 13 . However, contrary to our findings, He et al., (2018) proclaimed in their meta-analysis that vitamin D supplementation did not improve fasting glucose levels or insulin resistance, nor did it prevent T2DM in non-diabetics 31 . Furthermore, Moreira-Lucas et al., (2017) confirmed that vitamin D supplementation did not improve fasting or post challenge measures of insulin sensitivity, β-cell function or HbA1c 32 .
Among the limitations of the study were a small sample size compared to previous studies. Our study is the first to discuss the effect of vitamin D supplementation on peripheral neuropathy in prediabetic individuals whereas other studies have discussed the effect on diabetic patients. Finding prediabetic participants with peripheral neuropathy to include in the study was challenging.
Conclusion
This study found that vitamin D supplementation in prediabetics with peripheral neuropathy and hypovitaminosis D improves neuropathy in those patients as assessed by McGill and DN4 scores, as well as glycemic parameter namely HbA1c, FBG and 2h-PPG.
Data availability
Underlying data
Figshare: Underlying data for ‘The impact of vitamin D supplementation on peripheral neuropathy in a sample of Egyptian prediabetic individuals’, https://doi.org/10.6084/m9.figshare.16831858 33
This project contains the following underlying data:
Data file 1: prediabetic patients without peripheral neuropathy and their descriptive and laboratory data.
Data file 2: prediabetic with peripheral neuropathy and their descriptive, laboratory data, McGill Pain Questionnaire, clinical examination for neuropathy before vitamin D supplementation.
Data file 3: prediabetic with peripheral neuropathy and their descriptive and laboratory data, McGill Pain Questionnaire, clinical examination for neuropathy after Vitamin D supplementation.
Consent statement
Written informed consent was obtained from all individual participants included in our study.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 2; peer review: 2 approved]
References
- 1. Qu GB, Wang LL, Tang X, et al. : The association between vitamin D level and diabetic peripheral neuropathy in patients with type 2 diabetes mellitus: An update systematic review and meta-analysis. J Clin Transl Endocrinol. 2017;9:25–31. 10.1016/j.jcte.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. International Diabetes Federation: IDF diabetes atlas 9th edition.2019. Reference Source [Google Scholar]
- 3. Nebuchennykh M, Løseth S, Jorde R, et al. : Idiopathic polyneuropathy and impaired glucose metabolism in a Norwegian patient series. Eur J Neurol. 2008;15(8):810–816. 10.1111/j.1468-1331.2008.02197.x [DOI] [PubMed] [Google Scholar]
- 4. Holick MF: Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. 10.1056/NEJMra070553 [DOI] [PubMed] [Google Scholar]
- 5. Muñoz-Garach A, García-Fontana B, Muñoz-Torres M: Vitamin D Status, Calcium Intake and Risk of Developing Type 2 Diabetes: An Unresolved Issue. Nutrients. 2019;11(3):642. 10.3390/nu11030642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. American Diabetes Association: Criteria for testing for diabetes or prediabetes in asymptomatic adults. American Diabetes Association. 2019;42(Supp):1–17. [Google Scholar]
- 7. Bouhassira D, Attal N, Alchaar H, et al. : Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005;114(1–2):29–36. 10.1016/j.pain.2004.12.010 [DOI] [PubMed] [Google Scholar]
- 8. Hawker GA, Mian S, Kendzerska T, et al. : Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken). 2011;63 Suppl 11:S240–52. 10.1002/acr.20543 [DOI] [PubMed] [Google Scholar]
- 9. Hovsepian S, Amini M, Aminorroaya A, et al. : Prevalence of vitamin D deficiency among adult population of Isfahan City, Iran. J Health Popul Nutr. 2011;29(2):149–55. 10.3329/jhpn.v29i2.7857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Glendenning P: Measuring vitamin D. Aust Prescr. 2015;38(1):12–15. 10.18773/austprescr.2015.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oraby MI, Srie MA, Abdelshafy S, et al. : Diabetic peripheral neuropathy: the potential role of vitamin D deficiency. Egypt J Neurol Psychiatr Neurosurg. 2019;55:10. 10.1186/s41983-019-0058-y [DOI] [Google Scholar]
- 12. American Diabetes Association: 11. Microvascular complications and foot care: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43(Suppl 1):135–151. 10.2337/dc20-S011 [DOI] [PubMed] [Google Scholar]
- 13. Kuchay MS, Laway BA, Bashir MI, et al. : Effect of vitamin D supplementation on glycemic parameters and progression of prediabetes to diabetes: a 1-year, open-label randomized study. Indian J Endocrinol Metab. 2015;19(3):387–92. 10.4103/2230-8210.152783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rolim MC, Santos BM, Conceição G, et al. : Relationship between vitamin D status, glycemic control and cardiovascular risk factors in Brazilians with type 2 diabetes mellitus. Diabetol Metab Syndr. 2016;8:77. 10.1186/s13098-016-0188-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luo C, Wong J, Brown M, et al. : Hypovitaminosis D in Chinese type 2 diabetes: lack of impact on clinical metabolic status and biomarkers of cellular inflammation. Diab Vasc Dis Res. 2009;6(3):194–9. 10.1177/1479164109337974 [DOI] [PubMed] [Google Scholar]
- 16. Palomer X, González-Clemente JM, Blanco-Vaca F, et al. : Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obes Metab. 2008;10(3):185–197. 10.1111/j.1463-1326.2007.00710.x [DOI] [PubMed] [Google Scholar]
- 17. Alvarez JA, Ashraf A: Role of vitamin D in insulin secretion and insulin sensitivity for glucose homeostasis. Int J Endocrinol. 2010;2010:351385. 10.1155/2010/351385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shehab D, Al-Jarallah K, Mojiminiyi OA, et al. : Does Vitamin D deficiency play a role in peripheral neuropathy in Type 2 diabetes? Diabet Med. 2012;29(1):43–49. 10.1111/j.1464-5491.2011.03510.x [DOI] [PubMed] [Google Scholar]
- 19. Shillo P, Selvarajah PD, Greig M, et al. : Reduced vitamin D levels in painful diabetic peripheral neuropathy. Diabet Med. 2019;36(1):44–51. 10.1111/dme.13798 [DOI] [PubMed] [Google Scholar]
- 20. Basit A, Basit KA, Fawwad A, et al. : Vitamin D for the treatment of painful diabetic neuropathy. BMJ Open Diabetes Res Care. 2016;4(1):e000148. 10.1136/bmjdrc-2015-000148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Usluogullari CA, Balkan F, Caner S, et al. : The relationship between microvascular complications and vitamin D deficiency in type 2 diabetes mellitus. BMC Endocr Disord. 2015;15:33. 10.1186/s12902-015-0029-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. He R, Hu Y, Zeng H, et al. : Vitamin D deficiency increases the risk of peripheral neuropathy in Chinese patients with type 2 diabetes. Diabetes Metab Res Rev. 2017;33(2). 10.1002/dmrr.2820 [DOI] [PubMed] [Google Scholar]
- 23. Alkhatatbeh M, Abdul-Razzak KK: Neuropathic pain is not associated with serum vitamin D but is associated with female gender in patients with type 2 diabetes mellitus. BMJ Open Diabetes Res Care. 2019;7(1):e000690. 10.1136/bmjdrc-2019-000690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Płudowski P, Karczmarewicz E, Bayer M, et al. : Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe - recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol Pol. 2013;64(4):319–27. 10.5603/ep.2013.0012 [DOI] [PubMed] [Google Scholar]
- 25. Bell DS: Reversal of the symptoms of diabetic neuropathy through correction of vitamin D deficiency in a type 1 diabetic patient. Case Rep Endocrinol. 2012;2012:165056. 10.1155/2012/165056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shehab D, Al-Jarallah K, Abdella N, et al. : Prospective evaluation of the effect of short-term oral vitamin d supplementation on peripheral neuropathy in type 2 diabetes mellitus. Med Princ Pract. 2015;24(3):250–256. 10.1159/000375304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee P, Chen R: Vitamin D as an analgesic for patients with type 2 diabetes and neuropathic pain. Arch Intern Med. 2008;168(7):771–772. 10.1001/archinte.168.7.771 [DOI] [PubMed] [Google Scholar]
- 28. Fukuoka M, Sakurai K, Ohta T, et al. : Tacalcitol, an active vitamin D3, induces nerve growth factor production in human epidermal keratinocytes. Skin Pharmacol Appl Skin Physiol. 2001;14(4):226–33. 10.1159/000056351 [DOI] [PubMed] [Google Scholar]
- 29. Riaz S, Malcangio M, Miller M, et al. : A vitamin D(3) derivative (CB1093) induces nerve growth factor and prevents neurotrophic deficits in streptozotocin-diabetic rats. Diabetologia. 1999;42(11):1308–1313. 10.1007/s001250051443 [DOI] [PubMed] [Google Scholar]
- 30. Alam U, Arul-Devah V, Javed S, et al. : Vitamin D and diabetic complications: true or false prophet? Diabetes Ther. 2016;7(1):11–26. 10.1007/s13300-016-0159-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. He S, Yu S, Zhou Z, et al. : Effect of vitamin D supplementation on fasting plasma glucose, insulin resistance and prevention of type 2 diabetes mellitus in non-diabetics: A systematic review and meta-analysis. Biomed Rep. 2018;8(5):475–84. 10.3892/br.2018.1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moreira-Lucas TS, Duncan AM, Rabasa-Lhoret R, et al. : Effect of vitamin D supplementation on oral glucose tolerance in individuals with low vitamin D status and increased risk for developing type 2 diabetes (EVIDENCE): A double-blind, randomized, placebo-controlled clinical trial. Diabetes Obes Metab. 2017;19(1):133–41. 10.1111/dom.12794 [DOI] [PubMed] [Google Scholar]
- 33. Halawa MR, Ahmed IZ, Abouelezz NF, et al. : The impact of vitamin D supplementation on peripheral neuropathy in a sample of Egyptian prediabetic individuals. Figshare. Dataset,2021. 10.6084/m9.figshare.16831858 [DOI] [PMC free article] [PubMed] [Google Scholar]