This meta-analysis of 39 trials examines the efficacy and tolerability of antidepressant combination therapy compared with monotherapy in the treatment of acute depression.
Key Points
Question
What is the treatment efficacy and tolerability of antidepressant combination therapy compared with monotherapy in the treatment of acute depression, and are specific combinations preferable to others?
Findings
This meta-analysis of 39 trials comprising 6751 patients found that combination treatment using a reuptake inhibitor with an antagonist of presynaptic α2-autoreceptors (mianserin, mirtazapine, trazodone) was associated with significantly superior treatment outcomes compared with monotherapy, both as first-line treatment and for nonresponder populations. The dropout numbers did not differ between treatments.
Meaning
Combination therapy using an antagonist of presynaptic α2-autoreceptors may be an effective and safe antidepressant treatment option for patients who are nonresponders to monotherapy and as a potential first-line treatment in severe cases of depression.
Abstract
Importance
Combining antidepressants is frequently done in the treatment of acute depression, but studies have yielded conflicting results.
Objective
To conduct a systematic review and meta-analysis assessing efficacy and tolerability of combination therapy. Combinations using presynaptic α2-autoreceptor antagonists or bupropion were investigated separately.
Data Sources
MEDLINE, Embase, PsycINFO, and the Cochrane Central Register of Controlled Trials were systematically searched from each database inception through January 2020.
Study Selection
Randomized clinical trials (RCTs) comparing combinations of antidepressants with antidepressant monotherapy in adult patients with acute depression were included.
Data Extraction and Synthesis
Following guidelines from Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) and recommendations from the Cochrane Handbook, 2 reviewers independently performed a literature search, study selection, data extraction, and evaluation of risk of bias. Data were pooled in random-effects analyses.
Main Outcomes and Measures
Primary outcome was efficacy measured as standardized mean difference (SMD); secondary outcomes were response, remission, change from baseline in rating scale scores, number of dropouts, and number of dropouts due to adverse events.
Results
Thirty-nine RCTs including 6751 patients were eligible. Combination treatment was statistically significantly associated with superior treatment outcomes relative to monotherapy (SMD = 0.31; 95% CI, 0.19-0.44). Combining a reuptake inhibitor with an antagonist of presynaptic α2-autoreceptors was superior to other combinations (SMD = 0.37; 95% CI, 0.19-0.55). Bupropion combinations were not superior to monotherapy (SMD = 0.10; 95% CI, −0.07 to 0.27). Numbers of dropouts and dropouts due to adverse events did not differ between treatments. Studies were heterogeneous, and there was indication of publication bias (Egger test result was positive; P = .007, df = 36), but results remained robust across prespecified secondary outcomes and sensitivity and subgroup analyses, including analyses restricted to studies with low risk of bias.
Conclusions and Relevance
In this meta-analysis of RCTs comparing combinations of antidepressants with antidepressant monotherapy, combining antidepressants was associated with superior treatment outcomes but not with more patients dropping out of treatment. Combinations using an antagonist of presynaptic α2-autoreceptors may be preferable and may be applied as a first-line treatment in severe cases of depression and for patients considered nonresponders.
Introduction
Guidelines by the National Institute for Health and Care Excellence,1 American Psychological Association,2 and American Psychiatric Association,3 as well as the German National Clinical Practice Guideline4 recommend use of a single, non–monoamine oxidase inhibitor antidepressant as initial treatment in severe depression. Despite a host of antidepressant agents, response rates to initial antidepressant monotherapy hover at 60%, and remissions occur in only up to 40% of patients, even after 12 to 24 weeks of treatment.5
Guidelines advocate a number of second-step treatments for patients considered nonresponders, most prominently switching to a different monotherapy, dose escalation, augmentation (eg, with lithium or second-generation antipsychotics), or combining 2 antidepressants.1,2,6 Combining 2 antidepressants is a common next step, particularly in primary care settings,7,8 based on the assumption that combining 2 antidepressants with different modes of action increases clinical efficacy.
In a previous meta-analysis,9 we showed that, compared with monotherapy, combination therapy is more effective and comparably tolerable as a treatment for acute depression, most notably when applied as a first-line treatment. We also found that this was particularly the case for combinations that include monoamine reuptake inhibitors (selective serotonin reuptake inhibitor, serotonin-norepinephrine reuptake inhibitor, or tricyclic antidepressant) and antagonists of presynaptic α2-autoreceptors (mianserin, mirtazapine, trazodone). In the meantime, several important studies have been published, presenting partly contradictory results.10,11,12,13 Based on complementary mechanisms of action, combining mirtazapine or bupropion with reuptake inhibitors has been viewed as particularly promising, with regard to both efficacy and tolerability.9,14 In light of these recent developments, an updated synopsis of the evidence is warranted.
This systematic review and meta-analysis of randomized clinical trials (RCTs) comparing combinations of 2 antidepressants with antidepressant monotherapy in adults with acute depression addresses a number of questions. What is the efficacy of combination therapy, relative to monotherapy, both as first-line treatment and as treatment for nonresponders? Are combination treatments that include mirtazapine or bupropion particularly effective? What is the comparative tolerability of combination therapies?
Methods
The protocol of this study has been published on PROSPERO (CRD42020167739). We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines for systematic reviews15 and closely adhered to recommendations from the Cochrane Collaboration.16 The methods are described in detail in the eMethods and eAppendix in the Supplement. In brief, we searched MEDLINE, PsycINFO, Embase, and the Cochrane Central Register of Controlled Trials and selected RCTs meeting the following criteria: an intervention using a combination of 2 antidepressants, irrespective of dosage; a control group of patients taking antidepressant monotherapy; inclusion of participants 18 years or older; and depressive disorder diagnosed according to standard operationalized criteria. Comorbid medical conditions and concomitant diagnoses of other psychiatric disorders were not exclusion criteria. Studies solely focusing on bipolar depression were excluded. We also excluded trials of maintenance therapy. Trials of first-line treatment and trials with patients who had resistance to previous antidepressive treatments were eligible, including both initial combination therapy and adjunctive administration of a second antidepressant. In first-line studies, after randomization, monotherapy control groups received antidepressant monotherapy. In studies including patients resistant to previous antidepressive treatment, monotherapy control-group patients received either ongoing monotherapy with the same antidepressant (the same dose or an increased dose) or monotherapy with a different (switched) antidepressant.
Literature search, study selection, data extraction, and evaluation of risk of bias all were carried out independently by 2 reviewers (J.H. and D.A.) and followed the Cochrane Collaboration Handbook.16 The included studies were added to the trials retrieved by our previous systematic search,9 and all analyses were based on the combined set of studies, thus covering all available evidence from the inception of each database to January 1, 2020.
The primary outcome criterion was treatment efficacy measured as the standardized mean difference (SMD) between combination and monotherapy, on an intention-to-treat basis, if possible. Secondary outcome criteria were remission (score below predetermined thresholds, eg, ≤7 on the 17-item Hamilton Depression Rating Scale [HDRS]) and response (eg, ≥50% decrease on the 17-item HDRS or the Montgomery-Asberg Depression Rating Scale [MADRS]) as defined by the study authors, change from baseline on a rating scale score, and numbers of dropouts and dropouts due to adverse events.
Prespecified subgroup analyses included studies with nonresponders to previous treatment trials and with patients new to treatment, combinations including antagonists of presynaptic α2-autoreceptors and combinations including bupropion, and RCTs with low risk of bias. Following the Cochrane Handbook,16 RCTs were evaluated according to the Cochrane risk-of-bias tool, taking into account random sequence generation; allocation concealment; blinding of participants, personnel, and outcome assessors; incomplete outcome data; selective reporting; sponsorship; and other potential sources of bias. An overall assessment of risk of bias (low or unknown/high) was added. Summary SMDs and odds ratios (with 95% CI) were calculated in random-effects meta-analyses because included studies differed methodologically, eg, in regard to blinding or diagnostic criteria and the assessment scales used. Meta-regression analyses carried out post hoc investigated a possible association of baseline depression severity with effect size. Statistical significance was set at α = .05 (2-sided) for the primary, hypothesis-testing outcome. For all secondary outcomes and for all subgroup analyses, P values are presented, but not as a marker of statistical significance. Data analyses were carried out with Comprehensive Meta-analysis software (Version 3, Professional version; Biostat).
During screening of titles and abstracts, most articles were excluded because they did not report on combination treatment, RCTs, or clinical depression.
Results
Our database search retrieved 4244 different articles. During screening of titles and abstracts, most articles were excluded because they did not report on combination treatment, RCTs, or clinical depression. The full texts of 146 articles were read and 7 new studies included. In addition to the previously retrieved set of trials, this amounted to a final set of 39 studies as a basis for the analyses (Figure 1).
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Flowchart.
In total, trials included 6751 patients. Publication dates ranged from 1977 to 2020. Articles were published in English, Chinese (1 article), and Korean (1 article). Twenty-three studies (59%) were double-blind, 5 studies single-blind, and 11 studies open-label. Twenty-one trials (54%) recruited nonresponders to initial antidepressant treatment. (Table 1 lists study groups, trial size, and initial antidepressant pharmacotherapy in nonresponder studies.) According to the published reports, only 1 of the studies17 included patients previously exposed to antidepressant combination treatment.
Table 1. Characteristics of Trials.
| Source | Diagnosis | No. | Follow-up, wk | ITT population, No. | Blinding | Nonresponder only | Risk of bias | Depression severity at baselinea | |
|---|---|---|---|---|---|---|---|---|---|
| Combination | Monotherapy | ||||||||
| Bares et al,17 2013 | MDD, DSM-IV, at least stage 1 criteria for resistant depression (≥1 adequate antidepressant treatment in current episode), including prior combination treatment | 61 | 6 | Variable, n = 31 | Variable, n = 29 | Open | Y | Unknown/high | MADRS, C: 28.6 (3.2), M: 28.4 (3.2)b |
| Blier et al,18 2009 | MD, DSM-IV | 61 | 6 | Mirtazapine (15-45 mg/d) + paroxetine (10-30 mg/d), n = 21 | Paroxetine (10-30 mg), n = 19; or mirtazapine (15-45 mg), n = 21 | Double | N (first-line) | Low | MADRS, C: 34.4 (7.2), M: 32.2 (5.9); 32.0 (6.4)c |
| Blier et al,19 2010 | MDD, DSM-IV | 105 | 6 | Mirtazapine (30 mg/d) + fluoxetine (20 mg/d), n = 25; mirtazapine (30 mg/d) + venlafaxine (75 mg increased to 225 mg/d), n = 26; mirtazapine + bupropion (150 mg/d), n = 26 | Fluoxetine (20 mg/d), n = 28 | Double | N (first-line) | Low | MADRS, C: 32.4 (5); 31.7 (4.1); 31.0 (4.1); M: 31.8 (4.8). HAMD-17, C: 22.4 (3.5); 22.6 (3.1); 21.7 (2.6); M: 22.6 (3.0) |
| Carpenter et al,20 2002 | MD episode, DSM-IV; significant persistent depressive symptoms despite ≥4 wk of standard antidepressant monotherapy at maximum recommended or tolerated doses | 26 | 4 | Mirtazapine (15-30 mg/d) + primary antidepressant, n = 11 | Primary antidepressant agents continued at prestudy daily doses throughout augmentation trial (bupropion 450 mg, citalopram 30-60 mg, fluoxetine 40-50 mg, fluvoxamine 300 mg, paroxetine 30-40 mg, sertraline 100-200 mg, venlafaxine 200-300 mg), n = 15 | Double | Y | Low | HDRS-17, C: 21.9 (3.8), M: 22.5 (5.8) |
| Cha et al,21 1997 | DSM-IV | 36 | 6 | Dothiepin (75 mg/d, dose increase due to clinical state) + sertraline (75 mg/d, dose increase due to clinical state), n = 20 | Dothiepin (150 mg/d, dose increase due to clinical state), n = 16 | Open | N (first-line) | Unknown/high | HAMD-17, C: 22.9 (6.0), M: 21.0 (4.8)b |
| Dam et al,22 1998 | Depression, ICD-10, moderate to severe degree (corresponding to a DSM-III-R diagnosis of MD) | 34 | 6 | Mianserin (30 mg/d) + fluoxetine (20 mg/d), n = 16 | Fluoxetine (20 mg/d), n = 18 | Double | N (first-line) | Unknown/high | HAMD, median (range), C: 25.0 (20-34), M: 21.5 (17-30) |
| Fang et al,23,24 2010/2011 | MDD DSM-IV, stage 2 TRD, failed response to 2 or more adequate treatments from different classes of antidepressants in current depressive episode | 197 | 8 | Paroxetine (20 mg/d) + trazodone (100 mg/d), n = 47 | Venlafaxine XR (225 mg/d), n = 50; mirtazapine (45 mg/d), n = 55; paroxetine (20 mg/d), n = 45 | Double | Y | Unknown/high | HDRS-17, 24.6 (5.8) (only whole-sample data) |
| Fava et al,25 1994 | MDD, DSM-III-R-Patient Edition, refractoriness to initial fluoxetine 20 mg/d for 8 wk | 27 | 4 | Fluoxetine (20 mg/d) + desipramine (25-50 mg/d), n = 12 | Fluoxetine (40-60 mg/d), n = 15 | Double | Y | Unknown/high | HAMD-17, C: 17.5 (4.7), M: 16.2 (3.9) |
| Fava et al,26 2002 | MDD, DSM-III-R-Patient Edition, refractoriness to initial fluoxetine 20 mg/d for 8 wk | 67 | 4 | Fluoxetine (20 mg/d) + desipramine (25-50 mg/d), n = 34 | Fluoxetine (40-60 mg/d), n = 33 | Double | Y (+ partial) | Unknown/high | HAMD-17, C: 19.6 (3.1), M: 17.7 (3.4) |
| Ferreri et al,27 2001 | MDD, DSM III-R, nonresponders to 6 wk of fluoxetine 20 mg/d | 103 | 6 | Fluoxetine (20 mg/d) + mianserin (60 mg/d), n = 32 | Mianserin (60 mg/d), n = 33; fluoxetine (20 mg/d), n = 38 | Double | Y | Low | HAMD-17, C: 27.7 (1.9), M: 27.1 (2.52); 26.9 (1.9) |
| Fornaro et al,28 2014 | MD episode with atypical features, DSM-IV, SCID-I/P, and a HAMD-21 baseline score ≥14, documented TRD history concerning ≥1 previous “adequate” SSRI trial | 48 | 6 | Duloxetine (60-120 mg/d, mean dose: 86.09 mg/d) + bupropion (150-300 mg/d, mean dose: 215.22 mg/d), n = 23 | Duloxetine (60-120 mg/d, mean dose: 91.30 mg/d), n = 23 | Double | Y | Low | HAMD-21, C: 26.82 (6.15), M: 27.30 (7.71) |
| Gulrez et al,29 2012 | MDD, DSM-IV-TR, partial responders to SSRI treatment, ie, HDRS score ≥16 after 4 wk of SSRI | 60 | 4 | SSRI (escitalopram 10-30 mg/d, citalopram 20-60 mg/d, paroxetine 25-75 mg/d, sertraline 50-200 mg/d) + bupropion SR (300 mg/d), n = 30 | SSRI (dosing see left) + placebo, n = 30 | Single | Y | Unknown/high | HDRS, C: 17.80 (0.60), M: 17.57 (0.48)d |
| Jie et al,30 2019 | Depression, CCMD-3 diagnostic criteria for depression, ie, HAMD-17 score >18 | 104 | 12 | Fluoxetine (20 mg increased to 60 mg/d) + amitriptyline (≥150 mg/d), n = 52 | Fluoxetine (20 mg increased to 60 mg/d), n = 52 | Open | N (first-line) | Unknown/high | HAMD-17, C: 25.97 (3.75), M: 25.03 (3.35) |
| Kato et al,31 2017 | MDD DSM-IV, HAMD-17 > 14/17, inadequate response to 4 wk of SSRI or mirtazapine | 47 | 4 | SSRI (paroxetine or sertraline) + mirtazapine, n = 21 | SSRI (paroxetine 20-40 mg/d, mean dosee: 38.9 ± 5.8 mg/d, or sertraline 50-100 mg/d, mean dosee: 91.7 ± 22.9 mg/d), n = 13; mirtazapine 30-45 mg/d, mean dosee: 38.4 ± 10.1 mg/d, n = 13 | Open | Y | Unknown/high | HAMD-17, C: 19.3 (4.6), M: 14.4 (5.2); 13.7 (3.8) |
| Dosage of antidepressant added: SSRI (paroxetine 10-40 mg/d, mean dosee: 33.5 ± 4.8 mg/d, or sertraline 25-100 mg/d, mean dosee: 65.0 ± 33.5 mg/d) + mirtazapine 15-45 mg/d, mean dosee: 27.5 ± 10.8 mg/d | |||||||||
| Dosage of antidepressant continuously prescribed from step 1: SSRI (paroxetine 20-40 mg/d, mean dosee: 38.9 ± 5.8 mg/d, or sertraline 50-100 mg/d, mean dosee: 91.7 ± 22.9 mg/d) + mirtazapine 30-45 mg/d, mean dosee: 38.4 ± 10.1 mg/d | |||||||||
| Kato et al,10 2018 (SUN☺D) 6 wk | Primary diagnosis of a nonpsychotic unipolar MD episode within the past month, DSM-IV, PRIME-MD, nonremission (ie, PHQ-9 score >4) with 3 wk of sertraline | 1647 | 6 | Sertraline (25-100 mg/d) + mirtazapine (7.5-45 mg/d), n = 527 | Sertraline (25-100 mg/d), n = 537; mirtazapine (7.5-45 mg/d), n = 550 | Single (raters) | Y | Low | PHQ, C: 12.6 (5.1), M: 12.8 (5.2); 12.8 (5.2). BDI-II, C: 24.1 (10.7), M: 24.5 (10.7); 24.4 (10.9) |
| Kessler et al,12 2018 (MIR) 12 wk | Depression, ICD-10. TRD, BDI-II score ≥14, current treatment with an SSRI or SNRI at an adequate dose for ≥6 wk | 480 | 12 | SSRI/SNRI + mirtazapine (15 mg increased to 30 mg/d from week 3), n = 241 | SSRI/SNRI + placebo, n = 239 | Double | Y | Low | BDI-II score, C: 31.5 (10.2), M: 30.6 (9.6) |
| Lauritzen et al,32 1992 | HDRS >16 and or a Melancholia Scale score >15. Inclusion: depressed patients aged 18-67 y who were either too ill to go through a washout period of 7 days, or who had a depressive episode of more than 1 year (prolonged depression), and depressed patients aged >67 y | 40 | 6 | Imipramine (50-100 mg/d according to target plasma level 200 nmol/L) + mianserin (30 mg/d), n = 22 | Imipramine (50-100 mg/d according to target plasma level 200 nmol/L), n = 18 | Double | N (first-line) | Low | HAMD-17, median (IQR), C: 25.2 (21.0-27.0), M: 22.4 (19.0-29.0) |
| Leuchter et al,33,34 2009 | MDD, DSM-IV | 220 | 6 | Escitalopram (10 mg/d) + bupropion XL (300 mg/d), n = 74 (PP; ITT not indicated) | Escitalopram (10 mg/d), n = 73 (PP; ITT not indicated); bupropion XL (300 mg/d), n = 73 (PP; ITT not indicated) | Open | N (first-line) | Unknown/high | HAMD-17, C: 20.4 (4.3), M: 20.6 (4.4); 21.7 (4.0)b |
| Licht et al,35 2002 | MDD, DSM-IV, nonresponse to 4 wk of 50 mg/d and additional 2 wk of 100 mg/d sertraline | 293 | 5 | Sertraline (100 mg/d) + mianserin (30 mg/d), n = 98 | Sertraline (100 mg/d), n = 98; sertraline (200 mg/d), n = 97 | Double | Y | Low | HDRS-17 median (quartiles), C: 23 (21-26), M: 23 (21-26); 23 (22-26) |
| Maes et al,36 1996 | MD, DSM-III-R, nonresponse to ≥2 adequate trials with antidepressive agents from different classes, no prior antidepressant combination treatment | 22 | 4 | Fluoxetine (20 mg/d) + trazodone (100 mg/d), n = 12 | Trazodone (100 mg/d), n = 10 | Double | Both | Unknown/high | HAMD-17, no baseline data specified |
| Maes et al,37 1999 | MDD, DSM-III-R, TRD defined as nonresponse to prior treatment with a single adequate trial with antidepressants | 23 | 5 | Fluoxetine (20 mg/d) + mianserin (30 mg/d), n = 11 | Fluoxetine (20 mg/d), n = 12 | Double | Both | Low | HAMD-17, C: 23.7 (4.2), M: 21.0 (4.6) |
| Matreja et al,38 2012 | MDD, ICD-10 and DSM-IV; newly diagnosed patients, nonresponders, or partial responders to the earlier prescribed antidepressants | 60 | 6 | SSRI (daily escitalopram 10-30 mg, citalopram 20-60 mg, sertraline 50-150 mg, fluoxetine 20-40 mg, paroxetine 10-50 mg) + low-dose mirtazapine (7.5 mg/d), n = 30 | SSRI (daily dose of escitalopram 10-30 mg, citalopram 20-60 mg, sertraline 50-150 mg, fluoxetine 20-40 mg, paroxetine 10-50 mg), n = 30 | Open | Both | Unknown/high | HDRS-17, C: 20.53 (0.47), M: 20.76 (0.32)d |
| Medhus et al,39 1994 | Major depressive episode, DSM-III, nonresponse after treatment with adequate doses of a TCA for ≥4 wk | 37 | 3 | TCA (≥150 mg/d or maximum tolerable dose/range: 100-200 mg/d) + mianserin (60 mg/d), n = 18 | TCA (≥150 mg/d or maximum tolerable dose/range: 75-225 mg/d) + placebo, n = 19 | Double | Y | Unknown/high | MADRS, C: 32.6 (4.3), M: 31.7 (5.6) |
| Mohamed et al,40 2017 (VAST-D) | MDD DSM-IV-TR; PHQ-9; suboptimal response to SSRI, SNRI, or mirtazapin, ie, QIDS-C16 score ≥16 after ≥6 wk or a score ≥11 after ≥8 wk or treatment | 1017 | 12 | Augmentation of current antidepressive agent with bupropion SR (150 mg increased to 400 mg/d by 6 wk and kept at that level through 12 wk) + current antidepressive agent, n = 506 | Switch from current antidepressive agent to bupropion SR (150 mg increased to 400 mg/d by 6 wk and kept at that level through 12 wk), n = 511 | Single (raters) | Y | Unknown/high | QIDS-C16, C: 16.6 (3.2), M: 16.6 (3.3) |
| Murphy et al,41 1977 | Depressive illness of sufficient severity to justify treatment with TCA | 173 | 4 | Clomipramine (10 mg/d) + desipramine (25 mg/d), n = 58 | Clomipramine (10 mg/d), n = 57; desipramine (25 mg/d), n = 58 | Double | N (first-line) | Low | CDR scale-17 item, mean scores, C: 22.98; M: 24.06; 23.71 |
| Navarro et al,11 2019 | Moderate to severe MDD episode, DSM-IV, after nonresponse to 10 wk of venlafaxine ER (225-300 mg/d), pretreatment HDRS-17 score ≥21 | 112 | 10 | Add-on group: venlafaxine (225-300 mg/d) + mirtazapine (15 mg increased to 30 mg/d), n = 56 | Switching group (from venlafaxine): imipramine (50 mg increased to 150 mg/d), n = 56 | Open | Y | Unknown/high | HDRS-17, C: 28.55 (5.95), M: 27.89 (5.40) |
| Nelson et al,42 2004 | Yale Depression Inventory, unipolar nonpsychotic MD | 39 | 6 | Desipramine (target plasma level 160 ng/mL) + fluoxetine (20 mg/d), n = 13 | Fluoxetine (20 mg/d), n = 14; desipramine (target plasma level 160 ng/mL), n = 12 | Double | N (first-line) | Low | MADRS, C: 32 (6.3), M: 37.2 (7.1); 38.3 (7.5) |
| O’Brien et al,43 1993 | Research diagnostic criteria for MD | 79 | 6 | Amitriptyline (150 mg/d) + tranylcypromine (30 mg/d), n = 25 | Amitriptyline (150 mg/d), n = 28; tranylcypromine (30 mg/d), n = 26 | Double | N (first-line) | Unknown/high | HDRS-17: C: 22.0 (4.9), M: 24.1 (6.0); 20.8 (4.9) |
| Raisi et al,44 2007 | MDD, DSM-IV | 45 | 8 | Nortriptyline (50 mg/d) + citalopram (40 mg/d), n = 23 | Citalopram (40 mg/d), n = 22 | Double | N (first-line) | Unknown/high | HAMD-17, C: 30.80 (4.16); M: 31.2 (5.07) |
| Rush et al,45 2011 | DSM-IV-TR, recurrent MD or chronic MD (current episode lasting ≥2 y) | 665 | 12 | Bupropion SR (150-400 mg/d) + escitalopram (10-20 mg/d), n = 221; venlafaxine ER (150-300 mg/d) + mirtazapine (15-45 mg/d), n = 220 | Escitalopram (10-20 mg/d), n = 224 | Single | N (first-line) | Low | HAMD-17, C: 23.8 (4.6); 24.3 (5.0); M: 23.4 (4.9) |
| Stewart et al,46 2014 | MDD, DSM-IV-TR, MADRS ≥22 | 245 | 12 | Escitalopram (10 mg/d increased to 40 mg/d) + bupropion (150 mg/d increased to 450 mg/d), n = 78 | Escitalopram (10 mg/d increased to 40 mg/d), n = 84; bupropion (150 mg/d increased to 450 mg/d), n = 83 | Double | N (first-line) | Low | HAMD-17, C: 21 (5), M: 20 (5); 20 (5). MADRS, C: 30 (5), M: 29 (5); 29 (5). QIDS-SR-16, C: 22 (5), M: 21 (6); 21 (5) |
| Tanghe et al,47 1997 | DSM-III-R, MD episode, resistant to treatment with ≥2 separate antidepressants | 39 | 4 | Moclobemide (200-600 mg/d) + amitriptyline (increased to 280 mg/d), n = 20 | Moclobemide (200-600 mg/d), n = 19; amitriptyline (increased to 280 mg/d), n = 19 | Double | Y | Unknown/high | MADRS, C: 41.8 (6.08), M: 41.26 (8.22); 39.16 (5.1) |
| Vezmar et al,48 2009 | MD, DSM-IV | 22 | 2-6f | Amitriptyline (75 mg/d) + fluvoxamine (100 mg/d), n = 7 | Amitriptyline (75 mg/d), n = 9; fluvoxamine (100 mg/d), n = 6 | Open | N (first-line) | Unknown/high | HAM-D, C: 28.0 (5.5), M: 29.3 (5.8); 29.2 (8.1) |
| White et al,49 1980 | Major or minor depressive disorder | 30 | 4 | Amitriptyline (50-150 mg/d) + tranylcypromine (10-15 mg/d), n = 10 | Amitriptyline (75-300 mg/d), n = 9; tranylcypromine (10-20 mg/d), n = 11 | Open | N (first-line) | Unknown/high | HAMD, no baseline data specified |
| Xiao et al,13 2020 | MD episode, DSM-IV, HAMD-17 score ≥20, and ≥2 on item 1 (depressed mood), non-early response to paroxetine (≥20% decrease of HAMD-17 total score at wk 2) | 204 | 6 | Mirtazapine (30 mg/d) + paroxetine (20 mg/d), n = 68 | Mirtazapine (30 mg/d), n = 68; paroxetine (20 mg/d), n = 68 | Double | Y | Low | HAMD-17, C: 23.60 (3.24), M: 25.34 (4.31); 23.94 (3.47) |
| Xu et al,50 2002 | Depressive neurosis according to CCMD-2-R | 69 | 8 | Fluoxetine (20 mg/d) + amitriptyline (37.5 mg/d), n = 21 | Fluoxetine (20 mg/d), n = 21; amitriptyline (50 mg/d), n = 20 | Open | N (first-line) | Unknown/high | SDS, data not available |
| Yang et al,51 2005 | Refractory depression | 36 | 6 | Citalopram (20 mg increased to 40 mg/d) + amitriptylin (50 mg/d), n = 20 | Citalopram (20 mg/d increased to 40 mg/d), n = 16 | Single | Y | Unknown/high | HAMD, C: 28.9 (6.3), M: 28.5 (6.8) |
| Yazicioglu et al,52 2006 | MDD, DSM-IV | 43 | 10 | Reboxetine (4 mg/d increased to 8 mg/d) + sertraline (50 mg/d), n = 21 | Venlafaxine XR (75 mg/d increased to 150 mg/d), n = 22 | Open | N (first-line) | Unknown/high | HDRS-17, C: 18.1 (1.7), M: 18.3 (1.6) |
| Young et al,53 1979 | Mild or moderate depression not requiring ECT or inpatient admission | 135 | 6 | Trimipramine (mean: 102 mg/d) + phenelzine (mean: 44 mg/d); trimipramine (mean: 96 mg/d) + isocarboxazide (mean: 30 mg/d), n = 51 | Trimipramine (mean: 106 mg/d) + placebo; phenelzine (mean: 45 mg/d) + placebo; isocarboxazide (mean: 32 mg/d) + placebo, n = 84 | Double | N (first-line) | Unknown/high | HDRS, mean score, C: 22.9; 26.6, M: 22.5; 24.8; 24.8 |
Abbreviations: BDI, Beck Depression Inventory; C, combination therapy; CDR, Clinical Depression Rating scale; CCMD, Chinese Classification of Mental Disorders; ECT, electroconvulsive therapy; HDRS, Hamilton Depression Rating Scale (aka HAMD); ICD-10, International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; ITT, intent to treat; M, monotherapy; MADRS, Montgomery-Asberg Depression Rating Scale; MD, major depression; MDD, major depressive disorder; N, no; PHQ, Patient Health Questionnaire; PP, per protocol; PRIME-MD, Primary Care Evaluation of Mental Disorders; QIDS-C16, Quick Inventory of Depressive Symptomatology; SCID, Structured Clinical Interview for DSM-III-R; SDS, Self-Rating Depression Scale; SNRI, serotonin-norepinephrine reuptake inhibitor; SR, sustained release; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant; TRD, treatment-resistant depression; XL, extended release (aka XR); Y, yes.
Values are mean (SD) unless noted otherwise.
Not specified.
Mean (SEM).
Mean (SE).
In week 8.
Follow-up at 2 wk for response and at 4 wk and 6 wk for remission.
Primary Outcome
Of 39 studies included, 38 trial reports provided data on the primary outcome. The SMD was 0.31 (95% CI, 0.19-0.44) in favor of combination treatment (P < .001). Thirty-one of 38 studies (82%) suggested superior efficacy of combination treatments. Between-study heterogeneity was I2 = 77.5% and τ was 0.296 (Figure 2).
Figure 2. Primary Outcome: Efficacy Measured as Standardized Mean Difference (SMD).

Weighted according to random-effects analysis.
Combination therapy was associated with superior outcomes when analyses were restricted to studies of low risk of bias (SMD = 0.29; 95% CI, 0.15-0.42), among nonresponder populations (SMD = 0.18; 95% CI, 0.04-0.33), and when applied as a first-line treatment (SMD = 0.52; 95% CI, 0.24-0.79) (eFigure 1 and eFigure 2 in the Supplement).
Sensitivity and Subgroup Analyses
Results for sensitivity and subgroup analyses are presented in Table 2.
Table 2. Results: Outcomes Across Subgroup Analyses.
| Resultsa | Primary outcomeb | Secondary outcomes | |||||
|---|---|---|---|---|---|---|---|
| SMD (95% CI) | τ | OR (95% CI)c | Continuous change from baseline, SMD (95% CI) | OR (95% CI)d | |||
| Remission | Response | Dropouts | Dropouts due to AE | ||||
| Whole sample | 0.31 (0.19 to 0.44) [38 studies] | 0.296 | 1.52 (1.20 to 1.92) [25 studies] | 1.40 (1.15 to 1.69) [30 studies] | 0.38 (0.22 to 0.54) [28 studies] | 0.99 (0.86 to 1.14) [30 studies] | 1.17 (0.79 to 1.75) [17 studies] |
| I2, % | 77.52 | 63.38 | 44.11 | 84.21 | 3.66 | 20.87 | |
| Whole sample; low risk of bias | 0.29 (0.15 to 0.42) [15 studies] | 0.188 | 1.44 (1.12 to 1.85) [11 studies] | 1.46 (1.14 to 1.87) [14 studies] | 0.34 (0.17 to 0.50) [11 studies] | 1.09 (0.94 to 1.28) [12 studies] | 1.43 (0.87 to 2.34) [9 studies] |
| I2, % | 69.18 | 51.61 | 48.07 | 77.95 | 0 | 15.34 | |
| α2 + RI | 0.37 (0.19 to 0.55) [18 studies] | 0.305 | 1.42 (1.01 to 2.01) [12 studies] | 1.49 (1.18 to 1.87) [15 studies] | 0.44 (0.22 to 0.66) [15 studies] | 1.02 (0.86 to 1.21) [14 studies] | 1.06 (0.49 to 2.31) [10 studies] |
| I2, % | 80.58 | 74.27 | 47.77 | 85.10 | 0 | 34.82 | |
| α2 + RI; low risk of bias | 0.36 (0.19 to 0.53) [11 studies] | 0.221 | 1.39 (1.11 to 1.75) [9 studies] | 1.52 (1.15 to 2.00) [11 studies] | 0.36 (0.16 to 0.56) [9 studies] | 1.04 (0.88 to 1.24) [9 studies] | 1.17 (0.43 to 3.19) [6 studies] |
| I2, % | 75.28 | 37.42 | 53.44 | 78.26 | 0 | 49.63 | |
| α2 + RI; nonresponder/TRD | 0.24 (0.03 to 0.45) [12 studies] | 0.283 | 1.17 (0.82 to 1.67) [8 studies] | 1.35 (1.08 to 1.69) [10 studies] | 0.23 (−0.01 to 0.48) [9 studies] | 1.00 (0.83 to 1.20) [8 studies] | 0.75 (0.31 to 1.82) [6 studies] |
| I2, % | 79.52 | 68.26 | 34.11 | 82.55 | 0 | 0 | |
| α2 + RI; first-line | 0.64 (0.12 to 1.15) [5 studies] | 0.523 | 1.80 (0.74 to 4.37) [3 studies] | 1.54 (0.77 to 3.09) [3 studies] | 0.84 (0.30 to 1.38) [2 studies] | 0.81 (0.38 to 1.72) [3 studies] | 0.62 (0.15 to 2.62) [2 studies] |
| I2, % | 84.21 | 72.19 | 58.54 | 44.05 | 0 | 0 | |
| Combination with bupropion | 0.10 (−0.07 to 0.27) [7 studies] | 0.161 | 1.29 (0.95 to 1.74) [6 studies] | 1.06 (0.88 to 1.29) [5 studies] | 0.06 (−0.19 to 0.31) [4 studies] | 1.45 (0.95 to 2.23) [2 studies] | 1.79 (0.50 to 6.35) [2 studies] |
| I2, % | 59.48 | 45.52 | 0 | 74.07 | 0 | 29.45 | |
| Combination with bupropion; low risk of bias | 0.12 (−0.07 to 0.31) [4 studies] | 0.106 | 1.13 (0.80 to 1.59) [3 studies] | 1.05 (0.75 to 1.48) [3 studies] | 0.09 (−0.14 to 0.32) [2 studies] | 1.45 (0.95 to 2.23) [2 studies] | 1.79 (0.50 to 6.35) [2 studies] |
| I2, % | 30.22 | 13.78 | 0 | 60.35 | 0 | 29.45 | |
| Combination with bupropion; nonresponder/TRD | 0.17 (0.02 to 0.31) [3 studies] | 0.000 | 2.24 (0.61 to 8.26) [2 studies] | 1.15 (0.89 to 1.48) [2 studies] | 0.51 (−0.01 to 1.02) [1 study only] | No data | No data |
| I2, % | 0 | 80.93 | 0 | NA | |||
| Combination with bupropion; first-line | 0.04 (−0.20 to 0.29) [4 studies] | 0.200 | 1.09 (0.83 to 1.43) [4 studies] | 0.95 (0.70 to 1.28) [3 studies] | −0.01 (−0.26 to 0.23) [3 studies] | 1.45 (0.95 to 2.23) [2 studies] | 1.79 (0.50 to 6.35) [2 studies] |
| I2, % | 71.05 | 0 | 0 | 74.60 | 0 | 29.45 | |
Abbreviations: α2, antagonists of presynaptic α2-autoreceptors; AE, adverse events; NA, not applicable; OR, odds ratio; RI, (monoamine) reuptake inhibitor; SMD, standardized mean difference; TRD, treatment-resistant depression.
Of note, depending on design specifics, not all studies may be included in all outcome analyses (eg, only 17 randomized double-blind studies reported data on the number of dropouts due to AE).
For the primary outcome, SMD >0 in favor of combination.
For the secondary outcomes remission, response, and continuous change from baseline, OR >1 designates superiority of combination treatment; SMD >0 designates superiority of combination treatment.
For the secondary outcomes dropouts and dropouts due to AE, OR >1 designates superiority of monotherapy, ie, fewer dropouts in monotherapy groups.
Combination of a monoamine reuptake inhibitor with an antagonist of presynaptic α2-autoreceptors (RI+α2) was associated with superior outcomes relative to monotherapy: among all 18 RCTs (SMD = 0.37; 95% CI, 0.19-0.55) (Figure 3), among nonresponder populations (SMD = 0.24; 95% CI, 0.03-0.45), and in particular when applied as a first-line treatment (SMD = 0.64; 95% CI, 0.12-1.15).
Figure 3. Primary Outcome: Subgroup Analysis of Treatment With a Monoamine Reuptake Inhibitor Plus an Antagonist of Presynaptic α2-Autoreceptors.
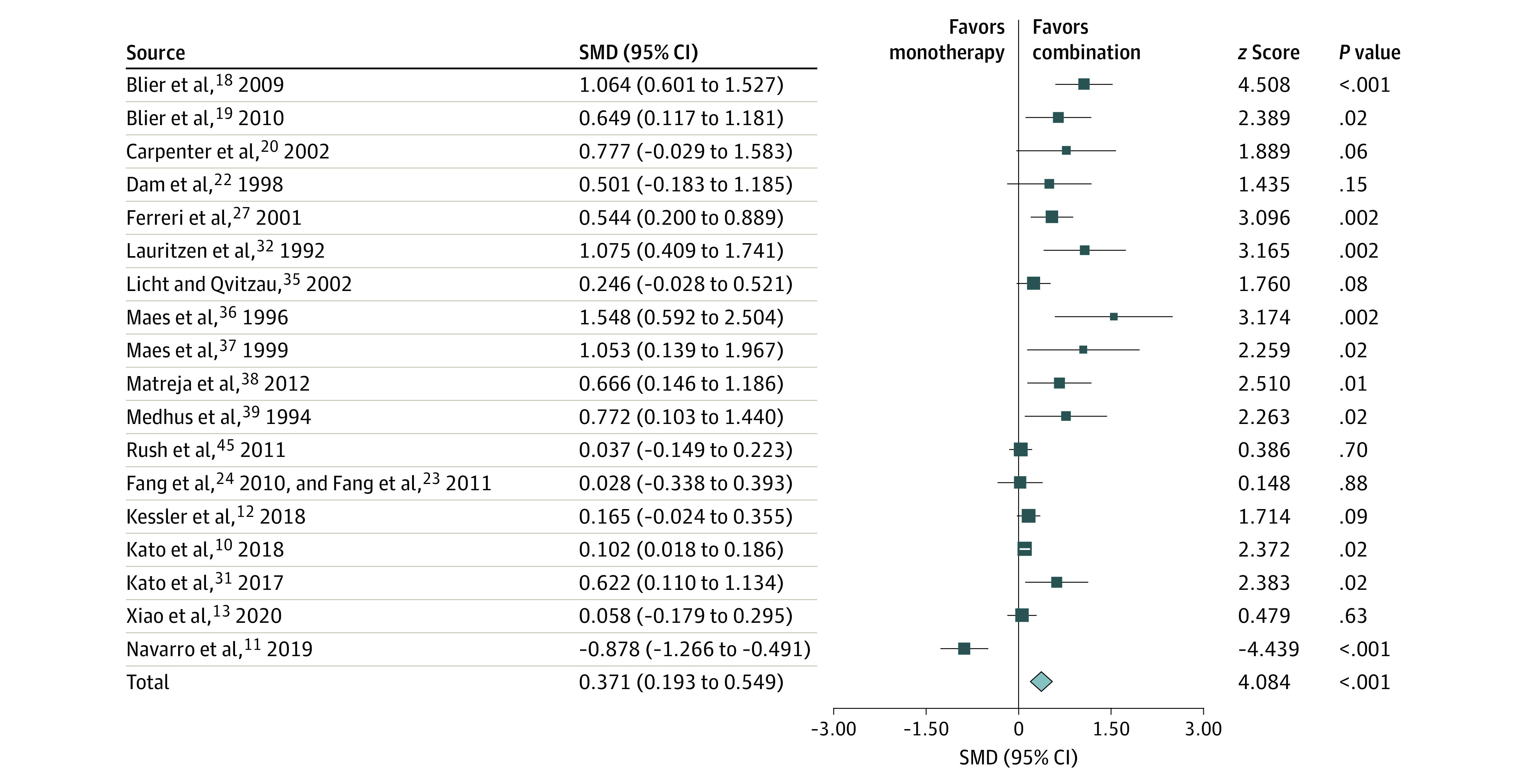
Efficacy was measured as standardized mean difference (SMD) and weighted according to random-effects analysis.
Combination therapy that included bupropion was not associated with superior outcomes compared with monotherapy. This applied to analyses among all 7 RCTs (SMD = 0.10; 95% CI, −0.07 to 0.27) (eFigure 3 in the Supplement), and to its application as first-line treatment (SMD = 0.04; 95% CI, −0.20 to 0.29). Among nonresponder populations, bupropion combinations were superior to monotherapy, with an SMD of 0.17 (95% CI, 0.02 to 0.31).
To avoid undue reliance on single studies, we removed each of the 38 studies in our primary outcome analysis 1 at a time from the calculation of the summary effect. None of the 38 rounds resulted in a substantial change of point estimate or significance for the primary outcome analysis of all RCTs. Effect sizes varied between 0.2 (after elimination of Xu et al50) and 0.34 (when Navarro et al11 was removed).
For RI+α2 analyses of RCTs, effect sizes varied between 0.32 (after elimination of Blier et al18) and 0.43 (when Kato et al10 was removed).
For bupropion combination analyses of RCTs, effect sizes varied between 0.06 (after elimination of Gulrez et al29) and 0.15 (when Leuchter et al33,34 was removed).
Post Hoc Analyses
In meta-regression, baseline HDRS scores were not associated with the SMD between combination treatment and mono-therapy (coefficient: 1.1; 95% CI, −1.3 to 3.5; P = .37; n = 26 studies). In a sensitivity analysis of 18 studies reporting outcome data that were based on follow-up examinations, such as remission rates, meta-regression returned similar results (coefficient: −2.6; 95% CI, −8.3 to 3.1; P = .37). Meta-regression based on MADRS baseline scores was not calculated as planned because the number of studies providing data was too small.
Secondary Outcomes
Primary and secondary outcomes as well as subgroup analyses are presented in Table 2.
Secondary outcome analyses of efficacy, based on remission and response rates as well as continuous data (change from baseline in rating scale scores), produced results that were generally in line with our primary outcome results (Table 2).
Tolerability
With respect both to patients dropping out of treatment for any reason and to dropouts due to adverse events, data for combination and monotherapy were similar (odds ratio = 0.99; 95% CI, 0.86-1.14; and odds ratio = 1.17; 95% CI, 0.79-1.75, respectively). Heterogeneity in these analyses was low (I2 = 3.66% and I2 = 20.87%, respectively).
Risk of Bias
Fifteen of the 39 included studies (38%) were considered to be of higher methodological rigor (“low” risk of bias). Summary ratings confirmed our primary outcome analysis and are displayed in Table 1 (also Figure 2 and Figure 3).
Heterogeneity
I2 statistics indicated substantial between-study heterogeneity in most of the primary outcome analyses, but significantly less so in most of the subgroup analyses, especially in analyses of response and dropouts (Table 2). Heterogeneity as measured by τ was substantially lower in sensitivity analyses (restricted to studies with low risk of bias), and τ indicated that the standard deviation of the weighted SMD estimate was approximately equal to or lower than the effect size.
Publication Bias
The funnel plot of studies included in the primary outcome analysis indicated small study effects (eFigure 4 in the Supplement). An Egger test result was positive (P = .007, df = 36). A trim-and-fill procedure (Duval and Tweedie) with 10 studies trimmed to the left of the mean resulted in a reduced effect size that was still statistically significant (0.13; 95% CI, 0.001-0.26). Twenty-two studies with an effect size of 0 would be necessary to reduce the overall effect to 0.1 (Orwin fail-safe N).
For RI+α2 analyses, an Egger test result was positive (P = .02, df = 16). A trim-and-fill procedure (Duval and Tweedie) with 6 studies trimmed to the left of the mean resulted in a reduced effect size that was still statistically significant (0.19; 95% CI, 0.01-0.36).
Discussion
This study yielded 2 main results. First, combination treatment as a general principle seems to be more effective than monotherapy without being associated with higher numbers of patients dropping out. Second, the combination of monoamine reuptake inhibitors (selective serotonin reuptake inhibitor, serotonin-norepinephrine reuptake inhibitor, or tricyclic antidepressant) and α2-adrenergic receptor antagonists (RI+α2) seems to be the most effective and preferable antidepressant combination.
Combination therapy may primarily be applied as a second-step treatment after insufficient response to initial monotherapy. Our findings suggest that using an RI+α2 combination is more effective in these cases compared with monotherapy. On the other hand, in a recent meta-analysis, switching antidepressant monotherapy for patients considered nonresponders was not more effective than sticking to the initial antidepressant.54 In the same vein, after nonresponse to a standard dose of selective serotonin reuptake inhibitor, a dose increase did not result in superior efficacy compared with continuation of the initial dose.55
Combination therapy was not associated with more dropouts or adverse events leading to discontinuation. It may thus be a safe treatment alternative when compared with other second-step strategies in treatment-resistant depression, such as augmenting monotherapy with lithium or atypical antipsychotics.56,57 Our analysis of the RI+α2 combination in nonresponders resulted in statistically significant but small effect sizes (SMD = 0.2). Still, patients who are resistant to treatment present a particular challenge, and effect sizes resulted from comparisons with active treatment (ongoing monotherapy, increasing the dose, or switching antidepressants). Such comparisons are likely to result in lower estimates of efficacy than contrasting combination and monotherapy in first-line treatment trials. Here, the RI+α2 combination seems to be particularly effective, with an effect size of SMD = 0.64. Antidepressant monotherapy itself has effect sizes of no more than about 0.3 compared with placebo.58,59 Of note, trials in our analysis also included populations with difficult-to-treat chronic depression.32,45
We have previously shown that the favorable treatment outcomes of combination therapy in comparison with monotherapy are not a dosage effect only.9 Also, some of the included trials found superior effects with subtherapeutic doses of a second antidepressant in RI+α2 combinations.32,36,37,38 Therefore, pharmacodynamic and clinical synergisms seem likely. For example, sedating α2-adrenergic receptor antagonists may counteract the restlessness, agitation, and sexual dysfunction associated with monoamine reuptake inhibitors. Reuptake inhibitors in monotherapy are likely to stimulate presynaptic α2-receptors by enhancing the intrasynaptic concentrations of serotonin and norepinephrine. However, combinations with blockers of presynaptic α2-receptors are supposed to prevent the negative feedback effect on neurotransmission induced by a stimulation of α2-receptors.
The relative tolerability of combination therapy and the modest response rates with initial antidepressant monotherapy also suggest considering RI+α2 combination therapy as a first-line treatment, at least in severe cases of depression.
On the whole, in our analysis, treatment effects of antidepressant combinations were not associated with baseline severity. According to these results, combination treatment is effective regardless of initial illness severity. Nevertheless, this finding must be viewed as preliminary because it rests on a subset of studies and only on outcomes ascertained by HDRS.
While the addition of bupropion has previously been shown to alleviate antidepressant-induced sexual dysfunction,14 and its addition to antidepressant monotherapy can be clinically sensible, our findings indicate that bupropion combinations in general are not associated with substantial enhancement of antidepressive efficacy compared with monotherapy. This result is counterintuitive because bupropion, with its dopaminergic properties, has a mechanism of action that may complement classical antidepressant pathways. Note that in nonresponder populations, the summary results for bupropion combinations remain inconclusive rather than negative, mainly because of the small number of methodologically sound studies existing to date: the CI spans a negative as well as a sizable positive effect.
Limitations
First, I2 values indicated substantial heterogeneity of effects. However, heterogeneity is known to increase with accumulating numbers. Additional τ statistics were calculated, indicating a spread of data not unfamiliar in medical studies: the standard deviation was lower than or had the same order of magnitude as the effect size. Nevertheless, as in most meta-analyses, included studies were not homogenous in their design, eg, with differences in blinding status or in the definition of nonresponse to previous antidepressant treatment. As a consequence, we applied random-effects models and showed that results remained robust after each study was left out. Further, dichotomizing criteria of treatment success in subgroup analyses, as in remission and response, supported the main results and explained large parts of the between-study heterogeneity. In the same vein, sensitivity analyses among studies of high methodological rigor (low risk of bias) and among double-blind studies (data not shown) also backed our main findings.
Second, funnel plot asymmetry indicated possible reporting bias. However, in combination treatment studies, reporting bias might not be as important as it is in placebo trials of antidepressant monotherapies because there is no negative result in the strict sense, and thus no disincentive to publish. Nevertheless, even when fully adjusting for possible publication bias, a reduced but still positive and statistically significant effect remained (for RI+α2 combination: SMD = 0.19; 95% CI, 0.01-0.36). Also, the observed funnel plot asymmetry may be caused by plot distortion associated with transforming a variety of outcomes into SMD, as has recently been emphasized.60 Reassuringly, therefore, sensitivity analyses using raw mean differences resulted in a substantially reduced funnel plot asymmetry (data not shown).
There also is considerable indication that the funnel plot asymmetry may represent a true asymmetry of observable effects. For example, many of the studies on bupropion combinations were recent and had large sample sizes but yielded only small effects. Low effect size and small-variance studies may distort funnel plots to the upper left quadrant. Besides, we observed funnel plot asymmetry only among studies of treatment-resistant depression and not among studies using a combination as a first-line treatment.
Third, true between-study heterogeneity may result not only from different study populations and combination treatments but also from different control groups. This particularly applies to studies of treatment-resistant depression, where active comparators were continuation, increased dose, or switching antidepressant. And yet, regardless of the kind of comparator, combination treatment was associated with higher efficacy (data not shown).
It is conceivable that antidepressant discontinuation syndromes may have interacted with outcomes. However, it has been shown that when the switch is between antidepressants, discontinuation syndromes rarely pose clinical problems.61
Conclusions
For clinical practice, physicians should be aware that combinations of reuptake inhibitors (selective serotonin reuptake inhibitor, serotonin-norepinephrine reuptake inhibitor, or tricyclic antidepressant) with α2-autoreceptor antagonists are a potent treatment option, associated with superior outcomes relative to monotherapy. Clinicians can inform patients that on average this advantage does not come at the cost of lower tolerability and that there is reason to believe in a synergistic therapeutic effect. While we did not find an association of outcome and severity of depression, we believe combination treatment particularly suggests itself in severe cases of depression and for patients resistant to standard treatment. Research should focus on the dearth of methodologically rigorous data on bupropion combinations for nonresponder populations.
eFigure 1. Forest Plot 3: Primary Outcome – Subgroup Analysis: First-line Studies Only
eFigure 2. Forest Plot 4: Primary Outcome – Subgroup Analysis: Nonresponder Studies Only
eFigure 3. Forest Plot 5: Primary Outcome – Subgroup Analysis: Bupropion Combinations
eFigure 4. Funnel Plot of Primary Outcome Analysis
eMethods
eReferences
eAppendix. Explicit Search Entry
References
- 1.National Collaborating Centre for Mental Health (UK) . Depression: The Treatment and Management of Depression in Adults (Updated Edition). Published online 2010. Accessed October 13, 2020. https://www.ncbi.nlm.nih.gov/books/NBK63748/
- 2.Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder: third edition. Published October 2010. Accessed January 11, 2022. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf
- 3.American Psychological Association. Depression treatments for adults. https://www.apa.org. Published August 2019. Accessed October 13, 2020. https://www.apa.org/depression-guideline/adults
- 4.DGPPN, BÄK, KBV, AWMF, AkdÄ, BPtK, BApK, DAGSHG, DEGAM, DGPM, DGPs, DGRW, eds; for the Guideline Group Unipolar Depression. S3 Guideline: National Care Guideline (NVL) Unipolar Depression, 2nd edition, Version 1. Published November 2015. Accessed April 12, 2021. http://www.depression.versorgungsleitlinien.de
- 5.Henssler J, Kurschus M, Franklin J, Bschor T, Baethge C. Trajectories of acute antidepressant efficacy: how long to wait for response? a systematic review and meta-analysis of long-term, placebo-controlled acute treatment trials. J Clin Psychiatry. 2018;79(3):17r11470. doi: 10.4088/JCP.17r11470 [DOI] [PubMed] [Google Scholar]
- 6.Schneider F, Härter M, Schorr S. S3-Leitlinie/Nationale VersorgungsLeitlinie Unipolare Depression. Published online March 3, 2017. doi: 10.1007/978-3-662-52906-5 [DOI]
- 7.Köhler S, Unger T, Hoffmann S, Steinacher B, Fydrich T, Bschor T. Comparing augmentation with non-antidepressants over sticking to antidepressants after treatment failure in depression: a naturalistic study. Pharmacopsychiatry. 2013;46(2):69-76. doi: 10.1055/s-0032-1323677 [DOI] [PubMed] [Google Scholar]
- 8.Valenstein M, McCarthy JF, Austin KL, Greden JF, Young EA, Blow FC. What happened to lithium? antidepressant augmentation in clinical settings. Am J Psychiatry. 2006;163(7):1219-1225. doi: 10.1176/ajp.2006.163.7.1219 [DOI] [PubMed] [Google Scholar]
- 9.Henssler J, Bschor T, Baethge C. Combining antidepressants in acute treatment of depression: a meta-analysis of 38 studies including 4511 patients. Can J Psychiatry. 2016;61(1):29-43. doi: 10.1177/0706743715620411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kato T, Furukawa TA, Mantani A, et al. ; SUN☺D Investigators . Optimising first- and second-line treatment strategies for untreated major depressive disorder, the SUN☺D study: a pragmatic, multi-centre, assessor-blinded randomised controlled trial. BMC Med. 2018;16(1):103. doi: 10.1186/s12916-018-1096-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navarro V, Boulahfa I, Obach A, et al. Switching to imipramine versus add-on mirtazapine in venlafaxine-resistant major depression: a 10-week randomized open study. J Clin Psychopharmacol. 2019;39(1):63-66. doi: 10.1097/JCP.0000000000000988 [DOI] [PubMed] [Google Scholar]
- 12.Kessler DS, MacNeill SJ, Tallon D, et al. Mirtazapine added to SSRIs or SNRIs for treatment resistant depression in primary care: phase III randomised placebo controlled trial (MIR). BMJ. 2018;363:k4218. doi: 10.1136/bmj.k4218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao L, Zhu X, Gillespie A, et al. Effectiveness of mirtazapine as add-on to paroxetine v. paroxetine or mirtazapine monotherapy in patients with major depressive disorder with early non-response to paroxetine: a two-phase, multicentre, randomized, double-blind clinical trial. Psychol Med. 2021;51(7):1166-1174. doi: 10.1017/S0033291719004069 [DOI] [PubMed] [Google Scholar]
- 14.Taylor MJ, Rudkin L, Bullemor-Day P, Lubin J, Chukwujekwu C, Hawton K. Strategies for managing sexual dysfunction induced by antidepressant medication. Cochrane Database Syst Rev. 2013;(5):CD003382. doi: 10.1002/14651858.CD003382.pub3 [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions, version 6.0. Updated July 2019. Accessed October 21, 2019. http://www.training.cochrane.org/handbook
- 17.Bares M, Novak T, Kopecek M, et al. Antidepressant monotherapy compared with combinations of antidepressants in the treatment of resistant depressive patients: a randomized, open-label study. Int J Psychiatry Clin Pract. 2013;17(1):35-43. doi: 10.3109/13651501.2012.674533 [DOI] [PubMed] [Google Scholar]
- 18.Blier P, Gobbi G, Turcotte JE, et al. Mirtazapine and paroxetine in major depression: a comparison of monotherapy versus their combination from treatment initiation. Eur Neuropsychopharmacol. 2009;19(7):457-465. doi: 10.1016/j.euroneuro.2009.01.015 [DOI] [PubMed] [Google Scholar]
- 19.Blier P, Ward HE, Tremblay P, Laberge L, Hébert C, Bergeron R. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry. 2010;167(3):281-288. doi: 10.1176/appi.ajp.2009.09020186 [DOI] [PubMed] [Google Scholar]
- 20.Carpenter LL, Yasmin S, Price LH. A double-blind, placebo-controlled study of antidepressant augmentation with mirtazapine. Biol Psychiatry. 2002;51(2):183-188. doi: 10.1016/S0006-3223(01)01262-8 [DOI] [PubMed] [Google Scholar]
- 21.Cha JH, Jung IK, Lee MS. Comparison between dothiepin-sertraline combination and dothiepin alone therapy in the treatment of depressive disorder. Korean J Biol Psychiatry. 1997;4(2):251-258. [Google Scholar]
- 22.Dam J, Ryde L, Svejsø J, Lauge N, Lauritsen B, Bech P. Morning fluoxetine plus evening mianserin versus morning fluoxetine plus evening placebo in the acute treatment of major depression. Pharmacopsychiatry. 1998;31(2):48-54. doi: 10.1055/s-2007-979298 [DOI] [PubMed] [Google Scholar]
- 23.Fang Y, Yuan C, Xu Y, et al. ; OPERATION Study Team . A pilot study of the efficacy and safety of paroxetine augmented with risperidone, valproate, buspirone, trazodone, or thyroid hormone in adult Chinese patients with treatment-resistant major depression. J Clin Psychopharmacol. 2011;31(5):638-642. doi: 10.1097/JCP.0b013e31822bb1d9 [DOI] [PubMed] [Google Scholar]
- 24.Fang Y, Yuan C, Xu Y, et al. ; OPERATION Study Team . Comparisons of the efficacy and tolerability of extended-release venlafaxine, mirtazapine, and paroxetine in treatment-resistant depression: a double-blind, randomized pilot study in a Chinese population. J Clin Psychopharmacol. 2010;30(4):357-364. doi: 10.1097/JCP.0b013e3181e7784f [DOI] [PubMed] [Google Scholar]
- 25.Fava M, Rosenbaum JF, McGrath PJ, Stewart JW, Amsterdam JD, Quitkin FM. Lithium and tricyclic augmentation of fluoxetine treatment for resistant major depression: a double-blind, controlled study. Am J Psychiatry. 1994;151(9):1372-1374. doi: 10.1176/ajp.151.9.1372 [DOI] [PubMed] [Google Scholar]
- 26.Fava M, Alpert J, Nierenberg A, et al. Double-blind study of high-dose fluoxetine versus lithium or desipramine augmentation of fluoxetine in partial responders and nonresponders to fluoxetine. J Clin Psychopharmacol. 2002;22(4):379-387. doi: 10.1097/00004714-200208000-00008 [DOI] [PubMed] [Google Scholar]
- 27.Ferreri M, Lavergne F, Berlin I, Payan C, Puech AJ. Benefits from mianserin augmentation of fluoxetine in patients with major depression non-responders to fluoxetine alone. Acta Psychiatr Scand. 2001;103(1):66-72. doi: 10.1111/j.1600-0447.2001.00148.x [DOI] [PubMed] [Google Scholar]
- 28.Fornaro M, Martino M, Mattei C, et al. Duloxetine-bupropion combination for treatment-resistant atypical depression: a double-blind, randomized, placebo-controlled trial. Eur Neuropsychopharmacol. 2014;24(8):1269-1278. doi: 10.1016/j.euroneuro.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 29.Gulrez G, Badyal DK, Deswal RS, Sharma A. Bupropion as an augmenting agent in patients of depression with partial response. Basic Clin Pharmacol Toxicol. 2012;110(3):227-230. doi: 10.1111/j.1742-7843.2011.00788.x [DOI] [PubMed] [Google Scholar]
- 30.Jie Y, Shuang D, Xinjie Z. Effects of concurrent treatment with amitriptyline hydrochloride tablets and fluoxetine hydrochloride on therapeutic indicator levels in patients with depression. Trop J Pharm Res. 2019;18(2):403-408. doi: 10.4314/tjpr.v18i2.26 [DOI] [Google Scholar]
- 31.Kato M, Takekita Y, Koshikawa Y, et al. Non response at week 4 as clinically useful indicator for antidepressant combination in major depressive disorder: a sequential RCT. J Psychiatr Res. 2017;89:97-104. doi: 10.1016/j.jpsychires.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 32.Lauritzen L, Clemmesen L, Klysner R, et al. Combined treatment with imipramine and mianserin: a controlled pilot study. Pharmacopsychiatry. 1992;25(4):182-186. doi: 10.1055/s-2007-1014403 [DOI] [PubMed] [Google Scholar]
- 33.Leuchter AF, Cook IA, Gilmer WS, et al. Effectiveness of a quantitative electroencephalographic biomarker for predicting differential response or remission with escitalopram and bupropion in major depressive disorder. Psychiatry Res. 2009;169(2):132-138. doi: 10.1016/j.psychres.2009.04.004 [DOI] [PubMed] [Google Scholar]
- 34.Leuchter AF, Cook IA, Marangell LB, et al. Comparative effectiveness of biomarkers and clinical indicators for predicting outcomes of SSRI treatment in major depressive disorder: results of the BRITE-MD study. Psychiatry Res. 2009;169(2):124-131. doi: 10.1016/j.psychres.2009.06.004 [DOI] [PubMed] [Google Scholar]
- 35.Licht RW, Qvitzau S. Treatment strategies in patients with major depression not responding to first-line sertraline treatment: a randomised study of extended duration of treatment, dose increase or mianserin augmentation. Psychopharmacology (Berl). 2002;161(2):143-151. doi: 10.1007/s00213-002-0999-0 [DOI] [PubMed] [Google Scholar]
- 36.Maes M, Vandoolaeghe E, Desnyder R. Efficacy of treatment with trazodone in combination with pindolol or fluoxetine in major depression. J Affect Disord. 1996;41(3):201-210. doi: 10.1016/S0165-0327(96)00089-4 [DOI] [PubMed] [Google Scholar]
- 37.Maes M, Libbrecht I, van Hunsel F, Campens D, Meltzer HY. Pindolol and mianserin augment the antidepressant activity of fluoxetine in hospitalized major depressed patients, including those with treatment resistance. J Clin Psychopharmacol. 1999;19(2):177-182. doi: 10.1097/00004714-199904000-00014 [DOI] [PubMed] [Google Scholar]
- 38.Matreja PS, Badyal DK, Deswal RS, Sharma A. Efficacy and safety of add on low-dose mirtazapine in depression. Indian J Pharmacol. 2012;44(2):173-177. doi: 10.4103/0253-7613.93843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medhus A, Heskestad S, Tjemsland L. Mianserin added to tricyclic antidepressants in depressed patients not responding to a tricyclic antidepressant alone: a randomized, placebo-controlled, double-blind study. Nord J Psychiatry. 1994;48(5):355-358. doi: 10.3109/08039489409081375 [DOI] [Google Scholar]
- 40.Mohamed S, Johnson GR, Chen P, et al. ; and the VAST-D Investigators . Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132-145. doi: 10.1001/jama.2017.8036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy JE. A comparative trial of anafranil, pertofran and an anafranil/pertofran combination. J Int Med Res. 1977;5(1)(suppl):16-23. [PubMed] [Google Scholar]
- 42.Nelson JC, Mazure CM, Jatlow PI, Bowers MB Jr, Price LH. Combining norepinephrine and serotonin reuptake inhibition mechanisms for treatment of depression: a double-blind, randomized study. Biol Psychiatry. 2004;55(3):296-300. doi: 10.1016/j.biopsych.2003.08.007 [DOI] [PubMed] [Google Scholar]
- 43.O’Brien S, McKeon P, O’Regan M. The efficacy and tolerability of combined antidepressant treatment in different depressive subgroups. Br J Psychiatry. 1993;162:363-368. doi: 10.1192/bjp.162.3.363 [DOI] [PubMed] [Google Scholar]
- 44.Raisi F, Habibi N, Nasehi AA, Akhondzadeh S. Combination of citalopram and nortriptyline in the treatment of severe major depression: a double-blind, placebo-controlled trial. Clin Pract. 2007;4(2):187. [Google Scholar]
- 45.Rush AJ, Trivedi MH, Stewart JW, et al. Combining medications to enhance depression outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. Am J Psychiatry. 2011;168(7):689-701. doi: 10.1176/appi.ajp.2011.10111645 [DOI] [PubMed] [Google Scholar]
- 46.Stewart JW, McGrath PJ, Blondeau C, et al. Combination antidepressant therapy for major depressive disorder: speed and probability of remission. J Psychiatr Res. 2014;52:7-14. doi: 10.1016/j.jpsychires.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 47.Tanghe A, Steeman J, Bollen E, van Renynghe de Voxvrie G, Dendooven M, Haazen L. Moclobemide and amitriptyline, alone or in combination, in therapy resistant depression. Hum Psychopharmacol Clin Exp. 1997;12(5):509-510. doi: [DOI] [Google Scholar]
- 48.Vezmar S, Miljkovic B, Vucicevic K, et al. Pharmacokinetics and efficacy of fluvoxamine and amitriptyline in depression. J Pharmacol Sci. 2009;110(1):98-104. doi: 10.1254/jphs.09013FP [DOI] [PubMed] [Google Scholar]
- 49.White K, Pistole T, Boyd JL. Combined monoamine oxidase inhibitor-tricyclic antidepressant treatment: a pilot study. Am J Psychiatry. 1980;137(11):1422-1425. doi: 10.1176/ajp.137.11.1422 [DOI] [PubMed] [Google Scholar]
- 50.Xu J, Tan J, Ou L. A comparative study of fluoxetine with a little dose of amitriptyline in treating depressive neurosis. Chin J Clin Rehabil. 2002;6(9):1312-1313. [Google Scholar]
- 51.Yang C, Wen Q, Wang X, Lu X. Comparative study of citalopram combined with amitriptyline for treatment of refractory depression. Int Med Health Guid News. 2005;11(4):69-70. [Google Scholar]
- 52.Yazicioglu B, Akkaya C, Sarandol A, Akgoz S, Saygin Eker S, Kirli S. A comparison of the efficacy and tolerability of reboxetine and sertraline versus venlafaxine in major depressive disorder: a randomized, open-labeled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(7):1271-1276. doi: 10.1016/j.pnpbp.2006.04.018 [DOI] [PubMed] [Google Scholar]
- 53.Young JP, Lader MH, Hughes WC. Controlled trial of trimipramine, monoamine oxidase inhibitors, and combined treatment in depressed outpatients. Br Med J. 1979;2(6201):1315-1317. doi: 10.1136/bmj.2.6201.1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bschor T, Kern H, Henssler J, Baethge C. Switching the antidepressant after nonresponse in adults with major depression: a systematic literature search and meta-analysis. J Clin Psychiatry. 2018;79(1):16r10749. doi: 10.4088/JCP.16r10749 [DOI] [PubMed] [Google Scholar]
- 55.Rink L, Braun C, Bschor T, Henssler J, Franklin J, Baethge C. Dose increase versus unchanged continuation of antidepressants after initial antidepressant treatment failure in patients with major depressive disorder: a systematic review and meta-analysis of randomized, double-blind trials. J Clin Psychiatry. 2018;79(3):17r11693. doi: 10.4088/JCP.17r11693 [DOI] [PubMed] [Google Scholar]
- 56.Strawbridge R, Carter B, Marwood L, et al. Augmentation therapies for treatment-resistant depression: systematic review and meta-analysis. Br J Psychiatry. 2019;214(1):42-51. doi: 10.1192/bjp.2018.233 [DOI] [PubMed] [Google Scholar]
- 57.Bschor T, Bauer M, Adli M. Chronic and treatment resistant depression: diagnosis and stepwise therapy. Dtsch Arztebl Int. 2014;111(45):766-775. doi: 10.3238/arztebl.2014.0766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5(2):e45. doi: 10.1371/journal.pmed.0050045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391(10128):1357-1366. doi: 10.1016/S0140-6736(17)32802-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zwetsloot PP, Van Der Naald M, Sena ES, et al. Standardized mean differences cause funnel plot distortion in publication bias assessments. Elife. 2017;6:e24260. doi: 10.7554/eLife.24260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Henssler J, Heinz A, Brandt L, Bschor T. Antidepressant withdrawal and rebound phenomena. Dtsch Arztebl Int. 2019;116(20):355-361. doi: 10.3238/arztebl.2019.0355 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Forest Plot 3: Primary Outcome – Subgroup Analysis: First-line Studies Only
eFigure 2. Forest Plot 4: Primary Outcome – Subgroup Analysis: Nonresponder Studies Only
eFigure 3. Forest Plot 5: Primary Outcome – Subgroup Analysis: Bupropion Combinations
eFigure 4. Funnel Plot of Primary Outcome Analysis
eMethods
eReferences
eAppendix. Explicit Search Entry



