Abstract
Objective:
To examine the association between air pollution and diabetes prevalence in the United States, 2002 to 2008.
Methods:
Annual average particulate matter (PM2.5) and ozone concentrations were calculated using daily county-level data from the CDC’s Tracking Network. Individual-level outcome and covariate data were obtained from the Centers for Disease Control and Prevention (CDC) Behavioral Risk Factor Surveillance System for 862,519 individuals. We used Poisson regression analyses to examine associations between each air pollutant (per 10-unit increase) with diabetes, including regional sub-analyses. Analyses were adjusted for year, age, sex, race, ethnicity, education, income, smoking status, body mass index, exercise, and asthma.
Results:
Positive associations between each pollutant and diabetes were found (PM2.5: prevalence ratio [PR] = 1.10; 95% confidence interval [CI] = 1.03, 1.17; ozone: PR = 1.06; 95% CI = 1.03, 1.09). There was limited evidence of effect modification by region.
Conclusions:
Interventions to reduce ambient air pollution may help alleviate the diabetes burden in the US.
Keywords: air pollution, diabetes, ozone, particulate matter
Diabetes mellitus (diabetes) is one of the most prevalent chronic diseases in the United States and adversely impacts individuals’ quality of life and the nation’s economy.1,2 In the United States, the estimated prevalence of diabetes is 9.4%.3 Of those with diabetes, less than 5% have Type 1, gestational, monogenic, or cystic fibrosis-related diabetes; the remaining 95% have Type 2 diabetes.3,4 Although established risk factors for Type 2 diabetes include age, race/ethnicity, obesity, physical inactivity, and an unhealthy diet,3,5,6 the potential impact of environmental exposures, such as air pollution, have not been thoroughly investigated.7
Two of the most ubiquitous air pollutants are fine particulate matter (PM2.5) and ozone, both of which may enter the alveoli of the respiratory system and induce adverse health effects.8–11 PM2.5 is a heterogeneous mix of particles less than 2.5 μm in diameter including both primary particles emitted directly into the environment (eg, via automobile exhaust) and secondary particles formed in the environment due to chemical reactions among atmospheric gases.11 The composition of PM2.5 varies by region and season, including components such as sulfates and nitrates, organic and elemental carbon, liquid droplets, metals, polycyclic aromatic hydrocarbons (PAHs), and even allergens.11,12 Unlike PM2.5, ozone is a secondary pollutant created from a chemical reaction between NOx and volatile organic compounds (VOCs) in the presence of sunlight; VOCs are released into the atmosphere from industrial, commercial, residential facilities, and traffic.12,13 Ozone concentrations also vary across US regions and seasons with higher concentrations occurring in regions with higher population density and during the summer months.12
In animal studies, exposure to PM2.5 and ozone have been associated with an array of effects including pro-inflammatory responses, increased insulin resistance, oxidative stress, and glucose intolerance which lead to metabolic dysregulation, a precursor to diabetes.8,14–19 Previous epidemiologic studies have also suggested that exposure to PM2.5 and ozone may increase the risk of diabetes. Multiple meta-analyses investigating the association between PM2.5 and diabetes report positive associations.20–26 Most of these studies focused on incident diabetes. Recent studies have also identified positive associations between exposure to residential-level ambient PM2.5 and increased clinical indicators of diabetes (eg, HbA1c and fasting blood glucose).27–32 Moreover, several studies investigating chronic exposure to ambient PM2.5 and diabetes also found positive associations,28,33–40 but not all associations were statistically significant.41 However, Coogan et al42 found little support for the association of PM2.5 exposure and diabetes incidence (hazard ratio [HR] = 0.99; 95% confidence interval [CI] = 0.90, 1.09). Similarly, Renzi et al43 also found little support of an association between PM2.5 and diabetes incidence (odds ratio [OR] = 0.996; 95% CI = 0.972, 1.020) or prevalence (OR 0.992; = 95% CI 0.970, 1.000). On the other hand, Pearson et al38 found an increase in diabetes prevalence associated with a 10 μg/m3 increase in ambient PM2.5 exposures for both 2004 (β = 0.77; 95% CI = 0.39, 1.25) and 2005 (β = 0.81; 95% CI = 0.48, 1.07). Overall, the evidence from prior studies suggests that increases in PM2.5 may have an impact on diabetes. Still, the evidence is, at best, inconclusive.
Fewer studies have investigated the association between ozone and diabetes. In a recent study, Jerrett et al44 identified a positive association between census tract-level long-term average ambient ozone and type 2 diabetes incidence among African American women in 56 metropolitan areas within the United States. While this study was novel, it was limited to only African American women. Although Renzi et al43 found no association between ozone and type 2 diabetes prevalence, they did report a positive association with incidence of type 2 diabetes. However, this study was conducted among an Italian cohort and may not be generalizable to US populations. Additional positive associations between acute area-level ozone exposures and diabetes mortality have been identified in at least two previous studies.45,46 One of these examined mortality from diabetes based on diagnosis prior to death as well as mortality records,45 while the other was based only on mortality records of individuals living in Eastern Massachusetts.46 Additionally, among a subgroup of non-hospitalized diabetics, Stafoggia et al47 reported increased diabetes-related mortality in association with increases in acute ambient ozone exposure. Using Medicare data to determine cause of mortality for a variety of chronic conditions, Zanobetti et al48 also found a positive association between long-term ozone exposure and diabetes mortality, but only between May and September. While these studies provide evidence of an association between ozone and diabetes mortality, the effects of pollution on prevalence may not necessarily be the same as those on mortality. To our knowledge, ours is the first study to explore the association between ozone and diabetes prevalence in the United States.
Given the burden of diabetes in the United States and the paucity of research addressing the association of PM2.5 and ozone with diabetes prevalence, further investigation of the relationship of PM2.5 and ozone with diabetes prevalence is merited. Therefore, we conducted an epidemiologic study with the following goals: (1) estimate the association of annual county-level average estimates of ambient PM2.5 and ozone concentrations with prevalent cases of diabetes in the United States, and (2) determine whether the association between annual county-level average estimates of PM2.5 and ozone concentrations with diabetes differs by US region.
MATERIALS AND METHODS
The present study includes data collected from 1,061,395 adults 18 years and older, from 372 unique US counties from 2002 to 2008, from the behavioral risk factor surveillance system (BRFSS) selected metropolitan/micropolitan area risk trends (SMART BRFSS) data, maintained by the US Centers for Disease Control and Prevention (CDC). Although BRFSS is a survey conducted at the state level, SMART BRFSS focuses on urban areas and only includes data for US counties and cities with 500 or more respondents.49,50 To conduct the survey, participants in each year between 2002 and 2008 were identified by using a disproportionate stratified sampling (DSS) design, an efficient type of complex random sampling.51 Given the random sample of participants each year, while it is possible that the same participants could be contacted, BRFSS data are not intended to have repeated measures on individuals, and it is more likely that different participants are selected each year. Ethical approval for this study was obtained from The University of Texas Health Science Center at Houston Committee for the Protection of Human Subjects.
Prevalent cases of diabetes were identified based on the standard core question in BRFSS: “Have you ever been told by a doctor that you have diabetes?”.51 If an individual responded “yes,” they were considered to have diabetes, with the exception of the following response: “yes, but female told only during pregnancy,” as this response is indicative of gestational diabetes. Data on pre-diabetes were also available for the years 2004 to 2008. During these years, “pre-diabetic” and “borderline diabetic” were volunteered response options to the question regarding diagnosis of diabetes. If a respondent indicated they were pre-diabetic or borderline diabetic, they were recorded as having pre-diabetes. For the present analysis, responses “don’t know/not sure” or “refused” were classified as missing.
Concentrations of air pollutants were obtained though the CDC’s Downscaler model which relates the US EPA’s Air Quality System (AQS) monitoring stations’ data to modeled 12 km grid cell data generated by the US EPA’s Community Multiscale Air Quality Modeling System (CMAQ).52–54 Although AQS data provides less biased estimates of ambient concentrations of pollutants where monitors are present, monitoring data are often sparse or not available, resulting in missing data.53 While CMAQ estimates are comprehensive and are available for the entire conterminous United States in 12 km grid cells, they provide more biased, uncertain results of the true concentration of ambient air pollutants.53 Thus, the Downscaler approach regresses observed AQS monitoring data on modeled CMAQ data using a Bayesian framework, accounting for spatio-temporal variation.53 Although the Downscaler model does not account for ambient temperature or land use, the inclusion of both the AQS and the CMAQ data improves upon use of either data source alone and provides less biased results for census tract centroids.53 These centroid measures can also be “upscaled” to larger spatial resolutions (eg, the county-level) and aggregated to any temporal summary without introducing additional bias.53 Additionally, when comparing the Downscaler model to other predictive models, the Downscaler model reduces the mean square error (MSE = 53.1) as compared with ordinary kriging (MSE = 60.9).52 Thus, using data from the Downscaler model, exposure data for each year from 2002 to 2008 in the current study were based on annual county-level averages of either: (1) daily estimates of PM2.5 or (2) daily estimates of the 8-hour maximum ozone concentration. To upscale the data to the county-level, daily census-tract centroid estimates were used to determine daily population-weighted county-level estimates at the centroid of the county using the following equation52:
where County estimatek = Daily Downscaler (DS) estimate for a pollutant at the county level for county k; ConcCT = Daily DS estimate for a pollutant at the census tract level for a tract j located within county k; and ; nk = number of census tracts in county k.52 These daily estimates taken over a 12-month period were then averaged across each year to calculate annual average concentrations of PM2.5 and ozone for each county. These annual county-level average estimates of PM2.5 and ozone concentrations were used to assign average annual exposure estimates to individuals in the present study.
We considered previously established risk factors for diabetes as covariates.21,34,38,47,48,55,56 The following individual-level variables were obtained from SMART BRFSS: sex (male, female), age (in years), race (white, black, Asian, other), ethnicity (Hispanic, non-Hispanic), education (more than high school, high school or GED, less than high school), annual household income (more than or equal to $75,000; $50,000 to $74,999; $35,000 to $49,999; less than $35,000), smoking status (never, former, current), exercise (yes, no), body mass index (BMI, in kg/m2), and current asthma (yes, no). Exercise was self-reported and based on whether the individual participated in any physical activity or exercise in the past month outside of their regular job and was recorded as “yes,” “no,” “don’t know/not sure,” or “refused.” (For the purposes of this study, “don’t know/not sure” or “refused” were classified as missing.) BMI was calculated using self-reported height (m) and weight (kg). A total of 314 individuals were excluded based on the following improbable height or weight values: height less than 1.2 m (n = 236) or more than 2.5 m (n = 1); weight less than 28 kg (n = 58) or more than 250 kg (n = 29). (Note that an individual may have been counted in more than one of these exclusion criterion.) Additionally, 197,985 individuals were excluded because they were missing data for diabetes status (n = 1,166), age (n = 10,628), race (n = 12,128), ethnicity (n = 4,766), education (n = 3,065), annual household income (n = 143,918), smoking status (n = 4,329), BMI (n = 52,453), exercise (n = 1,000), and/or asthma (n = 6,146). (Note that an individual may have been missing in one or more of the above exclusion categories.) After exclusions, a total of 862,519 (81.3% of the original sample) individuals remained for analyses. Of the 862,519 individuals remaining, 76,780 had diabetes. After accounting for the survey design and adjusting for age using the US Census 2000 population, the diabetes prevalence in the current study was 7.5%, which closely reflects the age-adjusted percentage of diabetes prevalence in the adult US population between 2002 and 2008 (6.6% to 7.9%, respectively).57
Poisson regression was used to analyze the association of diabetes prevalence with annual county-level average PM2.5 and ozone concentrations, separately. Since median annual county-level average air pollutant concentrations for each pollutant varied by year, the initial model was adjusted by year; subsequently, all covariates were added to the fully-adjusted model. All models were clustered by county to account for the potential within-county correlation of individual demographic characteristics. To determine if a robust variance estimator was necessary to account for over-dispersion, the mean and variance of our data was compared and model fit was assessed via a goodness-of-fit test. Since approximately 19% of our total observations included missing data, and the majority of the missing values arose due to income (~14%), we conducted a sensitivity analysis using multiple imputation (MI) whereby we imputed 20 datasets for missing values of income using a Markov Chain Monte Carlo procedure for categorical data58,59 and re-ran the adjusted models. To explore regional differences in the association between air pollution exposures and diabetes, we stratified the fully adjusted models by the four US regions defined by the US Census Bureau (ie, Northeast, Midwest, South, and West).60 We also assessed whether interaction was present between each region and PM2.5 or ozone by including interaction terms in the fully-adjusted models. To explore effect modification, we stratified the adjusted models by sex, race, and age (18 to 34, 35 to 44, 45 to 54, 55 to 64, more than or equal to 65). Prevalence ratios (PR) and 95% CI were computed. All results are based on a 10-unit increase in county-level estimated PM2.5 (μg/m3) or ozone (parts per billion, ppb) concentrations.
We also conducted several sensitivity analyses. We applied survey weights to the fully-adjusted model to account for the complex survey design of BRFSS. Since SMART BRFSS county-level weights for each respondent apply only to a particular year and sample, and the data in the present study spanned multiple years, new weights were created. To create new weights that would accommodate the multiple years, we followed recommendations from Texas BRFSS61 and recalculated the yearly SMART BRFSS weights by multiplying the quotient of the sample size of each year and the total sample size (2002 to 2008) by the original county weight in SMART BRFSS.
CVD and diabetes are often comorbid conditions, and as a result, CVD may influence the association between air pollution and diabetes.62 Thus, we analyzed the inclusion of CVD into the fully adjusted model. From 2002 to 2004, CVD questions were part of an optional module in BRFSS and were only asked in a limited number of states. From 2005 onwards the CVD questions were added to the core questionnaire for all states. Thus, CVD information was only available for a subset of BRFSS subjects in the study timeframe for which a composite CVD variable (yes, no) was created for the analysis. Among the subset of subjects with information on CVD and complete covariate data (n = 648,548), we examined whether the inclusion of this variable in the fully-adjusted model impacted the estimation of the association of either PM2.5 or ozone with diabetes.
We examined whether the addition of individuals classified as having pre-diabetes/borderline diabetes to the diabetes case group in the fully-adjusted model impacted results. Finally, we simultaneously included both PM2.5 and ozone in unweighted and weighted models to assess if the presence of one pollutant influenced the association of the other with diabetes.
Statistical significance was declared at P < 0.05 in all analyses. Analyses were conducted using Stata © (version 13.0, College Station, TX), including survey data commands to account for the complex survey design when appropriate. Figures were produced using ArcGIS © (version 10.2, ESRI, Redlands, CA).
RESULTS
The majority of individuals included in this study were female (61.8%), white (81.3%), non-Hispanic (91.8%), had greater than a high school education (64.3%), and were overweight or obese (57.2%) (Table 1). The distribution of characteristics was generally similar among subjects with diabetes, with some exceptions. For example, compared with the study population, a larger proportion of diabetics were 65 or older (45.5%), had less than a high school education (15.7%), had an annual household income of less than $35,000 (48.3%), had a BMI 30.00 kg/m2 or greater (46.9%), did not exercise within the past month (38.8%), or had CVD (27.6%). Additionally, fewer diabetics were in the 18 to 34 age group (3.0%), had an annual household income of $75,000 or greater (12.5%), or a BMI between 18.50 and 24.99 kg/m2 (15.3%) compared with the study population. Asthma and CVD were less prevalent in the total study population (8.7% and 10.0%, respectively) than among diabetics (12.5% and 27.6%, respectively). No marked differences in the distribution of covariates were observed between individuals with pre-diabetes and those with diabetes (data not shown).
TABLE 1.
Characteristics of Individuals in SMART BRFSS Counties* (n = 372) by Prevalent Diabetes Status, 2002–2008
| Characteristic |
n (%) n = 1,061,081 |
Diabetes n = 97,466 |
|---|---|---|
| Gender | ||
| Female | 656,184 (61.8) | 57,305 (58.8) |
| Male | 404,897 (38.2) | 40,161 (41.2) |
| Missing | 0 (0.0) | 0 (0.0) |
| Age | ||
| 18–34 | 190,512 (18.0) | 2,914 (3.0) |
| 35–44 | 192,678 (18.2) | 7,093 (7.3) |
| 45–54 | 217,571 (20.5) | 16,269 (16.7) |
| 55–64 | 190,865 (18.0) | 26,059 (26.7) |
| ≥65 | 258,827 (24.4) | 44,316 (45.5) |
| Missing | 10,628 (1.0) | 815 (0.8) |
| Race | ||
| White | 863,046 (81.3) | 73,074 (75.0) |
| Black | 107,765 (10.2) | 15,823 (16.2) |
| Asian | 18,480 (1.7) | 1,079 (1.1) |
| Other | 59,662 (5.6) | 6,239 (6.4) |
| Missing | 12,128 (1.1) | 1,251 (1.3) |
| Ethnicity | ||
| Non-Hispanic | 974,221 (91.8) | 88,772 (91.1) |
| Hispanic | 82,094 (7.7) | 8,075 (8.3) |
| Missing | 4,766 (0.5) | 619 (0.6) |
| Education | ||
| >High School | 682,125 (64.3) | 50,432 (51.7) |
| High School or GED | 284,026 (26.8) | 31,404 (32.2) |
| <High School | 91,865 (8.7) | 15,253 (15.7) |
| Missing | 3,065 (0.3) | 377 (0.4) |
| Annual household income | ||
| ≥$75,000 | 263,190 (24.8) | 12,154 (12.5) |
| $50,000–$74,999 | 160,009 (15.1) | 10,822 (11.1) |
| $35,000–$49,999 | 146,176 (13.8) | 12,427 (12.8) |
| <$35,000 | 347,788 (32.8) | 47,031 (48.3) |
| Missing | 143,918 (13.6) | 15,032 (15.4) |
| Smoking status | ||
| Never | 561,789 (52.9) | 44,715 (45.9) |
| Former | 303,864 (28.6) | 37,537 (38.5) |
| Current | 191,099 (18.0) | 14,752 (15.1) |
| Missing | 4,329 (0.4) | 462 (0.5) |
| BMI (kg/m2) | ||
| <18.50 | 17,468 (1.7) | 628 (0.6) |
| 18.50–24.99 | 383,768 (36.2) | 14,919 (15.3) |
| 25.00–29.99 | 365,092 (34.4) | 30,670 (31.5) |
| ≥30.00 | 242,300 (22.8) | 45,680 (46.9) |
| Missing | 52,453 (4.9) | 5,569 (5.7) |
| Exercise | ||
| No | 255,005 (24.03) | 37,809 (38.79) |
| Yes | 805,076 (75.87) | 59,520 (61.07) |
| Missing | 1,000 (0.09) | 137 (0.14) |
| Asthma | ||
| No | 962,133 (90.7) | 84,532 (86.7) |
| Yes | 92,802 (8.7) | 12,210 (12.5) |
| Missing | 6,146 (0.6) | 724 (0.8) |
| No | 714,552 (87.9) | 55,501 (69.7) |
| CVD† | ||
| Yes | 81,161 (10.0) | 22,024 (27.6) |
| Missing | 17,290 (2.1) | 2,156 (2.7) |
BMI, body mass index; BRFSS, behavioral risk factor surveillance system; CVD, cardiovascular disease; SMART, selected metropolitan/micropolitan area risk trends.
Only available for all states from 2004 to 2008.
Only available for six states in 2002, 20 states in 2003, nine states in 2004, and all states 2005–2008.
Figures 1 and 2 show the distribution of PM2.5 and ozone concentrations across the United States and in SMART counties included in this study. The overall median concentration of PM2.5 was 10.8 μg/m3 (interquartile range (IQR) = 8.6, 12.4) in the entire United States and 11.2 μg/m3 (IQR = 9.3, 13.1) among SMART counties. The median concentration of ozone was 40.9 ppb (IQR = 38.6, 43.1) in the entire United States and 39.7 ppb (IQR = 36.9, 42.4) among SMART counties.
FIGURE 1.
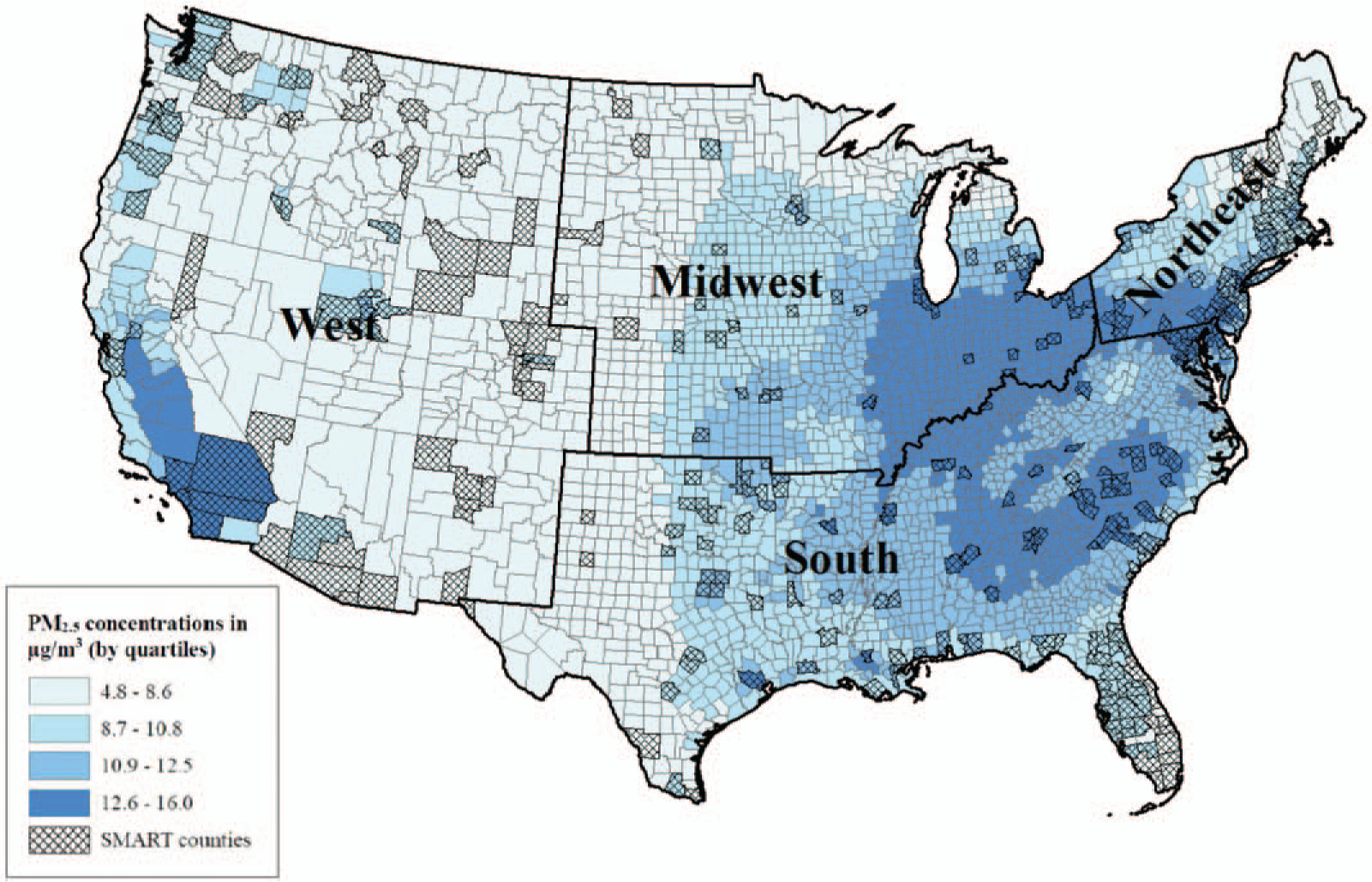
Median (IQR) annual county-level average estimates of daily PM2.5 concentrations (μg/m3) among contiguous US and SMART BRFSS counties within US Census Bureau regions, 2002 to 2008.
FIGURE 2.
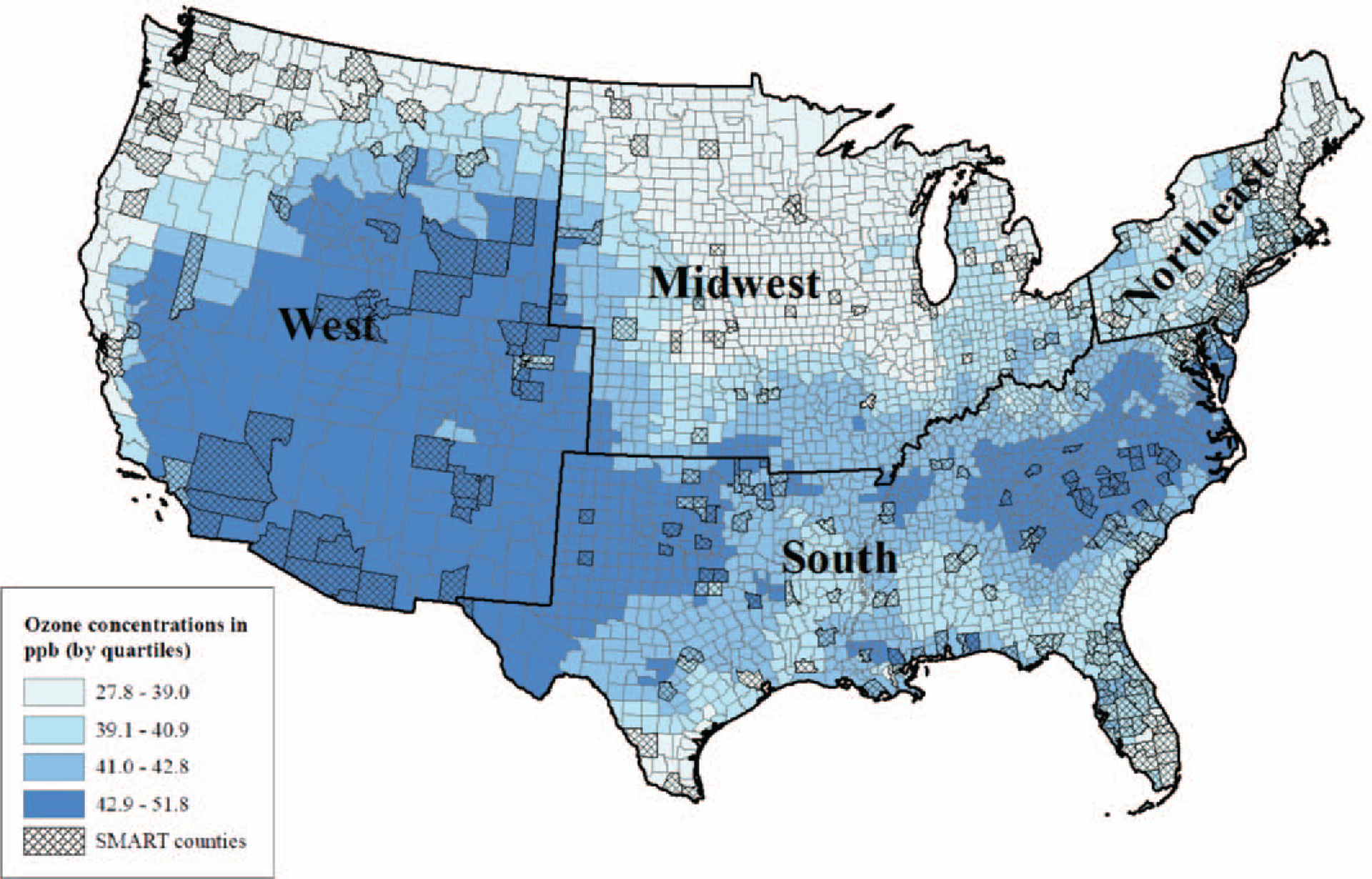
Median (IQR) annual county-level average estimates of daily 8-hour maximum ozone concentrations (ppb) among contiguous US and SMART BRFSS counties within US Census Bureau regions, 2002 to 2008.
When considering SMART counties only, we found statistically significant differences in median annual county-level average concentrations of PM2.5 and ozone concentrations by region (P < 0.001) (data not shown). Specifically, we found the median of the annual county-level average concentrations for PM2.5 was highest in the South (12.4 μg/m3) and ozone concentrations were highest in the West (42.2 ppb). The lowest concentrations among SMART counties were found in the West for PM2.5 (8.6 μg/m3) and the Midwest for ozone (36.6 ppb).
A positive association was found between annual county-level estimates of PM2.5 concentration and diabetes based on the initial model (PR = 1.32; CI = 1.19, 1.47) (Table 2). This association was attenuated in the fully-adjusted model (PR = 1.10; 95% CI = 1.03, 1.17). For PM2.5, we determined that the inclusion of race in the fully-adjusted model was driving the attenuation of the effect estimate. However, for ozone, the effect estimate was not appreciably affected by inclusion of any specific covariate into the model. A positive, but weak, association was found between annual county-level ozone concentrations and prevalence of diabetes in the initial model (PR = 1.05; 95% CI = 0.98, 1.12). The addition of covariates in the fully-adjusted model resulted in a similar, but more precise estimate of the association between ozone and diabetes (PR = 1.06; 95% CI = 1.03, 1.09). Since our data for diabetes indicated that the mean (0.092) approximately equaled the variance (0.084), including the robust variance estimator did not improve model fit (goodness-of-fit test P-value >0.05). As a result, we chose to present results from the most parsimonious model, the model without the robust option. Estimates from the multiple imputed dataset (imputing missing values of income) did not change the results for ozone (pooled MI estimate: PR = 1.06; 95% CI = 1.03, 1.09) and only marginally decreased the estimate for PM2.5 (pooled MI estimate: PR = 1.08; 95% CI = 1.02, 1.15).
TABLE 2.
Association of Annual County-Level Average Estimates of PM2.5 (μg/m3) and Ozone (ppb) Concentrations With Prevalent Diabetes in 372 SMART BRFSS Counties (n = 862,519), 2002–2008
| PM2.5 | Ozone | |
|---|---|---|
| Model | PR* (95% CI) | PR* (95% CI) |
| Initial† | 1.32 (1.19, 1.47) | 1.05 (0.98, 1.12) |
| Fully-adjusted† | 1.10 (1.03, 1.17) | 1.06 (1.03, 1.09) |
BRFSS, behavioral risk factor surveillance system; CI, confidence interval; CVD, cardiovascular disease; PM, particulate matter; ppb, parts per billion; PR, prevalence ratio; SMART, selected metropolitan/micropolitan area risk trends.
Per 10 unit increase in PM2.5 (μg/m3) or ozone (ppb).
Adjusted for year.
Adjusted for year, age, sex, race, ethnicity, education, annual household income, smoking status, body mass index, exercise and asthma.
Few regional differences in the associations between county-level estimates of air pollution and prevalence of diabetes were found, indicating there was limited evidence of effect modification by region (Table 3). In separate models, we assessed whether there was interaction between region and PM2.5 or ozone, however, the estimates for the interaction terms were not statistically significant (P > 0.05) (data not shown). Based on the stratified models, we found little evidence of effect modification of the association between ozone and diabetes for sex, race, or age (Table 4). Similarly, effect modification of the association between PM2.5 and diabetes was assessed by stratifying by sex, race, or age. We found only limited evidence of effect modification, with slightly stronger associations among males, white and Asian individuals, as well as the youngest age group18–34 and those aged 55 to 64, though many estimates were imprecise (Table 4).
TABLE 3.
Adjusted Association* of Annual County-Level Average Estimates of PM2.5 (μg/m3) and Ozone (ppb) Concentrations With Prevalent Diabetes Among SMART BRFSS Counties by US region, 2002–2008
| Northeast (N = 272,634; Counties = 81) |
Midwest (N = 139,907; Counties = 51) |
South (N = 234,058; Counties = 163) |
West (N = 215,920; Counties = 77) |
|
|---|---|---|---|---|
| PR† (95% CI) | PR† (95% CI) | PR† (95% CI) | PR† (95% CI) | |
| PM2.5 | 1.06 (0.89, 1.26) | 1.19 (1.07, 1.33) | 0.93 (0.82, 1.06) | 1.06 (0.95, 1.18) |
| Ozone | 1.12 (1.03, 1.22) | 1.12 (1.00, 1.26) | 1.06 (0.96, 1.18) | 1.00 (0.97, 1.05) |
BRFSS, behavioral risk factor surveillance system; CI, confidence interval; PM, particulate matter; ppb, parts per billion; PR, prevalence ratio; SMART, selected metropolitan/micropolitan area risk trends.
Adjusted for year, age, sex, race, ethnicity, education, annual household income, smoking status, body mass index, exercise, and asthma.
Per 10 unit increase in PM2.5 (μg/m3) or ozone (ppb).
TABLE 4.
Stratification by Sex, Race, and Age for the Adjusted Association of Annual County-Level Average Estimates of PM2.5 (μg/m3) and Ozone (ppb) Concentrations With Prevalent Diabetes Among SMART BRFSS Counties
| PM2.5 | Ozone | |||
|---|---|---|---|---|
| PR* | 95%CI | PR* | 95%CI | |
| Gender† | ||||
| Male | 1.13 | 1.05, 1.21 | 1.05 | 1.01, 1.09 |
| Female | 1.07 | 1.00, 1.15 | 1.07 | 1.03, 1.10 |
| Race‡ | ||||
| White | 1.14 | 1.07, 1.22 | 1.06 | 1.03, 1.10 |
| Black | 0.9 | 0.81, 1.00 | 1.06 | 1.00, 1.11 |
| Asian | 1.26 | 0.95, 1.69 | 0.99 | 0.88, 1.11 |
| Other | 0.93 | 0.81, 1.06 | 1.06 | 0.99, 1.13 |
| Age§ | ||||
| 18–34 | 1.22 | 1.02, 1.47 | 1.07 | 0.97, 1.19 |
| 35–44 | 1.02 | 0.89, 1.17 | 1.09 | 1.02, 1.16 |
| 45–54 | 1.12 | 1.02, 1.22 | 1.06 | 1.01, 1.11 |
| 55–64 | 1.19 | 1.10, 1.29 | 1.02 | 0.98, 1.06 |
| ≥65 | 1.07 | 1.00, 1.14 | 1.05 | 1.02, 1.09 |
Per 10-unit increase in PM2.5 (μg/m3) or ozone (ppb).
Adjusted for year, age, race, ethnicity, education, annual household income, smoking status, body mass index, exercise, and asthma.
Adjusted for year, age, sex, ethnicity, education, annual household income, smoking status, body mass index, exercise, and asthma.
Adjusted for year, sex, race, ethnicity, education, annual household income, smoking status, body mass index, exercise, and asthma.
The application of survey weights to the fully-adjusted model attenuated the association between annual estimated county-level PM2.5 and diabetes (see Table 5). Weighted analysis of the association between annual estimated county-level ozone concentrations and diabetes resulted in slightly stronger association (see Table 5). Additional sensitivity analyses including the analysis of the diabetes plus pre-diabetes group, adjusting for CVD, and investigating the association of each of the air pollutants with diabetes in unweighted and weighted models (data not shown) did not meaningfully deviate from the main results presented in Table 2.
TABLE 5.
Weighted Association of Annual County-Level Average Estimates of PM2.5 (μg/m3) and Ozone (ppb) Concentrations With Prevalent Diabetes in 372 SMART BRFSS Counties* (n = 862,519), 2002–2008
| Model | Diabetes PR† (95% CI) |
|
|---|---|---|
| PM2.5 | Initial‡ | 1.21 (1.11, 1.31) |
| Fully-adjusted§ | 1.05 (0.98, 1.13) | |
| Ozone | Initial‡ | 1.02 (0.97, 1.08) |
| Fully-adjusted§ | 1.08 (1.04, 1.12) |
BRFSS, behavioral risk factor surveillance system; CI, confidence interval; CVD, cardiovascular disease; PM, particulate matter; ppb, parts per billion; PR, prevalence ratio; SMART, selected metropolitan/micropolitan area risk trends.
Unique counties between 2002 and 2008.
Per 10 unit increase in PM2.5 (μg/m3) or ozone (ppb).
Adjusted for year.
Adjusted for year, age, sex, race, ethnicity, education, annual household income, smoking status, body mass index, exercise, and asthma.
DISCUSSION
We found evidence of a moderate positive association between annual county-level average PM2.5 concentration and diabetes prevalence in large urban areas of the contiguous United States, from 2002 to 2008, which is consistent with previous studies.27,28,35,38 We also found a weak positive association between annual county-level concentrations of ozone and prevalence of diabetes, an association that has been insufficiently studied in the existing literature.
Previous studies have investigated the association between PM2.5 and diabetes prevalence by relying on county-level estimates of covariates.35,38 However, more recent studies have investigated the association between PM2.5 concentrations and diabetes prevalence and/or related outcomes (eg, increased HbA1c or fasting blood glucose) using individual- and neighborhood-level covariates.27–29,39,43,63,64 Similarly, our study utilized individual-level diabetes and covariate data rather than area-level estimates, which reduces the potential for residual confounding.
Of the most recent studies investigating the association between PM2.5 and diabetes as well as diabetes-related outcomes, only two have focused on populations in the United States. Peng et al29 found an association between each interquartile increase in PM2.5 concentration and higher fasting blood glucose levels among non-diabetics, while Honda et al27 found associations between an interquartile range increase of PM2.5 and diabetes prevalence as well as increased levels of HbA1c. Although these are both longitudinal studies and the current study is cross-sectional, the larger sample size in our study allows for increased precision of the estimates in comparison to previous studies. The current study also has a broader age range (18 and older) than the Peng et al29 and Honda et al27 studies (for which the age was at least 57 and older). As a result, our findings may be more generalizable to US adults (18 or older), rather than only adults 57 and older as in these previous studies.
The literature also suggests positive associations between ozone and diabetes mortality.45–48 However, because deaths among diabetics often result from comorbidities, such as CVD, diabetes is not often listed as the primary cause of death.3 Thus, analyses utilizing mortality from diabetes may underestimate diabetes burden and potentially attenuate reported associations with risk factors such as air pollution. To our knowledge, this is the first study to explore the association between ambient ozone concentrations and diabetes prevalence in the US.
We provide some evidence of regional differences in the association between annual county-level estimates of PM2.5 with diabetes prevalence, which could be due to regional variations in the composition of PM2.5.12 Previously, using the National Climatic Data Center’s nine US regions, Chien et al35 found increases in PM2.5 led to the greatest spatial vulnerability of diabetes prevalence in the South, Central, and Southeast regions. Despite the more parsimonious regional classification used in the current study, it should nonetheless capture potential regional differences in PM2.5 composition.65,66 Additionally, the National Climatic Data Center regions identified as spatially vulnerable areas overlap with portions of the US Census Bureau’s Midwest region where we found the strongest regional association between county-level PM2.5 concentrations and diabetes prevalence. In the current study, although estimates of the association between ozone and diabetes prevalence were larger in the Northeast and Midwest, given the small effect sizes, the importance of these observed differences remains unclear.
While we found a positive association between both PM2.5 and ozone with diabetes, sensitivity analyses revealed similar, but more precise associations when we applied county-level weights to the fully-adjusted model. Weights used in BRFSS are intended to ensure that minorities and certain age and sex groups are accurately represented in the produced estimates since these populations are generally under-sampled.50 Given that the current study spanned multiple years and no weights exist for the combined population used in this study, new calculated county-level weights for the BRFSS data were created based on recommendations from BRFSS personnel.61 However, because we also used exposure data external to BRFSS, there is still some uncertainty regarding the correct application of these population weights. As a result, the weighted associations may have been exaggerated or attenuated, while the internal validity of the results using the unweighted models remains unaffected. Our sensitivity analyses also provided no indication that CVD acted as a confounder of the associations between either PM2.5 or ozone and diabetes. Similarly, adding the pre-diabetes group to the analyses did not change the results; however, this may be due to the small number of individuals who identified as pre-diabetic. Additionally, the multipollutant model did not reveal appreciable changes to the single-pollutant estimates, indicating that associations between PM2.5 and diabetes prevalence is not likely driving the small association observed with ozone. For PM2.5, stratification by sex, race, and age revealed stronger, positive associations among males, Asians, and 55 to 64 year olds, respectively. For ozone, stratification by sex and age revealed slightly stronger associations among females and 35 to 44 year olds, respectively. While there was no difference in the estimates between race groups of White, Black, or Other; the association between ozone and diabetes was nearest the null among Asians. For both PM2.5 and ozone, stratification by sex, race, and age revealed little evidence that effect modification of the association between PM2.5 and ozone with diabetes was present.
The present analysis had several limitations. Given the cross-sectional design of the currently study, temporality cannot be established and, as a result, the current study cannot suggest a causal relationship between PM2.5 and ozone with diabetes. Additionally, SMART BRFSS does not differentiate between Type 1 and Type 2 diabetes. However, at least 95% of all diabetes cases are Type 2 diabetes3 and thus, it is likely that our results reflect associations between ambient PM2.5 and ozone with Type 2 diabetes. It is possible that prevalence of diabetes was underreported in BRFSS since data on prescription medication use (eg, metformin or insulin) and fasting glucose data for diabetes were not available67,68 and participants may have been unaware of their diabetes status. Because our study relies on self-reported diabetes status, outcome misclassification is also possible. Schneider et al68 found excellent specificity (more than 95%) and moderate sensitivity (59% to 71%) between self-reported diabetes and various levels of fasting blood glucose among a subset of participants from the Atherosclerosis Risk in Communities (ARIC) study. Additionally, Okura et al69 reported substantial agreement (κ = 0.76; 95% CI = 0.70, 0.82) between self-reported diabetes status with medical records; however, Okura et al also stated their population may only be reflective of those with healthcare access. Healthcare access may play a role in the accuracy of self-reported diabetes status.70,71 Zhang et al71 and Selvin et al70 identified lack of healthcare access and insurance coverage as key factors that contribute to undiagnosed cases of type 2 diabetes. In our study, 89% (n = 946,111) of participants reported having at least some kind of health care insurance and 85% (n = 905,780) reported having a doctor; thus, the majority of participants appear to have healthcare access. Because self-report of diabetes substantially agrees with medical diagnoses of diabetes,69 we are confident that misclassification in 85% to 89% of our study population that have access to healthcare, if any, is minor. Still, some misclassification of diabetes among participants with no access to healthcare in the current study is possible, and more likely it would underestimate prevalent cases of diabetes.
Also, exercise was measured based on the past month and only recorded as a dichotomous yes/no variable which could result in residual confounding. Because SMART BRFSS only includes data for large, urban counties in the United States,49,51 the results of this study may not be generalizable to less populated, rural areas. The BRFSS data from 2002 to 2008 were collected via random-digit dialing of landline phones, excluding individuals with wireless only service (estimated to be less than 20% from 2003 to 2008) as well as an estimated 2% of individuals with no landline or cellphone service (data on wireless only service were not available for 2002).72 Compared with individuals using landline telephones, individuals who rely only on wireless phone service may be younger, Hispanic, male, and living in poverty,72,73 potentially introducing coverage bias. However, given the small percentage of individuals who relied on wireless only, we believe potential effects of coverage bias on our estimates are small. Because non-whites, younger individuals, and those who are less educated are less likely to respond to telephone surveys than their counterparts,74–76 survey non-response bias is also possible.
County-level exposure estimates used in the present study were obtained from an air pollution model which combines AQS monitoring data with CMAQ modeled data. However, ambient measures may not accurately reflect personal exposure. In a study conducted in Boston, MA, Brown et al. found seasonal variation played a role in the correlation between personal and ambient measures of PM2.5.77 For PM2.5, in the winter, ambient measures did not accurately reflect personal measures (median ρ=0.3), but in the summer the correlation between the two measures improved (median ρ = 0.6). Brown et al77 attributed the majority of these differences to seasonal changes in ventilation, but noted that the correlation could be influenced by personal behaviors. Additionally, in the winter, Brown found that the geometric mean (GM) of ambient measures was lower than personal measures (ambient: GM = 8.5 μg/m3; geometric standard deviation (GSD) = 1.8 μg/m3; personal: GM = 10.4 μg/m3; GSD = 1.8 μg/m3), but in the summer, ambient measures were higher (GM = 10.7 μg/m3; GSD = 1.6 μg/m3) than personal measures (GM = 8.5 μg/m3; GSD = 1.7 μg/m3). In a meta-analysis including 18 studies conducted by Avery et al,78 the findings were similar (r = 0.54; standard deviation = 0.12) with increased correlation among studies with higher mean ambient PM2.5, studies conducted in eastern North America, and areas with higher humidity. Given the varied results regarding agreement between ambient and personal PM2.5 exposures, the direction of the bias of the effect estimate in the current study cannot be determined.
Studies investigating the association between ambient and personal measures of ozone also have differing results. In a study conducted in Boston, MA, Brown et al79 did not find an association between personal and ambient measures of ozone during winter (β = −0.01; 95% CI = −0.14, 0.12); however, a small, but significant association was found during the summer months (β = 0.25; 95% CI = 0.14, 0.35). During the summer, the association was also increased with increased ventilation (windows open: β = 0.29; 95% CI = 0.19, 0.39; high air exchange rate: β = 0.30; 95% CI = 0.20, 0.40).79 Dimakopoulou et al80 also investigated the association between personal and ambient measures of ozone (from fixed monitoring sites) among school children in Athens and Thessaloniki, Greece and found a positive, significant association between personal and ambient measures of ozone (β = 0.042 95% CI = 0.007, 0.078). Given the different associations found between personal and ambient measures in the two studies, it is possible that ambient measures of ozone may not accurately reflect personal measures. Since ambient concentrations were higher as compared with personal measurements in both studies, it is possible that our estimates for ozone could be exaggerated.
Additionally, although the Downscaler approach is an improvement upon using AQS or CMAQ data alone, the Downscaler estimates may not be equivalent to monitoring data, particularly in areas where monitoring data are sparse.53 As a result, in the current study, estimates of PM2.5 and ozone using the Downscaler model may have more uncertainty in areas with fewer monitors. Due to the limitations of the Downscaler model, measurement error is possible. However, this error is expected to result in non-differential information bias.
County-level exposure data may not accurately reflect personal exposures. Large urban areas may have higher variability in particulate matter concentrations than rural areas,81,82 resulting in varied within-county, individual-level exposures. Individual mobility patterns are also not accounted for. However, because ambient air pollution interventions are likely to happen at the group-level (eg, policies are generally enacted by municipalities such as counties) rather than at the individual-level, the results of this study may still be used to inform community-level prevention and intervention efforts, even if they do not address the individual exposure–outcome response.
Our study provides evidence of moderate associations between area-level particulate matter and ozone with diabetes prevalence. While our results regarding PM2.5 are consistent with previous studies, our finding of a potential association between ambient ozone concentrations and diabetes prevalence is a novel one, though the strength of the association was weak. Even so, further examination is warranted. While evidence regarding the contribution of air pollution to diabetes burden in this country is mounting, lifestyle and diet remain primary determinants, although these could be influenced by place-based factors such as the built environment, availability and accessibility to healthy foods, as well as economic and health policies, at national and local levels. Although lifestyle and diet are important individual determinants, focusing on initiatives that address determinants of health at a community-level, such as reducing air pollution, provides a greater opportunity for a more sustainable, far-reaching impact on reducing the diabetes burden in the population. Additionally, our findings regarding regional differences in the associations between PM2.5 and ozone concentrations and diabetes prevalence highlight the need for future research opportunities. One aspect in which future studies should focus is the identification of specific PM2.5 components that may be driving the association with diabetes and their effects on human health.
Learning Objectives.
Become familiar with previously reported associations of diabetes with air pollution, specifically fine particulate matter (PM2.5) and ozone.
Summarize the new findings on the associations between PM2.5 and ozone and diabetes in US adults.
Discuss the study implications for efforts to reduce air pollution as part of policies to alleviate the US burden of diabetes.
Funding:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Drs David Gimeno Ruiz de Porras and Kristina Whitworth were partially supported by a Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health (CDC/NIOSH) Education and Research Center Grant No. 5T42OH008421 to the SWCOEH.
Footnotes
Authors Hernandez, Gimeno Ruiz de Porras, Marko, and Whitworth have no relationships/conditions/circumstances that present potential conflict of interest.
The JOEM editorial board and planners have no financial interest related to this research.
REFERENCES
- 1.Centers for Disease Control and Prevention. Diabetes Report Card 2014; 2015. Available at: http://www.cdc.gov/diabetes/pdfs/library/diabetesreport-card2014.pdf. Accessed March 24, 2017.
- 2.Gerteis J, Izrael D, Deitz D, et al. Multiple Chronic Conditions Chartbook. Rockville, MD: Agency for Health Care Research and Quality; 2014. [Google Scholar]
- 3.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017: Estimates of Diabetes and Its Burden in the United States; 2017. Available at: https://www.cdc.gov/diabetes/data/statistics/statistics-report.html. Accessed September 14, 2017. [Google Scholar]
- 4.National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). What is Diabetes? 2016. Available at: https://www.niddk.nih.gov/health-information/diabetes/overview/what-is-diabetes. Accessed February 8, 2017.
- 5.International Diabetes Federation. About Diabetes; 2014. Available at: http://www.idf.org/about-diabetes. Accessed April 19, 2015.
- 6.World Health Organization. Diabetes; 2015. Available at: http://www.who.int/mediacentre/factsheets/fs312/en/. Accessed April 15, 2017.
- 7.Bhatnagar A Could dirty air cause diabetes? Circulation. 2009;119: 492–494. [DOI] [PubMed] [Google Scholar]
- 8.Bass V, Gordon CJ, Jarema KA, et al. Ozone induces glucose intolerance and systemic metabolic effects in young and aged Brown Norway rats. Toxicol Appl Pharmacol. 2013;273:551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajagopalan S, Brook RD. Air pollution and type 2 diabetes: mechanistic insights. Diabetes. 2012;61:3037–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonnell WF, Raub JA, Spencer DC, Stone SL, Brown J, Wildermann E. Ozone and Your Patients’ Health Training for Health Care Providers; 2015. Available at: http://www.epa.gov/apti/ozonehealth/population.html#uptake. Accessed May 18, 2015.
- 11.World Health Organization. Health Effects of Particulate Matter: Policy Implications for Countries in Eastern Europe, Caucasus and Central Asia; 2013. Available at: http://www.euro.who.int/__data/assets/pdf_file/0006/189051/Health-effects-of-particulate-matter-final-Eng.pdf. Accessed March 24, 2017.
- 12.Dye T, MacDonald C, Anderson C, Hafner H, Wheeler N, Chan A. Guide-lines for Developing an Air Quality (Ozone and PM2.5) Forecasting Program; 2003. Available at: http://agris.fao.org/agris-search/search.do?recordID=US201300092899. Accessed April 21, 2017.
- 13.United States Environmental Protection Agency. Ground Level Ozone; 2014. Available at: http://www.epa.gov/airquality/ozonepollution/. Accessed February 8, 2015.
- 14.Liu C, Ying Z, Harkema J, Sun Q, Rajagopalan S. Epidemiological and experimental links between air pollution and type 2 diabetes. Toxicol Pathol. 2013;41:361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller DB, Snow SJ, Henriquez A, et al. Systemic metabolic derangement, pulmonary effects, and insulin insufficiency following subchronic ozone exposure in rats. Toxicol Appl Pharmacol. 2016;306:47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Q, Yue P, Deiuliis JA, et al. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119:538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Eeden SF, Tan WC, Suwa T, et al. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)). Am J Respir Crit Care Med. 2001;164:826–830. [DOI] [PubMed] [Google Scholar]
- 18.Xu X, Liu C, Xu Z, et al. Long-term exposure to ambient fine particulate pollution induces insulin resistance and mitochondrial alteration in adipose tissue. Toxicol Sci. 2011;124:88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong J, Allen K, Rao X, et al. Repeated ozone exposure exacerbates insulin resistance and activates innate immune response in genetically susceptible mice. Inhal Toxicol. 2016;28:383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balti EV, Echouffo-Tcheugui JB, Yako YY, Kengne AP. Air pollution and risk of type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2014;106:161–172. [DOI] [PubMed] [Google Scholar]
- 21.Eze IC, Hemkens LG, Bucher HC, et al. Association between ambient air pollution and diabetes mellitus in Europe and North America: systematic review and meta-analysis. Environ Health Perspect. 2015;123: 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He D, Wu S, Zhao H, et al. Association between particulate matter 2.5 and diabetes mellitus: a meta-analysis of cohort studies. J Diabetes Investig. 2017;8:687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park SK, Wang W. Ambient air pollution and Type 2 diabetes: a systematic review of epidemiologic research. Curr Environ Health Rep. 2014;1: 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang B, Xu D, Jing Z, Liu D, Yan S, Wang Y. Effect of long-term exposure to air pollution on type 2 diabetes mellitus risk: a systemic review and meta-analysis of cohort studies. Eur J Endocrinol. 2014;171:R173–R182. [DOI] [PubMed] [Google Scholar]
- 25.Janghorbani M, Momeni F, Mansourian M. Systematic review and meta-analysis of air pollution exposure and risk of diabetes. Eur J Epidemiol. 2014;29:231–242. [DOI] [PubMed] [Google Scholar]
- 26.Puett RC, Hart JE, Schwartz J, Hu FB, Liese AD, Laden F. Are particulate matter exposures associated with risk of type 2 diabetes? Environ Health Perspect. 2011;119:384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honda T, Pun VC, Manjourides J, Suh H. Associations between long-term exposure to air pollution, glycosylated hemoglobin and diabetes. Int J Hyg Environ Health. 2017;220:1124–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu C, Yang C, Zhao Y, et al. Associations between long-term exposure to ambient particulate air pollution and type 2 diabetes prevalence, blood glucose and glycosylated hemoglobin levels in China. Environ Int. 2016;92–93:416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng C, Bind MC, Colicino E, et al. Particulate air pollution and fasting blood glucose in nondiabetic individuals: associations and epigenetic mediation in the normative aging study, 2000–2011. Environ Health Perspect. 2016;124:1715–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yitshak Sade M, Kloog I, Liberty IF, Schwartz J, Novack V. The association between air pollution exposure and glucose and lipids levels. J Clin Endocrinol Metab. 2016;101:2460–2467. [DOI] [PubMed] [Google Scholar]
- 31.Teichert T, Vossoughi M, Vierkotter A, et al. Association between traffic-related air pollution, subclinical inflammation and impaired glucose metabolism: results from the SALIA study. PLoS ONE. 2013;8:e83042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thiering E, Cyrys J, Kratzsch J, et al. Long-term exposure to traffic-related air pollution and insulin resistance in children: results from the GINIplus and LISAplus birth cohorts. Diabetologia. 2013;56:1696–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brook RD, Cakmak S, Turner MC, et al. Long-term fine particulate matter exposure and mortality from diabetes in Canada. Diabetes Care. 2013;36:3313–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen H, Burnett RT, Kwong JC, et al. Risk of incident diabetes in relation to long-term exposure to fine particulate matter in Ontario, Canada. Environ Health Perspect. 2013;121:804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chien LC, Alamgir H, Yu HL. Spatial vulnerability of fine particulate matter relative to the prevalence of diabetes in the United States. Sci Total Environ. 2015;508:136–144. [DOI] [PubMed] [Google Scholar]
- 36.Hansen AB, Ravnskjaer L, Loft S, et al. Long-term exposure to fine particulate matter and incidence of diabetes in the Danish Nurse Cohort. Environ Int. 2016;91:243–250. [DOI] [PubMed] [Google Scholar]
- 37.Park SK, Adar SD, O’Neill MS, et al. Long-term exposure to air pollution and type 2 diabetes mellitus in a multiethnic cohort. Am J Epidemiol. 2015;181: 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearson JF, Bachireddy C, Shyamprasad S, Goldfine AB, Brownstein JS. Association between fine particulate matter and diabetes prevalence in the U.S. Diabetes Care. 2010;33:2196–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiu H, Schooling CM, Sun S, et al. Long-term exposure to fine particulate matter air pollution and type 2 diabetes mellitus in elderly: a cohort study in Hong Kong. Environ Int. 2018;113:350–356. [DOI] [PubMed] [Google Scholar]
- 40.To T, Zhu J, Villeneuve PJ, et al. Chronic disease prevalence in women and air pollution–A 30-year longitudinal cohort study. Environ Int. 2015;80:26–32. [DOI] [PubMed] [Google Scholar]
- 41.Weinmayr G, Hennig F, Fuks K, et al. Long-term exposure to fine particulate matter and incidence of type 2 diabetes mellitus in a cohort study: effects of total and traffic-specific air pollution. Environ Health. 2015;14:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coogan PF, White LF, Yu J, et al. PM2.5 and diabetes and hypertension incidence in the black Women’s Health Study. Epidemiology. 2016;27: 202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Renzi M, Cerza F, Gariazzo C, et al. Air pollution and occurrence of type 2 diabetes in a large cohort study. Environ Int. 2017;112:68–76. [DOI] [PubMed] [Google Scholar]
- 44.Jerrett M, Brook R, White LF, et al. Ambient ozone and incident diabetes: a prospective analysis in a large cohort of African American women. Environ Int. 2017;102:42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldberg MS, Burnett RT, Yale JF, Valois MF, Brook JR. Associations between ambient air pollution and daily mortality among persons with diabetes and cardiovascular disease. Environ Res. 2006;100:255–267. [DOI] [PubMed] [Google Scholar]
- 46.Ren C, Melly S, Schwartz J. Modifiers of short-term effects of ozone on mortality in eastern Massachusetts–a case-crossover analysis at individual level. Environ Health. 2010;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stafoggia M, Forastiere F, Faustini A, et al. Susceptibility factors to ozone-related mortality: a population-based case-crossover analysis. Am J Respir Crit Care Med. 2010;182:376–384. [DOI] [PubMed] [Google Scholar]
- 48.Zanobetti A, Schwartz J. Ozone and survival in four cohorts with potentially predisposing diseases. Am J Respir Crit Care Med. 2011;184:836–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System - SMART: BRFSS Frequently Asked Questions (FAQs); 2013. Available at: http://www.cdc.gov/brfss/smart/smart_faq.htm. Accessed November 17, 2015.
- 50.Centers for Disease Control and Prevention. SMART: BRFSS City and County Data and Documentation n.d. Available at: http://www.cdc.gov/brfss/smart/smart_data.htm. Accessed April 5, 2015.
- 51.Centers for Disease Control and Prevention. The BRFSS Data User Guide; 2013. Available at: http://www.cdc.gov/brfss/data_documentation/pdf/userguidejune2013.pdf. Accessed March 24, 2017.
- 52.Berrocal V, Gelfand A, Holland D. Space-time data fusion under error in computer model output: an application to modeling air quality. Biometrics. 2012;68:837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berrocal VJ, Gelfand AE, Holland DM. A spatio-temporal downscaler for output from numerical models. J Agric Biol Environ Stat. 2010;15:176–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Centers for Disease Control and Prevention. EPHTN Metadata Profile Report for Metadata ID: 7122; 2012. Available at: http://ephtracking.cdc.gov/showMetadataReport.action?id=aeff506a-3fc3-4878-9ebc-f2d2b3229d08#. Accessed June 26, 2015.
- 55.Kramer U, Herder C, Sugiri D, et al. Traffic-related air pollution and incident type 2 diabetes: results from the SALIA cohort study. Environ Health Perspect. 2010;118:1273–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Neill MS, Veves A, Zanobetti A, et al. Diabetes enhances vulnerability to particulate air pollution-associated impairment in vascular reactivity and endothelial function. Circulation. 2005;111:2913–2920. [DOI] [PubMed] [Google Scholar]
- 57.Centers for Disease Control and Prevention. US Diabetes Surveillance System n.d. Available at: http://www.cdc.gov/diabetes/data. Accessed September 14, 2017.
- 58.Allison PD. Missing Data. Thousand Oaks, Calif: Sage Publications; 2001. [Google Scholar]
- 59.StataCorp. Stata 14 Base Reference Manual. College Station, TX: Stata Press; 2015. [Google Scholar]
- 60.U.S. Census Bureau. Census Regions and Divisions of the United States. Available at: http://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf. Accessed January 26, 2016.
- 61.Wood R BRFSS SMART question. Texas Behavioral Risk Factor Surveillance System Center for Health Statistics; 2016. [Google Scholar]
- 62.International Diabetes Federation. Complications of Diabetes 2015. Available at: http://www.idf.org/complications-diabetes. Accessed June 29, 2016.
- 63.O’Donovan G, Chudasama Y, Grocock S, et al. The association between air pollution and type 2 diabetes in a large cross-sectional study in Leicester: The CHAMPIONS Study. Environ Int. 2017;104:41–47. [DOI] [PubMed] [Google Scholar]
- 64.Strak M, Janssen N, Beelen R, et al. Long-term exposure to particulate matter, NO2 and the oxidative potential of particulates and diabetes prevalence in a large national health survey. Environ Int. 2017;108:228–236. [DOI] [PubMed] [Google Scholar]
- 65.Bell ML, Ebisu K, Peng RD. Community-level spatial heterogeneity of chemical constituent levels of fine particulates and implications for epidemiological research. J Expo Sci Environ Epidemiol. 2011;21:372–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.United States Environmental Protection Agency. Report on the Environment: Ambient Concentrations of Particulate Matter; 2014. Available at: https://cfpub.epa.gov/roe/indicator.cfm?i=9. Accessed February 14, 2017.
- 67.Bielinski SJ, Pankow JS, Rasmussen-Torvik LJ, et al. Strength of association for incident diabetes risk factors according to diabetes case definitions: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2012;175: 466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schneider AL, Pankow JS, Heiss G, Selvin E. Validity and reliability of self-reported diabetes in the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2012;176:738–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57:1096–1103. [DOI] [PubMed] [Google Scholar]
- 70.Selvin E, Wang D, Lee AK, Bergenstal RM, Coresh J. Identifying trends in undiagnosed diabetes in U.S. adults by using a confirmatory definition: a cross-sectional study. Ann Int Med. 2017;167:769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang X, Geiss LS, Cheng YJ, Beckles GL, Gregg EW, Kahn HS. The missed patient with diabetes: how access to health care affects the detection of diabetes. Diabetes Care. 2008;31:1748–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blumberg SJ, Luke JV. Wireless substitution: Early release of estimates from the National Health Interview Survey, July-December 2008; 2009. Available at: http://www.cdc.gov/nchs/nhis/index.htm. Accessed August 1, 2016.
- 73.Blumberg SJ, Luke JV. Wireless substitution: Early release of estimates based on data from the National Health Interview Survey, July-December 2006; 2007. Available at: http://www.cdc.gov/nchs/nhis/index.htm. Accessed August 9, 2016.
- 74.Link MW, Mokdad AH, Stackhouse HF, Flowers NT. Race, ethnicity, and linguistic isolation as determinants of participation in public health surveillance surveys. Prev Chronic Dis. 2006;3:A09. [PMC free article] [PubMed] [Google Scholar]
- 75.Voigt LF, Koepsell TD, Daling JR. Characteristics of telephone survey respondents according to willingness to participate. Am J Epidemiol. 2003;157:66–73. [DOI] [PubMed] [Google Scholar]
- 76.Schneider KL, Clark MA, Rakowski W, Lapane KL. Evaluating the impact of non-response bias in the Behavioral Risk Factor Surveillance System (BRFSS). J Epidemiol Community Health. 2012;66:290–295. [DOI] [PubMed] [Google Scholar]
- 77.Brown KW, Sarnat JA, Suh HH, Coull BA, Spengler JD, Koutrakis P. Ambient site, home outdoor and home indoor particulate concentrations as proxies of personal exposures. J Environ Monit. 2008;10:1041–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Avery CL, Mills KT, Williams R, et al. Estimating error in using ambient PM2.5 concentrations as proxies for personal exposures: a review. Epidemiology. 2010;21:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brown KW, Sarnat JA, Suh HH, Coull BA, Koutrakis P. Factors influencing relationships between personal and ambient concentrations of gaseous and particulate pollutants. Sci Total Environ. 2009;407: 3754–3765. [DOI] [PubMed] [Google Scholar]
- 80.Dimakopoulou K, Grivas G, Samoli E, et al. Determinants of personal exposure to ozone in school children. Results from a panel study in Greece. Environ Res. 2017;154:66–72. [DOI] [PubMed] [Google Scholar]
- 81.Greco SL, Wilson AM, Hanna SR, Levy JI. Factors influencing mobile source particulate matter emissions-to-exposure relationships in the Boston urban area. Environ Sci Technol. 2007;41:7675–7682. [DOI] [PubMed] [Google Scholar]
- 82.Greco SL, Wilson AM, Spengler JD, Levy JI. Spatial patterns of mobile source particulate matter emissions-to-exposure relationships across the United States. Atmos Environ. 2007;41:1011–1025. [DOI] [PubMed] [Google Scholar]


