Abstract
Paget disease of the breast is an uncommon malignant tumor with an inferior outcome. Therefore, establishing nomograms to predict the survival outcomes of breast Paget disease patients is urgent. Clinicopathological and follow-up data of breast Paget disease patients diagnosed between 2010 and 2016 were retrieved through the Surveillance, Epidemiology, and End Result (SEER) database. The significant factors were screened out, and then those factors were utilized to build two valuable nomograms. The discriminative ability of nomograms was investigated using concordance-index (C-index), while the predictive accuracy and benefits were evaluated using calibration curves and decision curve analysis. Finally, a total of 417 breast Paget disease patients were enrolled. Tumor grade, histological type, American Joint Committee on Cancer (AJCC) stage, surgery, chemotherapy, and marital status were confirmed as independent overall survival (OS)-related factors; tumor grade, histological type, AJCC stage, and age were associated with independent cancer-specific survival (CSS)-related factors. The values of the C-index for OS nomogram acquired were 0.827 and 0.745 for training and validation cohorts, respectively. Meanwhile, the corresponding values of the C-index to CSS nomogram were 0.890 and 0.655, respectively. The calibration curves and decision curve analysis indicated that both nomograms had an excellent performance. Finally, the nomogram-based risk stratification system indicated that all breast Paget disease patients could be classified into low- and high-risk groups and showed distinct outcomes. In conclusion, two valuable nomograms incorporating various clinicopathological indicators were established for breast Paget disease patients. These prognostic nomograms provide accurate prognostic assessment for breast Paget disease patients and help clinicians select appropriate treatment strategies.
Keywords: Paget disease, breast cancer, overall survival, cancer-specific survival, nomogram
Impact statement
Breast Paget disease is a rare subtype of breast cancer. Unfortunately, its prognosis is worse than other types of breast cancers. Many risk factors and prognostic variables have been identified, but there is no thorough research focused on developing the prognostic prediction tools for breast Paget disease, which indicates that the probability of prognosis cannot be well assessed. In this population-based study, we established two effective nomograms to qualify the OS and CSS of breast Paget disease patients, and on this basis, we further established a risk stratification system. The nomogram scoring systems had better discriminative power and clinical application value compared with the prognostic factors alone. Besides, the results of the risk stratification system confirmed the powerful role of nomograms in distinguishing results and risk stratification.
Introduction
Breast cancer seriously affects the health of female patients, and breast cancer-specific deaths in 2018 accounted for approximately 15% of female cancer deaths. 1 Among them, Paget disease (PD) is extremely rare and accounts for 0.5–5% of all breast cancer cases.2–5 The breast PD is characterized by local erythema, scaling and ulcers of the nipple and can even extend to the areola.6–8 It is usually associated with carcinoma in situ or invasive carcinoma.9–11 Worse, breast PD showed poor prognosis than other types of breast cancers, and the five-year relapse-free survival rate is 52.2%.12–14
According to the degree of malignancy, breast PD can be grouped into three groups: PD with invasive ductal carcinoma (PD-IDC), PD with ductal carcinoma in situ (PD-DCIS), and PD of the nipple without concurrent breast cancer. 2 Considering that the prognosis of breast PD patients with different histological types is significantly different, and the traditional American Joint Committee on Cancer (AJCC) stage system cannot effectively predict the prognosis of patients, a robust prognostic biomarker and model is urgently needed. Recent work reported that many biomarkers and prognostic factors could effectively predict the prognosis of breast cancer, including c-erbB-2, Ki-67, Cyclin D1, Bcl-2, age, marital status, tumor size, and lymph node status.15–17 However, it is regrettable that no prognostic prediction model was established for breast PD patients.
Therefore, this study aimed to build two prognostic models to understand the prognosis of patients by analyzing a population-based breast PD cohort.
Materials and methods
Patients’ selection
Patients’ data were obtained using the SEER*Stat 8.3.6. Female patients who were diagnosed as all three kinds of breast PD (ICD-O-3: 8540/3, 8541/3, and 8543/3) between 2010 and 2016 were obtained. The exclusion criteria were shown as follows: (1) breast PD is not first primary tumor; (2) died but the cause of death is unclear; (3) unclear clinicopathological information, including age, tumor size, race, grade, histological type, AJCC TNM stage, surgery, radiotherapy, chemotherapy, breast subtype, estrogen receptor (ER) status, progesterone receptor (PR) status, human epidermal growth factor 2-neu (HER2) status, and marital status. All patients were randomly assigned to a training cohort (70%) and a validation cohort (30%). The training cohort and the validated cohort were used for internal development and external validation, respectively.
Data collection
Variables were classified according to demographic, cancer, and treatment data. Demographic variables included age, race, and marital status. The cancer characteristics included tumor size and grade, histological type, AJCC TNM stage, breast subtype, ER status, PR status, and HER2 status. Treatment characteristics included surgery, chemotherapy, and radiotherapy. Age and tumor size were recorded as continuous variables, but in our study, the X-tile software was utilized to confirm the optimal cutoff values of these two variables based on the prognostic data. 18 The optimal cutoff values of age for OS were 59 and 70 years, the best cutoff values of age for CSS were 51 and 80 years, and the best cutoff values of tumor size for OS and CSS were both 28 and 57 mm.
Statistical analysis
All statistical methods were employed using SPSS 25.0 (IBM) and R software (version 3.6.1). P < 0.05 (two-sided) was considered as statistically significant. Then, the optimal cutoff values of age and tumor size were obtained by performing X-tile software. Multivariate Cox analyses were used to screen factors that significantly affected the survival of breast PD patients and to construct predictive models further. The discriminative ability and the consistency were estimated using Concordance-index (C-index) and calibration curves, respectively. Furthermore, the range of threshold probabilities and the magnitude of benefit were identified by decision curve analysis (DCA). To verify the accuracy of comprehensive nomograms, we generated time-dependent receiver operating characteristic (ROC) curves of the prognostic nomograms and the areas under the ROC curves (AUCs) of the independent prognostic factors, and the nomograms were further compared. Finally, patients were roughly categorized into two risk groups based on the X-tile determined cutoff values, and then the value of nomograms for predicting prognosis was verified by survival curve and log-rank test. 19
Results
Patients’ baseline characteristics
The baseline information of 417 breast PD patients is listed in Table 1. The mean age of 417 patients was 59.2 ± 15.2 years old, and 326 (78.2%) were white. The majority grade is III–IV (61.2%), while 93.8% were in the M0 stage. The largest proportion of histological type was PD‐IDC (95.0%), and most breast PD patients underwent surgery. A total of 292 and 125 patients were incorporated into the training and validation cohort, respectively.
Table 1.
Clinical and pathological features of patients diagnosed as breast PD.
| Characteristics | Total cohort 417 | Training cohort 292 (70%) | Validation cohort 125 (30%) | Statistical value | P |
|---|---|---|---|---|---|
| Age, years | 59.2 ± 15.2 | 59.2 ± 15.0 | 59.2 ± 15.6 | –0.014 | 0.989 |
| Tumor size, mm | 26.5 ± 24.7 | 26.7 ± 25.9 | 26.0 ± 21.9 | 0.276 | 0.783 |
| Race | 0.217 | 0.897 | |||
| White | 326 (78.2%) | 230 (78.8%) | 96 (76.8%) | ||
| Black | 48 (11.5%) | 33 (11.3%) | 15 (12.0%) | ||
| Other | 43 (10.3%) | 29 (9.9%) | 14 (11.2%) | ||
| Grade | 0.009 | 0.923 | |||
| I–II | 162 (38.8%) | 113 (38.7%) | 49 (39.2%) | ||
| III–IV | 255 (61.2%) | 179 (61.3%) | 76 (60.8%) | ||
| Histological type | 0.629 | 0.799 | |||
| PD | 7 (1.7%) | 6 (2.1%) | 1 (0.8%) | ||
| PD-IDC | 396 (95.0%) | 276 (94.5%) | 120 (96.0%) | ||
| PD-DCIS | 14 (3.4%) | 10 (3.4%) | 4 (3.2%) | ||
| AJCC | 4.994 | 0.172 | |||
| I | 157 (37.6%) | 115 (39.4%) | 42 (33.6%) | ||
| II | 119 (28.5%) | 75 (25.7%) | 44 (35.2%) | ||
| III | 115 (27.6%) | 81 (27.7%) | 34 (27.2%) | ||
| IV | 26 (6.2%) | 21 (7.2%) | 5 (4.0%) | ||
| T stage | 0.569 | 0.903 | |||
| T1 | 208 (49.9%) | 148 (50.7%) | 60 (48.0%) | ||
| T2 | 113 (27.1%) | 76 (26.0%) | 37 (29.6%) | ||
| T3 | 31 (7.4%) | 22 (7.5%) | 9 (7.2%) | ||
| T4 | 65 (15.6%) | 46 (15.8%) | 19 (15.2%) | ||
| N stage | 0.595 | 0.898 | |||
| N0 | 201 (48.2%) | 142 (48.6%) | 59 (47.2%) | ||
| N1 | 132 (31.7%) | 92 (31.5%) | 40 (32.0%) | ||
| N2 | 50 (12.0%) | 36 (12.3%) | 14 (11.2%) | ||
| N3 | 34 (8.2%) | 22 (7.5%) | 12 (9.6%) | ||
| M stage | 1.525 | 0.217 | |||
| M0 | 391 (93.8%) | 271 (92.8%) | 120 (96.0%) | ||
| M1 | 26 (6.2%) | 21 (7.2%) | 5 (4.0%) | ||
| Surgery | 0.000 | 1.000 | |||
| No | 10 (2.4%) | 7 (2.4%) | 3 (2.4%) | ||
| Yes | 407 (97.6%) | 285 (97.6%) | 122 (97.6%) | ||
| Radiotherapy | 0.065 | 0.798 | |||
| No | 274 (65.7%) | 193 (66.1%) | 81 (64.8%) | ||
| Yes | 143 (34.3%) | 99 (33.9%) | 44 (35.2%) | ||
| Chemotherapy | 2.455 | 0.117 | |||
| No | 164 (39.3%) | 122 (41.8%) | 42 (33.6%) | ||
| Yes | 253 (60.7%) | 170 (58.2%) | 83 (66.4%) | ||
| Breast subtype | 5.923 | 0.115 | |||
| HR−/HER2− | 27 (6.5%) | 22 (7.5%) | 5 (4.0%) | ||
| HR−/HER2+ | 131 (31.4%) | 94 (32.2%) | 37 (29.6%) | ||
| HR+/HER2− | 137 (32.9%) | 100 (34.2%) | 37 (29.6%) | ||
| HR+/HER2+ | 122 (29.3%) | 76 (26.0) | 46 (36.8%) | ||
| ER | 0.131 | 0.718 | |||
| Negative | 169 (40.5%) | 120 (41.1%) | 49 (39.2%) | ||
| Positive | 248 (59.5%) | 172 (58.9%) | 76 (60.8%) | ||
| PR | 0.141 | 0.707 | |||
| Negative | 236 (56.6%) | 167 (57.2%) | 69 (55.2%) | ||
| Positive | 181 (43.4%) | 125 (42.8%) | 56 (44.8%) | ||
| HER2 | 2.455 | 0.117 | |||
| Negative | 164 (39.3%) | 122 (41.8%) | 42 (33.6%) | ||
| Positive | 253 (60.7%) | 170 (58.2%) | 83 (66.4%) | ||
| Marital status | 0.616 | 0.433 | |||
| No | 189 (45.3%) | 136 (46.6%) | 53 (42.4%) | ||
| Yes | 228 (54.7%) | 156 (53.4%) | 72 (57.6%) |
PD: Paget disease; PD-IDC: PD with invasive ductal carcinoma; PD-DCIS: PD with ductal carcinoma in situ; AJCC: American Joint Committee on Cancer; ER: estrogen receptor; PR: progesterone receptor; HER2: human epidermal growth factor 2-neu.
Identifying prognostic factors for PD patients
Through univariate Cox analyses, we determined that age, tumor size, grade, histological type, AJCC stage, T stage, N stage, surgery, and marital status were OS-related factors (Table 2), and the age, tumor size, grade, histological type, AJCC stage, T stage, N stage, and surgery were CSS-related factors (Table 3). Then, by integrating all OS- or CSS-related factors into the multivariate Cox analyses, grade, histological type, AJCC stage, surgery, chemotherapy, and marital status were determined as independent OS-related factors (Table 2). In addition, age, grade, histological type, and AJCC stage were confirmed as independent CSS-related factors (Table 3).
Table 2.
Univariate and multivariate Cox analysis of overall survival in breast PD patients.
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age, years | ||||
| <59 | ||||
| 59–70 | 2.422 (1.119–5.244) | 0.025 | ||
| >70 | 4.354 (2.250–8.427) | 0.001 | ||
| Tumor size, mm | ||||
| 28.0 | ||||
| 28.0–57.0 | 2.301 (1.144–4.629) | 0.019 | ||
| ≥57.0 | 6.431 (3.351–12.275) | 2.018 | ||
| Race | ||||
| White | ||||
| Black | 0.831 (0.372–1.856) | 0.652 | ||
| Other | 0.464 (0.120–1.797) | 0.266 | ||
| Grade | ||||
| I–II | ||||
| III–IV | 2.576 (1.288–5.153) | 0.007 | 2.697 (1.286–5.654) | 0.009 |
| Histological type | ||||
| PD | ||||
| PD-IDC | 0.239 (0.074–0.772) | 0.017 | 0.197 (0.054–0.726) | 0.015 |
| PD-DCIS | 0.383 (0.077–1.906) | 0.241 | 0.213 (0.036–1.251) | 0.087 |
| AJCC | ||||
| I | ||||
| II | 1.529 (0.574–4.074) | 0.396 | 1.124 (0.41–3.081) | 0.821 |
| III | 3.950 (1.749–8.920) | 0.001 | 7.308 (3.045–17.539) | 0.001 |
| IV | 19.774 (8.020–48.756) | 9.083 | 25.371 (9.56–67.33) | 0.001 |
| T stage | ||||
| T1 | ||||
| T2 | 1.962 (0.881–4.367) | 0.099 | ||
| T3 | 3.442 (1.291–9.173) | 0.013 | ||
| T4 | 7.188 (3.504–14.745) | 7.420 | ||
| N stage | ||||
| N0 | ||||
| N1 | 3.351 (1.615–6.951) | 0.001 | ||
| N2 | 4.041 (1.715–9.521) | 0.001 | ||
| N3 | 5.767 (2.318–14.352) | 0.001 | ||
| M stage | ||||
| M0 | ||||
| M1 | 9.863 (5.090–19.112) | 1.196 | ||
| Surgery | ||||
| No | ||||
| Yes | 0.235 (0.073–0.760) | 0.016 | 0.265 (0.071–0.994) | 0.049 |
| Radiotherapy | ||||
| No | ||||
| Yes | 0.787 (0.434–1.427) | 0.430 | ||
| Chemotherapy | ||||
| No | ||||
| Yes | 0.603 (0.346–1.050) | 0.074 | 0.279 (0.15–0.52) | <0.001 |
| Breast subtype | ||||
| HR–/HER2– | ||||
| HR–/HER2+ | 1.241 (0.359–4.286) | 0.733 | ||
| HR+/HER2– | 1.331 (0.388–4.570) | 0.649 | ||
| HR+/HER2+ | 1.726 (0.503–5.926) | 0.385 | ||
| ER | ||||
| Negative | ||||
| Positive | 1.314 (0.738–2.342) | 0.354 | ||
| PR | ||||
| Negative | ||||
| Positive | 0.670 (0.373–1.204) | 0.181 | ||
| HER2 | ||||
| Negative | ||||
| Positive | 1.148 (0.648–2.032) | 0.637 | ||
| Marital status | ||||
| No | ||||
| Yes | 0.388 (0.214–0.704) | 0.002 | 0.354 (0.186–0.674) | 0.002 |
PD: Paget disease; PD-IDC: PD with invasive ductal carcinoma; PD-DCIS: PD with ductal carcinoma in situ; HR: hazard ratio; CI: confidence interval; AJCC: American Joint Committee on Cancer; ER: estrogen receptor; PR: progesterone receptor; HER2: human epidermal growth factor 2-neu.
Table 3.
Univariate and multivariate Cox analysis of cancer-specific survival in breast PD patients.
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age, years | ||||
| 51 | ||||
| 51–80 | 2.667 (1.009–7.050) | 0.048 | 3.766 (1.326–10.694) | 0.013 |
| >80 | 4.637 (1.412–15.224) | 0.011 | 8.161 (2.246–29.65) | 0.001 |
| Tumor size, mm | ||||
| 28.0 | ||||
| 28.0–57.0 | 5.139 (1.955–13.505) | 0.001 | ||
| ≥57.0 | 16.606 (6.815–40.465) | 6.284 | ||
| Race | ||||
| White | ||||
| Black | 0.763 (0.293–1.988) | 0.580 | ||
| Other | 0.451 (0.087–2.325) | 0.341 | ||
| Grade | ||||
| I–II | ||||
| III–IV | 3.542 (1.368–9.175) | 0.009 | 5.019 (1.758–14.329) | 0.003 |
| Histological type | ||||
| PD | ||||
| PD-IDC | 0.157 (0.048–0.519) | 0.002 | 0.093 (0.024–0.356) | 0.001 |
| PD-DCIS | 0.266 (0.044–1.597) | 0.148 | 0.285 (0.043–1.887) | 0.193 |
| AJCC | ||||
| I | ||||
| II | 2.322 (0.388–13.896) | 0.356 | 1.745 (0.287–10.608) | 0.545 |
| III | 11.390 (2.604–49.815) | 0.001 | 13.82 (3.098–61.662) | 0.001 |
| IV | 69.977 (15.556–314.793) | 3.077 | 64.62 (13.859–301.293) | 0.001 |
| T stage | ||||
| T1 | ||||
| T2 | 5.222 (1.385–19.685) | 0.015 | ||
| T3 | 11.614 (2.775–48.611) | 0.001 | ||
| T4 | 23.599 (6.901–80.700) | 0.001 | ||
| N stage | ||||
| N0 | ||||
| N1 | 8.774 (2.539–30.315) | 0.001 | ||
| N2 | 13.232 (3.581–48.897) | 0.001 | ||
| N3 | 15.616 (3.902–62.488) | 0.001 | ||
| M stage | ||||
| M0 | ||||
| M1 | 15.972 (7.715–33.065) | 8.414 | ||
| Surgery | ||||
| No | ||||
| Yes | 0.166 (0.051–0.546) | 0.003 | ||
| Radiotherapy | ||||
| No | ||||
| Yes | 0.972 (0.478–1.977) | 0.938 | ||
| Chemotherapy | ||||
| No | ||||
| Yes | 0.788 (0.397–1.565) | 0.497 | ||
| Breast subtype | ||||
| HR–/HER2– | ||||
| HR–/HER2+ | 2.701 (0.349–20.925) | 0.341 | ||
| HR+/HER2– | 2.764 (0.357–21.415) | 0.330 | ||
| HR+/HER2+ | 3.208 (0.411–25.064) | 0.266 | ||
| ER | ||||
| Negative | ||||
| Positive | 1.303 (0.641–2.648) | 0.465 | ||
| PR | ||||
| Negative | ||||
| Positive | 0.765 (0.376–1.555) | 0.459 | ||
| HER2 | ||||
| Negative | ||||
| Positive | 1.212 (0.596–2.464) | 0.595 | ||
| Marital status | ||||
| No | ||||
| Yes | 0.540 (0.269–1.085) | 0.084 | ||
PD: Paget disease; PD-IDC: PD with invasive ductal carcinoma; PD-DCIS: PD with ductal carcinoma in situ; HR: hazard ratio; CI: confidence interval; AJCC: American Joint Committee on Cancer; ER: estrogen receptor; PR: progesterone receptor; HER2: human epidermal growth factor 2-neu.
Construction and verification of the OS and CSS nomograms
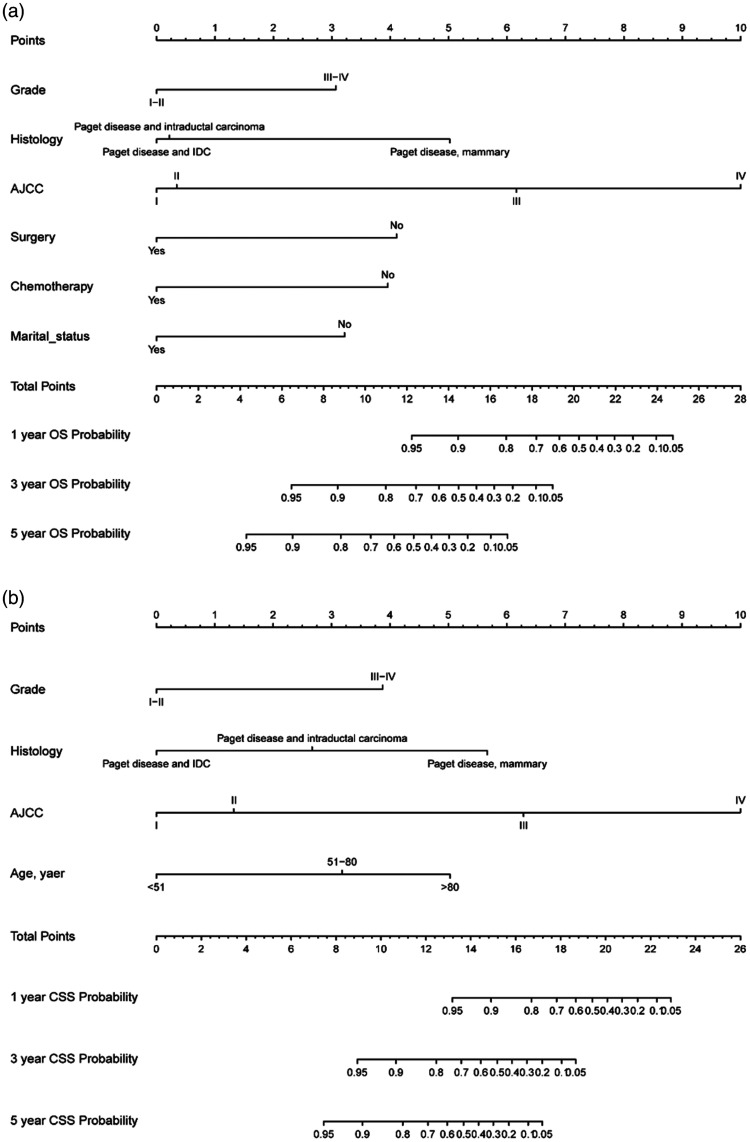
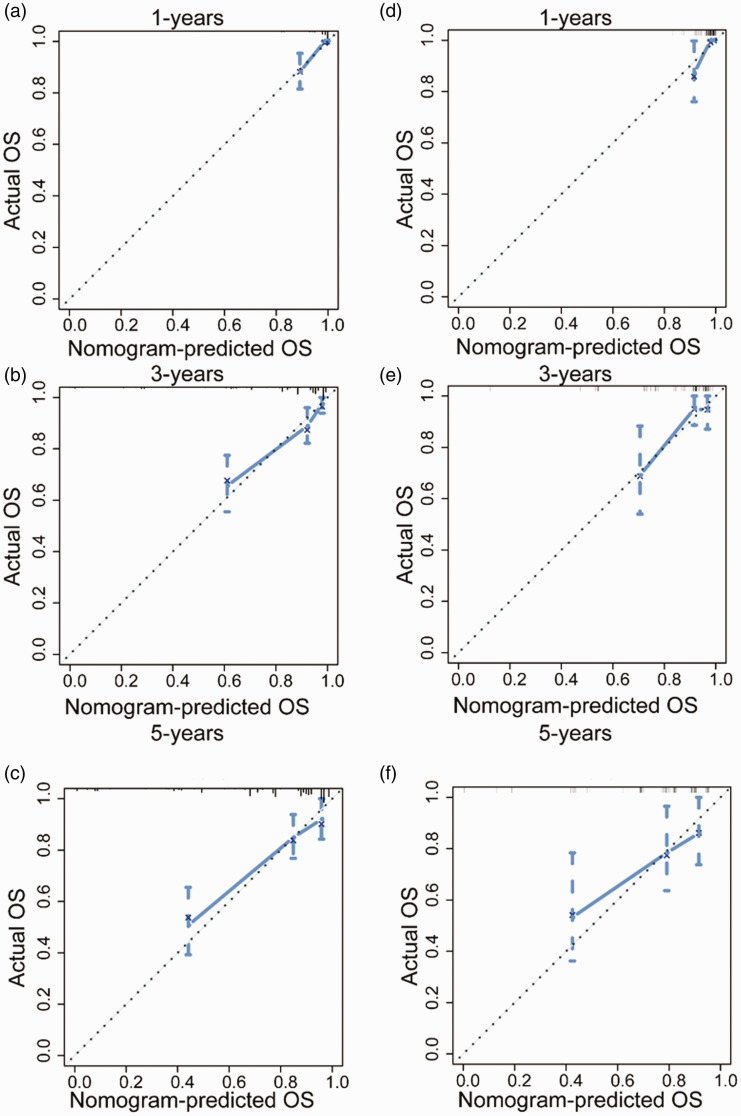
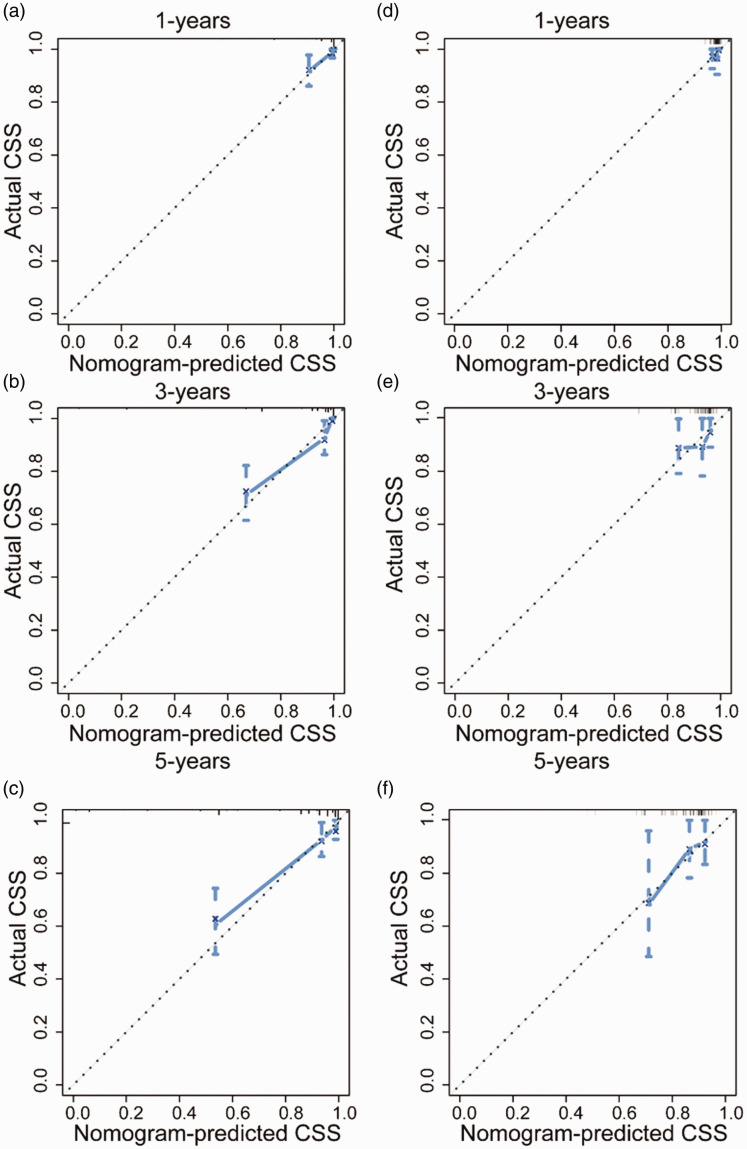
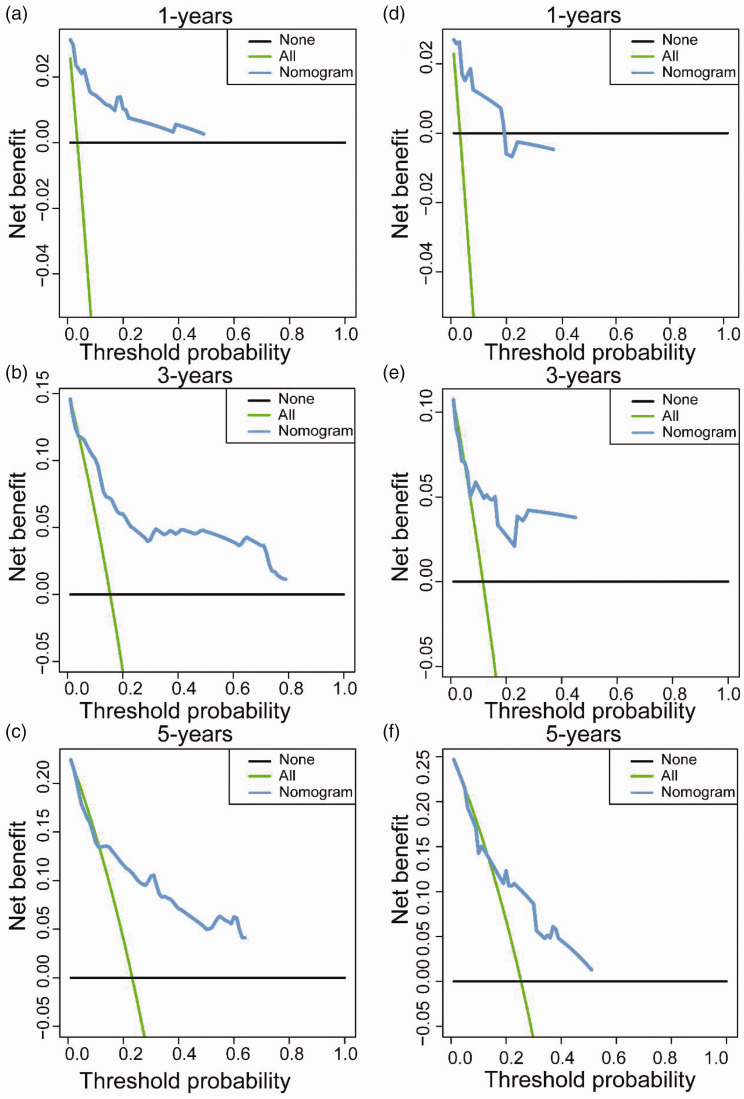
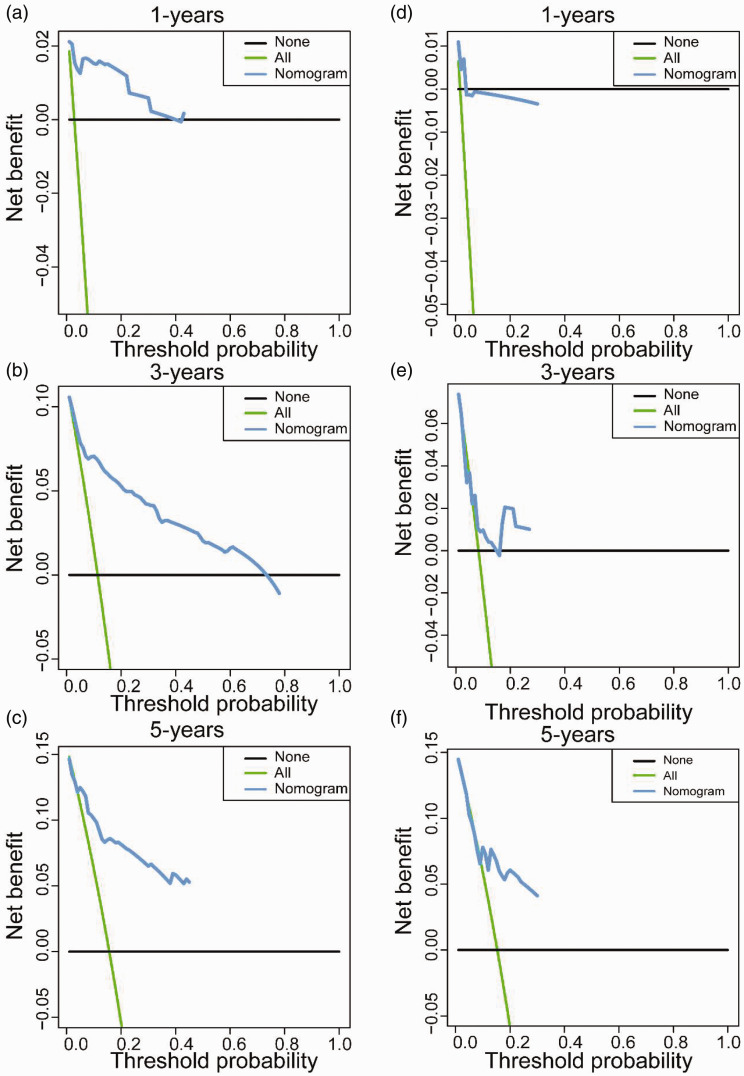
By incorporating corresponding independent prognostic factors, we established prognostic nomograms of OS and CSS (Figure 1). The OS and CSS nomograms were validated both internally and externally. In the training cohort, the values of the C-index were 0.827 and 0.890 for OS and CSS nomogram, respectively. In the validation cohort, the corresponding values of the C-index were 0.745 and 0.655, respectively. Meanwhile, the calibration curves indicated that the predicted outcome was close to the observed outcome (Figures 2 and 3). Besides, the DCA curves displayed that the nomograms have satisfactory clinical utility (Figures 4 and 5).
Figure 1.
Nomograms estimating one-year, three-year, and five-year OS (a) and CSS (b) rates of breast PD patients.
Figure 2.
The calibration curves of OS nomogram for one-year, three-years, and five-years in the training cohort (a–c) and validation cohort (d–f). (A color version of this figure is available in the online journal.)
Figure 3.
The calibration curves of CSS nomogram for one-year, three-years, and five-years in the training cohort (a–c) and validation cohort (d–f). (A color version of this figure is available in the online journal.)
Figure 4.
The decision curve analyses of OS nomogram for one-year, three-years, and five-years in the training cohort (a–c) and validation cohort (d–f). (A color version of this figure is available in the online journal.)
Figure 5.
The decision curve analyses of CSS nomogram for one-year, three-years, and five-years in the training cohort (a–c) and validation cohort (d–f). (A color version of this figure is available in the online journal.)
Comparison of discrimination between nomograms and independent prognostic factors
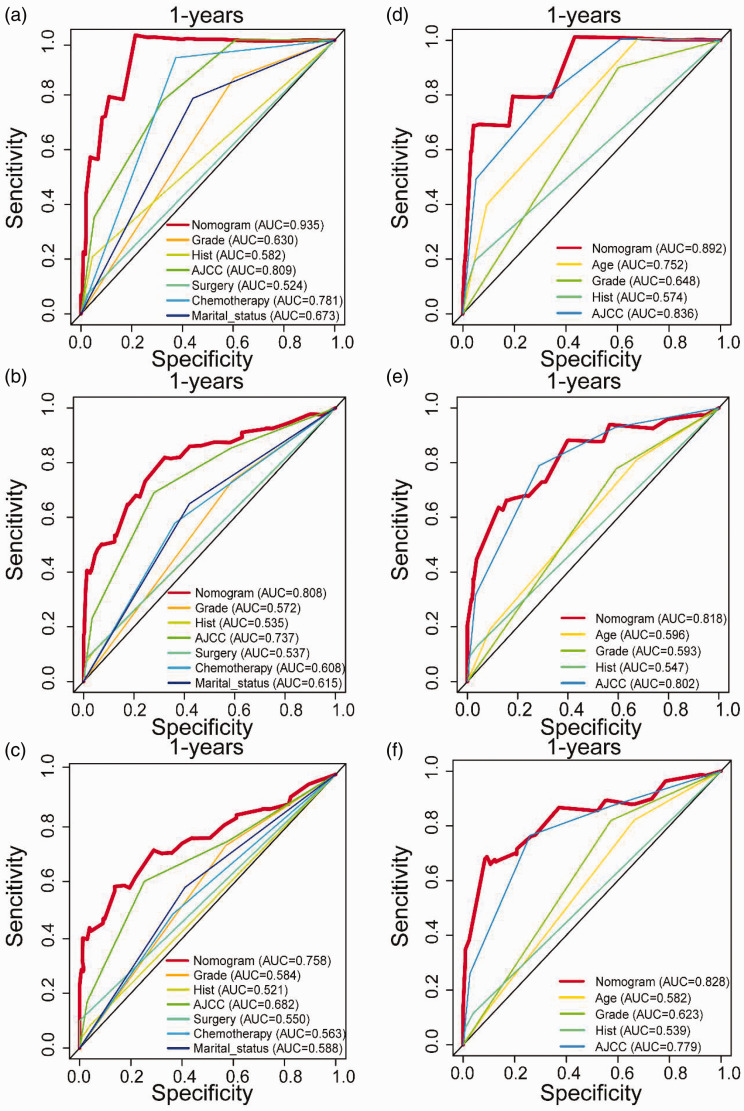
To further show the superior discrimination of our nomograms in assessing the survival of breast PD patients, we also generated the ROC curves of the nomograms and all independent prognostic factors. Collectively, the results verified that the AUCs of all prognostic factors alone were higher than 0.500, which means that all individual factors can serve as a reliable prognostic factor. AJCC stage has the largest AUCs, indicating that the AJCC stage is the most effective single indicator. However, all prognostic factors got lower AUCs than nomograms, including OS and CSS nomograms (Figure 6). Generally, we confirmed that the discrimination of the two nomograms were superior to all the independent prognostic factors.
Figure 6.
The receiver operating characteristic curves of OS nomogram for one-year, three-years, and five-years in the training cohort (a–c) and validation cohort (d–f). (A color version of this figure is available in the online journal.)
Nomogram-based risk stratification system for breast PD patients
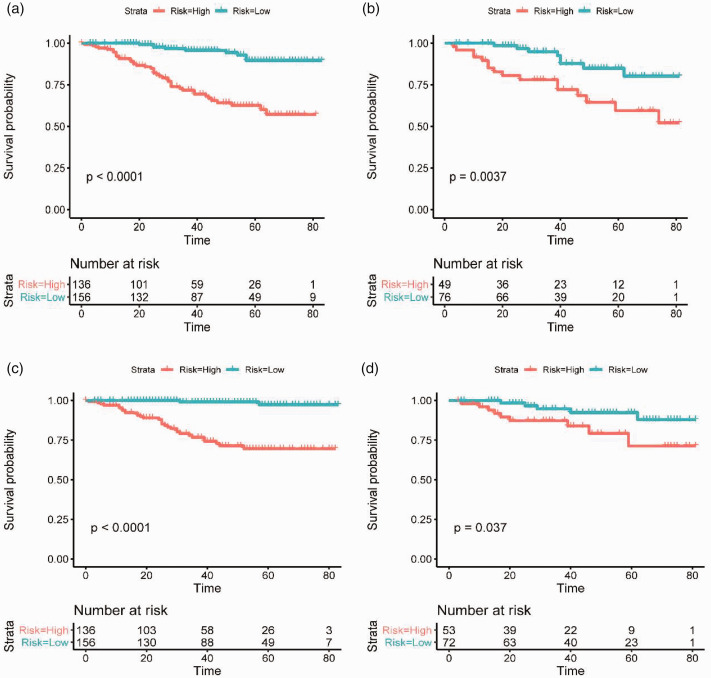
The total prognostic scores of all patients were calculated by the nomograms. Then, the Kaplan-Meier survival curves were further plotted for two groups based on the risk score and illustrated that nomograms had an excellent ability to distinguish (Figure 7). The cutoff values determined in the training cohort were used in the validation cohort. The prognosis of the two risk groups is significantly different. Generally, these results showed that our nomogram-based risk stratification system effectively stratifies patients into different prognostic groups.
Figure 7.
The Kaplan-Meier survival curves of OS nomograms for one-year, three-years, and five-years in the training cohort (a) and validation cohort (b). The Kaplan-Meier survival curves of CSS nomograms for one-year, three-years, and five-years in the training cohort (c) and validation cohort (d). (A color version of this figure is available in the online journal.)
Discussion
Due to the low incidence of breast PD, no studies explored the prognostic model of these patients, which means that the prognosis of breast PD patients cannot be estimated. This study constructed two prognostic nomograms incorporating corresponding independent prognostic clinical factors to better predict OS and CSS, respectively, for breast PD patients. Both nomograms performed well in discrimination and calibration. More importantly, the nomogram-based risk stratification systems were constructed to guide clinicians in decision making and disease monitoring.
In our research, age was confirmed as an independent prognostic factor for breast PD patients. As a clinical indicator not incorporated into the AJCC stage system, age is strongly associated with patients’ prognosis, including breast cancer. 20 The poor outcomes of elderly patients are related to the clinical course of the disease and related to comorbidities. In addition, due to poorly characterized functional status or weakened immune response, less active treatment was performed, resulting in a relatively poor prognosis.21,22 Marital status was confirmed as another independent prognostic factor for breast PD patients. Married breast PD patients showed a better prognosis. 23 Additionally, previous studies had also found that married cancer patients have a survival advantage and a reduced risk of death.19,24,25 This relationship may be attributed to the vital role of marital status in regulating the function of the endocrine system and immune system. 26
In terms of cancer characteristics, histological type, grade, and AJCC stage were related to the prognosis of breast PD patients. Zhao et al. 16 had reported that PD-IDC had the worst prognosis than other histological breast cancers. Conversely, unlike the previous study, our study showed that PD-IDC patients had the best prognosis, and PD patients had the worst prognosis. The reason for this result may be that almost all PD patients have underlying breast cancers and may be related to ductal carcinoma in situ or invasive cancer. 14 In the present study, compared with PD-IDC, PD-DCIS have a high-histological grade, advanced AJCC stage, and high HER2-positive ratios, which may lead to poor prognosis and reduce survival. Previous studies had identified that high grade is an indicator of a poor prognosis for breast cancer. 27 Higher grade showed the higher the degree of malignancy and the higher risk of metastasis and correlated with the poor prognosis. 28 In our study, the AJCC stage was confirmed as a strong predictor in breast PD patients, no matter OS or CSS. AJCC stage is a widely accepted prognostic factor for cancer patients. 29 Several studies had shown that the accuracy of the prognostic model can be significantly improved by integrating the AJCC stage and other clinical prognostic indicators.30,31 In this study, nomograms incorporated the AJCC stage and other prognostic clinicopathological parameters. We have observed that the AUCs of the nomograms were higher after integrating other indicators.
Regarding treatment factors, surgery and chemotherapy were independent prognostic factors for breast PD patients. Traditionally, mastectomy is the standard surgical treatment for breast PD patients.32,33 It is estimated that about two-thirds of PD patients have tumors confined to the central quadrant of the breast, and with the advancement of diagnostics, breast-conserving therapy has become a widely accepted treatment option. 34 Scholars had found that patients who underwent partial mastectomy had a better prognosis than patients who underwent a complete mastectomy, and the operation risk is lower.28,35,36 Most cases of breast PD can be classified as HER2 overexpression subtype.37,38 Therefore, considering this, chemotherapy and hormone therapy can be used as adjuvant therapy, 17 which can reduce cancer-related complications by killing or inhibiting cancer cells, thereby delaying disease recurrence and prolonging survival time. According to NCCN guidelines, chemotherapy can improve the prognosis of PD-IDC patients. However, chemotherapy is not recommended for PD-DCIS patients. 39 This may be one of the factors leading to a better prediction of chemotherapy. In general, our nomograms show that surgery and chemotherapy are beneficial to the survival of breast PD patients. Therefore, efforts to determine independent prognostic factors may help select high-risk patients and establish specific monitoring programs.
Inevitably, there are some limitations to our work. First, this was a retrospective study with a large sample, so potential selection bias was inevitable. Secondly, the SEER database lacked information, especially hormone therapy and HER2-targeted therapy, and could not fully cover the clinical situation. Thirdly, the construction and validation of the prognostic nomogram were carried out in a single institution, which may affect its clinical application to a certain extent. Therefore, it is necessary to calibrate the nomogram in the future further.
Conclusions
In summary, we used routine clinical data to construct and validate nomograms of PD patients at one year, three-, and five years. Compared with individual prognostic factors, the nomogram scoring systems had better distinguishing ability and clinical application value. This is very useful for promoting individualized therapy and management of breast PD patients.
Footnotes
AUTHORS’ CONTRIBUTIONS: TH and ZC contributed to the conception and design. TH drafted the article. MH and KL contributed with a critical revision of the article. The final article was carefully read and approved by all the authors.
Declaration OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Horizontal Research Project of Wenzhou Medical University (grant number KJHX2107).
ORCID iD: Tingting Hu https://orcid.org/0000-0003-4563-6486
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68:394–424 [DOI] [PubMed] [Google Scholar]
- 2.Sandoval-Leon AC, Drews-Elger K, Gomez-Fernandez CR, Yepes MM, Lippman ME. Paget's disease of the nipple. Breast Cancer Res Treat 2013; 141:1–12 [DOI] [PubMed] [Google Scholar]
- 3.Chaudary MA, Millis RR, Lane EB, Miller NA. Paget's disease of the nipple: a ten year review including clinical, pathological, and immunohistochemical findings. Breast Cancer Res Treat 1986; 8:139–46 [DOI] [PubMed] [Google Scholar]
- 4.Ascenso AC, Marques MS, Capitao-Mor M. Paget's disease of the nipple. Clinical and pathological review of 109 female patients. Dermatologica 1985; 170:170–9 [PubMed] [Google Scholar]
- 5.Nance FC, DeLoach DH, Welsh RA, Becker WF. Paget's disease of the breast. Ann Surg 1970; 171:864–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waldman RA, Finch J, Grant-Kels JM, Stevenson C, Whitaker-Worth D. Skin diseases of the breast and nipple: benign and malignant tumors. J Am Acad Dermatol 2019; 80:1467–81 [DOI] [PubMed] [Google Scholar]
- 7.Aguayo-Carreras P, Bonilla-Garcia L, Perez-Lopez I, Cuenca-Barrales C, Tercedor-Sanchez J. Paget's disease of the breast: a dangerous imitator of eczema. Sultan Qaboos Univ Med J 2017; 17:e487–e88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lohsiriwat V, Martella S, Rietjens M, Botteri E, Rotmensz N, Mastropasqua MG, Garusi C, De Lorenzi F, Manconi A, Sommario M, Barbieri B, Cassilha M, Minotti I, Petit JY. Paget's disease as a local recurrence after nipple-sparing mastectomy: clinical presentation, treatment, outcome, and risk factor analysis. Ann Surg Oncol 2012; 19:1850–5 [DOI] [PubMed] [Google Scholar]
- 9.Heyderman E. The histogenesis of mammary and extramammary Paget's disease. Histopathology 1989; 15:553–4 [DOI] [PubMed] [Google Scholar]
- 10.Sakorafas GH, Blanchard DK, Sarr MG, Farley DR. Paget's disease of the breast: a clinical perspective. Langenbecks Arch Surg 2001; 386:444–50 [DOI] [PubMed] [Google Scholar]
- 11.Fu W, Mittel VK, Young SC. Paget disease of the breast: analysis of 41 patients. Am J Clin Oncol 2001; 24:397–400 [DOI] [PubMed] [Google Scholar]
- 12.Durkan B, Bresee C, Bose S, Phillips EH, Dang CM. Paget's disease of the nipple with parenchymal ductal carcinoma in situ is associated with worse prognosis than Paget's disease alone. Am Surg 2013; 79:1009–12 [PubMed] [Google Scholar]
- 13.Ling H, Hu X, Xu X-L, Liu Z-B, Shao Z-M. Patients with nipple-areola Paget's disease and underlying invasive breast carcinoma have very poor survival: a matched cohort study. PLoS One 2013; 8:e61455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ortiz-Pagan S, Cunto-Amesty G, Narayan S, Crawford S, Derrick C, Larkin A, Khan A, Quinlan R, Layeequr Rahman R. Effect of Paget's disease on survival in breast cancer: an exploratory study. Arch Surg 2011; 146:1267–70 [DOI] [PubMed] [Google Scholar]
- 15.Fu W, Lobocki CA, Silberberg BK, Chelladurai M, Young SC. Molecular markers in Paget disease of the breast. J Surg Oncol 2001; 77:171–8 [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Sun H-F, Chen M-T, Gao S-P, Li L-D, Jiang H-L, Jin W. Clinicopathological characteristics and survival outcomes in Paget disease: a SEER population-based study. Cancer Med 2018; 7:2307–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S, Chen H, Yi Y, Jiang X, Lei H, Luo X, Chen Y, Liu S, Yuan D, Jia X, Li J. Comparative study of breast cancer with or without concomitant Paget disease: an analysis of the SEER database. Cancer Med 2019; 8:4043–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 2004; 10:7252–9 [DOI] [PubMed] [Google Scholar]
- 19.Eskander MF, Schapira EF, Bliss LA, Burish NM, Tadikonda A, Ng SC, Tseng JF. Keeping it in the family: the impact of marital status and next of kin on cancer treatment and survival. Am J Surg 2016; 212:691–9 [DOI] [PubMed] [Google Scholar]
- 20.Chen M-T, Sun H-F, Zhao Y, Fu W-Y, Yang L-P, Gao S-P, Li L-D, Jiang H-L, Jin W. Comparison of patterns and prognosis among distant metastatic breast cancer patients by age groups: a SEER population-based analysis. Sci Rep 2017; 7:9254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergman L, Dekker G, van Kerkhoff EH, Peterse HL, van Dongen JA, van Leeuwen FE. Influence of age and comorbidity on treatment choice and survival in elderly patients with breast cancer. Breast Cancer Res Treat 1991; 18:189–98 [DOI] [PubMed] [Google Scholar]
- 22.Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, Witkowski JM, Franceschi C. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front Immunol 2017; 8:1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst 2000; 92:994–1000 [DOI] [PubMed] [Google Scholar]
- 24.Goodwin JS, Hunt WC, Key CR, Samet JM. The effect of marital status on stage, treatment, and survival of cancer patients. Jama 1987; 258:3125–30 [PubMed] [Google Scholar]
- 25.Kravdal O. The impact of marital status on cancer survival. Soc Sci Med 2001; 52:357–68 [DOI] [PubMed] [Google Scholar]
- 26.Herberman RB, Ortaldo JR. Natural killer cells: their roles in defenses against disease. Science 1981; 214:24–30 [DOI] [PubMed] [Google Scholar]
- 27.Liu D, Wu J, Lin C, Andriani L, Ding S, Shen K, Zhu L. Breast subtypes and prognosis of breast cancer patients with initial bone metastasis: a population-Based study. Front Oncol 2020; 10:580112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sisti A, Huayllani MT, Restrepo DJ, Boczar D, Advani P, Lu X, Spaulding AC, Ball CT, McLaughlin SA, Forte AJ. Paget disease of the breast: a national retrospective analysis of the US population. Breast Dis 2020; 39:119–26 [DOI] [PubMed] [Google Scholar]
- 29.Giuliano AE, Edge SB, Hortobagyi GN. Eighth edition of the AJCC cancer staging manual: Breast cancer. Ann Surg Oncol 2018; 25:1783–5 [DOI] [PubMed] [Google Scholar]
- 30.Huang Y-Q, Liang C-H, He L, Tian J, Liang C-S, Chen X, Ma Z-L, Liu Z-Y. Development and validation of a radiomics nomogram for preoperative prediction of lymph node metastasis in colorectal cancer. Jco 2016; 34:2157–64 [DOI] [PubMed] [Google Scholar]
- 31.Kattan MW, Leung DHY, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. Jco 2002; 20:791–6 [DOI] [PubMed] [Google Scholar]
- 32.Ashikari R, Park K, Huvos AG, Urban JA. Paget's disease of the breast. Cancer 1970; 26:680–5 [DOI] [PubMed] [Google Scholar]
- 33. Classic articles in colonic and rectal surgery: Sir James Paget, 1814-1899, on disease of the mammary areola preceeding cancer of the mammary gland. Dis Colon Rectum 1980; 23:280–1 [PubMed] [Google Scholar]
- 34.Sheen-Chen SM, Chen HS, Chen WJ, Eng HL, Sheen CW, Chou FF. Paget disease of the breast – an easily overlooked disease? J Surg Oncol 2001; 76:261–5 [DOI] [PubMed] [Google Scholar]
- 35.Yao Y, Sun L, Meng Y, Zhuang Y, Zhao L, Yu Q, Si C. Breast-conserving surgery in patients with mammary Paget's disease. J Surg Res 2019; 241:178–87 [DOI] [PubMed] [Google Scholar]
- 36.Zurrida S, Veronesi U. Milestones in breast cancer treatment. Breast J 2015; 21:3–12 [DOI] [PubMed] [Google Scholar]
- 37.Arain SA, Arafah M, Said Raddaoui EM, Tulba A, Alkhawaja FH, Al Shedoukhy A. Immunohistochemistry of mammary Paget's disease. Cytokeratin 7, GATA3, and HER2 are sensitive markers. Saudi Med J 2020; 41:232–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park S, Suh Y-L. Useful immunohistochemical markers for distinguishing Paget cells from Toker cells. Pathology 2009; 41:640–4 [DOI] [PubMed] [Google Scholar]
- 39.Gradishar WJ, Anderson BO, Abraham J, Aft R, Agnese D, Allison KH, Blair SL, Burstein HJ, Dang C, Elias AD, Giordano SH, Goetz MP, Goldstein LJ, Isakoff SJ, Krishnamurthy J, Lyons J, Marcom PK, Matro J, Mayer IA, Moran MS, Mortimer J, O'Regan RM, Patel SA, Pierce LJ, Rugo HS, Sitapati A, Smith KL, Smith ML, Soliman H, Stringer-Reasor EM, Telli ML, Ward JH, Young JS, Burns JL, Kumar R. Breast cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2020; 18:452–78 [DOI] [PubMed] [Google Scholar]