Abstract
Neural tube defects (NTDs) are a classic example of preventable birth defects for which there is a proven-effective intervention, folic acid (FA); however, further methods of prevention remain unrealized. In the decades following implementation of FA nutritional fortification programs throughout at least 87 nations, it has become apparent that not all NTDs can be prevented by FA. In the United States, FA fortification only reduced NTD rates by 28–35% (Williams et al., 2015). As such, it is imperative that further work is performed to understand the risk factors associated with NTDs and their underlying mechanisms so that alternative prevention strategies can be developed. However, this is complicated by the sheer number of genes associated with neural tube development, the heterogeneity of observable phenotypes in human cases, the rareness of the disease, and the myriad of environmental factors associated with NTD risk. Given the complex genetic architecture underlying NTD pathology and the way in which that architecture interacts dynamically with environmental factors, further prevention initiatives will undoubtedly require precision medicine strategies that utilize the power of human genomics and modern tools for assessing genetic risk factors. Herein, we review recent advances in genomic strategies for discovering genetic variants associated with these defects, and new ways in which biological models, such as mice and cell culture-derived organoids, are leveraged to assess mechanistic functionality, the way these variants interact with other genetic or environmental factors, and their ultimate contribution to human NTD risk.
Keywords: anencephaly, genomics, in vitro models, mouse models, Myelomeningocele, spina bifida
1 ∣. INTRODUCTION
Neural tube defects arise early in human embryogenesis when the neural tube, the precursor to the brain and spinal cord, fails to properly close during neurulation. Failure to complete or maintain posterior closure causes myelomeningocele or spina bifida (Lee & Gleeson, 2020). Incomplete cranial closure results in anencephaly (Avagliano et al., 2019), while failed closure along the entire neural tube is referred to as craniorachischisis. Fetuses with anencephaly and craniorachischisis die in utero or are stillborn, while most spina bifida (SB) patients can survive well into adulthood, yet are likely to suffer from severe, life-long disabilities, and are at risk for psychosocial maladjustment. NTD birth prevalence ranges from one in 3,000 to one in 100, depending on global location, making NTDs the second most common structural malformation in humans (Ross, Mason, & Finnell, 2017). Despite their relative frequency of NTDs, treatment paradigms for SB have not evolved significantly in over 30 years, and we are only capable of imperfectly managing the spectrum of comorbidities associated with permanent and irreversible neurological damage resulting from these defects. As such, the efforts of public health research have generally focused on identifying the underlying risk factors and developing prevention strategies.
It is generally accepted that the origins of NTDs are multifactorial, in which both environmental (Brender, Suarez, Hendricks, Baetz, & Larsen, 2002; Brender & Suarez, 1990; Cabrera et al., 2019; Carmichael & Shaw, 2000; Carmichael, Shaw, Selvin, & Schaffer, 2003; Farley, Hambidge, & Daley, 2002; Lundberg, Wing, Xiong, Zhao, & Finnell, 2003; Sever, 1995; Shaw, Todoroff, et al., 1999; Shaw, Todoroff, Velie, & Lammer, 1998; Shaw, Velie, & Schaffer, 1996; Shaw, Wasserman, O'Malley, Nelson, & Jackson, 1999; Suarez, Cardarelli, & Hendricks, 2003; Waller et al., 2007; Wasserman, Shaw, Selvin, Gould, & Syme, 1998; White, Cohen, Sherman, & McCurdy, 1988) and genetic interactions contribute to the malformation (Figure 1) (Blom, Shaw, den Heijer, & Finnell, 2006; Campbell, Dayton, & Sohal, 1986; Shaw, Jensvold, Wasserman, & Lammer, 1994; Steele et al., 2019). Substantial evidence suggests that nonsyndromic NTDs are rarely monogenic disorders, and multiple pathological gene variants contribute to most human cases of failed neural tube closure (Chen, Lei, Cao, et al., 2018; Ross, 2010; Wallingford, Niswander, Shaw, & Finnell, 2013). Indeed, the genetic heritability of human NTDs approximates 70% (Jorde, Fineman, & Martin, 1983). Despite this genetic complexity, periconceptional use of folic acid (FA) prevents a significant percentage of the population's burden of NTDs (Berry et al., 1999; Canfield et al., 2005; Czeizel & Dudas, 1992; De Wals et al., 2007; Honein, Paulozzi, Mathews, Erickson, & Wong, 2001; MRC Vitamin Study Group, 1991; Williams et al., 2002). Nevertheless, significant numbers of NTDs are not preventable by FA supplementation, and these FA-resistant NTDs occur at an apparent baseline rate of 1 per 2,000 live births (Heseker, Mason, Selhub, Rosenberg, & Jacques, 2009). Despite the success of national folate fortification programs in reducing the overall prevalence of these malformations, complete prevention remains unrealized, and these defects still affect up to 2,300 US births annually and some 166,000 SB patients currently live in the US, more than half of whom are now adults. Given this reality, more targeted interventions developed through precision medicine approaches may prove effective at further reducing NTD prevalence and developing such approaches will necessarily leverage the power of novel genetic models and human genomic initiatives.
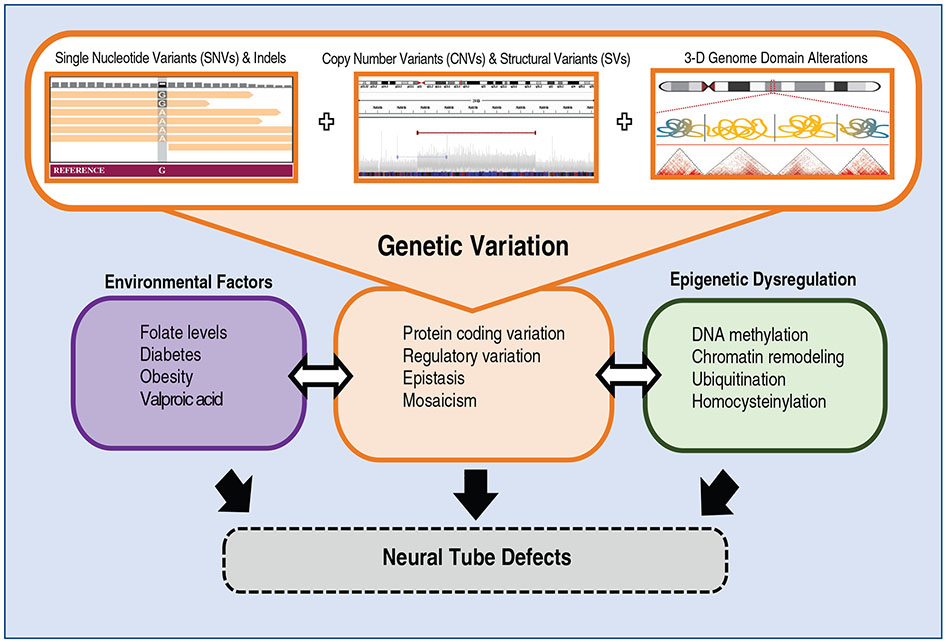
FIGURE 1.
Potential mechanisms underlying NTD pathophysiology. Genetic variants contributing to NTDs may include single nucleotide variants (SNVs) and indels as well as copy number variants (CNVs) and structural variants (SVs). These may disrupt protein-coding regions of the genome or noncoding regions, which may dysregulate gene regulatory networks. NTD risk is comprised of the interplay between these genetic variants with environmental and epigenetic factors
In this review, we discuss advances in mouse model studies of genetic and biological mechanisms leading to NTDs and how these insights have influenced investigations of genetic factors contributing to NTDs in humans. Animal modeling is further illuminating mechanisms that underlie comorbidities of SB. These recent technical innovations in genomics and biological insights offer promise for developing novel and effective intervention strategies and therapeutics for SB patients.
2 ∣. UTILIZING MOUSE KNOCKOUT MODELS TO ESTABLISH CANDIDATE NTD GENES
As an NTD model organism, the mouse has few rivals, as there are at least 250 NTD-causing mouse genetic mutations for hypothesis-generating investigations (Eppig et al., 2005; Harris & Juriloff, 2010), which underscores the genetic complexity underpinning these structural birth defects. The publications of mouse NTD models extend over three decades and are too extensive to comprehensively review here. However, studies that have, in aggregate, illuminated pathways that have guided human investigations deserve note. A number of clinical case–control studies focusing on one, or at most a few candidate genes, have found alleles suggesting an association with increased NTD risk. Many of the early studies were based on candidate genes identified in naturally occurring mouse models presenting with NTD phenotypes. The advent of gene editing and creation of transgenic mice with relative ease and efficiency has been critical to accelerating this gene discovery effort. The recognition that folic acid was a significant modifier of NTD prevalence (MRC Vitamin Study Group, 1991) drew attention to genes involved in the enzymatic biotransformation of folic acid as candidate genes for human NTDs. These models included gene inactivation for Mthfr, Mtrr, CBS, Mthfd1, Shmt1, Bhmt and many others (Beaudin & Stover, 2007, 2009).
The Rozen Laboratory inactivated the Mthfr gene and the nullizygous mutant mice presented with hyperhomocysteinemia and altered DNA methylation (Chen et al., 2001). Although these mice displayed an increased rate of resorptions and cardiac defects, the null mice lacked an NTD phenotype (Chen et al., 2001; Li et al., 2005). The frequency of neurological disorders in the Mthfr mutant mice could be increased either by altering the genetic background of the null allele (Lawrance et al., 2011), or by administering human and murine teratogens such as valproic acid (Roy et al., 2008). Many subsequent studies have confirmed the importance of the genetic background on the penetrance of NTDs in individual mutant mouse lines and modifier loci that have been mapped in several lines (Juriloff et al., 2001; Korstanje et al., 2008). In parallel clinical studies, the thermolabile variant (C677T) in the MTHFR gene was proposed to increase the NTD risk (Shields et al., 1999) in some, but not all human NTD cohorts. This was one of many candidate genes selected either based on mouse studies or initial trends gleaned from human genome sequences based on unfortunately small case numbers.
Rather than focusing on one-carbon metabolism (OCM) enzymes, other efforts pursued the idea that perhaps the link to NTDs was not so much insufficient dietary folate, or a defect in a critical metabolic enzyme, but rather that folate molecules were not being transported to the developing embryo due to defects in folate transport proteins (Finnell, Greer, Barber, & Piedrahita, 1998). By using gene targeting approaches to knockout the mouse ortholog (Folr1) of human folate receptor alpha gene (hFRα), highly penetrant anterior NTDs in the null embryos were observed (Barber, Lammer, Shaw, Greer, & Finnell, 1999; Piedrahita et al., 1999). The folate receptor 1 (Folr1) mutant mice have folate responsive NTDs (Piedrahita et al., 1999) and the folate-responsive gene expression profiles in the rostral portion of the neural tube of the mutant mice have been well described (Spiegelstein, Cabrera, Bozinov, Wlodarczyk, & Finnell, 2004). Previous work has emphasized the importance of considering the genetic background of mouse strains used in gene editing experiments, as it is likely to influence the development of diseases and congenital anomalies, including NTDs (Beck, 1999; Detrait et al., 2005). Leduc et al. (2017) demonstrated that background-dependent chromosomal linkage in Cecr2 mutants can impact the penetrance of NTD candidate genes.
To test the hypothesis that folate responsive defects are also largely influenced by gene–gene and gene-nutrient interactions, heterozygous Folr1+/− mice were crossed onto SWV-Fnn and LM/Bc-Fnn background mouse strains utilizing a speed congenic approach (Grove, Eckardt, & McLaughlin, 2016). Cabrera et al. (2019) reported that while both strains demonstrated a dose response to folinic acid supplementation, mutant mice on the SWV background had a significantly lesser reduction in NTD frequency at equal concentrations of folinic acid when compared to mutants on the LM/Bc genetic background. It is apparent that modifier genes within the LM/Bc strain contribute to the increased sensitivity of the Folr1 null embryos. Several examples show strain-dependent sensitivity to environmental factors observed in mouse NTD models, among them hyperthermia-induced NTDs. Pregnant dams were exposed for 10 min to a hyperthermic water bath during the critical window of neural tube closure to simulate maternal fever during pregnancy or exposure to occupational or environmental heat sources, such as dry cleaning facilities or recreational hot tubs. Swiss-Vancouver (SWV) offspring presented with a 44.3% occurrence of exencephaly, while less than 14% were affected in the other four strains tested (Finnell, Moon, Abbott, Golden, & Chernoff, 1986). Another example is exposure to the antiepileptic drug, valproic acid (VPA). SWV mice are similarly more sensitive to VPA than other highly inbred strains. For example, DBA/2J mice appear entirely resistant to VPA, while about 20% of LM/Bc and C57/BL6 embryos develop NTDs after VPA exposure at a particular dose during the critical window of neural tube closure. SWV mouse embryos had the highest susceptibility, displaying a 35% occurrence of NTDs (Finnell, Bennett, Karras, & Mohl, 1988; Lundberg et al., 2004). This sensitivity has gradually been mapped to a specific locus in SWV as genetic sequencing technologies have progressed over the years (Beck, 1999; Beck, 2001; Finnell et al., 1988; Finnell et al., 2000; Finnell, Wlodarczyk, Craig, Piedrahita, & Bennett, 1997; Lundberg et al., 2004). Thus, sensitivity to NTDs in some strains suggests that there are permissive genetic backgrounds rendering individuals more susceptible for a higher individual risk for NTDs given particular environmental exposures. These permissive backgrounds may result from multigenic variant interactions across the global genomic landscape or single gene variants in critical pathways. Mouse models have shown that genetic impairment of folate transport (Folr1 deficiency) or folate metabolism (Mthfr deficiency) increase NTD risk under conditions of maternal hyperglycemia in mouse models of diabetes (Lopez-Escobar et al., 2019).
Beyond an initial focus on OCM pathway genes, surprising connections between naturally occurring mouse NTD mutations and folate were exemplified in the Crooked tail mouse, for which folate supplementation protected homozygous animals from NTDs (Carter, Ulrich, Oofuji, Williams, & Ross, 1999). Positional cloning identified the mutation as a missense variant in the Lrp6 co-receptor needed for WNT signal transduction (Carter et al., 2005) and later work showed that while supplemental folate is protective against NTDs for Lrp6Cd/Cd mutants, excessive levels exacerbate embryopathy in the Lrp6−/−, null, mice (Gray et al., 2010). Moreover, although both Lrp6Cd/Cd and Lrp6−/− mutant cells display reduced canonical WNT signaling, the non-canonical pathway is upregulated only in Lrp6Cd/Cd animals, evidenced by elevated RhoA-GTP activation and rescue from NTDs by ROCK inhibition in mouse embryo culture (Gray et al., 2013). Thus, not only is the gene association with NTDs important, but also the functional consequence of the mutation (gain or loss) is critical to predicting genetic interactions with the environment.
3 ∣. PINPOINTING RELEVANT NTD PATHWAYS AND ASSOCIATED CHALLENGES
Investigators interested in developing murine NTD models were well aware of the role that Wnt signaling plays in neural development during early embryonic stages (Miller & McCrea, 2010). The planar cell polarity (PCP) (Curtin et al., 2003) genes in the non-canonical Wnt signaling pathway quickly became the focus of many genomic investigations for developing mouse models of neural tube closure defects. Multiple mouse PCP genes (Fz, Dvl, Vangl or Celsr) (Juriloff & Harris, 2012; Wallingford, 2012) result in an NTD phenotype when inactivated. Most of the NTDs associated with altered PCP pathway genes give rise to mice with craniorachischisis, secondary to defects in convergent extension during early embryogenesis (Wang, Hamblet, et al., 2006; Ybot-Gonzalez et al., 2007). By inactivating PCP genes, cells fail to interdigitate mediolaterally, and the resulting excessively wide neural plate tissue cannot properly fuse (Wallingford & Harland, 2001, 2002). Loss of function (LoF) alleles of the core components of the PCP pathway, including Celsr1 (Curtin et al., 2003) and Vangl2 (Greene, Gerrelli, Van Straaten, & Copp, 1998) result in craniorachischisis in mice. In addition to monogenic causes, double but not single knockouts of Fzd3 together with Fzd6 (Wang, Guo, & Nathans, 2006), as well as Dvls (Etheridge et al., 2008) also produce a craniorachischisis phenotype.
In addition to the many well described important developmental gene pathways, it is also important to interrogate mouse genes not intuitively relevant to neural tube closure. Such genes are often found by serendipity and will require functional analyses in models to document a potential value as human candidate NTD genes. One such example is Fkbp8 (also known as Fkbp38) (Bai et al., 2007; Lam, Martin, & Wiederrecht, 1995), a member of the FK506-binding protein family that functions in cell size regulation (Rosner, Hofer, Kubista, & Hengstschläger, 2003) and was noted to be the cellular inhibitory target of rapamycin (mTOR) signaling (Bai et al., 2007). FKBP8 is also an important inhibitor of apoptosis, through anchoring the anti-apoptotic proteins BCL2 and BCLXL to the mitochondrial membranes (Shirane & Nakayama, 2004). During morphogenesis, Fkbp8 controls neural cell fate through antagonism of SHH signaling, which is critical for proper neural tube closure (Bulgakov, Eggenschwiler, Hong, Anderson, & Li, 2004; Cho, Ko, & Eggenschwiler, 2008; Fong et al., 2003). Fkbp8 also contains functional tetratricopeptide-repeat (TPR) domains, a leucine zipper repeat, and a C-terminal transmembrane (TM) domain (De Cicco, Milroy, & Dames, 2018). The C-terminal TM domain facilitates FKBP8 protein anchorage to mitochondrial membranes (Shirane & Nakayama, 2003). Additionally, Fkbp8 is a co-chaperone involved in the regulation of adenylyl cyclase trafficking to cilia as well as to various heat shock proteins (Walker, Atanasiu, Lam, & Shrier, 2007; Wang, Venable, et al., 2006).
Fkbp8 mouse mutants have an isolated and completely penetrant SB, which are both folic acid and inositol-resistant (Wong et al., 2008). Unlike other NTD mouse mutants, the Fkbp8 nulls are not embryo lethal, enabling one to study both the evolution of the lesion from conception, past parturition into early life, as well as SB-associated comorbidities, such as urinary and fecal incontinence (Verhoef et al., 2004). The knockout mice share many features with human SB, including a dysplastic spinal cord, open neural canal and paralysis of limbs below the defect. There is increased apoptosis in the posterior neural tube, and expression of neural tube patterning genes is perturbed, suggesting that FKBP8 activity is critical in establishing the dorso-ventral patterning of the mouse neural tube. RNA sequencing on anterior and posterior tissues isolated from Fkbp8−/− and wild-type mice at E9.5 and E10.5 showed that Fkbp8−/− embryos have an abnormal expression profile within tissues harvested at posterior sites, thus leading to a posterior NTD. We found that Fkbp8 knockout mouse embryos display abnormal Wnt3a and Nkx2.9 expression during early stage neural tube development, perhaps accounting for the caudal location of its NTDs (Wong et al., 2008). Tsurubuchi et al. (2014) used neural stem cells from FKBP8−/− embryos and observed increased Noggin gene expression along with decreased Msx2 expression and premature differentiation—neurogenesis. The authors considered that the inability of folic acid to protect Fkbp8 nullizygous embryos from failed neural tube closure could be attributed in part to the increased noggin expression. In the presence of folic acid, noggin expression increases ventralization of the neural tube and further compromises proper fusion. These findings provide evidence that functional variants of FKBP8 are risk factors for SB and suggest a novel mechanism by which Fkbp8 mutations specifically cause SB in mice.
4 ∣. FROM MOUSE MODELS TO HUMAN GENETICS OF NTDS
The translation of these insights from mouse models to assessment of human genetic contributions to NTDs have been pursued for several decades. While putative risk genes and alleles have been identified, it has not been possible to define patterns of multiple gene interactions that determine individual risk. If permissive genetic backgrounds could be identified, they could be compensated for by providing evidence-based counseling to at risk families such that pregnancy outcomes can be optimized. A number of challenges derive from the complexity of NTD genetics and the rarity of the condition compared to more prevalent complex disorders such as Type II Diabetes, Alzheimer's Dementia or Autism Spectrum Disorder (Table 1). Several arguments favor the view that genetic contributions to NTDs with the largest effect size are likely to be rare variants, including the very fact that NTDs have an estimated base prevalence of folate resistant NTDs of 1 per 2,000 births (Heseker et al., 2009), rather than highly prevalent diseases associated with common genetic variants (i.e., minor allele frequency or MAF >1–5%) like diabetes and Alzheimer's. Not only do rare variants carry greater effect sizes than common variants (Park et al., 2011), but for diseases that are under strong negative selection like NTDs, restricting the analyses to variants that are most rare has revealed strong enrichments for causal mutations (Bomba, Walter, & Soranzo, 2017; Petrovski et al., 2017).
TABLE 1.
Recent point prevalence estimates of select complex genetic disorders
| United States |
Worldwide | |
|---|---|---|
| Autism spectrum disorder (ASD) | 1 in 54 | 1 in 160 |
| Type 2 diabetes (T2D) | 1 in 10 | 1 in 17 |
| Asthma | 1 in 13 | 1 in 26 |
| Alzheimer's disease (AD)a | 1 in 9 | 1 in 14 |
| Congenital heart Defects (CHD)b | 1 in 125 | 1 in 100 |
| Orofacial clefts (OFC)b | 1 in 940 | 1 in 679 |
| Spina bifida (SB)b | 1 in 2,750 | 1 in 100 to 1 in 3,000 |
Source: ASD: https://www.cdc.gov/ncbddd/autism/data.html, https://www.who.int/news-room/fact-sheets/detail/autism-spectrum-disorders; T2D: https://www.cdc.gov/diabetes/basics/type2.html, https://www.who.int/news-room/fact-sheets/detail/diabetes; Asthma: https://www.cdc.gov/asthma/asthmadata.htm, https://www.who.int/news-room/q-a-detail/chronic-respiratory-diseases-asthma; AD: https://www.cdc.gov/aging/aginginfo/alzheimers.htm, https://www.who.int/news-room/fact-sheets/detail/dementia; CHD: GBD 2017 Congenital Heart Disease Collaborators (2020). https://doi.org/10.1016/S2352-4642(19)30402-X; OFC: https://www.nidcr.nih.gov/research/data-statistics/craniofacial-birth-defects/prevalence, Panamonta, Pradubwong, Panamonta, and Chowchuen (2015); SB: https://www.cdc.gov/ncbddd/spinabifida/data.html, Atta et al. (2016). https://doi.org/10.2105/AJPH.2015.302902.
65 years and older.
Reported as birth prevalence.
Indeed, multiple reported human gene associations with NTDs have used some version of a search among candidate genes for predicted deleterious rare variants. This has included direct targeted sequencing of a single gene or a selected list of candidate genes. Although the initial studies of small NTD cohorts failed to identify a link between variants in the human folate receptor gene (hFRα) and NTD prevalence (Barber et al., 1998; Barber et al., 1999; Trembath et al., 1999), subsequent studies of human folate transport genes described 12 novel variants in FOLR1, FOLR2, and FOLR3 (Findley et al., 2017). This included four large insertion deletion variants in FOLR3 as well as a single stop gain variant. Their findings support the utility of using murine NTD genes involved in folate transport and investigating their impact on human susceptibility to NTDs. Based on animal studies like those discussed above, robust efforts in ever larger human NTD cohorts have now identified mutations in several PCP genes (Chen, Lei, Cao, et al., 2018; Kibar et al., 2007; Lei et al., 2019; Tian, Lei, et al., 2020; Wang, Marco, Capra, & Kibar, 2019). As the databases grow with ever more genomic studies of complex birth defects, it is important to mine this genotype/phenotype data from animal studies for genes such as those in the PCP/Wnt pathway that share specific developmental functions as a means of identifying candidate genes to explain the population burden of human NTDs.
The candidate gene approach to find human counterparts of NTD associated mutations in mouse continues to yield translational insight. For example, the first paper interrogating FKBP8 gene variants in human NTDs was only recently published (Tian, Cao, et al., 2020). These investigators used Sanger sequencing on genomic DNA samples from 472 SB and 565 control samples and identified five rare (MAF ≤ 0.001) deleterious variants in SB patients. Sequencing of the control cohort yielded no rare deleterious variants. One variant, p.Glu140*, was found to interfere with FKBP8 localization to the mitochondria as well as truncate the FKBP8 protein. The truncated protein was unable to interact with BCL2, resulting in an increase in cellular apoptosis. The p.Ser3Leu, p.Lys315Asn and p.Ala292Ser variants decreased the absolute concentration of the FKBP8 protein, while the p.Lys315Asn variant was shown to produce increased cellular apoptosis (Tian, Cao, et al., 2020). These functional assays, coupled with statistical argument for NTD association, remain a critically important avenue for the identification of relevant deleterious mutations.
A more recent strategy toward defining genetic contributions to NTD risk has employed whole exome sequencing (WES) for searches of protein coding regions of candidate genes or pathways coupled with gene collapsing strategies to detect putative NTD risk genes and variants. Examples include studies that examined 511 NTD cases for exon variants in glucose homeostasis/oxidative stress and OCM network (Hillman et al., 2020) or WNT signaling pathway genes (Hebert et al., 2020). Another study looked at the association between rare variants in specific functional pathways and in multiple subphenotypes of human neural tube defects (Zou et al., 2020). That study included 355 NTD cases manifesting a range of structural defects, including craniorachischisis, anencephaly, encephalocele, spina bifida aperta, spina bifida cystica and spina bifida occulta. Although the investigators reported potentially damaging rare variants in genes functioning in different pathways with different NTD phenotypes (e.g., chromatin modifications in anencephaly but not SB), this preliminary work paves the way for refined phenotyping in NTD cohorts. It also underscores the utility of investigating rare variants across all NTDs.
5 ∣. TOWARD GENOME-WIDE, UNTARGETED SEARCHES FOR HUMAN MUTATIONS ASSOCIATED WITH NTDS
It is increasingly apparent that if we are ever to understand the gene–gene interactions that interface with epigenetic and environmental influences contributing to human NTD risk, it will be necessary to devise means for looking at (a) the entire genome, not just the 2% represented by protein coding sequences, and (b) searching the genome in an untargeted manner. Whole genome sequencing (WGS) data provide an opportunity to interrogate DNA variants genome-wide and detect not only single nucleotide variants (SNVs) and indels, but also structural variants (SVs), whose breakpoints may fall in intergenic regions. Despite some known chromosomal abnormalities associated with NTDs, the effects of SVs on NTD susceptibility are not well understood. Rare SVs impact a greater number of nucleotides than rare SNVs and studies have shown that SVs can have numerous functional consequences, including disrupting the structure and dosage of genes (Ho, Urban, & Mills, 2020). Recent publications have also suggested that rare SVs may be causal at 3.5–6.8% of expression of quantitative trait loci (eQTL) sites (Chiang et al., 2017), which is much higher than previous estimates. Moreover, SVs are increasingly implicated in the genetic risk of several complex genetic disorders including schizophrenia (Caseras et al., 2021) and autism (Pavlides et al., 2016).
The difficulty with candidate gene or pathway interrogations is that the approach is inherently biased—one typically finds what one looks for. However, every genome differs from the reference on average at 4–5 million sites. Even if limited to rare variants with minor allele frequency (MAF) < 0.5% (0.005), the average genome contains 40,000–200,000 variants with respect to available databases. Our genome-wide investigations of exonic sequences established a threshold model of ultra-rare singleton loss of function variant (SloV) burden, replicated in three different populations, that found on average 9 SloVs per individual with SB, compared to 15 SloVs per genome in individuals with anencephaly (Chen, Lei, Zheng, et al., 2018). We recently reported an initial investigation of SVs through analysis of WGS from two ancestry balanced case–control cohorts (Wolujewicz et al., 2021). The study used a computational ensemble approach to identify copy number variants (CNV) with a MAF < 0.01%, relying on CNVs that met “high confidence” criteria. We found a statistically significant burden of rare gene disrupting CNVs among SB cases compared to controls, underscoring the importance of structural changes in intergenic regions to the genesis of human NTDs. These CNVs suggested new candidate genes within SB-associated pathways that are potential targets for biological investigation. However, none of the human genome studies mentioned in this section involve sufficient subject numbers to computationally identify statistically significant NTD associations at the single variant or gene level using an untargeted or completely unsupervised approach such as a genome-wide association study (GWAS). Nevertheless, these genome-wide, variant burden analyses illustrate why candidate gene approaches may fail to capture an adequate picture of the underlying mechanism and individual genetic predisposition to NTDs. There are several cautionary notes to consider for future genome-wide studies of NTDs, beyond a simple matter of accumulating larger cohorts. Population databases used for controls might not be representative and may skew frequency estimates for NTD risk alleles. Cohort stratification, especially by ancestral substructure, is a known confounder for genome-wide studies and rare variant interpretation (Jiang, Epstein, & Conneely, 2013; Ma & Shi, 2020; Mathieson & McVean, 2012). The prevalence of NTDs necessitates that large assembled cohorts will contain admixture and therefore must be adequately controlled through selection of unrelated control subjects of genetic background—based on ancestral markers, not geographic or self-identification—that is balanced with respect to cases (Elhaik & Ryan, 2019).
While amassing the thousands of NTD case and control data required to reach statistical significance in a GWAS, several computational strategies can be employed to minimize bias in a genome-wide search for novel genetic contributors to neurulation failure. One that we have recently explored is the use of a systems biology approach to NTD genetic factors (Aguiar-Pulido et al., in revision). Working with WGS data from 149 cases and 149 ancestry matched, unrelated controls, this strategy examined modestly rare single nucleotide variants (SNVs) in protein coding regions that represent a MAF < 1% that are predicted to be deleterious (stop loss/gain, frame shift, splice donor/acceptor, missense predicted to be deleterious by Sift/Polyphen/CADD). These variants were collapsed onto the gene level and submitted to training a random forest machine learning classifier to identify high discriminatory potential between SB cases from controls. 439 genes were then subjected to pathway analysis to obtain gene-enriched pathways that remained significant after FDR correction for multiple hypothesis testing. Remarkably, the pathways with highest significance were in Carbon Metabolism and Vitamin B12 Transport and Metabolism (adjusted p values 0.00081 and 0.00099, respectively). These genes, involved in glycolysis and fatty acid biosynthesis pathways, are linked to diabetes, obesity, metabolic syndrome and overlap with one-carbon transfer, reflecting major risk factors for NTD identified in epidemiological studies in the post-folate fortification era (Loeken, 2020). This result supports the validity of other pathways found to be significantly gene enriched in this study (Aguiar-Pulido et al., in revision). Further strategies to examine SNVs and SVs build a comprehensive picture of the entire genome landscape, including the 98% of the genome beyond protein coding sequences.
6 ∣. TRIO VERSUS CASE-CONTROL ANALYSES
Exome and genome sequencing of case-parent trios allow for de novo mutation (DNM) detection toward discovering genes with large effect size for neurodevelopmental disorders. The identification of DNMs from trio sequence data, requires comparing the observed rate of DNMs to the expected, and correlating the variant with phenotype based on biological function or pathway. Such analyses have identified new candidate NTD genes using 43 trios (Lemay et al., 2015).
Trio studies, aside from identifying DNMs, allow for use of transmission disequilibrium tests (TDTs) in order to assess whether alleles are overtransmitted to affected offspring, revealing patterns that may confer genetic risk. Furthermore, TDT results can be correlated with case–control association results using the ratio of the overtransmitted alleles compared to the undertransmitted alleles to serve as an approximation for the risk ratio. A recent study analyzed Irish and UK trios and identified a potential risk factor in dihydrofolate reductase 2 (DHFR2) with mixed results (Pangilinan et al., 2021). In their Irish cohort, which was comprised of 440 complete trios, the investigators observed a significant overtransmission of three alleles within DHFR2 from parents to affected offspring. Since these alleles share a high degree of linkage disequilibrium, the authors report that this is likely one signal that is associated with an increased case risk for NTD. Interestingly, they did not observe a statistically significant overtransmission of these alleles within DHFR2 in the UK trios, and it will be informative to see if these results are replicated in other cohorts.
A major advantage to conducting trio analyses is that they are generally resistant to confounding effects from population stratification. Moreover, extended family analyses can provide more statistical power (He et al., 2017). While the analysis of nuclear or extended families are critical to understanding the risk of de novo versus inherited variants in NTDs, there are certainly trade-offs. Statistical testing and power calculations between case–control and trio study designs should be considered at the time of recruitment. The statistical power of 500 trios is approximately the same as the p-value obtained from 500 cases and 1,000 controls, at considerably greater expense (Ahsan, Hodge, Heiman, Begg, & Susser, 2002; Hintsanen, Sevon, Onkamo, Eronen, & Toivonen, 2006). Given the challenges of trio recruitment, the statistical basis for a case–control study is appealing, provided that the controls are healthy and well matched in terms of population admixtures. Nevertheless, the judicious use of these study designs should help uncover genomic signals and variants that contribute risk toward NTD manifestation.
7 ∣. HUMAN NTD ASSOCIATED GENE VARIANTS CAN BE GERMLINE OR SOMATIC
As DNA sequencing technologies improve and the analysis of the trove of data these technologies create get ever more instructive, the number of potential candidate genes that contribute to human NTDs has grown increasingly large. Within these candidate genes are similarly large numbers of variants—both somatic and germline—that require functional analyses in order to validate their pathogenicity. Gene variants in fetuses may be inherited from parents or can occur de novo. These de novo variants may arise at a very early stage of embryonic development, affecting virtually all cells. It is also possible for these de novo variants to occur at a later developmental stage, affecting cells confined in a specific organ. These are referred to as somatic variants, which are postzygotic changes that can alter more than one group of cells with different genotypes in an organism, rather than a change in the DNA in a single fertilized egg. While somatic variants are well documented in cancer patients, they are also known to occur in several neurodevelopmental disorders, such as McCune–Albright syndrome (Weinstein et al., 1991), Sturge–Weber syndrome (Shirley et al., 2013), Proteus syndrome (Lindhurst et al., 2011) and select brain malformations (Poduri et al., 2012). These studies confirm that the nervous system is highly susceptible to somatic mutation (Muotri et al., 2005). With respect to congenital abnormalities such as NTDs, the existing literature concerning any association between somatic variants with the development of an NTD is quite limited. Galea et al. (2018, 2021) reported that a somatic Vangl2 deletion in murine neuroepithelial cells as well as in surface ectodermal cells causes SB in mice. In our recent publication, we described somatic variants in some key PCP genes (e.g., VANGL1 and FZD6) in neural tissue that are associated with human NTDs, suggesting a potentially important role somatic variants can play in the occurrence of human NTDs (Tian et al., 2021).
The Mediator complex (MED) is known to have significant regulatory effects on WNT signaling, which represents many candidate NTD genes as this pathway has critically important signaling functions during neural tube closure (Rocha, Scholze, Bleiss, & Schrewe, 2010). We recently considered the possibility that genetic variants in the Med12 and Med13 genes which serve as subunits in the regulatory domain of the complex, may be associated with increased NTD risks. We interrogated an initial cohort that comprised 48 pairs of neural lesion site and umbilical cord tissues from NTD affected fetuses. We performed DNA sequencing to identify potential NTD-related somatic variants which was subsequently validated by Sanger sequencing. We identified a heterozygous somatic variant of MED12 c.5344C>T (p.Arg1782Cys) in the neural lesion site tissue of a terminated craniorachischisis fetus. This variant was not found in any other normal tissues tested, indicating that this variant only occurred in neural tissue (Tian et al., 2021). This variant is not located within the functional domains of MED12, although the variant alters an amino acid at a tightly conserved position.
To examine germline mutations in the Med genes, we examined a second cohort comprised of 21 case-parent trios on which we performed WES to identify any de novo germline variants. As before, we also performed Sanger sequencing assays to validate any identified variants. In this cohort a heterozygous stop-gain variant of MED13L c.5278C>T (p.Arg1760*) was detected in an infant with myelomeningocele. Neither parent had this variant in the medPIWI domain, thus confirming its de novo nature. The mutant's affected amino acid is highly evolutionarily conserved among multiple species. With respect to functional studies, we performed subcellular localization on the MED12 p.Arg1782Cys variant by overexpressing it in MDCKII cell lines. Both the wild-type and MED12 mutant protein were expressed within the cell nucleus, indicating that the variant did not alter the subcellular location of MED12 protein, although it was determined that GFP signaling of mutated MED12 was reduced compared to the wild-type MED12 protein. The western blotting assay confirmed that the protein coded for by the MED12 variant p.Arg1782Cys was less abundant than wild-type MED12 (p < .05), indicating that this mutation may decrease MED12 expression or damage its protein stability.
We sought definitive evidence that the identified variant was related to NTDs by using CRISPR/Cas9 mutagenesis to generate knock-in mice with a homologous variant to that found in humans. Of the 192 injected embryos implanted into six recipients (each recipient carried 32 embryos), we collected 12 viable and 1 dead embryo. Of the 10 male embryos, 2 were MED p.Arg1784Cys knock-in hemizygotes that expressed NTD phenotypes (exencephaly and SB with curly tails), while the remaining viable embryos were normal. The result indicates that Med12 p.Arg1784Cys identified in a human NTD patient can also re-produce NTD phenotypes in mice.
8 ∣. VALIDATING HUMAN NTD ASSOCIATED VARIANTS IN MOUSE MODELS AND ORGANOID SYSTEMS
Another excellent demonstration of the functional pathogenicity of genetic variants identified in candidate NTD genes involves DNA damage response (DDR) genes that function in DNA repair, cell cycle control, and are essential for the rapid proliferation of neural progenitor cells. Using whole-genome sequencing and targeted sequencing, we identified significant enrichment of rare deleterious RAD9B variants in SB cases compared to unaffected control infants (8/409 vs. 0/298; p = .0241). This study yielded eight variants, and functional analyses determined that the two frameshift mutants and p.Gln146Glu affected RAD9B nuclear localization. Of the other identified variants, the two frameshift mutants decreased the protein level of RAD9B. p.Ser354Gly, as well as the two frameshift variants adversely affected the cell proliferation rate, while the p.Ser354Gly, p.Ser10Gly, p.Ile112Met, p.Gln146Glu, as well as the frameshift variants were less able to activate JNK phosphorylation (Cao et al., 2020). To better appreciate the potential roles of RAD9B during embryonic development, embryoid bodies (Fong et al.) were derived from hESCs. We were able to achieve a 60% reduction in RAD9B expression using small interfering RNAs (siRNAs) in an Oct4-GFP-hESC cell line (Cao et al., 2020). We focused on OCT4 expression as it reflects the pluripotency of stem cells. We determined that Oct4 expression was significantly downregulated in the RAD9B-KD EBs compared to controls. Additionally, flow cytometry studies confirmed that the Oct4 positive cell population in RAD9B-KD EBs was decreased by twofold compared to control EBs. Together, these data indicate that loss of RAD9B significantly impairs OCT4 expression in hESC derived EBs. Since Pax6 is known to restrict Oct4 expression as well as other pluripotency factors (Zhang et al., 2010) we predicted, and found, that both mRNA and protein levels of PAX6 were significantly elevated in the RAD9B-KD hESCs. Together, these observations demonstrate that loss of functional RAD9B disrupts early cell fate specification in differentiating EBs through abnormal regulation of PAX6 and OCT4 circuitry. We believe that the inhibition of RAD9B compromised the formation of the neural organoids in vitro. These results represent the first demonstration of a DDR gene as an NTD risk factor in humans and validated the pathogenicity of the observed RAD9B gene variants.
Animal models are not without their limitations, and they cannot exactly replicate human NTDs, as one might expect given that their genomes differ from humans, especially in the intergenic, 3-D architecture of their respective genomes. Moreover, the morphogenetic processes involved in neural tube closure are not identical among different model species (O'Rahilly & Müller, 2002). It is possible to carefully recapitulate human neural tube closure to better identify the underlying genetic architecture of NTDs by the use of three-dimensional in vitro cellular systems. In recent years, NTD studies have made excellent use of organoids, which are defined as 3-D cell aggregates generated from pluripotent stem cells or organ progenitor cells and consisting of organ-specific cell types that self-organize while maintaining restricted lineage commitment similar to that found in vivo (Lancaster & Knoblich, 2014; Runnels & Komiya, 2020). Organoids can be derived from embryonic stem cells (ESCs), adult stem cells (ASCs), and induced pluripotent stem cells (iPSCs). These cells, when provided the appropriate extracellular scaffolding and culture conditions with desired signaling factors, can be induced to self-organize and form distinct cell aggregates (Figure 2). Moreover, brain organoids can represent both the phenotypic and genetic characteristics of its in vivo counterparts and are capable of sustaining these features throughout their in vitro culturing process (Wu, Peng, Finnell, & Zheng, 2021). Thus, organoids represent a functional alternative to in vivo animal models as they can accurately recapitulate key elements of normal embryonic development. Neural tube organoids are often compared with in vivo neural tubes on the basis of their overall structure, neural fate commitment, apical–basal polarity, and anterior–posterior and dorsal–ventral patterns. Most neural tube organoids reported in the literature have a cystic shaped cavity rather than the long columnar shape cavity found in neural tubes developing in vivo (Abdel Fattah & Ranga, 2020).
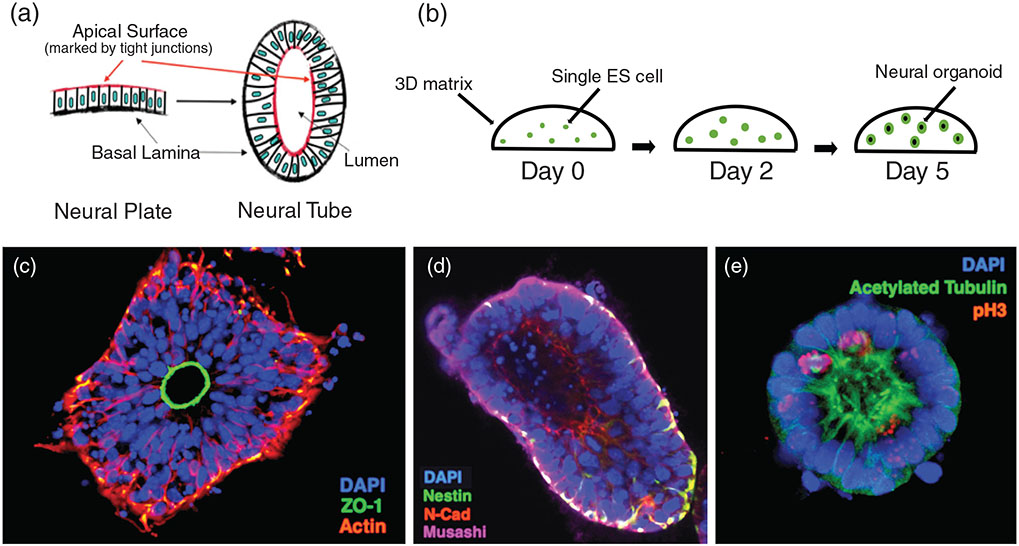
FIGURE 2.
Cell culture derived organoids as a tractable NTD model system. One example is the “neurocyst” model developed by Meinhardt et al. (1). Neural organoids are from single mouse embryonic stem (ES) cells to recapitulate aspects of neuroepithelial architecture in the developing neural plate and neural tube, such as an apical and basal surface, cell–cell junctions, and a hollow central lumen (a). Individual mouse ES cells are plated inside a matrigel 3D matrix, and cultured in neural differentiation media (b). After 5 days, each individual cell has formed a neural organoid with a hollow lumen. Neuroepithelial cells are apically oriented toward the lumen, evidenced by tight junction marker (ZO-1) (c). They express neural cell markers Nestin, Musashi, and N-cadherin (d). They also have apically localized primary cilia marked with acetylated tubulin, and display interkinetic nuclear migration, so that cells are dividing in metaphase at the apical surface as evidenced by immunostaining with phospho-histone H3 (pH 3) (e). Each of these attributes recapitulates some aspect of neural tube development, making them suitable for studying certain mechanisms as an NTD model. However, this model does have some limitations. For example, they do not recapitulate tissue fusion of neural folds or dorsal-lateral hinge point formation (Meinhardt et al., 2014)
Consistent with in vivo morphogenesis many aspects of neural tube closure are time-dependent; therefore, temporal information is often emphasized when describing individual aspects of neural tube organoids, especially when considering various pathological processes leading to the development of NTDs. Unlike using in vivo animal models, it is relatively easy to expose the developing organoids in vitro to study the potential deleterious impact of exogenous compounds such as suspected environmental teratogens. Furthermore, intracellular developmental abnormalities can be studied by interfering with the cells used to create the organoids. It is also possible to use human patient derived iPSCs or iPSCs in which known genetic variants have been edited into these cells to create genetically compromised organoids. That is, hPSCs can be edited by CRISPR/Cas9 methodologies to reflect known variants in human NTD candidate genes, and these hPSCs can serve as the initiating cells for creating neural tube organoids (Liu et al., 2019). By comparing these organoids to the organoids created from control, unaffected hPSCs, we can gain a better understanding of the functional impact NTD associated genetic variants have on critical morphological/biomechanical aspects of neural tube closure. Thus, neural tube organoids are excellent in vitro models for studying neural tube development, pathogenesis, and treatment of NTDs.
9 ∣. CONCLUSION
While prevention of thousands of NTDs through periconceptional FA supplementation and nationwide food fortification programs is a classic public health success story, full prevention of NTDs remains an unrealized goal. Undoubtedly, the underlying genetic complexity of these defects and the role of environmental interactions makes further progress in preventing the FA-non-responsive cases a daunting task. Future prevention strategies targeting these remaining non-FA responsive NTD cases will undoubtedly require modern precision medicine approaches that are capable of evaluating individual risks for mothers and infants using our increasing knowledge of the genomic landscape underlying these defects (Figure 3). Achieving such knowledge, while difficult, has become increasingly possible as the new boundaries in our technology have improved our ability to identify potentially damaging variants and dissect their mechanisms of action, or their interactions with other variants and environmental factors, by leveraging novel mouse and organoid models. In this review, we covered some examples of how the NTD research community has leveraged these technologies over recent years to continue advancing our knowledge of NTD etiology despite the challenges of working with such a rare and complex disease. We hope that by continuing to approach NTD prevention through these novel methodologies, and by developing new strategies for studying the genetic mechanisms of brain and spinal cord development, complete prevention of these debilitating defects can ultimately be achieved.
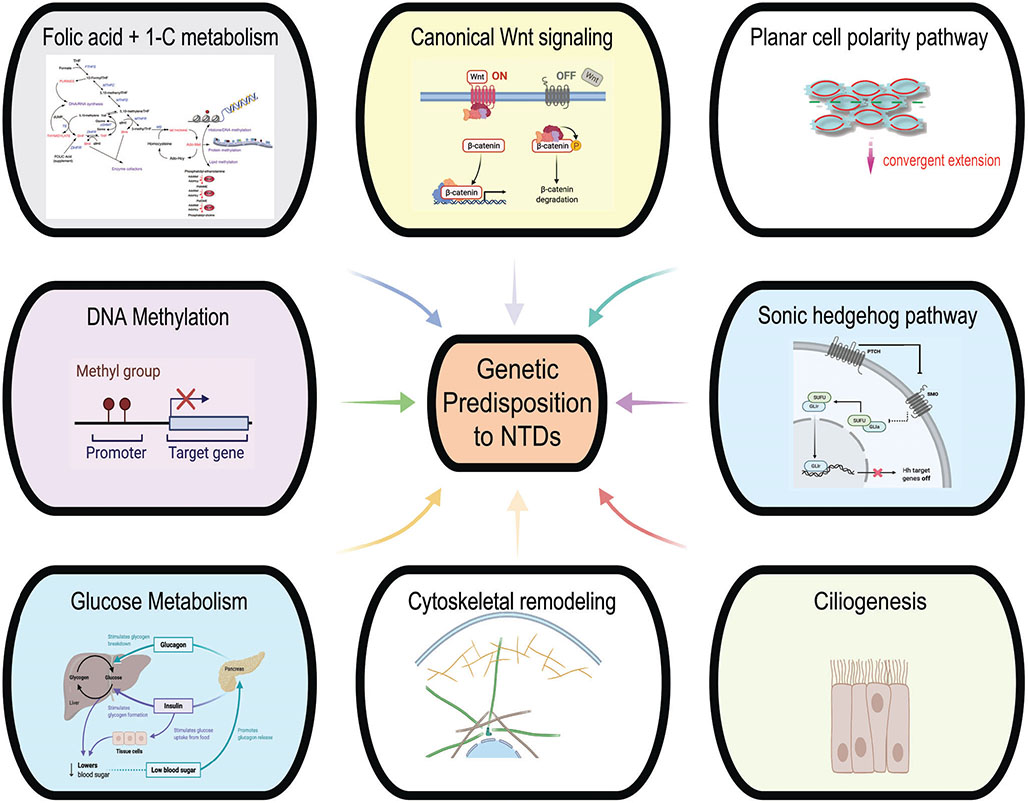
FIGURE 3.
Genetic predisposition to NTDs arises from a number of implicated signaling and metabolic pathways
ACKNOWLEDGMENTS
The authors graciously acknowledge the generous support from NICHD grants HD100535, HD098131, HD067244, HD100535, HD093758, and HD095520, in addition to NINDS NS083998, as well as T32 support to Mr. Wolujewicz (HD060600) and Dr. Steele (ES027801, from NIEHS through the Gulf Coast Consortium). Additional support to Dr. Finnell and Dr. Ross is provided by endowments from the William T. Butler M.D., Distinguished Chair and Nathan Cummings Professorship in Neurology and Neuroscience, respectively.
Footnotes
CONFLICT OF INTEREST
Dr. Finnell formerly held a leadership position in TeratOmic Consulting LLC, a now defunct organization. He also receives travel funds to attend editorial board meetings of the journal Reproductive and Developmental Medicine.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable as no new data were created or analyzed in this review.
REFERENCES
- Abdel Fattah AR, & Ranga A (2020). Nanoparticles as versatile tools for mechanotransduction in tissues and organoids. Frontiers in Bioengineering and Biotechnology, 8, 240. 10.3389/fbioe.2020.00240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahsan H, Hodge SE, Heiman GA, Begg MD, & Susser ES (2002). Relative risk for genetic associations: The case-parent triad as a variant of case-cohort design. International Journal of Epidemiology, 31(3), 669–678. 10.1093/ije/31.3.669 [DOI] [PubMed] [Google Scholar]
- Atta CA, Fiest KM, Frolkis AD, Jette N, Pringsheim T, St Germaine-Smith C, … Metcalfe A (2016). Global birth prevalence of Spina Bifida by folic acid fortification status: A systematic review and meta-analysis. American Journal of Public Health, 106(1), e24–e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avagliano L, Massa V, George TM, Qureshy S, Bulfamante GP, & Finnell RH (2019). Overview on neural tube defects: From development to physical characteristics. Birth Defects Research, 111(19), 1455–1467. 10.1002/bdr2.1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X, Ma D, Liu A, Shen X, Wang QJ, Liu Y, & Jiang Y (2007). Rheb activates mTOR by antagonizing its endogenous inhibitor, FKBP38. Science, 318(5852), 977–980. 10.1126/science.1147379 [DOI] [PubMed] [Google Scholar]
- Barber RC, Lammer EJ, Shaw GM, Greer KA, … Finnell RH (1999). The role of folate transport and metabolism in neural tube defect risk. Molecular Genetics and Metabolism, 66(1), 1–9. 10.1006/mgme.1998.2787 [DOI] [PubMed] [Google Scholar]
- Barber RC, Shaw GM, Lammer EJ, Greer KA, Biela TA, Lacey SW Finnell RH (1998). Lack of association between mutations in the folate receptor-alpha gene and spina bifida. American Journal of Medical Genetics, 76(4), 310–317. [PubMed] [Google Scholar]
- Beaudin AE, & Stover PJ (2007). Folate-mediated one-carbon metabolism and neural tube defects: Balancing genome synthesis and gene expression. Birth Defects Research. Part C, Embryo Today, 81(3), 183–203. 10.1002/bdrc.20100 [DOI] [PubMed] [Google Scholar]
- Beaudin AE, & Stover PJ (2009). Insights into metabolic mechanisms underlying folate-responsive neural tube defects: A minireview. Birth Defects Research. Part A, Clinical and Molecular Teratology, 85(4), 274–284. 10.1002/bdra.20553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck SL (1999). Contributions of dam and conceptus to differences in sensitivity to valproic acid among C57 black and SWV mice. Reproductive Toxicology, 13, 353–360. [DOI] [PubMed] [Google Scholar]
- Beck SL (2001). Does genomic imprinting contribute to valproic acid teratogenicity? Reproductive Toxicology, 15, 43–48. [DOI] [PubMed] [Google Scholar]
- Berry RJ, Li Z, Erickson JD, Li S, Moore CA, Wang H, … Correa A (1999). Prevention of neural-tube defects with folic acid in China. China-U.S. collaborative project for neural tube defect Prevention. New England Journal of Medicine, 341(20), 1485–1490. 10.1056/NEJM199911113412001 [DOI] [PubMed] [Google Scholar]
- Blom HJ, Shaw GM, den Heijer M, & Finnell RH (2006). Neural tube defects and folate: Case far from closed. Nature Reviews. Neuroscience, 7(9), 724–731. 10.1038/nrn1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomba L, Walter K, & Soranzo N (2017). The impact of rare and low-frequency genetic variants in common disease. Genome Biology, 18(1), 77. 10.1186/s13059-017-1212-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brender J, Suarez L, Hendricks K, Baetz RA, & Larsen R (2002). Parental occupation and neural tube defect-affected pregnancies among Mexican Americans. Journal of Occupational and Environmental Medicine, 44(7), 650–656. 10.1097/00043764-200207000-00011 [DOI] [PubMed] [Google Scholar]
- Brender JD, & Suarez L (1990). Paternal occupation and anencephaly. American Journal of Epidemiology, 131(3), 517–521. 10.1093/oxfordjournals.aje.a115526 [DOI] [PubMed] [Google Scholar]
- Bulgakov OV, Eggenschwiler JT, Hong DH, Anderson KV, & Li T (2004). FKBP8 is a negative regulator of mouse sonic hedgehog signaling in neural tissues. Development, 131(9), 2149–2159. 10.1242/dev.01122 [DOI] [PubMed] [Google Scholar]
- Cabrera RM, Souder JP, Steele JW, Yeo L, Tukeman G, Gorelick DA, & Finnell RH (2019). The antagonism of folate receptor by dolutegravir: Developmental toxicity reduction by supplemental folic acid. Aids, 33(13), 1967–1976. 10.1097/QAD.0000000000002289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell LR, Dayton DH, & Sohal GS (1986). Neural tube defects: A review of human and animal studies on the etiology of neural tube defects. Teratology, 34(2), 171–187. 10.1002/tera.1420340206 [DOI] [PubMed] [Google Scholar]
- Canfield MA, Collins JS, Botto LD, Williams LJ, Mai CT, Kirby RS, … National Birth Defects Preventin Network. (2005). Changes in the birth prevalence of selected birth defects after grain fortification with folic acid in the United States: Findings from a multistate population-based study. Birth Defects Research. Part A, Clinical and Molecular Teratology, 73(10), 679–689. 10.1002/bdra.20210 [DOI] [PubMed] [Google Scholar]
- Cao X, Tian T, Steele JW, Cabrera RM, Aguiar-Pulido V, Wadhwa S, … Lei Y (2020). Loss of RAD9B impairs early neural development and contributes to the risk for human spina bifida. Human Mutation, 41(4), 786–799. 10.1002/humu.23969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael SL, & Shaw GM (2000). Maternal life event stress and congenital anomalies. Epidemiology, 11(1), 30–35. 10.1097/00001648-200001000-00008 [DOI] [PubMed] [Google Scholar]
- Carmichael SL, Shaw GM, Selvin S, & Schaffer DM (2003). Diet quality and risk of neural tube defects. Medical Hypotheses, 60(3), 351–355. 10.1016/s0306-9877(02)00402-4 [DOI] [PubMed] [Google Scholar]
- Carter M, Chen X, Slowinska B, Minnerath S, Glickstein S, Shi L, … Ross ME (2005). Crooked tail (Cd) model of human folate-responsive neural tube defects is mutated in Wnt coreceptor lipoprotein receptor-related protein 6. Proceedings of the National Academy of Sciences of the United States of America, 102(36), 12843–12848. 10.1073/pnas.0501963102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter M, Ulrich S, Oofuji Y, Williams DA, & Ross ME (1999). Crooked tail (cd) models human folate-responsive neural tube defects. Human Molecular Genetics, 8(12), 2199–2204. 10.1093/hmg/8.12.2199 [DOI] [PubMed] [Google Scholar]
- Caseras X, Kirov G, Kendall KM, Rees E, Legge SE, Bracher-Smith M, … Murphy K (2021). Effects of genomic copy number variants penetrant for schizophrenia on cortical thickness and surface area in healthy individuals: Analysis of the UK biobank. The British Journal of Psychiatry, 218(2), 104–111. 10.1192/bjp.2020.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Karaplis AC, Ackerman SL, Pogribny IP, Melnyk S, Lussier-Cacan S, … Rozen R (2001). Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Human Molecular Genetics, 10(5), 433–443. 10.1093/hmg/10.5.433 [DOI] [PubMed] [Google Scholar]
- Chen Z, Lei Y, Cao X, Zheng Y, Wang F, Bao Y, … Wang H (2018). Genetic analysis of Wnt/PCP genes in neural tube defects. BMC Medical Genomics, 11(1), 38. 10.1186/s12920-018-0355-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Lei Y, Zheng Y, Aguiar-Pulido V, Ross ME, Peng R, … Wang H (2018). Threshold for neural tube defect risk by accumulated singleton loss-of-function variants. Cell Research, 28(10), 1039–1041. 10.1038/s41422-018-0061-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Scott AJ, Davis JR, Tsang EK, Li X, Kim Y, … Hall IM (2017). The impact of structural variation on human gene expression. Nature Genetics, 49(5), 692–699. 10.1038/ng.3834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho A, Ko HW, & Eggenschwiler JT (2008). FKBP8 cell-autonomously controls neural tube patterning through a Gli2- and Kif3a-dependent mechanism. Developmental Biology, 321(1), 27–39. 10.1016/j.ydbio.2008.05.558 [DOI] [PubMed] [Google Scholar]
- Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, … Murdoch JN (2003). Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Current Biology, 13(13), 1129–1133. 10.1016/s0960-9822(03)00374-9 [DOI] [PubMed] [Google Scholar]
- Czeizel AE, & Dudas I (1992). Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. The New England Journal of Medicine, 327(26), 1832–1835. 10.1056/NEJM199212243272602 [DOI] [PubMed] [Google Scholar]
- De Cicco M, Milroy LG, & Dames SA (2018). Target of rapamycin FATC domain as a general membrane anchor: The FKBP-12 like domain of FKBP38 as a case study. Protein Science, 27(2), 546–560. 10.1002/pro.3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wals P, Tairou F, Van Allen MI, Uh SH, Lowry RB, Sibbald B, … Niyonsenga T (2007). Reduction in neural-tube defects after folic acid fortification in Canada. The New England Journal of Medicine, 357(2), 135–142. 10.1056/NEJMoa067103 [DOI] [PubMed] [Google Scholar]
- Detrait ER, George TM, Etchevers HC, Gilbert JR, Vekemans M, & Speer MC (2005). Human neural tube defects: Developmental biology, epidemiology, and genetics. Neurotoxicology and Teratology, 27(3), 515–524. 10.1016/j.ntt.2004.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhaik E, & Ryan DM (2019). Pair matcher (PaM): Fast model-based optimization of treatment/case-control matches. Bioinformatics, 35(13), 2243–2250. 10.1093/bioinformatics/bty946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig JT, Bult CJ, Kadin JA, Richardson JE, Blake JA, Anagnostopoulos A, … Zhu Y (2005). The mouse genome database (MGD): From genes to mice—A community resource for mouse biology. Nucleic Acids Research, 33, D471–D475. 10.1093/nar/gki113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheridge SL, Ray S, Li S, Hamblet NS, Lijam N, Tsang M, … Wynshaw-Boris A (2008). Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genetics, 4(11), e1000259. 10.1371/journal.pgen.1000259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley TF, Hambidge SJ, & Daley MF (2002). Association of low maternal education with neural tube defects in Colorado, 1989–1998. Public Health, 116(2), 89–94. 10.1038/sj.ph.1900821 [DOI] [PubMed] [Google Scholar]
- Findley TO, Tenpenny JC, O'Byrne MR, Morrison AC, Hixson JE, Northrup H, & Au KS (2017). Mutations in folate transporter genes and risk for human myelomeningocele. American Journal of Medical Genetics. Part A, 173(11), 2973–2984. 10.1002/ajmg.a.38472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnell RH, Bennett GD, Karras SB, & Mohl VK (1988). Common hierarchies of susceptibility to the induction of neural tube defects in mouse embryos by valproic acid and its 4-propyl-4-pentenoic acid metabolite. Teratology, 38(4), 313–320. 10.1002/tera.1420380403 [DOI] [PubMed] [Google Scholar]
- Finnell RH, Gelineau-van Waes J, Bennett GD, Barber RC, Wlodarczyk B, Shaw GM, … Eberwine JH (2000). Genetic basis of susceptibility to environmentally induced neural tube defects. Annals of the new York Academy of Sciences, 919, 261–277. 10.1111/j.1749-6632.2000.tb06886.x [DOI] [PubMed] [Google Scholar]
- Finnell RH, Greer KA, Barber RC, & Piedrahita JA (1998). Neural tube and craniofacial defects with special emphasis on folate pathway genes. Critical Reviews in Oral Biology and Medicine, 9(1), 38–53. 10.1177/10454411980090010201 [DOI] [PubMed] [Google Scholar]
- Finnell RH, Moon SP, Abbott LC, Golden JA, & Chernoff GF (1986). Strain differences in heat-induced neural tube defects in mice. Teratology, 33(2), 247–252. 10.1002/tera.1420330213 [DOI] [PubMed] [Google Scholar]
- Finnell RH, Wlodarczyk BC, Craig JC, Piedrahita JA, & Bennett GD (1997). Strain-dependent alterations in the expression of folate pathway genes following teratogenic exposure to valproic acid in a mouse model. American Journal of Medical Genetics, 70(3), 303–311. [PubMed] [Google Scholar]
- Fong S, Mounkes L, Liu Y, Maibaum M, Alonzo E, Desprez PY, … Debs RJ (2003). Functional identification of distinct sets of antitumor activities mediated by the FKBP gene family. Proceedings of the National Academy of Sciences of the United States of America, 100(24), 14253–14258. 10.1073/pnas.2332307100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea GL, Nychyk O, Mole MA, Moulding D, Savery D, Nikolopoulou E, … Copp AJ (2018). Vangl2 disruption alters the biomechanics of late spinal neurulation leading to spina bifida in mouse embryos. Disease Models & Mechanisms, 11(3), dmm032219. 10.1242/dmm.032219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea GL, Maniou E, Edwards TJ, Marshall AR, Ampartzidis I, Greene N, & Copp AJ (2021). Cell non-autonomy amplifies disruption of neurulation by mosaic Vangl2 deletion in mice. Nature Communications, 12(1), 1159. 10.1038/s41467-021-21372-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2017 Congenital Heart Disease Collaborators. (2020). Global, regional, and national burden of congenital heart disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet. Child & Adolescent Health, 4(1), 185–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JD, Kholmanskikh S, Castaldo BS, Hansler A, Chung H, Klotz B, … Ross ME (2013). LRP6 exerts non-canonical effects on Wnt signaling during neural tube closure. Human Molecular Genetics, 22(21), 4267–4281. 10.1093/hmg/ddt277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JD, Nakouzi G, Slowinska-Castaldo B, Dazard JE, Rao JS, Nadeau JH, & Ross ME (2010). Functional interactions between the LRP6 WNT co-receptor and folate supplementation. Human Molecular Genetics, 19(23), 4560–4572. 10.1093/hmg/ddq384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene ND, Gerrelli D, Van Straaten HW, & Copp AJ (1998). Abnormalities of floor plate, notochord and somite differentiation in the looptail (Lp) mouse: A model of severe neural tube defects. Mechanisms of Development, 73(1), 59–72. 10.1016/s0925-4773(98)00029-x [DOI] [PubMed] [Google Scholar]
- Grove E, Eckardt S, & McLaughlin KJ (2016). High-speed mouse back-crossing through the female germ line. PLoS One, 11(12), e0166822. 10.1371/journal.pone.0166822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MJ, & Juriloff DM (2010). An update to the list of mouse mutants with neural tube closure defects and advances toward a complete genetic perspective of neural tube closure. Birth Defects Research. Part A, Clinical and Molecular Teratology, 88(8), 653–669. 10.1002/bdra.20676 [DOI] [PubMed] [Google Scholar]
- He Z, Zhang D, Renton AE, Li B, Zhao L, Wang GT, … Leal SM (2017). The rare-variant generalized disequilibrium test for association analysis of nuclear and extended pedigrees with application to Alzheimer disease WGS data. American Journal of Human Genetics, 100(2), 193–204. 10.1016/j.ajhg.2016.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert L, Hillman P, Baker C, Brown M, Ashley-Koch A, Hixson JE, … Au KS (2020). Burden of rare deleterious variants in WNT signaling genes among 511 myelomeningocele patients. PLoS One, 15(9), e0239083. 10.1371/journal.pone.0239083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heseker HB, Mason JB, Selhub J, Rosenberg IH, & Jacques PF (2009). Not all cases of neural-tube defect can be prevented by increasing the intake of folic acid. The British Journal of Nutrition, 102(2), 173–180. 10.1017/S0007114508149200 [DOI] [PubMed] [Google Scholar]
- Hillman P, Baker C, Hebert L, Brown M, Hixson J, Ashley-Koch A, … Au KS (2020). Identification of novel candidate risk genes for myelomeningocele within the glucose homeostasis/oxidative stress and folate/one-carbon metabolism networks. Molecular Genetics & Genomic Medicine, 8(11), e1495. 10.1002/mgg3.1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintsanen P, Sevon P, Onkamo P, Eronen L, & Toivonen H (2006). An empirical comparison of case-control and trio based study designs in high throughput association mapping. Journal of Medical Genetics, 43(7), 617–624. 10.1136/jmg.2005.036020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SS, Urban AE, & Mills RE (2020). Structural variation in the sequencing era. Nature Reviews. Genetics, 21(3), 171–189. 10.1038/s41576-019-0180-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, & Wong LY (2001). Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA, 285(23), 2981–2986. 10.1001/jama.285.23.2981 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Epstein MP, & Conneely KN (2013). Assessing the impact of population stratification on association studies of rare variation. Human Heredity, 76(1), 28–35. 10.1159/000353270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorde LB, Fineman RM, & Martin RA (1983). Epidemiology and genetics of neural tube defects: An application of the Utah Genealogical Data Base. American Journal of Physical Anthropology, 62(1), 23–31. 10.1002/ajpa.1330620106 [DOI] [PubMed] [Google Scholar]
- Juriloff DM, Gunn TM, Harris MJ, Mah DG, Wu MK, & Dewell SL (2001). Multifactorial genetics of exencephaly in SELH/Bc mice. Teratology, 64(4), 189–200. 10.1002/tera.1064 [DOI] [PubMed] [Google Scholar]
- Juriloff DM, & Harris MJ (2012). A consideration of the evidence that genetic defects in planar cell polarity contribute to the etiology of human neural tube defects. Birth Defects Research. Part A, Clinical and Molecular Teratology, 94(10), 824–840. 10.1002/bdra.23079 [DOI] [PubMed] [Google Scholar]
- Kibar Z, Torban E, McDearmid JR, Reynolds A, Berghout J, Mathieu M, … Gros P (2007). Mutations in VANGL1 associated with neural-tube defects. New England Journal of Medicine, 356(14), 1432–1437. 10.1056/NEJMoa060651 [DOI] [PubMed] [Google Scholar]
- Korstanje R, Desai J, Lazar G, King B, Rollins J, Spurr M, … Millonig JH (2008). Quantitative trait loci affecting phenotypic variation in the vacuolated lens mouse mutant, a multigenic mouse model of neural tube defects. Physiological Genomics, 35(3), 296–304. 10.1152/physiolgenomics.90260.2008 [DOI] [PubMed] [Google Scholar]
- Lam E, Martin M, & Wiederrecht G (1995). Isolation of a cDNA encoding a novel human FK506-binding protein homolog containing leucine zipper and tetratricopeptide repeat motifs. Gene, 160(2), 297–302. 10.1016/0378-1119(95)00216-s [DOI] [PubMed] [Google Scholar]
- Lancaster MA, & Knoblich JA (2014). Generation of cerebral organoids from human pluripotent stem cells. Nature Protocols, 9(10), 2329–2340. 10.1038/nprot.2014.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrance AK, Racine J, Deng L, Wang X, Lachapelle P, & Rozen R (2011). Complete deficiency of methylenetetrahydrofolate reductase in mice is associated with impaired retinal function and variable mor-tality, hematological profiles, and reproductive outcomes. Journal of Inherited Metabolic Disease, 34(1), 147–157. 10.1007/s10545-010-9127-1 [DOI] [PubMed] [Google Scholar]
- Lee S, & Gleeson JG (2020). Closing in on Mechanisms of Open Neural Tube Defects. Trends in Neurosciences, 43(7), 519–532. 10.1016/j.tins.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Kim SE, Chen Z, Cao X, Zhu H, Yang W, … Finnell RH (2019). Variants identified in PTK7 associated with neural tube defects. Molecular Genetics & Genomic Medicine, 7(4), e00584. 10.1002/mgg3.584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemay P, Guyot MC, Tremblay É, Dionne-Laporte A, Spiegelman D, Henrion É, … Kibar Z (2015). Loss-of-function de novo mutations play an important role in severe human neural tube defects. Journal of Medical Genetics, 52(7), 493–497. 10.1136/jmedgenet-2015-103027 [DOI] [PubMed] [Google Scholar]
- Leduc RY, Singh P, & McDermid HE (2017). Genetic backgrounds and modifier genes of NTD mouse models: An opportunity for greater understanding of the multifactorial etiology of neural tube defects. Birth Defects Research, 109(2), 140–152. 10.1002/bdra.23554 [DOI] [PubMed] [Google Scholar]
- Li D, Pickell L, Liu Y, Wu Q, Cohn JS, & Rozen R (2005). Maternal methylenetetrahydrofolate reductase deficiency and low dietary folate lead to adverse reproductive outcomes and congenital heart defects in mice. American Journal of Clinical Nutrition, 82(1), 188–195. 10.1093/ajcn.82.1.188 [DOI] [PubMed] [Google Scholar]
- Lindhurst MJ, Sapp JC, Teer JK, Johnston JJ, Finn EM, Peters K, … Biesecker LG (2011). A mosaic activating mutation in AKT1 associated with the Proteus syndrome. New England Journal of Medicine, 365(7), 611–619. 10.1056/NEJMoa1104017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Huang J, Zhang L, Chen J, Zeng Y, Tang Y, & Liu Z (2019). Advances in cerebral organoid systems and their application in disease modeling. Neuroscience, 399, 28–38. 10.1016/j.neuroscience.2018.12.013 [DOI] [PubMed] [Google Scholar]
- Loeken MR (2020). Mechanisms of congenital malformations in pregnancies with pre-existing diabetes. Current Diabetes Reports, 20(10), 54. 10.1007/s11892-020-01338-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Escobar B, Wlodarczyk BJ, Caro-Vega J, Lin Y, Finnell RH, & Ybot-Gonzalez P (2019). The interaction of maternal diabetes with mutations that affect folate metabolism and how they affect the development of neural tube defects in mice. Developmental Dynamics, 248(10), 900–917. 10.1002/dvdy.92 [DOI] [PubMed] [Google Scholar]
- Lundberg YW, Cabrera RM, Greer KA, Zhao J, Garg R, & Finnell RH (2004). Mapping a chromosomal locus for valproic acid-induced exencephaly in mice. Mammalian Genome, 15(5), 361–369. 10.1007/s00335-004-2345-9 [DOI] [PubMed] [Google Scholar]
- Lundberg YW, Wing MJ, Xiong W, Zhao J, & Finnell RH (2003). Genetic dissection of hyperthermia-induced neural tube defects in mice. Birth Defects Research. Part A, Clinical and Molecular Teratology, 67(6), 409–413. 10.1002/bdra.10044 [DOI] [PubMed] [Google Scholar]
- Ma S, & Shi G (2020). On rare variants in principal component analysis of population stratification. BMC Genetics, 21(1), 34. 10.1186/s12863-020-0833-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieson I, & McVean G (2012). Differential confounding of rare and common variants in spatially structured populations. Nature Genetics, 44(3), 243–246. 10.1038/ng.1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt A, Eberle D, Tazaki A, Ranga A, Niesche M, Wilsch-Brauninger M, … Tanaka EM (2014). 3D reconstitution of the patterned neural tube from embryonic stem cells. Stem Cell Reports, 3(6), 987–999. 10.1016/j.stemcr.2014.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RK, & McCrea PD (2010). Wnt to build a tube: Contributions of Wnt signaling to epithelial tubulogenesis. Developmental Dynamics, 239(1), 77–93. 10.1002/dvdy.22059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MRC Vitamin Study Group. (1991). Prevention of neural tube defects: Results of the Medical Research Council vitamin study. Lancet, 338(8760), 131–137. [PubMed] [Google Scholar]
- Muotri AR, Chu VT, Marchetto MC, Deng W, Moran JV, & Gage FH (2005). Somatic mosaicism in neuronal precursor cells mediated by L1 retrotransposition. Nature, 435(7044), 903–910. 10.1038/nature03663 [DOI] [PubMed] [Google Scholar]
- O'Rahilly R, & Müller F (2002). The two sites of fusion of the neural folds and the two neuropores in the human embryo. Teratology, 65(4), 162–170. 10.1002/tera.10007 [DOI] [PubMed] [Google Scholar]
- Panamonta V, Pradubwong S, Panamonta M, & Chowchuen B (2015). Global birth prevalence of orofacial clefts: A systematic review. Journal of the Medical Association of Thailand, 98, S11–S21. [PubMed] [Google Scholar]
- Pangilinan F, Finlay EK, Molloy AM, Abaan HO, Shane B, Mills JL, … Parle-McDermott A (2021). A dihydrofolate reductase 2 (DHFR2) variant is associated with risk of neural tube defects in an Irish cohort but not in a United Kingdom cohort. American Journal of Medical Genetics. Part A, 185(4), 1307–1311. 10.1002/ajmg.a.62090 [DOI] [PubMed] [Google Scholar]
- Park JH, Gail MH, Weinberg CR, Carroll RJ, Chung CC, Wang Z, … Chatterjee N (2011). Distribution of allele frequencies and effect sizes and their interrelationships for common genetic susceptibility variants. Proceedings of the National Academy of Sciences of the United States of America, 108(44), 18026–18031. 10.1073/pnas.1114759108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides JM, Zhu Z, Gratten J, McRae AF, Wray NR, & Yang J (2016). Predicting gene targets from integrative analyses of summary data from GWAS and eQTL studies for 28 human complex traits. Genome Medicine, 8(1), 84. 10.1186/s13073-016-0338-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovski S, Todd JL, Durheim MT, Wang Q, Chien JW, Kelly FL, … Goldstein DB (2017). An exome sequencing study to assess the role of rare genetic variation in pulmonary fibrosis. American Journal of Respiratory and Critical Care Medicine, 196(1), 82–93. 10.1164/rccm.201610-2088OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedrahita JA, Oetama B, Bennett GD, van Waes J, Kamen BA, Richardson J, … Finnell RH (1999). Mice lacking the folic acid-binding protein Folbp1 are defective in early embryonic development. Nature Genetics, 23(2), 228–232. 10.1038/13861 [DOI] [PubMed] [Google Scholar]
- Poduri A, Evrony GD, Cai X, Elhosary PC, Beroukhim R, Lehtinen MK, … Walsh CA (2012). Somatic activation of AKT3 causes hemispheric developmental brain malformations. Neuron, 74(1), 41–48. 10.1016/j.neuron.2012.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha PP, Scholze M, Bleiss W, & Schrewe H (2010). Med12 is essential for early mouse development and for canonical Wnt and Wnt/PCP signaling. Development, 137(16), 2723–2731. 10.1242/dev.053660 [DOI] [PubMed] [Google Scholar]
- Rosner M, Hofer K, Kubista M, & Hengstschläger M (2003). Cell size regulation by the human TSC tumor suppressor proteins depends on PI3K and FKBP38. Oncogene, 22(31), 4786–4798. 10.1038/sj.onc.1206776 [DOI] [PubMed] [Google Scholar]
- Ross ME (2010). Gene-environment interactions, folate metabolism and the embryonic nervous system. Wiley Interdisciplinary Reviews. Systems Biology and Medicine, 2(4), 471–480. 10.1002/wsbm.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross ME, Mason CE, & Finnell RH (2017). Genomic approaches to the assessment of human spina bifida risk. Birth Defects Research, 109(2), 120–128. 10.1002/bdra.23592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Leclerc D, Wu Q, Gupta S, Kruger WD, & Rozen R (2008). Valproic acid increases expression of methylenetetrahydrofolate reductase (MTHFR) and induces lower teratogenicity in MTHFR deficiency. Journal of Cellular Biochemistry, 105(2), 467–476. 10.1002/jcb.21847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runnels LW, & Komiya Y (2020). TRPM6 and TRPM7: Novel players in cell intercalation during vertebrate embryonic development. Developmental Dynamics, 249(8), 912–923. 10.1002/dvdy.182 [DOI] [PubMed] [Google Scholar]
- Sever LE (1995). Looking for causes of neural tube defects: Where does the environment fit in? Environmental Health Perspectives, 103, 165–171. 10.1289/ehp.95103s6165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw GM, Jensvold NG, Wasserman CR, & Lammer EJ (1994). Epidemiologic characteristics of phenotypically distinct neural tube defects among 0.7 million California births, 1983–1987. Teratology, 49(2), 143–149. 10.1002/tera.1420490210 [DOI] [PubMed] [Google Scholar]
- Shaw GM, Todoroff K, Finnell RH, Lammer EJ, Leclerc D, Gravel RA, & Rozen R (1999). Infant methionine synthase variants and risk for spina bifida. Journal of Medical Genetics, 36(1), 86–87. [PMC free article] [PubMed] [Google Scholar]
- Shaw GM, Todoroff K, Velie EM, & Lammer EJ (1998). Maternal illness, including fever and medication use as risk factors for neural tube defects. Teratology, 57(1), 1–7. [DOI] [PubMed] [Google Scholar]
- Shaw GM, Velie EM, & Schaffer D (1996). Risk of neural tube defect-affected pregnancies among obese women. JAMA, 275(14), 1093–1096. 10.1001/jama.1996.03530380035028 [DOI] [PubMed] [Google Scholar]
- Shaw GM, Wasserman CR, O'Malley CD, Nelson V, & Jackson RJ (1999). Maternal pesticide exposure from multiple sources and selected congenital anomalies. Epidemiology, 10(1), 60–66. [PubMed] [Google Scholar]
- Shields DC, Kirke PN, Mills JL, Ramsbottom D, Molloy AM, Burke H, … Whitehead AS (1999). The “thermolabile” variant of methylenetetrahydrofolate reductase and neural tube defects: An evaluation of genetic risk and the relative importance of the genotypes of the embryo and the mother. American Journal of Human Genetics, 64(4), 1045–1055. 10.1086/302310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirane M, & Nakayama KI (2003). Inherent calcineurin inhibitor FKBP38 targets Bcl-2 to mitochondria and inhibits apoptosis. Nature Cell Biology, 5(1), 28–37. 10.1038/ncb894 [DOI] [PubMed] [Google Scholar]
- Shirane M, & Nakayama KI (2004). Immunophilin FKBP38, an inherent inhibitor of calcineurin, targets Bcl-2 to mitochondria and inhibits apoptosis. Nihon Rinsho, 62(2), 405–412. [PubMed] [Google Scholar]
- Shirley MD, Tang H, Gallione CJ, Baugher JD, Frelin LP, Cohen B, … Pevsner J (2013). Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. New England Journal of Medicine, 368(21), 1971–1979. 10.1056/NEJMoa1213507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelstein O, Cabrera RM, Bozinov D, Wlodarczyk B, & Finnell RH (2004). Folate-regulated changes in gene expression in the anterior neural tube of folate binding protein-1 (Folbp1)-deficient murine embryos. Neurochemical Research, 29(6), 1105–1112. 10.1023/b:nere.0000023597.37698.13 [DOI] [PubMed] [Google Scholar]
- Steele JW, Bayliss S, Bayliss J, Lin YL, Wlodarczyk BJ, Cabrera RM, … George TM (2019). Heritable Spina bifida in sheep: A potential model for fetal repair of Myelomeningocele. Journal of Pediatric Surgery, 55(3), 475–481. 10.1016/j.jpedsurg.2019.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez L, Cardarelli K, & Hendricks K (2003). Maternal stress, social support, and risk of neural tube defects among Mexican Americans. Epidemiology, 14(5), 612–616. 10.1097/01.ede.0000073270.39780.e9 [DOI] [PubMed] [Google Scholar]
- Tian T, Cao X, Chen Y, Jin L, Li Z, Han X, … Lei Y (2021). Somatic and de novo germline variants of MEDs in human neural tube defects. Frontiers in Cell and Development Biology, 9, 641831. 10.3389/fcell.2021.641831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T, Cao X, Kim SE, Lin YL, Steele JW, Cabrera RM, … Lei Y (2020). FKBP8 variants are risk factors for spina bifida. Human Molecular Genetics, 29(18), 3132–3144. 10.1093/hmg/ddaa211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurubuchi T, Allender EV, Siddiqui MR, Shim KW, Ichi S, Boshnjaku V, … Mayanil CS (2014). A critical role of noggin in developing folate-nonresponsive NTD in Fkbp8 −/− embryos. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery, 30(8), 1343–1353. 10.1007/s00381-014-2428-1 [DOI] [PubMed] [Google Scholar]
- Tian T, Lei Y, Chen Y, Guo Y, Jin L, Finnell RH, … Ren A (2020).Rare copy number variations of planar cell polarity genes are associated with human neural tube defects. Neurogenetics, 21(3), 217–225. 10.1007/s10048-020-00613-6 [DOI] [PubMed] [Google Scholar]
- Trembath D, Sherbondy AL, Vandyke DC, Shaw GM, Todoroff K, Lammer EJ, … Murray JC (1999). Analysis of select folate pathway genes, PAX3, and human T in a Midwestern neural tube defect population. Teratology, 59(5), 331–341. [DOI] [PubMed] [Google Scholar]
- Verhoef M, Barf HA, Post MW, van Asbeck FW, Gooskens RH, & Prevo AJ (2004). Secondary impairments in young adults with spina bifida. Developmental Medicine and Child Neurology, 46(6), 420–427. 10.1017/s0012162204000684 [DOI] [PubMed] [Google Scholar]
- Walker VE, Atanasiu R, Lam H, & Shrier A (2007). Co-chaperone FKBP38 promotes HERG trafficking. Journal of Biological Chemistry, 282(32), 23509–23516. 10.1074/jbc.M701006200 [DOI] [PubMed] [Google Scholar]
- Waller DK, Shaw GM, Rasmussen SA, Hobbs CA, Canfield MA, Siega-Riz AM, … National Birth Defects Prevention, S. (2007). Pre-pregnancy obesity as a risk factor for structural birth defects. Archives of Pediatrics & Adolescent Medicine, 161(8), 745–750. 10.1001/archpedi.161.8.745 [DOI] [PubMed] [Google Scholar]
- Wallingford JB (2012). Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Annual Review of Cell and Developmental Biology, 28, 627–653. 10.1146/annurev-cellbio-092910-154208 [DOI] [PubMed] [Google Scholar]
- Wallingford JB, & Harland RM (2001). Xenopus Dishevelled signaling regulates both neural and mesodermal convergent extension: Parallel forces elongating the body axis. Development, 128(13), 2581–2592. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, & Harland RM (2002). Neural tube closure requires Dishevelled-dependent convergent extension of the midline. Development, 129(24), 5815–5825. 10.1242/dev.00123 [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Niswander LA, Shaw GM, & Finnell RH (2013). The continuing challenge of understanding, preventing, and treating neural tube defects. Science, 339(6123), 1222002. 10.1126/science.1222002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, … Wynshaw-Boris A (2006). Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development, 133(9), 1767–1778. 10.1242/dev.02347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Marco P, Capra V, & Kibar Z (2019). Update on the role of the non-canonical Wnt/planar cell polarity pathway in neural tube defects. Cells, 8(10), 1198. 10.3390/cells8101198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Venable J, LaPointe P, Hutt DM, Koulov AV, Coppinger J, … Balch WE (2006). Hsp90 cochaperone Aha1 down-regulation rescues misfolding of CFTR in cystic fibrosis. Cell, 127(4), 803–815. 10.1016/j.cell.2006.09.043 [DOI] [PubMed] [Google Scholar]
- Wang Y, Guo N, & Nathans J (2006). The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. The Journal of Neuroscience, 26(8), 2147–2156. 10.1523/jneurosci.4698-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman CR, Shaw GM, Selvin S, Gould JB, & Syme SL (1998). Socioeconomic status, neighborhood social conditions, and neural tube defects. American Journal of Public Health, 88(11), 1674–1680. 10.2105/ajph.88.11.1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, & Spiegel AM (1991). Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. New England Journal of Medicine, 325(24), 1688–1695. 10.1056/nejm199112123252403 [DOI] [PubMed] [Google Scholar]
- White FM, Cohen FG, Sherman G, & McCurdy R (1988). Chemicals, birth defects and stillbirths in New Brunswick: Associations with agricultural activity. CMAJ, 138(2), 117–124. [PMC free article] [PubMed] [Google Scholar]
- Williams J, Mai CT, Mulinare J, Isenburg J, Flood TJ, Ethen M, … Centers for Disease Control and Prevention. (2015). Updated estimates of neural tube defects prevented by mandatory folic acid fortification – United States, 1995–2011. Morbidity and Mortality Weekly Report, 64(1), 1–5. [PMC free article] [PubMed] [Google Scholar]
- Williams LJ, Mai CT, Edmonds LD, Shaw GM, Kirby RS, Hobbs CA, … Levitt M (2002). Prevalence of spina bifida and anencephaly during the transition to mandatory folic acid fortification in the United States. Teratology, 66(1), 33–39. 10.1002/tera.10060 [DOI] [PubMed] [Google Scholar]
- Wolujewicz P, Aguiar-Pulido V, AbdelAleem A, Nair V, Thareja G, Suhre K, … Ross ME (2021). Genome-wide investigation identifies a rare copy-number variant burden associated with human spina bifida. Genetics in Medicine, 23, 1211–1218. 10.1038/s41436-021-01126-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RL, Wlodarczyk BJ, Min KS, Scott ML, Kartiko S, Yu W, … Finnell RH (2008). Mouse Fkbp8 activity is required to inhibit cell death and establish dorso-ventral patterning in the posterior neural tube. Human Molecular Genetics, 17(4), 587–601. 10.1093/hmg/ddm333 [DOI] [PubMed] [Google Scholar]
- Wu Y, Peng S, Finnell RH, & Zheng Y (2021). Organoids as a new model system to study neural tube defects. FASEB Journal, 35(4), e21545. 10.1096/fj.202002348R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Savery D, Gerrelli D, Signore M, Mitchell CE, Faux CH, … Copp AJ (2007). Convergent extension, planar-cell-polarity signalling and initiation of mouse neural tube closure. Development, 134(4), 789–799. 10.1242/dev.000380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Huang CT, Chen J, Pankratz MT, Xi J, Li J, … Zhang SC (2010). Pax6 is a human neuroectoderm cell fate determinant. Cell Stem Cell, 7(1), 90–100. 10.1016/j.stem.2010.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Wang F, Yang X, Wang H, Niswander L, Zhang T, & Li H (2020). Association between rare variants in specific functional pathways and human neural tube defects multiple subphenotypes. Neural Development, 15(1), 8. 10.1186/s13064-020-00145-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable as no new data were created or analyzed in this review.