Key Points
Question
What are the associations between Alzheimer disease (AD) and related dementias pathologies and the clinical presentation of common neuropsychiatric symptoms (NPS)?
Findings
In this cohort study of 1808 brains from the National Alzheimer Coordinating Center, hallucinations were more common in individuals with AD and Lewy body disease (LBD) neuropathology compared with LBD alone; apathy and disinhibition were common in individuals with behavioral variant frontotemporal lobar degeneration; and apathy and disinhibition frequently occurred in individuals with hippocampal sclerosis. Increased severity of neuropathology was associated with increased NPS across diagnoses.
Meaning
An association between severity of neuropathology and severity of NPS suggests that NPS may reflect underlying neuropathology; the findings of increased apathy and disinhibition in individuals with hippocampal sclerosis merit further investigation.
This study evaluates the association of Alzheimer disease and related dementias with common neuropsychiatric symptoms.
Abstract
Importance
Understanding associations of Alzheimer disease (AD) and related dementias (ADRD) pathologies with common neuropsychiatric symptoms (NPS) may have implications for diagnosis and management.
Objective
To evaluate ADRD neuropathological diagnoses and NPS without consideration of clinical diagnosis.
Design, Setting, and Participants
This retrospective cohort study evaluated 1808 brains from 39 sites in the US National Alzheimer Coordinating Center v. 10 collection for participants among whom the Neuropsychiatric Inventory Questionnaire (NPIQ) was administered annually. Brain autopsy diagnoses of AD, Lewy body disease (LBD), cerebral amyloid angiopathy, frontotemporal lobar degeneration, cerebrovascular disease, hippocampal sclerosis, and no known pathology were examined. Autopsy data collected from January 2012 to January 2018 were deidentified and compiled into the publicly available v. 10 database. Data were analyzed from February 2021 to August 2021.
Main Outcomes and Measures
The primary outcome was NPIQ domain score, if present at any time point, and mean NPIQ domain score during follow-up was secondary. Associations of ADRD diagnoses with 12 NPIQ symptom domains were examined in regression analyses, correcting for multiple comparisons.
Results
The study sample of 1808 adults had a mean (SD) age of 80.0 (11.0) years, and 987 participants (54.6%) were male. Apathy was the most prevalent NPS, reaching 80% (203 of 254 individuals) in those with hippocampal sclerosis. Cerebrovascular disease showed few NPS associations. Frontotemporal lobar degeneration was associated with increased apathy, increased disinhibition, and decreased psychosis and agitation compared with AD. Hippocampal sclerosis was associated with increased apathy (odds ratio, 2.60; 95% CI; 1.86-3.66, false discovery rate controlled P < .001) and disinhibition (odds ratio, 2.15; 95% CI, 1.63-2.84; false discovery rate controlled P < .001). In multiple regression analyses that included concomitant neuropathologies, the main findings remained. More severe pathology was consistently associated with increased NPS (eg, LBD was associated with an increase in hallucinations from brain stem [β, 0.23; 95% CI, 0.07-0.76; P = .02] to limbic [β, 1.69; 95% CI, 1.27-2.27; P < .001] to neocortical [β, 4.49; 95% CI, 3.27-6.16; P < .001] pathology). Hallucinations were more common in participants with AD and LBD (168 of 534 [31.5%]) compared with those with AD without LBD (152 of 704 [21.6%]) and those with LBD without AD (23 of 119 [19.6%]).
Conclusions and Relevance
In this cohort study of 1808 brains from the US National Alzheimer Coordinating Center, patients with LBD and AD showed a higher prevalence of hallucinations compared with those with LBD without AD. Neuropsychiatric symptom criteria of apathy and disinhibition in behavioral variant frontotemporal lobar degeneration were supported in this study. In hippocampal sclerosis, the findings of increased apathy and disinhibition merit further investigation. Severity of neuropathology was associated with NPS severity, indicating that NPS may reflect underlying ADRD pathology and highlighting the importance of diagnosing and treating NPS.
Introduction
Neuropsychiatric symptoms (NPS) frequently occur in individuals with Alzheimer disease (AD) and related dementias (ADRD).1,2 Psychosis, agitation, aggression, depression, anxiety, and apathy are common NPS in people with ADRD. The prevalence of psychosis, defined by the presence of delusions or hallucinations, differs by clinically diagnosed subtype of dementia: 10% to 15% in those with behavioral variant frontotemporal dementia and vascular dementia, 20% to 45% in those with AD, and 30% to 80% in those with dementia with Lewy bodies.1,2,3,4 Agitation occurs in nearly one-third of patients with AD at the mild to moderate stage of illness and increases with disease severity.1,2 Symptoms of psychosis, agitation, and aggression are associated with more rapid cognitive decline, increased caregiver burden, institutionalization, and mortality.5,6 Anxiety and depression are common in patients across the ADRD spectrum.1,2 Apathy has been reported in most patients with AD and behavioral variant frontotemporal dementia.7,8,9 NPS are part of the diagnostic criteria for specific neurodegenerative disorders, including visual hallucinations for Dementia with Lewy bodies and apathy and disinhibition for behavioral variant frontotemporal dementia.7,10,11
Neuropathology remains the criterion standard for the diagnosis of ADRD. In a study from the National Alzheimer Coordinating Center (NACC) autopsy database,12 psychosis, particularly hallucinations, was associated with more frequent neuritic plaques, higher Braak staging of neurofibrillary tangles, and Lewy body disease (LBD). Visual hallucinations have been shown to be associated with the neuropathology of both AD and LBD.13 Studies indicate that psychosis is associated with an increase in abnormally phosphorylated tau protein and neurofibrillary tangles, which are characteristic of AD, at autopsy.14,15 Increased agitation and aggression scores on the Neuropsychiatric Inventory Questionnaire (NPIQ) have been associated with transactive response DNA-binding protein 43 (TDP-43) pathology, a common cause of frontotemporal lobar degeneration (FTLD).16 There is inconsistent evidence on whether depression is associated with neuritic plaque and neurofibrillary tangle burden in AD.17,18 To our knowledge, associations of hippocampal sclerosis with specific NPS have not been reported.
We examined the associations of specific neuropathologies with NPS in the large NACC v. 10 database,19 which uses standardized neuropathological diagnostic criteria. We hypothesized that each ADRD dementia subtype would be associated with specific NPS, indicating different disease mechanisms; that AD and LBD pathology would predict hallucinations and delusions; and that FTLD pathology would predict apathy and disinhibition. Understanding the pathological mechanisms underlying specific NPS may help to improve diagnostic criteria for specific ADRD and eventually clinical management.
Methods
Data Source
At 39 US Alzheimer Disease Center sites, autopsies were conducted locally using the NACC Coding Guidebook protocol (January 2014) for uniform collection and ratings of neuropathological data.19 Institutional review board approval for clinical and autopsy procedures were obtained locally. Autopsy data collected from January 2012 to January 2018 were compiled into the publicly available v. 10 database.19 Data were analyzed from February 2021 to August 2021.
Study Sample
After exclusion of the neuropathological diagnoses of brain injury, CNS neoplasm, Down syndrome, Huntington disease, and prion disease, we included all brains for which the NPIQ was administered to a caregiver at least once during life.
As per NACC protocol, additional semiquantitative ratings were made by immunohistochemistry, histochemistry, microscopic visualization, or visual inspection with appropriate categorization of brain regions. For AD, ABC criteria for severity were rated.20 Criteria A, diffuse amyloid plaque (Thal stage), is a measure of spread of plaque, and higher scores indicate greater spread. Criteria B, Braak stage, is a measure of progression of neurofibrillary tangles, and higher scores indicate spread of pathology to neocortex. In criteria C, neuritic plaques, higher scores indicate greater density of pathology. Intersite agreement for ABC pathological criteria was high (κ, 0.88) and individual A, B, and C scores had agreement κ values ranging from 0.70 to 0.84.21 As recommended by the National Institute on Aging–Alzheimer's Association consortium, intermediate to high probability ABC criteria defined Alzheimer disease pathology.22 LBD was rated as present or absent and by severity on a scale from 1 to 3 based on α-synuclein aggregations in brain stem (1), limbic (2), and neocortical regions (3), with the highest rating used for each brain.11 Frontotemporal lobar degeneration (FTLD) was defined as present or absent based on any of the following diagnoses: FTLD-TDP-43 (sporadic, with motor neuron disease, and with associated variants resulting in FTLD-TDP-43, including GRN variants and C9ORF72 expansions), 3 repeat (3R) tauopathies (Pick disease, non-Pick 3R predominant FTLD, associated with MAPT variants resulting in 3R tauopathies), 4 repeat (4R) tauopathies (corticobasal degeneration, progressive supranuclear palsy, globular glial tauopathy, argyrophilic grain disease, associated with MAPT variants leading to 4R tauopathies), 3R and 4R tauopathies (tangle dominant disease, chronic traumatic encephalopathy, amyotrophic lateral sclerosis/Guam Parkinsonism-dementia complex, associated with MAPT variants leading to 3R and 4R tauopathies), and all other subtypes of FTLD-TDP and FTLD–fused in sarcoma, FTLD–ubiquitin-proteasome system, and FTLD–nitric oxide synthase. The NACC protocol required brains to be characterized for TDP-43 staining. Present and absent ratings for the pathologies of cerebral amyloid angiopathy, cerebrovascular disease (CVD) defined as multiple microinfarcts or infarcts or lacunes, and hippocampal sclerosis were also evaluated. We did not use clinical or neuroimaging-based diagnoses. This novel, unbiased, and powerful approach avoids diagnostic errors that may be inconsistent with neuropathology, which remains the criterion standard for ADRD diagnoses.10
Each NPIQ domain was examined separately in analyses: delusions, hallucinations, agitation and aggression, dysphoria and depression, anxiety, euphoria and elation, apathy and indifference, disinhibition, irritability and lability, aberrant motor behavior, sleep and nighttime behavior, and appetite and eating. For each NPIQ domain, present and absent and, if present, severity rating (1 to 3) were analyzed. Each symptom domain occurring at least once (ever present) was the primary neurobehavioral measure, as published elsewhere.12,16 The mean NPIQ domain severity score across annual study visits was the secondary measure. Patient demographic characteristics were collected at baseline and all time points; these measures were defined at the last clinic visit prior to death (Table 1). Race and ethnicity data were collected via self-report based on NACC protocol.
Table 1. Demographic and Clinical Characteristics by Neuropathological Diagnosis.
| Characteristic | No. (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Total (N = 1808)a | AD (n = 1246) | LBD (n = 658) | CAA (n = 1117) | CVD (n = 568) | FTLD (n = 303) | HS (n = 254) | NP (n = 131) | |
| Age at death, mean (SD), y | 80.0 (11.0) | 80.8 (10.4) | 79.6 (9.9) | 80.6 (10.6) | 84.4 (9.4) | 77.5 (11.3) | 82.3 (10.0) | 76.2 (13.3) |
| Age at last visit, mean (SD), y | 78.7 (11.2) | 79.4 (10.7) | 78.1 (10.2) | 79.2 (10.9) | 82.9 (9.7) | 76.2 (11.5) | 80.8 (10.3) | 75.2 (13.3) |
| Female | 821 (45.4) | 602 (48.3) | 277 (42.1) | 523 (46.8) | 282 (49.6) | 126 (41.6) | 128 (50.4) | 42 (32.1) |
| Male | 987 (54.6) | 644 (51.7) | 381 (57.9) | 594 (53.2) | 286 (50.4) | 177 (58.4) | 126 (49.6) | 89 (67.9) |
| Education, mean (SD), y | 15.51 (3.11) | 15.42 (3.16) | 15.70 (3.05) | 15.44 (3.19) | 15.37 (3.14) | 15.87 (2.99) | 15.60 (3.22) | 15.84 (2.99) |
| Whiteb | 1710 (94.6) | 1177 (94.5) | 627 (95.3) | 1051 (94.1) | 539 (94.9) | 290 (95.7) | 238 (93.7) | 126 (96.2) |
| Total NPIQ score, mean (SD)c | 5.41 (4.49) | 5.37 (4.36) | 5.67 (4.41) | 5.30 (4.35) | 4.85 (4.25) | 6.08 (4.57) | 5.36 (3.95) | 6.22 (5.69) |
| APOE ε4 positive | 707 (44.9) | 606 (55.4) | 310 (53.4) | 556 (56.7) | 207 (42.1) | 76 (28.4) | 110 (49.8) | 20 (18.5) |
| ChEI or memantine use | 1114 (61.6) | 886 (71.1) | 475 (72.2) | 774 (69.3) | 325 (57.2) | 142 (46.9) | 194 (76.4) | 42 (32.1) |
| Antipsychotic use | 391 (21.6) | 302 (24.2) | 165 (25.1) | 249 (22.3) | 104 (18.3) | 55 (18.2) | 50 (19.7) | 23 (17.6) |
| Anxiolytic or sedative use | 523 (28.9) | 345 (27.7) | 193 (29.3) | 310 (27.8) | 145 (25.5) | 88 (29.0) | 62 (24.4) | 43 (32.8) |
| Antidepressant use | 983 (54.4) | 700 (56.2) | 381 (57.9) | 616 (55.1) | 268 (47.2) | 156 (51.5) | 146 (57.5) | 73 (55.7) |
| Time from final visit to death, mean (SD), y | 1.79 (1.92) | 1.87 (2.00) | 1.97 (2.03) | 1.91 (1.98) | 1.95 (2.03) | 1.82 (1.87) | 2.02 (2.02) | 1.51 (1.65) |
Abbreviations: AD, Alzheimer disease (defined by intermediate or high ABC criteria); APOE, apolipoprotein E; CAA, cerebral amyloid angiopathy; ChEI, cholinesterase inhibitor; CVD, cerebrovascular disease; FTLD, frontotemporal lobar degeneration; HS, hippocampal sclerosis; LBD, Lewy body disease; NP, no known pathology; NPIQ, Neuropsychiatric Inventory Questionnaire.
Number of participants in each category does not add up to the total because some participants had more than 1 neuropathology diagnosis.
Race and ethnicity data were collected via self-report as per National Alzheimer Coordinating Center protocol. Categories other than White are not reported because of low numbers.
NPIQ mean total score across assessment time points. Medication use at any time point was rated.
Statistical Analyses
Statistical analysis was performed using R version 4.0.2 (R Foundation). For descriptive statistics, means and SDs and frequency and percentage were reported for continuous and categorical variables, respectively. For statistical testing, χ2 test or Fischer exact test and t tests were performed as appropriate. For all analyses evaluating neuropathology and NPIQ domains, we corrected for multiple comparisons, controlling for false discovery rate.23 For post hoc and sensitivity analyses, uncorrected P values are reported.
In all analyses, the relevant NPIQ domain score was the dependent variable and the neuropathology subtype (AD, LBD, cerebral amyloid angiopathy [CAA], CVD, FTLD, hippocampal sclerosis, and no known pathology) was the independent variable while adjusting for age at death, sex, and time in years between last available NPIQ assessment and death.
First, we examined the overall effect of each neuropathology on NPIQ using pairwise regression analysis. For each NPIQ endorsement (ever present vs always absent), separate logistic regression analyses with each neuropathology diagnosis as the independent variable were performed. We then examined the mean severity across all time points of each NPIQ domain by neuropathology diagnosis using linear regression. Further, we tested the independent effect of the neuropathology diagnoses by performing multiple regression, including all neuropathology diagnoses as independent variables for each NPIQ domain (ever present).
For sensitivity analyses, we repeated the analyses, adjusting for the presence of at least 1 apolipoprotein E ε4 allele. We also examined in exploratory analyses the type of psychotropic medication as a covariate (eg, antidepressants for depression, antipsychotics and antidepressants for psychosis and agitation and aggression, cholinesterase inhibitors, and memantine).
Results
Participants
A total of 1808 participants with available NPIQ and neuropathological data were included. The mean (SD) age was 80.0 (11.0) years, and 987 participants (54.6%) were male. Nearly all (1710 [94.6%]) individuals self-identified as White (Table 1); data for other racial and ethnic groups are not reported owing to small numbers. At the final clinic visit, the no known pathology group of 131 study participants was heterogenous with global Clinical Dementia Rating scores of 0 for no dementia in 32 participants (24.4%), 0.5 for questionable dementia in 28 (21.4%), 1 for mild dementia in 18 (13.7%), 2 for moderate dementia in 19 (14.5%), and 3 for severe dementia in 34 (26.0%). In this group, 17 individuals (13.0%) had missing data for TDP-43 and 8 (6.1%) had missing data for α-synuclein.
Frequencies of NPS by Type of Neuropathology
In frequency comparisons of NPIQ domains (ever present vs always absent) and specific neuropathologies (present vs absent), delusions occurred in 454 individuals with AD (36.4%) vs 77 without (17.0%) and hallucinations occurred in 325 with AD (26.1%) vs 54 without (10.4%). Delusions occurred in 238 with LBD (36.2%) vs 308 without (27.1%), and hallucinations occurred in 192 with LBD (29.2%) vs 189 without (16.6%). The highest prevalence for any NPIQ symptom domain was apathy, which reached 80% (203 of 254 individuals) in hippocampal sclerosis. Overall, delusions and hallucinations were more common in AD and LBD and less common in FTLD, which was associated with greater disinhibition and apathy (Figure; eTable 1 in the Supplement).
Figure. Frequency of Neuropsychiatric Inventory Questionnaire (NPIQ) Domain Scores by Neuropathology Subtype.
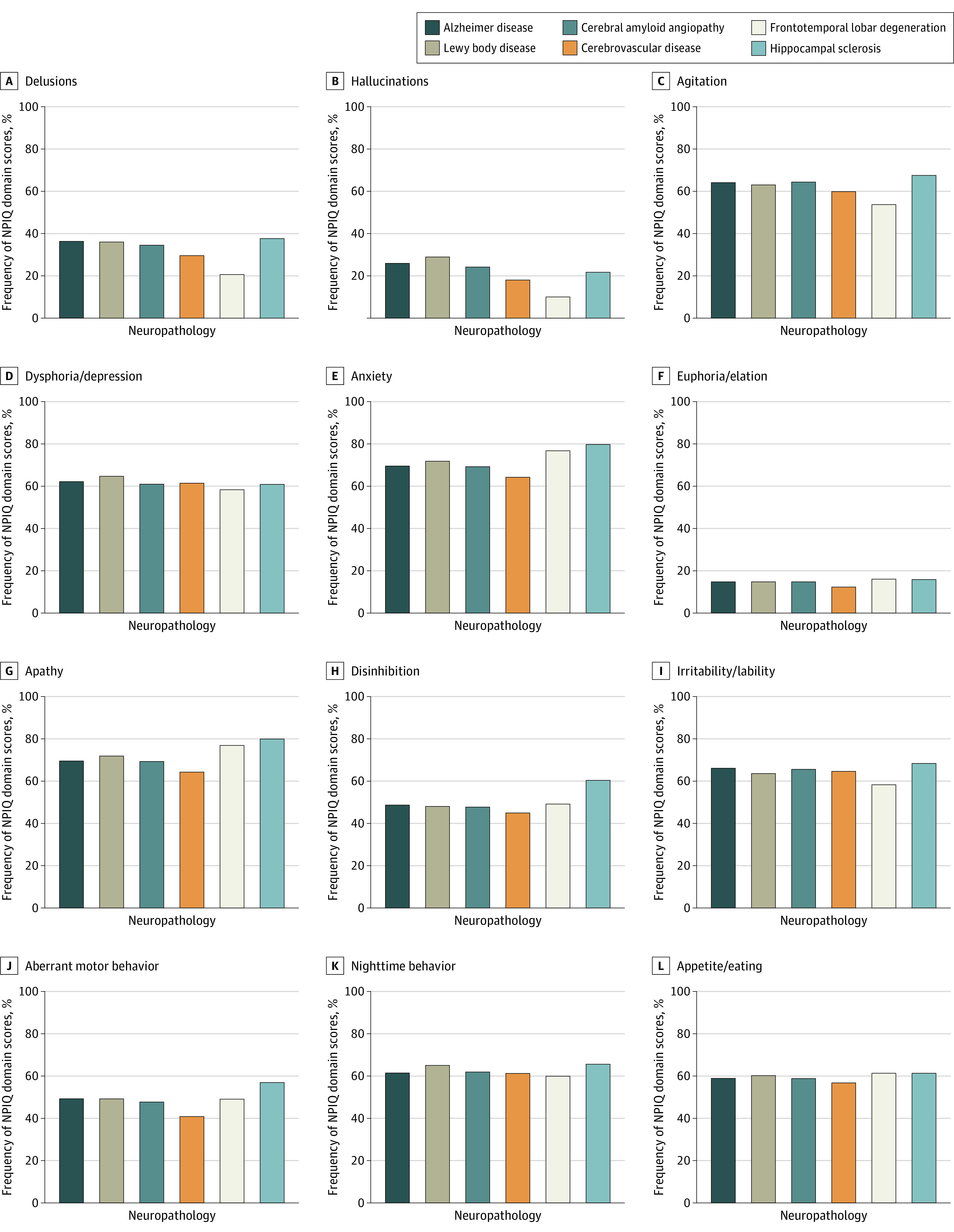
NPIQ domain scores defined as ever present vs always absent. Alzheimer disease defined by intermediate or high ABC criteria. Subtypes not controlled for the presence of other neuropathologies.
Association of Specific Neuropathologies With NPS
Separate logistic regression analyses were conducted with each NPIQ endorsement as the dependent variable and each neuropathology diagnosis as the independent variable, controlling for age at death, sex, and time in years between last NPIQ assessment and death. AD was associated with increased symptoms for all domains except for euphoria and elation, sleep and nighttime behaviors, and appetite and weight change; both delusions (odds ratio [OR], 3.04; 95% CI, 2.34-3.95) and hallucinations (OR, 3.49; 95% CI, 2.55-4.79) demonstrated an OR greater than 3 (Table 2). LBD was associated with increased delusions and hallucinations, but not agitation and aggression, and with increased depression, anxiety, apathy, and sleep and nighttime behaviors. For CAA, associations were similar to AD. For FTLD, delusions, hallucinations, agitation and aggression, anxiety, and irritability decreased, and apathy increased (Table 2). For hippocampal sclerosis, several domain scores increased, with the highest OR of 2.60 (95% CI, 1.86-3.66; false discovery rate controlled P > .001) for apathy (Table 2). In linear regression analyses conducted using mean NPIQ domain scores (range 0 to 3) as the dependent variable and each neuropathology diagnosis (present vs absent) as the independent variable, controlling for the same covariates led to similar results to those obtained in logistic regression analyses (eTable 2 in the Supplement).
Table 2. Pairwise Logistic Regression Analyses for Alzheimer Diseasea and Related Dementia (ADRD) Diagnoses on Neuropsychiatric Inventory Questionnaire (NPIQ) Domain Scoresb During Follow-up.
| Diagnosis | OR (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| ADb | LBDb | CAAb | CVDb | FTLDb | HSb | NPb | |
| Delusions | 3.04 (2.34-3.95)c | 1.59 (1.29-1.96)c | 1.85 (1.48-2.30)c | 1.01 (0.81-1.27) | 0.54 (0.40-0.73)c | 1.56 (1.18-2.07)c | 0.43 (0.27-0.69)c |
| Hallucinations | 3.49 (2.55-4.79)c | 2.20 (1.74-2.77)c | 1.77 (1.38-2.26)c | 0.84 (0.65-1.09) | 0.34 (0.23-0.51)c | 1.17 (0.84-1.63) | 0.52 (0.31-0.86)c |
| Agitation/aggression | 1.88 (1.52-2.33)c | 1.22 (1.0-1.49) | 1.72 (1.41-2.1)c | 1.16 (0.94-1.44) | 0.67 (0.52-0.86)c | 1.65 (1.24-2.20)c | 0.67 (0.46-0.96) |
| Dysphoria/depression | 1.40 (1.13-1.73)c | 1.41 (1.15-1.72)c | 1.14.(0.94-1.39) | 1.21 (0.98-1.50) | 0.90 (0.70-1.16) | 1.08 (0.82-1.43) | 0.71 (0.50-1.02) |
| Anxiety | 2.30 (1.85-2.87)c | 1.46 (1.19-1.80)c | 1.64 (1.34-2.01)c | 1.18 (0.95-1.46) | 0.66 (0.51-0.86)c | 1.22 (0.92-1.62) | 0.39 (0.27-0.57)c |
| Euphoria/elation | 1.35 (0.99-1.84) | 1.05 (0.80-1.39) | 1.21 (0.91-1.61) | 1.12 (0.82-1.53) | 0.98 (0.69-1.38) | 1.39 (0.95-2.02) | 0.64 (0.37-1.12) |
| Apathy | 1.6 (1.27-2.01)c | 1.31 (1.05-1.63)c | 1.33 (1.07-1.64)c | 1.08 (0.86-1.35) | 1.50 (1.12-2.03)c | 2.60 (1.86-3.66)c | 0.49 (0.33-0.73)c |
| Disinhibition | 1.37 (1.11-1.70)c | 1.05 (0.87-1.28) | 1.14 (0.93-1.38) | 1.09 (0.88-1.34) | 0.98 (0.76-1.26) | 2.15 (1.63-2.84)c | 0.64 (0.44-0.92)c |
| Irritability/lability | 1.71 (1.38-2.12)c | 1.06 (0.86-1.29) | 1.46 (1.20-1.79)c | 1.29 (1.04-1.60)c | 0.75 (0.58-0.96)c | 1.46 (1.10-1.95)c | 0.53 (0.37-0.77)c |
| Aberrant motor behavior | 1.81 (1.44-2.26)c | 1.19 (0.97-1.46) | 1.29 (1.05-1.58)c | 1.02 (0.82-1.26) | 0.95 (0.73-1.24) | 2.08 (1.56-2.76)c | 0.55 (0.37-0.81)c |
| Sleep/nighttime behaviors | 1.22 (0.99-1.52) | 1.41 (1.15-1.73)c | 1.28 (1.05-1.56)c | 1.17 (0.94-1.46) | 0.93 (0.72-1.21) | 1.42 (1.07-1.90)c | 0.75 (0.52-1.08) |
| Appetite/eating disturbances | 1.06 (0.86-1.32) | 1.12 (0.91-1.36) | 1.11 (0.91-1.36) | 1.11 (0.90-1.37) | 1.04 (0.80-1.34) | 1.29 (0.97-1.70) | 0.77 (0.53-1.11) |
Abbreviations: AD, Alzheimer disease (defined as intermediate or high ABC criteria); CAA, cerebral amyloid angiopathy; CVD, cerebrovascular disease; FTLD, frontotemporal lobar degeneration; HS, hippocampal sclerosis; LBD, Lewy body disease; NP, no known pathology; OR, odds ratio.
Defined as intermediate or high ABC criteria.
Defined as ever present or always absent.
P < .05 after multiple comparison correction to control for false discovery rate.
Severity of Neuropathology and Severity of NPS
For LBD, brain stem, limbic, and neocortical α-synucleinopathy, rated as 1, 2 and 3, indicate progressive regional involvement. Delusions (ever present vs absent) increased from brain stem (β, 0.53; 95% CI, 0.27-1.02) to limbic (β, 1.48; 95% CI, 1.14-1.91; P = .002) to neocortical pathology (β, 2.34; 95% CI, 1.73-3.15; P < .001), as did hallucinations (β, 0.23; 95% CI, 0.07-0.76; P = .02 to β, 1.69; 95% CI, 1.27-2.27; P < .001 to β, 4.49; 95% CI, 3.27-6.16; P < .001). Depression was significant for limbic (β, 1.35; 95% CI, 1.05-1.72; P = .02) and neocortical (β, 1.67; 95% CI, 1.23-2.28; P = .001) pathology; anxiety showed similar results for limbic (β, 1.43; 95% CI, 1.11-1.85; P = .006) and neocortical (β, 1.75; 95% CI, 1.27-1.43; P < .001) pathology. Apathy increased from brain stem to limbic to neocortical pathology with neocortical pathology being significant (β, 1.55; 95% CI, 1.10-2.17; P = .01). Sleep and nighttime behavior was significant for neocortical pathology only (β, 2.21; 95% CI, 1.59-3.08; P < .001). In the 2 other pathologies that also had semiquantitative ratings, AD and CAA, more severe pathology was associated consistently with increased NPIQ domain scores.
Multiple Neuropathologies
Multiple logistic regression was performed after including all neuropathology variables in the same model with correction for multiple comparisons. In these analyses, 1672 participants had data available for all neuropathologies (Table 3). Most of the associations observed in the pairwise regression analysis remained significant in the multiple regression analyses (Table 3). The proportion of NPS by multiple pathologies with analyses restricted to pathologies present in at least 40 brains is described in Table 4.
Table 3. Multiple Logistic Regression of Neuropathology Subtypes on Neuropsychiatric Inventory Questionnaire (NPIQ) Domain Scores During Follow-up (N = 1672)a.
| Diagnosis | OR (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| AD | LBD | CAA | CVD | FTLD | HS | NP | |
| Delusions | 2.78 (1.96-3.94)b | 1.31 (1.04-1.64) | 1.16 (0.89-1.52) | 1.12 (0.88-1.42) | 0.86 (0.61-1.21) | 1.63 (1.21-2.21)b | 1.19 (0.65-2.15) |
| Hallucinations | 3.13 (2.03-4.82)b | 1.81 (1.4-2.33)b | 0.98 (0.73-1.33) | 0.95 (0.72-1.26) | 0.61 (0.39-0.95) | 1.14 (0.8-1.62) | 1.22 (0.6-2.48) |
| Agitation/aggression | 1.57 (1.18-2.1)b | 1.07 (0.86-1.33) | 1.36 (1.06-1.74) | 1.25 (0.99-1.57) | 0.83 (0.62-1.13) | 1.8 (1.33-2.44)b | 1.11 (0.69-1.78) |
| Dysphoria/depression | 1.38 (1.04-1.83) | 1.35 (1.09-1.68)b | 0.96 (0.75-1.22) | 1.18 (0.94-1.48) | 1.06 (0.79-1.42) | 1.11 (0.83-1.48) | 0.97 (0.61-1.55) |
| Anxiety | 1.82 (1.36-2.43)b | 1.24 (0.99-1.55) | 1.07 (0.83-1.37) | 1.14 (0.9-1.44) | 0.78 (0.58-1.05) | 1.24 (0.91-1.67) | 0.59 (0.37-0.96) |
| Euphoria/elation | 1.32 (0.85-2.06) | 1.01 (0.75-1.36) | 1.03 (0.72-1.47) | 1.06 (0.76-1.48) | 0.98 (0.64-1.51) | 1.36 (0.91-2.01) | 0.83 (0.41-1.69) |
| Apathy | 1.7 (1.24-2.31)b | 1.23 (0.97-1.56) | 1.07 (0.82-1.4) | 1.09 (0.85-1.39) | 1.82 (1.29-2.57)b | 2.7 (1.89-3.85)b | 0.92 (0.56-1.53) |
| Disinhibition | 1.46 (1.09-1.95) | 0.96 (0.77-1.18) | 0.91 (0.71-1.16) | 1.13 (0.9-1.41) | 1.09 (0.81-1.47) | 2.17 (1.62-2.90)b | 0.88 (0.55-1.42) |
| Irritability/lability | 1.48 (1.11-1.98)b | 0.89 (0.72-1.11) | 1.13 (0.88-1.44) | 1.34 (1.06-1.69) | 0.88 (0.65-1.18) | 1.48 (1.09-2.01) | 0.68 (0.42-1.09) |
| Aberrant motor behavior | 1.94 (1.42-2.64)b | 1.1 (0.88-1.37) | 0.92 (0.72-1.19) | 1.06 (0.84-1.34) | 1.25 (0.91-1.70) | 2.22 (1.65-3.00)b | 0.88 (0.53-1.46) |
Abbreviations: AD, Alzheimer disease (defined as intermediate or high ABC criteria); CAA, cerebral amyloid angiopathy; CVD, cerebrovascular disease; FTLD, frontotemporal lobar degeneration; HS, hippocampal sclerosis; LBD, Lewy body disease; NP, no known pathology; OR, odds ratio.
All regression analyses were adjusted for age at death, sex, and difference in years between NPIQ assessment and death.
P < .05 after multiple comparisons correction to control for false discovery rate.
Table 4. Neuropsychiatric Symptoms (NPS) by Multiple Pathologies With Analyses Restricted to Pathologies Present in at Least 40 Brains.
| Pathology | No. | No. (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delusions | Hallucinations | Agitation/aggression | Dysphoria/depression | Anxiety | Euphoria/elation | Apathy | Disinhibition | Irritability/lability | Aberrant motor behavior | Sleep/night/behavior | Appetite/eating | ||
| No known pathology | 131 | 23 (17.6) | 19 (14.5) | 72 (55.0) | 70 (53.4) | 62 (47.3) | 17 (13.0) | 78 (59.5) | 54 (41.2) | 68 (51.9) | 53 (40.5) | 75 (57.3) | 74 (56.5) |
| FTLD only | 90 | 15 (16.7) | 4 (4.4) | 44 (48.9) | 48 (53.3) | 55 (61.1) | 16 (17.8) | 72 (80.0) | 50 (55.6) | 50 (55.6) | 54 (60.0) | 57 (63.3) | 60 (66.7) |
| CVD only | 49 | 8 (16.3) | 3 (6.1) | 27 (55.1) | 28 (57.1) | 25 (51.0) | 3 (6.1) | 25 (51.0) | 21 (42.9) | 30 (61.2) | 9 (18.4) | 26 (53.1) | 29 (59.2) |
| AD only | 93 | 30 (32.3) | 25 (26.9) | 48 (51.6) | 67 (72.0) | 63 (67.7) | 11 (11.8) | 59 (63.4) | 40 (43.0) | 62 (66.7) | 44 (47.3) | 51 (54.8) | 51 (54.8) |
| AD and CAA | 288 | 98 (34.0) | 70 (24.3) | 189 (65.6) | 167 (58.0) | 201 (69.8) | 44 (15.3) | 189 (65.6) | 138 (47.9) | 200 (69.4) | 134 (46.5) | 172 (59.7) | 175 (60.8) |
| AD, CAA, and HS | 41 | 19 (46.3) | 9 (22.0) | 31 (75.6) | 24 (58.5) | 24 (58.5) | 4 (9.8) | 32 (78.0) | 23 (56.1) | 26 (63.4) | 24 (58.5) | 25 (61.0) | 19 (46.3) |
| AD, CAA, and CVD | 140 | 47 (33.6) | 29 (20.7) | 86 (61.4) | 85 (60.7) | 89 (63.6) | 19 (13.6) | 88 (62.9) | 56 (40.0) | 92 (65.7) | 55 (39.3) | 81 (57.9) | 76 (54.3) |
| AD and LBD | 64 | 21 (32.8) | 20 (31.2) | 36 (56.2) | 33 (51.6) | 41 (64.1) | 9 (14.1) | 46 (71.9) | 29 (45.3) | 39 (60.9) | 33 (51.6) | 37 (57.8) | 34 (53.1) |
| AD, LBD, and CAA | 235 | 91 (38.7) | 84 (35.7) | 159 (67.7) | 152 (64.7) | 171 (72.8) | 43 (18.3) | 178 (75.7) | 122 (51.9) | 145 (61.7) | 135 (57.4) | 146 (62.1) | 142 (60.4) |
| AD, LBD, CAA, and CVD | 105 | 44 (41.9) | 26 (24.8) | 76 (72.4) | 74 (70.5) | 82 (78.1) | 13 (12.4) | 72 (68.6) | 57 (54.3) | 73 (69.5) | 49 (46.7) | 71 (67.6) | 64 (61.0) |
| Total | 1808 | 551 (30.5) | 388 (21.5) | 1094 (60.5) | 1086 (60.1) | 1153 (63.8) | 264 (14.6) | 1234 (68.3) | 858 (47.5) | 1141 (63.1) | 848 (46.9) | 1098 (60.7) | 1065 (58.9) |
Abbreviations: AD, Alzheimer disease (defined as intermediate or high ABC criteria); CAA, cerebral amyloid angiopathy; CVD, cerebrovascular disease; FTLD, frontotemporal lobar degeneration; HS, hippocampal sclerosis; LBD, Lewy body disease.
Hippocampal sclerosis may be associated with CVD or FTLD.24,25 In sensitivity analyses, after excluding all participants with FTLD or CVD both separately and together, the associations of hippocampal sclerosis with specific NPIQ domains remained at similar levels of significance. For patients with hippocampal sclerosis as the only identified neuropathology with no missing data for the other neuropathologies, 14 of 138 (10.1%) had missing data for TDP-43 assessment. FTLD and hippocampal sclerosis did not demonstrate significant interactions for the domains of disinhibition (β, 0.11; 95% CI, −0.6 to 0.85; P = .76) and apathy (β, 0.05; 95% CI, −0.87 to 1.11; P = .92) (eTable 3 in the Supplement). Further, FTLD had a lower rate of hallucinations than AD (OR, 0.19; 95% CI, 0.15-0.33; P < .001), as did CVD compared with AD (OR, 0.30; 95% CI, 0.24-0.50; P < .001). FTLD was associated with less agitation and aggression than AD (OR, 0.53; 95% CI, 0.45-0.75; P < .001). However, FTLD and CVD did not differ significantly from the no known pathology group on hallucinations or agitation and aggression.
AD and LBD were the most common comorbid neuropathologies.26 The prevalence of hallucinations was higher in participants with AD and LBD (168 of 534 [31.5%]) compared with those AD without LBD (152 of 704 [21.6%]) and those with LBD without AD (23 of 119 [19.6%]) (χ23 = 78.02; P < .001) and was lowest for participants with neither AD nor LBD (30 of 397 [7.6%]). Hallucinations increased from brain stem (1.5%) to limbic (8.5%) to neocortical (16.8%) LBD; delusions did not consistently increase from brain stem (13.2%) to limbic (18.4%) to neocortical (16.8%) LBD. Neocortical LBD and AD showed higher prevalence of psychotic symptoms than neocortical LBD without AD: delusions 48.1% vs 31.4%, hallucinations 47% vs 37.1%, and agitation and aggression 64.9% vs 51.4%. In logistic regression analyses, apolipoprotein E ε4 genotype and use of psychotropic medications (overall or individual classes), cholinesterase inhibitors, or memantine were not significant covariates.
Discussion
To our knowledge, this is the largest study examining the associations of neuropathological diagnoses with NPS. The study design used a direct and unbiased transdiagnostic approach without the filter or bias of clinical diagnosis of dementia classification; we used a similar approach to examine apolipoprotein E ε2 associations with neuropathological diagnoses.27,28 Another strength is that NACC data were collected prospectively without regard for specific hypotheses.
The neuropathological diagnosis of AD was associated with increased delusions, hallucinations, and agitation and aggression, consistent with prior studies.12,15,16 The association of LBD with hallucinations was stronger in the LBD with AD group than in the LBD without AD group; neocortical LBD showed a high prevalence of hallucinations.29,30 A recent study in a smaller independent sample found that AD with LBD was associated with more hallucinations and moderate increase in delusions compared with AD without LBD.4 Nearly half of individuals clinically diagnosed with AD show some degree of LBD pathology at autopsy.31,32 This range of findings from independent autopsy series suggests that visual hallucinations, which are in the consensus diagnostic criteria for dementia with Lewy bodies, are more characteristic of neocortical than limbic LBD and may be more common in LBD with AD than LBD without AD.10,11 These findings are intriguing and need to be replicated. CAA and AD showed similar associations with NPS which may be an artifact of CAA being closely associated with AD; CAA did not separate from AD on frequency of NPIQ domains in multiple regression analyses.
CVD showed weak associations with mood symptoms, consistent with diagnostic criteria for vascular dementia.33 FTLD was associated with decreased psychotic features and agitation. Most individuals with FTLD had sporadic FTLD; these results differ from previous reports of a higher prevalence of psychotic symptoms in FTLD owing to C9ORF72 expansions, the most common genetic cause of FTLD.4,8 In a recent autopsy series from a specialized FTLD clinic, delusions were present in one-third of the FTLD-TDP subsample but were less common in other FTLD subtypes and hallucinations were uncommon.4 In our study, FTLD demonstrated a strong association with apathy, which is a clinical diagnostic criterion for behavioral variant FTD.7 The results indirectly support the inclusion of apathy and disinhibition as NPS criteria for behavioral variant FTD.7
Hippocampal Sclerosis
To our knowledge, the associations between hippocampal sclerosis and specific NPS have not been published previously. Hippocampal sclerosis was associated with a significant increase in apathy, disinhibition, and aberrant motor behavior, and additional associations with several NPS were found in multiple regression analyses. Both FTLD and CVD are known to occur in conjunction with hippocampal sclerosis, but the associations of hippocampal sclerosis with specific NPS were not altered by exclusion of individuals with FTLD and CVD. The histopathologic diagnosis of hippocampal sclerosis is based on neuronal loss and chronic fibrillary gliosis centered on the pyramidal cell layer; other features include granule cell dispersion, mossy fiber sprouting and alterations to interneurons.24 Hippocampal sclerosis is often associated with seizures, particularly status epilepticus, and less commonly with traumatic brain injury, inflammation, or other brain lesions.25 The current findings may have clinical implications. We speculate that the relatively poor response rates to antidepressants in placebo-controlled clinical trials of depression in dementia may be owing to apathy being misdiagnosed as depression; apathy occurred in 80% of individuals with hippocampal sclerosis in this sample.34 This possibility requires further investigation.
Limbic-Predominant Age-Related TDP-43 Neuropathological Diagnosis
Limbic-predominant age-related TDP-43 encephalopathy (LATE) is an amnestic dementia syndrome that occurs primarily after the eighth decade of life.35 Hippocampal sclerosis occurs in LATE with TDP-43 proteinopathy that is restricted largely to medial temporal and frontal regions. Brains with hippocampal sclerosis that lack stereotypic TDP-43 proteinopathy are not currently considered to represent LATE-neuropathological change (LATE-NC).35 In this NACC sample, the associations between hippocampal sclerosis and specific NPS remained after excluding individuals with FTLD, suggesting that LATE by itself cannot explain the observed associations. Hippocampal sclerosis, FTLD, and LATE, which have overlapping pathologies, may also have overlapping NPS phenotypes.
There was considerable overlap among AD, LBD, and CAA in this study, while FTLD showed the least overlap with these pathologies. Multiple neurodegenerative pathologies are known to occur in the same individual with dementia,36 but the observed associations between each neuropathology and individual NPS were largely maintained even after controlling for concomitant neuropathologies. Apolipoprotein E ε4 genotype has been associated with psychosis in some studies,37 but apolipoprotein E e4 was not a significant covariate in this study.
Limitations
This study has limitations. The case series has an ascertainment bias because a large proportion met pathological criteria for intermediate to high AD neuropathological change, which was expected because the NACC sites focused on monitoring patients with clinical AD dementia. The no known pathology group was cognitively heterogenous and more than half were rated as having clinical dementia, reflecting the inaccuracy of clinical diagnosis compared with criterion standard neuropathology. The NPIQ relies on informant report that is not always reliable. Visual hallucinations are known to be associated with α-synucleinopathy in dementia with Lewy bodies, AD, and Parkinson disease with psychosis.13 However, in the NACC database, NPIQ scores for hallucinations do not separate visual and auditory hallucinations. Therefore, only the impact of specific neuropathologies on hallucinations, but not visual hallucinations specifically, could be assessed. Intersite and interrater reliability for NPIQ administration, which relies on informant report, was not assessed, but strong reliability was established for neuropathological diagnostic assessment.19 NPS fluctuate during the course of illness in subcategories of dementia1,2; wide variability in follow-up duration did not permit systematic examination of NPS trajectories in association with neuropathology. Nevertheless, for neuropathologies rated on a continuous scale (eg, AD and LBD), increasing pathology was associated with greater neurobehavioral disturbance.
Conclusions
In this cohort study, patients with LBD and AD showed a higher prevalence of delusions, hallucinations, and agitation and aggression compared with those with LBD without AD. The findings on apathy and disinhibition in hippocampal sclerosis are novel and unique, and remained after excluding patients with CVD and FTLD, which are known to impact medial temporal lobe structure and function. Severity of neuropathology being related to severity of NPS across ADRD diagnoses suggests that manifestations of NPS may reflect underlying neuropathology and that identification and treatment of NPS are clinically important.
eTable 1. Frequencies of NPI-Q domain scores (present at any time-point) and neuropathology subtypes (present/absent)
eTable 2. Linear regression analyses of neuropathology subtypes (present/absent) on mean NPI-Q scores during follow-up
eTable 3. Prevalence of single vs concurrent apathy and disinhibition in Frontotemporal Lobar Degeneration and Hippocampal Sclerosis
References
- 1.Devanand DP, Jacobs DM, Tang M-X, et al. The course of psychopathologic features in mild to moderate Alzheimer disease. Arch Gen Psychiatry. 1997;54(3):257-263. doi: 10.1001/archpsyc.1997.01830150083012 [DOI] [PubMed] [Google Scholar]
- 2.Lyketsos CG, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JC. Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry. 2000;157(5):708-714. doi: 10.1176/appi.ajp.157.5.708 [DOI] [PubMed] [Google Scholar]
- 3.Nagahama Y, Okina T, Suzuki N, Matsuda M. Neural correlates of psychotic symptoms in dementia with Lewy bodies. Brain. 2010;133(Pt 2):557-567. doi: 10.1093/brain/awp295 [DOI] [PubMed] [Google Scholar]
- 4.Naasan G, Shdo SM, Rodriguez EM, et al. Psychosis in neurodegenerative disease: differential patterns of hallucination and delusion symptoms. Brain. 2021;144(3):999-1012. doi: 10.1093/brain/awaa413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scarmeas N, Brandt J, Albert M, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62(10):1601-1608. doi: 10.1001/archneur.62.10.1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scarmeas N, Brandt J, Blacker D, et al. Disruptive behavior as a predictor in Alzheimer disease. Arch Neurol. 2007;64(12):1755-1761. doi: 10.1001/archneur.64.12.1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(Pt 9):2456-2477. doi: 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw SR, El-Omar H, Roquet D, et al. Uncovering the prevalence and neural substrates of anhedonia in frontotemporal dementia. Brain. 2021;144(5):1551-1564. doi: 10.1093/brain/awab032 [DOI] [PubMed] [Google Scholar]
- 9.Manera V, Fabre R, Stella F, et al. A survey on the prevalence of apathy in elderly people referred to specialized memory centers. Int J Geriatr Psychiatry. 2019;34(10):1369-1377. [DOI] [PubMed] [Google Scholar]
- 10.Cummings J. The role of neuropsychiatric symptoms in research diagnostic criteria for neurodegenerative diseases. Am J Geriatr Psychiatry. 2020;29(4):375-383. doi: 10.1002/gps.5125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88-100. doi: 10.1212/WNL.0000000000004058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer CE, Qian W, Schweizer TA, et al. Lewy bodies, vascular risk factors, and subcortical arteriosclerotic leukoencephalopathy, but not Alzheimer pathology, are associated with development of psychosis in Alzheimer’s disease. J Alzheimers Dis. 2016;50(1):283-295. doi: 10.3233/JAD-150606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobson SA, Morshed T, Dugger BN, et al. ; Arizona Parkinson’s Disease Consortium . Plaques and tangles as well as Lewy-type alpha synucleinopathy are associated with formed visual hallucinations. Parkinsonism Relat Disord. 2014;20(9):1009-1014. doi: 10.1016/j.parkreldis.2014.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballard CG, Jacoby R, Del Ser T, et al. Neuropathological substrates of psychiatric symptoms in prospectively studied patients with autopsy-confirmed dementia with lewy bodies. Am J Psychiatry. 2004;161(5):843-849. doi: 10.1176/appi.ajp.161.5.843 [DOI] [PubMed] [Google Scholar]
- 15.Koppel J, Sunday S, Buthorn J, Goldberg T, Davies P, Greenwald B; Alzheimer’s Disease Neuroimaging Initiative . Elevated CSF Tau is associated with psychosis in Alzheimer’s disease. Am J Psychiatry. 2013;170(10):1212-1213. doi: 10.1176/appi.ajp.2013.13040466 [DOI] [PubMed] [Google Scholar]
- 16.Sennik S, Schweizer TA, Fischer CE, Munoz DG. Risk factors and pathological substrates associated with agitation/aggression in Alzheimer’s disease: a preliminary study using NACC data. J Alzheimers Dis. 2017;55(4):1519-1528. doi: 10.3233/JAD-160780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rapp MA, Schnaider-Beeri M, Purohit DP, Perl DP, Haroutunian V, Sano M. Increased neurofibrillary tangles in patients with Alzheimer disease with comorbid depression. Am J Geriatr Psychiatry. 2008;16(2):168-174. doi: 10.1097/JGP.0b013e31816029ec [DOI] [PubMed] [Google Scholar]
- 18.McCutcheon ST, Han D, Troncoso J, et al. Clinicopathological correlates of depression in early Alzheimer’s disease in the NACC. Int J Geriatr Psychiatry. 2016;31(12):1301-1311. doi: 10.1002/gps.4435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Besser LM, Kukull WA, Teylan MA, et al. The revised National Alzheimer’s Coordinating Center’s neuropathology form—available data and new analyses. J Neuropathol Exp Neurol. 2018;77(8):717-726. doi: 10.1093/jnen/nly049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montine TJ, Monsell SE, Beach TG, et al. Multisite assessment of NIA-AA guidelines for the neuropathologic evaluation of Alzheimer’s disease. Alzheimers Dement. 2016;12(2):164-169. doi: 10.1016/j.jalz.2015.07.492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. J Neuropathol Exp Neurol. 2012;71(4):266-273. doi: 10.1097/NEN.0b013e31824b211b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montine TJ, Phelps CH, Beach TG, et al. ; National Institute on Aging; Alzheimer’s Association . National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123(1):1-11. doi: 10.1007/s00401-011-0910-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289-300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 24.Malmgren K, Thom M. Hippocampal sclerosis—origins and imaging. Epilepsia. 2012;53(suppl 4):19-33. doi: 10.1111/j.1528-1167.2012.03610.x [DOI] [PubMed] [Google Scholar]
- 25.Walker MC. Hippocampal sclerosis: causes and prevention. Semin Neurol. 2015;35(3):193-200. doi: 10.1055/s-0035-1552618 [DOI] [PubMed] [Google Scholar]
- 26.DeTure MA, Dickson DW. The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener. 2019;14(1):32. doi: 10.1186/s13024-019-0333-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldberg TE, Huey ED, Devanand DP. Association of APOE e2 genotype with Alzheimer’s and non-Alzheimer’s neurodegenerative pathologies. Nat Commun. 2020;11(1):4727. doi: 10.1038/s41467-020-18198-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldberg TE, Huey ED, Devanand DP. Associations of APOE e2 genotype with cerebrovascular pathology: a postmortem study of 1275 brains. J Neurol Neurosurg Psychiatry. 2020;jnnp-2020-323746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malek-Ahmadi M, Beach TG, Zamrini E, et al. Faster cognitive decline in dementia due to Alzheimer disease with clinically undiagnosed Lewy body disease. PLoS One. 2019;14(6):e0217566. doi: 10.1371/journal.pone.0217566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferman TJ, Aoki N, Crook JE, et al. The limbic and neocortical contribution of α-synuclein, tau, and amyloid β to disease duration in dementia with Lewy bodies. Alzheimers Dement. 2018;14(3):330-339. doi: 10.1016/j.jalz.2017.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hansen L, Salmon D, Galasko D, et al. The Lewy body variant of Alzheimer’s disease: a clinical and pathologic entity. Neurology. 1990;40(1):1-8. doi: 10.1212/WNL.40.1.1 [DOI] [PubMed] [Google Scholar]
- 32.Outeiro TF, Koss DJ, Erskine D, et al. Dementia with Lewy bodies: an update and outlook. Mol Neurodegener. 2019;14(1):5. doi: 10.1186/s13024-019-0306-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Román GC. Facts, myths, and controversies in vascular dementia. J Neurol Sci. 2004;226(1-2):49-52. doi: 10.1016/j.jns.2004.09.011 [DOI] [PubMed] [Google Scholar]
- 34.Nelson JC, Devanand DP. A systematic review and meta-analysis of placebo-controlled antidepressant studies in people with depression and dementia. J Am Geriatr Soc. 2011;59(4):577-585. doi: 10.1111/j.1532-5415.2011.03355.x [DOI] [PubMed] [Google Scholar]
- 35.Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2019;142(6):1503-1527. doi: 10.1093/brain/awz099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Power MC, Mormino E, Soldan A, et al. Combined neuropathological pathways account for age-related risk of dementia. Ann Neurol. 2018;84(1):10-22. doi: 10.1002/ana.25246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim J, Fischer CE, Schweizer TA, Munoz DG. Gender and pathology-specific effect of apolipoprotein E genotype on psychosis in Alzheimer’s disease. Curr Alzheimer Res. 2017;14(8):834-840. doi: 10.2174/1567205014666170220150021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Frequencies of NPI-Q domain scores (present at any time-point) and neuropathology subtypes (present/absent)
eTable 2. Linear regression analyses of neuropathology subtypes (present/absent) on mean NPI-Q scores during follow-up
eTable 3. Prevalence of single vs concurrent apathy and disinhibition in Frontotemporal Lobar Degeneration and Hippocampal Sclerosis


