Abstract
Optogenetic stimulation of the locus coeruleus noradrenergic neurons can increase wakefulness, and high-frequency stimulation decreases noradrenaline levels and produces loss of muscle tone similar to that seen in cataplexy.
The ‘blue spot’, or locus coeruleus (in Latin), visible in fresh tissue of the dorsal pontine brainstem was first described in the eighteenth century by Félix Vicq-d’Azyr. It is the principal brain site for the synthesis of noradrenaline and its cluster of neurons has widespread ascending and descending projections. It has been implicated in arousal, motor control, plasticity, blood flow control, mood regulation and addiction1, although identification of its precise role in any of these functions has remained elusive. In this issue, Carter et al.2 bring the new technique of optogenetic stimulation to bear on this question. They found that levels of arousal and muscle tone can be regulated by varying the frequency of activation of locus coeruleus neurons and discovered a surprising loss of muscle tone triggered by high-frequency stimulation of this nucleus.
Optogenetic stimulation uses genetic techniques to insert light-sensitive ion channels into cell membranes. To selectively target locus coeruleus neurons, Carter et al.2 micro-injected a Cre-dependent adeno-associated virus into the locus coeruleus of mice engineered to express Cre recombinase in tyrosine hydroxylase–positive neurons. Depending on the optogenetic transgene inserted into the virus, laser illumination of the cells with light of the appropriate color will either excite or inhibit these neurons. Carter et al.2 delivered the light to the locus coeruleus of the unrestrained, behaving mouse through a fiber optic cable. The authors used immunohistochemical, fluorescence and in vitro studies to confirm that the ion channels were inserted into locus coeruleus cells and that laser stimulation activated the channels.
The selectivity of optogenetic stimulation contrasts with the effect of electrical stimulation. Electrical stimulation will activate all neurons in the vicinity of the electrode, not just those of a particular phenotype. It is well known that the functional effect of such stimulation may be analogous to that of a lesion. For example, stimulation of Broca’s area does not elicit speech, even though lesion of this area severely impairs speech. Neurosurgeons attempting to precisely localize and thereby prevent damage to Broca’s area commonly ask the subject to talk while they stimulate in the vicinity of the structure. If the stimulation stops speech, they have located Broca’s area. In a similar manner, deep brain stimulation of the globus pallidus or the subthalamic nucleus is used clinically to functionally inactivate these structures in Parkinson’s disease.
Optogenetic stimulation, as applied here, allows for the activation of the population of locus coeruleus neurons that have absorbed the transgene and are illuminated by the appropriate laser light. As with electrical stimulation, any pattern of pulses can be applied. Some of the caveats that apply to electrical stimulation also apply to in vivo optogenetic stimulation. Activated cells will include locus coeruleus cells that have different patterns of projections and that might not normally be activated together. It can be expected that all neurons with the implanted light-sensitive channels will respond in synchrony to the applied light stimulation. Such a pattern of activity is not commonly seen in brain regions, except during seizures, which can cause ‘drop attacks’. The normal asynchronous activity of neuronal populations contains information beyond the ‘on’ and ‘off’ state of the neurons. This activity normally drives and is driven by feedback from the activated cell populations. This feedback inhibition and excitation may be lost with applied stimulation trains. A prior unit-recording study monitoring as many as four locus coeruleus simultaneously across the sleep-wake cycle found that these cells do not fire in synchrony3.
It has long been known that electrical stimulation of large portions of the brainstem and diencephalic reticular formation produces rapid arousal4. Carter et al.2 found that optogenetic activation restricted to locus coeruleus could rapidly awaken mice that were asleep. Further research is needed to determine whether this arousal differs in threshold or time course from arousal triggered by stimulation of other brainstem areas. It is also unknown whether inactivation of locus coeruleus would prevent or raise the threshold for triggering arousal by activation of other waking-related cell groups. Optogenetic inhibition of locus coeruleus, as used by Carter et al.2, could be employed to address this question.
The most interesting of Carter et al.’s findings2 was that high-frequency stimulation of locus coeruleus caused a loss of muscle tone or behavioral arrest resembling that seen in human and animal cataplexy, a symptom of narcolepsy in which laughter or other sudden onset emotions cause loss of tone while consciousness is maintained. This finding seemed inconsistent with earlier findings that locus coeruleus cells cease discharge in cataplexy and in rapid eye movement (REM) sleep, both states in which muscle tone is blocked. In both REM sleep and cataplexy, the cessation occurs without prior burst discharge of locus coeruleus neurons3. Carter et al.2 carried out microdialysis sampling and high-performance liquid chromatography to determine whether their high-frequency stimulation caused high levels of noradrenaline release in vivo. They discovered that it did just the reverse. High-frequency stimulation decreased release of noradrenaline, presumably because it caused a depolarization block or depleted the cells’ stores of noradrenaline. Administration of noradrenaline reuptake inhibitors during stimulation reduced behavioral arrests, again indicating that the arrests were a result of noradrenaline depletion. This illustrates the need for any in vivo stimulation technique to be carried out in the context of neuronal recording, immediate early gene activation data or microdialysis measurement of release to allow for comparison of the stimulated activity with the normal activity of the investigated cells.
Clearly, muscle tone and alerting are not generated by the locus coeruleus in isolation from other neural systems. But when Carter et al.’s findings2 are combined with prior data, a clearer picture emerges of why cataplexy occurs in narcoleptics and why it does not occur in normal individuals (Fig. 1). It appears that muscle tone regulation across the sleep-wake cycle is a function of coordinated facilitation and inhibition of motoneurons. The locus coeruleus provides a critical source of facilitation5. Other monoaminergic, glutamatergic and hypocretinergic (orexinergic) neurons provide additional facilitatory input to motoneurons6. Inhibition is provided by glycinergic and GABAergic neurons7–9. Inhibition and disfacilitation combine to produce the muscle atonia of REM sleep and cataplexy3,10. The critical link between the inhibitory and facilitatory systems is a projection from the region in the pontine inhibitory area to GABA interneurons that project to locus coeruleus. These pontine neurons also activate neurons in the medullary inhibitory area. Thus, this circuit inhibits locus coeruleus neurons, removing their facilitation of motoneruons11 at the same time as neurons containing GABA and glycine are activated, inhibiting these same motoneurons12. The result is muscle tone suppression. This circuit is responsible for suppressing muscle tone in REM sleep to prevent dream enactment. When it fails, REM sleep behavior disorder, an often injurious acting out of dream mentation, occurs. Carter et al.’s findings2 suggest that optogenetic inhibition of locus coeruleus might reverse this pathology.
Figure 1.
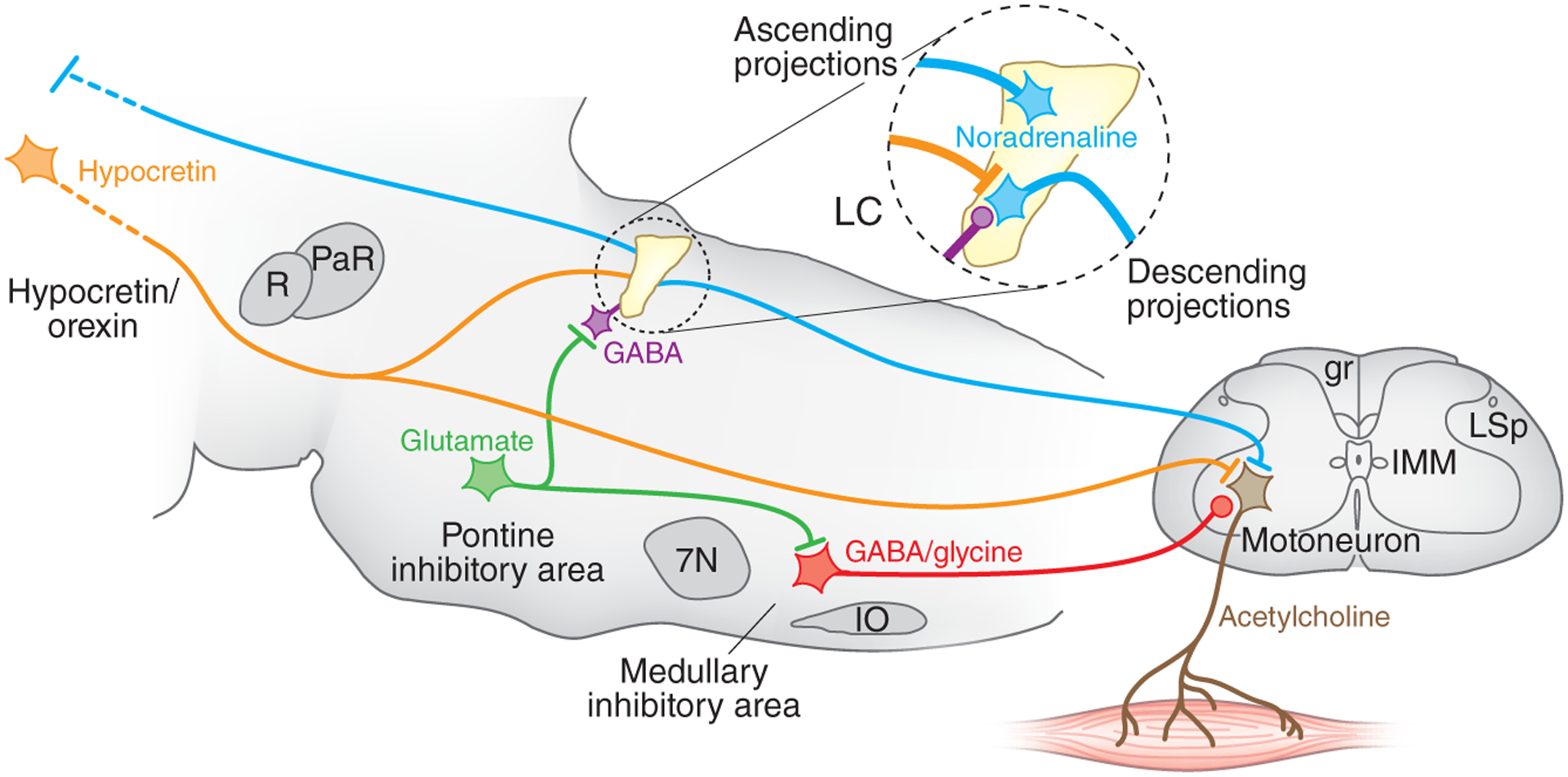
The locus coeruleus and muscle tone regulation. Shown is a sagittal section of the brainstem modeling some important elements of the circuit regulating muscle tone. Circled region shows the inputs and outputs to the locus coeruleus, which contains noradrenergic cells. The locus coeruleus projects ascending axons to the forebrain, where it can increase alertness, and to spinal motoneurons, where it facilitates muscle tone. Locus coeruleus cells shut off before and during REM sleep and before and during cataplexy. Muscle tone is lost in REM sleep. It is also lost in cataplexy, but in cataplexy waking is maintained. The cessation of noradrenaline release disfacilitates muscle tone. Carter et al.2 found that depletion of noradrenaline by high-frequency stimulation also produces a loss of muscle tone. Hypocretin (orexin) neurons, located in the hypothalamus and inactive in sleep, excite both locus coeruleus and motoneurons. Loss of muscle tone in REM sleep and cataplexy results not only from disfacilitation but also from active inhibition by glycine and GABA. The glutamatergic cells in the pontine inhibitory region, which are selectively active in REM sleep, inhibit locus coeruleus neurons through a GABAergic interneuron and excite cells in the medullary inhibitory region, which directly or indirectly release GABA and glycine onto motoneurons, depicted at the L7 level. Lines at axon terminations indicate excitatory connections and circles indicate inhibitory connections. gr, gracile fasciculus; IMM, intermedio-medial nucleus; IO, inferior olive; LC, locus coeruleus; LSp, lateral spinal nucleus; PaR, pararubral nucleus; R, red nucleus; 7N, seventh nerve nucleus.
Normal individuals experience mild muscle weakness when laughing and with certain other strong, sudden onset emotions. This weakness is limited by a massive phasic excitatory hypocretin projection to the locus coeruleus13. In narcoleptics, the loss of hypocretin neurons allows this transient weakness to progress to the complete loss of muscle tone that constitutes cataplexy. It is logically possible that phasic optogenetic or electrical stimulation of locus coeruleus at a critical frequency might prevent or attenuate cataplexy in narcoleptics. Smaller changes in the activity of the hypocretin system, acting through the locus coeruleus and other brainstem regions, undoubtedly contribute to postural control throughout waking13,14. Locus coeruleus cells also project rostrally, causing forebrain arousal1. Therefore, activation of this structure will not only enhance muscle tone but also produce alerting, an adaptive combination.
Rapid progress in laser control technology will allow greater control of the light stimulus used in optogenetic stimulation. This can be coupled with increasingly sophisticated genetic manipulations to target specific neuronal subtypes that are not necessarily limited to the genetic identity of the targeted cells15. This combination will surely continue to reveal new phenomena and insights into neuronal function. The selectivity of optogenetic activation will be particularly valuable when trying to dissect the functional roles of intermixed cell types that cannot be selectively activated with electrical stimulation techniques or readily identified with unit-recording techniques.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Aston-Jones G & Cohen JD J. Comp. Neurol 493, 99–110 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Carter ME et al. Nat. Neurosci 13, 1526–1533 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu MF et al. Neuroscience 91, 1389–1399 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moruzzi G & Magoun HW Electroencephalogr. Clin. Neurophysiol 1, 455–473 (1949). [PubMed] [Google Scholar]
- 5.Lai YY, Kodama T & Siegel JM J. Neurosci 21, 7384–7391 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peever JH, Lai YY & Siegel JM J. Neurophysiol 89, 2591–2600 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Chase MH & Morales FR in Principles of Sleep Medicine (eds. Kryger MH, Roth T & Dement WC) 154–168 (Elsevier Saunders, Philadelphia, 2005). [Google Scholar]
- 8.Kodama T, Lai YY & Siegel JM J. Neurosci 23, 1548–1554 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooks PL & Peever JH Adv. Exp. Med. Biol 669, 259–262 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Siegel JM et al. Science 252, 1315–1318 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mileykovskiy BY, Kiyashchenko LI, Kodama T, Lai YY & Siegel JM J. Neurosci 20, 8551–8558 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai YY, Kodama T, Schenkel E & Siegel JM J. Neurophysiol 104, 2024–2033 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mileykovskiy BY, Kiyashchenko LI & Siegel JM Neuron 46, 787–798 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siegel JM Exp. Neurol 65, 691–698 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gradinaru V et al. Cell 141, 154–165 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]


