Abstract
Purpose of Review
Provide an updated review of the clinical management and diagnosis of Kawasaki disease with inclusion of potential diagnostic difficulties with multisystem inflammatory syndrome in children (MIS-C) given the ongoing COVID-19 pandemic.
Recent Findings
Adjunctive corticosteroid therapy has been shown to reduce the rate of coronary artery dilation in children at high risk for IVIG resistance in multiple Japanese clinical studies (most notably RAISE study group). Additional adjunctive therapies (etanercept, infliximab, cyclosporin) may also provide limited benefit, but data is limited to single studies and subgroups of patients with cardiac abnormalities. The efficacy of other agents (atorvastatin, doxycycline) is currently being investigated. MIS-C is a clinically distinct entity from KD with broad clinical manifestations and multiorgan involvement (cardiac, GI, hematologic, dermatologic, respiratory, renal). MIS-C with Kawasaki manifestations is more commonly seen in children < 5 years of age.
Summary
The 2017 American Heart Association (AHA) treatment guidelines have included changes in aspirin dosing (including both 80–100 mg/kg/day and 30–50 mg/kg/day treatment options), consideration of the use of adjuvant corticosteroid therapy in patients at high risk of IVIG resistance, and the change in steroid regimen for refractory KD to include both pulse-dose IVMP and longer course of prednisolone with an oral taper. A significant proportion of children diagnosed with MIS-C, a post-infectious syndrome of SARS-CoV-2 infection, meet criteria for Kawasaki disease. Further investigation is warranted to further delineate these conditions and optimize treatment of these conditions given the ongoing COVID-19 pandemic.
Keywords: Kawasaki disease, MIS-C, Update, Pathogenesis, Etiology, Treatment
Introduction
Kawasaki disease (KD), also known as mucocutaneous lymph node syndrome, is an acute self-limited vasculitis of unknown etiology with characteristic clinical features first described in Japanese children in 1967 [1]. These characteristic clinical features of ≥ 5 days of fever, bilateral non-purulent conjunctivitis, rash, cervical lymphadenopathy, and mucocutaneous changes remain the mainstay of diagnosis of KD [2••]. However, the diagnosis of KD can be challenging given that the clinical features overlap with many common pediatric febrile illnesses and the wide spectrum of clinical presentations which include both incomplete clinical features and atypical presentations. However, prompt diagnosis and treatment are essential in reducing the significant cardiac morbidity associated with KD. Untreated cases are associated development of coronary artery aneurysms (CAA) in approximately 25% of patients, and KD is now the leading cause of acquired pediatric heart disease in the USA [3]. Treatment with high-dose intravenous immunoglobulin (IVIG) significantly decreases the incidence of cardiac involvement (25% to 3–5%) [4].
Epidemiology
The significant variation in the worldwide incidence of KD is a reflection upon the differing racial and ethnic composition around the globe. Japan has the highest incidence of KD of any country in children < 5 years (369/100,000 in 2018) [5], followed by Korea (195/100,000 in 2014) [6], and Taiwan (75/100,000 in 2011) [7]. By comparison, the incidence of KD is significantly lower in Western countries varying from 4 to 25/100,000 in children < 5 years (17.5–20.8/100,000 in the USA) [8]. The overall incidence of KD in the West has plateaued in the last decade following an initial increase attributed to initial diagnosis [9], while the incidence in East Asian countries continues to increase [6, 7, 10, 11].
The racial variation in the incidence of KD in the Western countries suggests genetic susceptibility as the driving factor behind the higher prevalence of KD in East Asia. Compared to Caucasians, the incidence of KD is significantly higher in Americans of Asian/Pacific Islander (2.5 times) and African American (1.5 times) descent [8]. Indeed, Americans of Japanese ancestry in Hawaii exhibit a similar incidence of KD to that of the general population of Japan (> 200/100,000 in children < 5 years) [12].
KD predominantly affects males (1.5:1 male:female ratio in all countries) and young children (80% of KD in children under 5 years and 50% of those in children under 2 years). The seasonality of KD varies by region (winter peaks in Japan and winter-spring peaks in the USA) [13].
Etiology
Despite significant investigations since its discovery in 1967, a putative agent has not been identified. Epidemiological features strongly suggest the presence of an inciting infection or agent that is etiologically linked to KD. The correlation of the seasonality of KD with tropospheric winds from northeastern China to Japan suggests a possible windborne pathogen [14]. Outbreaks of KD in Japan with simultaneous or sequential cases in siblings, twins, or other contacts can suggest both genetic susceptibility and common exposure to potentially infectious agents [13].
Bacterial and viral pathogens which have been isolated from KD patients include Staphylococcus aureus, Streptococcus pyogenes, Epstein-Barr virus, adenovirus, parvovirus B19, human herpes virus 6, measles, rotavirus, parainfluenza type 3, dengue virus, varicella-zoster virus, 2009 H1N1 pandemic influenza, human coronavirus NL63, and bocavirus [15]. However, the etiologic importance of these pathogens is unclear given the breadth of infectious pathogens isolated. A bacterial toxin/superantigen etiology has been suggested given the similar desquamation seen in scarlet fever, toxic shock syndrome, and KD. However, no bacterial toxin has been detected in the peripheral blood of KD patients [15].
The clinical features which comprise KD may be the result of a common pathway of immune-mediated vascular inflammation after a variety of inciting infections rather than infection with a specific pathogen [16]. Similar clinical features are seen in multi-system inflammatory syndrome in children (MIS-C), a post-infectious syndrome of SARS-CoV-2 infection. A total of 10–20% children diagnosed with MIS-C met the criteria for Kawasaki disease in a case series from the UK [17•].
Diagnosis
KD is diagnosed thorough the presence of clinical criteria. A thorough history and physical examination is essential as the characteristic clinical features of KD may not be present concurrently. The classic clinical criteria for the diagnosis of KD require the presence of ≥ 5 days of fever and ≥ 4/5 clinical features (Table 1). Alternatively, KD can be diagnosed with only 4 days of fever and the presence of ≥ 4 clinical criteria (particularly when redness and swelling of the hands and feet are present) and some experienced clinicians have treated KD with only 3 days of fever [2••].
Table 1.
Kawasaki diagnostic criteria
| Classic clinical criteria | |
|---|---|
| Fever persisting for at least 5 days | |
| Plus | |
| At least 4 of the following principle features: | |
| 1. Extremity changes | Erythema of palms/soles, edema of hands/feet, periungual peeling of fingers, toes in weeks 2–3 |
| 2. Rash | Polymorphous exanthema, NOT bullous/vesicular |
| 3. Changes in lips and oral cavity | Erythema, cracked lips, strawberry tongue, diffuse injection of oral and pharyngeal mucosa |
| 4. Conjunctival injection | Bilateral, bulbar sparing limbus, non-purulent |
| 5. Cervical lymphadenopathy | > 1.5 cm in diameter, usually unilateral |
| Supplemental laboratory criteria | |
| Hypoalbuminia | < 3.0 mg/dL |
| Alanine aminotransferase (ALT) | Elevation above reference range (varies by laboratory) |
| Anemia for age | Decreased in hemoglobin below reference range for age |
| Leukocytosis | White blood cells > 15,000/mm3 |
| Sterile pyuria (urinalysis) | > 10 WBC/HPF (high power field) |
| Thrombocytosis after 7 days | Platelets > 450,000/mm3 after 7 days |
Fever
KD is typically characterized by high spiking (> 39–40 °C) and remittent fever. Untreated fever can last for 1-3 weeks but can spontaneously resolve after 1 week. Fever will typically resolve within 36 h after treatment with IVIG.
Conjunctivitis
Bilateral conjunctival injection which spares the limbus typically begins shortly after fever onset. Anterior uveitis (during the first week of fever), subconjunctival hemorrhage, and punctate keratitis can also be observed.
Extremity Changes
In the acute phase of KD, extremity changes present as erythema, induration, and swelling of the hands and/or feet. Later findings after fever resolution include desquamation of the fingers and toes (2–3 weeks) and deep traverse grooves across the nails known as Beau’s lines (1–2 months).
Oral Cavity Changes
Oral changes in KD include generalized erythema of the lips and oropharyngeal mucosa, peeling/cracking/fissuring of the lips, and “strawberry tongue” (erythema and prominent fungiform papillae on the tongue). However, strawberry tongue is not unique to KD as it is also associated with streptococcal scarlet fever. Oral ulcers and pharyngeal exudates are not seen in KD.
Rash
The polymorphous exanthem of KD usually appears within 5 days of fever onset. Rash pattern includes diffuse maculopapular (most common), scarlatiniform erythroderma/erythema multiforme (common), to urticarial/fine micro-pustular eruptions (less common). KD is not associated with bullous, vesicular, and petechial rashes. Psoriasis with plaques and pustules can rarely occur during or after acute KD [18]. Early desquamation and accentuation in the groin region are a characteristic feature and strong indicator for KD.
Cervical Lymphadenopathy
Cervical lymphadenopathy is the least commonly observed clinical criteria. Diagnostic criteria require ≥ 1 lymph node which is > 1.5 cm in diameter. Presentation is usually unilateral and classically located in the anterior cervical triangle.
Incomplete KD
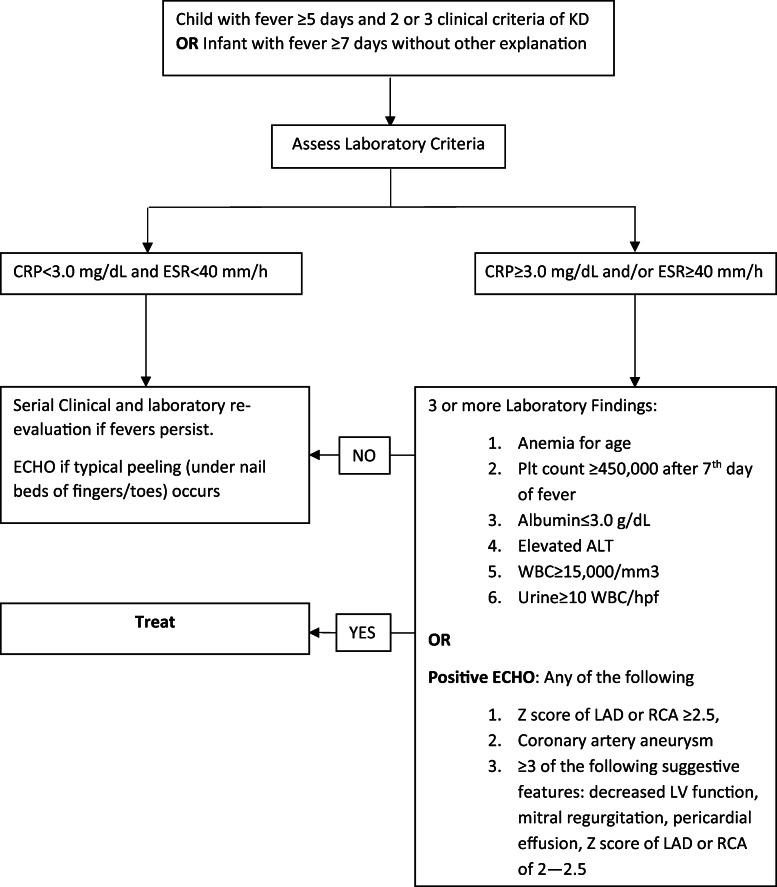
Incomplete KD can be diagnosed in patients who do not completely fulfill the classic clinical criteria and in young children with unexplained fever. Children under 6 months of age may have only prolonged fever as the sole clinical finding and are at substantial risk for development of CAA. Therefore, the 2017 AHA guidelines were updated to recommend evaluation of incomplete KD in infants with ≥ 7 days of unexplained fever and in children with ≥ 5 days in the presence of 2–3 clinical features of KD [2••]. The evaluation of incomplete KD includes initial screening for elevation in inflammatory markers, followed by evaluation for supplementary laboratory criteria, ECHO, and serial clinical and laboratory re-examination (Fig. 1).
Fig. 1.
Incomplete Kawasaki algorithm
The presence of 3 supplementary laboratory criteria and nonspecific laboratory findings of elevated markers of inflammation (erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP)) are considered a positive evaluation for incomplete KD. Supplementary laboratory criteria include hypoalbuminemia (< 3.0 mg/dL), anemia for age, elevation of alanine aminotransferase (ALT), thrombocytosis after 7 days (≥ 450,000/mm3), leukocytosis (≥ 15,000/mm3), and sterile pyuria (≥ 10 WBC/hpf). Elevation of ESR and CRP is nearly universally present, but CRP is generally more useful given its faster elevation/normalization. Thrombocytosis is characteristic late finding, occurring in the 2nd week of disease and resolving by weeks 4–6. Thrombocytopenia can be seen in acute KD (first 1–2 weeks) and is a risk factor for the development of CAA. A total of 40–60% of patients will have mild transaminitis or gamma glutamyl transpeptidase elevation, and 10% present with mild hyperbilirubinemia. Sterile pyuria is present in up to 80% of children, but this finding lacks specificity [19]. Approximately 30% of children who undergo LP have CSF pleocytosis with mononuclear predominance and normal glucose/protein [20].
Atypical KD
Atypical KD, in which patients present with symptoms typically not seen in KD, should be considered a separate entity to incomplete KD. These symptoms include transient unilateral peripheral facial nerve palsy, transient high-frequency sensorineural hearing loss, hepatic enlargement with jaundice, and acalculous gallbladder distention. Testicular swelling, pulmonary nodules, pleural effusions, and hemophagocytic syndromes have also been reported but are less common. Kawasaki shock syndrome, the presentation of KD disease with systolic hypotension or clinical signs of poor perfusion, has also been described as a rare presentation of KD [21].
Multiorgan Involvement
KD is a vasculitis that affects all medium-sized arteries in multiple organs/tissues, resulting in systemic inflammation and pathology can develop in multiple organ systems. Cardiac complications include CAA, pericarditis, myocarditis, valvulitis, new valvular regurgitation, and cardiogenic shock. Arrhythmia, prolonged PR interval, and nonspecific ST and T wave changes may be present on EKG. Additional organ involvement includes liver (hepatitis), GI (gallbladder hydrops/biliary sludging, abdominal pain), pancreas (pancreatitis), lymph nodes (lymphadenopathy), lung (interstitial pneumonitis), and urinary tract (sterile pyuria).
Diagnostic Challenges
The diagnosis of KD can often be challenging due to both its broad spectrum of clinical features and atypical presentations. Incomplete KD is of considerable concern given the lack of enough diagnostic clinical features can ultimately result is both misdiagnosis and delayed diagnosis of KD. Additionally, the overlap between KD and MIS-C continues to confound the diagnosis of KD, especially given the current SARS-CoV-2 pandemic.
MIS-C
At the end of March 2020, a variety of European countries (the UK, France, Italy), Middle East (Saudi Arabia, Israel), and North America (the USA, Canada) began to report increased hospitalizations of children hospitalized with fever and multisystem inflammation with a history of SARS-CoV-2 infection [22], and the CDC case definition for MIS-C has been published (Table 2). In contrast to KD, MIS-C occurs in non-Asian countries with no significant outbreaks in East Asian countries reported. These symptoms were often similar to KD or KD shock syndrome [23, 24], including the presence of CAA. American case series have demonstrated that the peak of SARS-CoV-2 infection rate precedes the peak of MIS-C cases by 25–31 days [25, 26]. This temporal relationship and the high prevalence of positive IgG (83%) in these patients with only intermittent nasal PCR positivity (26%) [17•] suggest that MIS-C is likely due to a post-infectious syndrome from prior SARS-CoV-2 infection.
Table 2.
CDC MIS-C case definition
| Criteria | Specifications |
|---|---|
| Age | < 21 years |
| AND | |
| Fever | > 38.0 °C for ≥ 24 h or report of subjective fever ≥ 24 h |
| AND | |
| Laboratory evidence of inflammation | ≥ 1 of the following: CRP, ESR, fibrinogen, procalcitonin, d-dimer, ferritin, lactic acid dehydrogenase, IL-6, elevated neutrophils, reduced lymphocytes, low albumin |
| AND | |
| Evidence of clinically significant illness requiring hospitalization with multisystem organ involvement | > 2 organ involvement (cardiac, kidney, respiratory, hematologic, gastrointestinal, dermatologic, neurological) |
| AND | |
| Evidence of recent or current SARS-CoV-2 infection | Positive RT-PCR, serology, antigen test, or COVID-19 exposure within the 4 weeks prior to the onset of symptoms |
| AND | |
| No alternative plauusible diagnoses |
MIS-C is a clinically distinct entity from KD. Case series have shown significant divergence in age, sex, and race of MIS-C patients. Whittaker et al. reported a case series of 58 MIS-C patients from 8 UK hospitals. These patients were predominantly Black (38%), followed by Asian (31%), Caucasian (21%) and other (10%). Of the 58 patients, 3 distinct clinical patterns were described: fever and elevated inflammatory markers (23/58), fever and shock +/− cardiac dysfunction (29/58), and meeting criteria for KD (7/59, but 13/59 met criteria when CAA were included). When compared to a historical cohort of KD and KD shock syndrome patients from Rady Children’s from 2002 to 2019, patients were found to have older age (median age MIS-C 9, KD 2.7 years, KD shock syndrome 3.8 years), with higher neutrophil count, CRP, more profound lymphopenia, and anemia. Additional laboratory differences include lower platelet counts, higher fibrinogen levels, greater elevation of troponin, and BNP. While children in the KD patient group were on average 4 years younger than the other groups of MIS-C patients, CAA abnormalities were found in a subset of all 3 groups of MIS-C patients. Additional comparison of children with MIS-C who developed CAA did not show any difference in clinical or laboratory markers [17•, 25, 26•]
Clinical manifestations of MIS-C are broad and often affect more than 1 organ system. A US case series showed that up to 80% of all patients had some sort of cardiac involvement which included elevated troponin (50%), BNP > 400 pg/mL (73%), pericarditis or pericardial effusion (26%), ejection fraction < 55% (38%), abnormal LAD or RAD Z score ≥ 2.5 (8%), and arrhythmia (12%). Non-cardiovascular involvement includes GI (hepatitis, pancreatitis, gallbladder hydrops), hematologic involvement (DVT, coagulopathy), dermatological manifestations (rash), respiratory involvement (respiratory insufficiency, CXR infiltrate, pleural effusions), musculoskeletal involvement (myositis, myalgia, arthritis, arthralgia), neurologic involvement (headache, confusion, altered mental status), and renal involvement (acute kidney injury) [26•]. Additionally, clinical manifestations of MIS-C vary with age. A case series of 95 children in New York State showed that the 0–5 years group had distinctly higher rates of dermatological manifestations (87% 0–5 years vs 78.6% in 6–12 years, 61 % in 13–20 years), higher rates of KD or atypical KD features (58.4% 0–5 years, 43% 6–12 years, 12% 13–20 years), lower rates of GI symptoms (74.2% 0–5 years, 83% 6–12 years, 81% 13–20 years) and lower rates of cardiomyopathy (38.7% 0–5 years, 50% 6–12 years, 73% 13–20 years) and neurological symptoms (13% 0–5 years, 38% 6–12 years, 39% 13–20 years) [25].
The diagnosis of MIS-C should be considered in any patient with clinical features of KD and history of SARS-CoV-2 infection or known exposure to SARS-CoV-2. Suspicion of MIS-C should be particularly high in older patients with atypical laboratory findings (significant lymphopenia, profound elevation of CRP, high fibrinogen, troponin elevation), gastrointestinal complaints, or significant myocardial dysfunction/shock.
Node-First Presentation of KD
NFKD occurs when fever and cervical adenopathy present prior to other clinical features of KD. As such, NFKD is often misdiagnosed as bacterial cervical adenitis. Patients with NFKD are typically older (median 4.2 years vs 1.6 years) with higher inflammatory markers (median CRP 13.7 mg/dL vs 6.1 mg/dL) than cervical adenitis. Bacterial cervical adenitis tends to present as a single node or conglomerations/suppuration of nodes rather than the multiple discrete nodes of NFKD [27].
Kawasaki Shock Syndrome
Kawasaki shock syndrome (KSS) is a rare manifestation of KD which is characterized by systolic hypotension or clinical signs of poor perfusion. When compared to classic KD, patients with KSS tended to have higher CRP; lower hemoglobin; more frequent hyponatremia, hypoalbuminemia, and coagulopathy; increase in cardiac troponins; and elevations in cytokines (IL-6, IL-10, IFN-γ) [21, 28]. There is a significant overlap between KSS with both MIS-C (discussed previously) and toxic shock syndrome (TSS). Anemia, thrombocytosis, and presence of cardiac abnormalities are more suggestive of KD [29].
Adenovirus Infection
Adenoviral infection and KD can both present with conjunctivitis in conjunction with fever and elevated inflammatory markers. Adenovirus conjunctivitis typically presents with unilateral onset, prominence of tearing, and follicular hyperplasia, while KD conjunctivitis is bilateral with limbal sparing. Additionally, pharyngoconjunctival fever (conjunctivitis and nonexudative or exudative pharyngitis) is seen with adenovirus infections but not KD. Extremity changes and the unilateral cervical lymphadenopathy of KD are not typically seen in adenovirus.
Retropharyngeal Abscess
The retropharyngeal edema that is a rare presentation of KD can be misdiagnosed as retropharyngeal abscess. This has resulted in delayed diagnosis as well as unnecessary needle aspiration/antibiotic therapy [30]. Therefore, a high index of suspicion of KD should be maintained in patients with sterile culture results from aspiration. Additionally, clinical symptoms such as stridor, neck pain, dysphagia, and radiographic findings of ring enhancement/mass effect on CT are uncommon in KD and are suggestive of retropharyngeal abscess [31].
Systemic onset juvenile idiopathic arthritis
Early clinical features of systemic onset juvenile idiopathic arthritis (SoJIA) have significant overlap with KD (fever, rash, thrombocytosis, increased inflammatory markers, arthritis). Features suggestive of SoJIA include more severe arthritis, intermittent rash, early thrombocytosis, and IVIG resistance. The arthritis of SoJIA is persistent and severe, requiring immunosuppressive agent treatment for resolution, while arthritis in KD is mild and self-limited [32]. The rash of SoJIA is more intermittent and often presents with fever spikes rather than the persistent rash of KD. SoJIA should be considered in patients diagnosed with KD refractory to IVIG with the appropriate symptoms. Compared to the late thrombocytosis of KD (typically in the 2nd week), thrombocytosis is typically found in the early stages of SoJIA. Mucocutaneous findings are also more suggestive of KD [33].
Treatment
Primary Treatment Regimen
The goal of initial treatment of acute KD is the reduction of inflammation (and subsequently arterial damage) and the prevention of potentially catastrophic thrombosis associated with coronary artery abnormalities. The mainstay of therapy is currently high-dose intravenous immune globulin (IVIG) together with aspirin (ASA).
Intravenous Immune Globulin
IVIG is a biological product made from the pooled donor plasma. The exact mechanism of action remains unknown but the generalized anti-inflammatory effect of IVIG appears to inhibit the progression of KD with subsequently preventing the development of coronary artery aneurysms. Treatment with IVIG within the first 10 days of illness reduces the risk of development of coronary artery abnormalities to approximately 5% from 25% [4, 34, 35].
A single infusion of IVIG (dosed at 2 mg/kg over 10–12 h) should be given together with ASA as the initial treatment for KD [2••]. While IVIG should ideally be administered within the first 10 days of illness, treatment should still be administered to children diagnosed with KD after the 10th day of illness if persistent signs of inflammation are present (persistent fever without explanation, CAA with ongoing levitations in inflammatory markers) [2••]. Patients should be closely monitored for adverse reactions during infusion, including aseptic meningitis, Coombs-positive hemolytic anemia, and generalized infusion reactions.
Live vaccines (particularly measles, mumps, varicella) should be deferred for 11 months after receiving high-dose IVIG. Children at high risk for measles may receive the vaccination earlier but will require re-immunization 11 months after IVIG administration.
Aspirin
ASA is administered at moderate to high doses during the acute phase of KD (80–100 mg/kg/day divided every 6 h in the USA, 30–50 mg/kg/day in Japan and Western Europe) and subsequently transitioned to a low dose (3–5 mg/kg/day) until the patient has no evidence of CAA by 6–8 weeks after onset of illness. Low-dose ASA can be initiated after the child has been afebrile for 48–72 hours, although some clinicians will continue high-dose ASA until the 14th day of illness and at least of 48–72 hours without fever.
There is currently no data to suggest that which of the 2 high-dose ASA regimens (80–100 mg/kg/day vs 30–50 mg/kg/day) is superior. Despite the lack of comparative trials, the rates of CAA are essentially identical despite these differences in ASA dosing [36]. Some experts recommend the use of ASA at the lower of the two dosing regimens (30–50 mg/kg/day divided q6h) [37] given concerns for side effects (GI bleed, Reye’s syndrome).
Children are at risk for development of Reye syndrome when on prolonged high-dose ASA, but low-dose ASA has not been associated with Reye’s syndrome. In patients who present with influenza or varicella and KD, ASA should be replaced with an alternative antiplatelet agent for a minimum of 2 weeks. Children on long-term ASA therapy should receive influenza vaccine, but ASA should be avoided for 6 weeks after administration of varicella vaccine. Many clinicians will substitute another antiplatelet medication for ASA in that period [2••].
Adjunctive Therapy
Corticosteroids
The use of adjunctive corticosteroids as initial therapy for KD remains controversial. Newburger et al. conducted a randomized, double-blind, placebo control trial which compared the efficacy of adding a single-dose pulsed IV methylprednisolone (IVMP) dosed at 30 mg/kg to the standard regimen of IVIG (2 mg/kg) and ASA. This study did not show any reduction in the prevalence of CAA or total length of hospital stay. However, children with persistent fever requiring repeat IVIG did have significantly lower rates of CAA [38].
The use of corticosteroids as adjunctive therapy for children at high risk for IVIG resistance is supported by multiple Japanese clinical studies. These include the Okada et al. which compared the effectiveness of a single dose of 30 mg/kg IVMP plus IVIG and ASA as primary treatment for high-risk Japanese children described by the Sano score in a multicenter study [39], Egami et al. and Ogata et al. which compared the effectiveness of the same single-dose 30 mg/kg IVMP plus IVIG and ASA as primary treatment for high-risk Japanese children described by the Egami score in a single-center study [40, 41], and the RAISE study group (Kobayashi et al.) compared the effectiveness of an IV prednisolone 2 mg/kg/day for 5 days followed by an oral taper over weeks plus IVIG and ASA in patients predicted to have IVIG resistance based on the Kobayashi score in a multicenter, prospective, randomized, open-label, blinded-end point trial [42••]. Despite the varying steroid regimens used, a meta-analysis including these trials found that the use of corticosteroid with standard-dose IVIG as initial treatment in high-risk patients reduced the rate of CAA [43].
The AHA currently recommends that a longer course of adjunct corticosteroid therapy (tapered over 2–3 weeks) may be considered for treatment of KD patients at high risk for IVIG resistance when such a risk can be identified in patients before initiation of treatment [2••]. This treatment regimen most closely resembles the regimen of the RAISE study group based on the strength of its study design. However, it should be noted that the sensitivity of Japanese risk-scoring systems for predicting IVIG resistance has been found to be low when used in different ethnic groups, most notably in North America [44].
Infliximab
Infliximab is a chimeric monoclonal antibody which inhibits inflammation through TNF-α blockade. Currently, infliximab adjunct therapy is not routinely indicated for the initial treatment of acute KD. This recommendation based upon a 2-center randomized, double-blind, placebo-controlled trial conducted by Tremoulet et al. The addition of infliximab to IVIG for initial treatment shortened fever duration and inflammatory marker normalization rate but did not decrease the rate of CAA or rate of IVIG resistance [45]. However, there was a significant decrease in the Z score for the LAD in the infliximab treatment group which suggests infliximab may inhibit progression of CAA but not prevent development of CAA.
Etanercept
Etanercept is a soluble TNF receptor inhibitor that was recently completed a multicenter double-blind, placebo-controlled trial comparing the effectiveness of adjunctive etanercept to IVIG alone. Etanercept did not show significant reduction in IVIG resistance in the entire population. However, benefit in IVIG resistance in patients > 1 year of age (p = 0.03) and reduction in progression of coronary artery dilation in patients with baseline abnormalities was noted [46]. The AHA has not yet updated the 2017 AHA guidelines to incorporate this study, and the use of etanercept as adjunctive therapy is not currently recommended [2••].
Cyclosporin
Cyclosporin is a calcineurin inhibitor, which targets the Ca2+/NFAT signaling pathway which is associated with immune hyper-reactivity and has been implicated in both KD susceptibility and CAA formation [47]. The KAICA trial, a phase III multicenter, randomized, open-label, blinded-end point trial to evaluate the efficacy of primary treatment with IVIG and cyclosporin vs IVIG alone for the prevention of CAA in patients identified as high risk for IVIG resistance demonstrated significantly lower rates of CAA in the cyclosporin group (14% cyclosporin vs 31% in IVIG, p = 0.01). The dose of cyclosporin was started at 5 mg/kg/day with a target trough of 60–200 ng/mL and continued for 5 days [48]. The AHA has not yet updated the 2017 AHA guidelines to incorporate this study, and therefore, the use of cyclosporin as adjunctive therapy is not currently recommended [2••].
Refractory Kawasaki Disease
Refractory KD is defined as the development of recurrent or persistent fever 36 h after the completion of their initial IVIG infusion and occurs approximately 10–20% of patients [49, 50]. The most likely explanation for persistent fever in these patients is failure of initial IVIG to abort the disease process of KD. These patients have also been classified as IVIG-resistant.
The most common treatment regimens for refractory KD are a repeat infusion of IVIG, retreatment with IVIG plus prednisone, or infliximab.
IVIG Retreatment
Patients with refractory KD should receive a repeat infusion of IVIG at the same dose (2 mg/kg as a single infusion). Retrospective series have suggested efficacy in this approach [51, 52].
Corticosteroids
There is no current consensus on the optimal steroid regimen for refractory KD. High-dose pulse steroids (IVMP 20–30 mg/kg IV for 3 days with or without PO prednisolone taper) may be considered an alternative to a 2nd infusion of IVIG or for retreatment of patients with recurrent fever after additional IVIG infusions. Alternatively, administration of a longer course of prednisolone (2 mg/kg/day IV divided q8h until afebrile then PO prednisolone until CRP normalized followed by 2–3-week taper) with IVIG and ASA is also an acceptable regimen [2••]. The regimen closely resembles a retrospective study by Kobayashi et al. who found significantly lower rates of failure to respond, CAA in the IV prednisolone group [53].
Infliximab
Infliximab may be considered to be an alternative to a 2nd infusion of IVIG or corticosteroids for refractor KD. Son et al. conducted a retrospective review of 2 centers that consistently administered 2nd-dose IVIG or infliximab to IVIG-resistant patients which showed shorter hospitalization and fever duration be similar outcomes with CAA [54].
The Kawasaki Disease Comparative Effectiveness Trial (KIDCARE) is a phase III, 2-arm, randomized, multicenter, superiority treatment study to compare infliximab to the 2nd-dose IVIG for treatment of children with IVIG resistance that was recently completed with results to be published. (Clinicaltrials.gov ID NCT03065244). In patients with KD, IVIG administration was shown to influence the pharmacokinetics of infliximab, resulting in lower volume of distribution [55]. Therefore, this trial currently uses a higher dose of infliximab (10 mg/kg) than the currently recommended 5 mg/kg.
Cyclosporin
Cyclosporin (IV 3 mg/kg/day divided q12h, PO 4–8 mg/kg/day divided q12h; target trough 50–150 ng/mL; 2 h peak 300–600 ng/mL) may be considered in patients with refractory KD in which the 2nd IVIG infusion, infliximab, or corticosteroids have failed. Cyclosporin should be continued until the patient is afebrile and clinically improving with CRP < 12 mg/dL or after 2 weeks of therapy. The dose should then be tapered by 10% every 3 days until the dose has reached 1 mg/kg/day [2••].
Limited clinical data for the use of cyclosporin in the treatment of refractory KD exists. Tremoulet et al. demonstrated successful use of cyclosporin or tacrolimus in a small study group of 10 IVIG-resistant children [56]. A small Japanese single-arm pilot trial in Japan with 28 IVIG-resistant children showed a response in 78% of patients treated with 4–6 mg/kg/day of PO cyclosporin [57].
Anakinra
Anakinra (2–6 mg/kg/day given by subcutaneous injection) is an IL-1 receptor antagonist that has been used in cases of highly refractory KD. However, data is limited to 2 successful case reports [58, 59]. Clinical trials with anakinra are currently recruiting. This includes the ANAKID trial, phase I/II trial of anakinra in KD patients < 2 years of age with coronary artery abnormalities is ongoing in the USA. Subjects will receive a 2- or 6-week course of daily anakinra injections. The study aims to evaluate the safety and tolerability of anakinra to prevent/attenuate coronary artery damage in patients with KD (Clinical Trials.gov ID NCT021798953).
The results of the KAWAKINRA trial, a phase IIa multicenter trial of anakinra in KD patients who fail to respond to initial IVIG therapy (persistent fever up to 48 hours after infusion) (Clinicaltrials.gov ID NCT02390596), have not yet been published. However, an oral presentation noted that 8/14 patients had CAA at inclusion with resolution of all but 3 cases by day 14. Further reduction in the Z score was decreased at day 45 [60].
Other Treatment Modalities
Plasma exchange [61] and cytotoxic agents [62] such as cyclophosphamide have been used in particularly refractory acute KD. However, given the limited data and risks of these agents, use should be reserved for patients for which other modalities have failed.
Ongoing Clinical Trials
Atorvastatin
Statins have been associated with anti-inflammatory and anti-oxidant effects, in particular with lower inflammatory markers such as CRP [63]. The anti-inflammatory, anti-oxidant, and endothelial healing properties of statins have been postulated to be beneficial in blocking the progression of coronary artery abnormalities in KD [64], and improvement in endothelial function and reduction in CRP was noted in 13 children with KD with medium to giant CAA treated with pravastatin [65]. Recently, a PK/Safety Study of Atorvastatin in Children with KD and CAA was completed (ClinicalTrials.gov ID NCT01431105). Additionally, a clinical trial for the adjunctive use of statins in children with severe CA abnormalities is currently recruiting in China (ClinicalTrials.gov ID NCT03915795).
Doxycycline
Doxycycline is an antibiotic that inhibits matrix metalloproteinase-9 (MMP-9). MMP-9 has been found at elevated serum levels during acute KD [66] and may play a significant role in the development of CAA [67]. A phase II study to assess the efficacy and safety of doxycycline in preventing coronary artery aneurysm formation and progression is currently recruiting (ClinicalTrials.gov ID NCT01917721).
Conclusion
The diagnosis and initial treatment of KD with IVIG and aspirin remains unchanged in the most recent 2017 American Heart Association (AHA) treatment guidelines. Prompt treatment with IVIG and aspirin remains the mainstay of treatment. However, attention should be paid to the minor changes in treatment regimens for ASA (dosing can now be either 80–100 mg/kg/day or 30–50 mg/kg/day), consideration of the use of adjuvant corticosteroid therapy in patients at high risk of IVIG resistance, and the change in steroid regimen for refractory KD to include both pulse-dose IVMP and longer course of prednisolone with oral taper. Additionally, the overlap of clinical features of KD with other common pediatric illnesses continues to provide significant diagnostic challenges. This is illustrated in the diagnostic and treatment dilemmas due to the overlap of KD and MIS-C in the ongoing COVID-19 pandemic. Further investigation is warranted to optimize treatment of these conditions as the number of COVID-19 cases continues to increase.
Declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Pediatric Infectious Diseases
The original online version of this article was revised due to a retrospective Open Access cancellation and incorrect data in Fig. 1.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/3/2021
A Correction to this paper has been published: 10.1007/s11908-021-00754-1
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. 1967;16(3):178–222. [PubMed] [Google Scholar]
- 2.•• McCrindle, B.W., et al., Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation, 2017. 135(17): p. e927-e999. This is the current guideline for diagnosis and management of KD. It is published by the American Heart Association and endorsed by the American Academy of Pediatrics. [DOI] [PubMed]
- 3.Taubert KA, Rowley AH, Shulman ST. Nationwide survey of Kawasaki disease and acute rheumatic fever. J Pediatr. 1991;119(2):279–282. doi: 10.1016/S0022-3476(05)80742-5. [DOI] [PubMed] [Google Scholar]
- 4.Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE, Glode MP, Mason WH, Reddy V, Sanders SP, Shulman ST, Wiggins JW, Hicks RV, Fulton DR, Lewis AB, Leung DYM, Colton T, Rosen FS, Melish ME. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315(6):341–347. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- 5.Ae R, Makino N, Kosami K, Kuwabara M, Matsubara Y, Nakamura Y. Epidemiology, treatments, and cardiac complications in patients with Kawasaki disease: the nationwide survey in Japan, 2017-2018. J Pediatr. 2020;225:23–29.e2. doi: 10.1016/j.jpeds.2020.05.034. [DOI] [PubMed] [Google Scholar]
- 6.Kim GB, Park S, Eun LY, Han JW, Lee SY, Yoon KL, Yu JJ, Choi JW, Lee KY. Epidemiology and clinical features of Kawasaki disease in South Korea, 2012-2014. Pediatr Infect Dis J. 2017;36(5):482–485. doi: 10.1097/INF.0000000000001474. [DOI] [PubMed] [Google Scholar]
- 7.Huang YH, Lin KM, Ho SC, Yan JH, Lo MH, Kuo HC. Increased incidence of Kawasaki disease in Taiwan in recent years: a 15 years nationwide population-based cohort study. Front Pediatr. 2019;7:121. doi: 10.3389/fped.2019.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uehara R, Belay ED. Epidemiology of Kawasaki disease in Asia, Europe, and the United States. J Epidemiol. 2012;22(2):79–85. doi: 10.2188/jea.JE20110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh S, Vignesh P, Burgner D. The epidemiology of Kawasaki disease: a global update. Arch Dis Child. 2015;100(11):1084–1088. doi: 10.1136/archdischild-2014-307536. [DOI] [PubMed] [Google Scholar]
- 10.Makino N, Nakamura Y, Yashiro M, Ae R, Tsuboi S, Aoyama Y, Kojo T, Uehara R, Kotani K, Yanagawa H. Descriptive epidemiology of Kawasaki disease in Japan, 2011-2012: from the results of the 22nd nationwide survey. J Epidemiol. 2015;25(3):239–245. doi: 10.2188/jea.JE20140089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim GB, Han JW, Park YW, Song MS, Hong YM, Cha SH, Kim DS, Park S. Epidemiologic features of Kawasaki disease in South Korea: data from nationwide survey, 2009-2011. Pediatr Infect Dis J. 2014;33(1):24–27. doi: 10.1097/INF.0000000000000010. [DOI] [PubMed] [Google Scholar]
- 12.Holman RC, Christensen KY, Belay ED, Steiner CA, Effler PV, Miyamura J, Forbes S, Schonberger LB, Melish M. Racial/ethnic differences in the incidence of Kawasaki syndrome among children in Hawaii. Hawaii Med J. 2010;69(8):194–197. [PMC free article] [PubMed] [Google Scholar]
- 13.Rowley AH, Shulman ST. The epidemiology and pathogenesis of Kawasaki disease. Front Pediatr. 2018;6:374. doi: 10.3389/fped.2018.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodo X, Curcoll R, Robinson M, Ballester J, Burns JC, Cayan DR, Lipkin WI, Williams BL, Couto-Rodriguez M, Nakamura Y, Uehara R, Tanimoto H, Morgui JA. Tropospheric winds from northeastern China carry the etiologic agent of Kawasaki disease from its source to Japan. Proc Natl Acad Sci U S A. 2014;111(22):7952–7957. doi: 10.1073/pnas.1400380111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowley AH. Kawasaki disease: novel insights into etiology and genetic susceptibility. Annu Rev Med. 2011;62:69–77. doi: 10.1146/annurev-med-042409-151944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi K, Oharaseki T, Yokouchi Y. Update on etio and immunopathogenesis of Kawasaki disease. Curr Opin Rheumatol. 2014;26(1):31–36. doi: 10.1097/BOR.0000000000000010. [DOI] [PubMed] [Google Scholar]
- 17.• Whittaker, E., et al., Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA, 2020. The first major case series of MIS-C with comparison of laboratory values with historical cohort of KD. [DOI] [PMC free article] [PubMed]
- 18.Eberhard BA, Sundel RP, Newburger JW, Baker A, Fuhlbrigge RC, Burns JC, Gellis SE. Psoriatic eruption in Kawasaki disease. J Pediatr. 2000;137(4):578–580. doi: 10.1067/mpd.2000.107840. [DOI] [PubMed] [Google Scholar]
- 19.Shike H, Kanegaye JT, Best BM, Pancheri J, Burns JC. Pyuria associated with acute Kawasaki disease and fever from other causes. Pediatr Infect Dis J. 2009;28(5):440–443. doi: 10.1097/INF.0b013e318193ec8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dengler LD, et al. Cerebrospinal fluid profile in patients with acute Kawasaki disease. Pediatr Infect Dis J. 1998;17(6):478–481. doi: 10.1097/00006454-199806000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Taddio A, Rossi ED, Monasta L, Pastore S, Tommasini A, Lepore L, Bronzetti G, Marrani E, Mottolese BD’A, Simonini G, Cimaz R, Ventura A. Describing Kawasaki shock syndrome: results from a retrospective study and literature review. Clin Rheumatol. 2017;36(1):223–228. doi: 10.1007/s10067-016-3316-8. [DOI] [PubMed] [Google Scholar]
- 22.Paediatric inflammatory multisystem syndrome and SARS-CoV-2 infection in children – 15 May 2020, E.C.f.D.P.a. Control, Editor. 2020: Stockholm.
- 23.Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, Bonanomi E, D'Antiga L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viner RM, Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. 2020;395(10239):1741–1743. doi: 10.1016/S0140-6736(20)31129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, Barranco MA, Maxted AM, Rosenberg ES, Easton D, Udo T, Kumar J, Pulver W, Smith L, Hutton B, Blog D, Zucker H, New York State and Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome in Children Investigation Team Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383(4):347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.• Feldstein, L.R., et al., Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med, 2020. 383(4): p. 334-346. This is the most comprehensive report on epidemiology, clinical course of MIS-C in the USA. This paper has excellent description of the clinical characteristics of patients with MIS-C who presented with features of KD. [DOI] [PMC free article] [PubMed]
- 27.Kanegaye, J.T., et al., Lymph-node-first presentation of Kawasaki disease compared with bacterial cervical adenitis and typical Kawasaki disease. J Pediatr, 2013. 162(6): p. 1259-63, 1263 e1-2. [DOI] [PMC free article] [PubMed]
- 28.Li Y, Zheng Q, Zou L, Wu J, Guo L, Teng L, Zheng R, Jung LKL, Lu M. Kawasaki disease shock syndrome: clinical characteristics and possible use of IL-6, IL-10 and IFN-gamma as biomarkers for early recognition. Pediatr Rheumatol Online J. 2019;17(1):1. doi: 10.1186/s12969-018-0303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanegaye JT, Wilder MS, Molkara D, Frazer JR, Pancheri J, Tremoulet AH, Watson VE, Best BM, Burns JC. Recognition of a Kawasaki disease shock syndrome. Pediatrics. 2009;123(5):e783–e789. doi: 10.1542/peds.2008-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hung MC, Wu KG, Hwang B, Lee PC, Meng CCL. Kawasaki disease resembling a retropharyngeal abscess--case report and literature review. Int J Cardiol. 2007;115(2):e94–e96. doi: 10.1016/j.ijcard.2006.08.095. [DOI] [PubMed] [Google Scholar]
- 31.Nomura O, Hashimoto N, Ishiguro A, Miyasaka M, Nosaka S, Oana S, Sakai H, Takayama JI. Comparison of patients with Kawasaki disease with retropharyngeal edema and patients with retropharyngeal abscess. Eur J Pediatr. 2014;173(3):381–386. doi: 10.1007/s00431-013-2179-0. [DOI] [PubMed] [Google Scholar]
- 32.Dogra S, Gehlot A, Suri D, Rawat A, Kumar RM, Singh S. Incomplete Kawasaki disease followed by systemic onset juvenile idiopathic arthritis - the diagnostic dilemma. Indian J Pediatr. 2013;80(9):783–785. doi: 10.1007/s12098-012-0893-7. [DOI] [PubMed] [Google Scholar]
- 33.Kumar S, Vaidyanathan B, Gayathri S, Rajam L. Systemic onset juvenile idiopathic arthritis with macrophage activation syndrome misdiagnosed as Kawasaki disease: case report and literature review. Rheumatol Int. 2013;33(4):1065–1069. doi: 10.1007/s00296-010-1650-8. [DOI] [PubMed] [Google Scholar]
- 34.Furusho K, Kamiya T, Nakano H, Kiyosawa N, Shinomiya K, Hayashidera T, Tamura T, Hirose O, Manabe Y, Yokoyama T. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet. 1984;2(8411):1055–1058. doi: 10.1016/S0140-6736(84)91504-6. [DOI] [PubMed] [Google Scholar]
- 35.Durongpisitkul K, Gururaj VJ, Park JM, Martin CF. The prevention of coronary artery aneurysm in Kawasaki disease: a meta-analysis on the efficacy of aspirin and immunoglobulin treatment. Pediatrics. 1995;96(6):1057–1061. doi: 10.1542/peds.96.6.1057. [DOI] [PubMed] [Google Scholar]
- 36.Ogata S, Tremoulet AH, Sato Y, Ueda K, Shimizu C, Sun X, Jain S, Silverstein L, Baker AL, Tanaka N, Ogihara Y, Ikehara S, Takatsuki S, Sakamoto N, Kobayashi T, Fuse S, Matsubara T, Ishii M, Saji T, Newburger JW, Burns JC. Coronary artery outcomes among children with Kawasaki disease in the United States and Japan. Int J Cardiol. 2013;168(4):3825–3828. doi: 10.1016/j.ijcard.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burns JC. Frequently asked questions regarding treatment of Kawasaki disease. Glob Cardiol Sci Pract. 2017;2017(3):e201730. doi: 10.21542/gcsp.2017.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newburger JW, Sleeper LA, McCrindle BW, Minich LLA, Gersony W, Vetter VL, Atz AM, Li JS, Takahashi M, Baker AL, Colan SD, Mitchell PD, Klein GL, Sundel RP. Randomized trial of pulsed corticosteroid therapy for primary treatment of Kawasaki disease. N Engl J Med. 2007;356(7):663–675. doi: 10.1056/NEJMoa061235. [DOI] [PubMed] [Google Scholar]
- 39.Okada K, Hara J, Maki I, Miki K, Matsuzaki K, Matsuoka T, Yamamoto T, Nishigaki T, Kurotobi S, Sano T, For the Osaka Kawasaki Disease Study Group Pulse methylprednisolone with gammaglobulin as an initial treatment for acute Kawasaki disease. Eur J Pediatr. 2009;168(2):181–185. doi: 10.1007/s00431-008-0727-9. [DOI] [PubMed] [Google Scholar]
- 40.Ogata S, Ogihara Y, Honda T, Kon S, Akiyama K, Ishii M. Corticosteroid pulse combination therapy for refractory Kawasaki disease: a randomized trial. Pediatrics. 2012;129(1):e17–e23. doi: 10.1542/peds.2011-0148. [DOI] [PubMed] [Google Scholar]
- 41.Egami K, Muta H, Ishii M, Suda K, Sugahara Y, Iemura M, Matsuishi T. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr. 2006;149(2):237–240. doi: 10.1016/j.jpeds.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 42.•• Kobayashi, T., et al., Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open-label, blinded-endpoints trial. Lancet, 2012. 379(9826): p. 1613-20. This study showed benefit of adjunctive corticosteroids in patients predicted to have IVIG resistance in the initial treatment of KD. The current AHA recommendation for consideration of adjunct corticosteroid therapy in patients at high risk for IVIG resistance resembles this study’s regimen based on strength of this study’s design and findings. [DOI] [PubMed]
- 43.Chen S, Dong Y, Yin Y, Krucoff MW. Intravenous immunoglobulin plus corticosteroid to prevent coronary artery abnormalities in Kawasaki disease: a meta-analysis. Heart. 2013;99(2):76–82. doi: 10.1136/heartjnl-2012-302126. [DOI] [PubMed] [Google Scholar]
- 44.Sleeper LA, Minich LL, McCrindle B, Li JS, Mason W, Colan SD, Atz AM, Printz BF, Baker A, Vetter VL, Newburger JW, Pediatric Heart Network Investigators Evaluation of Kawasaki disease risk-scoring systems for intravenous immunoglobulin resistance. J Pediatr. 2011;158(5):831–835. doi: 10.1016/j.jpeds.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tremoulet AH, Jain S, Jaggi P, Jimenez-Fernandez S, Pancheri JM, Sun X, Kanegaye JT, Kovalchin JP, Printz BF, Ramilo O, Burns JC. Infliximab for intensification of primary therapy for Kawasaki disease: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet. 2014;383(9930):1731–1738. doi: 10.1016/S0140-6736(13)62298-9. [DOI] [PubMed] [Google Scholar]
- 46.Portman, M.A., et al., Etanercept with IVIg for acute Kawasaki disease: a randomized controlled trial. Pediatrics, 2019. 143(6). [DOI] [PMC free article] [PubMed]
- 47.Shimizu C, Eleftherohorinou H, Wright VJ, Kim J, Alphonse MP, Perry JC, Cimaz R, Burgner D, Dahdah N, Hoang LT, Khor CC, Salgado A, Tremoulet AH, Davila S, Kuijpers TW, Hibberd ML, Johnson TA, Takahashi A, Tsunoda T, Kubo M, Tanaka T, Onouchi Y, Yeung RS, Coin LJ, Levin M, Burns JC, International Kawasaki Disease Genetics Consortium Genetic variation in the SLC8A1 calcium signaling pathway is associated with susceptibility to Kawasaki disease and coronary artery abnormalities. Circ Cardiovasc Genet. 2016;9(6):559–568. doi: 10.1161/CIRCGENETICS.116.001533. [DOI] [PubMed] [Google Scholar]
- 48.Hamada H, Suzuki H, Onouchi Y, Ebata R, Terai M, Fuse S, Okajima Y, Kurotobi S, Hirai K, Soga T, Ishiguchi Y, Okuma Y, Takada N, Yanai M, Sato J, Nakayashiro M, Ayusawa M, Yamamoto E, Nomura Y, Hashimura Y, Ouchi K, Masuda H, Takatsuki S, Hirono K, Ariga T, Higaki T, Otsuki A, Terauchi M, Aoyagi R, Sato T, Fujii Y, Fujiwara T, Hanaoka H, Hata A, KAICA trial Investigators Efficacy of primary treatment with immunoglobulin plus ciclosporin for prevention of coronary artery abnormalities in patients with Kawasaki disease predicted to be at increased risk of non-response to intravenous immunoglobulin (KAICA): a randomised controlled, open-label, blinded-endpoints, phase 3 trial. Lancet. 2019;393(10176):1128–1137. doi: 10.1016/S0140-6736(18)32003-8. [DOI] [PubMed] [Google Scholar]
- 49.Bar-Meir M, Kalisky I, Schwartz A, Somekh E, Tasher D, Israeli Kawasaki Group Prediction of resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatric Infect Dis Soc. 2018;7(1):25–29. doi: 10.1093/jpids/piw075. [DOI] [PubMed] [Google Scholar]
- 50.Tremoulet AH, Best BM, Song S, Wang S, Corinaldesi E, Eichenfield JR, Martin DD, Newburger JW, Burns JC. Resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatr. 2008;153(1):117–121. doi: 10.1016/j.jpeds.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burns JC, et al. Intravenous gamma-globulin treatment and retreatment in Kawasaki disease. US/Canadian Kawasaki Syndrome Study Group. Pediatr Infect Dis J. 1998;17(12):1144–1148. doi: 10.1097/00006454-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 52.Sundel RP, Burns JC, Baker A, Beiser AS, Newburger JW. Gamma globulin re-treatment in Kawasaki disease. J Pediatr. 1993;123(4):657–659. doi: 10.1016/S0022-3476(05)80972-2. [DOI] [PubMed] [Google Scholar]
- 53.Kobayashi T, Kobayashi T, Morikawa A, Ikeda K, Seki M, Shimoyama S, Ishii Y, Suzuki T, Nakajima K, Sakamoto N, Arakawa H. Efficacy of intravenous immunoglobulin combined with prednisolone following resistance to initial intravenous immunoglobulin treatment of acute Kawasaki disease. J Pediatr. 2013;163(2):521–526. doi: 10.1016/j.jpeds.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 54.Son MB, Gauvreau K, Burns JC, Corinaldesi E, Tremoulet AH, Watson VE, Baker A, Fulton DR, Sundel RP, Newburger JW. Infliximab for intravenous immunoglobulin resistance in Kawasaki disease: a retrospective study. J Pediatr. 2011;158(4):644–649. doi: 10.1016/j.jpeds.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 55.Vande Casteele N, Oyamada J, Shimizu C, Best BM, Capparelli EV, Tremoulet AH, Burns JC. Infliximab pharmacokinetics are influenced by intravenous immunoglobulin administration in patients with Kawasaki disease. Clin Pharmacokinet. 2018;57(12):1593–1601. doi: 10.1007/s40262-018-0653-6. [DOI] [PubMed] [Google Scholar]
- 56.Tremoulet AH, Pancoast P, Franco A, Bujold M, Shimizu C, Onouchi Y, Tamamoto A, Erdem G, Dodd D, Burns JC. Calcineurin inhibitor treatment of intravenous immunoglobulin-resistant Kawasaki disease. J Pediatr. 2012;161(3):506–512. doi: 10.1016/j.jpeds.2012.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suzuki H, Terai M, Hamada H, Honda T, Suenaga T, Takeuchi T, Yoshikawa N, Shibuta S, Miyawaki M, Oishi K, Yamaga H, Aoyagi N, Iwahashi S, Miyashita R, Onouchi Y, Sasago K, Suzuki Y, Hata A. Cyclosporin A treatment for Kawasaki disease refractory to initial and additional intravenous immunoglobulin. Pediatr Infect Dis J. 2011;30(10):871–876. doi: 10.1097/INF.0b013e318220c3cf. [DOI] [PubMed] [Google Scholar]
- 58.Shafferman A, Birmingham JD, Cron RQ. High dose Anakinra for treatment of severe neonatal Kawasaki disease: a case report. Pediatr Rheumatol Online J. 2014;12:26. doi: 10.1186/1546-0096-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohen S, Tacke CE, Straver B, Meijer N, Kuipers IM, Kuijpers TW. A child with severe relapsing Kawasaki disease rescued by IL-1 receptor blockade and extracorporeal membrane oxygenation. Ann Rheum Dis. 2012;71(12):2059–2061. doi: 10.1136/annrheumdis-2012-201658. [DOI] [PubMed] [Google Scholar]
- 60.Koné-Paut I, et al. OP0147 KAWAKINRA: A PHASE IIA MULTICENTER TRIAL TO ASSESS THE EFFICACY, AND SAFETY OF ANAKINRA IN PATIENTS WITH INTRAVENOUS IMMUNOGLOBULIN-RESISTANT KAWASAKI DISEASE. Annals of the Rheumatic Diseases. 2019;78(Suppl 2):149. [Google Scholar]
- 61.Hokosaki T, Mori M, Nishizawa T, Nakamura T, Imagawa T, Iwamoto M, Yokota S. Long-term efficacy of plasma exchange treatment for refractory Kawasaki disease. Pediatr Int. 2012;54(1):99–103. doi: 10.1111/j.1442-200X.2011.03487.x. [DOI] [PubMed] [Google Scholar]
- 62.Wallace CA, French JW, Kahn SJ, Sherry DD. Initial intravenous gammaglobulin treatment failure in Kawasaki disease. Pediatrics. 2000;105(6):E78. doi: 10.1542/peds.105.6.e78. [DOI] [PubMed] [Google Scholar]
- 63.Ridker PM, Rifai N, Pfeffer MA, Sacks FM, Moye LA, Goldman S, Flaker GC, Braunwald E. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1998;98(9):839–844. doi: 10.1161/01.CIR.98.9.839. [DOI] [PubMed] [Google Scholar]
- 64.Tremoulet AH. The role of statins in inflammatory vasculitides. Autoimmunity. 2015;48(3):177–180. doi: 10.3109/08916934.2015.1027818. [DOI] [PubMed] [Google Scholar]
- 65.Duan C, du ZD, Wang Y, Jia LQ. Effect of pravastatin on endothelial dysfunction in children with medium to giant coronary aneurysms due to Kawasaki disease. World J Pediatr. 2014;10(3):232–237. doi: 10.1007/s12519-014-0498-5. [DOI] [PubMed] [Google Scholar]
- 66.Takeshita S, Tokutomi T, Kawase H, Nakatani K, Tsujimoto H, Kawamura Y, Sekine I. Elevated serum levels of matrix metalloproteinase-9 (MMP-9) in Kawasaki disease. Clin Exp Immunol. 2001;125(2):340–344. doi: 10.1046/j.1365-2249.2001.01608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Senzaki H. The pathophysiology of coronary artery aneurysms in Kawasaki disease: role of matrix metalloproteinases. Arch Dis Child. 2006;91(10):847–851. doi: 10.1136/adc.2005.087437. [DOI] [PMC free article] [PubMed] [Google Scholar]