This genetic association study uses next-generation sequencing technology to assess the prevalence of deleterious sequence variations in patients with pancreatic ductal adenocarcinoma in China.
Key Points
Question
What is the prevalence of germline sequence variations among patients with pancreatic ductal adenocarcinoma (PDAC) in China?
Findings
In this genetic association study of 1009 Chinese patients with PDAC and 885 with non-PDAC diseases, pathogenic sequence variations were detected in 6.2% of patients with PDAC; SPINK1 and CFTR variations were associated with higher risk of PDAC. Variations in the pancreatic secretory enzyme genes CPA1 and CPB1 were not detected in the study cohort.
Meaning
In this study, sporadic pancreatic cancer in a cohort from China was associated with pathogenic germline variations.
Abstract
Importance
A higher incidence of pancreatic cancer has been reported in the Chinese population compared with the White population, but genetic differences are unknown to date. Large-sample germline testing for both familial and sporadic pancreatic cancers has been conducted predominantly in White populations, whereas similar studies in Chinese populations are limited.
Objective
To assess the prevalence of germline sequence variations in patients with pancreatic diseases in China.
Design, Setting, and Participants
This genetic association study was a case series that included genetic data from patients with pancreatic ductal adenocarcinoma (PDAC) or non-PDAC pancreatic diseases seen at The First Affiliated Hospital of Nanjing Medical University in Nanjing, China, between January 2006 and December 2017 (Nanjing cohort). Comparator group data were obtained for a US cohort from Johns Hopkins Hospital (JHH), a population from East Asia from the Exome Aggregation Consortium (ExAC) database, and the larger population from China from the ChinaMAP database. Data were updated and analyzed in July 2021.
Main Outcomes and Measures
Next-generation sequencing technology was used to examine the prevalence of deleterious variations in 59 genes of the included Chinese patients with DNA extracted from peripheral blood samples. The Fisher exact test was used to assess differences among the frequencies of germline variations in the study patients vs the comparator groups.
Results
A total of 1009 patients with PDAC (627 [62.1%] male; mean [SD] age, 62.8 [10.2] years) and 885 with non-PDAC diseases (477 [53.9%] male; mean [SD] age, 52.0 [15.9] years) from the Nanjing cohort were included for genetic analysis; all were Han Chinese individuals. Pathogenic variations were detected in 63 patients with PDAC (6.2%; 95% CI, 4.7%-7.7%). Variations in BRCA2 (odds ratio [OR], 3.2; 95% CI, 1.4-7.7; P = .008) and PALB2 (OR, 5.2; 95% CI, 1.6-17.0; P = .007) were significantly associated with pancreatic risk in the Nanjing cohort. Pathogenic variants of genes associated with homologous recombination DNA damage repair, including ATM, BRCA1/2, PALB2, BRIP1, FANCA, FANCC, RAD51D, and XRCC2, were found in 34 patients with PDAC (3.4%). No Ashkenazi Jewish–specific BRCA2 variation (p.Ser1982fs) was detected. The odds ratio of a SPINK1 variation in patients with PDAC was 3.2 (95% CI, 1.8-5.7; P < .001) in the Nanjing cohort compared with the ExAC cohort. Variations in the pancreatic secretory enzyme genes CPA1 and CPB1 were not detected in the Nanjing cohort.
Conclusions and Relevance
In this genetic association study, sporadic pancreatic cancer was associated with pathogenic germline variations in a cohort from China. These findings provide insights into the genetic background of pancreatic cancer in the Han Chinese population with PDAC.
Introduction
Pancreatic cancer is a deadly disease with poor prognoses. There are approximately 95 000 new cases of pancreatic ductal adenocarcinoma (PDAC) per year in China, making it the sixth leading cause of cancer-related death in China.1 Despite improvements in surgical and systemic treatment, the 5-year survival rate remains only 9%.1 In its early stages, pancreatic cancer is largely asymptomatic, and approximately 80% of patients are not diagnosed until the cancer has reached an advanced stage that limits therapeutic options.2 Therefore, the early detection of pancreatic cancer is essential to improvement in its prognosis.
Pathogenic germline sequence variations in patients with pancreatic cancer mainly occur in patients with familial pancreatic cancer; however, only approximately 10% of patients with pancreatic cancer have an associated family history.3 Carriers of variations may have access to more targeted drugs for cancer-associated genes.4 Previous studies5,6 showed that BRCA carriers from the US, mainly White individuals with the BRCA1/2 variation with sporadic PDAC, had worse survival after pancreatectomy than did their counterparts with wild-type BRCA. These BRCA1/2 carriers also had markedly improved survival if treated with platinum-based chemotherapy.5,6 In addition, the Pancreas Cancer Olaparib Ongoing trial7 showed that patients with metastatic cancer who had germline BRCA1/2 variations tended to have improved progression-free survival when treated with maintenance olaparib after first-line platinum-based chemotherapy.
A study8 in 2019 showed that native Hawaiian (relative risk [RR], 1.60; 95% CI, 1.30-1.98), Japanese American (RR, 1.33; 95% CI, 1.15-1.54), and African American (RR, 1.20; 95% CI, 1.01-1.42) individuals had a higher risk of developing PDAC compared with non-Hispanic White individuals in the US. However, the reports of germline variants for PDAC were mostly from the non-Hispanic White population. The prevalence of germline variations in the Chinese population and racial and ethnic disparities of germline variations between Chinese patients and the US population are unknown. We conducted a study using targeted next-generation sequencing technology to assess the prevalence of deleterious variations in Chinese patients and explored whether there were differences in sequence variations associated with pancreatic cancer between the Nanjing cohort and cohorts from the US, East Asia, and a larger sample from China.
Methods
Patients and Specimens
This genetic association study included patients seen at the Pancreas Center of The First Affiliated Hospital of Nanjing Medical University in Nanjing, China, between January 2006 and December 2017. All peripheral blood samples for DNA extraction were collected and stored in the center’s Pancreas Biobank after written informed consent was obtained from participants.9 Samples were collected preoperatively in the operation room or at the clinic visit. All the available samples in the Pancreas Biobank during the study period were included. All patients in the Nanjing cohort were from the Han Chinese population. Personal and family history was obtained from medical records. This study was approved by the institutional review board at The First Affiliated Hospital of Nanjing Medical University and followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) and Strengthening the Reporting of Genetic Association Studies (STREGA) reporting guidelines.
DNA Isolation
According to the manufacturer’s instructions, genomic DNA was extracted from the peripheral blood samples obtained from all patients using the QIAamp DNA Mini Kit (QIAGEN). The DNA samples were quantified using Qubit assay kits and a Qubit fluorometer (ThermoFisher Scientific).
Targeted DNA Sequencing
Fifty-nine genes were sequenced using a 59-gene AmpliSeq custom panel (ThermoFisher Scientific). Next-generation sequencing was performed using the Ion GeneStudio S5 System (ThermoFisher Scientific) according to the manufacturer’s protocol. These genes were either known pancreatic cancer susceptibility genes, known cancer susceptibility genes, or candidate pancreatic cancer susceptibility genes. Sixteen of these genes (ARID1A, ATM, BRCA1/2, PALB2, BRIP1, ERCC4, FANCA, FANCC, FANCG, FANCL, FANCJ, RAD51C, RAD51D, RECQL4, XRCC2, and XRCC3) are associated with the homologous recombination DNA damage repair (HR-DDR) pathway. Pancreatitis-associated genes were also included. Amplicon coverage is shown in eTable 1 in the Supplement. Next-generation sequencing details are described in the eAppendix in the Supplement. Deleterious variations were compared with those in the ChinaMAP database,10 which included whole-genome sequencing data for 10 588 individuals from the population of China.11
Sanger Sequencing
We downloaded the target sequence from the University of California, Santa Cruz, Genome Browser,12 inputted the target sequence into Primer Premier 5 software (PREMIER Biosoft), and selected the appropriate and synthesized primers for the Sanger sequencing. All deleterious variations were confirmed and validated by the ABI3730 sequencer (ThermoFisher Scientific). The Sanger sequencing primer list is shown in eTable 2 in the Supplement.
Statistical Analysis
Analyses were conducted using SPSS, version 21.0 (IBM). The 95% CIs for the percentages of samples with a germline variation were calculated with the modified Wald method. Because of the small number of patients with germline variations, we used the Fisher exact test to assess the difference between the frequencies of germline variations in the study patients and in individuals without cancer in the population from East Asia in the Exome Aggregation Consortium (ExAC)13 database, US patients with sporadic PDAC from Johns Hopkins Hospital (JHH),5 and individuals without pancreatic cancer from the population of China from the ChinaMAP database. Odds ratios (ORs), SEs, and 95% CIs were calculated according to Altman.14 In the JHH cohort,5 patients with a pathologic diagnosis of PDAC were sequenced with a germline panel via the same platform used in this study. A cohort with pancreatic cancer from The Cancer Genome Atlas (TCGA) with sequence variation data was also used as comparison group. Two-tailed P < .05 was considered to be statistically significant. Bonferroni correction was used when multiple testing was performed. Data were updated and analyzed in July 2021.
Results
Patient Characteristics
In the Nanjing cohort, 1009 patients were diagnosed with PDAC (627 [62.1%] male; mean [SD] age, 62.8 [10.2] years) and 885 patients were diagnosed with non-PDAC diseases (477 [53.9%] male; mean [SD] age, 52.0 [15.9] years), including pancreatic neuroendocrine tumors (119 [13.4%]), gastrointestinal stromal tumors (11 [1.2%]), acute (355 [37.8%]) or chronic pancreatitis (98 [11.1%]), intraductal papillary mucinous neoplasms (IPMNs) (70 [7.9%]), mucinous cystic neoplasms (44 [5.0%]), serous cystic neoplasms (32 [3.6%]), solid pseudopapillary tumors (56 [6.3%]), ampullary carcinoma (31 [3.5%]), cholangiocarcinoma (35 [3.9%]), and duodenal adenocarcinoma (34 [3.8%]) (Table 1). Only 9 of the patients with PDAC (0.9%) had at least 1 first-degree relative diagnosed with pancreatic cancer, and 1000 patients (99.1%) had sporadic pancreatic cancer. Thirty-two patients (3.2%) had a history of other tumors, such as breast, prostate, or colon cancer.
Table 1. Characteristics of Patients in the Nanjing and JHH Cohortsa.
| Characteristic | Nanjing cohort | JHH cohort | |||
|---|---|---|---|---|---|
| Patients with PDAC (n = 1009) | Patients with non-PDAC diseases (n = 885) | Patients who underwent pancreatectomy (n = 939) | Patients with PDAC (n = 854) | Patients with non-PDAC diseases (n = 339) | |
| Age, y | |||||
| Mean (SD) | 62.8 (10.2) | 52.0 (15.9) | NA | 65.0 (10.9) | 60.1 (14.1) |
| Median (range) | 65 (16-82) | 65 (10-85) | NA | NA | NA |
| Sex | |||||
| Female | 382 (37.9) | 408 (46.1) | NA | 399 (46.7) | 158 (46.6) |
| Male | 627 (62.1) | 477 (53.9) | NA | 455 (53.3) | 181 (53.4) |
| Disease | |||||
| PDAC | 690 (68.4) | NA | NA | 854 (100) | NA |
| Acute pancreatitis | NA | 355 (37.8) | 0 | NA | NA |
| Chronic pancreatitis | NA | 98 (11.1) | 65 (6.9) | NA | NA |
| Ampullary carcinoma | NA | 31 (3.5) | 29 (3.1) | NA | NA |
| Cholangiocarcinoma | NA | 35 (3.9) | 26 (2.8) | NA | 47 (13.9) |
| Duodenal adenocarcinoma | NA | 34 (3.8) | 32 (3.4) | NA | 54 (15.9) |
| Pancreatic neuroendocrine tumor | NA | 119 (13.4) | 112 (11.9) | NA | NA |
| Gastrointestinal stromal tumor | NA | 11 (1.2) | 9 (1.0) | NA | NA |
| Intraductal papillary mucinous neoplasm | NA | 70 (7.9) | 67 (7.1) | NA | NA |
| Mucinous cystic neoplasm | NA | 44 (5.0) | 43 (4.6) | NA | NA |
| Serous cystic neoplasm | NA | 32 (3.6) | 28 (3.0) | NA | NA |
| Solid pseudopapillary tumor | NA | 56 (6.3) | 56 (6.0) | NA | 4 (1.2) |
| History of other cancers | 32 (3.2) | 14 (1.6) | NA | NA | NA |
| First-degree relative with pancreatic cancer | 9 (0.9) | 0 | NA | 77 (9.0) | NA |
Abbreviations: JHH, Johns Hopkins Hospital; NA, not available; PDAC, pancreatic ductal adenocarcinoma.
Data are presented as the number (percentage) of individuals unless otherwise indicated.
Deleterious Sequence Variations in Chinese Patients With PDAC
In the Nanjing cohort, deleterious variations were detected in 63 patients with PDAC (6.2%; 95% CI, 4.7%-7.7%) (Figure, A). Deleterious variations associated with cancer according to the ClinVar database of the National Center for Biotechnology Information15 were detected in 35 patients (3.5%; 95% CI, 2.3%-4.6%) (Figure, A, and Table 2). Variations of pancreatic cancer susceptibility genes were detected in 24 patients (2.4%; 95% CI, 1.4%-3.3%). These variants included 9 BRCA2 variations (0.9%). No Ashkenazi Jewish–specific BRCA2 variations (p.Ser1982fs) were detected. BRCA1 variations were detected in 3 patients (0.3%); the total number of BRCA variation carriers was 12 (1.2%). ATM variations were detected in 5 patients (0.5%). PALB2 variations were detected in 6 patients (0.6%). The prevalence of variations in HR-DDR pathway–related genes (ATM, BRCA1/2, PALB2, BRIP1, FANCA, FANCC, RAD51D, and XRCC2) was 3.4% (34 patients) (Table 3). Another gene with a variation, TP53, was detected in 1 patient with PDAC (0.1%). In addition, variations in the pancreatitis-related genes CFTR (8 cases [0.8%]) and SPINK1 (21 cases [2.1%]) were frequent in patients with PDAC (Table 2). All SPINK1 variations were c.194 + 2T>C. The recurrent variations in the CFTR gene included p.Arg74Trp (2 cases [0.2%]) and p.Gly970Asp (3 cases [0.3%]). Most of these variations were absent or rare in the 10 588 individuals in the ChinaMap cohort (6864 [64.8%] male; mean age, 54.2 years); for example, only 114 patients [1.1%] in the ChinaMap cohort had a SPINK1 c.194 + 2T>C variation (eTable 3 in the Supplement). Numerous variants of uncertain significance were also identified in the ChinaMap cohort (eTable 4 in the Supplement).
Figure. Distribution of Pathogenic Germline Variants Across Genes in Patients With Pancreatic Ductal Adenocarcinoma (PDAC) and With Non-PDAC Diseases.
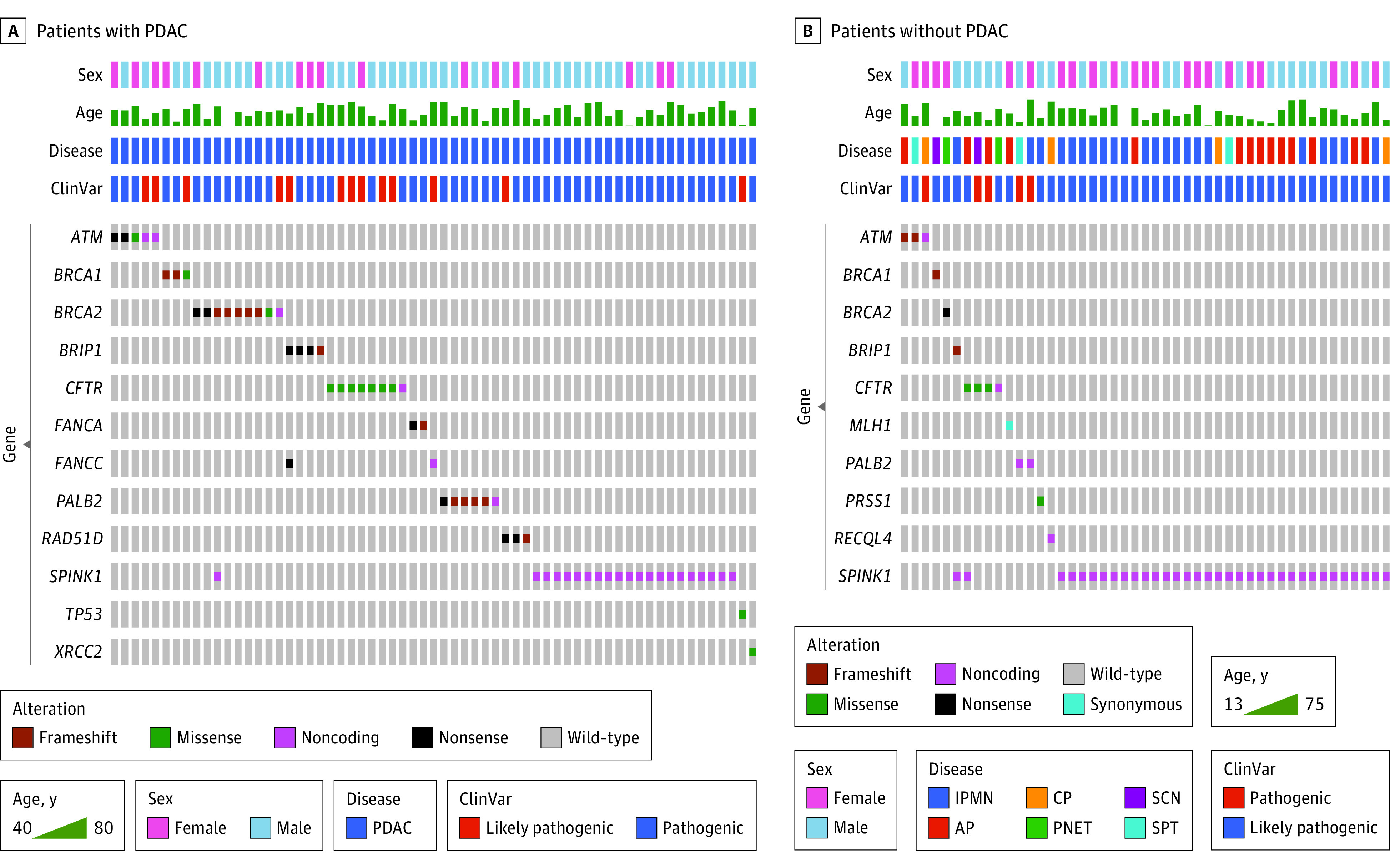
ClinVar indicates the pathogenic classification according to the ClinVar database.15 AP, acute pancreatitis; CP, chronic pancreatitis; IPMN, intraductal papillary mucinous neoplasm; PNET, pancreatic neuroendocrine tumor; SCN, serous cystic neoplasm; and SPT, solid pseudopapillary tumor.
Table 2. Cancer-Associated Germline Sequence Variations in Patients With PDAC in the Nanjing Cohort.
| Case ID | Chromosome position | Sex | Age, y | Gene | Amino acid change | Nucleotide change | Function | ClinVara | Personal history of other disease | Family history of cancer |
|---|---|---|---|---|---|---|---|---|---|---|
| P1720 | chr11:108106561 | Female | 71 | ATM | p.Glu166Gln | c.496G>C | Missense | Pathogenic | Negative | Negative |
| P0251 | chr11:108153468 | Female | 65 | ATM | p.Tyr1203Ter | c.3609delT | Nonsense | Pathogenic | Negative | Negative |
| P1659 | chr11:108170440 | Male | 51 | ATM | Splice | c.5006-1G>A | Noncoding | Likely pathogenic | Negative | Negative |
| P2296 | chr11:108178646 | Male | 64 | ATM | p.Cys1899Ter | c.5697C>A | Nonsense | Pathogenic | Negative | Negative |
| P2281 | chr11:108186639 | Female | 60 | ATM | Splice | c.6095 + 1G>A | Noncoding | Likely pathogenic | Negative | Negative |
| P0537 | chr17:41197809 | Female | 66 | BRCA1 | p.Ile1845fs | c.5533_5540delATTGGGCA | Frameshift | Pathogenic | Breast cancer, lymphoma | Negative |
| P2289 | chr17:41219625 | Male | 66 | BRCA1 | p.Asp1713Asn | c.5137G>A | Missense | Likely pathogenic | Negative | Negative |
| P1565 | chr17:41245390 | Male | 47 | BRCA1 | p.Glu720fs | c.2157_2158insA | Frameshift | Pathogenic | Negative | Negative |
| P0907 | chr13:32900280 | Male | 70 | BRCA2 | p.Lys157fs | c.470_474delAGTCA | Frameshift | Pathogenic | Negative | Negative |
| P1473 | chr13:32906915 | Male | 55 | BRCA2 | p.Lys437fs | c.1310_1313delAAGA | Frameshift | Pathogenic | Negative | Negative |
| P2349 | chr13:32907029 | Male | 51 | BRCA2 | p.Gln472Ter | c.1414C>T | Nonsense | Pathogenic | Negative | Negative |
| P0418 | chr13:32907371 | Female | 74 | BRCA2 | p.Lys586Ter | c.1756A>T | Nonsense | Pathogenic | Lung cancer | Negative |
| P1449 | chr13:32911228 | Male | 61 | BRCA2 | p.Thr915fs | c.2743_2747delACTTG | Frameshift | Pathogenic | Negative | Negative |
| P0796 | chr13:32912337 | Male | 40 | BRCA2 | p.Val1283fs | c.3847_3848delGT | Frameshift | Pathogenic | Negative | Negative |
| P1579 | chr13:32914102 | Female | 63 | BRCA2 | p.Lys1872fs | c.5616_5620delAGTAA | Frameshift | Pathogenic | Ovarian cancer | Negative |
| P0703 | chr13:32921033 | Male | 61 | BRCA2 | p.Arg2336His | c.7007G>A | Missense | Pathogenic | Negative | Negative |
| P2247 | chr13:32944538 | Male | 64 | BRCA2 | Splice | c.8332-1G>T | Noncoding | Likely pathogenic | Negative | Negative |
| P2372 | chr16:23614914 | Male | 48 | PALB2 | p.Leu1143fs | c.3426_3427insA | Frameshift | Pathogenic | Negative | Negative |
| P0375 | chr16:23619181 | Female | 67 | PALB2 | Splice | c.3350 + 4A>G | Noncoding | Pathogenic | Negative | Negative |
| P1670 | chr16:23635403 | Male | 66 | PALB2 | p.Gln921fs | c.2760_2761insA | Frameshift | Pathogenic | Negative | Negative |
| P1649 | chr16:23641218 | Male | 77 | PALB2 | p.Arg753Ter | c.2257C>T | Nonsense | Pathogenic | Negative | Negative |
| P0757 | chr16:23641306 | Female | 69 | PALB2 | p.Met723fs | c.2167_2168delAT | Frameshift | Pathogenic | Negative | Negative |
| P0732 | chr16:23646660 | Male | 56 | PALB2 | p.Leu403fs | c.1206delT | Frameshift | Pathogenic | Negative | Negative |
| P0214 | chr17:7577545 | Male | 42 | TP53 | p.Met246Val | c.736A>G | Missense | Likely pathogenic | Negative | Negative |
| P0493 | chr17:33428225 | Male | 68 | RAD51D | p.Arg320Ter | c.958C>T | Nonsense | Likely pathogenic | Negative | Father with pancreatic cancer |
| P0774 | chr17:33430317 | Female | 80 | RAD51D | p.Arg252Ter | c.754C>T | Nonsense | Pathogenic | Colon polyps | Negative |
| P1831 | chr17:33434458 | Male | 68 | RAD51D | p.Lys111fs | c.331_332insTA | Frameshift | Pathogenic | Negative | Negative |
| P0417 | chr17:59857633 | Female | 75 | BRIP1 | p.Asn643fs | c.1923delT | Frameshift | Pathogenic | Negative | Negative |
| P0437 | chr17:59858254 | Male | 59 | BRIP1 | p.Arg581Ter | c.1741C>T | Nonsense | Pathogenic | Negative | Negative |
| P0581 | chr17:59858254 | Female | 69 | BRIP1 | p.Arg581Ter | c.1741C>T | Nonsense | Pathogenic | Negative | Negative |
| P2568 | chr17:59878688 | Female | 60 | BRIP1 | p.Arg356Ter | c.1066C>T | Nonsense | Pathogenic | Negative | Negative |
| P0519 | chr16:89833603 | Male | 57 | FANCA | p.Ser849fs | c.2546delC | Frameshift | Pathogenic | Negative | Negative |
| P1545 | chr16:89866028 | Male | 49 | FANCA | p.Gln271Ter | c.811C>T | Nonsense | Pathogenic | Negative | Negative |
| P0763 | chr9:98002929 | Male | 77 | FANCC | Splice | c.345 + 2GT>T | Noncoding | Likely pathogenic | Esophageal cancer | Negative |
| P0437 | chr9:98002937 | Male | 59 | FANCC | p.Trp113Ter | c.339G>A | Nonsense | Likely pathogenic | Negative | Negative |
| P1410 | chr7:152346380 | Male | 68 | XRCC2 | p.Arg64Ter | c.190C>T | Missense | Pathogenic | Negative | Negative |
| P0201 | chr5:147207583 | Male | 51 | SPINK1 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P0212 | chr5:147207583 | Male | 57 | SPINK1 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P0322 | chr5:147207583 | Male | 68 | SPINK1 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P0328 | chr5:147207583 | Male | 73 | SPINK1 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P0357 | chr5:147207583 | Male | 54 | SPINK1 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Chronic pancreatitis | Negative |
| P0572 | chr5:147207583 | Male | 75 | SPINK1 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P0727 | chr5:147207583 | Male | 77 | SPINK1 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P0754 | chr5:147207583 | Male | 56 | SPINK1 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P0907 | chr5:147207583 | Male | 70 | SPINK1 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P1048 | chr5:147207583 | Male | 65 | SPINK1 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P1435 | chr5:147207583 | Female | 41 | SPINK1 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P1439 | chr5:147207583 | Male | 54 | SPINK1 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P1501 | chr5:147207583 | Male | 65 | SPINK1 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Father with gastric cancer |
| P1513 | chr5:147207583 | Female | 57 | SPINK1 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P1536 | chr5:147207583 | Female | 77 | SPINK1 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P1555 | chr5:147207583 | Male | 72 | SPINK1 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P1610 | chr5:147207583 | Male | 59 | SPINK1 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P1732 | chr5:147207583 | Male | 61 | SPINK1 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P2209 | chr5:147207583 | Male | 69 | SPINK1 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P2519 | chr5:147207583 | Male | 78 | SPINK1 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P2556 | chr5:147207583 | Male | 64 | SPINK1 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Chronic pancreatitis | Father with lung cancer |
| P1689 | chr7:117149143 | Female | 70 | CFTR | p.Arg74Trp | c.220C>T | Missense | Likely pathogenic | Negative | Negative |
| P2293 | chr7:117149143 | Male | 49 | CFTR | p.Arg74Trp | c.220C>T | Missense | Likely pathogenic | Negative | Negative |
| P1714 | chr7:117232086 | Male | 55 | CFTR | p.Gly622Asp | c.1865G>A | Missense | Pathogenic | Negative | Negative |
| P1674 | chr7:117246728 | Male | 73 | CFTR | p.Gly970Asp | c.2909G>A | Missense | Likely pathogenic | Negative | Negative |
| P1677 | chr7:117246728 | Male | 77 | CFTR | p.Gly970Asp | c.2909G>A | Missense | Likely pathogenic | Negative | Negative |
| P2370 | chr7:117246728 | Male | 74 | CFTR | p.Gly970Asp | c.2909G>A | Missense | Likely pathogenic | Lymphosarcoma | Negative |
| P0533 | chr7:117251704 | Male | 73 | CFTR | p.Arg1070Gln | c.3209G>A | Missense | Pathogenic | Negative | Negative |
| P1681 | chr7:117254665 | Male | 66 | CFTR | Splice | c.3368-2A>G | Noncoding | Pathogenic | Negative | Negative |
Abbreviation: PDAC, pancreatic ductal adenocarcinomas.
Pathogenic classification according to the ClinVar database.15
Table 3. Prevalence of Sequence Variations Among Patients With PDAC in the Nanjing Cohort and Individuals Without Cancer in the ExAC Cohorta.
| Gene | Nanjing cohort (n = 1009) | ExAC population (n = 4327) | Nanjing cohort vs ExAC population, OR (95% CI) | P value | ||
|---|---|---|---|---|---|---|
| Individuals with variation, No. (%) | Individuals sequenced, No. | Individuals with variation, No. (%) | Individuals sequenced, No., meanb | |||
| Hereditary cancer genes | 35 (3.5)c | 1009 | 94 (9.3) | 4154 | 1.6 (1.0-2.3) | .03 |
| Pancreatic cancer susceptibility genes | 24 (2.4) | 1009 | 40 (1.0) | 4154 | 2.5 (1.5-4.2) | <.001d |
| HR-DDR genes | 34 (3.4)c | 1009 | 79 (1.7) | 4154 | 1.8 (1.2-2.7) | <.001 |
| ATM | 5 (0.5) | 1009 | 16 (0.4) | 4313 | 1.3 (0.5-3.7) | .57 |
| BRCA1 | 3 (0.3) | 1009 | 6 (0.1) | 4317 | 2.1 (0.5-8.1) | .28 |
| BRCA2 | 9 (0.9) | 1009 | 12 (0.3) | 4305 | 3.2 (1.4-7.7) | .008 |
| BRIP1 | 4 (0.4) | 1009 | 18 (0.4) | 4198 | 0.7 (0.2-2.4) | .56 |
| FANCA | 2 (0.2) | 1009 | 10 (0.2) | 4119 | 0.8 (0.2-3.7) | .79 |
| FANCC | 2 (0.2) | 1009 | 1 (0.02) | 4321 | 8.6 (0.8-94.6) | .08 |
| PALB2 | 6 (0.6) | 1009 | 5 (0.1) | 4321 | 5.2 (1.6-17.0) | .007 |
| RAD51D | 3 (0.3) | 1009 | 11 (0.3) | 4321 | 1.2 (0.3-4.2) | .81 |
| XRCC2 | 1 (0.1) | 1009 | 0 (0) | 4288 | 12.8 (0.5-313.2) | .19 |
| SPINK1 | 21 (2.1) | 1009 | 26 (0.7) | 3958 | 3.2 (1.8-5.7) | <.001d |
| CFTR | 8 (0.8) | 1009 | 6 (0.1) | 4266 | 5.7 (2.0-16.4) | .001d |
| TP53 | 1 (0.1) | 1009 | 1 (0.03) | 3122 | 3.1 (0.2-49.5) | .42 |
Abbreviations: ExAC, Exome Aggregation Consortium; HR-DDR, homologous recombination DNA damage repair; OR, odds ratio; PDAC, pancreatic ductal adenocarcinoma.
All patients in the Nanjing cohort were from the Han Chinese population, and patients in the ExAC cohort were from East Asia.
The number of individuals sequenced varied for different loci.
One patient with 2 variants was included as 1 observation.
Remained significant at P < .003 if Bonferroni correction was used.
The cohort from TGCA included 150 patients with PDAC. In the TCGA cohort, the most common genes with variations were BRCA2, ATM, and CHEK2 (eFigure in the Supplement). There was no overlap for BRCA2 germline variants among the JHH, TGCA, and Nanjing cohorts (eFigure in the Supplement). These variations were also investigated in the healthy individuals from the ChinaMap database; most were not detected (eTable 3 in the Supplement). Advanced disease stage, tumor location, younger age, and sex were not associated with germline variations (eTable 5 in the Supplement).
Of note, only 13 of the 59 genes (22.0%) were detected with deleterious variations in the 1009 patients in the Nanjing cohort. Variation-free genes in the panel included known pancreatic cancer susceptibility genes, such as CDKN2A, MLH1, MSH2, PRSS1, and STK11; candidate pancreatic cancer susceptibility genes, such as FANCG, FANCL, RECQL4, XRCC3, ERCC4, TERT, BRIP1, BAP1, BUB1, BUB3, and RNF43; and most of the pancreatitis-associated genes except SPINK1 and CFTR.
Prevalence of Deleterious Sequence Variations in Patients With PDAC and non-PDAC in the Nanjing Cohort and the JHH Cohort and Patients Without Cancer in the ExAC Cohort
The JHH cohort included 854 patients with PDAC (455 [53.3%] male; mean (SD) age, 65.0 [10.9] years) and 339 patients with non-PDAC diseases (181 [53.4%] male; mean [SD] age, 60.1 [14.1] years); the ExAC cohort included 4327 patients without cancer from East Asia (demographic data not available). The odds of germline deleterious variations involving BRCA2, PALB2, SPINK1, and CFTR were significantly greater in patients with pancreatic cancer in the Nanjing cohort than in those without pancreatic cancer from the ExAC cohort (control) (BRCA2: OR, 3.2 [95% CI, 1.4-7.7; P = .008]; PALB2: OR, 5.2 [95% CI, 1.6-17.0; P = .007]; SPINK1: OR, 3.2 [95% CI, 1.8-5.7; P < .001]; and CFTR: 5.7 [95% CI, 2.0-16.4; P = .001]) (Table 3); the odds of SPINK1 and CFTR stayed significant after Bonferroni correction. Prevalence of variations in pancreatic cancer susceptibility genes, hereditary cancer genes, and HR-DDR pathway–related genes in the Nanjing cohort was significantly higher than that in the ExAC cohort (Table 3). Comparing the pathogenic germline variations found in this study with those in the JHH cohort, we found no significant difference in the prevalence of germline pathogenic variations in most of the genes between the 2 cohorts (eTable 6 in the Supplement). The germline variation rate of PALB2 was more prevalent in the Nanjing cohort compared with the JHH cohort (6 of 1009 [0.6%] vs 2 of 854 [0.2%]), but the difference was not significant. Of note, sequence variations in the pancreatic secretory enzyme genes CPA1 and CPB1 were not detected in the Nanjing cohort.
Deleterious Sequence Variations in Patients With Non-PDAC Diseases in the Nanjing Cohort
Among patients with non-PDAC diseases in the Nanjing cohort (Table 4, Figure, B, and eTable 7 in the Supplement), 20 of 98 patients with chronic pancreatitis (20.4%) were found to have pathogenic germline variations, including 18 patients (18.4%) with a recurrent variation of SPINK1 c.194 + 2T>C, 1 patient (1.0%) with both SPINK1 c.194 + 2T>C and BRIP1 p.Leu43fs variations, and 1 patient (1.0%) with a PRSS1 p.Arg122His variation. Four patients (4.1%), 3 (3.1%) with a SPINK1 c.194 + 2T>C variation and 1 (1.0%) with a PRSS1 p.Arg122His variation, experienced multiple recurrent pancreatitis episodes after surgical resection for chronic pancreatitis. Germline pathogenic variations were detected in 15 of 355 patients with acute pancreatitis (4.2%), including a recurrent variation of SPINK1 c.194 + 2T>C in 11 patients (3.1%), CFTR variations (p.Arg74Trp and p.Gly970Asp) in 2 patients (0.6%), and cancer susceptibility gene variations (MLH1 p.Ser577 = and ATM p.Phe2558fs) in 2 patients (0.6%). The patient with an MLH1 p.Ser577 = variation had a history of colorectal cancer, and the patient with an ATM p.Phe2558fs variation had a history of gastric cancer. None of these patients had a family history of pancreatitis. In addition, 2 of 70 patients with IPMN (2.8%) had cancer-related variations, and both were splice-site variations (RECQL4 c.2755 + 1G>A and ATM c.3576 + 1G>A). Of note, the patient with IPMN who had an ATM variation (c.3576 + 1G>A) was found to have an invasive carcinoma 9 years after initial surgical resection. A cancer-associated variation (BRCA1 p.Lys1290fs) was detected in 1 of 119 patients with pancreatic neuroendocrine tumors (0.8%), and this patient was diagnosed at the age of 13 years. Variations were detected in 2 patients (6.3%) with serous cystic neoplasms (BRCA2 p.Tyr1655Ter and CFTR c.580-1G>T). Cancer-associated germline variations, namely ATM p.Lys468fs and PALB2 c.2835-1G>C, were detected in 2 of 56 patients with solid pseudopapillary tumors (3.6%). The ChinaMAP database showed that most variations in patients with non-PDAC diseases were not detected in healthy Chinese individuals.
Table 4. Germline Sequence Variations in Patients With Non-PDAC Diseases in the Nanjing Cohort.
| Case ID | Sex | Age, y | Disease | Gene | Chromosome position | Amino acid change | Nucleotide change | Function | ClinVara | History of other disease | Family history of cancer |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P1864 | Female | 67 | IPMNb | ATM | chr11:108151896 | Splice | c.3576 + 1G>A | Noncoding | Likely pathogenic | Negative | Negative |
| P1056 | Male | 70 | IPMN | RECQL4 | chr8:145738229 | Splice | c.2755 + 1G>A | Noncoding | Pathogenic | Rectal cancer | Father with liver cancer |
| P1067 | Male | 48 | IPMN | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P2558 | Male | 27 | IPMN | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P0871 | Male | 64 | AP | ATM | chr11:108202643 | p.Phe2558fs | c.7671_7674delGTTT | Frameshift | Pathogenic | Gastric cancer | Negative |
| P1313 | Male | 27 | AP | CFTR | chr7:117149143 | p.Arg74Trp | c.220C>T | Missense | Likely pathogenic | Negative | Negative |
| P1277 | Male | 37 | AP | CFTR | chr7:117246728 | p.Gly970Asp | c.2909G>A | Missense | Likely pathogenic | Negative | Negative |
| P1290 | Female | 42 | AP | MLH1 | chr3:37083822 | p.Ser577 = | c.1731G>A | Synonymous | Pathogenic | Colorectal cancer | Negative |
| P0862 | Female | 55 | AP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P1122 | Male | 36 | AP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P1221 | Female | 29 | AP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P1249 | Female | 24 | AP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P1265 | Male | 20 | AP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P1271 | Male | 51 | AP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P1277 | Male | 37 | AP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P1280 | Male | 73 | AP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P1783 | Male | 34 | AP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P2494 | Female | 30 | AP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P2509 | Male | 44 | AP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P2453 | Male | 49 | CP | BRIP1 | chr17:59937230 | p.Leu43fs | c.128_131delTGTT | Frameshift | Pathogenic | Negative | Negative |
| P0811 | Male | 31 | CP | PRSS1 | chr7:142459789 | p.Arg122His | c.365G>A | Missense | Pathogenic | Negative | Negative |
| P0802 | Female | 55 | CP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P0808 | Male | 53 | CP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P0810 | Female | 38 | CP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P0814 | Male | 56 | CP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P0815 | Female | 62 | CP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P0829 | Male | 13 | CP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P1014 | Female | 27 | CP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P1018 | Female | 44 | CP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P1021 | Male | 52 | CP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P1029 | Male | 37 | CP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P1036 | Female | 41 | CP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P1045 | Female | 62 | CP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P1046 | Female | 15 | CP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P1632 | Male | 75 | CP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P1806 | Male | 55 | CP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P1811 | Female | 52 | CP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P1993 | Male | 39 | CP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P2453 | Male | 49 | CP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P2512 | Female | 68 | CP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P2545 | Female | 75 | CP | PALB2 | chr16:23625413 | Splice | c.3114-1G>A | Noncoding | Likely pathogenic | Negative | Negative |
| P0626 | Female | 54 | CP | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
| P2445 | Female | 13 | PNET | BRCA1 | chr17:41243677 | p.Lys1290fs | c.3869_3870delAA | Frameshift | Pathogenic | Negative | Negative |
| P1124 | Male | 45 | PNET | CFTR | chr7:117246728 | p.Gly970Asp | c.2909G>A | Missense | Likely pathogenic | Negative | Negative |
| P1862 | Female | 35 | SCN | BRCA2 | chr13:32913456 | p.Tyr1655Ter | c.4965delC | Nonsense | Pathogenic | Negative | Negative |
| P2592 | Male | 53 | SCN | CFTR | chr7:117175301 | Splice | c.580-1G>T | Noncoding | Pathogenic | Negative | Negative |
| P0980 | Female | 36 | SPT | ATM | chr11:108121593 | p.Lys468fs | c.1402_1403delAA | Frameshift | Pathogenic | Negative | Negative |
| P1924 | Male | 22 | SPT | PALB2 | chr16:23634452 | Splice | c.2835-1G>C | Noncoding | Likely pathogenic | Negative | Negative |
| P1119 | Female | 39 | SPT | SPINK1 | chr5:147207583 | Splice | c.194 + 2T>C | Noncoding | Pathogenic | Negative | Negative |
Abbreviations: AP, acute pancreatitis; CP, chronic pancreatitis; IPMN, intraductal papillary mucinous neoplasm; PDAC, pancreatic ductal adenocarcinomas; PNET, pancreatic neuroendocrine tumor; SCN, serous cystic neoplasm; SPT, solid pseudopapillary tumor.
Pathogenic classification according to the ClinVar database.15
This patient was diagnosed with IPMN, underwent resection, and developed an invasive cancer during surveillance.
Discussion
To our knowledge, this study is the first to provide a large sample of data on germline variations in a Chinese population with sporadic pancreatic cancer. Germline deleterious variations in cancer susceptibility genes, known pancreatic cancer susceptibility genes, and HR-DDR pathway–related genes were found in the study cohort. Rates of deleterious variations in patients with PDAC were significantly higher than those in patients without pancreatic cancer from the ExAC and ChinaMAP databases. In addition to pancreatic cancer, this study also reported germline variations in patients with noncancer pancreatic diseases such as pancreatic neuroendocrine tumors, pancreatitis, and pancreatic cystic tumors; to our knowledge, this is the first study in which most of these data have been reported.
Among the HR-DDR genes, the genes that most frequently have variations include ATM, BRCA1/2, and PALB2.6 Germline variations in the BRCA1/2 genes may cause double-strand DNA damage repair deficiency and are associated with risk for pancreatic cancer.16 The Pancreas Cancer Olaparib Ongoing trial7 detected BRCA1/2 variations in 2167 patients with metastatic pancreatic cancer. The prevalence of variations was higher among Black individuals (10.7%) than among White individuals (6.1%), Asian individuals (5.0%), and individuals of other racial and ethnic groups (1.6%), indicating racial and ethnic differences in the variations associated with pancreatic cancer. Studies by Chang et al17 and Grant et al18 showed that no significant BRCA2 susceptibility was found in the population of China after comparing the variations of pancreatic cancer susceptibility genes with those obtained from a large sample study in Europe using a whole-exome association analysis. Studies19,20,21 from Japan and South Korea have shown the importance of genetic testing for pancreatic cancer, but the sample sizes were small (<100 patients). The present study provides data on germline variations of BRCA1/2 and HR-DDR genes in a large sample of Han Chinese patients with pancreatic cancer. Of interest, no Ashkenazi Jewish–specific BRCA2 variation (p.Ser1982fs) was found in this cohort, which suggests that the BRCA variation is associated with race and ethnicity, similar to the findings of another study.22 In the JHH cohort,5 BRCA2 and ATM variations were associated with higher pancreatic cancer risk in the US population, whereas BRCA2 and PALB2 variations were significantly associated with pancreatic cancer risk in the Nanjing cohort. SPINK1 and CFTR variations were also associated with higher risk of PDAC among the Chinese population, whereas variations of these 2 genes were not associated with pancreatic cancer in patients in a study from Germany.23 Another study24 from JHH showed that variations in the pancreatic secretory enzyme genes CPA1 and CPB1 were associated with pancreatic cancer, whereas no variations of these 2 genes were detected in the Nanjing cohort. Of importance, variations in the Nanjing cohort and JHH cohort in this study were detected with the same sequencing platform and strategy, which enhanced the credibility of the findings.
A retrospective study6 showed that patients with pancreatic cancer who had germline variations in BRCA1/2 were more sensitive to platinum-based chemotherapy. A follow-up study of that cohort showed that a deleterious variant in DNA-damage-repair genes was associated with improved survival after resection and adjuvant chemotherapy for pancreatic ductal adenocarcinoma.25 Olaparib is a poly(adenosine diphosphate ribose) polymerase inhibitor that interferes with DNA repair in tumor cells and is more effective in patients with BRCA variations with homologous recombination repair deficiency.26 The Pancreas Cancer Olaparib Ongoing trial7 showed that olaparib substantially improved disease-free survival among patients with BRCA variations who had PDAC that did not progress after platinum-based first-line chemotherapy. Our study may provide a foundation for the treatment of Chinese patients who have germline variations in the HR-DDR pathway and may be used as a reference for further clinical research and clinical trials of personalized medicine for patients with pancreatic cancer. Our findings suggest that patients with sporadic pancreatic cancer in whom germline variations are detected should undergo familial surveillance and screening. In this study, patients who had a germline variation had a higher incidence of sporadic pancreatic cancer. In addition, genes that were not detected with variations were also of importance when considering cost-effectiveness during the panel design. Large-panel germline testing for sporadic PDACs in Chinese patients should be reconsidered.
Our study also showed deleterious germline variants in patients with noncancer pancreatic diseases. Variations were detected in pancreatic cystic tumors, including IPMNs (ATM, RECQL4, and SPINK1), serous cystic neoplasms (BRCA2 and CFTR), and solid pseudopapillary tumors (ATM, PALB2, and SPINK1). Skaro et al27 reported that deleterious germline variations of hereditary cancer genes (eg, ATM, BRCA2, PALB2) were found in patients with IPMN associated with concurrent invasive cancer. In the Nanjing cohort, 1 patient with IPMN with an ATM splice-site variation (c.3576 + 1G>A) developed an invasive IPMN during long-term follow-up, suggesting that careful lifelong surveillance is needed for all resected IPMN diseases. The outcomes of germline variants for serous cystic neoplasms and solid pseudopapillary tumors was unclear in the present study. Additional large-sample studies of pancreatic cystic tumors are needed to investigate the prevalence of germline variations and their associations with patient outcomes.
Genetic factors are among the main factors associated with chronic pancreatitis.28 Hereditary pancreatitis is often accompanied by germline variations in the PRSS, SPINK1, and CFTR genes.29 There is a lack of relevant data among the Chinese population. A sequencing study from China30 explored the germline variation of patients with idiopathic chronic pancreatitis, showing that the SPINK1 c.194 + 2T>C variation was present in 56.2% of adolescent and 42.0% of adult patients with idiopathic chronic pancreatitis. Patients with variations are more likely to develop recurrent pancreatitis,31 as did some of the patients in the present study. Of patients diagnosed with acute pancreatitis, 3.1% also carried the SPINK1 c.194 + 2T>C variation, which suggests that in some patients, a new diagnosis of acute pancreatitis might need to be distinguished from idiopathic chronic pancreatitis. An association of the SPINK1 c.194 + 2T>C variation with chronic pancreatitis has been widely discussed.32 However, an association between a SPINK1 variation and pancreatic cancer has been less frequently reported, especially in the Chinese population. In the Nanjing cohort, 21 patients with PDAC (2.1%) had a SPINK1 c.194 + 2T>C variation, which was significantly more frequent than in the ChinaMap population (114 patients [1.1%]). Of note, only 2 of the 21 patients (9.5%) had a history of chronic pancreatitis, suggesting that a SPINK1 variation might be directly associated with pancreatic cancer and not necessarily associated with chronic pancreatitis.33
Limitations
This study has limitations. First, owing to the study’s retrospective nature, clinical information collection was based on medical records. Second, public databases such as ExAC and ChinaMAP were used as controls for this study instead of sequencing the Chinese population with the same sequencing platform and methods. Third, because this study included patients from a surgical center, patients with PDAC included in this study were mostly initialized for radical resection, which does not represent the whole group of patients with PDAC disease, including those with unresectable PDAC. Fourth, although this is the first study, to our knowledge, to report germline variations in Chinese patients with noncancer pancreatic diseases, the sample sizes of these diseases were small, which requires further exploration of their prevalence and clinical significance in a better designed study with a larger patient cohort.
Conclusions
This is, to our knowledge, the first large-sample study of Chinese patients with pancreatic cancer using next-generation sequencing technology to detect germline variations. We found that sporadic pancreatic cancer in a population from China was associated with pathogenic germline variations. There were small differences in germline variants of PDAC. This is also the first study, to our knowledge, to report genetic data on noncancer pancreatic diseases. Germline variation detection might be useful for the early detection of pancreatic cancer in patients with high-risk disease, such as IPMN, during surveillance and also might provide supportive information for precision medicine in pancreatic cancer to optimize chemotherapeutic treatments and improve outcomes.
eAppendix. Supplementary Materials and Methods
eFigure. Comparison of Pathogenic Germline Variants Between Nanjing Cohort and TCGA Cohort
eTable 1. Amplicons Coverage of Panel
eTable 2. Primers for Sanger Sequencing
eTable 3. Prevalence of Pathogenic Germline Mutations in PDAC and Chinese Population
eTable 4. Variants of Uncertain Significance and Novel Variants Detected
eTable 5. Clinicopathologic Characteristics of Patients With Germline Mutation and Wild-Type Cases
eTable 6. Comparison of Nanjing Cohort and Johns Hopkins Cohort on the Pathogenic Germline Mutations of PDAC Patients
eTable 7. Prevalence of Pathogenic Germline Mutations in Non-PDAC and Chinese Population
References
- 1.Zheng RS, Sun KX, Zhang SW, et al. Report of cancer epidemiology in China, 2015. Article in Chinese. Zhonghua Zhong Liu Za Zhi. 2019;41(1):19-28. [DOI] [PubMed] [Google Scholar]
- 2.Denbo JW, Fleming JB. Definition and management of borderline resectable pancreatic cancer. Surg Clin North Am. 2016;96(6):1337-1350. doi: 10.1016/j.suc.2016.07.008 [DOI] [PubMed] [Google Scholar]
- 3.Tempero MA. NCCN guidelines updates: pancreatic cancer. J Natl Compr Cancer Netw. 2019;17(5.5):603-605. doi:10.6004/jnccn.2019.5007 [DOI] [PubMed]
- 4.Knudsen ES, O’Reilly EM, Brody JR, Witkiewicz AK. Genetic diversity of pancreatic ductal adenocarcinoma and opportunities for precision medicine. Gastroenterology. 2016;150(1):48-63. doi: 10.1053/j.gastro.2015.08.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shindo K, Yu J, Suenaga M, et al. Deleterious germline mutations in patients with apparently sporadic pancreatic adenocarcinoma. J Clin Oncol. 2017;35(30):3382-3390. doi: 10.1200/JCO.2017.72.3502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blair AB, Groot VP, Gemenetzis G, et al. BRCA1/BRCA2 germline mutation carriers and sporadic pancreatic ductal adenocarcinoma. J Am Coll Surg. 2018;226(4):630-637.e1. doi: 10.1016/j.jamcollsurg.2017.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381(4):317-327. doi: 10.1056/NEJMoa1903387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang BZ, Stram DO, Le Marchand L, et al. Interethnic differences in pancreatic cancer incidence and risk factors: the Multiethnic Cohort. Cancer Med. 2019;8(7):3592-3603. doi: 10.1002/cam4.2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian L, Zhang K, Wang X, et al. Utilization of mobile application for better implementation of good clinical practice in a biorepository sample collection process: functions of PancMoBio in biobanking. Biopreserv Biobank. 2020;18(2):46-52. doi: 10.1089/bio.2019.0025 [DOI] [PubMed] [Google Scholar]
- 10.National Clinical Research Center for Metabolic Diseases . ChinaMAP. Accessed August 1, 2020. http://www.mbiobank.com/
- 11.Cao Y, Li L, Xu M, et al. ; ChinaMAP Consortium . The ChinaMAP analytics of deep whole genome sequences in 10,588 individuals. Cell Res. 2020;30(9):717-731. doi: 10.1038/s41422-020-0322-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genomics Institute, University of California, Santa Cruz . Genome browser. Accessed March 20, 2020. https://genome.ucsc.edu/
- 13.Broad Institute . Genome aggregation database. Accessed January 17, 2022. https://gnomad.broadinstitute.org/
- 14.Altman DG. Practical Statistics for Medical Research.Chapman & Hall/CRC; 1991. [Google Scholar]
- 15.National Center for Biotechnology Information . ClinVar. US National Library of Medicine, National Institutes of Health. Accessed January 17, 2022. https://www.ncbi.nlm.nih.gov/clinvar/
- 16.Thompson D, Easton DF; Breast Cancer Linkage Consortium . Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94(18):1358-1365. doi: 10.1093/jnci/94.18.1358 [DOI] [PubMed] [Google Scholar]
- 17.Chang J, Tian J, Zhu Y, et al. Exome-wide analysis identifies three low-frequency missense variants associated with pancreatic cancer risk in Chinese populations. Nat Commun. 2018;9(1):3688. doi: 10.1038/s41467-018-06136-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant RC, Denroche RE, Borgida A, et al. Exome-wide association study of pancreatic cancer risk. Gastroenterology. 2018;154(3):719-722.e3. doi: 10.1053/j.gastro.2017.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeuchi S, Doi M, Ikari N, Yamamoto M, Furukawa T. Mutations in BRCA1, BRCA2, and PALB2, and a panel of 50 cancer-associated genes in pancreatic ductal adenocarcinoma. Sci Rep. 2018;8(1):8105. doi: 10.1038/s41598-018-26526-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohmoto A, Morizane C, Kubo E, et al. Germline variants in pancreatic cancer patients with a personal or family history of cancer fulfilling the revised Bethesda guidelines. J Gastroenterol. 2018;53(10):1159-1167. doi: 10.1007/s00535-018-1466-y [DOI] [PubMed] [Google Scholar]
- 21.Lee K, Yoo C, Kim KP, et al. Germline BRCA mutations in Asian patients with pancreatic adenocarcinoma: a prospective study evaluating risk category for genetic testing. Invest New Drugs. 2018;36(1):163-169. doi: 10.1007/s10637-017-0497-1 [DOI] [PubMed] [Google Scholar]
- 22.Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93-99. [DOI] [PubMed] [Google Scholar]
- 23.Schubert S, Traub F, Brakensiek K, et al. CFTR, SPINK1, PRSS1, and CTRC mutations are not associated with pancreatic cancer in German patients. Pancreas. 2014;43(7):1078-1082. doi: 10.1097/MPA.0000000000000166 [DOI] [PubMed] [Google Scholar]
- 24.Tamura K, Yu J, Hata T, et al. Mutations in the pancreatic secretory enzymes CPA1 and CPB1 are associated with pancreatic cancer. Proc Natl Acad Sci U S A. 2018;115(18):4767-4772. doi: 10.1073/pnas.1720588115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu H, Zhu Y, Pu N, et al. Association of germline variants in human DNA damage repair genes and response to adjuvant chemotherapy in resected pancreatic ductal adenocarcinoma. J Am Coll Surg. 2020;231(5):527-535.e14. doi: 10.1016/j.jamcollsurg.2020.06.019 [DOI] [PubMed] [Google Scholar]
- 26.Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33(3):244-250. doi: 10.1200/JCO.2014.56.2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skaro M, Nanda N, Gauthier C, et al. Prevalence of germline mutations associated with cancer risk in patients with intraductal papillary mucinous neoplasms. Gastroenterology. 2019;156(6):1905-1913. doi: 10.1053/j.gastro.2019.01.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss FU, Skube ME, Lerch MM. Chronic pancreatitis: an update on genetic risk factors. Curr Opin Gastroenterol. 2018;34(5):322-329. doi: 10.1097/MOG.0000000000000461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh VK, Yadav D, Garg PK. Diagnosis and management of chronic pancreatitis: a review. JAMA. 2019;322(24):2422-2434. doi: 10.1001/jama.2019.19411 [DOI] [PubMed] [Google Scholar]
- 30.Liu M, Xia T, Zhang D, et al. Genetic background and clinical characters of pediatric chronic pancreatitis: data and implications from the East. Gastroenterol Res Pract. 2017;2017:7548753. doi: 10.1155/2017/7548753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Del Vecchio Blanco G, Gesuale C, Varanese M, Monteleone G, Paoluzi OA. Idiopathic acute pancreatitis: a review on etiology and diagnostic work-up. Clin J Gastroenterol. 2019;12(6):511-524. doi: 10.1007/s12328-019-00987-7 [DOI] [PubMed] [Google Scholar]
- 32.Tang X-Y, Zou W-B, Yu F-F, et al. Meta-analysis of the impact of the SPINK1 c.194 + 2T > C variant in chronic pancreatitis. Dig Liver Dis. 2020;52(2):143-148. doi: 10.1016/j.dld.2019.07.004 [DOI] [PubMed] [Google Scholar]
- 33.Suzuki M, Shimizu T. Is SPINK1 gene mutation associated with development of pancreatic cancer? new insight from a large retrospective study. EBioMedicine. 2019;50:5-6. doi: 10.1016/j.ebiom.2019.10.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Supplementary Materials and Methods
eFigure. Comparison of Pathogenic Germline Variants Between Nanjing Cohort and TCGA Cohort
eTable 1. Amplicons Coverage of Panel
eTable 2. Primers for Sanger Sequencing
eTable 3. Prevalence of Pathogenic Germline Mutations in PDAC and Chinese Population
eTable 4. Variants of Uncertain Significance and Novel Variants Detected
eTable 5. Clinicopathologic Characteristics of Patients With Germline Mutation and Wild-Type Cases
eTable 6. Comparison of Nanjing Cohort and Johns Hopkins Cohort on the Pathogenic Germline Mutations of PDAC Patients
eTable 7. Prevalence of Pathogenic Germline Mutations in Non-PDAC and Chinese Population


