Thioredoxins (Trxs) are proteins that can receive or donate electrons via reduction or oxidation, respectively, of their redox-active cysteine pair, converting a disulfide to thiol or vice versa. They are involved in multiple biological processes throughout all kingdoms of life, modulating the activity of enzymes that function, for example, in ribonucleotide reduction, nutrient assimilation, detoxification of reactive oxygen species, cell signaling, or photosynthesis (1). In plant biology, classic Trxs are especially well known for their role in reducing and thereby activating the enzymes of the Calvin–Benson cycle (refer to https://blog.aspb.org/calvin-cycle-calvin-benson-cycle-or-other/ for an explanation about not including Bassham in the name of this cycle), such as ribulose-1,5-bisphosphate carboxylase/oxygenase activase (RCA), fructose-1,6-bisphosphatase (FBPase), or phosphoribulokinase (PRK) for CO2 fixation (2). The reducing power comes from light-dependent reactions through photosystem I, ferredoxin (Fd), and ferredoxin-thioredoxin reductase (FTR) or Fd-NADP+ reductase for NADPH production. Hence, the division of chloroplast reactions into light and carbon reactions (please, correct textbooks and stop teaching it as “dark” reactions!) is due to the carbon reactions relying on illumination for reducing power to activate the Calvin–Benson cycle enzymes (3). In the recent years, an outstanding question was regarding their deactivation. What factors in the dark oxidize the Calvin–Benson enzymes? An important milestone was the in vitro demonstration that Trx-like proteins, such as Trx-like 2 (TrxL2) (4, 5), atypical Cys His-rich thioredoxin (ACHT) (6), and Trx-f (7), can play this role. The in vivo demonstration was eagerly awaited (8), and this knowledge gap is now filled by Yokochi et al. (9). Another part of the puzzle concerned the oxidation of the oxidizing factors themselves. Where do the electrons ultimately go? Pérez-Ruiz et al. (10) showed that both chloroplast redox systems, the Fd-FTR-Trx and the NADPH-dependent thioredoxin reductase C (NTRC), are donating electrons to 2-Cys peroxiredoxin (2-Cys Prx) with final acceptor hydrogen peroxide (H2O2) yielding H2O. The long-standing issue of how photosynthetic organisms rest their photosynthetic activity at night and/or upon light–dark transitions is reviewed here (11, 12).
In PNAS, Yokochi et al. (9) present the characterization of Arabidopsis mutant lines affected in genes whose protein product is predicted to act as an oxidizing factor of Calvin–Benson cycle enzymes. Based on in vitro data showing their capacity for oxidation of target proteins and reduction of 2-Cys Prx, a set of “priority” proteins was chosen to test their function in vivo, namely TrxL2.1 and TrxL2.2, ACHT1 and ACHT2, and Trx-f1 and Trx-f2. Using CRISPR-Cas9, the knockout mutants trxl2.1 and acht (mutated in both genes) were generated, and trx-f insertional mutants (mutated in both genes) were used (trxl2.2 could not be obtained). Of note, TrxL2.1 and ACHT2 display higher expression in leaves than their counterpart and may be the dominant isoforms. The trxl2.1 and acht mutants displayed wild-type growth, chlorophyll content, and photosynthetic parameters under the conditions tested (long day in low light). However, upon light–dark transitions, the in vivo redox status of Trx-targeted proteins, monitored using a thiol-labeling agent that increases the size of reduced protein, was affected in the mutants. This work clearly demonstrates that in vivo TrxL2.1 and Trx-f act as oxidation factors for the γ-subunit of adenosine triphosphate (ATP) synthase (CF1-γ) and RCA, respectively, and to a lesser extent, that ACHT1 and ACHT2 play a role in oxidizing FBPase; oxidation factors for PRK are yet to be found (Fig. 1).
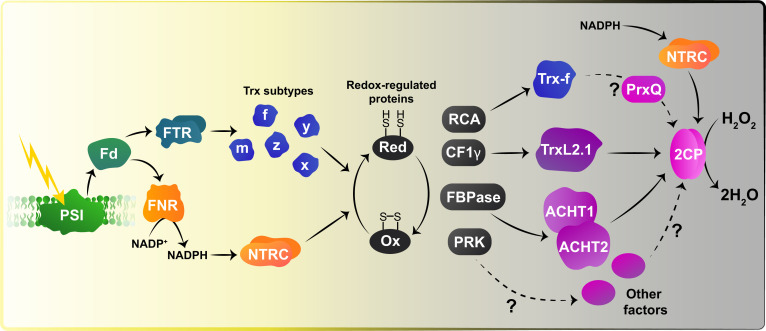
Fig. 1.
Updated model for the redox-regulatory systems in the chloroplast. Activation by light-dependent reduction (Red) of target proteins in the light (yellow background) and deactivation by oxidation (Ox) of target proteins with different specificities in the dark (gray background) are shown. Arrows indicate the flow of reducing power. PrxQ, peroxiredoxin Q; 2CP, 2-Cys Prx; FNR, ferredoxin-NADP+ reductase; PSI, photosystem I. Image credit: Daria Chrobok (DC SciArt). Adapted with permission from ref. 11.
The stunted growth and pale green phenotype of ntrc were largely rescued to wild-type levels in the ntrc trxl2.1 and ntrc acht mutants, and the redox status of target proteins returned to being relatively reduced. Yokochi et al. (9) conclude that 2-Cys Prx receives electrons from both NTRC and the TrxL2.1 and ACHT oxidation factors and that growth defects in the ntrc mutant are due to overoxidation of target proteins (and not redox imbalance in 2-Cys Prx and lack of H2O2 scavenging). Less reduction of 2-Cys Prx is observed in the ntrc acht compared with ntrc trxl2.1, implying that ACHT may play an important role in 2-Cys Prx reduction during light-to-dark transitions. Interestingly, the authors discover a positive correlation between the amount of ACHT specifically (and not TrxL2.1) and nonphotochemical quenching (NPQ), with ACHT2 overexpression leading to more NPQ and ACHT knockout in an ntrc mutant background suppressing the higher NPQ phenotype.
NPQ comprises processes that dissipate excess light energy as heat and thereby are photoprotective (13). The higher level of NPQ observed in low light in the ntrc mutant may be due to a buildup in the proton motive force from impaired reduction of CF1-γ (14). In light of the results shown here (9), an overoxidation of CF1-γ by TrxL2.1 could have been proposed, but that does not explain the higher NPQ phenotype as this phenotype remains in the ntrc trxl2.1. Recently uncovered was a possible role for NTRC in regulating proton gradient regulator 5 (PGR5) activity, which could also explain the higher NPQ phenotype of ntrc (15). However, Okegawa et al. (16) found that the phenotypic rescue of ntrc by pgr5 would be rather caused by the partial restoration of Trx-dependent reduction of thiol enzymes due to altered electron partitioning. In the same line, the NPQ phenotypic rescue of ntrc by acht may be due to partial restoration of Trx targets staying reduced. The high NPQ phenotype of ntrc might be indirect and due to overoxidation of ACHT targets rendering the Calvin–Benson inactive, diminishing ATP consumption and causing the buildup in proton motive force; more discussion is in ref. 16.
In PNAS, Yokochi et al. present the characterization of Arabidopsis mutant lines affected in genes whose protein product is predicted to act as an oxidizing factor of Calvin–Benson cycle enzymes.
The picture of the interplay between the different redox systems in the chloroplast is becoming more complete, and yet, knowledge of transmembrane regulation across the thylakoid membrane is still somewhat lacking (17). The chloroplast redox regulatory network impacts not only the Calvin–Benson cycle but also NPQ, the malate valve, and other metabolic processes such as diurnal starch accumulation and the oxidative pentose phosphate pathway, and its fine-tuning is critical for photosynthetic organisms acclimation to a fluctuating environment (18). A recent review and a special issue assessed the prospect of improving plant photosynthetic efficiency by manipulating the chloroplast redox systems (19) and demonstrated the centrality of redox signaling and emerging tools for a “plant redox biology” field definitely “on the move” (20), respectively.
As FBPase oxidation still occurred in the acht mutant, albeit at a slower rate, it could be that other factors can additionally act as oxidation factors in this case. In the future, it will be of interest to investigate the specificity of these oxidation factors, examine the role of other Trx (m, x, y, z) or ACHT isoforms in vivo for deactivation of enzymes by oxidation as well as the role of glutaredoxin [it has a higher midpoint potential than classical Trx, similar to TrxL2 (8)], and test the conserved function of oxidation factors throughout the photosynthetic lineages.
Acknowledgments
My research is supported by European Commission Marie Skłodowska-Curie Actions Individual Fellowship Reintegration Panel Grant 845687, Swedish Research Council Vetenskapsrådet Starting Grant 2018-04150, and Swedish Foundation for Strategic Research Consortium Grant ARC19-0051.
Footnotes
The author declares no competing interest.
See companion article, “Oxidative regulation of chloroplast enzymes by thioredoxin and thioredoxin-like proteins in Arabidopsis thaliana,” 10.1073/pnas.2114952118.
Change History
February 2, 2022: The title has been updated.
References
- 1.Meyer Y., Buchanan B. B., Vignols F., Reichheld J.-P., Thioredoxins and glutaredoxins: Unifying elements in redox biology. Annu. Rev. Genet. 43, 335–367 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Michelet L., et al. , Redox regulation of the Calvin-Benson cycle: Something old, something new. Front Plant Sci 4, 470 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchanan B. B., The carbon (formerly dark) reactions of photosynthesis. Photosynth. Res. 128, 215–217 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Yoshida K., Hara A., Sugiura K., Fukaya Y., Hisabori T., Thioredoxin-like2/2-Cys peroxiredoxin redox cascade supports oxidative thiol modulation in chloroplasts. Proc. Natl. Acad. Sci. U.S.A. 115, E8296–E8304 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaseghi M. J., et al. , The chloroplast 2-cysteine peroxiredoxin functions as thioredoxin oxidase in redox regulation of chloroplast metabolism. eLife 7, e38194 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eliyahu E., Rog I., Inbal D., Danon A., ACHT4-driven oxidation of APS1 attenuates starch synthesis under low light intensity in Arabidopsis plants. Proc. Natl. Acad. Sci. U.S.A. 112, 12876–12881 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yokochi Y., et al. , Impact of key residues within chloroplast thioredoxin-f on recognition for reduction and oxidation of target proteins. J. Biol. Chem. 294, 17437–17450 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacquot J.-P., Dark deactivation of chloroplast enzymes finally comes to light. Proc. Natl. Acad. Sci. U.S.A. 115, 9334–9335 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yokochi Y., Fukushi Y., Wakabayashi K., Yoshida K., Hisabori T., Oxidative regulation of chloroplast enzymes by thioredoxin and thioredoxin-like proteins in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 118, e2114952118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pérez-Ruiz J. M., Naranjo B., Ojeda V., Guinea M., Cejudo F. J., NTRC-dependent redox balance of 2-Cys peroxiredoxins is needed for optimal function of the photosynthetic apparatus. Proc. Natl. Acad. Sci. U.S.A. 114, 12069–12074 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshida K., Yokochi Y., Hisabori T., New light on chloroplast redox regulation: Molecular mechanism of protein thiol oxidation. Front Plant Sci 10, 1534 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cejudo F. J., Ojeda V., Delgado-Requerey V., González M., Pérez-Ruiz J. M., Chloroplast redox regulatory mechanisms in plant adaptation to light and darkness. Front. Plant Sci. 10, 380 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bassi R., Dall’Osto L., Dissipation of light energy absorbed in excess: The molecular mechanisms. Annu. Rev. Plant Biol. 72, 47–76 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Carrillo L. R., Froehlich J. E., Cruz J. A., Savage L. J., Kramer D. M., Multi-level regulation of the chloroplast ATP synthase: The chloroplast NADPH thioredoxin reductase C (NTRC) is required for redox modulation specifically under low irradiance. Plant J. 87, 654–663 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Naranjo B., Penzler J. F., Rühle T., Leister D., NTRC effects on non-photochemical quenching depends on PGR5. Antioxidants 10, 900 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okegawa Y., Tsuda N., Sakamoto W., Motohashi K., Maintaining the chloroplast redox balance through the PGR5-dependent pathway and the Trx system is required for light-dependent activation of photosynthetic reactions. Plant Cell Physiol., 10.1093/pcp/pcab148 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Buchanan B. B., The path to thioredoxin and redox regulation in chloroplasts. Annu. Rev. Plant Biol. 67, 1–24 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Thormählen I., et al. , Thioredoxins play a crucial role in dynamic acclimation of photosynthesis in fluctuating light. Mol. Plant 10, 168–182 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Nikkanen L., Rintamäki E., Chloroplast thioredoxin systems dynamically regulate photosynthesis in plants. Biochem. J. 476, 1159–1172 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geigenberger P., Smirnoff N., Van Breusegem F., Dietz K.-J., Noctor G., Plant redox biology-on the move. Plant Physiol. 186, 1–3 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]