Abstract
Introduction:
In 2011, the International Agency for Research on Cancer evaluated the epidemiological evidence for the association between occupational exposure to radiofrequency (RF) electromagnetic fields (EMF) and cancer as inadequate, due in part to limitations in exposure assessment. This study examines the relation between both occupational RF and intermediate frequency (IF) EMF exposure and brain tumour risk in the INTEROCC multinational case-control study population (nearly 10,000 subjects), using a novel exposure assessment approach.
Methods:
Individual indices of cumulative exposure to RF and IF-EMF were assigned to study participants using a source-exposure matrix and detailed interview data on work with or nearby EMF sources, both overall and in specific exposure time windows. Conditional logistic regression models were used to investigate associations with glioma and meningioma risk.
Results:
Overall, there was no clear evidence for an association between RF or IF-EMF and the brain tumours studied, with the vast majority of analyses showing no associations and in fact odds ratios (ORs) below one. The largest adjusted ORs were obtained for RF magnetic fields in the highest exposed category for the most recent exposure time window (1–4 years before the diagnosis or reference date) for both glioma (OR=1.62 (95% confidence interval (CI): 0.86, 3.01)) and meningioma (1.52 (CI: 0.65, 3.55)). Similar results were obtained using a continuous analysis.
Conclusion:
Despite the individualized approach used in this study, the largest case-control on brain tumours and EMF occupational exposures, no clear associations were identified. However, RF magnetic fields should be further investigated using more exposed participants and alternative exposure/dose metrics.
Keywords: brain tumours, electromagnetic fields, intermediate frequency, occupational exposure, radiofrequency, source-exposure matrix
INTRODUCTION
Glioma and meningioma are the most frequent primary brain tumour types in adults. Gliomas originate in the glial tissue and are mostly malignant, representing around 80% of all malignant brain tumours (1–3). Meningiomas are commonly benign, although approximately 5% are malignant (1). The etiologies of these diseases remain largely unknown. The only well-established risk factors, ionizing radiation and genetic disorders, account for a small portion of cases (2,4). The current evidence for other possible risk factors, such as non-ionizing radiation (mostly for extremely-low frequency and radiofrequency electromagnetic fields) and certain chemicals, is inconclusive (2,5–7).
High-frequency electromagnetic fields (EMF) are a form of non-ionizing radiation and comprise, as defined in the international INTEROCC study, intermediate frequency (IF) EMF, between 3 kHz and 10 MHz, and radiofrequency (RF) EMF, between 10 MHz and 300 GHz. The International Agency for Research on Cancer (IARC) classified RF-EMF as possibly carcinogenic to humans (group 2B) in 2011, based on limited animal evidence, mostly from co-carcinogenicity experiments, and limited epidemiological evidence, mainly based on associations between use of cellular telephones and glioma and acoustic neuroma risk (8). The limited evidence from animal experiments was partly based on studies that examined RF-EMF exposure in combination with known carcinogens whose results, recently replicated (9), suggested that RF-EMF may act in the promotion and/or progression of already initiated tumours.
The biophysical mechanism(s) by which RF-EMF might play a role in brain tumours are not clear. Both thermal effects, caused by the absorption of RF energy at a rate greater than the body’s cooling mechanism (10), and non-thermal hypotheses (11–15) have been proposed, including oxidative stress, due to the formation of radical pairs, or calcium efflux due to activation of voltage-gated calcium channels. For IF-EMF, only very limited evidence exists from some available in vivo studies while no specific epidemiologic study of IF has been conducted (7,16).
Epidemiologic evidence on brain cancer risk from occupational exposure to RF-EMF is inadequate and few studies have been performed (17–30). Exposure assessment in most of these studies was mainly based on exposure surrogates, such as distance to the source or specific job titles or groups of workers thought to be exposed to RF fields, using occupational duties, qualitative exposure estimates assigned by hygienists (19) or job-exposure matrices based on expert judgments (22,23,26,27). Only a few studies, involving military personnel (28), radio and telegraph operators (30) or embassy employees (25) used quantitative exposure estimates based on measurements of RF field intensities. However, exposure estimates were generally based on small number of measurements and changes in exposure levels over time were not considered. Sample sizes in these studies were also small (31).
As part of the INTEROCC studýs aim to improve upon the exposure assessments in previous studies, a source-exposure matrix (SEM) was developed (32) containing confidence-weighted mean estimates, based mainly on measurements collected from the literature (33), for the EMF sources reported by the study participants. In the current paper, we used the SEM, together with detailed information collected through interviews on work with or nearby occupational EMF sources to derive individual indices of cumulative RF and IF exposure. These indices were used to analyze the possible association between cumulative occupational exposure to RF or IF-EMF and glioma and meningioma risk, both overall and in specific exposure time windows.
METHODS
Study population
The INTEROCC study comprises data from seven of the thirteen countries included in the international case-control study on mobile phone use and brain cancer risk, INTERPHONE (34). In these seven countries, detailed occupational information was collected from study participants. Incident cases of primary brain tumours (i.e. glioma and meningioma) were identified between 2000 and 2004 in participating hospitals in the study regions of Australia, Canada, France, Germany, Israel, New Zealand and the United Kingdom. The core INTERPHONE protocol (34) included cases aged 30 to 59 years of age, though several countries included cases from a broader age range, including up to 69 years in Germany, 18 years and above in Israel and 18 to 69 years in the United Kingdom. Controls were randomly selected from population registries and electoral lists in most countries. Patient lists were used in the UK and random digit dialling in Ottawa (Canada). To control for confounding, controls were frequency matched to cases by age (5-year groups), sex, study region and country. Case-control ratios were about 1:1 in all countries but Germany (1:2). All potential participants identified were contacted, informed about the study and asked whether they wanted to participate. For subjects who agreed, a signed informed consent was obtained before the interview process.
In total, the INTEROCC study comprises 2,054 glioma cases, 1,924 meningioma cases, and 5,601 controls. Overall participation rates for cases in INTERPHONE were 65% for glioma and 78% for meningioma cases, although numbers varied considerably by tumour type and centre (34). Participation among glioma cases for low- and high-grade tumours was similar (71% and 67%, respectively). The most frequent reasons for non-participation were refusal (64%) and inability to contact (27%). Only a few subjects were interviewed by telephone. Proxy respondents were allowed (e.g. 13% for glioma cases overall) if the participant had died or was unable to participate. Ethics approval was obtained from the Ethics Committee of the International Agency for Research on Cancer (IARC) and appropriate ethics committees in all participating countries for the INTERPHONE study, as well as from the Ethics Committee of the Municipal Institute for Medical Investigation (IMIM) in Barcelona, Spain, for use of the anonymised INTERPHONE and INTEROCC data.
Data collection
The interviews took place between 2000 and 2004 and lifetime occupational history data were collected back to the 1970s. A full occupational calendar for all jobs held for at least six months (including job title, start and stop date, and company name and description) was completed. In addition, the occupational questionnaire included screening questions designed to identify subjects with potential EMF exposure. The screening questions focused on work with or in the proximity of specific EMF sources with frequencies from 0Hz to 300GHz. A positive response to any of the screening questions led to more specific questions concerning the job in which this exposure occurred, including the tasks and work organization involved, as well as start and stop years, and the number of hours per week/month in relation to the EMF sources reported. A detailed description of the screening questions is provided elsewhere (33).
Exposure assessment
The source-exposure matrix (SEM) was used to assign average exposure levels to each RF and IF source reported by the study participants. Of the twelve occupational sections in the questionnaire, seven of them entailed work with sources of RF and/or IF-EMF. These sections involved work with or nearby 1) radars, 2) telecommunication antennas, 3) transmitters (e.g. walkie talkies), or equipment for 4) semiconductors manufacturing, 5) medical diagnosis and treatment (e.g. hyperthermia), 6) industrial heating (e.g. induction furnaces) or 7) food heating (e.g. bulk microwave drying ovens). Cumulative exposure algorithms were developed for each occupational section by combining the SEM’s arithmetic mean for each reported source, adjusted by information on automation, distance to the source and other modifiers depending on the specific section, with information on exposure duration (in years) and exposure rate (i.e. number of hours per day or week). Details of the cumulative exposure algorithms used will be published elsewhere (Vila et al., in preparation).
The quality of the data collected on EMF sources and ancillary information was assessed through comparisons with the data in the full occupational histories. Errors identified, such as incongruent dates or responses not obeying the questionnaire logic, were corrected. Imputation of missing data was performed using median values from the controls. Subjects for which imputation or correction of unreliable data was not possible, and participants with insufficient information to assign an exposure estimate (i.e. because of unclear EMF source and/or exposure duration), were excluded from the analysis.
To combine exposures from different-frequency sources, field intensities for each EMF source in the SEM were weighted using the frequency-dependent reference levels issued by the International Commission on Non-Ionizing Radiation Protection (ICNIRP) for occupational exposure (10). ICNIRP reference levels for frequencies above 10 MHz are obtained from basic restrictions for Specific Absorption Rate (SAR). Thus, since squared fields are proportional to power density (35), E-field squared ICNIRP ratios (equation 1) can be assumed to correlate well with SAR, and their cumulative exposures correlate with the specific absorbed energy. Reference levels for frequencies below 10 MHz are obtained from basic restrictions for internal E-field (and SAR above 100 kHz); hence ICNIRP ratios may be considered proportional to internal E-fields (equation 2). Moreover, since measurement surveys of IF sources (10,36) have shown that magnetic fields emitted by these sources tend to prevail over electric fields, the H-field ICNIRP ratio was selected to assess cumulative exposure to IF sources.
| (1) |
| (2) |
where represents the mean electric field strength (E, in V/m) for the RF source s from the SEM; represents the mean magnetic field strength (H, in A/m) for the IF source s from the SEM. ERL and HRL are the ICNIRP frequency-dependent reference levels for occupational exposure (10) ; and f is the frequency.
Since internal magnetic fields are proportional to incident magnetic fields and their penetration into the body has little frequency dependence (12), we used the (frequency-unweighted) RF H-fields directly from the SEM as the metric to assess cumulative exposure to RF magnetic fields.
Statistical analysis
Although the INTERPHONE study generally included one matched control per case, all eligible controls were included in INTEROCC to maximize statistical power. Controls were frequency matched to cases on age (5-year groups), sex, study region and country. The date of diagnosis was the reference date for cases. The reference date for controls was the date of interview minus the median difference between diagnosis and the case interview date.
Conditional logistic regression models using strata defined by the matching factors were used to calculate adjusted odds ratios (ORs) and 95% confidence intervals for the association between cumulative exposure to RF E- or H-fields, or IF H-fields and glioma or meningioma risk. All models were stratified by age (5-year groups), sex, study region and country, and adjusted for education. Associations of lifetime cumulative exposure (1-year lag), cumulative exposure at 5- and 10-year lags were examined, as well as cumulative exposure in different time windows defined a priori (1–4, and 5–9 years before the reference date). These time windows were chosen to assess the hypothesis that recent exposures to RF and/or IF-EMF may entail different risks, possibly related to tumour promotion or progression (31), than exposures further in the past.
Both categorical and continuous indicators of RF and IF-EMF cumulative exposure were examined. Due to skewed distributions of exposure data, irregular cut-points (i.e. 50th, 75th and 90th percentiles) were used to define categories to span the range of the exposure distribution. For IF, due to the small number of exposed subjects, the median value (i.e. 50th percentile) was used as the only cut point. The reference category for the main analysis was subjects never exposed to occupational RF or IF-EMF.
For the continuous analyses, exposure was modelled linearly and departure from linearity was tested using polynomials and logarithmic transformation of exposure. Models adequacy, in terms of goodness of fit, was evaluated using the Akaike Information Criterion (AIC) and the Bayesian Information Criterion (BIC) (38,39). The symmetry of the log-likelihood function in each model was assessed to confirm the adequacy of Wald-type confidence intervals (40,41). Due to the small number of subjects exposed to IF fields, the continuous analysis was only performed using RF E- and H-fields.
Potential confounding by cigarette smoking, any allergy history (42), and mobile phone use (never vs regular user) was also examined using a 10% change in the risk estimate criterion. Analyses were also conducted for high- and low-grade glioma types, separately. Potential effect modification by matching variables was assessed by including in the models cross-product terms between exposure and these variables and assessing the significance of the likelihood ratio test between models with and without the interaction term (40).
Sensitivity analyses were performed by using the lowest exposed group rather than the unexposed group as the reference category, or excluding proxy interviewees, participants who were judged by the interviewers as non-collaborative, participants aged >60 years, participants with very high (>99th percentile of cumulative exposure) or very low (<1st percentile of cumulative exposure) exposure levels, and participants with a history of neurofibromatosis or tuberous sclerosis.
All analyses and graphics were performed using R version 3.2.3 (43). Regression models were created using the “clogit” function (44).
RESULTS
In total, 1,943 glioma cases, 1,862 meningioma cases, and 5,387 controls were included in the analysis. Several participants were excluded due to insufficient information on exposure intensity (i.e. EMF source(s) not clearly identified) and/or exposure duration (n=355), or missing data on education (n=32). Table 1 describes cases and controls included in the analysis. Meningioma cases tended to be slightly older on average than glioma cases, and were mainly (74%) women, compared to 40% for glioma. In general, more glioma cases reported working with transmitters and telecommunication antennas than meningioma cases or controls. More meningioma cases reported working with sources used for heating food than glioma cases or controls. The reported sources with the highest exposure levels were “RF sealers/welders for plastic & rubber”, for RF, and “Electronic Article Surveillance (EAS) system”, for IF (Table 2). The RF and IF sources most frequently reported were “walkie-talkie” and “induction heater”, respectively. The mean (SD) number of sources per subject was 1.33 (0.83) for glioma cases and 1.31 (0.65) for meningioma cases, and 1.35 (0.92) for controls. Overall, approximately 10% of participants were ever exposed to RF E- or H-fields, while only 1% were ever exposed to IF H-fields.
Table 1.
Distribution of included study participants by age, sex, education, country, region and occupational section. INTEROCC study. Data from Australia, Canada, France, Germany, Israel, New Zealand, and United Kingdom, 1970s-2004.
| Glioma cases | Controls | Meningioma cases | Controls | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | n | % | n | % | n | % | n | % | |
| 1,943 | 100 | 5,387 | 100 | 1,862 | 100 | 5,387 | 100 | ||
| Age a | |||||||||
| <35 | 214 | 11% | 413 | 8% | 82 | 4% | 415 | 8% | |
| 35–39 | 171 | 9% | 454 | 8% | 97 | 5% | 457 | 9% | |
| 40–44 | 216 | 11% | 624 | 12% | 166 | 9% | 622 | 12% | |
| 45–49 | 239 | 12% | 726 | 14% | 269 | 14% | 730 | 14% | |
| 50–54 | 347 | 18% | 955 | 18% | 370 | 20% | 963 | 18% | |
| 55–59 | 310 | 16% | 992 | 18% | 319 | 17% | 980 | 18% | |
| 60–64 | 190 | 10% | 501 | 9% | 189 | 10% | 499 | 9% | |
| 65–69 | 140 | 7% | 433 | 8% | 170 | 9% | 434 | 8% | |
| 70+ | 116 | 6% | 289 | 5% | 200 | 11% | 287 | 5% | |
| Sex | |||||||||
| Male | 1,162 | 60% | 2,350 | 44% | 485 | 26% | 2,350 | 44% | |
| Female | 781 | 40% | 3,037 | 56% | 1,377 | 74% | 3,037 | 56% | |
| Education b | |||||||||
| High school or less | 1,034 | 53% | 2,914 | 54% | 1,125 | 60% | 2,914 | 54% | |
| Medium-level technical school | 376 | 19% | 1,004 | 19% | 357 | 19% | 1,004 | 19% | |
| University | 533 | 27% | 1,469 | 27% | 380 | 20% | 1,469 | 27% | |
| Country | Region | ||||||||
| Australia | 1 | 135 | 15% | 305 | 12% | 121 | 13% | 305 | 12% |
| 2 | 147 | 337 | 120 | 337 | |||||
| Canada | 1 | 63 | 8% | 225 | 12% | 47 | 5% | 225 | 12% |
| 2 | 21 | 172 | 14 | 172 | |||||
| 3 | 78 | 229 | 31 | 229 | |||||
| France | 1 | 28 | 5% | 112 | 9% | 41 | 8% | 112 | 9% |
| 2 | 63 | 351 | 103 | 351 | |||||
| Germany | 1 | 99 | 18% | 438 | 28% | 100 | 20% | 438 | 28% |
| 2 | 179 | 709 | 206 | 709 | |||||
| 3 | 77 | 360 | 70 | 360 | |||||
| Israel | 1 | 16 | 22% | 31 | 18% | 38 | 39% | 31 | 18% |
| 2 | 402 | 927 | 684 | 927 | |||||
| New Zealand | 1 | 80 | 4% | 158 | 3% | 50 | 3% | 158 | 3% |
| United Kingdom | 1 | 139 | 29% | 269 | 19% | 62 | 13% | 269 | 19% |
| 2 | 121 | 232 | 60 | 232 | |||||
| 3 | 105 | 182 | 24 | 182 | |||||
| 4 | 190 | 350 | 91 | 350 | |||||
| Occupational section c | |||||||||
| Diagnosis & Treatment | 12 | 2% | 83 | 5% | 14 | 4% | 83 | 5% | |
| Heating Food & Medical-Dental | 75 | 12% | 268 | 16% | 85 | 22% | 268 | 16% | |
| Heating Industrial | 160 | 26% | 441 | 27% | 111 | 29% | 441 | 27% | |
| Radars | 18 | 3% | 81 | 5% | 19 | 5% | 81 | 5% | |
| Semiconductors | 6 | 1% | 17 | 1% | 3 | 1% | 17 | 1% | |
| Telecommunication Antennas | 38 | 6% | 61 | 4% | 11 | 3% | 61 | 4% | |
| Transmitters | 299 | 49% | 674 | 41% | 137 | 36% | 674 | 41% | |
5-year age groups as used for the matching of cases and controls in the recruitment.
A total of 16 cases and 11 controls were removed due to missing information for Education.
Subjects not assigned to any section were considered unexposed and are not included here.
Table 2.
RF and IF sources most frequently reported by the INTEROCC study participants and sources reported with the highest levels of exposure. INTEROCC study. Data from Australia, Canada, France, Germany, Israel, New Zealand, and United Kingdom, 2000–2004.
| Most frequent sources | Sources with highest mean exposure levelsa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Radiofrequency (10 MHz - 300 GHz) | Intermediate frequency (3 kHz - 10 MHz) | Radiofrequency (10 MHz - 300 GHz) | Intermediate frequency (3 kHz - 10 MHz) | ||||||||
| Source | E-field (V/m)a | Nb | Source | H-field (A/m)a | N | Source | E-field (V/m)a | Nb | Source | H-field (A/m)a | N |
| walkie-talkie | 335 | 411 | induction heater/furnace for metals | 38,6 | 56 | RF sealers/welders for plastic & rubber | 459 | 27 | Electronic Article Surveillance (EAS) |
24,5 | 3 |
| microwave heating | 23,4 | 174 | dielectric heater/plastic & rubber | 333 | 22 | walkie-talkie | 335 | 411 | plasma etcher/metal etcher/dry plasma etcher | 4,99 | 15 |
| two-way radio/on motorcycle | 14 | 174 | glue heater curer/wood & fiber glass | 19 | 16 | TV/VHF/mast/worked on | 310 | 1 | induction welding | 3,55 | 4 |
| CB radio | 244 | 153 | surgical diathermy equipment/surgeon | 12 | 12 | continuous short wave diathermy | 299 | 12 | electrical resistance furnaces/metals | 3,1 | 32 |
| RF sealers/welders for plastic & rubber | 459 | 27 | ultrasound diathermy/physiotherapist | 1,1 | 12 | shuttle tray machine for plastic & rubber | 264 | 2 | dielectric heater/plastic & rubber | 1,8 | 28 |
| Telecomm. and personal services misc./ground/surrounding | 11,1 | 24 | plasma etcher/metal etcher/dry plasma etcher | 0,17 | 9 | CB radio | 244 | 168 | metal detectors | 1,27 | 4 |
| dielectric heater/plastic & rubber | 102 | 22 | plasma-enhanced chemical vapor deposition (CVD) | 11 | 4 | TV/UHF/mast/worked on | 210 | 8 | induction heater/furnace for metals | 0,73 | 60 |
| navigation radar/work surrounded | 1,23 | 20 | induction welding/metal | 42 | 4 | microwave diathermy/physiotherapist | 106 | 7 | dielectric heater/wood & fiber glass | 0,72 | 1 |
| mobile phone base station antennas/ground/worked on | 0,36 | 16 | gluing press/wood & fiber glass | 100 | 4 | dielectric heater/plastic & rubber | 102 | 28 | gluing press/wood & fiber glass | 0,72 | 6 |
| pulsed short wave diathermy | 60,1 | 16 | Electronic Article Surveillance (EAS) | 24,5 | 3 | Radio/FM/mast/worked on | 92 | 1 | induction welding / metal | 0,72 | 4 |
Mean exposure levels from the SEM.
N: Number of subjects who reported work with or nearby the EMF source.
Figure 1 and Table S1 presents levels of cumulative exposure to RF E- and H-fields, and IF H-fields of exposed subjects, overall (1-year lag) and for the 1- to 4-year exposure time window. Exposure distributions for all fields and exposure lags and time windows were strongly right-skewed. There was generally little difference in exposure distributions between cases and controls, except cumulative exposure overall and in the 1- to 4-year exposure time window for RF E-fields, where cases had slightly higher median levels.
Figure 1.
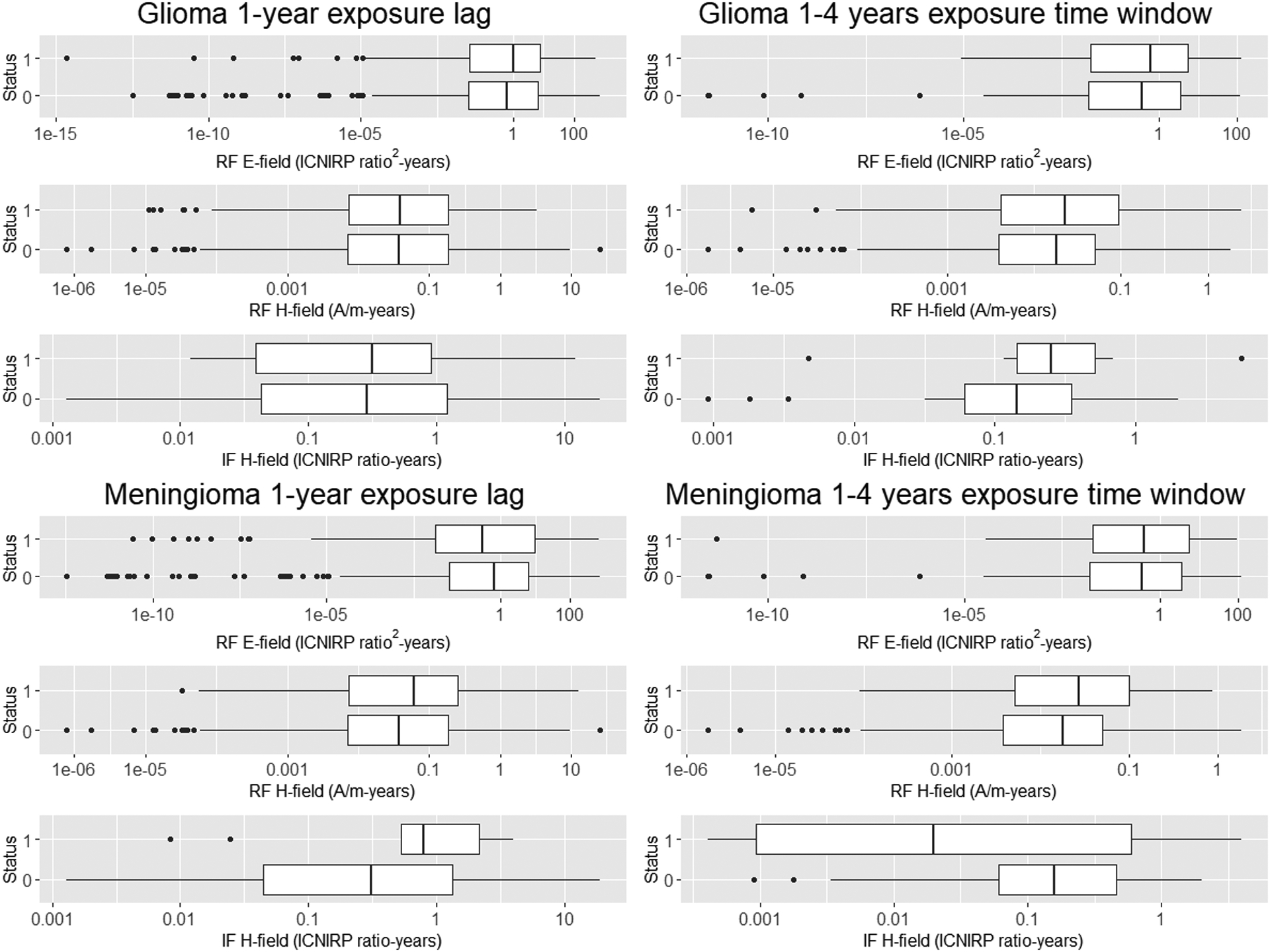
Cumulative exposure to radiofrequency (RF) and intermediate frequency (IF) electric and magnetic fields (various metrics) by brain tumour type and exposure lag (overall exposure, 1-year lag) and time window (1–4 years exposure time window).
Status (1=Cases; 0=Controls).
There was no clear evidence for an association between cumulative exposure to RF E-fields (Table 3) or H-fields (Table 4) and glioma or meningioma risk overall (1-year lag) or in any other exposure lags or time windows, using categorical classifications of cumulative exposure. In both analyses, there were reduced ORs in most exposure categories; the reduction was statistically significant in the lowest exposure categories in some groups. However, there were some positive ORs in the highest exposure categories in the 1–4 year exposure time window for glioma and RF E- and H-fields, and in several groups for meningioma. The highest OR was obtained in the analysis of glioma and RF H-fields in the highest exposure category (>90th percentile) for the 1–4 year exposure time window: OR=1.62 (95% confidence interval (CI): 0.86, 3.01, based on 19 exposed cases). The OR for meningioma in the same category was 1.52 (95% CI: 0.65, 3.55, based on eight exposed cases). Analyses using RF E-fields provided somewhat smaller estimates, although the largest OR was again in the highest exposure category: OR= 1.38 (95% CI: 0.75, 2.54) for glioma, and OR=1.30 (95% CI: 0.58, 2.91) for meningioma. There was no clear evidence for an exposure-response trend in any of the time-windows studied.
Table 3.
Adjusted odds ratios (OR) and 95% confidence intervals for glioma or meningioma risk and cumulative RF E-field exposure (ICNIRP ratio2-years) for various exposure lags and time windows. INTEROCC study: Data from Australia, Canada, France, Germany, Israel, New Zealand and United Kingdom, 2000–2004.
| Glioma | Meningioma | |||||
|---|---|---|---|---|---|---|
| RF E-field cumulative exposure (ICNIRP ratio2-years)b | Cases (n) | Controls (n) | ORa (95% confidence interval) | Cases (n) | Controls (n) | ORa (95% confidence interval) |
| 1-year lag | ||||||
| Non exposedc | 1,718 | 4,734 | 1.00 (ref.) | 1,744 | 4,566 | 1.00 (ref.) |
| <0.63 | 102 | 277 | 0.75 (0.59, 0.96) | 63 | 251 | 0.84 (0.62, 1.13) |
| 0.63≥6.22 | 57 | 135 | 0.82 (0.59, 1.14) | 18 | 111 | 0.69 (0.41, 1.16) |
| 6.22≥40.7 | 40 | 82 | 0.92 (0.62, 1.37) | 20 | 72 | 1.24 (0.73, 2.10) |
| ≥40.7 | 24 | 55 | 0.87 (0.53, 1.44) | 13 | 49 | 1.08 (0.57, 2.06) |
| 5-year lag | ||||||
| Non exposed | 1,718 | 4,734 | 1.00 (ref.) | 1,744 | 4,563 | 1.00 (ref.) |
| <0.46 | 82 | 246 | 0.69 (0.53, 0.90) | 54 | 223 | 0.84 (0.61, 1.15) |
| 0.46≥5.66 | 53 | 122 | 0.83 (0.59, 1.17) | 20 | 103 | 0.80 (0.48, 1.31) |
| 5.66≥40.7 | 33 | 72 | 0.89 (0.58, 1.37) | 20 | 61 | 1.51 (0.88, 2.57) |
| ≥40.7 | 20 | 49 | 0.81 (0.47, 1.39) | 11 | 44 | 1.01 (0.50, 2.03) |
| 10-year lag | ||||||
| Non exposed | 1,718 | 4,734 | 1.00 (ref.) | 1,744 | 4,563 | 1.00 (ref.) |
| <0.27 | 66 | 212 | 0.64 (0.48, 0.86) | 47 | 191 | 0.86 (0.61, 1.21) |
| 0.27≥4.93 | 38 | 104 | 0.73 (0.50, 1.08) | 18 | 91 | 0.84 (0.50, 1.43) |
| 4.93≥34.7 | 30 | 63 | 0.92 (0.58, 1.45) | 15 | 52 | 1.36 (0.74, 2.49) |
| ≥34.7 | 17 | 43 | 0.79 (0.44, 1.42) | 9 | 38 | 0.95 (0.44, 2.05) |
| 5–9 years | ||||||
| Non exposed | 1,718 | 4,721 | 1.00 (ref.) | 1,744 | 4,550 | 1.00 (ref.) |
| <0.42 | 58 | 139 | 0.84 (0.61, 1.17) | 22 | 124 | 0.60 (0.38, 0.97) |
| 0.42≥4.48 | 32 | 67 | 0.93 (0.60, 1.44) | 18 | 53 | 1.48 (0.84, 2.61) |
| 4.48≥18.9 | 18 | 40 | 0.82 (0.46, 1.47) | 8 | 38 | 1.08 (0.66, 2.39) |
| ≥18.9 | 12 | 27 | 0.90 (0.44, 1.83) | 6 | 23 | 1.03 (0.45, 2.63) |
| 1–4 years | ||||||
| Non exposed | 1,718 | 4,721 | 1.00 (ref.) | 1,744 | 4,553 | 1.00 (ref.) |
| <0.36 | 48 | 139 | 0.69 (0.49, 0.98) | 23 | 123 | 0.60 (0.38, 0.96) |
| 0.36≥3.46 | 30 | 69 | 0.85 (0.54, 1.35) | 13 | 51 | 1.13 (0.60, 2.14) |
| 3.46≥13.7 | 18 | 42 | 0.77 (0.44, 1.37) | 6 | 37 | 0.86 (0.35, 2.13) |
| ≥13.7 | 19 | 27 | 1.38 (0.75, 2.54) | 9 | 26 | 1.30 (0.58, 2.91) |
Odds ratio (OR) from conditional logistic regression models, matched by 5-year age group, sex, country, and region, and adjusted by education. Confidence intervals (CIs) based on Wald test.
Exposure categories based on irregular quantiles of the cumulative exposure distribution of controls (i.e. 50th, 75th, and 90th percentiles).
Figures for non-exposed cases and controls include only subjects who were never exposed (i.e. 1-year lag). Numbers do not coincide if strata without cases or controls were excluded.
Table 4.
Adjusted odds ratios (OR) and 95% confidence intervals for glioma or meningioma risk and cumulative RF H-field exposure (A/m-years) for various exposure lags and time windows. INTEROCC study: Data from Australia, Canada, France, Germany, Israel, New Zealand and United Kingdom, 1970s-2004.
| Glioma | Meningioma | |||||
|---|---|---|---|---|---|---|
| RF H-field cumulative exposure (A/m-years)b | Cases (n) | Controls (n) | ORa (95% confidence interval) | Cases (n) | Controls (n) | ORa (95% confidence interval) |
| 1-year lag | ||||||
| Non exposedc | 1,740 | 4,803 | 1.00 (ref.) | 1,756 | 4,629 | 1.00 (ref.) |
| <0.04 | 99 | 243 | 0.77 (0.60, 1.00) | 45 | 210 | 0.78 (0.56, 1.10) |
| 0.04≥0.19 | 52 | 119 | 0.85 (0.59, 1.17) | 23 | 101 | 0.87 (0.55, 1.40) |
| 0.19≥0.66 | 29 | 69 | 0.91 (0.58, 1.43) | 21 | 64 | 1.25 (0.74, 2.09) |
| ≥0.66 | 21 | 49 | 0.92 (0.54, 1.57) | 13 | 45 | 1.24 (0.64, 2.40) |
| 5-year lag | ||||||
| Non exposed | 1,740 | 4,803 | 1.00 (ref.) | 1,756 | 4,626 | 1.00 (ref.) |
| <0.04 | 83 | 213 | 0.74 (0.56, 0.97) | 40 | 184 | 0.83 (0.58, 1.19) |
| 0.04≥0.19 | 44 | 106 | 0.81 (0.56, 1.17) | 23 | 93 | 0.92 (0.57, 1.49) |
| 0.19≥0.64 | 29 | 62 | 1.00 (0.63, 1.59) | 18 | 55 | 1.24 (0.71, 2.17) |
| ≥0.64 | 14 | 43 | 0.75 (0.40, 1.41) | 11 | 40 | 1.25 (0.61, 2.55) |
| 10-year lag | ||||||
| Non exposed | 1,740 | 4,803 | 1.00 (ref.) | 1,756 | 4,626 | 1.00 (ref.) |
| <0.04 | 69 | 182 | 0.75 (0.56, 1.01) | 34 | 159 | 0.83 (0.56, 1.23) |
| 0.04≥0.17 | 30 | 89 | 0.65 (0.42, 1.01) | 17 | 77 | 0.91 (0.52, 1.57) |
| 0.17≥0.63 | 27 | 54 | 1.08 (0.67, 1.75) | 17 | 48 | 1.34 (0.74, 2.41) |
| ≥0.63 | 9 | 37 | 0.54 (0.26, 1.15) | 8 | 34 | 0.92 (0.41, 2.09) |
| 5–9 years | ||||||
| Non exposed | 1,740 | 4,789 | 1.00 (ref.) | 1,756 | 4,616 | 1.00 (ref.) |
| <0.03 | 57 | 124 | 0.90 (0.65, 1.26) | 25 | 103 | 0.88 (0.56, 1.40) |
| 0.03≥0.10 | 25 | 61 | 0.80 (0.49, 1.30) | 12 | 57 | 0.89 (0.47, 1.69) |
| 0.10≥0.30 | 22 | 36 | 1.22 (0.71, 2.11) | 9 | 32 | 1.17 (0.54, 2.54) |
| ≥0.30 | 8 | 25 | 0.72 (0.31, 1.63) | 6 | 23 | 1.24 (0.48, 3.22) |
| 1–4 years | ||||||
| Non exposed | 1,740 | 4,789 | 1.00 (ref.) | 1,756 | 4,616 | 1.00 (ref.) |
| <0.02 | 49 | 124 | 0.79 (0.55, 1.11) | 18 | 100 | 0.61 (0.36, 1.02) |
| 0.02≥0.05 | 21 | 65 | 0.64 (0.38, 1.06) | 17 | 58 | 1.37 (0.78, 2.43) |
| 0.05≥0.13 | 17 | 34 | 0.94 (0.51, 1.71) | 7 | 31 | 0.89 (0.37, 2.10) |
| ≥0.13 | 19 | 25 | 1.62 (0.86, 3.01) | 8 | 23 | 1.52 (0.65, 3.55) |
Odds ratio (OR) from conditional logistic regression models, matched by 5-year age group, sex, country, and region, and adjusted by education. Confidence intervals (CIs) based on Wald test.
Exposure categories based on irregular quantiles of the cumulative exposure distribution of controls (i.e. 50th, 75th, and 90th percentiles).
Figures for non-exposed cases and controls include only subjects who were never exposed (i.e. 1-year lag). Numbers do not coincide if strata without cases or controls were excluded.
In the continuous analyses, no associations were found between lifetime cumulative exposure to RF E-fields (Table S2) and glioma or meningioma risk in any exposure lag or time window (only results for 1-year lag and 1–4 years exposure time window shown). Analyses using polynomials with or without log transformation of exposure did not indicate evidence of departure from linearity according to model fit results. Analyses using RF H-fields (Table 5) for the 1–4 years exposure time window showed increased ORs for both glioma: OR=1.82 (95% confidence interval: 0.75, 4.42), and meningioma: OR=1.46 (95% confidence interval: 0.37, 5.74), while results for other exposure metrics (only results for the 1-year lag shown) gave mostly no association. Results for non-linear models gave larger AIC/BIC values and/or wide confidence intervals (only results for the log-log models are shown since more complex models were unstable due to limited data).
Table 5.
Adjusted ORs for glioma and meningioma using continuous RF H-field cumulative exposure for 1-year lag and 1- to 4-year exposure time window, and two polynomial models (log-linear and log-log). INTEROCC study: Data from Australia, Canada, France, Germany, Israel, New Zealand and United Kingdom, 2000–2004.
| Model # | Model form | Odds Ratioa | 95%CIb | AICc | BICd |
|---|---|---|---|---|---|
| GLIOMA | |||||
| RF H-field cumulative exposure, 1-year lag | |||||
| 1 | Log-linear | 0.91 | 0.74–1.13 | 7164.97 | 7181.69 |
| 2 | Log-log | 0.84 | 0.51–1.37 | 7165.58 | 7182.30 |
| RF H-field cumulative exposure, 1- to 4-years exposure time window | |||||
| 1 | Log-linear | 1.82 | 0.75–4.42 | 7164.44 | 7181.16 |
| 2 | Log-log | 2.14 | 0.54–8.45 | 7164.93 | 7181.65 |
| MENINGIOMA | |||||
| RF H-field cumulative exposure, 1-year lag | |||||
| 1 | Log-linear | 0.99 | 0.87–1.13 | 6752.52 | 6769.11 |
| 2 | Log-log | 1.10 | 0.65–1.86 | 6752.43 | 6769.02 |
| RF H-field cumulative exposure, 1- to 4-years exposure time window | |||||
| 1 | Log-linear | 1.46 | 0.37–5.74 | 6752.28 | 6768.87 |
| 2 | Log-log | 1.98 | 0.29–13.3 | 6752.09 | 6768.68 |
ORs from conditional logistic regression models, matched by 5-year age group, sex, country, and region, and adjusted by education.
Confidence intervals (CI) based on Wald test.
AIC: Akaike Information Criterion.
BIC: Bayesian Information Criterion.
Figure 2 shows the exposure-response relationship between RF E-fields cumulative exposure (overall and in the 1- to 4-year exposure time window) for glioma and meningioma, based on predicted risk estimates from two models (log-linear and translog-quadratic) as well as the ORs and 95% confidence intervals from the categorical analysis for the same exposure lag or time window. While predicted ORs in the 1–4 years exposure time window tended to be above one for both glioma and meningioma, overall exposure (1-year lag) provided ORs near one or below. Overall, the ORs obtained in the categorical analysis gave a similar pattern as the continuous models, where the similarities with the translog-quadratic model are especially apparent. However, the modelś wide confidence intervals highlight the large uncertainties embedded in this analysis. Similar results were obtained using RF H-fields for both glioma and meningioma data (Figure S1). This figure only shows risk predictions for the linear model since the unstable results from the translog-quadratic model using RF H-fields were considered unreliable.
Figure 2.
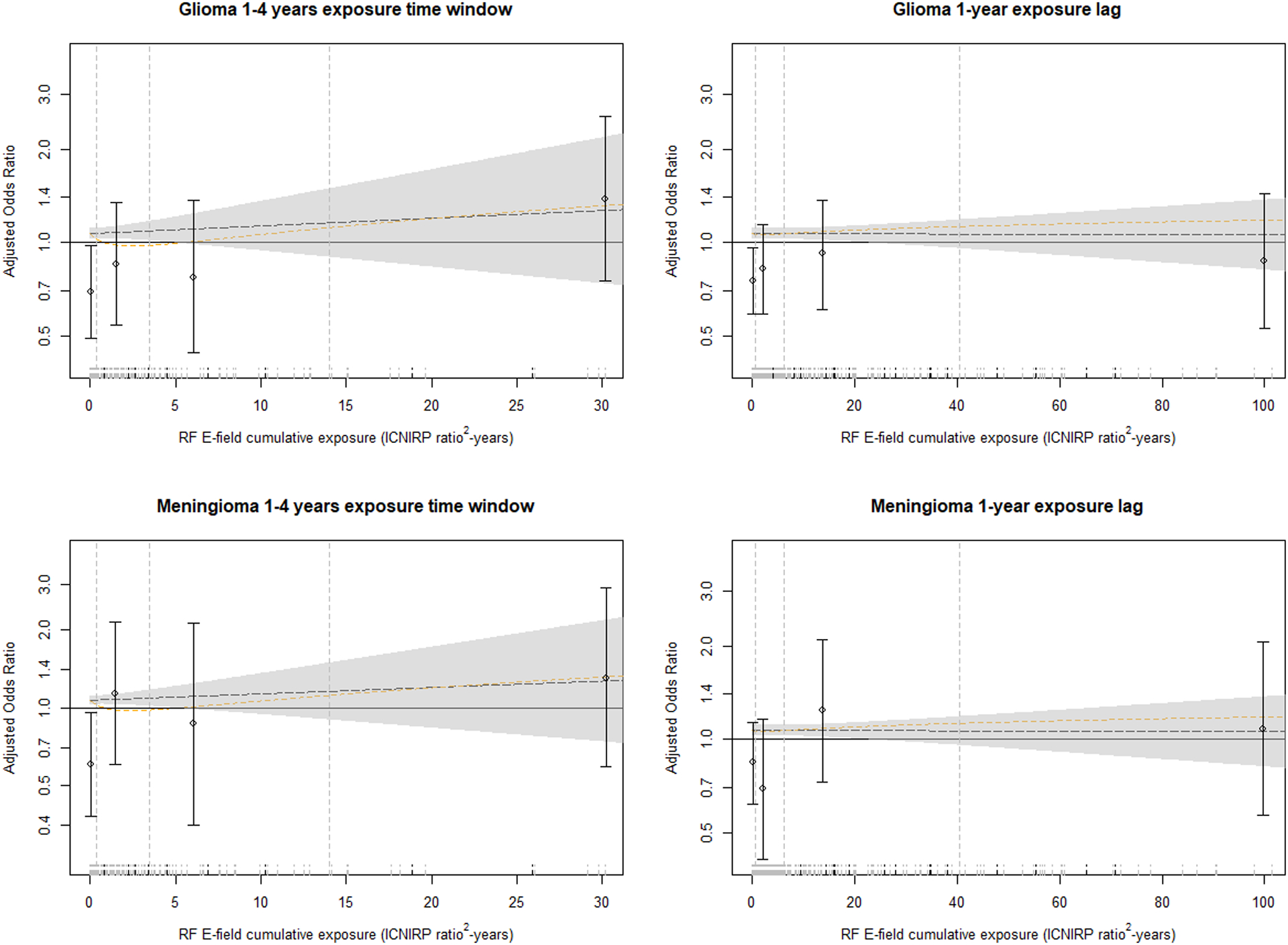
Exposure-response relationship between risk of glioma and meningioma (ORs based on conditional logistic regression models, matched by 5-year age group, sex, country, and region, and adjusted by education) and RF E-field cumulative exposure (ICNIRP ratio2-years) for overall exposure (1-year lag) and 1- to 4-year exposure time window. The dashed lines indicate the linear model (black line) and a quadratic model with log-transformed exposure (yellow line). The grey shadow indicates Wald-type 95% CIs for the linear model. Vertical dashed lines on the left side of the plot indicate the cut points used in the categorical analysis (i.e. 50th, 75th and 90th percentiles of the cumulative exposure distribution of controls). Points and error bars indicate adjusted ORs and Wald-type 95% CIs for the exposure categories based on these cut points. The ORs and CIs from the categorical analysis are positioned at the median exposure for each interval. Plot rug are cases (dashed short vertical black lines) and controls (dashed short vertical grey lines). Plots truncated at the 95th percentile of cumulative exposure to improve visibility.
Results for IF H-fields showed no clear suggestion of an association with either glioma or meningioma risk and were based on very small number of exposed participants (Table S3). ORs were also generally <1 in the lowest exposure category (i.e. < median) for both glioma and meningioma. ORs above 1 were only found in the highest exposure categories for the 1–4 years and 5–9 years exposure time windows for glioma, while results for meningioma were not consistent.
In sensitivity analyses, removal of unresponsive subjects, subjects aged >60 years, subjects with very low or very high cumulative exposure levels, proxy interviewees, and participants with a history of neurofibromatosis or tuberous sclerosis had little effect (<10% change) on the ORs obtained (results not shown); neither did analysis of high- and low-grade glioma separately. There was no evidence of confounding or effect modification by any of the factors studied. Restricting the analyses to exposed subjects only (Tables S4 and S5), using the lowest exposed category as the reference group for either RF E- and H-fields, resulted in positive ORs in most exposure categories, without evidence of an exposure-response trend.
DISCUSSION
This study, based on the analysis of nearly 4,000 brain tumour cases and over 5,000 controls, is the largest case-control study of brain tumours and occupational RF and IF exposure to date. The work on exposure assessment, based on a detailed source-based questionnaire and a source-exposure matrix specifically developed for the project is, to our knowledge, the most comprehensive effort aimed at estimating occupational exposure to RF and IF-EMF in an epidemiological study. Overall, despite the major improvement in exposure assessment and risk modelling, the study provided no clear evidence for an association between cumulative RF or IF-EMF exposure and either glioma or meningioma risk, with risk estimates mostly below 1 and only some non-significant positive ORs in some exposure time windows.
Previous studies examining associations of brain tumours and exposure to RF fields have not found clear results. A non-significant increase of brain cancer risk was observed in a study of radio operators (45), while studies of police officers (46) and naval and aviation personnel (22) found non-statistically reduced risks for brain tumours. However, these studies had small numbers of cases and none of them looked at risk by level of exposure. In one case-control study (21), semi-quantitative exposure estimates were assigned to male air force workers based on a detailed occupational history obtained through questionnaire. Although no overall association was found for exposure level and risk of brain cancer, a small excess risk was seen when comparing ever versus never exposed. Another case-control study (29), in which workerś exposure was classified by expert industrial hygienists, found a significant increased risk among men exposed to RF for more than twenty years. A study in Australia in which researchers assessed glioma and RF exposure data (23), using a general job-exposure matrix, found many reduced ORs.
An INTEROCC sub-study using the German population (19) classified the subjects according to their RF-EMF exposure likelihood based on their job-title. This study found several non-significant positive associations for occupational RF exposure and brain tumour risk. The OR for glioma in highly exposed subjects was 1.22 (95% confidence interval: 0.69, 2.15) overall and 1.39 (95% confidence interval 0.67, 2.88) when only jobs conferring high exposure for more than ten years were considered. Similar results were obtained for meningioma, with ORs of 1.34 (95% confidence interval 0.61, 2.96) for overall exposure and 1.55 (95% confidence interval 0.52, 4.62) for ten years or more of high exposure jobs. Our results with the categorical analysis are similar, with non-significantly increased ORs for highly exposed groups. However, while the German study observed the increase in the 10+ years exposure lag, we observed an increase only in the most recent exposure time window.
Weaknesses of this study include possible recall and selection biases. It is possible that our results could have been affected by recall error, particularly with regard to the impact of the disease on cases’ ability to remember details of past jobs. Unlike the recall bias on details of past mobile phone use in the INTERPHONE studies (47–49), we may assume it is easier for workers to recall the type of machinery used during their working lifetimes. Moreover, since subjects were generally unaware of exposure levels associated with the reported EMF sources, any recall bias is probably small, though random error could occur. Another weakness is the low participation rates among controls (55% vs 65% for glioma cases and 78% for meningiomas), raising concerns about potential selection bias. Control participation was associated with socioeconomic status and with being mobile phone user (Ref), and both could be associated with certain EMF sources. Such biases could be aggravated by the different protocols for subject selection and staff training among the INTERPHONE countries (34).
Exposure assessment of study participants was aimed at brain exposure through the confidence weighting of the SEM estimates (32), which strengthens the validity of our results to assess brain cancer risks. However, information bias may be a major source of uncertainty in our study. Thus, although individual exposure assessment may reduce between-subject misclassification (i.e. Berkson error) it can also increase classical error (51,52). Nevertheless, as with the use of job-exposure matrices (31,37,53), there is possibly residual Berkson error due to the many assumptions and expectations used. These biases are likely to be non-differential, possibly attenuating ORs towards no effect. Hence, further improvements are advisable on the individualized EMF cumulative exposure assessment performed in our study.
Very little information exists in the literature regarding exposure to high-frequency magnetic fields (H-fields) and risk of brain or other cancer types (16,54,55). However, recent studies have described possible mechanisms by which weak RF H-fields, together with static fields, could be responsible for the formation of reactive oxygen species, also called the radical pair mechanism, which may lead to cellular proliferation and eventually to cancer (11,12,56). Thus, because the absorbed energy hypothesis alone ignores other possible mechanisms by which RF and/or IF-EMF could cause cancer, we also used RF H-fields in our analyses. The results obtained using this metric differed from those obtained using E-fields, which could reflect the lack of proportionality in the near field (10). These results, although imprecise, provided the highest ORs. Results from analyzing risk to IF H-fields were inconsistent since they were based on low number of exposed subjects. However, given the skewed exposure distributions, there was little contrast between below and over the median, which makes these results difficult to interpret.
Several studies have used ICNIRP-based metrics to assess cumulative/integrative exposure to RF fields (58–60). However, ICNIRP-based exposure indices have several limitations. The term “ICNIRP ratio” is a metric for compliance with a regulatory limit, rather than an exposure metric with biophysical meaning, and there is little evidence to support that frequency-adjusted EMF using ICNIRP reference levels are a good exposure metric. Moreover, for the many different EMF exposure conditions found in workplaces, the cumulative ICNIRP metric is biased towards higher levels, since exposure limits are based on worst-case scenarios rather than average exposures (61). Therefore, although ICNIRP linear and squared ratios could be highly correlated with dose, it is possible that exposure levels assigned to study participants were overestimated, which may have underestimated our risk results.
In the statistical analyses, we used both categorical and continuous exposure variables, including polynomials to test for departures from linearity (62,63). The linear model provided the best fit for our data based on AIC/BIC criteria (40,64), For the categorical analysis, considering the skewed nature of our data, we used irregular cut-points based on the 50th, 75th, and 90th percentiles of the distribution, as the best method to represent the spread of the EMF distribution (38). This approach allowed us to compare groups of subjects with similar exposure variability (GSD ≈ 2 in most groups). However, since the majority of participants were considered unexposed (~90% for RF-EMF), the highest 10% of exposed subjects (>90th percentile) represents <1% of the study population. This limitation was also present in the German INTEROCC sub-study (19), where only ~11% of subjects were considered exposed. Moreover, although the German data are included in our analyses, the number of exposed subjects is still small and statistical power remains limited, hence the lack of significance of our study may have been due to the effect being smaller than detectable with our study size and exposure prevalence (31,37).
In conclusion, despite the improved quantitative exposure assessment used in this study, the results do not support a positive association between occupational exposure to high-frequency EMF and the brain tumours studied. However, the non-significant positive ORs found in the highest exposed groups for the most recent exposures, both in the continuous and categorical analyses for glioma and meningioma, suggests the need for further research investigating differences in exposure over time and focusing on RF magnetic fields and tumour promotion. This is particularly important considering the limited statistical power of our study, due to the small number of exposed participants.
Traditional exposure-response analysis remains a powerful tool to identify health risks (67). Therefore, future studies may use similar exposure metrics, such as combinations of static and RF magnetic fields (11), as well as the source-based methodology developed in INTEROCC. Future efforts should identify ways to account for differences between near-field (e.g. transmitters) and far-field exposures (e.g. radars), particularly with regard to RF H-fields characteristics. Furthermore, despite the limitations associated with dosimetric modelling (e.g. required assumptions may add more uncertainty to available exposure data), the development of biology-based dose metrics may provide further insights on the potential biophysical mechanism(s), other than heating and nerve electro-stimulation (10,68), by which long-term exposure to high-frequency EMF may damage health.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to give special thanks to Dr. Dave Conover (deceased), USA, for his contribution in the assignment of RF and IF-EMF sources to study subjects, as well as to Taichi Murata, Japan, and Martin Doczkat, USA, for their work on exposure models for military radars and antennas. We also would like to thank Myles Capstick, UK, and Wout Joseph, Belgium, for their input on the calculation of cumulative exposure to electromagnetic fields.
Financial support:
This work was funded by the European Commission grant 603794 (GERoNiMO project). The conduct of the INTEROCC study was funded by the National Institutes for Health (NIH) Grant No. 1R01CA124759-01. The work on the French occupational data was in part funded by AFSSET (Convention N° ST-2005-004). The INTERPHONE study was supported by funding from the European Fifth Framework Program, ‘Quality of Life and Management of Living Resources’ (contract 100 QLK4-CT-1999901563) and the International Union against Cancer (UICC). The UICC received funds for this purpose from the Mobile Manufacturers’ Forum (MMF), now Mobile & Wireless Forum (MWF), and the GSM Association. Provision of funds to the INTERPHONE study investigators via the UICC was governed by agreements that guaranteed INTERPHONE’s complete scientific independence (http://interphone.iarc.fr/interphone_funding.php). In Australia, funding was received from the Australian National Health and Medical Research 5 Council (EME Grant 219129) with funds originally derived from mobile phone service licence fees; a University of Sydney Medical Foundation Program; the Cancer Council NSW and The Cancer Council Victoria. In Montreal, Canada, funding was received from the Canadian Institutes of Health Research (project MOP-42525); the Canada Research Chair programme; the Guzzo-CRS Chair in Environment and Cancer; the Fonds de la recherche en santé du Quebec; the Société de recherche sur le cancer; in Ottawa and Vancouver, Canada, from the Canadian Institutes of Health Research (CIHR), the latter including partial support from the Canadian Wireless Telecommunications Association; the NSERC/SSHRC/McLaughlin Chair in Population Health Risk Assessment at the University of Ottawa. In France, funding was received by l’Association pour la Recherche sur le Cancer (ARC) (Contrat N85142) and three network operators (Orange, SFR, Bouygues Telecom). In Germany, funding was received from the German Mobile Phone Research Program (Deutsches Mobilfunkforschungsprogramm) of the German Federal Ministry for the Environment, Nuclear Safety, and Nature Protection; the Ministry for the Environment and Traffic of the state of Baden- Wurttemberg; the Ministry for the Environment of the state of North Rhine-Westphalia; the MAIFOR Program (Mainzer Forschungsforderungsprogramm) of the University of Mainz. In New Zealand, funding was provided by the Health Research Council, Hawkes Bay Medical Research Foundation, the Wellington Medical Research Foundation, the Waikato Medical Research Foundation and the Cancer Society of New Zealand. Additional funding for the UK study was received from the Mobile Telecommunications, Health and Research (MTHR) program, funding from the Health and Safety Executive, the Department of Health, the UK Network Operators (O2, Orange, T-Mobile, Vodafone, ‘3’) and the Scottish Executive. All industry funding was governed by contracts guaranteeing the complete scientific independence of the investigators. MCT was funded by a Canadian Institutes of Health Research Fellowship and the Departament de Salut, Generalitat de Catalunya. ISGlobal is a member of the CERCA Programme, Generalitat de Catalunya.
Footnotes
Conflict of interest: The authors have no conflicts of interest to declare.
Publisher's Disclaimer: DISCLAIMER
Publisher's Disclaimer: The findings and conclusions in this paper have not been formally disseminated by the National Institute for Occupational Safety and Health and should not be construed to represent any agency determination or policy.
REFERENCES
- 1.Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010. Sep;99(3):307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bondy ML, Scheurer ME, Malmer B, Barnholtz-Sloan JS, Davis FG, Il’yasova D, et al. Brain tumour epidemiology: consensus from the Brain Tumour Epidemiology Consortium. Cancer. 2008. Oct 1;113(7 Suppl):1953–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006. Sep;2(9):494–503; quiz 1 p following 516. [DOI] [PubMed] [Google Scholar]
- 4.Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, et al. The epidemiology of glioma in adults: a ‘state of the science’ review. Neuro-Oncol. 2014. Jul;16(7):896–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braganza MZ, Kitahara CM, Berrington de Gonzalez A, Inskip PD, Johnson KJ, Rajaraman P. Ionizing radiation and the risk of brain and central nervous system tumours: a systematic review. Neuro-Oncol. 2012. Sep 5;14(11):1316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quach P, El Sherif R, Gomes J, Krewksi D. A systematic review of the risk factors associated with the onset and progression of primary brain tumours. Neurotoxicology. 2016. May 17; [DOI] [PubMed] [Google Scholar]
- 7.SCENIHR. Opinion on: Potential health effects of exposure to electromagnetic fields (EMF) [Internet]. Luxembourg: Scientific Committee on Emerging and Newly Identified Health Risks; 2015. [cited 2017 Aug 2]. Available from: https://ec.europa.eu/health/sites/health/files/scientific_committees/emerging/docs/scenihr_o_041.pdf [Google Scholar]
- 8.IARC. Non-ionizing radiation, Part 2: Radiofrequency electromagnetic fields. Int Agency Res Cancer Work Group Eval Carcinog Risks Hum. 2013;102(2):1–460. [PMC free article] [PubMed] [Google Scholar]
- 9.Lerchl A, Klose M, Grote K, Wilhelm AFX, Spathmann O, Fiedler T, et al. Tumour promotion by exposure to radiofrequency electromagnetic fields below exposure limits for humans. Biochem Biophys Res Commun. 2015. Apr 17;459(4):585–90. [DOI] [PubMed] [Google Scholar]
- 10.ICNIRP. Guidelines for limiting exposure to time-varying electric, magnetic, and electromagnetic fields (up to 300 GHz). International Commission on Non-Ionizing Radiation Protection. Health Phys. 1998. Apr;74(4):494–522. [PubMed] [Google Scholar]
- 11.Barnes F, Greenebaum B. Some Effects of Weak Magnetic Fields on Biological Systems: RF fields can change radical concentrations and cancer cell growth rates. IEEE Power Electron Mag. 2016. Mar;3(1):60–8. [Google Scholar]
- 12.Barnes FS, Greenebaum B. The effects of weak magnetic fields on radical pairs. Bioelectromagnetics. 2015. Jan;36(1):45–54. [DOI] [PubMed] [Google Scholar]
- 13.Blackman CF, Benane SG, Elder JA, House DE, Lampe JA, Faulk JM. Induction of calcium-ion efflux from brain tissue by radiofrequency radiation: effect of sample number and modulation frequency on the power-density window. Bioelectromagnetics. 1980;1(1):35–43. [DOI] [PubMed] [Google Scholar]
- 14.Castello PR, Hill I, Sivo F, Portelli L, Barnes F, Usselman R, et al. Inhibition of cellular proliferation and enhancement of hydrogen peroxide production in fibrosarcoma cell line by weak radio frequency magnetic fields. Bioelectromagnetics. 2014. Dec;35(8):598–602. [DOI] [PubMed] [Google Scholar]
- 15.Rao VS, Titushkin IA, Moros EG, Pickard WF, Thatte HS, Cho MR. Nonthermal effects of radiofrequency-field exposure on calcium dynamics in stem cell-derived neuronal cells: elucidation of calcium pathways. Radiat Res. 2008. Mar;169(3):319–29. [DOI] [PubMed] [Google Scholar]
- 16.Sienkiewicz Z, Schüz J, Cardis E. Risk analysis of human exposure to electromagnetic fields (revised). Deliverable Report D2 of EHFRAN project [Internet]. European Health Risk Assessment Network on Electromagnetic Fields Exposure; 2010. Available from: http://efhran.polimi.it/docs/EFHRAN_D2_final.pdf [Google Scholar]
- 17.Armstrong B, Thériault G, Guénel P, Deadman J, Goldberg M, Héroux P. Association between Exposure to Pulsed Electromagnetic Fields and Cancer in Electric Utility Workers in Quebec, Canada, and France. Am J Epidemiol. 1994. Nov 1;140(9):805–20. [DOI] [PubMed] [Google Scholar]
- 18.Baldi I, Coureau G, Jaffré A, Gruber A, Ducamp S, Provost D, et al. Occupational and residential exposure to electromagnetic fields and risk of brain tumours in adults: a case-control study in Gironde, France. Int J Cancer. 2011. Sep 15;129(6):1477–84. [DOI] [PubMed] [Google Scholar]
- 19.Berg G, Spallek J, Schüz J, Schlehofer B, Böhler E, Schlaefer K, et al. Occupational exposure to radio frequency/microwave radiation and the risk of brain tumours: Interphone Study Group, Germany. Am J Epidemiol. 2006. Sep 15;164(6):538–48. [DOI] [PubMed] [Google Scholar]
- 20.Degrave E, Meeusen B, Grivegnée A-R, Boniol M, Autier P. Causes of death among Belgian professional military radar operators: a 37-year retrospective cohort study. Int J Cancer. 2009. Feb 15;124(4):945–51. [DOI] [PubMed] [Google Scholar]
- 21.Grayson JK. Radiation exposure, socioeconomic status, and brain tumour risk in the US air force: a nested case-control study. Am J Epidemiol. 1996;143(5):480–6. [DOI] [PubMed] [Google Scholar]
- 22.Groves FD, Page WF, Gridley G, Lisimaque L, Stewart PA, Tarone RE, et al. Cancer in Korean war navy technicians: mortality survey after 40 years. Am J Epidemiol. 2002. May 1;155(9):810–8. [DOI] [PubMed] [Google Scholar]
- 23.Karipidis KK, Benke G, Sim MR, Kauppinen T, Giles G. Occupational exposure to ionizing and non-ionizing radiation and risk of glioma. Occup Med Oxf Engl. 2007. Oct;57(7):518–24. [DOI] [PubMed] [Google Scholar]
- 24.Lagorio S, Rossi S, Vecchia P, De Santis M, Bastianini L, Fusilli M, et al. Mortality of plastic-ware workers exposed to radiofrequencies. Bioelectromagnetics. 1997;18(6):418–21. [PubMed] [Google Scholar]
- 25.Lilienfeld A Foreign service health status study: evaluation of health status of foreign service and other employees from selected Eastern European posts : final report [Internet]. Baltimore, Md: Dept. of Epidemiology, School of Hygiene and Public Health, Johns Hopkins University; 1978. 250 p. Available from: https://catalyst.library.jhu.edu/catalog/bib_1807 [Google Scholar]
- 26.Morgan RW, Kelsh MA, Zhao K, Exuzides KA, Heringer S, Negrete W. Radiofrequency exposure and mortality from cancer of the brain and lymphatic/hematopoietic systems. Epidemiol Camb Mass. 2000. Mar;11(2):118–27. [DOI] [PubMed] [Google Scholar]
- 27.Robinette CD, Silverman C, Jablon S. Effects upon health of occupational exposure to microwave radiation (radar). Am J Epidemiol. 1980. Jul;112(1):39–53. [DOI] [PubMed] [Google Scholar]
- 28.Szmigielski S Cancer morbidity in subjects occupationally exposed to high frequency (radiofrequency and microwave) electromagnetic radiation. Sci Total Environ. 1996. Feb 2;180(1):9–17. [DOI] [PubMed] [Google Scholar]
- 29.Thomas TL, Stolley PD, Stemhagen A, Fontham ET, Bleecker ML, Stewart PA, et al. Brain tumour mortality risk among men with electrical and electronics jobs: a case-control study. J Natl Cancer Inst. 1987. Aug;79(2):233–8. [PubMed] [Google Scholar]
- 30.Tynes T, Hannevik M, Andersen A, Vistnes A, Haldorsen T. Incidence of breast cancer in Norwegian female radio and telegraph operators. Cancer Causes Control. 1996;7(2):197–204. [DOI] [PubMed] [Google Scholar]
- 31.Smith TJ, Kriebel DA. A Biological Approach to Environmental Assessment and Epidemiology. New York: Oxford University Press; 2010. [Google Scholar]
- 32.Vila J, Bowman JD, Figuerola J, Moriña D, Kincl L, Richardson L, et al. Development of a source-exposure matrix for occupational exposure assessment of electromagnetic fields in the INTEROCC study. J Expo Sci Environ Epidemiol. 2017;27(4):398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vila J, Bowman JD, Richardson L, Kincl L, Conover DL, McLean D, et al. A Source-based Measurement Database for Occupational Exposure Assessment of Electromagnetic Fields in the INTEROCC Study: A Literature Review Approach. Ann Occup Hyg. 2016. Mar;60(2):184–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cardis E, Richardson L, Deltour I, Armstrong B, Feychting M, Johansen C, et al. The INTERPHONE study: design, epidemiological methods, and description of the study population. Eur J Epidemiol. 2007. Sep 1;22(9):647–64. [DOI] [PubMed] [Google Scholar]
- 35.Hitchcock RT. Radio-Frequency Radiation. In: Hamilton and Hardy’s Industrial Toxicology. Edited by Harbison Raymond D.,Bourgeois Marie M.,Johns Giffe T.. John Wiley and Sons, Inc; 2015. (Sixth edition). [Google Scholar]
- 36.Joseph W, Vermeeren G, Verloock L, Goeminne F. In situ magnetic field exposure and ICNIRP-based safety distances for electronic article surveillance systems. Radiat Prot Dosimetry. 2012. Mar;148(4):420–7. [DOI] [PubMed] [Google Scholar]
- 37.Rothman K, Greenland S. Modern epidemiology. Philadelphia: Lippincott-Raven; 1998. [Google Scholar]
- 38.Röösli M, Vienneau D. Epidemiological Exposure Assessment. In: Epidemiology of Electromagnetic Fields. CRC Press; 2014. p. 368. (Biological Effects of Electromagnetics). [Google Scholar]
- 39.Vrieze SI. Model selection and psychological theory: a discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Psychol Methods. 2012. Jun;17(2):228–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hosmer D, Lemeshow S, Sturdivant R. Applied Logistic Regression, 3rd Edition. Wiley; 2013. [Google Scholar]
- 41.Pek J, Wu H. Profile Likelihood-Based Confidence Intervals and Regions for Structural Equation Models. Psychometrika. 2015. Dec;80(4):1123–45. [DOI] [PubMed] [Google Scholar]
- 42.Turner MC, Krewski D, Armstrong BK, Chetrit A, Giles GG, Hours M, et al. Allergy and brain tumours in the INTERPHONE study: pooled results from Australia, Canada, France, Israel, and New Zealand. Cancer Causes Control CCC. 2013. May;24(5):949–60. [DOI] [PubMed] [Google Scholar]
- 43.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/. 2014. [Google Scholar]
- 44.Therneau T A Package for Survival Analysis in S. version 2.38, http://CRAN.R-project.org/package=survival. 2015.
- 45.Milham S Mortality by license class in amateur radio operators. Am J Epidemiol. 1988. Nov;128(5):1175–6. [DOI] [PubMed] [Google Scholar]
- 46.Finkelstein MM. Cancer incidence among Ontario police officers. Am J Ind Med. 1998. Aug;34(2):157–62. [DOI] [PubMed] [Google Scholar]
- 47.Cardis E, Armstrong BK, Bowman JD, Giles GG, Hours M, Krewski D, et al. Risk of brain tumours in relation to estimated RF dose from mobile phones: results from five Interphone countries. Occup Environ Med. 2011. Sep;68(9):631–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vrijheid M, Richardson L, Armstrong BK, Auvinen A, Berg G, Carroll M, et al. Quantifying the impact of selection bias caused by nonparticipation in a case-control study of mobile phone use. Ann Epidemiol. 2009. Jan;19(1):33–41. [DOI] [PubMed] [Google Scholar]
- 49.Vrijheid M, Deltour I, Krewski D, Sanchez M, Cardis E. The effects of recall errors and of selection bias in epidemiologic studies of mobile phone use and cancer risk. J Expo Sci Environ Epidemiol. 2006. Jul;16(4):371–84. [DOI] [PubMed] [Google Scholar]
- 50.Pearce N, Checkoway H, Kriebel D. Bias in occupational epidemiology studies. Occup Environ Med. 2007. Aug;64(8):562–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tielemans E, Kupper LL, Kromhout H, Heederik D, Houba R. Individual-based and group-based occupational exposure assessment: some equations to evaluate different strategies. Ann Occup Hyg. 1998. Feb;42(2):115–9. [DOI] [PubMed] [Google Scholar]
- 52.Kim H-M, Richardson D, Loomis D, Van Tongeren M, Burstyn I. Bias in the estimation of exposure effects with individual- or group-based exposure assessment. J Expo Sci Environ Epidemiol. 2011. Apr;21(2):212–21. [DOI] [PubMed] [Google Scholar]
- 53.Sorahan T, Swanson J. Does ‘job’ predict exposure to magnetic fields? Occup Environ Med. 2017. Jun 13; [DOI] [PubMed] [Google Scholar]
- 54.Ahlbom A, Bridges J, de Seze R, Hillert L, Juutilainen J, Mattsson M-O, et al. Possible effects of electromagnetic fields (EMF) on human health--opinion of the scientific committee on emerging and newly identified health risks (SCENIHR). Toxicology. 2008. Apr 18;246(2–3):248–50. [DOI] [PubMed] [Google Scholar]
- 55.SCENIHR. Possible Effects of Electromagnetic Fields (EMF) on Human Health. Brussles: European Commission: Health and Consumer Protection; Directorate General; 2007. (Scientific Committee on Emerging and Newly Identified Health Risks). [Google Scholar]
- 56.Usselman RJ, Hill I, Singel DJ, Martino CF. Spin Biochemistry Modulates Reactive Oxygen Species (ROS) Production by Radio Frequency Magnetic Fields. PLoS ONE [Internet]. 2014. Mar 28 [cited 2017 Aug 7];9(3). Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3969378/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hitchcock RT, Patterson RM. Radio-Frequency and ELF Electromagnetic Energies: A Handbook for Health Professionals. New York: Van Nostrand Reinhold; 1995. [Google Scholar]
- 58.Baste V, Mild KH, Moen BE. Radiofrequency exposure on fast patrol boats in the Royal Norwegian Navy—an approach to a dose assessment. Bioelectromagnetics. 2010. Jul 1;31(5):350–60. [DOI] [PubMed] [Google Scholar]
- 59.Heinrich S, Thomas S, Heumann C, von Kries R, Radon K. Association between exposure to radiofrequency electromagnetic fields assessed by dosimetry and acute symptoms in children and adolescents: a population based cross-sectional study. Environ Health Glob Access Sci Source. 2010. Nov 25;9:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomas S, Kühnlein A, Heinrich S, Praml G, Nowak D, von Kries R, et al. Personal exposure to mobile phone frequencies and well-being in adults: a cross-sectional study based on dosimetry. Bioelectromagnetics. 2008. Sep;29(6):463–70. [DOI] [PubMed] [Google Scholar]
- 61.Roach W Radiofrequency radiation dosimetry handbook [Internet]. Brooks Air Force Base, Texas, USAF, School of Aerospace Medicine; 2009. (Report AFRL-RH-BR-TR-2010–0065). Available from: http://www.agriculturedefensecoalition.org/sites/default/files/file/pge_smart_meters/18J_2009_U.S._Air_Force_Directed_Energy_Bioeffects_Divsion_Radio_Frequency_Radiation_Branch_Handbook_5th_Editor_July_2009.pdf [Google Scholar]
- 62.Royston P, Sauerbrei W, Altman DG. Modeling the effects of continuous risk factors. J Clin Epidemiol. 2000. Feb;53(2):219–21. [DOI] [PubMed] [Google Scholar]
- 63.Royston P, Sauerbrei W. Multivariable Model-Building: A Pragmatic Approach to Regression Analysis Based on Fractional Polynomials for Modelling Continuous Variables. Chichester, UK: John Wiley & Sons Ltd; 2008. [Google Scholar]
- 64.Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. New York: Springer; 2001. [Google Scholar]
- 65.Greenland S, Senn SJ, Rothman KJ, Carlin JB, Poole C, Goodman SN, et al. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol. 2016. Apr;31(4):337–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rothman KJ. Disengaging from statistical significance. Eur J Epidemiol. 2016. May;31(5):443–4. [DOI] [PubMed] [Google Scholar]
- 67.Kriebel D, Checkoway H, Pearce N. Exposure and dose modelling in occupational epidemiology. Occup Environ Med. 2007. Jul;64(7):492–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.IEEE. IEEE Standard for Safety Levels with Respect to Human Exposure to Radio Frequency Electromagnetic Fields, 3 kHz to 300 GHz. IEEE Std C951–2005 Revis IEEE Std C951–1991. 2006;1–238. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


