Significance
Soil organic carbon (C) responses to agricultural management are highly uncertain, hindering our ability to assess the C sequestration potential of croplands and develop sound policies to mitigate climate change while enhancing other ecosystem services. Combining experimental evidence from a long-term field experiment and a meta-analysis of published literature, we show that the accrual of mineral-associated soil C in intensively managed Mollisols was only achieved by managing ruminant grazing on perennial grasslands. Although modifying dominant grain-based systems with reduced tillage, diversified rotations, and legumes and manure additions improve soil health metrics—which is critical to soil, nutrient, and water conservation—they are unlikely to enhance persistent forms of soil C in Mollisols to help drawdown atmospheric C and stabilize climate.
Keywords: soil organic carbon, agricultural practices, Mollisols, soil microbes, managed grasslands
Abstract
Intensive crop production on grassland-derived Mollisols has liberated massive amounts of carbon (C) to the atmosphere. Whether minimizing soil disturbance, diversifying crop rotations, or re-establishing perennial grasslands and integrating livestock can slow or reverse this trend remains highly uncertain. We investigated how these management practices affected soil organic carbon (SOC) accrual and distribution between particulate (POM) and mineral-associated (MAOM) organic matter in a 29-y-old field experiment in the North Central United States and assessed how soil microbial traits were related to these changes. Compared to conventional continuous maize monocropping with annual tillage, systems with reduced tillage, diversified crop rotations with cover crops and legumes, or manure addition did not increase total SOC storage or MAOM-C, whereas perennial pastures managed with rotational grazing accumulated more SOC and MAOM-C (18 to 29% higher) than all annual cropping systems after 29 y of management. These results align with a meta-analysis of data from published studies comparing the efficacy of soil health management practices in annual cropping systems on Mollisols worldwide. Incorporating legumes and manure into annual cropping systems enhanced POM-C, microbial biomass, and microbial C-use efficiency but did not significantly increase microbial necromass accumulation, MAOM-C, or total SOC storage. Diverse, rotationally grazed pasture management has the potential to increase persistent soil C on Mollisols, highlighting the key role of well-managed grasslands in climate-smart agriculture.
Modern agriculture depleted soil organic carbon (SOC) from much of the world’s arable lands over the past 150 y (1). Building soil organic matter (SOM) in agricultural soils is crucial to our ability to counteract this trend and provide for our wants and needs (2). Grassland-derived Mollisols cover ∼916 million ha of Earth’s surface and are now the agricultural heartlands of North America, South America, and Eurasia. Intensive agriculture on these Mollisols has liberated ∼2 Pg C since cultivation began (3, 4). Enhancing SOM in Mollisols would not only offset a portion of global greenhouse gas emissions but also improve soil health that underpins vital ecosystems services including food sovereignty, clean water, flood reduction, and biodiversity, and therefore contribute to achieving sustainable development goals of the 2030 Agenda of the United Nations (5).
Simulation and conceptual modeling indicate a large potential for SOM accumulation on Mollisols (6, 7), but uncertainty remains about whether intensive agricultural use of these soils can accrue carbon (C) and maintain it for relatively long periods (8, 9). SOM is comprised of fractions that differ in formation, persistence, and function (10). Reducing tillage, diversifying crop rotations, and adding legumes and manure are touted as promising strategies to regenerate SOM in croplands (11–13). Whereas they appear to increase the relatively undecomposed particulate organic matter (POM) fraction (14, 15), which is directly associated with improved soil health, their ability to build more persistent mineral-associated organic matter (MAOM) and enhance total C stock and persistence in Mollisols has been debated (16–19).
Growing evidence suggests MAOM is formed mainly when microbial products associate with mineral surfaces (20–22), so practices that promote efficient microbial growth and necromass production should drive persistent C accrual in soils with high mineral capacity for organomineral associations (23–25). In the North Central United States, an important dairy-producing region, agricultural operations in past decades have incorporated more forage legumes such as alfalfa into crop rotations (26), which may result in MAOM accumulation from enhanced soil microbial growth and necromass accumulation because of more low C:nitrogen (N) plant inputs and periodic manure additions (27). The fine texture of Mollisols should favor the physical protection of newly synthesized microbially derived C (23, 28). However, the expected C accrual has not been observed consistently in field studies (15, 29).
To investigate how agricultural management geared toward improving soil health affect soil C accrual on Mollisols, we explored soils from the Wisconsin Integrated Cropping Systems Trial (WICST), a long-term field experiment in the North Central United States. The WICST was established in 1989 on land that was tallgrass prairie until late 19th century, when it was plowed and converted to annual grain and forage production through most of the 20th century. The WICST consists of six side-by-side conventional and alternative cropping systems, including three cash-grain systems: 1) continuous monoculture maize (Maize) system with annual tillage, 2) no-till maize-soybean (MS) rotation, and 3) organically managed maize-soybean-wheat (MSW) rotation that has a legume cover crop after wheat, and three dairy forage systems: 4) maize-alfalfa-alfalfa-alfalfa (MAAA) rotation, 5) organic maize-oats/alfalfa/alfalfa (MOA) rotation, and 6) diverse, rotationally grazed cool-season pasture (Pasture) with mixed legumes and grasses (SI Appendix, Fig. S1 and Table S1) (30). We expected that systems with reduced tillage or inclusion of cover or forage crops (i.e., increased presence of living roots) would increase POM-C. We also expected that systems with the incorporation of low C:N legumes and livestock excreta would lead to increased MAOM-C as a result of the higher microbial C-use efficiency (CUE) and microbial necromass production (23, 27, 31). To test these hypotheses, we studied the quantity and composition of SOM and their relationships with soil microbial traits after 29 y of management. We also compiled data from published literature addressing the effects of various agricultural practices on the SOC content and distribution between POM and MAOM in Mollisols worldwide to explore the generality of our findings at WICST.
Results and Discussion
Total and Mineral-Associated Soil C Highest under Well-Managed Pastures.
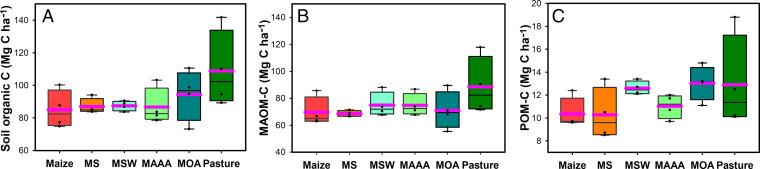
Our results suggest that reducing tillage, diversifying crop rotations, or adding legumes or manure on these Mollisols is unlikely to build MAOM-C and SOC, whereas managing the land as rotationally grazed, diverse pastures has the capacity to do so. SOC stocks (0 to 30 cm) were 15 to 28% greater in Pasture than Maize, MS, MSW, and MAAA (Fig. 1A), similar to the 20-y SOC change findings at WICST of Sanford et al. (29). There was no difference in POM-C, MAOM-C, or SOC between Maize and no-till MS. There were also no significant differences in MAOM-C and N among all systems except Pasture, which showed 18 to 29% greater MAOM-C than all other systems (SI Appendix, Table S2 and Fig. 1B). On the contrary, POM-C and N were significantly higher in the alfalfa-based systems (MOA and MAAA) compared to Maize and MS and were greatest in Pasture (Fig. 1C and SI Appendix, Table S2), aligning with previous results of Cates et al. (14). The C:N ratio of POM was significantly lower in the alfalfa-based systems than Maize and MS. Notably, the organic grain system (MSW), which had a legume cover crop and manure addition but less total and belowground C input, also had greater POM-C and N and a lower POM C:N ratio than Maize and MS (Fig. 1C and SI Appendix, Table S2). The meta-analysis we conducted based on data from 17 published articles on Mollisols of the world (SI Appendix, Table S3) aligned with the results from WICST. Out of 28 paired comparisons of 1) reduced or no tillage and conventional tillage, 2) diversified crop rotations with legumes and monocultures, 3) manure and synthetic fertilizers, or 4) cover crops and no cover crops, only two comparisons showed higher MAOM-C under soil health practices, whereas 26 reported no differences (SI Appendix, Fig. S2 and Table S3). Although trends at deeper depths might differ from the surface layers (32), these results suggest that effects of these soil health management practices on MAOM-C and total SOC on Mollisols are typically small in intensive annual cropping systems.
Fig. 1.
SOC stocks across WICST cropping systems. (A) Organic carbon stocks in bulk soils, (B) MAOM, and (C) POM in the surface 30 cm after 29 y of Maize, MS, MSW, MAAA, and MOA rotation and well-managed Pasture. Box boundaries indicate the 25th and 75th percentiles. Black lines indicate medians, and pink lines indicate means.
Incorporating Legumes and Manure in Annual Cropping Systems Enhanced Microbial Growth But Not Microbial Necromass or MAOM.
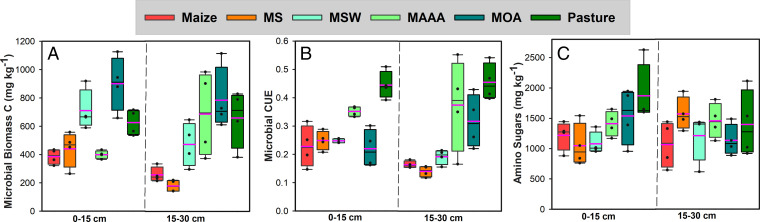
We observed no differences between Maize and no-till MS in CUE, microbial biomass C (MBC), or the content of amino sugars (biomarkers of microbial necromass). Alfalfa-based systems had higher microbial CUE and MBC, but not higher microbial necromass accumulation relative to grain-based systems (Fig. 2 and SI Appendix, Table S4). The negative relationship between CUE and POM C:N (SI Appendix, Table S5) suggested that microbial physiological potential in using C substrate was regulated by the C:N ratio of inputs. Low C:N legume and manure inputs can enhance soil microbial growth and necromass production (33). POM is largely comprised of partially decomposed plant polymers after initial depolymerization and serves as the major energy source for heterotrophic microbes in the soil (10). Our results indicate that by incorporating legumes and/or manure the alfalfa-based systems can lower the C:N ratio of POM therefore stimulate microbial CUE by meeting the microbial stoichiometric demand (33).
Fig. 2.
Soil microbial traits across WICST cropping systems. (A) Microbial biomass C, (B) microbial CUE, and (C) total amino sugars of 0- to 15- and 15- to 30-cm soil at the WICST after 29 y of Maize, MS, MSW, MAAA, and MOA rotation and well-managed Pasture. Box boundaries indicate the 25th and 75th percentiles. Black lines indicate medians, and pink lines indicate means.
However, similar to the trend of MAOM-C, the content of microbial necromass biomarkers (amino sugars) was highest in Pasture, but not significantly different across other systems (Fig. 2C). The positive correlation between MAOM-C and the aliphatic C content of MAOM (SI Appendix, Tables S5 and S6) and between MAOM-C and microbial necromass (SI Appendix, Fig. S3 and Table S5) indicated that microbially derived C is the main contributor to MAOM (21, 34). Piecewise structural equation modeling (SEM) showed that MAOM-C was directly related to the amount of microbial necromass accumulated in the soil, but not microbial biomass or CUE (SI Appendix, Fig. S3), indicating that adding low C:N legumes and manure to enhance microbial CUE may not always lead to increased accumulation of microbial necromass or MAOM-C.
More Efficient Formation and Slower Mineralization of MAOM in Well-Managed Pastures than Annual Cropping Systems.
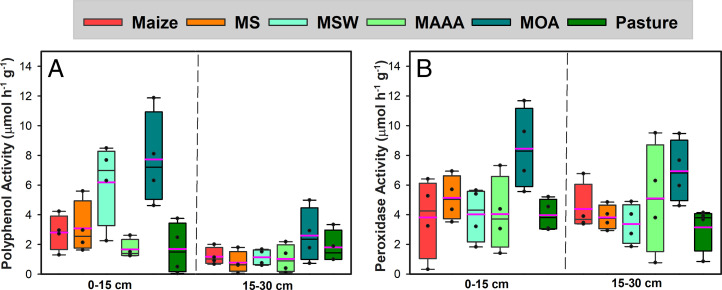
SOC storage is the balance between formation and mineralization. While MAOM-C is protected through association with soil minerals, a fraction of it may decompose quickly. We measured the activities of polyphenol oxidase (PPO) and peroxidase (PER), which were produced by soil microbes to catalyze SOM decomposition and nutrient provision (35). Higher PPO activity in MSW and MOA and higher PER activity in MOA (SI Appendix, Table S7 and Fig. 3 A and B) indicated faster SOM decomposition in these low fertilizer-input systems than high fertilizer-input systems and Pasture, suggesting that microbial necromass or MAOM may be subject to quick mineralization in these systems (36, 37). Similar to most cropped soils, these soils have very little POM, indicating that MAOM might be an important source of nutrients (38). MAOM is more N-enriched than POM and therefore a better match for microbial stoichiometric needs (39). Once liberated after desorption from mineral associations, microbially derived organic matter may be quickly mineralized to meet crop nutrient demands (40), which may be especially true for maize growing on Mollisols where the N demand in the peak growing season is high (41). High C:N maize residues are also more likely to induce the priming of extant SOM because of increased microbial nutrient demand, especially when maize is in rotation with N-rich soybean or alfalfa which would enhance microbial growth, leading to increased microbial population size and substrate demand in the maize phase (42). On the other hand, when tillage is used to control weeds in these organically managed systems, newly formed MAOM can be exposed to microbial degradation because of soil disturbance. Although unlikely to increase SOC stock on Mollisols, incorporating legume cover and forage crops in maize/soybean dominated systems do have meaningful agronomic and environmental benefits, including reduced synthetic fertilizer needs, improved soil health, and greater yield stability (43).
Fig. 3.
Activities of soil oxidative enzymes across WICST cropping systems. (A) PPO activity and (B) PER activity of 0- to 15- and 15- to 30-cm soil at the WICST after 29 y of different management systems of Maize, MS, MSW, MAAA, and MOA rotation and well-managed Pasture. Box boundaries indicate the 25th and 75th percentiles. Black lines indicate medians, and pink lines indicate means.
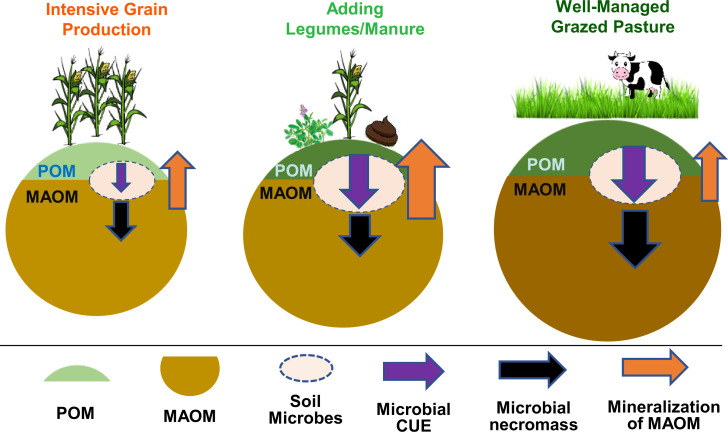
Although the precise mechanisms for greater MAOM-C in Pasture require further research, the enhanced formation and reduced mineralization of MAOM may have contributed to the SOC accrual under well-managed pastures relative to annual cropping systems (Fig. 4). In diverse, rotationally grazed pastures that more closely resemble the structure and function of the bison-grazed tallgrass prairie compared to annual grain and dairy forage cropping systems, continuous low C:N plant and animal inputs can promote microbial CUE, microbial necromass production, and MAOM formation (31). Lower oxidative enzyme activities in Pasture also indicated a slower rate of SOM decomposition in the peak growing season when temperature and precipitation favor microbial activity. In addition to greater microbial necromass accumulation (24), the combination of abundant, diverse root architecture, phenology, production, turnover, and exudation rates and compounds (44, 45) may be conspiring to support soil C building under diverse, perennial grasslands than annual crops (46). Future research should focus on unraveling the plant–microbe–soil associations and associated changes in root architectures and soil microstructures related to enhanced SOC and MAOM-C accumulation in perennial grasslands under a range of grazing management.
Fig. 4.
Conceptual model of how agricultural systems affect microbially regulated SOM dynamics. The size of the POM, persistent MAOM, and soil microbes within each circle indicate the relative sizes of C pools. Different colors of labile organic matter fractions indicate the quality of C input (light green = higher C:N ratio of labile fractions, dark green = lower C:N ratio of labile fractions). CUE, microbial biomass production/C assimilated. The size of arrows indicates the relative magnitude of C fluxes. Adding legume cover or forage crops and/or adding manure in the systems supply higher-quality C input (reflected by lower C:N ratio of the POM), which increases microbial CUE and necromass production. However, the oxidative loss of MAOM induced by tillage or nutrient requirement (mostly from maize) results in a faster mineralization of MAOM. As a result, only the rotationally grazed pasture systems led to meaningful C accrual.
Conclusions
Implementing no-till, crop rotations, and legumes and manure additions in annual grain or semiannual forage systems on Mollisols may improve soil health but are not likely to make their topsoil (0 to 30 cm) atmospheric C sinks. Incorporating low C:N inputs may enhance POM and microbial C cycling but not lead to increases in microbial necromass and MAOM-C, possibly because of higher SOM mineralization. In addition to reducing erosion and nutrient loss, increasing infiltration and water storage, and enhancing biodiversity (47, 48), well-managed grazed perennial grasslands have the potential to build persistent soil C in Mollisols making them a critical part of climate-smart agriculture.
Materials and Methods
Site Description and Sampling.
Established in 1990, the WICST is located at the University of Wisconsin–Madison Agricultural Research Station (43°17′45″N, 89°22′48″W, 315 m above sea level) in Arlington, WI. The soil is Plano silt loam (fine-silty, mixed, superactive, mesic Typic Argiudoll, US Department of Agriculture [USDA] Soil Taxonomy) with 6% sand, 72% silt, and 22% clay. The mean annual temperature at Arlington is 6.9 °C and mean annual precipitation is 898 mm (1981 to 2010, National Oceanic and Atmospheric Administration).
WICST is a randomized complete block design with four blocks, with all phases of the six cropping systems represented each year (details are shown in SI Appendix, Table S1). A complete site and study description can be found in Posner et al. (30). Plots are 0.3 ha, and commercial farm-scale equipment is used for all field work. The six cropping systems represent grain (Maize, MS, and MSW) and forage (MOA, MAAA, and Pasture) enterprises. Pastures are comprised of cool-season grasses Timothy (Phleum pratense L.), Kentucky bluegrass (Poa pratensis L.), orchardgrass (Dactylis glomerata L.), ryegrass (Lolium perenne L.), and festulolium (×Festulolium Asch. & Graebn.), dandelion (Taraxacum officinale F.H. Wigg.), and clover (Trifolium pratense L. and T. repens L.). Pastures are rotationally grazed by six heifers each year between 1 May and 10 October for a stocking rate of ∼14 A.U. ha−1 y−1.
Maize in Maize, MS, and MAAA receives commercial fertilizer at recommended rates. In MSW, pelletized composted poultry manure is applied prior to maize (2.2 Mg ha−1) and wheat (1.6 Mg ha−1) crops, and a green manure cover crop of oats and clover is sown after wheat harvest. Dairy slurry is applied in the fall prior to maize and first year alfalfa seeding in MAAA and MOA systems. The Maize, MSW, MAAA, and MOA systems are chisel-plowed in the fall prior to maize planting, and MS is strip-tilled in the fall prior to maize planting. A field cultivator is used in the spring just prior to planting maize and soybeans in Maize, MSW, MAAA, and MOA. Soybeans in MS are planted using a no-till drill. A chisel plow is also used prior to soybean planting in MSW and alfalfa seeding in MAAA and MOA. Additional cultivation, including use of a tine weeder, a rotary hoe, and Danish tine and/or disk hiller row cultivator for weed control, is performed as needed in maize, soybean, and wheat of MSW and MOA systems, whereas in Maize, MS, MAAA, and Pasture weeds are chemically controlled.
Soil sampling was conducted in July 2018, during the peak growing season in the maize phase of each rotation except the rotationally grazed Pasture system. Soil samples (0 to 15 and 15 to 30 cm) were collected from eight locations in each plot using a zigzag sampling pattern (to equally sample across the maize row). A soil probe (diameter, 3.5 cm) was used to take three in-row and three between-row soil samples at each location. Soil samples collected from all locations (n = 48) were composited into one soil sample per plot. Samples were transported to the laboratory immediately, passed through a 2-mm sieve, and subsampled into two portions. One subsample was kept at 4 °C for soil microbial and enzyme assays within 1 wk, the other subsample was air-dried for physical fractionation, C and N, and spectroscopy analyses.
SOM Fractionation.
All soils were separated after aggregate dispersion into two size fractions: POM (>53 μm) and MAOM (<53 μm) by wet sieving (49). Sodium hexametaphosphate solution (80 mL) was added to 10 g of air-dried, 2-mm-sieved soil and shaken for 18 h to disperse soil aggregates. MilliQ water was used to wash the contents through a 53-μm sieve. The two fractions that were separated by the sieve were dried to constant mass at 70 °C. All POM and MAOM fractions were ground to homogeneity by hand with a coffee grinder. A subsample of all homogenized samples was analyzed for C and N content on an elemental analyzer (PDZ-Europa ANCA-GSL).
MAOM Composition.
We used diffuse reflectance infrared Fourier transform spectroscopy analysis in the midinfrared range to characterize functional groups of MAOM samples (50, 51). Spectra were obtained using an X,Y Autosampler (Pike Technologies Inc.) coupled with a Nicolet iS50 spectrometer equipped with a diffuse reflectance accessory (Thermo Fisher Scientific Inc.). Four spectral readings were performed for each subsample using the random oversampling motion function of the X,Y Autosampler (within a 3-mm diameter of the sample cup’s centroid) configured in AutoProTM software (Pike Technologies Inc.). We used anodized aluminum plates that fit 24 polystyrene sample cups (5.5-mL volume and 10-mm top opening diameter), each loaded with a subsample, dried for >48 h at 40 °C and 12 to 14% relative humidity. All measurements were conducted from 4,000 to 400 cm−1 at 4 cm−1 wavenumber resolution using 24 coadded scans (52). Spectra absorbance peaks were integrated using the local baseline technique, as described by Demyan et al. (50) and Deiss et al. (52), and local peak areas were determined using the triangle method available in the “geometry” package in R. We evaluated relative peak areas, or proportion of a specific peak area relative to the total peak areas for the following organic functional groups: aliphatic C-H functional group of methyl and methylene groups (wavenumber 3,010 to 2,800 cm−1), aromatic C = C stretch and/or asymmetric -COO- stretch (wavenumber 1,660 to 1,580 cm−1), aromatic C = C stretch (wavenumber 1,546 to 1,520 cm−1), and C-O in both poly-alcoholic and ether functional groups (wavenumber 1,170 to 1,148 cm−1) (50). Our analysis focused on the aliphatic functional groups (peak 2,930 cm−1; from microbial cell components and plant wax layers) and aromatic functional groups (peaks 1,530 and 1,620 cm−1; from lignin-derived products).
Microbial Biomass and CUE Characterization.
We estimated microbial CUE by the 13C glutamic acid tracing method. Because glutamic acid is taken up directly into microbial cells, this approach allows us to directly compare CUE across treatments since substrate-C allocation toward enzyme production is minimized (21). Fresh soil samples (20 g dry weight) were amended in the laboratory with 50 μg C g−1 dry soil of 25 atom% labeled 13C glutamic acid (<1% total soil C) and incubated at 45% water holding capacity for 22 h at 25 °C (27). An additional set of soils received only deionized H2O to serve as controls. After incubation, a 12-mL CO2 sample was collected using airtight plastic syringes for determining 13CO2-C respiration on a gas chromatography (Hewlett-Packard Model 6890)-isotope ratio mass spectrometer (PDZ-Europa Model 20-20) and MBC was determined by the fumigation-extraction method with a Shimadzu TOC Analyzer (Shimadzu Corp.) (53). 13C incorporation in MBC was determined by an isotope ratio mass spectrometer (PDZ-Europa Model 20-20) after aliquots of K2SO4 extracts from both fumigated and unfumigated soils were dried at 60 °C in tin capsules. Microbial CUE was calculated as [MB13C/(MB13C + 13CO2-C) × 100], where MB13C and 13CO2-C are determined using a standard isotope mixing model equation and represent the amount of substrate incorporated into MBC and the substrate-C respired as CO2, respectively (27). Geyer et al. (54) demonstrated that a significant amount (∼1/3) of glutamic acid C can be released by microbes into the soil as microbial residues within 6 h of addition, which would result in underestimation of CUE with this method, but there is no evidence that this bias would be different across plant communities.
Microbial Necromass Assay.
We assayed four amino sugars by gas chromatography after their conversion to aldonitrile acetates (55) using a procedure modified from Liang et al. (56). The procedure was based on the extraction of signature amino sugar biomarkers from the cell wall of microorganisms. Approximately 1 g finely ground air-dried soil samples were hydrolyzed with 6 M HCl at 105 °C for 8 h to release the amino sugar monomers. After purification and derivatization, we analyzed extracts with an Agilent 6890 GC (Agilent Technologies) equipped with a J&W Scientific Ultra-2 column (25 m × 0.2 mm × 0.33 μm) and flame ionization detector. The individual amino sugar derivatives were identified by comparing their retention time with those of authentic standards. Quantification from peak areas to mass per mass of soil (μg/g) was gained relative to the internal standard myo-inositol, which was added to the samples prior to purification. We also used the recovery standard N-methylglucamine, added before derivatization to assess the efficiency of the derivatization step. Muramic acid (MurA) is found in the bacterial cell wall peptidoglycan and is not produced by eukaryotic cells (57). Equal amounts of MurA and glucosamine (GluN) are found in bacterial peptidoglycan, but in soils GluN predominantly originates from fungal chitin rather than bacterial peptidoglycan (57). The origin of galactosamine or mannosamine is currently debated (58, 59).
Soil Extracellular Enzyme Assays.
The hydrolytic enzymes, α-glucosidase, acid phosphatase, β-1,4-glucosidase, β-xylosidase, cellobiohydrolase, and N-acetyl-β-d-glucosaminidase of fresh soil samples were measured fluorometrically using methylumbelliferone-labeled substrates (60, 61). Specifically, each equivalent of 1.0 g dry mass of fresh soil was added into a 100-mL centrifuge tube, homogenized with 50 mL of 50-mM acetate buffer using a polytron homogenizer, then the mixture was poured into a round wide-mouth beaker. An additional 50 mL of acetate buffer was used to wash the centrifuge tube and poured into the same beaker. A magnetic stirrer was used to maintain a uniform suspension. The buffer, sample suspension, 10-μM references and 200-μM substrates were dispensed into the wells of a black 96-well microplate in the volume and order described by DeForest (60). The microplates were covered and incubated in the dark at 25 °C for 4 h and the fluorescence quantified using a microplate fluorometer with 365-nm excitation and 450-nm emission filters (61).
The nonfluorometric enzymes, polyphenol oxidase and peroxidase (breakdown of lignin and other aromatic compounds), were measured spectrophotometrically in the clear 96-well microplate using the substrate of l-3,4-dihydroxyphenylalanine (l-DOPA). The dispensed volume and the order of buffer, sample suspension, 25-mM l-DOPA, and 0.3% H2O2 were the same as for the fluorometric enzymes (60). The microplates were covered and incubated in the dark at 25 °C for 20 h, and the activities were assayed by measuring the absorbance at 450 nm using the microplate reader and expressed in units of μmol h−1 g−1.
Literature Data.
We used the Web of Science to search for papers containing words such as “Mollisols” (or Chernozem, Kastanozem, and Phaeozem, based on the World Reference Base classification), “soil organic matter fractions,” “particulate organic matter,” or “mineral-associated organic matter” published. We selected the field-based studies that have compared at least one “alternative practice” with conventional practices. We chose studies that have used aggregate dispersion and wet sieving methods to separate POM and MAOM by using the 53-μm sieve. In total, we found 17 research articles that studied the response of either POM or MAOM fractions of the world’s Mollisols. The alternative practices used in these studies include reduce or no tillage vs. conventional tillage, crop rotation vs. monoculture, adding manure vs synthetic fertilizers, cover crops vs. no cover crops, or a combination of these practices. The experiment length lasted from 4 to 60 y. Several studies have investigated deeper depths, but to be able to compare with data from WICST, we extracted data only from soils within 0 to 30 cm deep. We selected the natural log of the response ratio (alternative/conventional) as our meta-analytic effect size as commonly used in agroecological meta-analysis.
Statistical Analysis.
Data including soil total organic C stock and POM-C and MAOM-C (0 to 30 cm), C and N content and their ratios of bulk soil, POM and MAOM (0 to 15 and 15 to 30 cm), microbial CUE, MBC, activities of extracellular enzymes, total and individual amino sugar concentrations, and C functional groups of MAOM were analyzed with linear mixed-effects ANOVA models to compare the effects of different cropping systems and depths. We used cropping system and depth as fixed effects and replicate blocks as random effect. Univariate responses including SOM fractions, microbial traits (CUE, MBC, and amino sugars), enzyme activities, and the relative stability of MAOM were analyzed with linear mixed-effects ANOVA models to compare system and depth effects. The relationship between microbial traits and soil properties was analyzed by Pearson’s correlation analysis. All ANOVA and correlation analyses were performed in SAS v.9.4 (SAS Institute) using PROC MIXED and PROC CORR. Significance for all analyses was determined at P < 0.05.
We also used piecewise SEM (62) to examine the direct and indirect relationships between POM C, POM C:N ratio, the microbial traits, and MAOM-C. Piecewise SEM takes a local estimation approach and analyzes the components of the path diagram individually as a set of linear equations (62) rather than a global estimation of parameters that best capture the observed variance-covariance matrix (63). This allows for smaller sample sizes than are typically required by traditional SEM. Each endogenous variable was examined using a generalized linear model, specifying a Gaussian distribution. We hypothesized that POM C and POM C:N ratio both had direct effects on microbial biomass, CUE, and MAOM-C. Microbial biomass and CUE, in turn had direct effects on microbial necromass, which directly affected MAOM-C. We also specified a correlation between POM C and POM C:N ratio based on an initial correlation analysis. Analyses were conducted using the R package piecewiseSEM v2.1.0 (62).
Supplementary Material
Acknowledgments
We would like to thank Dr. Josh Posner (1949 to 2012), in great memory, for his foresight and leadership in setting up the Wisconsin Integrated Cropping Systems Trial in 1989. We thank Dr. Cynthia Kallenbach for her advice with lab analysis; Chelsea Zegler, Harry Read, and Jaimie West for their help with field sampling and lab analyses; and Dr. Andong Cai for his advice with data analysis. We also would like to acknowledge those who maintain and facilitate research at the trial—namely James Sustachek, Mark Walsh, and the staff of the University of Wisconsin–Madison’s Arlington Agricultural Research Station. This study was made possible by financial support from the USDA National Institute of Food and Agriculture (NIFA) Agriculture and Food Research Initiative (Grants 2013-68002-20525 and 2020-67019-31160) and USDA NIFA Sustainable Agricultural Systems (Grants 2019-68012-29852 and 2019-67013-29202). The grant support to Chao Liang from the National Natural Science Foundation of China (31930070) is also acknowledged.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2118931119/-/DCSupplemental.
Data Availability
All study data that have been used to generate figures and tables in the article and/or SI Appendix are available from the Dryad database (DOI: 10.5061/dryad.h44j0zpn2).
References
- 1.Sanderman J., Hengl T., Fiske G. J., Soil carbon debt of 12,000 years of human land use. Proc. Natl. Acad. Sci. U.S.A. 114, 9575–9580 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lal R., Negassa W., Lorenz K., Carbon sequestration in soil. Curr. Opin. Environ. Sustain. 15, 79–86 (2015). [Google Scholar]
- 3.Liu X., et al. , Overview of Mollisols in the world: Distribution, land use and management. Can. J. Soil Sci. 92, 383–402 (2012). [Google Scholar]
- 4.David M. B., McIsaac G. F., Darmody R. G., Omonode R. A., Long-term changes in Mollisol organic carbon and nitrogen. J. Environ. Qual. 38, 200–211 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Rumpel C., et al. , Put more carbon in soils to meet Paris climate pledges. Nature 564, 32–34 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Xu X., Pei J., Xu Y., Wang J., Soil organic carbon depletion in global Mollisols regions and restoration by management practices: A review. J. Soils Sediments 20, 1173–1181 (2020). [Google Scholar]
- 7.Smith J., et al. , Projected changes in the organic carbon stocks of cropland mineral soils of European Russia and the Ukraine, 1990–2070. Glob. Chang. Biol. 13, 342–356 (2007). [Google Scholar]
- 8.Ogle S. M., Breidt F. J., Easter M., Williams S., Paustian K., An empirically based approach for estimating uncertainty associated with modelling carbon sequestration in soils. Ecol. Modell. 205, 453–463 (2007). [Google Scholar]
- 9.Smith P., Carbon sequestration in croplands: The potential in Europe and the global context. Eur. J. Agron. 20, 229–236 (2004). [Google Scholar]
- 10.Lavallee J. M., Soong J. L., Cotrufo M. F., Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Chang. Biol. 26, 261–273 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Uri N. D., Conservation practices in U.S. agriculture and their impact on carbon sequestration. Environ. Monit. Assess. 70, 323–344 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Paustian K., et al. , Climate-smart soils. Nature 532, 49–57 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Lal R., Griffin M., Apt J., Lave L., Morgan M. G., Managing soil carbon. Science 304, 393 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Cates A. M., Ruark M. D., Hedtcke J. L., Posner J. L., Long-term tillage, rotation and perennialization effects on particulate and aggregate soil organic matter. Soil Tillage Res. 155, 371–380 (2016). [Google Scholar]
- 15.Lazicki P. A., Liebman M., Wander M. M., Root parameters show how management alters resource distribution and soil quality in conventional and low-input cropping systems in central Iowa. PLoS One 11, e0164209 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart C. E., Paustian K., Conant R. T., Plante A. F., Six J., Soil carbon saturation: Evaluation and corroboration by long-term incubations. Soil Biol. Biochem. 40, 1741–1750 (2008). [Google Scholar]
- 17.Castellano M. J., Mueller K. E., Olk D. C., Sawyer J. E., Six J., Integrating plant litter quality, soil organic matter stabilization, and the carbon saturation concept. Glob. Chang. Biol. 21, 3200–3209 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Ogle S. M., Swan A., Paustian K., No-till management impacts on crop productivity, carbon input and soil carbon sequestration. Agric. Ecosyst. Environ. 149, 37–49 (2012). [Google Scholar]
- 19.Puget P., Lal R., Soil organic carbon and nitrogen in a Mollisol in central Ohio as affected by tillage and land use. Soil Tillage Res. 80, 201–213 (2005). [Google Scholar]
- 20.Hassink J., The capacity of soils to preserve organic C and N by their association with clay and silt particles. Plant Soil 191, 77–87 (1997). [Google Scholar]
- 21.Kallenbach C. M., Frey S. D., Grandy A. S., Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat. Commun. 7, 13630 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleber M., et al. , Old and stable soil organic matter is not necessarily chemically recalcitrant: Implications for modeling concepts and temperature sensitivity. Glob. Chang. Biol. 17, 1097–1107 (2011). [Google Scholar]
- 23.Cotrufo M. F., Wallenstein M. D., Boot C. M., Denef K., Paul E., The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 19, 988–995 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Zhu X., Jackson R. D., DeLucia E. H., Tiedje J. M., Liang C., The soil microbial carbon pump: From conceptual insights to empirical assessments. Glob. Chang. Biol. 26, 6032–6039 (2020). [DOI] [PubMed] [Google Scholar]
- 25.C. Liang, J. P. Schimel, J. D. Jastrow, The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2, 1–6 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Sulc R. M., Franzluebbers A. J., Exploring integrated crop-livestock systems in different ecoregions of the United States. Eur. J. Agron. 57, 21–30 (2014). [Google Scholar]
- 27.Kallenbach C. M., Grandy A. S., Frey S. D., Diefendorf A. F., Microbial physiology and necromass regulate agricultural soil carbon accumulation. Soil Biol. Biochem. 91, 279–290 (2015). [Google Scholar]
- 28.Creamer C. A., et al. , Is the fate of glucose-derived carbon more strongly driven by nutrient availability, soil texture, or microbial biomass size? Soil Biol. Biochem. 103, 201–212 (2016). [Google Scholar]
- 29.Sanford G. R., et al. , Soil carbon lost from Mollisols of the North Central U.S.A. with 20 years of agricultural best management practices. Agric. Ecosyst. Environ. 162, 68–76 (2012). [Google Scholar]
- 30.Posner J. L., Casler M. D., Baldock J. O., The Wisconsin integrated cropping systems trial: Combining agroecology with production agronomy. Am. J. Altern. Agric. 10, 98–107 (1995). [Google Scholar]
- 31.Mosier S., et al. , Adaptive multi-paddock grazing enhances soil carbon and nitrogen stocks and stabilization through mineral association in southeastern U.S. grazing lands. J. Environ. Manage. 288, 112409 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Tautges N. E., et al. , Deep soil inventories reveal that impacts of cover crops and compost on soil carbon sequestration differ in surface and subsurface soils. Glob. Chang. Biol. 25, 3753–3766 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Manzoni S., Taylor P., Richter A., Porporato A., Ågren G. I., Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol. 196, 79–91 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Dungait J. A. J., Hopkins D. W., Gregory A. S., Whitmore A. P., Soil organic matter turnover is governed by accessibility not recalcitrance. Glob. Chang. Biol. 18, 1781–1796 (2012). [Google Scholar]
- 35.Sinsabaugh R. L., Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol. Biochem. 42, 391–404 (2010). [Google Scholar]
- 36.Daly A. B., et al. , A holistic framework integrating plant-microbe-mineral regulation of soil bioavailable nitrogen. Biogeochemistry 154, 211–229 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jilling A., et al. , Minerals in the rhizosphere: Overlooked mediators of soil nitrogen availability to plants and microbes. Biogeochemistry 139, 103–122 (2018). [Google Scholar]
- 38.Lugato E., Lavallee J. M., Haddix M. L., Panagos P., Cotrufo M. F., Different climate sensitivity of particulate and mineral-associated soil organic matter. Nat. Geosci. 14, 295–300 (2021). [Google Scholar]
- 39.Hall S. J., McNicol G., Natake T., Silver W. L., Large fluxes and rapid turnover of mineral-associated carbon across topographic gradients in a humid tropical forest: Insights from paired 14C analysis. Biogeosciences 12, 2471–2487 (2015). [Google Scholar]
- 40.Cui J., et al. , Carbon and nitrogen recycling from microbial necromass to cope with C:N stoichiometric imbalance by priming. Soil Biol. Biochem. 142, 107720 (2020). [Google Scholar]
- 41.Stanford G., Rationale for optimum nitrogen fertilization in corn production. J. Environ. Qual. 2, 159–166 (1973). [Google Scholar]
- 42.Hall S. J., Russell A. E., Moore A. R., Do corn-soybean rotations enhance decomposition of soil organic matter? Plant Soil 444, 427–442 (2019). [Google Scholar]
- 43.Sanford G. R., Jackson R. D., Booth E. G., Hedtcke J. L., Picasso V., Perenniality and diversity drive output stability and resilience in a 26-year cropping systems experiment. Field Crops Res. 263, 108071 (2021). [Google Scholar]
- 44.Sprunger C. D., Oates L. G., Jackson R. D., Robertson G. P., Plant community composition influences fine root production and biomass allocation in perennial bioenergy cropping systems of the upper Midwest, USA. Biomass Bioenergy 105, 248–258 (2017). [Google Scholar]
- 45.Sprunger C. D., Martin T., Mann M., Systems with greater perenniality and crop diversity enhance soil biological health. Agric. Environ. Lett. 5 (2020). [Google Scholar]
- 46.Yang Y., Tilman D., Soil and root carbon storage is key to climate benefits of bioenergy crops. Biofuel Res. J. 7, 1143–1148 (2020). [Google Scholar]
- 47.Spratt E., et al. , Accelerating regenerative grazing to tackle farm, environmental, and societal challenges in the upper Midwest. J. Soil Water Conserv. 76, 15A–23A (2021). [Google Scholar]
- 48.Franzluebbers A. J., et al. , Well-managed grazing systems: A forgotten hero of conservation. J. Soil Water Conserv. 67, 100A–104A (2012). [Google Scholar]
- 49.Bradford M. A., Fierer N., Reynolds J. F., Soil carbon stocks in experimental mesocosms are dependent on the rate of labile carbon, nitrogen and phosphorus inputs to soils. Funct. Ecol. 22, 964–974 (2008). [Google Scholar]
- 50.Demyan M. S., et al. , Use of specific peaks obtained by diffuse reflectance Fourier transform mid-infrared spectroscopy to study the composition of organic matter in a Haplic Chernozem. Eur. J. Soil Sci. 63, 189–199 (2012). [Google Scholar]
- 51.Margenot A. J., Calderón F. J., Bowles T. M., Parikh S. J., Jackson L. E., Soil organic matter functional group composition in relation to organic carbon, nitrogen, and phosphorus fractions in organically managed tomato fields. Soil Sci. Soc. Am. J. 79, 772–782 (2015). [Google Scholar]
- 52.Deiss L., Culman S. W., Demyan M. S., Grinding and spectra replication often improves mid-DRIFTS predictions of soil properties. Soil Sci. Soc. Am. J. 84, 914–929 (2020). [Google Scholar]
- 53.Vance E. D., Brookes P. C., Jenkinson D. S., An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 19, 703–707 (1987). [Google Scholar]
- 54.Geyer K., Schnecker J., Grandy A. S., Richter A., Frey S., Assessing microbial residues in soil as a potential carbon sink and moderator of carbon use efficiency. Biogeochemistry 151, 237–249 (2020). [Google Scholar]
- 55.Zhang X., Amelung W., Gas chromatographic determination of muramic acid, glucosamine, mannosamine, and galactosamine in soils. Soil Biol. Biochem. 28, 1201–1206 (1996). [Google Scholar]
- 56.Liang C., Read H. W., Balser T. C., GC-based detection of aldononitrile acetate derivatized glucosamine and muramic acid for microbial residue determination in soil. J. Vis. Exp. 63, e3767 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amelung W., Miltner A., Zhang X., Zech W., Fate of microbial residues during litter decomposition as affected by minerals. Soil Sci. 166, 598–606 (2001). [Google Scholar]
- 58.Glaser B., Turrión M. B., Alef K., Amino sugars and muramic acid – Biomarkers for soil microbial community structure analysis. Soil Biol. Biochem. 36, 399–407 (2004). [Google Scholar]
- 59.Engelking B., Flessa H., Joergensen R. G., Shifts in amino sugar and ergosterol contents after addition of sucrose and cellulose to soil. Soil Biol. Biochem. 39, 2111–2118 (2007). [Google Scholar]
- 60.DeForest J. L., The influence of time, storage temperature, and substrate age on potential soil enzyme activity in acidic forest soils using MUB-linked substrates and l-DOPA. Soil Biol. Biochem. 41, 1180–1186 (2009). [Google Scholar]
- 61.Saiya-Cork K. R., Sinsabaugh R. L., Zak D. R., The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 34, 1309–1315 (2002). [Google Scholar]
- 62.Lefcheck J. S., piecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol. Evol. 7, 573–579 (2016). [Google Scholar]
- 63.Grace J. B., Structural Equation Modeling and Natural Systems (Cambridge University Press, 2006). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data that have been used to generate figures and tables in the article and/or SI Appendix are available from the Dryad database (DOI: 10.5061/dryad.h44j0zpn2).