Abstract
Background
Carbamazepine and phenytoin are potent inducers of enzymes that metabolize oral anticoagulants.
Objectives
To determine the clinical impact of drug‐drug interactions between these anticonvulsants and oral anticoagulants, and whether they affect the treatment with direct oral anticoagulants (DOACs) or vitamin K antagonists (VKAs).
Material and methods
Data on patients cotreated with carbamazepine or phenytoin and an oral anticoagulant were retrospectively retrieved from medical records from 2011 to 2020. Outcomes were time in therapeutic range (TTR), DOAC levels, thromboembolic events, major bleeding, and all‐cause mortality.
Results
Among 85 patients (37% female, median age 68 years) treated with carbamazepine (n = 43 [51%]) or phenytoin (n = 42 [49%]), 53 (62%) were initially treated with VKAs and 32 (38%) with DOACs. TTR in VKA patients was 63%, which improved in year 2. Four of seven trough and five of 12 peak DOAC plasma levels were lower than expected. The incidence rate (95% confidence interval) per 100 person‐years for thromboembolism was 3.6 (3.1‐4.2) for VKA patients and 4.4 (3.5‐5.6) for DOAC patients; for major bleeding 1.8 (1.5‐2.1) and 1.5 (1.2‐1.9), and for all‐cause mortality 3.6 (3.1‐4.2) and 1.5 (1.2‐1.9), respectively. Incidence rates between VKAs and DOACs and between carbamazepine and phenytoin were similar.
Conclusion
There was a high incidence of thromboembolism in patients cotreated with anticoagulants and carbamazepine or phenytoin. The incidence rates of thrombotic and bleeding events were similar between VKA and DOAC patients. DOAC levels were lower than expected in 47% of cases tested, without correlation with clinical outcomes.
Keywords: anticoagulants, carbamazepine, hemorrhage, phenytoin, thrombosis
Essentials.
Carbamazepine and phenytoin may interact with oral blood thinners.
We analyzed data from 85 patients on carbamazepine/phenytoin in combination with blood thinners.
The occurrence of thrombotic events, but not bleeding, was higher than expected.
There was no difference between vitamin K antagonists and direct oral blood thinners.
1. BACKGROUND
Carbamazepine and phenytoin are potent inducers of enzymes that metabolize drugs, including the microsomal enzymes, cytochrome P (CYP) 1A2, 2C9, and 3A4, as well as the efflux transporter P‐glycoprotein (P‐gp). The metabolism of warfarin is mainly through CYP 2C9, whereas the direct oral anticoagulants (DOACs) apixaban and rivaroxaban are partially dependent on CYP 3A4 for their elimination. Edoxaban undergoes very limited CYP 3A4 metabolism (<10% of oral dose) to active metabolites with anti‐Xa activity, unlike apixaban and rivaroxaban, which do not have active circulating metabolites. 1 All DOACs are substrates for P‐gp. Thus, cotreatment with carbamazepine or phenytoin will speed up the elimination of all oral anticoagulants.
Because warfarin is monitored with prothrombin time and the dose is adjusted to keep the international normalized ratio (INR) in the therapeutic range, typically INR 2.0–3.0, the antithrombotic effect should be maintained when patients are treated with carbamazepine or phenytoin, although it may require some time after initiation or dose change, before the new equilibrium has been achieved. A nationwide Swedish study demonstrated that comedication with carbamazepine and warfarin required on average an increase of the dose of warfarin of 49% to maintain therapeutic INR. 2
DOACs are given at a fixed dose, whereas a reduced dose is used in the presence of renal dysfunction, advanced age, low body weight, and/or concomitant CYP 3A4 inhibitors. For CYP 3A4/P‐gp inducers, the recommendation is to avoid use of DOACs, and vitamin K antagonists often become the default choice. Case reports highlight the possibility of low DOAC levels in patients treated with rivaroxaban or apixaban who are taking carbamazepine but, when switched to edoxaban, a DOAC with minimal hepatic metabolism, adequate edoxaban levels have been observed. 3 However, the clinical significance of the interaction remains unclear. 3 , 4 , 5 In an analysis of the US Food and Drug Administration (FDA) adverse event reporting system, Perlman et al. identified 1423 ischemic or thrombotic reports among 9693 reported events between 2013 and 2018 in patients that received direct factor Xa inhibitors (mainly rivaroxaban or apixaban) with antiepileptic drugs (AEDs) (including carbamazepine, phenytoin, phenobarbital, valproic acid, lamotrigine, and levetiracetam). Compared with other AEDs, patients cotreated with the first three AEDs, which are CYP 3A4 enzyme inducers, had about a 1.8‐fold increased reporting odds ratio of thrombosis or ischemia. 6
It is unclear how the difficulties to achieve therapeutic INR with warfarin or how the potential for low DOAC levels might affect clinical outcomes in patients treated with previously mentioned comedications. Moreover, most of the previously mentioned studies analyzed data on different types of anticonvulsants, so that estimates specifically for carbamazepine or phenytoin are still lacking.
In a retrospective cohort of patients receiving either warfarin or a DOAC, and who are cotreated with an enzyme‐inducing AED, we (1) evaluate how carbamazepine or phenytoin affect the quality of the anticoagulation with warfarin, measured as proportion of time in therapeutic range (TTR); (2) describe the prevalence of low DOAC levels; and (3) provide best estimates of bleeding and ischemic event rates for warfarin and DOACs, respectively.
2. METHODS
This was a retrospective, single‐center, observational study conducted at the Thrombosis Service, Hamilton General Hospital, Ontario, Canada. The center monitors approximately 2000 adult patients on warfarin and follows a variable number of patients on DOACs with complicating factors (e.g., concomitant treatment with potentially interacting drugs, morbid obesity, high bleeding risk, decreasing renal function). The most common indications are stroke prophylaxis in atrial fibrillation, followed by mechanical heart valves (only vitamin K antagonists [VKAs]) and venous thromboembolism. All patients older than 18 years on active treatment with an anticoagulant (VKA or DOAC) and comedicated with carbamazepine or phenytoin were included. The concomitant treatment had to start between January 1, 2012, and December 31, 2020, with the start date of the combination defined as index date. The exclusion criteria were lack of clinical information or absence of any follow up.
The clinical records were manually examined. Data on demographics (age, sex), clinical information (medical history, indication for the anticoagulant and the anticonvulsant(s), type, and posology of anticonvulsant therapy) were retrieved.
Proportion of TTR per year of treatment was calculated by linear interpolation between successive INR results for up to 3 years, if available, starting from the index date, and the method has previously been described in detail by us. 7 Furthermore, the mean VKA dose was obtained calculating the average of all available weekly doses for each year.
Because of the retrospective study design, DOAC plasma levels were not systematically requested by the physicians. Levels were mainly performed because the patient was on concomitant anticonvulsant therapy, occasionally because the physician was interested in intraindividual variability over time, but never following a bleeding or thrombotic event or for worsening clinical condition of the patient. Any DOAC plasma levels collected as part of clinical care were retrieved. As previously published, 8 DOAC plasma levels were measured with a specifically calibrated anti‐factor Xa assay (Liquid anti Xa, Diagnostica Stago, Asnieres‐sur‐Seine, France) for apixaban, rivaroxaban, and edoxaban and a specific dilute thrombin time (Hemoclot Thrombin Inhibitors, Hyphen BioMed, Neuville sur Oise, France) on a STA‐R Evolution coagulometer. Lower limits of detection are 20 ng/ml for apixaban, edoxaban, and dabigatran, and 25 ng/ml for rivaroxaban. There is no upper limit of detection. Blood samples for peak level assessment were taken 2–3 h after ingestion of the drug, whereas blood samples for trough levels were taken within 1 h before the next DOAC dose. The DOAC levels were then classified as “low,” “normal,” or “high” after comparing them with the expected ranges as proposed by the current European Heart Rhythm Association Practical Guide. 9 Patients were seen routinely once a year in the outpatient clinic and asked about thromboembolic or bleeding complications. In addition, the electronic medical records were reviewed for such outcomes. Thromboembolism included objectively verified acute myocardial infarction, stroke, deep vein thrombosis, pulmonary embolism, and acute peripheral ischemia. Major bleeding was defined according to the International Society on Thrombosis and Haemostasis. 10 Death of any cause was also recorded.
Clinical outcomes were captured from the index date and until the last informative visit recorded, or until one of the following events: discontinuation of anticoagulant or anticonvulsant therapy, occurrence of one of the clinical outcomes or up to December 31, 2020. The follow‐up visit was considered informative if both anticoagulant and anticonvulsant therapy were explicitly reported in the document.
The study was approved as a retrospective chart review without need for informed consent by the Hamilton Integrated Research Ethics Board.
3. STATISTICAL ANALYSIS
Continuous variables are presented as median and interquartile range (IQR) and categorical as number and percentage. Differences between groups were tested with Fisher exact test and Mann‐Whitney test as appropriate.
Variation in VKA dose and median TTR across the 3 years were tested with Wilcoxon test for paired samples for the carbamazepine‐only and phenytoin‐only subgroups.
The possible association between TTR, VKA dose, and anticonvulsant drugs was verified through multivariable linear regression models taking the TTR as dependent variable and the interaction between VKA dose (log transformed) and carbamazepine or phenytoin dose (as continuous variable) as independent factor. Each model was adjusted for sex and age.
The clinical outcomes for the overall cohort and for VKA and DOAC subgroups were reported as number and percentage and as incidence rate expressed as new cases per 100 persons‐years and relative 95% confidence interval (CI). For patients that switched anticoagulant therapy during the study, the person‐year time in the subgroup analysis was calculated considering the interval between drug initiation and discontinuation as the exposure period. Incidence rate ratios between VKA and DOAC and relative 95% CIs were also calculated.
Kaplan‐Meier curves for major bleeding, thromboembolic events, and all‐cause mortality were built accordingly with the anticoagulant therapy at baseline. Patients were censored when they switched anticoagulant therapy, at the event time or at the end of the observation without events. Groups were compared with log‐rank test.
A two‐sided p value less than 0.05 was considered to indicate statistical significance. All analyses were performed using R software, version 4.0.4, and Rstudio version 1.1.423 (2009–2018 RStudio, Inc.).
4. RESULTS
Among 116 patients retrieved, 28 were excluded because of a lack of clinical information and three because of absence of any follow‐up. Eventually, a total of 85 patients (female sex, n = 31; 37%) were included in the study. Of those, 43 (51%) patients were on carbamazepine and 42 (49%) were on phenytoin. Two additional patients (2%) on carbamazepine also received phenytoin for a short time during the observation period. Baseline characteristics at index date are shown in Table 1. The main indication for anticoagulation was atrial fibrillation with or without additional indications (54 patients, 65%), whereas for anticonvulsant treatment it was epilepsy (76 patients, 89%). Among the latter, 58 (76%) had a primary diagnosis of seizure, whereas 18 (24%) started the treatment as seizure prophylaxis after a cerebrovascular accident or a neurosurgical procedure. The median daily dose was 400 mg (range, 100–1000 mg) for carbamazepine and 300 mg (range, 200–750 mg) for phenytoin.
TABLE 1.
Baseline characteristics
| Overall | Carbamazepine | Phenytoin | VKA | DOACs | |
|---|---|---|---|---|---|
| N | 85 | 43 | 42 | 53 | 32 |
| Age, median (IQR) | 68 (59, 79) | 66 (58, 80) | 71 (62, 79) | 66 (57, 74) | 75 (66, 84) |
| Female sex, n (%) | 31 (37) | 18 (42) | 13 (31) | 33 (62.3) | 21 (65.6) |
| New anticoagulant users, n (%) | 49 (58) | 27 (63) | 22 (52) | 26 (49.1) | 23 (71.9) |
| Anticoagulant indication, n (%) | |||||
| Atrial fibrillation | 49 (59) | 27 (63) | 22 (52) | 28 (52.8) | 21 (65.6) |
| Atrial fibrillation + mechanical heart valve | 3 (4) | 3 (7) | ‐ | 3 (5.7) | ‐ |
| Atrial fibrillation + venous thromboembolism | 2 (2) | 1 (2) | 1 (2) | 1 (1.9) | 1 (3.1) |
| Arterial thromboembolism | 1 (1) | 1 (2) | ‐ | 1 (1.9) | ‐ |
| Mechanical heart valve | 4 (5) | ‐ | 4 (10) | 4 (7.5) | ‐ |
| Patent foramen ovale + stroke | 1 (1) | 1 (2) | ‐ | 1 (1.9) | ‐ |
| Venous thromboembolism | 23 (27) | 10 (23) | 13 (31) | 13 (24.5) | 10 (31.2) |
| Other a | 2 (2.4) | ‐ | 2 (5.0) | 2 (3.8) | ‐ |
| Carbamazepine/phenytoin indication, n (%) | |||||
| Epilepsy | 76 (89) | 34 (79) | 42 (100) | 48 (90.6) | 28 (87.5) |
| Pain management | 7 (8) | 7 (16) | ‐ | 5 (9.4) | 2 (6.2) |
| Psychiatric treatment | 2 (2) | 2 (5) | ‐ | ‐ | 2 (6.2) |
| Anticoagulant therapy | |||||
| Vitamin K antagonist, n (%) b | 56 (66) | 32 (74) | 24 (57) | ||
| Direct oral anticoagulant, n (%) b | 39 (46) | 19 (44) | 20 (48) | ||
| Dabigatran | 6 (15) | 4 (20) | 2 (10) | 1 (2) | 5 (16) |
| Apixaban | 18 (46) | 10 (53) | 8 (40) | 3 (6) | 15 (47) |
| Edoxaban | 3 (8) | 1 (5) | 2 (10) | 1 (2) | 2 (6) |
| Rivaroxaban | 12 (31) | 4 (21) | 8 (40) | 2 (4) | 10 (31) |
| Switched anticoagulant therapy, n (%) | |||||
| DOAC → VKA | 3 (4) | 2 (5) | 1 (2) | 3 (9.4) | |
| VKA → DOAC | 7 (8) | 6 (14) | 1 (2) | 7 (13.2) | |
| Concomitant antiplatelet therapy, n (%) | |||||
| Single | 21 (25) | 17 (40) | 4 (10) | 19 (35.8) | 2 (6.2) |
| Dual | 4 (5) | 2 (5) | 2 (5) | 3 (5.7) | 1 (3.1) |
| Co‐morbidities | |||||
| Hypertension, n (%) | 63 (74) | 35 (81) | 28 (67) | 41 (77.4) | 22 (68.8) |
| Diabetes, n (%) | 20 (24) | 10 (23) | 10 (24) | 15 (28.3) | 5 (15.6) |
| Active cancer, n (%) | 14 (17) | 7 (16) | 7 (17) | 8 (15.1) | 6 (18.8) |
| History of cancer, n (%) | 10 (12) | 5 (12) | 5 (12) | 6 (11.3) | 4 (12.5) |
| Prior thromboembolism, n (%) | 52 (61) | 24 (56) | 28 (67) | 35 (66.0) | 17 (53.1) |
| Prior major bleeding, n (%) | 12 (14) | 3 (7) | 9 (21) | 8 (15.1) | 4 (12.5) |
| CHA2DS2‐VASc, median (IQR) b | 5 (4, 6) | 4 (3, 6) | 5 (4, 5) | 5 (3, 6) | 4 (4, 5) |
Abbreviations: CHA2DS2‐VASc, congestive heart failure, hypertension, age >75 years, diabetes mellitus, stroke, vascular disease, age 65–years, sex category; DOAC, direct oral anticoagulant; IQR, interquartile range; VKA, vitamin K antagonist.
Tissue aortic valve replacement and ascending aorta replacement with tube graft; acute myocardial infarction with placement of drug‐eluting stent.
Includes patients who switched anticoagulant therapy; CHA2DS2‐VASc was calculated only for patients with atrial fibrillation.
Fifty‐three (62%) patients were initially treated with a VKA and 32 (38%) with a DOAC. During the observation period, seven patients were switched from a VKA to a DOAC, whereas three switched from a DOAC to a VKA. The only VKA used was warfarin. Forty‐nine patients (58%) started the anticoagulation therapy (VKA or DOAC) at the index date, whereas the remainder started the anticonvulsant drug at the index date.
4.1. VKA therapy and carbamazepine/phenytoin interaction
Annual TTR was available for 54/56 (96%), 40/46 (87%), and 34/34 (100%) patients for the first, second, and third year, respectively. The corresponding median TTR was 63% (IQR 43%, 75%), 73% (IQR 62%, 79%), and 67% (IQR 51%, 77%), and the corresponding median weekly VKA dose was 47 mg (IQR 35 mg, 54 mg), 42 mg (IQR 31 mg, 58 mg), and 42 mg (IQR 28 mg, 52 mg) (Figure 1, Table S1). Patients treated with carbamazepine experienced a progressive lowering of the VKA dose across the years (Figure 1) that seemed relevant (p = 0.032) between the first and the second year of treatment. On the other hand, the median values of TTR tended to increase, especially between the first and the second year (p = 0.022). Regarding the patients treated with phenytoin, the results were similar across the 3 years of observation.
FIGURE 1.
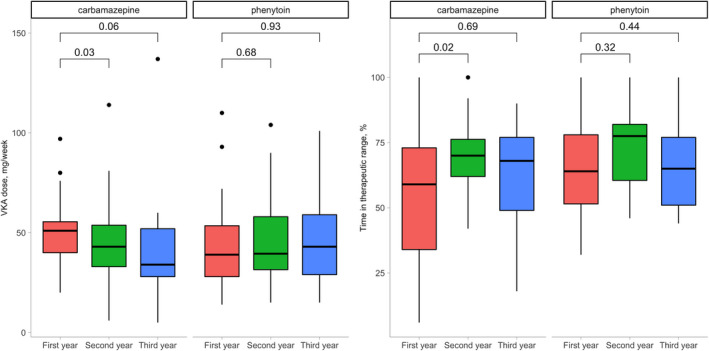
Vitamin K antagonist doses and time in therapeutic range across 3 years of observation
None of the multivariable regression models showed an association between VKA dose, anticonvulsant drug dose, and their interaction with TTR except for carbamazepine at year 2, where an inverse interaction between VKA dose and carbamazepine dose was found (β for interaction = −0.07; p = 0.01; adjusted R 2 = 0.25). This means that with increasing doses of carbamazepine the effect of the dose of VKA on the TTR will tend to decrease and vice versa.
4.2. DOAC patients and carbamazepine/phenytoin interaction
Plasma DOAC levels were available for 19/39 (49%) patients. When compared with the expected peak and trough levels, 9 nine (47%) patients appeared to have a “low,” nine (47%) had a “normal,” and one (6%) had a “high” DOAC level (Table 2). Two of the low DOAC levels were undetectable (<20 ng/ml). Four of eight (50%) DOAC patients comedicated with phenytoin showed low plasma DOAC levels, whereas the corresponding figure for carbamazepine was 5/11 (45%).
TABLE 2.
DOAC levels according to increasing maintenance doses of the anticonvulsant drug
| Drug type | Dose (mg) | DOAC type | DOAC dose (mg) a | Timing | DOAC level (ng/ml) | Classification |
|---|---|---|---|---|---|---|
| Carbamazepine | 200 | Dabigatran | 75 | Trough | 24 | Low |
| Carbamazepine | 200 | Apixaban | 5 | Peak | 179 | Normal |
| Carbamazepine | 300 | Apixaban | 5 | Peak | 466 | High |
| Carbamazepine | 400 | Apixaban | 5 | Peak | 67 | Low |
| Carbamazepine | 400 | Apixaban | 5 | Peak | 108 | Normal |
| Carbamazepine | 400 | Rivaroxaban | 20 once a day | Peak | 161 | Low |
| Carbamazepine | 400 | Rivaroxaban | 20 once a day | Peak | 236 | Normal |
| Carbamazepine | 400 | Apixaban | 2.5 | Trough | Undetectable | Low |
| Carbamazepine | 400 | Apixaban | 5 | Peak | 51 | Low |
| Carbamazepine | 800 | Apixaban | 5 | Peak | 102 | Normal |
| Carbamazepine | 1000 | Apixaban | 5 | Peak | 223 | Normal |
| Phenytoin | 200 | Rivaroxaban | 20 once a day | Trough | 39 | Normal |
| Phenytoin | 200 | Edoxaban | 30 once a day | Trough | 20 | Normal |
| Phenytoin | 200 | Dabigatran | 110 | Trough | Undetectable | Low |
| Phenytoin | 300 | Apixaban | 5 | Peak | 23 | Low |
| Phenytoin | 400 | Apixaban | 5 | Trough | 32 | Low |
| Phenytoin | 400 | Apixaban | 5 | Peak | 32 | Low |
| Phenytoin | 400 | Rivaroxaban | 15 | Trough | 77 | Normal |
| Phenytoin | 750 | Apixaban | 5 | Peak | 171 | Normal |
Abbreviation: DOAC, direct oral anticoagulant.
Doses are twice a day unless otherwise specified.
4.3. Clinical outcomes
After a median observation time of 28 months (IQR 8, 48), nine patients (11%) suffered thromboembolic events for an incidence rate of 3.8 per 100 persons‐years (95% CI, 3.5–4.5) (Tables 2, 3 and 4). The main thrombotic event was stroke (n = 4), followed by acute myocardial infarction (n = 2), deep vein thrombosis (n = 2), and mechanical heart valve thrombus (n = 1). One stroke was fatal and occurred in a patient on a VKA.
TABLE 3.
Thromboembolic and bleeding events according to anticoagulant treatment and antiseizure drugs
| Sex a | Age, y | OAC a | Indication a | DOAC level classification/median TTR (%) a | Antiepileptic drug | Dose (mg) | Indication | Outcome |
|---|---|---|---|---|---|---|---|---|
| F | 71 | Apixaban 5 mg BID | AF | ‐ | Carbamazepine | 600 | Epilepsy | Stroke |
| M | 71 | Rivaroxaban 20 mg | VTE | ‐ | Phenytoin | 300 | Epilepsy | Deep vein thrombosis |
| F | 63 | Rivaroxaban 15 mg BID | VTE | Normal | Phenytoin | 400 | Epilepsy | Deep vein thrombosis |
| F | 89 | Apixaban 5 mg BID | AF | Normal | Phenytoin | 750 | Epilepsy | Intracranial hemorrhage |
| F | 89 | Warfarin | AF | 56 | Carbamazepine | 200 | Pain management | Fatal stroke |
| M | 61 | Warfarin | MHV | 49 | Phenytoin | 200 | Epilepsy | Aortic mechanical valve thrombosis |
| F | 73 | Warfarin | AF | 59 | Phenytoin | 300 | Epilepsy | Acute myocardial infarction |
| M | 77 | Warfarin | VTE | 78 | Phenytoin | 400 | Epilepsy | Stroke |
| M | 76 | Warfarin | AF | 90 | Phenytoin | 400 | Epilepsy | Stroke |
| M | 69 | Warfarin | AF | 94 | Phenytoin | 400 | Epilepsy | Acute myocardial infarction |
| M | 84 | Warfarin | AF | 75 | Carbamazepine | 400 | Epilepsy | Fatal subdural hematoma |
| M | 58 | Warfarin | AF | 67 | Carbamazepine | 400 | Epilepsy | Rectal bleeding |
| F | 72 | Warfarin | AF | 70 | Phenytoin | 200 | Epilepsy | Intracranial hemorrhage |
Abbreviations: AF, atrial fibrillation; BID, twice per day; DOAC, direct oral anticoagulant; F, female; M, male; MHV, mechanical heart valve; OAC, oral anticoagulant; TTR, time in therapeutic range; VTE, venous thromboembolism.
The median TTR corresponds to the median of all the annual TTRs available for each patient.
TABLE 4.
Clinical outcomes
|
All patients N = 85 n (%) |
Cases per 100 p‐y (95% CI) |
VKA N = 56 a n (%) |
Cases per 100 p‐y (95% CI) |
DOAC N = 39 a n (%) |
Cases per 100 p‐y (95% CI) |
Incidence rate ratio VKA/DOAC (95% CI) |
|
|---|---|---|---|---|---|---|---|
| Thromboembolism | 9 (11) | 3.8 (3.5–4.5) | 6 (11) | 3.6 (3.1–4.2) | 3 (8) | 4.4 (3.5–5.6) | 0.8 (0.2–3.3) |
| Major bleeding | 4 (5) | 1.7 (1.9–2.5) | 3 (5) | 1.8 (1.5–2.1) | 1 (3) | 1.5 (1.2–1.9) | 1.2 (0.1–11.5) |
| All‐cause death | 7 (8) | 3.0 (2.6–3.4) | 6 (11) | 3.6 (3.1–4.2) | 1 (3) | 1.5 (1.2–1.9) | 2.4 (0.3–19.9) |
Abbreviations: CI, confidence interval; DOAC, direct oral anticoagulant; p‐y, person‐years; VKA, vitamin K antagonist.
These groups include patients who switched the anticoagulant.
There were four (5%) patients with major bleeding (one of which was fatal) and seven (8%) died, which corresponded to incidence rates of 1.7 per 100 person‐years (95% CI, 1.9–2.5) and 3.0 per 100 person‐years (95% CI, 2.6–3.4), respectively (Tables 2, 3 and 4).
There were no differences regarding incidence of the clinical outcomes between carbamazepine and phenytoin. Overall, one bleeding event, three thrombotic events, and three deaths occurred in patients on concomitant antiplatelet treatment. Moreover, among 11 (13%) patients who were taking potentially interacting drugs such as amiodarone (n = 2), phenobarbital (n = 3), or topiramate (n = 8), none experienced clinical outcomes.
Taking separately, the VKA patients and DOAC patients contributed to the study with 166 and 68 person‐years, respectively, and the incidence rate for thromboembolism was 3.6 per 100 person‐years (95% CI, 3.1–4.2) and 4.4 per 100 person‐years (95% CI, 3.5–5.6), respectively (Table 4). The VKA patients that suffered thrombotic events had a median TTR (i.e., the median of all available annual TTRs) of 49%, 56%, 59%, 78%, 90%, and 94%. Among the three DOAC patients who experienced a thrombotic event, the DOAC plasma level was available only for one and it was “normal.” Thus, none of the nine patients with lower‐than‐expected DOAC levels suffered any thromboembolic event. One of the major bleeds was fatal, a subdural hematoma in a patient on VKA and carbamazepine 400 mg (Table 3).
Except for the two deaths mentioned, the remaining deaths were not related to either thrombotic or bleeding events.
Eventually, there were no statistically significant differences between incidence rates of any of the clinical outcomes for treatment with VKA vs. DOAC, as attested by the wide incidence rate ratio's CIs and the Kaplan‐Meier curves (Table 2; Figure 2).
FIGURE 2.
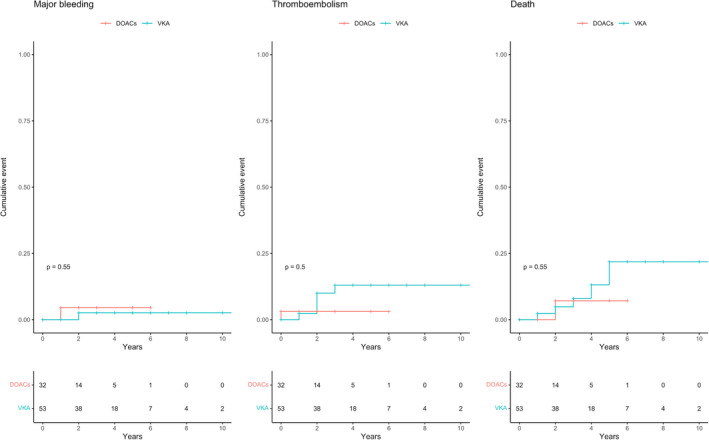
Kaplan‐Meier curves for major bleeding events, thromboembolic events, and all‐cause mortality according to the anticoagulant treatment at baseline. Patients were censored at the time of the event, at the end of observation in absence of events, or at change of anticoagulant
5. DISCUSSION
In this retrospective cohort of patients taking carbamazepine or phenytoin with warfarin or DOACs, we found that (1) the TTR with warfarin was acceptable, with potential improvement with increasing dose and time on warfarin; (2) DOAC levels were lower than the expected range in 47% of patients tested, and incidence rate of thrombotic events was high, irrespective of the type of anticoagulant; and (3) the overall incidence rate of thrombotic events (3.8 per 100 persons‐years), major bleeding events (1.7 per 100 person‐years), and all‐cause mortality (3.0 per 100 person‐years) was comparable between patients on VKAs and DOACs.
The pivotal trials that evaluated VKAs versus DOACs in atrial fibrillation reported a median TTR for the VKA patients ranging from 58% to 68.4%. 11 , 12 , 13 , 14 The median TTR in our carbamazepine/phenytoin‐treated cohort was aligned with these results (Table S1). The mean TTR during the second and third year of cotreatment, 73% and 67%, respectively, is similar to our mean center TTR, which consistently is at 72%. It has previously been reported that patients on carbamazepine and VKAs require higher doses of the latter to achieve optimal treatment. 2 Indirectly, the inverse interaction we found between carbamazepine doses and VKA doses would confirm this finding. The effect of phenytoin treatment was more complex. In fact, although an interaction between VKA dose and phenytoin dose was not found, the TTR seemed to improve in patients taking higher doses of phenytoin (Figure S1).
DOACs do not require routine coagulation monitoring, and there is still no standardization or consensus regarding therapeutic plasma DOAC level ranges. 9 , 15 , 16 , 17 We compared the plasma DOAC levels with the expected range, as published in the recent practical guide update by European Heart Rhythm Association 9 but, because of the small sample size, we were not able to perform any inference between DOAC plasma levels and clinical outcomes or carbamazepine/phenytoin dose. Sennesael et al. found in a retrospective study that, among seven patients cotreated with carbamazepine/phenytoin and DOACs, two had DOAC plasma levels below the expected range, whereas five showed normal levels. 18 These data highlight that the measurement of plasma DOAC levels in patients treated with carbamazepine/phenytoin show a great variability. Considering the sensitivity of this laboratory analysis both to the temporal distance from drug administration and to factors related to the subject (e.g., renal clearance, absorption, missed doses), 16 repeated measurements of the concentration of DOACs are probably necessary to attest the real DOAC plasma levels over time. Whether DOAC dose adjustments based on DOAC plasma level could result in improved therapeutic efficacy remains unclear and needs further investigation.
A recent retrospective study found a higher risk of stroke or systemic embolism among patients with atrial fibrillation cotreated with carbamazepine (n = 89), phenytoin (n = 71), and DOACs (rivaroxaban, apixaban, and dabigatran) when compared with the rest of the study population on DOACs (n = 22 801). 19 In this study, the authors used logistic regression models adjusted for the propensity of receiving carbamazepine or phenytoin but not for patient clinical characteristics. This reduces the power of the results because patient clinical characteristics also impact the drug‐drug interaction. 15 , 16 Moreover, a prospective study involving 91 patients cotreated with various anticonvulsants (nine patients were on carbamazepine) and DOACs reported an incidence rate of 5.7 per 100 patient‐years for thromboembolic events and of 1.9 per 100 patient‐years for major bleeding. 20 Finally, another study linked phenytoin with higher bleeding risk when coadministered with DOACs, 21 suggesting the presence of unmeasured confounders in observational studies, which lowers the confidence in these findings increasing doubts about the real risks associated with such comedication.
The incidence rate of thromboembolic events in the phase 3 trials in atrial fibrillation for VKA patients ranged between 1.5 and 2.4 per 100 patient‐years (pooled mean, 1.8), whereas for patients on standard dose of DOACs, it was between 1.1 and 2.1 per 100 patient‐years (pooled mean, 1.4). 11 , 12 , 13 , 14 The same figure for major bleeding ranged between 3.1 and 3.4 per 100 patient‐years (pooled mean, 3.3) for VKA patients and 2.1–3.6 per 100 patient‐years (pooled mean, 2.9) for DOAC patients, respectively (Table S2). Thus, whereas we found similar incidence rates regarding major bleeding, our rates of thromboembolic events of 3.8 per 100 persons‐years (95% CI, 3.5–4.5) or, specifically 3.6 per 100 person‐years (95% CI, 3.1–4.2) for VKA and 4.4 per 100 person‐years (95% CI, 3.5–5.6) for DOACs, are higher. For completeness, it is necessary to underline that our study also considered patients with mechanical heart valve and patients treated for venous thromboembolism. Additionally, the high prevalence of prior thromboembolism and prior stroke (frequently the cause of the consequent antiseizure treatment) as well as of cancer could suggest that this is a cohort of patients with a higher thromboembolic risk despite therapeutic anticoagulation (Table 4).
These original data add support to the possible clinical relevance of the drug‐drug interaction between carbamazepine/phenytoin and oral anticoagulants. Our findings of a high incidence of thromboembolic events in patients taking the studied AEDs are in line with those from the analysis of the FDA Adverse Event Reporting System, 6 but treatment with dose adjusted VKA was not associated with lower thromboembolic risk. Perhaps the best solution, if allowed by the indication, is to switch to the newer nonenzyme‐inducing antiseizure medication such as levetiracetam, lamotrigine, or valproic acid. The FDA study renders support for such a change, but it suffers from a risk of selection bias and lack of detailed patient data. 6
Our study has several limitations that need to be discussed. First, the retrospective design does not allow the exclusion of all possible confounding factors. Second, information on creatinine clearance was not available for all patients but, on the other hand, we have not done any analysis where clearance of creatinine may have played a role. Third, the relatively small study population results in imprecision of our risk estimates, and the statistical power is not such that any definitive conclusions can be drawn, but at the same time it is sufficient to suggest a more critical approach to the problem. Fourth, we only compared our clinical event rates with those of the pivotal trials in atrial fibrillation but not with a control group from our center. With the small number of clinical events and imprecision in risk estimates in the patients comedicated with carbamazepine or phenytoin, a comparison with a matched control group would still be unreliable. Fifth, the management of VKA in community practice is generally of lower quality than at specialized anticoagulation clinics, 22 , 23 and therefore our single‐center data on TTR and clinical outcomes with VKA are not generalizable to all settings of care. On the other hand, this is the first study that provides clinical event estimates, based on follow‐up during a median of 28 months in a cohort of carbamazepine/phenytoin‐ and VKA/DOAC‐treated patients and concomitant data on the quality of anticoagulation with TTR for VKA patients and drug levels for DOAC patients.
In conclusion, starting from the well‐known pharmacological interaction between carbamazepine/phenytoin and oral anticoagulants (both VKAs and DOACs), we observed a higher‐than‐expected incidence of thrombotic events when the previously mentioned drugs were coadministered. Furthermore, the incidence rates of thrombotic events and bleeding events were similar between VKA and DOAC patients. Half of the DOAC plasma levels tested were lower than expected. For patients on VKAs and for patients on DOACs, the TTR or the plasma levels, respectively, did not appear to predict thromboembolic events. These results should ideally be confirmed in a sufficiently large, prospective study with a control population on the newer AEDs.
CONFLICT OF INTEREST
Drs. Candeloro and Chan have no conflict of interest to declare. Dr. Schulman has received research funding from Boehringer Ingelheim and Octapharma and honoraria from Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi‐Sankyo, Pfizer, and Sanofi. Dr. Bhagirath has received honoraria from Bayer and Pfizer, and an unrestricted educational grant from Pfizer. Dr. Eikelboom has received consulting/honoraria from Astra‐Zeneca, Bayer, Boehringer‐Ingelheim, Bristol‐Myer‐Squibb, Daiichi‐Sankyo, Eli‐Lilly, Glaxo‐Smith‐Kline, Pfizer, Janssen, and Sanofi‐Aventis and Servier grants and/or in‐kind support from Astra‐Zeneca, Bayer, Boehringer‐Ingelheim, Bristol‐Myer‐Squibb, Glaxo‐Smith‐Kline, Pfizer, Janssen, and Sanofi‐Aventis. Dr. Douketis has received consulting fees and/or honoraria from Bayer, BMS, Janssen, Leo Pharma, Pfizer, and Servier.
AUTHOR CONTRIBUTIONS
Study conception and design: Sam Schulman; data acquisition: Matteo Candeloro, Sam Schulman; statistical analysis: Matteo Candeloro; interpretation of the data: all authors; drafting of the manuscript: Matteo Candeloro, Sam Schulman; critical revision of the manuscript for important intellectual content: all authors; final approval of the manuscript: all authors.
Supporting information
Fig S1
Table S1‐S2
Candeloro M, Eikelboom JW, Chan N, Bhagirath V, Douketis JD, Schulman S. Carbamazepine, phenytoin, and oral anticoagulants: Drug‐drug interaction and clinical events in a retrospective cohort. Res Pract Thromb Haemost. 2022;6:e12650. doi: 10.1002/rth2.12650
Handling Editor: Dr Cihan Ay
Funding information
The study was performed with internal funding. M.C. receives a salary from the University “G d'Annunzio” for his research fellowship and doctoral work.
Contributor Information
Matteo Candeloro, Email: matteo.candeloro@unich.it, @Matteocandeloro.
Sam Schulman, @SamSchulman6.
REFERENCES
- 1. Bathala MS, Masumoto H, Oguma T, He L, Lowrie C, Mendell J. Pharmacokinetics, biotransformation, and mass balance of edoxaban, a selective, direct factor Xa inhibitor, in humans. Drug Metab Dispos. 2012;40:2250‐2255. [DOI] [PubMed] [Google Scholar]
- 2. Mannheimer B, Andersson ML, Järnbert‐pettersson H, Lindh JD. The effect of carbamazepine on warfarin anticoagulation: a register‐based nationwide cohort study involving the Swedish population. J Thromb Haemost. 2016;14:765‐771. doi: 10.1111/jth.13268 [DOI] [PubMed] [Google Scholar]
- 3. Di Gennaro L, Lancellotti S, De Cristofaro R, De Candia E. Carbamazepine interaction with direct oral anticoagulants: help from the laboratory for the personalized management of oral anticoagulant therapy. J Thromb Thrombolysis. 2019;48:528‐531. [DOI] [PubMed] [Google Scholar]
- 4. Stöllberger C, Finsterer J. Recurrent venous thrombosis under rivaroxaban and carbamazepine for symptomatic epilepsy. Neurol Neurochir Pol. 2017;51:194‐196. [DOI] [PubMed] [Google Scholar]
- 5. Byon W, Sweeney K, Frost C, Boyd R. Population pharmacokinetics, pharmacodynamics, and exploratory exposure‐response analyses of apixaban in subjects treated for venous thromboembolism. CPT: Pharmacomet Syst Pharmacol. 2017;6:340‐349. doi: 10.1002/psp4.12184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perlman A, Wanounou M, Goldstein R, Choshen Cohen L, Singer DE, Muszkat M. Ischemic and thrombotic events associated with concomitant Xa‐inhibiting direct oral anticoagulants and antiepileptic drugs: analysis of the FDA adverse event reporting system (FAERS). CNS Drugs. 2019;33:1223‐1228. doi: 10.1007/s40263-019-00677-5 [DOI] [PubMed] [Google Scholar]
- 7. Schulman S, Parpia S, Stewart C, Rudd‐Scott L, Julian JA, Levine M. Warfarin dose assessment every 4 weeks versus every 12 weeks in patients with stable international normalized ratios. Ann Intern Med. 2011;155:653. [DOI] [PubMed] [Google Scholar]
- 8. Piran S, Traquair H, Chan N, Bhagirath V, Schulman S. Peak plasma concentration of direct oral anticoagulants in obese patients weighing over 120 kilograms: a retrospective study. Res Pract Thrombos Haemos. 2018;2:684‐688. doi: 10.1002/rth2.12146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Steffel J, Collins R, Antz M, et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non‐Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. EP Europace; 2021. https://academic.oup.com/europace/advance‐article/doi/ 10.1093/europace/euab065/6247378 [DOI] [PubMed] [Google Scholar]
- 10. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3:692‐694. doi: 10.1111/j.1538-7836.2005.01204.x [DOI] [PubMed] [Google Scholar]
- 11. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883‐891. doi: 10.1056/NEJMoa1009638 [DOI] [PubMed] [Google Scholar]
- 12. Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093‐2104. doi: 10.1056/NEJMoa1310907 [DOI] [PubMed] [Google Scholar]
- 13. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139‐1151. doi: 10.1056/NEJMoa0905561 [DOI] [PubMed] [Google Scholar]
- 14. Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981‐992. doi: 10.1056/NEJMoa1107039 [DOI] [PubMed] [Google Scholar]
- 15. Eikelboom JW, Quinlan DJ, Hirsh J, Connolly SJ, Weitz JI. Laboratory monitoring of non‐vitamin K antagonist oral anticoagulant use in patients with atrial fibrillation. JAMA Cardiol. 2017;2:566. doi: 10.1001/jamacardio.2017.0364 [DOI] [PubMed] [Google Scholar]
- 16. Samuelson BT, Cuker A, Siegal DM, Crowther M, Garcia DA. Laboratory assessment of the anticoagulant activity of direct oral anticoagulants. Chest. 2017;151:127‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Douxfils J, Adcock DM, Bates SM, et al. 2021 Update of the International Council for Standardization in Haematology recommendations for laboratory measurement of direct oral anticoagulants. Thromb Haemost. 2021;121:1008‐1020. doi: 10.1055/a-1450-8178 [DOI] [PubMed] [Google Scholar]
- 18. Sennesael A‐L, Larock A‐S, Hainaut P, et al. The impact of strong inducers on direct oral anticoagulant levels. Am J Med. 2021;134(10):1295‐1299. [DOI] [PubMed] [Google Scholar]
- 19. Gronich N, Stein N, Muszkat M. Association between use of pharmacokinetic‐interacting drugs and effectiveness and safety of direct acting oral anticoagulants: nested case‐control study. Clin Pharmacol Ther. 2021;110(6):1526‐1536. doi: 10.1002/cpt.2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Giustozzi M, Mazzetti M, Paciaroni M, Agnelli G, Becattini C, Vedovati MC. Concomitant use of direct oral anticoagulants and antiepileptic drugs: a prospective cohort study in patients with atrial fibrillation. Clin Drug Invest. 2021;41:43‐51. doi: 10.1007/s40261-020-00982-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang C‐L, Wu VC‐C, Chang K‐H, et al. Assessing major bleeding risk in atrial fibrillation patients concurrently taking non‐vitamin K antagonist oral anticoagulants and antiepileptic drugs. Eur Heart J ‐ Cardiovasc Pharmacother. 2020;6:147‐154. [DOI] [PubMed] [Google Scholar]
- 22. Han SY, Palmeri ST, Broderick SH, et al. Quality of anticoagulation with warfarin in patients with nonvalvular atrial fibrillation in the community setting. J Electrocardiol. 2013;46:45‐50. [DOI] [PubMed] [Google Scholar]
- 23. McAuliffe GN, de Silva F, Upton A, Chan G. International normalised ratio monitoring in the community populations of the Auckland and Northland regions of New Zealand: time in therapeutic range and frequency of testing. Intern Med J. 2018;48:1487‐1491. doi: 10.1111/imj.14032 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1‐S2


