Abstract
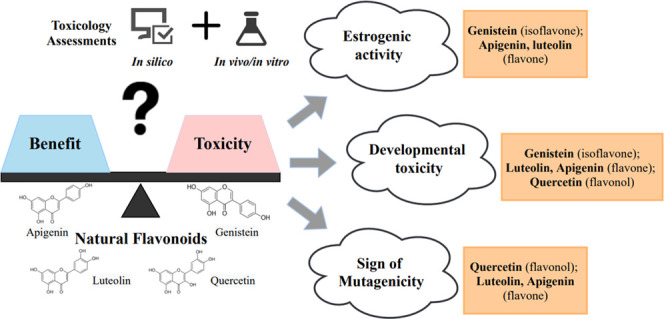
Flavonoids are bioactive phenolic compounds widely present in plant food and used in various nutraceutical, pharmaceutical, and cosmetic products. However, recent studies showed rising concerns of endocrine disruptions and developmental toxicities for many flavonoids. To understand the impacts of flavonoid structure on toxicity, we used a new multitiered platform to investigate the toxicities of four common flavonoids, luteolin, apigenin, quercetin, and genistein, from flavone, flavonol, and isoflavone. Weak estrogenic activity was detected for four flavonoids (genistein, apigenin, quercetin, and luteolin) at 10–12 to 10–7 M by the MCF-7 cell proliferation assay, which agreed with the molecular docking results. Consistent with the simulation results of Toxicity Estimation Software Tool, genistein and luteolin showed high developmental toxicity in the chicken embryonic assay (45–477 μg/kg) with mortality rate up to 50%. Luteolin, quercetin, and apigenin showed signs of mutagenicity at 5 × 10–3 pmol/plate. The findings showed nonmonotonic dose responses for the chemicals.
1. Introduction
Flavonoids are a large class of naturally occurring secondary plant metabolites with extensive bioactivities. They are widely found in fruits and vegetables and have been used in nutraceutical, pharmaceutical, and cosmetic products due to their health benefits.1 Among them, luteolin, apigenin, quercetin, and genistein are four typical naturally plant-derived dietary flavonoids. Quercetin can be found in basically all kinds of the berries such as whortleberry (158 mg/kg fresh weight) and chokeberry (89 mg/kg), and an average of 5320 mg apigenin glycosides were found per 100 g of dried chamomile flowers.2,3 Genistein is predominantly present in soy-based foods, with 5.6 to 276 mg/100 g in mature soybeans.4 Both aglycone and glycoside forms of flavonoids may exist in foods. The enzymes in human small intestines and the gut microflora can effectively convert flavonoid glycoside to aglycone. The estimated amounts of genistein and genistin (its β-glycoside) were 4.6 and 200.6 μg/g beans, respectively. The higher genistein level, 38.5–229.1 μg/g food, was detected in fermented soybean products (e.g., miso and natto).5 Additionally, genistein glycoside was readily converted to its aglycone form and exerted its biological activities after ingestion.6 On average, humans consume approximately 1 g of flavonoids in their daily diet.7 The estimated intake of these flavonoids via food, commonly fruits and vegetables, is between 0.02 and 3 mg/kg bw/day, but the supplementary intake can increase it up to 23 mg/kg bw/day.8
The promising biological activities of natural flavonoids make them receive increased attention,9,10 while the “natural” term of flavonoids has occasionally misled the consumers’ perceptions to overlook their possible adverse effects. Researchers already reported that some of these plant-derived flavonoids exhibited hepatotoxicity, pro-oxidant activity, and potential estrogenic activity (EA).11 Flavonoids could have potential EA since most of them have similar structures to the major female sex hormone-17β-estradiol (E2). Some flavonoids, especially isoflavones, are also called phytoestrogens and may disrupt the normal hormone balance in adolescents or children.12 One possible negative health outcome from the disrupting of hormone balance is the impairment of reproductive functions, and the antifertility potential has been reported for quercetin or quercetin-rich extracts (Thevetia peruviana) with a reduced progesterone production in a female Sprague-Dawley rat uterus model.13 Additionally, the adverse effect of flavonoids on early life stage has been reported using a zebrafish model, with 15 out of 24 flavonoids including apigenin and genistein showing developmental toxicity at 1–50 μM.14 The mutagenicity of quercetin and the risk of mutagenicity of luteolin and fisetin were reported using the Ames test.15 Most of the previous findings were related to high exposure levels.
Because of their wide presence in fruits and vegetables, different flavonoids are important components of our daily diets and many people consider that consumption of these natural flavonoids can benefit human health due to their beneficial bioactivities.16 However, the relationships and mechanisms between their chemical structures and potential toxicities are not well studied and only a few studies focused on their potential adverse effects on human health using higher exposure levels. Bioavailable flavonoids are in the nanomolar to low micromolar ranges, and their plasma concentrations are less than 1 μM.9 To understand how the chemical structures and low exposure levels of flavonoids impact their potential toxicities, we selected four common flavonoids in this study, from three subclasses (flavone: apigenin and luteolin; flavonol: quercetin; isoflavone: genistein, Figure 1) and investigated their EA, developmental toxicity, and mutagenicity. These flavonoids have low bioavailability making low exposure levels as used in our study highly possible. We included two in silico simulations as the first toxicity evaluation to choose the chemicals before the experimental approaches, due to the low cost and fast speed of the simulations. We assessed the binding affinities of these flavonoids to 14 human nuclear receptors that are the common targets of endocrine-disrupting (ED) chemicals.14,15 Recently, the chicken embryo is recognized as a model to bridge the gap between cell-based and animal-based methods and has become an attractive alternative to in vivo assays under the 3Rs (Replacement, Reduction, and Refinement) guidance.18,19 We also utilized Toxicity Estimation Software Tool (T.E.S.T.) for the prediction of developmental toxicity and mutagenicity. To confirm the findings of the computational methods, we used the MCF-7 cell proliferation assay for EA, chicken embryonic assay for developmental toxicity, and Ames test for mutagenicity. This study is important to evaluate the efficacy of our new toxicity method for the common natural compounds with similar structures. After validating the effectiveness of our new approach, we will study other flavonoids more efficiently in the future.
Figure 1.
Chemical structures of flavonoids.
2. Results
2.1. Four Flavonoids Had High or Medium Level of Binding Affinities to Several EDCs’ Targeting Receptors, Developmental Toxicity, and Mutagenicity When Predicated Using Two In Silico Simulations
In this study, molecular docking was conducted to target 14 potential nuclear receptors of EDCs, including four antagonist conformations (AR an, ERα an, ERβ an, and GR an). E2, the major type of female sex hormone, served as the positive control. Compared to E2, the four flavonoids had similar binding affinities to the androgen receptor (AR) and two thyroid receptors (TRs), and only quercetin showed slightly lower binding affinities to the TRs (Table S1). Genistein had the highest binding affinities to ERα and ERβ, and apigenin had the second highest affinities, but both showed lower binding levels compared to the affinities of E2. Genistein and apigenin had good binding of ERβ an, while having medium binding of ERα an and ERβ. For ERα, genistein had good binding and apigenin had medium binding. Luteolin had medium binding of ERα, ERα an, and ERβ an, as well as low binding of ERβ, while quercetin showed low binding of these four ER conformations. Moreover, these four flavonoids all had higher binding affinities to GR and MR than the findings of E2, with medium binding for GR, while high binding for MR.
When the oral rat LD50 value was between 300 and 2000 mg/kg, the chemical belongs to class 4,20 with class 1 representing the most severe toxicity. In our T.E.S.T. study, the E2, apigenin, and genistein all belonged to class 4, with a higher acute toxicity level than quercetin and luteolin (both in class 5). All four chemicals along with E2 were classified as developmental toxicants, and luteolin showed the highest level of developmental toxicity followed by quercetin and genistein (Table S2). For mutagenicity, quercetin and luteolin were reported as mutagenicity-positive.
2.2. Four Flavonoids Demonstrated Consistent EA Results from the MCF-7 Cell Proliferation Assay Compared with the Finding of Molecular Docking to ERs
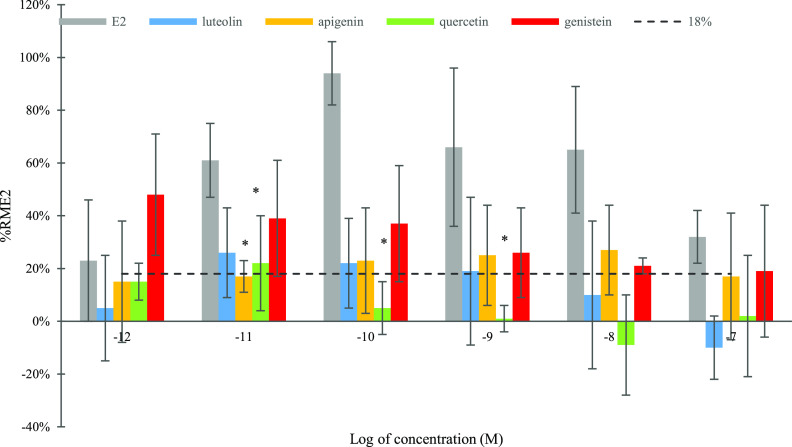
The chemicals were considered to have detectable EA levels, with the absolute % RME2 higher than vehicle control (VC) + 3 standard deviation (SD).21 In our study, VC + 3 SD was 18%. E2 demonstrated the highest EA at 10–10 M and EC50 at 1.0 × 10–11 M. Genistein showed the highest EA level (from 19 to 48% RME2, Figure 2) among test flavonoids except for 10–8 M, with the highest EA at 10–12 M (48%, no statistical significance). Apigenin had the second nonstatistically higher EA value (from 15 to 27%) than the findings of luteolin and quercetin except for 10–11 M (p > 0.05). Compared with quercetin, luteolin had a higher % RME2 level at four out six test concentrations (10–11 to 10–8 M) without statistical significance. Quercetin showed significantly less EA values than E2 at three concentrations (10–11, 10–10, and 10–9 M) (p < 0.05). Quercetin had no detectable EA (<18%) at all test concentrations except at 10–11 M.
Figure 2.
Estrogenic activity of E2, luteolin, apigenin, quercetin, and genistein. Data represented as mean ± SDof at least three independent trials with triplicates in each trial; % RME2 indicates the relative maximum % E2. Differences were evaluated using one-way analysis of variance (ANOVA) followed by Tukey’s test; * indicates significant difference between test compounds and E2 at the same concentration (p < 0.05). The % RME2 value in the control group was set to 0%, and the SD value was set to 6%. The broad line shown indicates VC + 3 SD = 18% (VC: 0.1% dimethyl sulfoxide (DMSO) dissolved in cell medium).
2.3. Four Flavonoids Affected Chicken Embryonic Development Differently
In this test, 1% DMSO in phosphate-buffered solution (PBS) served as VC and two doses of E2 were used as positive controls. A higher dose (450 μg/kg) of genistein had the highest mortality rate (50%), which was followed by the higher dose (477 μg/kg) of luteolin with 43.8% mortality (Table 1). The two doses of E2 (9.1 and 91 μg/kg) and the lower dose (45 μg/kg) of genistein had mortality rate ∼30%. Apigenin and quercetin groups showed lower mortality rates (<25.0%) than the findings of the other two flavonoids, which agreed with the developmental toxicity and rat acute toxicity data from T.E.S.T. The highest malformation rate was found in the 47.7 μg/kg luteolin group at 18.8% with three stunting embryos. The deformed embryo (deformed claw or stunting) was also observed in a high dose of luteolin and quercetin and a low dose of apigenin groups (Figure S1). The lowest value of REEW (0.33) was detected in the low dose (47.7 μg/kg) of the luteolin group, which was significantly lower than the value of the VC group (Table 1, p < 0.05). Interestingly, the higher dose (450 μg/kg) of genistein had a significantly increased REEW at 0.45 (p < 0.05) than the value of the VC group. These results reflect that the exposure to the luteolin impacted mostly the chicken embryonic development; in contrast, the genistein treatment might cause other problems in chicken embryo growth, such as edema. Except for the quercetin 50 μg/kg treatment, the VC group showed the highest LSI value at 2.33%. The LSI values of two apigenin treatments were <2.00%, with a significant decrease at the lower dosage (p < 0.05). Compared with the VC, the higher-dose apigenin treatment had a significantly lower (∼20%) fetal liver weight (p < 0.05). The embryonic heart weight for treatments and controls was similar at approximately 0.19 g.
Table 1. Mortality Rate, Malformation Rate, Ratio of Embryo to Egg Weight (REEW), Liver Somatic Index (LSI) (%), and Weight of Embryo and Organs of Chicken Embryo on Day 18, after Injection of E2, Luteolin, Apigenin, Quercetin, and Genisteina.
| weights (g) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| treatments | injection concentration (mM) | final dose (μg/kg) | mortality rate | malformation rate | REEW | LSI (%) | embryo | liver | heart |
| VC | 1% DMSO | N/A | 6.25% (1) | 0.0% (0) | 0.39 ± 0.063 | 2.33 ± 0.04 | 23.18 ± 3.45 | 0.52 ± 0.03 | 0.21 ± 0.03 |
| E2 | 0.01 | 9.1 | 31.8% (7) | 0.0% (0) | 0.39 ± 0.001 | 2.18 ± 0.01 | 20.24 ± 3.53 | 0.54 ± 0.01 | 0.18 ± 0.03 |
| 0.1 | 91 | 33.3% (6) | 0.0% (0) | 0.38 ± 0.021 | 2.06 ± 0.38 | 22.89 ± 0.87 | 0.47 ± 0.07 | 0.18 ± 0.01 | |
| luteolin | 0.05 | 47.7 | 12.5% (2) | 21.43% (3) | 0.33 ± 0.012* | 2.14 ± 0.30 | 19.75 ± 0.75 | 0.44 ± 0.05 | 0.19 ± 0.05 |
| 0.5 | 477 | 43.75% (7) | 11.11% (1) | 0.35 ± 0.017 | 2.19 ± 0.18 | 20.70 ± 0.00 | 0.49 ± 0.05 | 0.18 ± 0.05 | |
| apigenin | 0.05 | 45 | 10.0% (1) | 11.11% (1) | 0.37 ± 0.007 | 1.81 ± 0.23* | 24.29 ± 0.03 | 0.44 ± 0.02 | 0.18 ± 0.02 |
| 0.5 | 450 | 10.0% (1) | 0.0% (0) | 0.41 ± 0.021 | 1.94 ± 0.24 | 21.49 ± 1.44 | 0.41 ± 0.02* | 0.17 ± 0.02 | |
| quercetin | 0.05 | 50 | 25.0% (2) | 0.0% (0) | 0.41 ± 0.035 | 2.52 ± 0.34 | 22.11 ± 2.26 | 0.56 ± 0.13 | 0.20 ± 0.02 |
| 0.5 | 500 | 12.5% (1) | 25.0% (1) | 0.43 ± 0.057 | 1.85 ± 0.21 | 24.06 ± 2.35 | 0.44 ± 0.01 | 0.21 ± 0.05 | |
| genistein | 0.05 | 45 | 40.0% (4) | 0.0% (0) | 0.41 ± 0.049 | 2.05 ± 0.44 | 21.96 ± 0.73 | 0.46 ± 0.10 | 0.17 ± 0.01 |
| 0.5 | 450 | 50.0% (5) | 0.0% (0) | 0.45 ± 0.085* | 2.12 ± 0.14 | 23.88 ± 2.02 | 0.50 ± 0.09 | 0.20 ± 0.03 | |
REEW: Ratio of embryo to egg weight, LSI: liver somatic index. The chemical solution (0.1 and 0.01 mM for E2, 0.5 and 0.05 mM for four flavonoids) was injected at 0.2 mL into the egg (average weight, 60 g) yielding a final dose in egg: 91 μg E2/kg, 9.1 μg E2/kg, 47.7 μg luteolin/kg, 477 μg luteolin/kg, 45 μg apigenin/kg, 450 μg apigenin/kg, 50 μg quercetin/kg, 500 μg quercetin/kg, 45 μg genistein/kg, and 450 μg genistein/kg (VC: 1% DMSO dissolved in PBS). The number in parentheses represents the number of dead chicken embryo or malformation chicken embryo. All values are expressed as mean ± standard deviation (SD) from two independent trials. Differences were evaluated using ANOVA followed by Turkey’s test, and statistical significance was indicated by p < 0.05 (*p < 0.05). *Statistically significant difference compared to VC.
The thiobarbituric acid-reactive substance (TBAR) level reflected the lipid peroxidation in chicken fetal liver. As shown in Figure 3, the VC group had the second lowest value of TBARs at 67.87 nmol/g and the higher dose of the quercetin group had the lowest value at 62.48 nmol/g. Significantly increased TBAR values were observed in two doses of apigenin (45 and 450 μg/kg) groups and the lower dose of luteolin (47.7 μg/kg) group than the values of VC, with values of 103.73, 112.31, and 142.56 nmol/g, respectively (p < 0.01). Within the two doses, a significantly higher TBARs level was detected in the luteolin treatment at the lower dose (p < 0.05). Generally, the quercetin and genistein groups had lower TBARs levels than the findings for apigenin and luteolin.
Figure 3.
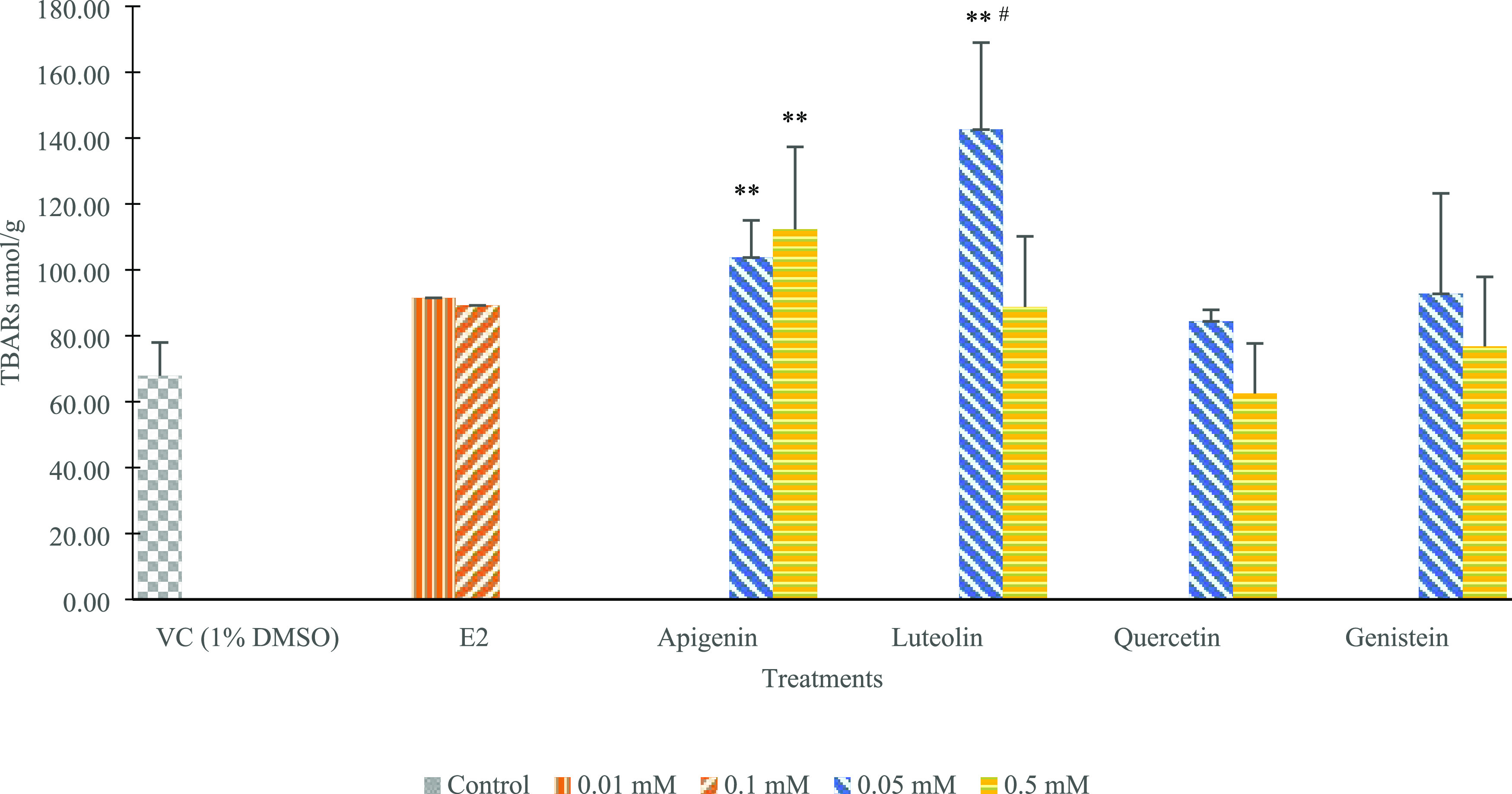
Impacts of E2, luteolin, apigenin, quercetin, and genistein on indications of TBARs value for each treatment group (each compound included two inject concentrations). Values are expressed as mean ± SD from two independent trials performed in triplicate (N = 6). The 0.1 and 0.01 mM for E2, while 0.5 and 0.05 mM for luteolin, apigenin, quercetin, and genistein resulted in the following final concentrations in egg (average of 60 g per egg, each injection at 0.2 mL): 91 μg E2/kg, 9.1 μg E2/kg, 47.7 μg luteolin/kg, 477 μg luteolin/kg, 45 μg apigenin/kg, 450 μg apigenin/kg, 50 μg quercetin/kg, 500 μg quercetin/kg, 45 μg genistein/kg, and 450 μg genistein/kg. Differences were evaluated using one-way ANOVA and followed by Tukey’s test, and statistical significance was indicated by p < 0.05 or p < 0.01; ** indicates statistically significant difference at p < 0.01 compared to VC (1% DMSO dissolved in PBS), and # indicates difference between two doses within one treatment p < 0.05.
2.4. Different Mutagenic Index (MI) Revealed for Four Flavonoids Using the Ames Test
Table 2 shows that the positive control of each bacterial strain with or without S9 produced a statistically significant increase in the number of revertant colonies, and the VC of each test compound (0.1% DMSO in PBS) had a number within our historical ranges, which confirmed the sensitivity and accuracy of the test system. Because these four compounds were conducted in two separated periods, two different sets of negative and positive controls were included for comparison. A significant increase in the number of revertants was observed for all three strains (TA98, TA100, and TA102) after exposure to luteolin. For TA98 strain, without S9 mixture, luteolin increased the number of revertants at 50, 5, and 0.5 pmol/plate with MI at 1.9, 1.9, and 1.8, which were very close to the critical MI value of 2.0. A significant increase of revertants was also observed in TA100 and TA102 strains after exposure to luteolin at 0.05–50 pmol/plate with MI up to 1.4. After exposure to the apigenin, the significant increase in the revertants was only observed at TA100 (+S9) and TA102 strains (−S9) at 0.5–50 pmol/plate, with the MI up to 1.4. Exposure to quercetin leads to the significantly increased number of revertants of TA100 and TA102 strains at four concentrations (5 × 10–3, 0.5, 5, and 50 pmol/plate) (p < 0.05) compared with VC, with MI up to 1.4. Interestingly, genistein did not significantly increase numbers at any strains and test concentrations. Compared with the T.E.S.T. data, only discrepancy was determined in apigenin. It was classified as mutagenicity-negative in T.E.S.T. but showed signs of mutagenic activity in the Ames test.
Table 2. Impacts of Luteolin, Apigenin, Quercetin, and Genistein on Mutagenicity Using the Ames Testa.
| number
of revertants/plate in S. typhimurium strains (M ± SD) and (MI) |
||||||
|---|---|---|---|---|---|---|
| treatments | TA98 (±) | TA100 (±) | TA102 (±) | |||
| Luteolin (pmol/plate) | ||||||
| 0b | 10 ± 1 | 74 ± 10 | 93 ± 6 | 306 ± 7 | 110±11 | 302±21 |
| 50 | 19 ± 4** (1.9) | 88 ± 14 (1.2) | 83 ± 6 (0.9) | 427 ± 7** (1.4) | 150 ± 7** (1.4) | 409 ± 7** (1.4) |
| 5 | 19 ± 2** (1.9) | 80 ± 3 (1.1) | 97 ± 4 (1.0) | 371 ± 21 (1.2) | 135 ± 9 (1.2) | 422 ± 13** (1.4) |
| 0.5 | 18 ± 4** (1.8) | 85 ± 6 (1.1) | 95 ± 6 (1.0) | 387 ± 15* (1.3) | 144 ± 8* (1.3) | 394 ± 10* (1.3) |
| 5 × 10–2 | 13 ± 5 (1.3) | 75 ± 8 (1.0) | 90 ± 4 (1.0) | 357 ± 15 (1.2) | 126 ± 8 (1.1) | 388 ± 14* (1.3) |
| 5 × 10–3 | 12 ± 1 (1.2) | 79 ± 1 (1.1) | 76 ± 13 (0.8) | 338 ± 8 (1.1) | 129 ± 13 (1.2) | 377 ± 15 (1.2) |
| 5 × 10–4 | 11 ± 5 (1.1) | 75 ± 13 (1.0) | 86 ± 11 (0.9) | 349 ± 6 (1.1) | 126 ± 6 (1.1) | 372 ± 37 (1.2) |
| 5 × 10–5 | 7 ± 1 (0.7) | 64 ± 4 (0.9) | 86 ± 4 (0.9) | 371 ± 8 (1.2) | 113 ± 14 (1.0) | 361 ± 25 (1.2) |
| positive control | 723 ± 64c** | 846 ± 47e** | 860 ± 52d** | 941 ± 56e** | 854 ± 32f** | 997 ± 35e** |
| Apigenin (pmol/plate) | ||||||
| 0b | 10 ± 1 | 74 ± 10 | 93 ± 6 | 306 ± 7 | 110 ± 11 | 302 ± 21 |
| 50 | 11 ± 4 (1.1) | 75 ± 20 (1.0) | 96 ± 8 (1.0) | 400 ± 2* (1.3) | 147 ± 8* (1.3) | 360 ± 13 (1.1) |
| 5 | 13 ± 1 (1.3) | 80 ± 10 (1.1) | 96 ± 4 (1.0) | 390 ± 4* (1.3) | 154 ± 11** (1.4) | 364 ± 4 (1.1) |
| 0.5 | 9 ± 1 (0.9) | 85 ± 6 (1.1) | 94 ± 4 (1.0) | 415 ± 4** (1.4) | 142 ± 15 (1.3) | 308 ± 19 (1.0) |
| 5 × 10–2 | 10 ± 4 (1.0) | 87 ± 6 (1.2) | 85 ± 6 (0.9) | 377 ± 15 (1.2) | 131 ± 11 (1.2) | 303 ± 19 (1.0) |
| 5 × 10–3 | 10 ± 3 (1.0) | 80 ± 18 (1.1) | 75 ± 13 (0.8) | 349 ± 10 (1.1) | 124 ± 12 (1.1) | 290 ± 20 (0.9) |
| 5 × 10–4 | 7 ± 3 (0.7) | 83 ± 9 (1.1) | 83 ± 6 (0.9) | 342 ± 14 (1.1) | 114 ± 13 (1.0) | 288 ± 14 (0.9) |
| 5 × 10–5 | 10 ± 3 (1.0) | 68 ± 2 (0.9) | 87 ± 6 (0.9) | 358 ± 6 (1.2) | 126 ± 8 (1.1) | 316 ± 19 (1.0) |
| positive control | 723 ± 64c** | 846 ± 47e** | 860 ± 52d** | 941 ± 56e** | 854 ± 32f** | 997 ± 35e** |
| Quercetin (pmol/plate) | ||||||
| 0b | 22 ± 5 | 23 ± 13 | 101 ± 3 | 322 ± 14 | 264 ± 28 | 306 ± 8 |
| 50 | 30 ± 2 (1.3) | 18 ± 13 (0.8) | 88 ± 8 (0.9) | 369 ± 12 (1.1) | 347 ± 10* (1.4) | 310 ± 31 (1.0) |
| 5 | 27 ± 11 (1.2) | 19 ± 10 (0.8) | 104 ± 8 (1.0) | 386 ± 12* (1.2) | 312 ± 13 (1.2) | 279 ± 21 (0.9) |
| 0.5 | 28 ± 1 (1.3) | 25 ± 13 (1.1) | 105 ± 11 (1.0) | 398 ± 8* (1.2) | 267 ± 23 (1.0) | 326 ± 19 (1.1) |
| 5 × 10–2 | 19 ± 6 (0.8) | 23 ± 17 (1.0) | 113 ± 6 (1.1) | 366 ± 14 (1.1) | 202 ± 14 (1.2) | 337 ± 7 (1.1) |
| 5 × 10–3 | 31 ± 6 (1.4) | 27 ± 16 (1.2) | 103 ± 7 (1.0) | 367 ± 5 (1.1) | 325 ± 10* (1.3) | 369 ± 13* (1.2) |
| 5 × 10–4 | 27 ± 3 (1.2) | 18 ± 13 (0.8) | 98 ± 7 (1.0) | 349 ± 8 (1.1) | 301 ± 16 (1.2) | 312 ± 16 (1.0) |
| 5 × 10–5 | 25 ± 2 (1.1) | 17 ± 8 (0.7) | 97 ± 4 (1.0) | 358 ± 14 (1.1) | 284 ± 15 (1.1) | 291 ± 11 (0.9) |
| positive control | 634 ± 16c** | 806 ± 25e** | 743 ± 28d** | 845 ± 45e** | 740 ± 23f** | 757 ± 35e** |
| Genistein (pmol/plate) | ||||||
| 0b | 22 ± 5 | 23 ± 13 | 101 ± 3 | 322 ± 14 | 264 ± 28 | 306 ± 8 |
| 50 | 13 ± 4 (0.6) | 28 ± 16 (1.2) | 80 ± 12 (0.8) | 296 ± 11 (0.9) | 215 ± 27 (0.8) | 324 ± 28 (1.1) |
| 5 | 18 ± 6 (0.8) | 23 ± 14 (1.0) | 96 ± 4 (0.9) | 301 ± 13 (0.9) | 240 ± 8 (0.9) | 299 ± 4 (1.0) |
| 0.5 | 15 ± 1 (0.7) | 26 ± 13 (1.1) | 103 ± 10 (1.0) | 307 ± 7 (1.0) | 247 ± 18 (1.0) | 336 ± 17 (1.1) |
| 5 × 10–2 | 16 ± 2 (0.7) | 28 ± 5 (1.2) | 97 ± 11 (0.9) | 308 ± 8 (1.0) | 222 ± 30 (0.9) | 311 ± 18 (1.0) |
| 5 × 10–3 | 11 ± 1 (0.5) | 28 ± 10 (1.2) | 100 ± 11 (1.0) | 313 ± 11 (1.0) | 198 ± 8 (0.8) | 312 ± 13 (1.0) |
| 5 × 10–4 | 13 ± 6 (0.6) | 24 ± 8 (1.0) | 94 ± 8 (0.9) | 301 ± 4 (0.9) | 196 ± 20 (0.8) | 285 ± 10 (0.9) |
| 5 × 10–5 | 15 ± 4 (0.7) | 19 ± 6 (0.8) | 98 ± 6 (1.0) | 294 ± 6 (0.9) | 207 ± 5 (0.8) | 290 ± 4 (0.9) |
| positive control | 634 ± 16c** | 806 ± 25e** | 743 ± 28d** | 845 ± 45e** | 740 ± 23f** | 757 ± 35e** |
10–12 to 10–6 M at 0.05 mL to yield final concentrations from 5 × 10–5 to 50 pmol/plate. The 0.1% DMSO in PBS was used as a negative control and was the solvent for dissolving test chemicals (VC). Differences were evaluated using one-way ANOVA followed by Tukey’s test, and statistical significance was indicated by p < 0.05 and p < 0.01 compared to the negative control, VC. Data were shown as mean ± SD revertants/plate from two independent trials performed in triplicate (N = 6).
VC (0.1% DMSO in PBS); positive controls.
2-NF (1 μg/plate).
NaN3 (1 μg/plate).
2-AA (5 μg/plate).
Mitomycin C (1 μg/plate).
2.5. Structure–Activity Relationships (SARs) of Four Flavonoids
The four flavonoids possessed a similar structure backbone with different number of phenolic hydroxyl groups and different locations of the hydroxybenzene ring B (Table 3 and Figure 1). Compared to the structure of the other three flavonoids, genistein had the hydroxybenzene ring B at position R2 rather than R1, as the characteristic structure for isoflavones. Apigenin and luteolin belonged to the flavone subgroup, and luteolin had one more hydroxyl group at the R3 position in ring B. In this study, the EA results showed that genistein had the highest % RME2 value than other three flavonoids at five test concentrations. From 10–12 to 10–7 M, the highest % RME2 value of luteolin and quercetin was observed at 10–11 M (10 pM), while the values of apigenin and genistein were reported at 10–8 M (10 nM) and 10–12 M (1 pM), respectively. The ranking of max % RME2 values was consistent with the findings of the docking results to two ERs, with genistein showing the highest max % RME2, followed by apigenin and luteolin, while apigenin and luteolin had similar values. The high EA for genistein indicates the significant impacts from the hydroxybenzene ring B at position R2. When comparing the EC50 and estradiol equivalent factor (EEF) values, apigenin had the highest values, followed by genistein. The EC50 values of apigenin, genistein, and luteolin were 6.2 × 10–11, 1.2 × 10–10, and 8.4 × 10–9 M, respectively (Table 3). Apigenin and genistein had the top two highest EEF values at 0.16 and 0.08, respectively. The EC50 value was not available for quercetin since it only had EA at one test concentration 10–11 M. Besides the potential effects of the B ring’s location, the numbers of hydroxyl substitution also play a role in EA potency (from EC50 and EEF values) following the order: apigenin (three hydroxyl groups) > genistein (three hydroxyl groups) > luteolin (four hydroxyl groups) > quercetin (five hydroxyl groups).
Table 3. Estrogenic, Developmental, and Mutagenic SARs of Four Flavonoids.
| Flavonoids | luteolin | apigenin | quercetin | genistein | ||
|---|---|---|---|---|---|---|
| Subgroup | flavone | flavone | flavonol | isoflavone | ||
| Structure | R1 | ring B | ring B | ring B | H | |
| R2 | H | H | OH | ring B | ||
| R3 | OH | H | OH | H | ||
| Experimental tests | MCF-7 cell proliferation | max % RME2a | 26% | 27% | 22% | 48% |
| EC50 (M)b | 8.4 × 10–9 | 6.2 × 10–11 | NA | 1.2 × 10–10 | ||
| EEFc | 0.0012 | 0.16 | NA | 0.08 | ||
| Chicken embryonic assay | maximum mortality rated | 43.8% | 10.0% | 25.0% | 50.0% | |
| maximum TBARs valuee (nmol/g) | 142.56 | 112.31 | 84.37 | 92.73 | ||
| Ames test | max MI valuef | 1.9 | 1.4 | 1.4 | 1.2 | |
| In silico simulation | Docking to ERs | ER α | –8.6 | –8.8 | –8.2 | –9.2 |
| binding class | medium | medium | low | good | ||
| ER β | –7.6 | –8.2 | –7.1 | –8.7 | ||
| binding class | low | medium | low | medium | ||
| T.E.S.T. results | oral rat LD50 (mg/kg) | 2175.63 | 1707.99 | 2782.81 | 1172.86 | |
| development value | 0.88 | 0.65 | 0.77 | 0.76 | ||
| mutagenicity value | 0.53 | 0.29 | 0.55 | 0.23 | ||
The highest % RME2 from a test range at 10–12–10–7 M measured by MCF-7 cell proliferation assay.
EC50 of test compounds was calculated using GraphPad Prism. “NA” means unavailable data since detectable EA of quercetin was only observed in one test dose.
EEF was calculated as the EC50 of E2 divided by that of the test compounds. NA means unavailable data since detectable EA of quercetin is only observed in one test dose.
The highest mortality rate detected in chicken embryonic assay (detected at a higher injection concentration (0.5 mM) for luteolin, apigenin, and genistein; at a lower injection concentration (0.05 mM) for quercetin).
The highest TBARs value detected in chicken embryonic assay (detected at a lower injection concentration (0.05 mM) for luteolin, quercetin, and genistein; at a higher injection concentration (0.5 mM) for apigenin).
The highest MI value from the test range 5 × 10–5 to 50 pmol/plate measured by Ames test.
The presence of hydroxyl group at 3′ position in B ring (e.g., luteolin) increased the developmental toxicity in the chicken embryo model and the MI value in the Ames test, compared to findings of apigenin, which were also consistent with the T.E.S.T. results. Quercetin belonged to the flavonol group with an extra hydroxyl group at position R2. This 2-hydroxyl substitution in the C ring deceased the developmental toxicity in the chicken embryo model and T.E.S.T. simulation. Compared to apigenin, quercetin had two additional hydroxyl groups at 3-position in C ring and 3′-position in B ring (R2 and R3), resulting in comparable developmental toxicity but higher mutagenic activity in T.E.S.T. (Table 3). Genistein had a B ring at 3-position in C ring (R2), which increased the developmental toxicity and decreased MI level, compared to the findings of apigenin.
3. Discussion
3.1. Binding Affinities to EDCs’ Targeting Receptors and EA Results from MCF-7 Cell Proliferation Assay Indicated Endocrine Disruption Potentials of the Test Flavonoids
In this study, we applied for the first time a multitiered platform consisting of in silico, in vitro, and in vivo tests and compared the in vitro or in vivo results to the in silico data on three toxicity endpoints (EA, developmental toxicity, and mutagenicity) for understanding the impacts of flavonoid structure on toxicities. A few studies investigated the potential toxicity of flavonoids systematically, especially at the low exposure range. Our study is the first to investigate EA, mutagenicity, and developmental toxicity at low and human exposure-related concentrations for different flavonoids subgroups. Our results demonstrated that the EA effect, the sign of mutagenicity, and chicken developmental toxicity were detected at concentrations <1 μM for different flavonoids. Flavonoids have high potentials to disrupt hormone pathways in the endocrine system, even in low exposure levels and these are not well studied. Considering the binding to the different targeted receptors and the further alternation of the transcription levels as a crucial underlying mechanism of ED, we first used the molecular docking of four flavonoids to 14 nuclear receptors to reveal the potential ED of these flavonoids. The ARs, ERs, and TRs with vital roles in the development, growth, and function of reproductive and nonreproductive tissues were the major targets for EDCs. Our results indicated that the four test flavonoids showed high binding affinities to the two conformational AR structures, which was similar to the findings of E2. Compared with the finding of E2, four test flavonoids showed higher binding affinity levels to MR and similar levels to the GR, which are members of the steroid receptor subfamily and regarded as potential targets for EDCs.22 Both are the receptors to adrenal cortical steroid hormones (e.g., aldosterone) and play essential roles in the immune, metabolic, endocrine, and nervous systems. Therefore, in addition to AR, ERs, and TRs, it is highly possible for the flavonoids to exert endocrine disruption effects through other steroid receptors.
Because of the similar structure to 17β-estradiol, some flavonoids (e.g., genistein) are named phytoestrogens, which can bind to ERα and ERβ and exert estrogenic or/and antiestrogenic effects in mammals. Genistein was reported to compete with E2 for the ERs with a higher binding affinity for ERβ (87%) and lower binding affinity for ERα (4%).23 Apigenin is also a weak phytoestrogen with binding affinities of 0.3 and 6% for ERα and ERβ, respectively.24 Genistein can induce proliferative activity in MCF-7 cells at 10–7 to 10–5 M with the maximum value at 10–6 M.25 Apigenin also demonstrated strong activating ability for ERα or ERβ than luteolin in ERα or ERβ SK-NBE-derived cells, while luteolin only had a slight activating effect on ERβ.26 The EA of quercetin in the previous MCF-7 proliferation assay was contradictory. Quercetin dramatically inhibited MCF-7 cell growth at concentration > 2.5 μM, while the stimulation effect was not detected at low concentration ranges (0.5–2.5 μM).27 In another study,28 quercetin only slightly increased cell proliferation in the MCF-7 cells (<120%) at 0.001–1 μM when tested from 0.001 to 50 μM. In our study, quercetin had a weak EA of 22% only at one concentration, 10–11 M. Most of these previous findings were obtained at higher concentrations than the levels used in our work, yet the highest % RME2 of 48% at 10–12 M was still detected for genistein. In addition, it had EA at a wider range from 10–12 to 10–7 M, compared with findings of apigenin, luteolin, and quercetin.
It is noteworthy that several flavonoids such as genistein and apigenin were reported to have biphasic effects on the proliferation of estrogen-dependent MCF-7 cells in a concentration-dependent manner.29,30 High concentrations of genistein (>10 μM) were associated with tumor suppression, whereas a low concentration range (0.01–1 μM) had a proliferation effect in estrogen receptor-positive cells.30,31 Apigenin is able to stimulate ER-positive breast cancer cell lines (e.g., MCF-7 and T47D cells) with less potent activities than that of genistein from 10 nM to 10 μM.32 Similarly, luteolin also acts as a partial agonist that stimulates the MCF-7 cell’s proliferation at 1 nM to 10 μM.33
In our study, the four flavonoids all showed weak EA (max % RME2 from 22 to 48%; Figure 2 and Table 3) in MCF-7 cells from 10–12 to 10–7 M. The highest EA results from the MCF-7 cell proliferation assay were consistent with the molecular docking results to two ERs, following the order genistein > apigenin > luteolin > quercetin, which confirmed that binding to the ERs was one critical pathway in EA. Interestingly, genistein had a bigger max % RME2, 21% more than the value of apigenin, but it had half values of EEF or EC50 of apigenin (Table 3). Even though apigenin and luteolin had similar max % RME2 values, EEF or EC50 values for both chemicals varied by 100 times. This indicated that EEF or EC50 used in previous publications for ranking the EA effects might not be sufficient as they did not consider the different patterns of response curves.34,35 It was noteworthy that genistein had different dose–response curve patterns in the calculation of EC50 and EEF, compared with the response curves for apigenin and luteolin using the MCF-7 cell proliferation from 10–12 to 10–7 M. The highest EA (48%) of genistein was determined at the lowest concentration, while apigenin showed the highest effect (27%) at 10–8 M. For luteolin and quercetin, the highest EA values existed in 10–11 M at 26 and 22%, respectively. The maximum EA of E2 (94%) was obtained at 10–10 M. The nonmonotonic dose–response (NMDR) effect, which has been widely proved in endocrine-disrupting chemicals, was detected for E2, apigenin, luteolin, and quercetin, with the highest EA detected at the middle concentrations.
3.2. Four Flavonoids Showed Developmental Toxicity by In Silico Simulation T.E.S.T. and Chicken Embryonic Assay
Another in silico simulation T.E.S.T. result showed that E2, apigenin, and genistein had a stronger acute toxicity class (class 4) than the classification for luteolin and quercetin (class 5, less toxicity).20 Furthermore, E2 and four test flavonoids were classified as developmental toxicants with the order E2 > luteolin > quercetin > genistein > apigenin. To validate the in silico simulation in the T.E.S.T., we assessed the developmental toxicity of four flavonoids using comparable dosages with human daily exposure levels in a chicken embryo model. To derive human equivalent dose (mg/kg/day) from chicken, a conversion factor of 18.5 and a safety factor were used based on the recommendation by the Agency for Toxic Substances and Disease Registry (ATSDR).36,37 Thus, the dosages (45–500 μg/kg) used in this study can be converted to 83.25 to 925 μg/kg (human equivalent dose = (chemical dose in chicken embryo × 18.5)/10), which agreed well with the published human estimated daily intake level of flavonoids at 20–3000 μg/kg.8 Genistein at 45 and 450 μg/kg, along with luteolin at 477 μg/kg, exerted detrimental effects on chicken embryo development, with mortality rates higher than 40%. Low-dose luteolin treatment (47.7 μg/kg) induced a 12.5% death rate but a relatively high malformation rate at 18.8% of stunting. On the other hand, the mortality rates and deformation rates were lower after exposure to apigenin and quercetin. Furthermore, other developmental indexes, including the REEW, LSI, and organs weight, were impacted by luteolin, apigenin, and genistein treatments. Our findings on the developmental toxicity of flavonoids confirmed the previously reported results on zebrafish embryo-larval developmental toxicity of apigenin and genistein.14 Consistent with T.E.S.T. results on the classification of developmental toxicants for the four flavonoids, all of them showed adverse effects on the chicken embryogenesis. Luteolin, which had the highest developmental toxicity in T.E.S.T., showed a higher mortality rate than apigenin and quercetin.
Significantly increased TBAR values were detected after exposure to apigenin and luteolin (p < 0.05) in our study. As the primary final product of lipid peroxidation, the TBAR level was commonly used as a biomarker to evaluate oxidative damage and has been reported as an important contributor to DNA damage and mutation.38 Our results agreed with the previous finding that apigenin at 100 and 200 mg/kg led to hepatotoxicity in the Swiss mice model, even though our dosages were 1000 times lower. The mouse toxicity included increased levels of MDA and ROS, along with altered gene expression levels related to oxidative stress and apoptosis.39 High TBAR values in the apigenin treatment might be one mechanism to impact the developmental toxicity related to the LSI and liver weight.
Luteolin and genistein showed a high and similar death rate (43.75 and 50%) on chicken embryos especially at the higher dosage groups while the TBARs levels in these two groups were not statistically different (p > 0.05). Oxidative stress might not be the only pathway to induce toxicity. In previous studies, luteolin (at 10 and 20 μM) and genistein showed antiangiogenic activity by inhibiting the Gas6/Axl signaling pathway or targeting at angiostatin,40,41 and the relationship between the antiangiogenic activity and teratogenic effects in the development of chicken embryos included the primary types of twisting in the spinal cord, which caused a delay in chicken development.42 Additionally, exposure to genistein at 0.025 to 0.1 mM significantly decreased the survival rate for zebrafish embryos, which might be related to the inhibition of tyrosine kinase and the disruption of several ionic channels in the organism.43 Interestingly, the lowest TBAR level was found in the quercetin group at 62.48 nmol/g, even lower than the finding of VC (67.87 nmol/g), which might attribute to the reported potent antioxidant effects of quercetin with decreased levels of oxidative stress markers and increased antioxidant enzyme activities in mice and rat models.44,45 Quercetin demonstrated lower chicken embryonic mortality and deformation rate in our study compared with the findings of luteolin and genistein, which could be associated with the lowest TBARs levels. In the chicken embryonic assay, even though the high mortality rates of E2 and flavonoids (except quercetin) were detected in higher-dose treatments, NMDR was revealed for luteolin and apigenin in developmental indexes and TBAR levels. A significantly decreased REEW level was detected in the 47.7 μg/kg luteolin group but not for the 477 μg/kg group. Only 45 μg/kg (but not 450 μg/kg) apigenin treatment had a significantly lower LSI%.
3.3. Luteolin, Apigenin, and Quercetin Had the Sign of Mutagenicity by Ames Test at a Low Concentration Range
We determined the mutagenicity in the low exposure range from 5 × 10–5 to 50 pmol/plate of these four flavonoids as a few studies evaluated the mutagenic activity of the flavonoids at these exposures. The exposures were easily overlooked, but they were possible exposure levels for humans due to the low bioavailability of these flavonoids. The revertant number of VC and positive controls in our study were similar to the previous findings,46 showing the reliability of our study. In our Ames test, luteolin, apigenin, and quercetin showed signs of mutagenicity in two or three test strains, but genistein did not demonstrate such a sign. This result partly agreed with the QSRA simulation results, in which luteolin and quercetin were classified as mutagenicity-positive while apigenin and genistein were mutagenicity-negative. Our results were also in good agreement with the previous research that luteolin showed signs of mutagenicity in the TA102 strain at 116.4 and 174.7 nmol/plate, and quercetin showed mutagenicity for three strains TA98, TA100, and TA102 at 12.1 to 147.8 nmol/plate,15 even though our test levels are much lower. The potential mutagenicity of flavonoids is mainly due to their pro-oxidant activity, which will produce free radicals, cause DNA damage, and lead to mutagenesis.47 No mutagenic activity was reported for genistein by others when using the incorporation or the preincubation Ames assays at concentrations 10–3333 μg/plate.48 Interestingly, the NMDR effect was still found in the Ames test for some flavonoids because the highest MI level was observed at 5 × 10–3 pmol/plate for quercetin in TA102 strain (with S9) among levels ranging from 5 × 10–5 to 50 pmol/plate.
3.4. SARs of Four Flavonoids on Three Toxicity Endpoints Revealed Some Small Structural Differences with Big Toxicity Impacts of the Test Flavonoids
In general, these four phenolic compounds had similar structures with only a difference in the number of hydroxyl groups or location of the B ring; however, they showed different toxicity responses in several endpoints. Quercetin, belonging to the flavonols with the hydroxyl group at position 3 in ring C, showed low developmental toxicity, low binding affinity to major hormone receptors (such as ERs and TRs), and high mutagenic activity even at low concentrations. As an essential compound in the isoflavones group, genistein showed high binding ability to estrogen receptors and other hormone-related receptors, high acute toxicity and developmental toxicity, and low mutagenicity. For the compounds in the flavone group, apigenin and luteolin showed a moderate binding affinity in EDC target receptors, a high developmental toxicity, and a high risk of mutagenicity. Our findings generally showed a similar pattern to that reported in previous findings on the correlation between their structures and cytotoxicity. In a previous study, the 3′- hydroxyl substitution in the B ring (R3 position) played an important role in inhibiting the growth of HL-60 cells, which made luteolin the most cytotoxic flavone among 14 tested flavonoids.49 A SAR study of the effects of flavonoids on the apoptosis of HL-60 cells indicated that apigenin had a higher potency than quercetin in inducing cellular DNA fragmentation and ROS generation.50 In our study, apigenin showed the highest lipid peroxidation (TBARs) level in fetal chicken livers, and the TBAR values decreased as the number of hydroxyl groups increased in luteolin (B-3′) at the higher dosage group or in quercetin (B-3′ and C-3) at both dosage groups. Compared to apigenin, luteolin and quercetin have an ortho-dihydroxy structure in the B ring. The number and position of hydroxy groups in polyphenol structures have been reported to play a critical role in the antioxidant activity of flavonoids.51−53 Thus, the lower TBAR value for quercetin group might be associated with its higher antioxidant capacity. The additional hydroxyl group at C-3 for quercetin showed an effect in reducing oxidative stress in chicken fetal liver. Several studies reported that the presence of a C-2,3 double bond increased the cytotoxic effects, while the presence of a 3-hydroxyl group in the C ring lowered the cytotoxicity.54,55 In our study, all four flavonoids had the C-2,3 double bond and quercetin had a 3-hydroxyl group in the C ring. Indeed, quercetin with the C-3 hydroxyl group was attributed to the lower developmental toxicity in chicken embryos and the lower acute rat toxicity from the T.E.S.T. simulation. Our work demonstrated that a small structural difference could have big impacts on different toxicity endpoints, and further studies are still needed to have a more comprehensive understanding of the impacts of different structural changes on various toxicities. The absorption and metabolism of flavonoids after human consumption also need to be considered to understand the contribution of bioavailability to their underlying toxicology mechanisms. These four flavonoids (luteolin, apigenin, quercetin, and genistein) possessed similar pharmacokinetics in both animals and humans, and they were extensively metabolized. Different phase II metabolites have been detected in plasma and urine samples in human, among which glucuronides and sulfates were two primary types.56−58 It is important in the future to evaluate the toxicities of these glucuronides and sulfates.
4. Conclusions
Our study applied a new multitiered method consisting of in silico, in vitro, and in vivo tests to estimate the potential toxicity of four common flavonoids (luteolin, apigenin, quercetin, and genistein) effectively. The results indicated that except for their therapeutic potential and chemoprotective ability, they demonstrated toxicity concerns, including developmental toxicity, endocrine disruption, and mutagenicity. The toxic concerns are especially big when young population are exposed to the chemicals. In addition, the two in silico simulations, molecular docking and T.E.S.T., could provide insightful information in assessing endocrine-disrupting activity, acute toxicity, developmental toxicity, and mutagenicity of phenolic compounds. The high binding affinity was observed of these four compounds to AR, ER, GR, MR, and TR in the molecular docking. The order of binding affinity to ERs was consistent with the EA results found in the MCF-7 cell proliferation assay. The T.E.S.T. simulation results agreed well with the findings from the chicken embryo model and bacterial reverse mutation test. The SAR results showed that genistein (isoflavone) possessed high developmental toxicity and EA, along with low TBARs and MI levels. For two flavones, luteolin showed higher developmental toxicity and signs of genotoxicity than apigenin. Quercetin (flavonol) with 2-hydroxyl substitution in the C ring had lower developmental toxicity and EA among the test flavonoids. Our approach can be used as a valuable alternative toxicity assessment platform for natural compounds, following the guiding principles published by the Society of Toxicology to use alternative ways to reduce animal number and refine or replace whole animals.
5. Experimental Section
5.1. Chemicals and Cell Lines
Luteolin, apigenin, quercetin, genistein, E2, DMSO (D1391), and PBS (Gibco, 20-012-027) were purchased from Fisher Scientific (Waltham, MA). The three Salmonella typhimurium tester strains (TA98, TA100, and TA102), top agar, Oxoid Nutrient Broth No.2, and S9 mixture solutions were purchased from Molecular Toxicology, Inc. (Boone, NC). MCF-7 cells were purchased from American Type Culture Collection (ATCC No. HTB-22). Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Cat. No.: 12-430-054), phenol red-free DMEM (Gibco, 21-063-029), fetal bovine serum (FBS) (Gibco, 16-140-071), charcoal-stripped FBS (Gibco, 12-676-029), and penicillin–streptomycin (Gibco, 15-140-148) were purchased from Fisher Scientific, along with the cell culture 96-Well clear polypropylene microplates (Corning, 05-539-200) and polystyrene T-25 flasks (Corning, 08-772-45).
5.2. In Silico Simulations
5.2.1. Molecular Docking
The endocrine-disrupting potential of four common flavonoids (luteolin, apigenin, quercetin, and genistein) was estimated by the docking Interface for Target Systems (DoTS) platform (named endocrine disruptome tool) via AutoDock Vina.17,59 The SMILES (Simplified Molecular-Input Line Entry-System) files for each test chemicals (ligands) were used for simulating the binding affinity to 14 nuclear receptors described in our previous study60 and are listed in Table S1.
5.2.2. Toxicity Estimation Software Tool (T.E.S.T.)
T.E.S.T. has been developed by the United States Environmental Protection Agency (U.S. EPA) to allow users to easily estimate toxicity and physical properties using a variety of QSAR methodologies. The predicted toxicity data presented in this study was generated from the Consensus method, which was estimated by an average of the predicted toxicities of five QSAR methods, including hierarchical, FDA, single model, group contribution, and nearest-neighbor method. The endpoints included: 96 h fathead minnow LC50, 48 h Daphnia magna LC50, Tetrahymena pyriformis IGC 50, oral rat LD50, bioaccumulation factor, developmental toxicity, and mutagenicity.
5.3. MCF-7 Cell Proliferation Assay
The MCF-7 cell proliferation assay was performed as previously described.60,61 The cells were treated with five chemicals (luteolin, apigenin, quercetin, genistein, and E2) at six different concentrations ranging from 10–12 to 10–7 M in EA-free culture medium. The proliferation rate was quantified by measuring the absorbance of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) products at 570 nm using a microplate reader (BioTek Synergy2). Each test was repeated in three independent trials and in triplicate for each trial. The EA of test chemicals was shown as the relative maximum %E2 (% RME2) calculated from the following equation: 100 × (OD of test – OD of VC)/(MAX OD of E2 – OD of VC). The half-maximal effective concentration (EC50, by Prism 8) value and the estradiol equivalent factor (EEF) (EC50 of E2 divided by EC50 of the sample) were calculated for each compound.
5.4. Chicken Embryonic Assay
5.4.1. Egg Treatment
In total, 144 fertilized Leghorn eggs were obtained from the University of Delaware research farm. The eggs were weighed and divided into 11 groups: VC and two dosages of each of five compounds. On day 7, the eggs were candled and a hole was drilled for injection of each chemical solution or VC at 0.2 mL. The chemical solution concentrations were 0.1 and 0.01 mM for E2, and 0.5 and 0.05 mM for luteolin, apigenin, quercetin, and genistein respectively. The final doses in egg (average of 60 g per egg) included 91 μg E2/kg, 9.1 μg E2/kg, 476.5 μg luteolin/kg, 47.7 μg luteolin/kg, 450 μg apigenin/kg, 45 μg apigenin/kg, 500 μg quercetin/kg, 50 μg quercetin/kg, 450 μg genistein/kg, and 45 μg genistein/kg. The eggs were randomly assigned on day 0 to each treatment and control group, with 16 eggs for each of control and two luteolin groups, 22 eggs for each of two E2 groups, and 10 eggs for each of apigenin, quercetin, and genistein groups. More eggs were used in luteolin groups to validate the findings. On the injection day (day 7), the four unfertilized eggs were removed from E2 91 μg/kg and two from each quercetin group (50 and 500 μg/kg), and not recorded, resulting in different total numbers of embryos in Table 1. The hole was sealed with Duco Cement, and the eggs were placed back in the egg incubator at 38 °C and 60% relative humidity. Each test was repeated at least in two independent trials. All of the experiments on the chicken embryos were performed in accordance with all national or local guidelines and regulations.
5.4.2. General Toxicity
The number of dead and deformed embryos was recorded during the tests. The incubation was terminated on day 18 by placing the embryos in the refrigerator overnight. All embryos were dissected and evaluated for deformation, embryo mass, liver mass, heart mass, ratio of embryo to egg weight (RREW), and liver somatic index (LSI). The TBAR level was evaluated using the TBARS assay kit (from Cayman Chemical, Item No. 700870) following the manufacturer’s instructions. Each test was repeated in two independent trials and in duplicate for each trial.
5.5. Ames Test
The Ames test using three S. typhimurium tester strains (TA98, TA100, and TA102) was conducted by the preincubation method as previously described.60 Briefly, 0.05 mL of tested compounds, 0.5 mL of S9 metabolic activation mixture (or 0.5 mL PBS), and 0.1 mL of bacterial culture were mixed. After 30 min incubation at 37 °C, 2 mL of top agar was added and the mixture was poured onto a plate containing minimal agar. The His+ revertant colonies on plates were counted manually after 48 h incubation. The chemical concentrations from 10–12 to 10–6 M at 0.05 mL in each plate yielded the final concentration of 5 × 10–5 to 50 pmol/plate. Each test was repeated in two independent trials and in duplicate for each trial.
5.6. Data Analysis
The results were analyzed with the statistical software package JMP (JMP PRO 13). In the MCF-7 cell proliferation assay, the cell proliferation rates were analyzed by one-way ANOVA followed by Dunnett’s test to compare to the E2 group at each concentration (JMP PRO 13).
In the chicken embryonic assay, the morphological, developmental endpoints among groups, and TBARs levels were all determined using a one-way ANOVA followed by Tukey’s test for multiple comparisons versus the vehicle control (JMP PRO 13). Changes were considered statistically significant if p < 0.05 or p < 0.01. In the Ames test, the data (revertants/plate) were assessed by ANOVA, followed by Tukey’s test. The MI was also calculated for each concentration using the mean number of revertants per plate with the test compound divided by the mean number of revertants per plate with the negative control (VC). A tested compound was considered mutagenic if a 2-fold increase in the number of mutants (MI ≥ 2) was observed in at least one concentration.15 Signs of mutagenicity means that the compound that did not reach the 2-fold increase but showed statistical significance (p < 0.05) of revertant number compared to the VC.
Acknowledgments
Part of this material is based upon work supported by the National Science Foundation Growing Convergence Research Big Idea under Grant No. GCR CMMI 1934887 and US Department of Agriculture/National Institute of Food and Agriculture No. NI20HFPXXXXG001. The authors gratefully acknowledge Jinglin Zhang for her assistance in revising the manuscript.
Glossary
Abbreviations Used
- EA
estrogenic activity
- ED
endocrine-disrupting
- T.E.S.T.
Toxicity Estimation Software Tool
- E2
17β-estradiol
- % RME2
the relative maximum % E2
- EC50
half-maximal effective concentration
- EEF
estradiol equivalent factor
- DMSO
dimethyl sulfoxide
- PBS
phosphate-buffered solution
- VC
vehicle control
- LSI
liver somatic index
- RREW
ratio of embryo to egg weight
- TBARs
thiobarbituric acid-reactive substances
- MI
mutagenic index
- ANOVA
analysis of variance
- ER
estrogen receptor
- AR
androgen receptor
- TR
thyroid receptor
- GR
glucocorticoid receptor
- LXR
liver X receptor
- MR
mineralocorticoid receptor
- PR
progesterone receptor
- PPAR
peroxisome proliferator-activated receptor
- SARs
structure–activity relationships
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c04239.
Binding energy of E2, luteolin, apigenin, quercetin, and genistein for 14 nuclear receptors using endocrine disruptome tool (Table S1); toxicity data of E2, luteolin, apigenin, quercetin, and genistein by T.E.S.T. software (Table S2); and different types of malformation found in chicken embryo (Figure S1) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Patel D.; Shukla S.; Gupta S. Apigenin and Cancer Chemoprevention: Progress, Potential and Promise (Review). Int. J. Oncol. 2007, 30, 233–245. 10.3892/ijo.30.1.233. [DOI] [PubMed] [Google Scholar]
- Hostetler G. L.; Ralston R. A.; Schwartz S. J. Flavones: Food Sources, Bioavailability,. Adv Nutr. 2017, 8, 423–435. 10.3945/an.116.012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhanpal P.; Rai D. K. Quercetin: A Versatile Flavonoid. Internet J. Med. Updat. 2007, 2, 22–37. 10.4314/ijmu.v2i2.39851. [DOI] [Google Scholar]
- Nabavi S. F.; Devi K. P.; Loizzo M. R.; Tundis R.; Nabavi S. M.; et al. Genistein and Cancer: Current Status, Challenges. Adv. Nutr. 2015, 6, 408–419. 10.3945/an.114.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukutake M.; Takahashi M.; Ishida K.; Kawamura H.; Sugimura T.; Wakabayashi K. Quantification of Genistein and Genistin in Soybeans and Soybean Products. Food Chem. Toxicol. 1996, 34, 457–461. 10.1016/0278-6915(96)87355-8. [DOI] [PubMed] [Google Scholar]
- Mizushina Y.; Shiomi K.; Kuriyama I.; Takahashi Y.; Yoshida H. Inhibitory Effects of a Major Soy Isoflavone, Genistein, on Human DNA Topoisomerase II Activity and Cancer Cell Proliferation. Int. J. Oncol. 2013, 43, 1117–1124. 10.3892/ijo.2013.2032. [DOI] [PubMed] [Google Scholar]
- Formica J. V.; Regelson W. Review of the Biology of Quercetin and Related Bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. 10.1016/0278-6915(95)00077-1. [DOI] [PubMed] [Google Scholar]
- Manach C.; Scalbert A.; Morand C.; Rémésy C.; Jiménez L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- Tresserra-Rimbau A.; Lamuela-Raventos R. M.; Moreno J. J. Polyphenols, Food and Pharma. Current Knowledge and Directions for Future Research. Biochem. Pharmacol. 2018, 156, 186–195. 10.1016/j.bcp.2018.07.050. [DOI] [PubMed] [Google Scholar]
- Tarragon E.; Moreno J. J. Polyphenols and Taste 2 Receptors. Physiological, Pathophysiological and Pharmacological Implications. Biochem. Pharmacol. 2020, 178, 114086 10.1016/j.bcp.2020.114086. [DOI] [PubMed] [Google Scholar]
- Galati G.; O’Brien P. J. Potential Toxicity of Flavonoids and Other Dietary Phenolics: Significance for Their Chemopreventive and Anticancer Properties. Free Radicals Biol. Med. 2004, 37, 287–303. 10.1016/j.freeradbiomed.2004.04.034. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Hu Y.; Yang C.; Chen C.; Huang W.; Dang Z. Aggregation Kinetics of UV Irradiated Nanoplastics in Aquatic Environments. Water Res. 2019, 163, 114870 10.1016/j.watres.2019.114870. [DOI] [PubMed] [Google Scholar]
- Samanta J.; Bhattacharya S.; Rana A. C. Antifertility Activity of Thevetia peruviana (Pers.) K. Schum Leaf in Female Sprague-Dawley Rat. Indian J. Pharmacol. 2016, 48, 669 10.4103/0253-7613.194861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugel S. M.; Bonventre J. A.; Tanguay R. L. Comparative Developmental Toxicity of Flavonoids Using an Integrative Zebrafish System. Toxicol. Sci. 2016, 154, 55–68. 10.1093/toxsci/kfw139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resende F. A.; Vilegas W.; Dos Santos L. C.; Varanda E. A. Mutagenicity of Flavonoids Assayed by Bacterial Reverse Mutation (Ames) Test. Molecules 2012, 17, 5255–5268. 10.3390/molecules17055255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui C.; Qi X.; Qianyong Z.; Xiaoli P.; Jundong Z.; Mantian M. Flavonoids, Flavonoid Subclasses and Breast Cancer Risk: A Meta-Analysis of Epidemiologic Studies. PLoS One 2013, 8, e54318 10.1371/journal.pone.0054318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolšek K.; Mavri J.; Sollner Dolenc M.; Gobec S.; Turk S. Endocrine Disruptome—An Open Source Prediction Tool for Assessing Endocrine Disruption Potential through Nuclear Receptor Binding. J. Chem. Inf. Model. 2014, 54, 1254–1267. 10.1021/ci400649p. [DOI] [PubMed] [Google Scholar]
- Törnqvist E.; Annas A.; Granath B.; Jalkesten E.; Cotgreave I.; Öberg M. Strategic Focus on 3R Principles Reveals Major Reductions in the Use of Animals in Pharmaceutical Toxicity Testing. PLoS One 2014, 9, e101638 10.1371/journal.pone.0101638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca B. B.; da Silva M. V.; de Morais Ribeiro L. N. The Chicken Embryo as an in Vivo Experimental Model for Drug Testing: Advantages and Limitations. Lab Anim. 2021, 50, 138–139. 10.1038/s41684-021-00774-3. [DOI] [PubMed] [Google Scholar]
- UNECE . Part 3 Health Hazards; UNECE, 2015; pp 107–216. [Google Scholar]
- Yang C. Z.; Casey W.; Stoner M. A.; Kollessery G. J.; Wong A. W.; Bittner G. D. A Robotic MCF-7: WS8 Cell Proliferation Assay to Detect Agonist and Antagonist Estrogenic Activity. Toxicol. Sci. 2014, 137, 335–349. 10.1093/toxsci/kft250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Sanchez E.; Gomez-Sanchez C. E. The Multifaceted Mineralocorticoid Receptor. Compr. Physiol. 2011, 4, 965–994. 10.1002/cphy.c130044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S.; Li Y.; Wang Z.; Sarkar F. H. Multi-Targeted Therapy of Cancer by Genistein. Cancer Lett. 2008, 269, 226–242. 10.1016/j.canlet.2008.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper G. G. J. M.; Lemmen J. G.; Carlsson B.; Corton J. C.; Safe S. H.; Van Der Saag P. T.; Van Der Burg B.; Gustafsson J. Å. Interaction of Estrogenic Chemicals and Phytoestrogens with Estrogen Receptor β. Endocrinology 1998, 139, 4252–4263. 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Murata M.; Midorikawa K.; Koh M.; Umezawa K.; Kawanishi S. Genistein and Daidzein Induce Cell Proliferation and Their Metabolites Cause Oxidative DNA Damage in Relation to Isoflavone-Induced Cancer of Estrogen-Sensitive Organs. Biochemistry 2004, 43, 2569–2577. 10.1021/bi035613d. [DOI] [PubMed] [Google Scholar]
- Innocenti G.; Vegeto E.; Dall’Acqua S.; Ciana P.; Giorgetti M.; Agradi E.; Sozzi A.; Fico G.; Tomè F. In Vitro Estrogenic Activity of Achillea Millefolium L. Phytomedicine 2007, 14, 147–152. 10.1016/j.phymed.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Miodini P.; Fioravanti L.; Di Fronzo G.; Cappelletti V. The Two Phyto-Oestrogens Genistein and Quercetin Exert Different Effects on Oestrogen Receptor Function. Br. J. Cancer 1999, 80, 1150–1155. 10.1038/sj.bjc.6690479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Woude H.; Ter Veld M. G. R.; Jacobs N.; Van Der Saag P. T.; Murk A. J.; Rietjens I. M. C. M. The Stimulation of Cell Proliferation by Quercetin Is Mediated by the Estrogen Receptor. Mol. Nutr. Food Res. 2005, 49, 763–771. 10.1002/mnfr.200500036. [DOI] [PubMed] [Google Scholar]
- Lucki N. C.; Sewer M. B. Genistein Stimulates MCF-7 Breast Cancer Cell Growth by Inducing Acid Ceramidase (ASAH1) Gene Expression. J. Biol. Chem. 2011, 286, 19399–19409. 10.1074/jbc.M110.195826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allred C. D.; Allred K. F.; Ju Y. H.; Virant S. M.; Helferich W. G. Soy Diets Containing Varying Amounts of Genistein Stimulate Growth of Estrogen-Dependent (MCF-7) Tumors in a Dose-Dependent Manner. Cancer Res. 2001, 61, 5045–5050. [PubMed] [Google Scholar]
- Hsieh C.-Y.; Santell R. C.; Haslam S. Z.; Helferich W. G. Estrogenic Effects of Genistein on the Growth of Estrogen Receptor-Positive Human Breast Cancer (MCF-7) Cells in Vitro and in Vivo. Cancer Res. 1998, 58, 3833–3838. [PubMed] [Google Scholar]
- Seo H. S.; DeNardo D. G.; Jacquot Y.; Laïos I.; Vidal D. S.; Zambrana C. R.; Leclercq G.; Brown P. H. Stimulatory Effect of Genistein and Apigenin on the Growth of Breast Cancer Cells Correlates with Their Ability to Activate ER Alpha. Breast Cancer Res. Treat. 2006, 99, 121–134. 10.1007/s10549-006-9191-2. [DOI] [PubMed] [Google Scholar]
- Resende F. A.; de Oliveira A. P. S.; de Camargo M. S.; Vilegas W.; Varanda E. A. Evaluation of Estrogenic Potential of Flavonoids Using a Recombinant Yeast Strain and MCF7/BUS Cell Proliferation Assay. PLoS One 2013, 8, e74881 10.1371/journal.pone.0074881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isidori M.; Cangiano M.; Palermo F. A.; Parrella A. E-Screen and Vitellogenin Assay for the Detection of the Estrogenic Activity of Alkylphenols and Trace Elements. Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol. 2010, 152, 51–56. 10.1016/j.cbpc.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Omoruyi I. M.; Pohjanvirta R. Estrogenic Activities of Food Supplements and Beers as Assessed by a Yeast Bioreporter Assay. J. Diet. Suppl. 2018, 15, 665–672. 10.1080/19390211.2017.1380104. [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) . Agency for Toxic Substances and Disease Registry (ATSDR) EPA Reportable Quantity Methodology Used to Establish Toxicity; EPA, 2011; pp 1–6. [Google Scholar]
- Nair A.; Jacob S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm. 2016, 7, 27 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedernhofer L. J.; Daniels J. S.; Rouzer C. A.; Greene R. E.; Marnett L. J. Malondialdehyde, a Product of Lipid Peroxidation, Is Mutagenic in Human Cells. J. Biol. Chem. 2003, 278, 31426–31433. 10.1074/jbc.M212549200. [DOI] [PubMed] [Google Scholar]
- Singh P.; Mishra S. K.; Noel S.; Sharma S.; Rath S. K. Acute Exposure of Apigenin Induces Hepatotoxicity in Swiss Mice. PLoS One 2012, 7, e31964 10.1371/journal.pone.0031964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S. J.; Yeh T. M.; Chuang W. J.; Ho C. L.; Chang K. L.; Cheng H. L.; Liu H. S.; Cheng H. L.; Hsu P. Y.; Chow N. H. The Novel Targets for Anti-Angiogenesis of Genistein on Human Cancer Cells. Biochem. Pharmacol. 2005, 69, 307–318. 10.1016/j.bcp.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Li X.; Chen M.; Lei X.; Huang M.; Ye W.; Zhang R.; Zhang D. Luteolin Inhibits Angiogenesis by Blocking Gas6/Axl Signaling Pathway. Int. J. Oncol. 2017, 51, 677–685. 10.3892/ijo.2017.4041. [DOI] [PubMed] [Google Scholar]
- Beedie S. L.; Mahony C.; Walker H. M.; Chau C. H.; Figg W. D.; Vargesson N. Shared Mechanism of Teratogenicity of Anti-Angiogenic Drugs Identified in the Chicken Embryo Model. Sci. Rep. 2016, 6, 30038 10.1038/srep30038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. J.; Seok S. H.; Baek M. W.; Lee H. Y.; Na Y. R.; Park S. H.; Lee H. K.; Dutta N. K.; Kawakami K.; Park J. H. Developmental Toxicity and Brain Aromatase Induction by High Genistein Concentrations in Zebrafish Embryos. Toxicol. Mech. Methods 2009, 19, 251–256. 10.1080/15376510802563330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerin F.; Sener U.; Erman H.; Yilmaz A.; Aydin B.; Armutcu F.; Gurel A. The Effects of Quercetin on Acute Lung Injury and Biomarkers of Inflammation and Oxidative Stress in the Rat Model of Sepsis. Inflammation 2016, 39, 700–705. 10.1007/s12640-012-9351-6. [DOI] [PubMed] [Google Scholar]
- Sharma D. R.; Wani W. Y.; Sunkaria A.; Kandimalla R. J. L.; Verma D.; Cameotra S. S.; Gill K. D. Quercetin Protects against Chronic Aluminum-Induced Oxidative Stress and Ensuing Biochemical, Cholinergic, and Neurobehavioral Impairments in Rats. Neurotoxic. Res. 2013, 23, 336–357. 10.1007/s12640-012-9351-6. [DOI] [PubMed] [Google Scholar]
- Mortelmans K.; Zeiger E. The Ames Salmonella/Microsome Mutagenicity Assay. Mutat. Res., Fundam. Mol. Mech. Mutagen. 2000, 455, 29–60. 10.1016/S0027-5107(00)00064-6. [DOI] [PubMed] [Google Scholar]
- Eghbaliferiz S.; Iranshahi M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phyther. Res. 2016, 30, 1379–1391. 10.1002/ptr.5643. [DOI] [PubMed] [Google Scholar]
- Michael McClain R.; Wolz E.; Davidovich A.; Bausch J. Genetic Toxicity Studies with Genistein. Food Chem. Toxicol. 2006, 44, 42–55. 10.1016/j.fct.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Chen L.-X.; He H.; Xia G.-Y.; Zhou K.-L.; Qiu F. A New Flavonoid from the Aerial Parts of Andrographis Paniculata. Nat. Prod. Res. 2014, 28, 138–143. 10.1080/14786419.2013.856907. [DOI] [PubMed] [Google Scholar]
- Wang I.-K.; Lin-Shiau S.-Y.; Lin J.-K. Induction of Apoptosis by Apigenin and Related Flavonoids through Cytochrome c Release and Activation of Caspase-9 and Caspase-3 in Leukaemia HL-60 Cells. Eur. J. Cancer 1999, 35, 1517–1525. 10.1016/S0959-8049(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Murias M.; Jäger W.; Handler N.; Erker T.; Horvath Z.; Szekeres T.; Nohl H.; Gille L. Antioxidant, Prooxidant and Cytotoxic Activity of Hydroxylated Resveratrol Analogues: Structure–Activity Relationship. Biochem. Pharmacol. 2005, 69, 903–912. 10.1016/j.bcp.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Wu C.; Chen F.; Wang X.; Kim H.-J.; He G.; Haley-Zitlin V.; Huang G. Antioxidant Constituents in Feverfew (Tanacetum parthenium) Extract and Their Chromatographic Quantification. Food Chem. 2006, 96, 220–227. 10.1016/j.foodchem.2005.02.024. [DOI] [Google Scholar]
- Storniolo C. E.; Moreno J. J. Resveratrol Analogs with Antioxidant Activity Inhibit Intestinal Epithelial Cancer Caco-2 Cell Growth by Modulating Arachidonic Acid Cascade. J. Agric. Food Chem. 2019, 67, 819–828. 10.1021/acs.jafc.8b05982. [DOI] [PubMed] [Google Scholar]
- Rusak G.; Gutzeit H. O.; Müller J. L. Structurally Related Flavonoids with Antioxidative Properties Differentially Affect Cell Cycle Progression and Apoptosis of Human Acute Leukemia Cells. Nutr. Res. 2005, 25, 143–155. 10.1016/j.nutres.2004.12.003. [DOI] [Google Scholar]
- Menezes J. C.; Orlikova B.; Morceau F.; Diederich M. Natural and Synthetic Flavonoids: Structure–Activity Relationship and Chemotherapeutic Potential for the Treatment of Leukemia. Crit. Rev. Food Sci. Nutr. 2016, 56, S4–S28. 10.1080/10408398.2015.1074532. [DOI] [PubMed] [Google Scholar]
- Almeida A. F.; Borge G. I. A.; Piskula M.; Tudose A.; Tudoreanu L.; Valentová K.; Williamson G.; Santos C. N. Bioavailability of Quercetin in Humans with a Focus on Interindividual Variation. Compr. Rev. Food Sci. Food Saf. 2018, 17, 714–731. 10.1111/1541-4337.12342. [DOI] [PubMed] [Google Scholar]
- Hostetler G. L.; Ralston R. A.; Schwartz S. J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. 10.3945/an.116.012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L.; House S. E.; Prior R. L.; Fang N.; Ronis M. J. J.; Clarkson T. B.; Wilson M. E.; Badger T. M. Metabolic Phenotype of Isoflavones Differ among Female Rats, Pigs, Monkeys, and Women. J. Nutr. 2006, 136, 1215–1221. 10.1093/jn/136.5.1215. [DOI] [PubMed] [Google Scholar]
- Devillers J.; Bro E.; Millot F. Prediction of the Endocrine Disruption Profile of Pesticides. SAR QSAR Environ. Res. 2015, 26, 831–852. 10.1080/1062936X.2015.1104809. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Peng Y.; Wu C. Chicken Embryonic Toxicity and Potential in Vitro Estrogenic and Mutagenic Activity of Carvacrol and Thymol in Low Dose/Concentration. Food Chem. Toxicol. 2021, 150, 112038 10.1016/j.fct.2021.112038. [DOI] [PubMed] [Google Scholar]
- Peng Y.; Nicastro K. H.; Epps T. H.; Wu C. Evaluation of Estrogenic Activity of Novel Bisphenol A Alternatives, Four Bioinspired Bisguaiacol F Specimens, by in Vitro Assays. J. Agric. Food Chem. 2018, 66, 11775–11783. 10.1021/acs.jafc.8b03746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.