Abstract
Adrenal myelolipomas are benign, lipomatous tumours with elements of myeloid cells, most of which present as adrenal incidentalomas and comprise 3.3–6.5% of all adrenal masses. Adrenal myelolipomas are usually unilateral (95%), variable in size, most often found in midlife, and affect both sexes almost equally. On imaging, adrenal myelolipomas show pathognomonic imaging features consistent with the presence of macroscopic fat. Large adrenal myelolipomas may cause symptoms of mass effect, and occasionally be complicated by haemorrhage. In the event of a concomitant adrenal cortical adenoma or hyperplasia, adrenal hormone excess may be detected in patients with adrenal myelolipoma. Patients with congenital adrenal hyperplasia demonstrate a higher prevalence of adrenal myelolipomas, and are at risk to develop large and bilateral lesions.
Introduction
Myelolipomas were first described in 1905 as an adrenal tumour composed of mature fat mixed with myeloid and erythroid cells.1 Adrenal myelolipoma is the second most common benign tumour in the adrenals following after adrenocortical adenoma.2,3 On imaging, adrenal myelolipomas appear as rounded tumours containing macroscopic fat and varying amounts of myeloid components.4 The majority of myelolipomas are indolent slow growing tumours, but may occasionally cause pressure symptoms.5,6 Seldomly, the patients may present to the emergency room due to haemorrhage or rupture of the myelolipoma.
Despite being so common, the entity is still unfamiliar for many physicians, and there is therefore a need to make the diagnosis better known. This review discusses the adrenal myelolipoma pathogenesis, epidemiology, clinical presentation, association with congenital adrenal hyperplasia (CAH), histology, differential diagnosis, radiological features, management and outcome, and additionally addresses clinically important questions on follow up and gaps of knowledge.
Pathogenesis
Adrenal myelolipomas consist of adipose tissue and haematopoietic cells arising in patients with a healthy bone marrow. This extra-medullary haematopoiesis is physiologic in the foetus until the bone marrow matures. In the foetus, erythropoietin receptors are expressed in the adrenal cortex.7 Extra-medullary haematopoiesis also occurs in adult life secondary to defective haemoglobin synthesis.8 In rare instances, patients with adrenal myelolipoma may have a concomitant hematologic disease, such as thalassemia.9 In patients with chronic anaemia, elevated erythropoietin may stimulate the development of adrenal myelolipoma, similarly to the reported bilateral large myelolipomas secondary to erythropoietin administration.10
The bulk of scientific data point towards an association between elevated adrenocorticotropic hormone (ACTH) concentrations and the increased risk of developing adrenal myelolipomas, as these lesions are frequently reported in patients with Cushing disease or CAH.11–13 Although the levels of ACTH can be very high in primary adrenal insufficiency such as Addison’s disease, adrenal myelolipomas have not been reported in these patients. In Thomas Addison’s original paper from 1855, one of the eleven patients had tuberculosis deposits to the adrenals, but also “opaque matter exhibited a copious amount of fatty matters, but no nucleated cells”.14 This may have been a first description of adrenal myelolipoma driven by the elevated ACTH in a patient with primary adrenal insufficiency. Sufficient amounts of remaining adrenocortical tissue in patients with autoimmune Addison`s disease could make them susceptible to developing myelolipomas. However, autoimmune Addison`s disease causes atrophy and fibrotic derangement of the adrenal glands, which may explain why adrenal myelolipomas have not been reported in this setting, despite chronically elevated ACTH.
Interestingly, pituitary extracts injected in rat adrenals gave rise to a myeloid phenotype, thereby possibly arguing that ACTH could influence the development of myelolipomas.15 Even so, the few available ACTH receptor expression studies in adrenal myelolipomas are not consistent, with some reporting overexpression and others a complete lack of ACTH receptor immunoreactivity.16–18 However, the majority of patients with myelolipoma have normal levels of ACTH, indicating other drivers.
Other postulated mechanisms are metaplasia of reticuloendothelial cells in the adrenal glands due to stress, infection or trauma, or via embolism of bone marrow cells.19 It has also been speculated that myelolipomas result from adrenocortical adipocytes derived from mesenchymal stem cells and circulating bone marrow released and recruited by granulocyte stimulating factor.20
Molecular and genetic characteristics
Apart from the potential physiological association of ACTH and erythropoietin with myelolipoma development, little is known about the underlying molecular events driving the formation of an adrenal myelolipoma. To our knowledge, next-generation DNA sequencing studies on these tumours have yet to be published, and hence the bulk of genetic information are based on targeted gene sequencing analyses. The first somatic genetic aberrancy associated to adrenal myelolipoma was the finding of a balanced translocation between chromosomes 3q25 and 21p11 in a single case, thereby providing the earliest genetic evidence for a tumoral origin.21 Other mechanism could be non-random X chromosome inactivation in haematopoietic and fat cells, which could suggest a clonal origin.22 Moreover, initial molecular studies involved the investigation of potential Multiple Endocrine Neoplasia type 1 (MEN1) gene dysregulation, a theory based on the fact that a subset of adrenal myelolipomas could have hormonal activity, and were also reported in conjunction to other endocrine tumours in the same patient.23–26 However, no MEN1 gene mutations or MEN1 loss of heterozygosity were noted.27 In addition, the micro-RNA (miR) landscape of adrenal myelolipoma was recently characterized, in which a myelolipoma-specific miRNA signature was found when compared to adrenocortical tumours.28 Specifically, hsa-miR-451a, hsa-miR-486–5p, hsa-miR-363–3p, and hsa-miR-150–5p were overexpressed in myelolipomas, of which has-mIR-451a overexpression was also reproduced in plasma of patients with myelolipomas. The observed miRNA dysregulation indicates that one or several of the aberrantly expressed miRs could influence tumour development through translational regulation of various messenger RNAs (mRNAs).28 These biomarkers need further validation and may be useful in making non-invasive diagnosis of adrenal myelolipoma.
Histopathological aspects
Adrenal myelolipomas display a characteristic gross appearance, with a yellowish to brownish red cut surface representing adipose tissue with scattered areas of haemorrhage reflecting the dispersed marrow tissue. Histologically, adrenal myelolipomas are well circumscribed and heterogenous, with variable amount of mature adipose tissue admixed with an extramedullary trilinear haematopoiesis with full maturation (Figure 1). The pathologist should thus be able to identify megakaryocytes as markers of thrombocytopoesis, various erythroid cells as evidence of erythropoiesis and granulocytic cells as part of the myeloid lineage. Occasionally, areas of degeneration (haemorrhage and calcifications) are seen. Immunohistochemical verification of the diagnosis is usually not required as the histological appearance is straightforward on routine haematoxylin-eosin staining, but rare differentials with lipomatous appearances of clinical importance, such as liposarcoma, myxoid liposarcoma and myofibrosarcoma should not be overlooked.29–31 In these instances, the identification of high-grade nuclear changes and increased mitotic activity are clues to the diagnosis, although well-differentiated liposarcomas may be devoid of lipoblasts and only show adipocytes with scarce amounts of nuclear atypia. As immunohistochemical analyses are of little value in differentiating between sarcomas and myelolipomas, molecular testing for MDM2/CDK4 amplifications (liposarcomas) or CHOP gene fusions (myxoid liposarcomas) could be considered.32–35 Other fatty adrenal tumours such as lipoma, teratoma and angiomyolipoma are also potential confounders, but display favourable prognosis and are not alarming in the same sense as sarcomas, if misclassified.29,36 Of note, angiomyolipoma is a triphasic tumour consisting of dysmorphic blood vessels, smooth muscle elements admixed with mature adipose tissue, and positive smooth muscle actin (SMA) and in these cases desmin immunostainings could be of help in pinpointing the diagnosis.29 Moreover, subsets of adrenocortical tumours can also exhibit myelolipomatous change, and should not be confused with bona fide adrenal myelolipomas.37,38
Figure 1.
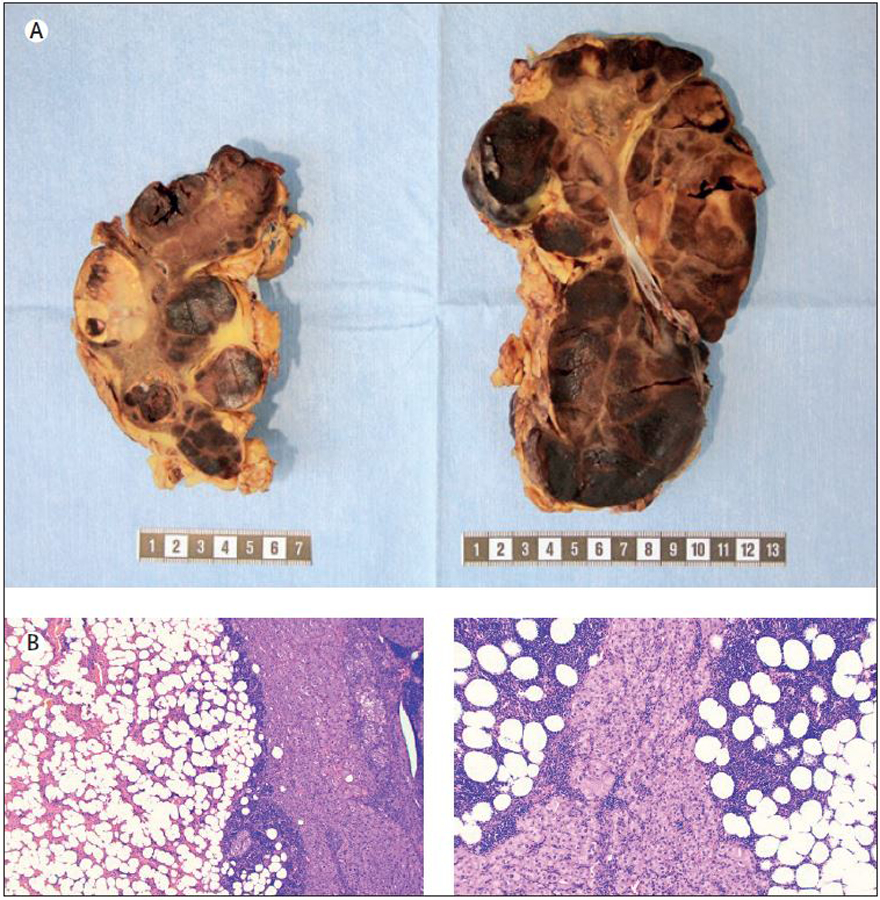
Macroscopic and histological attributes of a bilateral adrenalectomy specimen in a patient with congenital adrenal hyperplasia. Top row: Gross image of the resected adrenals displaying multiple foci of adrenal myelolipoma (dark brownish-red areas) intermingled with hyperplastic adrenal cortical tissue (bright yellow). Metric ruler is depicted for size estimations. Bottom row: Representative photomicrographs of haematoxylin-eosin stained sections from each adrenal specimen (magnified x40 and x100 respectively) displaying myelolipoma and hyperplastic cortical tissue.
Epidemiology
The majority of adrenal myelolipomas are found incidentally and comprise 3.3–3.6% of all adrenal tumours in a population, and up to 6–6.5% of all adrenal tumours seen in the endocrine clinics.2,3,13,39–41 In the adrenalectomy cohorts, the prevalence of myelolipomas was 4–10% overall, and represented 15–20% in tumours >4cm (Table 1).3,13,29,42–51 Prevalence of adrenal myelolipoma in patients undergoing computed tomography (CT) was 0.24%,6 and in the general population at age 40 the prevalence has been estimated to be 0.32%.11 Notably, as discussed in detail below, patients with CAH present with a much higher prevalence of adrenal myelolipomas of 8.6%.11
Table 1.
Epidemiology and clinical presentation of patients with adrenal myelolipomas.
| Population-based or nationwide cohorts2,3 | Endocrine clinic and radiology referral cohort13,39–41 | Adrenalectomy cohort3,13,29,42–51 | Congenital adrenal hyperplasia11 | |
|---|---|---|---|---|
| Proportion of all adrenal tumours | 3.3–3.6% | 1.8–6.5% | 4–10% of all adrenal tumours 15–20% of large adrenal tumours > 4 cm |
25.4% (all CAH) 36.6% (genetically verified CAH) |
| Proportion of benign adrenal tumours | 3.7% | - | - | Same as above |
| Median age at diagnosis, years | - | 60–65 | 50–55 | 44 |
| Female sex | - | 45% | 35–75% | 35.7% |
|
Mode of discovery:
Incidental Symptoms of mass effect Other |
95% 0% 5% |
86% 5% 9% |
35–70% 20–50% 2–5% |
Almost all occur due to poor hormonal control with either incidental discovery, or based on symptoms of mass effect |
| Median tumour size at diagnosis | - | 2 – 4 cm | 5–7 cm | 10.2 cm |
|
Bilateral
Unilateral |
- | 5% 95% |
5–10% 90–95% |
59.6% 40.4% |
CAH, congenital adrenal hyperplasia.
Clinical presentation
Adrenal myelolipomas are usually diagnosed in adults, at a median age of 55–65 years, and affect both sexes almost equally (Table 1).2,3,6,13,42,47,49 Most adrenal myelolipomas (85–90%) are incidentalomas, diagnosed on imaging performed for reasons other than adrenal disease, or during cancer staging (5–10%).2,6,13,39,46 Symptoms of adrenal mass effect with abdominal discomfort leads to eventual discovery of a myelolipoma in 5% of cases.13,47 Rarely, symptoms of overt hormone excess, due to concomitant primary aldosteronism or Cushing syndrome, can lead to a discovery of an adrenal myelolipoma along with an adrenal cortical adenoma.13 Notably, 6% of patients with adrenal myelolipomas demonstrate a concomitant functioning or non-functioning adrenal cortical adenoma.13 Only a minority of patients with adrenal myelolipomas undergo work up with dexamethasone suppression test, thus the true prevalence of autonomous cortisol secretion in these patients is unknown.
At the time of initial diagnosis, patients usually present with a unilateral adrenal myelolipoma (95%) with a median tumour size of 2–2.5 cm, however, the size ranges widely, between 0.5 and >10 cm.3,6,47 In comparison to patients with smaller tumours, those with large adrenal myelolipomas (>6) cm are more likely to present with bilateral disease (21% vs 3%) and report symptoms of mass effect (32% vs 0%).13 Symptoms of mass effect include abdominal, back and flank pain and positional shortness of breath.13 In addition, acute haemorrhage necessitating surgery was reported only in patients with large adrenal myelolipomas, occurring in 6.8% of cases.13 Rupture of adrenal myelolipoma is exceedingly rare, described to occur mainly in tumours >8–10 cm.52–54
Association with congenital adrenal hyperplasia
CAH is a group of autosomal recessive disorders affecting the steroid synthesis in the adrenal cortex.55 As a response to this hormone deficiency, an increased ACTH secretion from the pituitary gland ensues due to a reduction in negative feedback. CAH can be classified as classic, including the salt-wasting and simple virilizing phenotype, and the non-classic (NC) phenotype.56 Since the introduction of neonatal screening of CAH in many countries during the last few decades, almost all classic cases are diagnosed in the neonatal period.57,58 The NC phenotype has 20–70% residual enzyme activity and therefore has less symptoms and signs than classic CAH.59 Since NCCAH often is not picked up by neonatal screening, most patients, especially males, are probably never diagnosed.59
ACTH as an adrenal growth stimulating factor, may result in large adrenals (adrenal cortical hyperplasia) in untreated or poorly managed patients with CAH. Whether continuous high levels of ACTH also can cause tumour growth is less clear. In a recent meta-analysis of adrenal tumours in patients with CAH, 29.3% of patients were affected, and when only patients with genetically confirmed CAH were included, the prevalence was 23.6% (Table 1).11 Moreover, in another meta-analysis undiagnosed CAH was the cause of adrenal incidentalomas in 5.9% of patients when only biochemical diagnosis with 17-hydroxyprogesterone (17OHP) was made, and in 0.8% if genetic confirmation had been performed.62 When analysing already diagnosed CAH cases with subsequent adrenal tumours, 25.4% harboured adrenal myelolipomas which increased to 36.6% when only studies with genetically confirmed CAH were included (Table 1).11 Of the patients with CAH and concomitant adrenal myelolipoma, 46% were asymptomatic and 33% experienced abdominal or flank pain.11 Patients with CAH and adrenal myelolipomas were older than those with CAH and a non-myelolipoma adrenal tumour (44 vs 33 years), the adrenal tumours were larger (10.2 vs 1 cm) and were more frequently bilateral (59.6% vs 10.7%). Those with adrenal myelolipomas had been diagnosed late with CAH or had been poorly managed in the vast majority (93.5%),11 further indicating that prolonged periods of increased ACTH play a role in the development of adrenal myelolipomas.
In a review of literature that included 440 patients with adrenal myelolipomas, 10% had concomitant CAH.9 Whether CAH should be screened for with 17OHP in all cases with a myelolipoma is, however, unclear. The Endocrine Society Guidelines do not recommend routine screening for adrenal masses, including myelolipomas, in CAH.57
Association with adrenal hormone excess
In a single centre study of 126 patients with adrenal myelolipomas who were evaluated for adrenal hormone excess, autonomous cortisol secretion and primary aldosteronism was diagnosed in 3% and 12%, respectively.13 In another study of 65 patients with a surgically treated adrenal myelolipoma, adrenal hormone excess was found in 4.6%, although the diagnostic work up was incompletely described.47 Another study of 150 patients with adrenal myelolipoma, in which only 20 patients were tested for endocrine dysfunction, reported 3 patients with autonomous cortisol secretion and one patient with primary aldosteronism.6 A small case series of concomitant aldosterone, cortisol, or androgen excess in patients with myelolipomas have demonstrated adrenal cortical hyperplasia on pathology.54 Adrenal myelolipoma was also reported in Carney complex.63,64 Other very rare reported coincidental associations with adrenal myelolipoma included an aldosterone secreting adrenocortical carcinoma,65 pheochromocytoma66,67 and also adrenal medullary hyperplasia.68
Association with other disorders
Adrenal myelolipomas associated with haematologic diseases have sometimes been reported, and these patients often have chronic anaemia. Patients with various forms of thalassemias and myelolipomas have been described,69 but also hereditary spherocytosis,70,71 and sickle cell disease.72 In 27 patients with thalassemia, sickle cell anaemia or myelofibrosis five myelolipomas were found (19%).73 Lin et al. found 9% (2/23) patients with plasma cell myeloma within myelolipomas.74 These adrenal myelolipomas contained huge aggregates of dysplastic plasma cells in individuals who previously did not have a myeloma diagnosis.
DIAGNOSIS OF ADRENAL MYELOLIPOMA
Imaging of myelolipomas
The typical CT/MRI appearance of an adrenal myelolipoma is a rounded tumour mainly comprising macroscopic fat (Figure 2A),4 but with also a higher attenuating myeloid component, either appearing as a cloudy pattern (Figure 2B), as “solid strands” (Figure 2C), or forming a separate “solid nodule” within the fat (Figure 2D).75 There is, however, a huge variation in the proportions of fat and myeloid tissue between myelolipomas. In those almost totally comprising macroscopic fat, the CT attenuation is very low (pure fat −100 Hounsfield Units) and appear dark on CT and white on T1- and T2-weighted MRI (Supplemental Figure S1A).75 With increasing myeloid component (Figure 2D), the CT attenuation increases and in many myelolipomas measure between −50 and −20 Hounsfield Units).46 Sometimes the myeloid and fat components are distinctly separated and of approximately similar proportions (Figure 2E) and in other myelolipomas the myeloid component may be very dominating with merely one or a few small islands of macroscopic fat (Supplemental Figure S1B) in an otherwise “solid” myeloid tumour. Sometimes the fat component is very small to non-existent and does not allow for supporting the CT/MRI diagnosis of myelolipoma by attenuation measurement of the fat component. The solid myeloid component is typically contrast-enhancing (Figure 2H, Supplemental Figure S1C).76 The myelolipomas are generally clearly demarcated which is facilitated by the fairly frequent appearance of a pseudocapsule.77 Adrenal myelolipomas may be bilateral, typically when they are large (Figure 2I). Sometimes calcifications, usually small, are found within the myelolipoma (Figure 2J).78 Differential diagnoses constitute adrenocortical carcinomas with macroscopic fat or retroperitoneal liposarcoma,9,79,80 both of which are extremely unusual.
Figure 2.
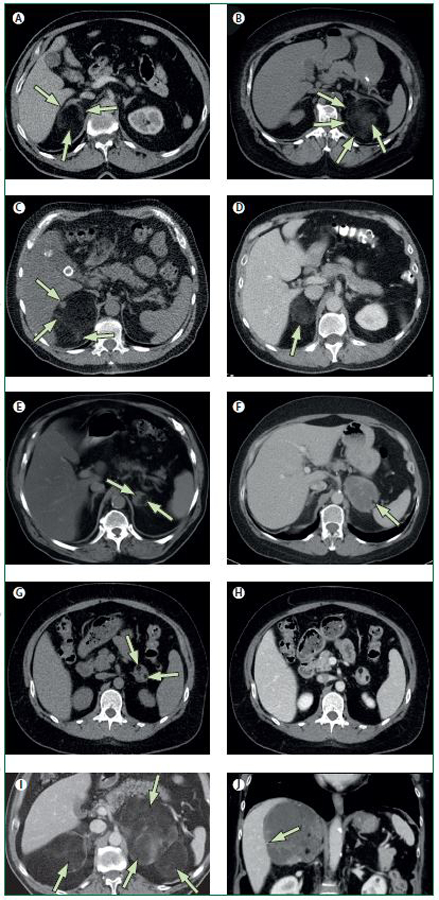
A-I. Transversal computed tomography (CT) images. J. Coronal CT image.
A. Typical myelolipoma in the right adrenal gland (long arrow), comprising almost exclusively macroscopic fat, spreading the lateral and medial adrenal limbs apart (short arrows), extending posteriorly but demarcated from the periadrenal fat by a thin capsule.
B. The myelolipoma in the left adrenal gland is dislocating the lateral adrenal limb ventrally and extends laterally to the spleen. The capsule is clearly seen (short arrows), and the myeloid component shows a cloudy appearance (long arrow).
C. In the myelolipoma in the right adrenal gland, the myeloid components are seen as a nodule (long arrow) in the ventral-medial part of the tumour and as strands (small arrows) in its posterior aspects.
D. In this myelolipoma in the right adrenal gland (arrow), the myeloid and fat components are mixed and are evenly spread throughout the whole tumour.
E. By contrast, in this myelolipoma in the left adrenal gland, the myeloid (short arrow) and fat components (long arrow) are distinctly separated and are located in the medial and lateral part of the tumour, respectively.
F. The myelolipoma in the left adrenal gland comprise almost exclusively myeloid tissue in which there is a small island of macroscopic fat in the lateral-posterior aspect of the tumour (arrow).
G. CT without intravenous contrast-enhancement. In this myelolipoma in the left adrenal gland, with a similar appearance there are two small fatty islands (short arrows).
H. CT with intravenous contrast-enhancement in the venous phase shows in the same tumour contrast-enhancement of the myeloid component, similar to that of the liver and spleen.
I. Myelolipomas are sometimes bilateral, especially when they are large (arrows).
J. Sometimes calcifications are seen in myelolipomas and they are usually small (arrow).
Adrenal biopsy
Adrenal biopsy (either fine needle aspiration biopsy or core needle biopsy) is not recommended in the diagnostic work up of adrenal myelolipoma, mainly due to the high accuracy of imaging.
Adrenalectomy
Adrenalectomy is usually reserved to a minority of patients with adrenal myelolipomas, more common in patients with large tumours, those with tumour growth, acute haemorrhage, symptoms of abdominal mass effect, or uncontrolled CAH13,47,49 In several case series of surgically treated myelolipomas, the median tumour size was around 5–8 cm.13,45,47–49 Adrenalectomy was also the treatment of choice in patients with ipsilateral concomitant adrenal hormone excess.13,44,54 In a series of 305 patients with adrenal myelolipomas, surgery was done in 37 (12%) patients because of increasing tumour size in a large myelolipoma, symptoms of mass effect, ipsilateral adenoma with adrenal hormone excess, acute haemorrhage, concomitant resection for other reasons, and to confirm a questionable diagnosis on imaging.13 Patients undergoing adrenalectomy were younger, had larger tumours with accelerated tumour growth and a higher likelihood of haemorrhagic changes on imaging than those who were managed conservatively.13
Long-term outcomes
Most patients with myelolipomas are asymptomatic, do not demonstrate tumour growth or develop adrenal malignancy. In a series of 163 patients with myelolipomas followed with imaging over a median of 7 years (range 0.5–20 years), the overall median tumour growth was 0 cm, ranging from −1 cm (tumour shrinkage) to 11 cm tumour growth.13 The maximum tumour growth per year was 1.4 cm. Only 16% of patients demonstrated an overall tumour growth of at least 1 cm.13 A larger tumour size at diagnosis was reported to be a risk factor for future tumour growth. In another study of 69 patients with adrenal myelolipomas followed for a median of 3.9 years, 11 (16%) demonstrated a median tumour growth of 1.1 cm (range 0.6–8.4), a median growth per year of 0.16 cm/year (range 0.08–0.71).6 In this study, younger age, and duration of follow up, but not the initial tumour size were associated with tumour growth.6 In another series of 15 patients followed for an average of 3.2 years (0.3–10.8 years), 13 remained asymptomatic, and 2 continued to have some abdominal discomfort during follow up.44 Imaging follow up demonstrated a minor tumour size increase in 6 patients.44 Patients with large myelolipomas may develop new onset symptoms of mass effect when tumour growth continues. Acute haemorrhage and tumour rupture are very rare events, and almost always occur in very large myelolipomas, usually >8–10 cm. When adrenalectomy is performed, it is a definite treatment for myelolipoma, with no recurrence reported.13,29,46
Management
Any patient with a newly discovered adrenal mass needs to undergo a parallel work up to determine the aetiology of the adrenal mass, and the presence of hormonal excess. Due to scarcity of original literature on management of myelolipomas, guidance is mostly based on expert opinion.
After determining the likelihood of myelolipoma based on the imaging characteristics (most with pathognomonic features), we also recommend to consider hormonal work up informed by the clinical presentation (Figure 3). Patients with suspected hormone excess should be investigated with a 1 mg overnight dexamethasone suppression test, and measurements of aldosterone, renin plasma activity, and potassium (Figure 3). In very rare situations of diagnosed adrenal hormone excess, management should be targeted to the type and site of adrenal hormone excess, which could be ipsilateral or contralateral to myelolipoma (Figure 3).
Figure 3.
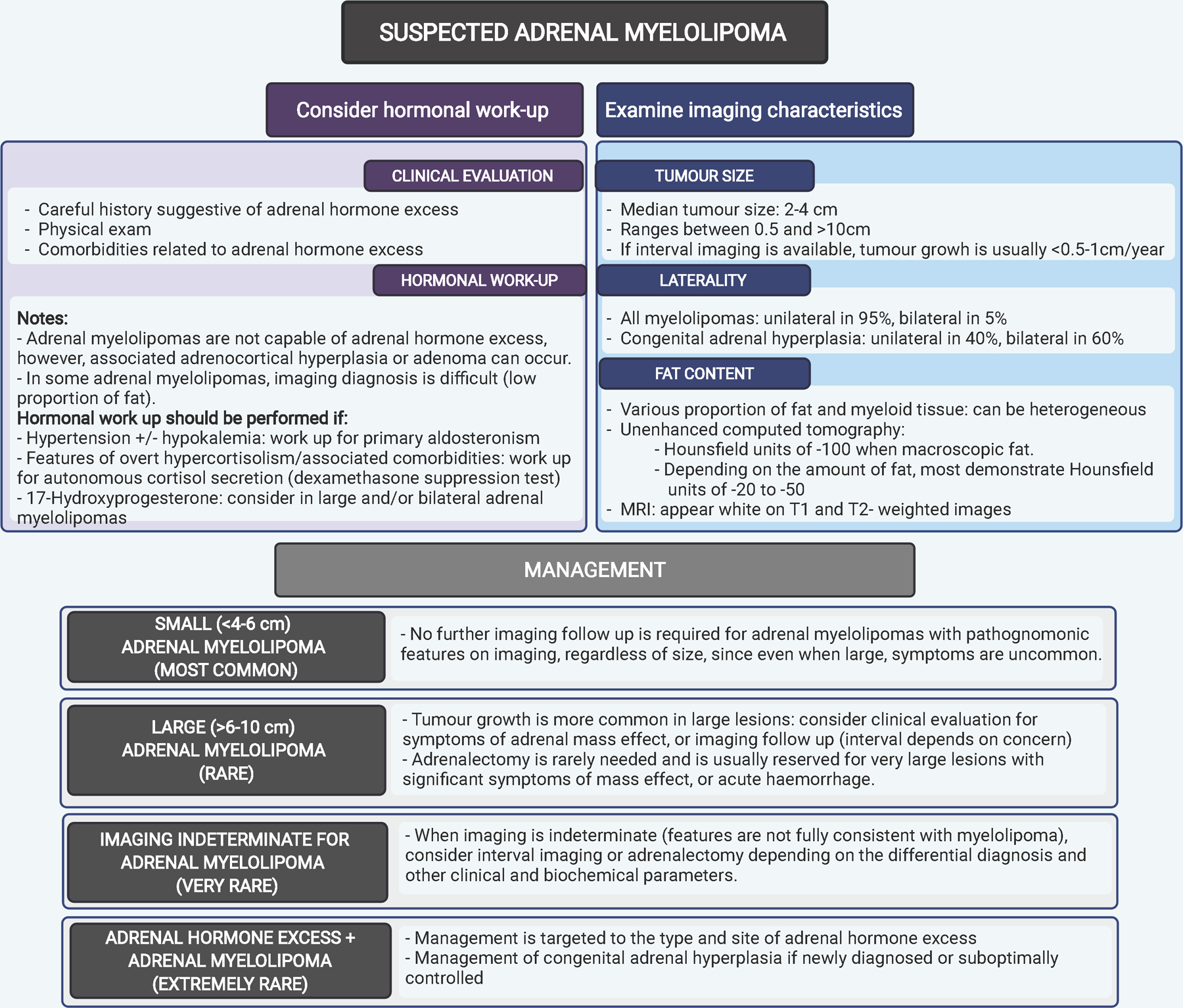
An algorithm on imaging characteristics, investigations and management of adrenal myelolipomas.
Patients with large and/or bilateral myelolipomas should be investigated for a possibility of CAH with measurement of 17OHP. Bilateral adrenalectomy is occasionally used in poorly controlled CAH,81 sometimes with concomitant bilateral adrenal myelolipomas. ACTH levels usually increase after the procedure which may stimulate the growth of ectopic adrenal rest tumors.82 Since adrenal rest tumours, especially in the testicles, may impair fertility,83 surgical removal of adrenal myelolipomas in CAH should only be done after careful consideration.In the vast majority of patients with adrenal myelolipoma, imaging diagnosis is clear and no further imaging follow-up or adrenalectomy is required (Figure 3). In rare selected cases of very large myelolipomas, clinical or imaging follow up may be considered.
However, adrenalectomy in these cases is usually reserved only if symptoms of mass effect or acute hemorrhage is present (Figure 3). In other rare instances when imaging is not completely typical of myelololipoma, such as tumors with a high abundance of calcifications, or when it is difficult to identify macroscopic fat, management should be discussed at a multidisciplinary meeting with options including imaging follow up, another type of imaging, or adrenalectomy. Since most myelolipomas are discovered as incidentalomas, in the occasional instances of atypical imaging findings they are frequently managed as such, whereby the patient may be discharged when the tumour size and appearance is unchanged on follow-up imaging at 6 months (or in comparison to imaging ≥ 6 moths previously).
Gaps in knowledge
Most adrenal myelolipomas are small, non-functioning, and asymptomatic. A minority of myelolipomas may grow, and rarely cause symptoms of mass effect. However, the predictors of growth, or development of haemorrhage are not clear, and as such, management should be individualized. The adequate duration and interval of imaging monitoring in patients with atypical imaging features are unknown. Future studies should clarify the approach to hormonal assessment in patients with myelolipomas. The pathogenic drivers are not known, but there is an association between myelolipoma development and elevation of ACTH and possibly of erythropoietin, but other paracrine drivers may well be discovered in the future. Adrenal tumours may occasionally display local ACTH secretion,62,84 causing Cushing syndrome and potentially myelolipomas. Genetic explanations may also be discovered as well as differently expressed various messenger RNAs.
Conclusion
Adrenal myelolipomas are indolent tumours that are usually discovered incidentally. Imaging presents a tumour with fat and myeloid components. Patients with adrenal myelolipoma may seldom demonstrate adrenal hormone excess due to concomitant adrenocortical adenoma or hyperplasia, and decision on hormonal work up should be individualized. Association with CAH needs to be considered in any patient with adrenal myelolipoma, but especially in those with large and bilateral lesions. Large lesions may warrant follow-up as growth could develop, which rarely may cause mass effect symptoms. In tumours with atypical appearance on imaging as low fat content repeated imaging should be considered. Adrenalectomy may very rarely be discussed in selected patients.
Supplementary Material
Footnotes
Declaration of interests
IB is supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (NIH) USA under award K23DK121888 (IB). The views expressed are those of the author(s) and not necessarily those of the NIH. IB reports consulting with Strongbridge, HRA Pharma, Corcept, CinCor, and Sparrow Pharmaceutics outside the submitted work, and serves on the data safety board for Adrenas Therapeutics. HF has consulted for Neurocrine Biosciences Inc, Diurnal Ltd, Roche Diagnostics International Ltd and Adrenas Therapeutics.
References
- 1.Gierke E Über Knochenmarksgewebe in der Nebenniere. Zeiglers Beitr Pathol Anat 1905; 7: 311–24. [Google Scholar]
- 2.Ebbehoj A, Li D, Kaur RJ, et al. Epidemiology of adrenal tumours in Olmsted County, Minnesota, USA: a population-based cohort study. Lancet Diabetes Endocrinol 2020; 8(11): 894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ichijo T, Ueshiba H, Nawata H, Yanase T. A nationwide survey of adrenal incidentalomas in Japan: the first report of clinical and epidemiological features. Endocr J 2020; 67(2): 141–52. [DOI] [PubMed] [Google Scholar]
- 4.Reginelli A, Vacca G, Belfiore M, et al. Pitfalls and differential diagnosis on adrenal lesions: current concepts in CT/MR imaging: a narrative review. Gland surgery 2020; 9(6): 2331–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daneshmand S, Quek ML. Adrenal myelolipoma: diagnosis and management. Urol J 2006; 3(2): 71–4. [PubMed] [Google Scholar]
- 6.Campbell MJ, Obasi M, Wu B, Corwin MT, Fananapazir G. The radiographically diagnosed adrenal myelolipoma: what do we really know? Endocrine 2017; 58(2): 289–94. [DOI] [PubMed] [Google Scholar]
- 7.Juul SE, Yachnis AT, Christensen RD. Tissue distribution of erythropoietin and erythropoietin receptor in the developing human fetus. Early Hum Dev 1998; 52(3): 235–49. [DOI] [PubMed] [Google Scholar]
- 8.Taher A, Vichinsky E, Musallam K, Cappellini MD, Viprakasit V. In: Weatherall D, ed. Guidelines for the Management of Non Transfusion Dependent Thalassaemia (NTDT) Nicosia (Cyprus); 2013. [PubMed] [Google Scholar]
- 9.Decmann A, Perge P, Toth M, Igaz P. Adrenal myelolipoma: a comprehensive review. Endocrine 2018; 59(1): 7–15. [DOI] [PubMed] [Google Scholar]
- 10.Civrilli K, Damry N, Steppe R, Efira A, Mathieu J. Bilateral adrenal myelolipomas. JBR-BTR 2008; 91(3): 90–1. [PubMed] [Google Scholar]
- 11.Nermoen I, Falhammar H. Prevalence and Characteristics of Adrenal Tumors and Myelolipomas in Congenital Adrenal Hyperplasia: A Systematic Review and Meta-Analysis. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists 2020; 26(11): 1351–65. [DOI] [PubMed] [Google Scholar]
- 12.Boudreaux D, Waisman J, Skinner DG, Low R. Giant adrenal myelolipoma and testicular interstitial cell tumor in a man with congenital 21-hydroxylase deficiency. The American journal of surgical pathology 1979; 3(2): 109–23. [DOI] [PubMed] [Google Scholar]
- 13.Hamidi O, Raman R, Lazik N, et al. Clinical course of adrenal myelolipoma: A long-term longitudinal follow-up study. Clinical endocrinology 2020; 93(1): 11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Addison T On the constitutional and local effects of disease of the suprarenal capsule London: Samuel Highley; 1855. [Google Scholar]
- 15.Selye H, Stone H. Hormonally induced transformation of adrenal into myeloid tissue. Am J Pathol 1950; 26(2): 211–33. [PMC free article] [PubMed] [Google Scholar]
- 16.Hagiwara H, Usui T, Kimura T, et al. Lack of ACTH and androgen receptor expression in a giant adrenal myelolipoma associated with 21-hydroxylase deficiency. Endocr Pathol 2008; 19(2): 122–7. [DOI] [PubMed] [Google Scholar]
- 17.Larose S, Bondaz L, Mermejo LM, et al. Coexistence of Myelolipoma and Primary Bilateral Macronodular Adrenal Hyperplasia With GIP-Dependent Cushing’s Syndrome. Front Endocrinol (Lausanne) 2019; 10: 618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almeida MQ, Kaupert LC, Brito LP, et al. Increased expression of ACTH (MC2R) and androgen (AR) receptors in giant bilateral myelolipomas from patients with congenital adrenal hyperplasia. BMC Endocr Disord 2014; 14: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shenoy VG, Thota A, Shankar R, Desai MG. Adrenal myelolipoma: Controversies in its management. Indian J Urol 2015; 31(2): 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng C, Jiang H, Ding Q, Wen H. Adrenal myelolipoma: a mingle of progenitor cells? Med Hypotheses 2013; 80(6): 819–22. [DOI] [PubMed] [Google Scholar]
- 21.Chang KC, Chen PI, Huang ZH, Lin YM, Kuo PL. Adrenal myelolipoma with translocation (3;21)(q25;p11). Cancer Genet Cytogenet 2002; 134(1): 77–80. [DOI] [PubMed] [Google Scholar]
- 22.Yildiz L, Akpolat I, Erzurumlu K, Aydin O, Kandemir B. Giant adrenal myelolipoma: case report and review of the literature. Pathol Int 2000; 50(6): 502–4. [DOI] [PubMed] [Google Scholar]
- 23.Banik S, Hasleton PS, Lyon RL. An unusual variant of multiple endocrine neoplasia syndrome: a case report. Histopathology 1984; 8(1): 135–44. [DOI] [PubMed] [Google Scholar]
- 24.Saunders RN, Koch CA, Brown KB, et al. Bilateral adrenal myelolipomas in a woman with chronic anticoagulation, postmenopausal uterine bleeding, primary hyperparathyroidism and hyperthyroidism. Am J Med Sci 2013; 346(1): 82–5. [DOI] [PubMed] [Google Scholar]
- 25.Hegstrom JL, Kircher T. Alimentary tract ganglioneuromatosis-lipomatosis, adrenal myelolipomas, pancreatic telangiectasias, and multinodular thyroid goiter. A possible neuroendocrine syndrome. Am J Clin Pathol 1985; 83(6): 744–7. [DOI] [PubMed] [Google Scholar]
- 26.Yoshioka M, Fujimori K, Wakasugi M, et al. Cushing’s disease associated with adrenal myelolipoma, adrenal calcification and thyroid cancer. Endocrine journal 1994; 41(4): 461–6. [DOI] [PubMed] [Google Scholar]
- 27.Schulte KM, Heinze M, Mengel M, Scheuring S, Kohrer K, Roher HD. Complete sequencing and mRNA expression analysis of the MEN-I gene in adrenal myelolipoma. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme 2000; 32(5): 169–73. [DOI] [PubMed] [Google Scholar]
- 28.Decmann A, Perge P, Nyiro G, et al. MicroRNA Expression Profiling in Adrenal Myelolipoma. J Clin Endocrinol Metab 2018; 103(9): 3522–30. [DOI] [PubMed] [Google Scholar]
- 29.Lam KY, Lo CY. Adrenal lipomatous tumours: a 30 year clinicopathological experience at a single institution. J Clin Pathol 2001; 54(9): 707–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho J, Kinsey D, Kimchi ET, et al. Retroperitoneal extra-adrenal myelolipoma misdiagnosed as liposarcoma: A case report. Radiol Case Rep 2021; 16(2): 364–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLaughlin SA, Schmitt TM, Huguet KL, Menke DM, Nguyen JH. Myofibrosarcoma of the adrenal gland. The American surgeon 2005; 71(3): 191–3. [PubMed] [Google Scholar]
- 32.Dei Tos AP, Doglioni C, Piccinin S, et al. Coordinated expression and amplification of the MDM2, CDK4, and HMGI-C genes in atypical lipomatous tumours. The Journal of pathology 2000; 190(5): 531–6. [DOI] [PubMed] [Google Scholar]
- 33.Sirvent N, Coindre JM, Maire G, et al. Detection of MDM2-CDK4 amplification by fluorescence in situ hybridization in 200 paraffin-embedded tumor samples: utility in diagnosing adipocytic lesions and comparison with immunohistochemistry and real-time PCR. The American journal of surgical pathology 2007; 31(10): 1476–89. [DOI] [PubMed] [Google Scholar]
- 34.Rabbitts TH, Forster A, Larson R, Nathan P. Fusion of the dominant negative transcription regulator CHOP with a novel gene FUS by translocation t(12;16) in malignant liposarcoma. Nat Genet 1993; 4(2): 175–80. [DOI] [PubMed] [Google Scholar]
- 35.Crozat A, Aman P, Mandahl N, Ron D. Fusion of CHOP to a novel RNA-binding protein in human myxoid liposarcoma. Nature 1993; 363(6430): 640–4. [DOI] [PubMed] [Google Scholar]
- 36.Zhou L, Pan X, He T, et al. Primary adrenal teratoma: A case series and review of the literature. Mol Clin Oncol 2018; 9(4): 437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perna V, Taylor NF, Dworakowska D, et al. Adrenocortical adenomas with regression and myelolipomatous changes: urinary steroid profiling supports a distinctive benign neoplasm. Clinical endocrinology 2014; 81(3): 343–9. [DOI] [PubMed] [Google Scholar]
- 38.Stenman A, Shabo I, Ramstrom A, Zedenius J, Juhlin CC. Synchronous aldosterone- and cortisol-producing adrenocortical adenomas diagnosed using CYP11B immunohistochemistry. SAGE Open Med Case Rep 2019; 7: 2050313X19883770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song JH, Chaudhry FS, Mayo-Smith WW. The incidental adrenal mass on CT: prevalence of adrenal disease in 1,049 consecutive adrenal masses in patients with no known malignancy. AJR Am J Roentgenol 2008; 190(5): 1163–8. [DOI] [PubMed] [Google Scholar]
- 40.Yeomans H, Calissendorff J, Volpe C, Falhammar H, Mannheimer B. Limited value of long-term biochemical follow-up in patients with adrenal incidentalomas-a retrospective cohort study. BMC endocrine disorders 2015; 15: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patrova J, Jarocka I, Wahrenberg H, Falhammar H. Clinical Outcomes in Adrenal Incidentaloma: Experience from One Center. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists 2015; 21(8): 870–7. [DOI] [PubMed] [Google Scholar]
- 42.Bezjak M, Sesar P, Ulamec M, et al. [Adrenal myelolipoma--report of 15 patients]. Acta Med Croatica 2013; 67(3): 255–8. [PubMed] [Google Scholar]
- 43.Bin X, Qing Y, Linhui W, Li G, Yinghao S. Adrenal incidentalomas: experience from a retrospective study in a Chinese population. Urol Oncol 2011; 29(3): 270–4. [DOI] [PubMed] [Google Scholar]
- 44.Han M, Burnett AL, Fishman EK, Marshall FF. The natural history and treatment of adrenal myelolipoma. J Urol 1997; 157(4): 1213–6. [PubMed] [Google Scholar]
- 45.Mantero F, Terzolo M, Arnaldi G, et al. A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. The Journal of clinical endocrinology and metabolism 2000; 85(2): 637–44. [DOI] [PubMed] [Google Scholar]
- 46.Gadelkareem RA, Moeen AM, Khalil M, et al. Experience of a Tertiary-Level Urology Center in Clinical Urological Events of Rare and Very Rare Incidence. V. Urological Tumors: 1. Adrenal Myelolipoma. Curr Urol 2020; 14(2): 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao J, Sun F, Jing X, et al. The diagnosis and treatment of primary adrenal lipomatous tumours in Chinese patients: A 31-year follow-up study. Can Urol Assoc J 2014; 8(3–4): E132–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gershuni VM, Bittner JGt, Moley JF, Brunt LM. Adrenal myelolipoma: operative indications and outcomes. J Laparoendosc Adv Surg Tech A 2014; 24(1): 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yin L, Teng J, Zhou Q, et al. A 10-year single-center experience with surgical management of adrenal myelolipoma. J Endourol 2014; 28(2): 252–5. [DOI] [PubMed] [Google Scholar]
- 50.Ahn SH, Kim JH, Baek SH, et al. Characteristics of Adrenal Incidentalomas in a Large, Prospective Computed Tomography-Based Multicenter Study: The COAR Study in Korea. Yonsei Med J 2018; 59(4): 501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iniguez-Ariza NM, Kohlenberg JD, Delivanis DA, et al. Clinical, Biochemical, and Radiological Characteristics of a Single-Center Retrospective Cohort of 705 Large Adrenal Tumors. Mayo Clin Proc Innov Qual Outcomes 2018; 2(1): 30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amano T, Takemae K, Niikura S, Kouno M, Amano M. Retroperitoneal hemorrhage due to spontaneous rupture of adrenal myelolipoma. Int J Urol 1999; 6(11): 585–8. [DOI] [PubMed] [Google Scholar]
- 53.Machuca Santa-Cruz F, Perez Rodriguez D, Julve Villalta E, et al. [Spontaneous retroperitoneal hematoma secondary to the rupture of suprarenal myelolipoma]. Arch Esp Urol 2000; 53(8): 724–5. [PubMed] [Google Scholar]
- 54.Su HC, Huang X, Zhou WL, et al. Pathologic analysis, diagnosis and treatment of adrenal myelolipoma. Can Urol Assoc J 2014; 8(9–10): E637–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Merke DP, Auchus RJ. Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency. The New England journal of medicine 2020; 383(13): 1248–61. [DOI] [PubMed] [Google Scholar]
- 56.Nordenstrom A, Lajic S, Falhammar H. Clinical outcomes in 21-hydroxylase deficiency. Current opinion in endocrinology, diabetes, and obesity 2021. [DOI] [PubMed]
- 57.Speiser PW, Arlt W, Auchus RJ, et al. Congenital Adrenal Hyperplasia Due to Steroid 21-Hydroxylase Deficiency: An Endocrine Society Clinical Practice Guideline. The Journal of clinical endocrinology and metabolism 2018; 103(11): 4043–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zetterstrom RH, Karlsson L, Falhammar H, Lajic S, Nordenskjold A. Update on the Swedish Newborn Screening for Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency. Int J Neonatal Screen 2020; (6): 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nordenstrom A, Falhammar H. MANAGEMENT OF ENDOCRINE DISEASE: Diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency. European journal of endocrinology / European Federation of Endocrine Societies 2019; 180(3): R127–R45. [DOI] [PubMed] [Google Scholar]
- 60.Falhammar H, Wedell A, Nordenstrom A. Biochemical and genetic diagnosis of 21-hydroxylase deficiency. Endocrine 2015; 50(2): 306–14. [DOI] [PubMed] [Google Scholar]
- 61.Falhammar H Non-functioning adrenal incidentalomas caused by 21-hydroxylase deficiency or carrier status? Endocrine 2014; 47(1): 308–14. [DOI] [PubMed] [Google Scholar]
- 62.Falhammar H, Torpy DJ. Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency Presenting as Adrenal Incidentaloma: A Systematic Review and Meta-Analysis. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists 2016; 22(6): 736–52. [DOI] [PubMed] [Google Scholar]
- 63.Lowe KM, Young WF Jr., Lyssikatos C, Stratakis CA, Carney JA. Cushing Syndrome in Carney Complex: Clinical, Pathologic, and Molecular Genetic Findings in the 17 Affected Mayo Clinic Patients. The American journal of surgical pathology 2017; 41(2): 171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reza-Albarran AA, Gomez-Perez FJ, Lopez JC, et al. Myelolipoma: A New Adrenal Finding in Carney’s Complex? Endocrine pathology 1999; 10(3): 251–7. [DOI] [PubMed] [Google Scholar]
- 65.Sun X, Ayala A, Castro CY. Adrenocortical carcinoma with concomitant myelolipoma in a patient with hyperaldosteronism. Archives of pathology & laboratory medicine 2005; 129(6): e144–7. [DOI] [PubMed] [Google Scholar]
- 66.Ukimura O, Inui E, Ochiai A, Kojima M, Watanabe H. Combined adrenal myelolipoma and pheochromocytoma. J Urol 1995; 154(4): 1470. [PubMed] [Google Scholar]
- 67.Jakka N, Venkateshwarlu J, Satyavani N, Neelaveni K, Ramesh J. Functioning adrenal myelolipoma: A rare cause of hypertension. Indian J Endocrinol Metab 2013; 17(Suppl 1): S249–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ishay A, Dharan M, Luboshitzky R. Combined adrenal myelolipoma and medullary hyperplasia. Horm Res 2004; 62(1): 23–6. [DOI] [PubMed] [Google Scholar]
- 69.Gamberini MR, Prandini N, Chiodi E, Farneti C, Garani MC. Adrenal incidentaloma in thalassemia: a case report and literature review. Pediatr Endocrinol Rev 2011; 8 Suppl 2: 324–30. [PubMed] [Google Scholar]
- 70.Calhoun SK, Murphy RC, Shariati N, Jacir N, Bergman K. Extramedullary hematopoiesis in a child with hereditary spherocytosis: an uncommon cause of an adrenal mass. Pediatr Radiol 2001; 31(12): 879–81. [DOI] [PubMed] [Google Scholar]
- 71.Salemme M, Rodella R, Fisogni S, Facchetti F. Nodular extramedullary hematopoiesis involving the adrenal gland. An uncommon cause of adrenal “incidentaloma”. Pathologica 2011; 103(2): 46–9. [PubMed] [Google Scholar]
- 72.Gamss C, Chia F, Chernyak V, Rozenblit A. Giant hemorrhagic myelolipoma in a patient with sickle cell disease. Emerg Radiol 2009; 16(4): 319–22. [DOI] [PubMed] [Google Scholar]
- 73.Papavasiliou C, Gouliamos A, Deligiorgi E, Vlahos L, Cambouris T. Masses of myeloadipose tissue: radiological and clinical considerations. International journal of radiation oncology, biology, physics 1990; 19(4): 985–93. [DOI] [PubMed] [Google Scholar]
- 74.Lin CY, Levy D, Higgins JPT, Kunder CA, Kao CS. Adrenal Myelolipomas Involved by Plasma Cell Myeloma. Am J Clin Pathol 2018; 150(5): 406–14. [DOI] [PubMed] [Google Scholar]
- 75.d’Amuri FV, Maestroni U, Pagnini F, et al. Magnetic resonance imaging of adrenal gland: state of the art. Gland surgery 2019; 8(Suppl 3): S223–S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bokhari MR, Zulfiqar H, Garla VV. Adrenal Myelolipoma. StatPearls. Treasure Island (FL); 2021. [PubMed]
- 77.Shaaban AM, Rezvani M, Tubay M, Elsayes KM, Woodward PJ, Menias CO. Fat-containing Retroperitoneal Lesions: Imaging Characteristics, Localization, and Differential Diagnosis. Radiographics 2016; 36(3): 710–34. [DOI] [PubMed] [Google Scholar]
- 78.Low G, Dhliwayo H, Lomas DJ. Adrenal neoplasms. Clin Radiol 2012; 67(10): 988–1000. [DOI] [PubMed] [Google Scholar]
- 79.Craig WD, Fanburg-Smith JC, Henry LR, Guerrero R, Barton JH. Fat-containing lesions of the retroperitoneum: radiologic-pathologic correlation. Radiographics 2009; 29(1): 261–90. [DOI] [PubMed] [Google Scholar]
- 80.Ferrozzi F, Bova D. CT and MR demonstration of fat within an adrenal cortical carcinoma. Abdom Imaging 1995; 20(3): 272–4. [DOI] [PubMed] [Google Scholar]
- 81.MacKay D, Nordenstrom A, Falhammar H. Bilateral Adrenalectomy in Congenital Adrenal Hyperplasia: A Systematic Review and Meta-Analysis. The Journal of clinical endocrinology and metabolism 2018. [DOI] [PubMed]
- 82.Burman P, Falhammar H, Waldenström E, Sundin A, Bitzén U. 11C-metomidate PET/CT detected multiple ectopic adrenal rest tumors in a woman with congenital adrenal hyperplasia The Journal of clinical endocrinology and metabolism 2020. [DOI] [PubMed]
- 83.Claahsen-van der Grinten HL, Stikkelbroeck N, Falhammar H, Reisch N. MANAGEMENT OF ENDOCRINE DISEASE: Gonadal dysfunction in congenital adrenal hyperplasia. European journal of endocrinology / European Federation of Endocrine Societies 2021; 184(3): R85–R97. [DOI] [PubMed] [Google Scholar]
- 84.Saeger W Pathology of adrenal neoplasms. Minerva endocrinologica 1995; 20(1): 1–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


