Abstract
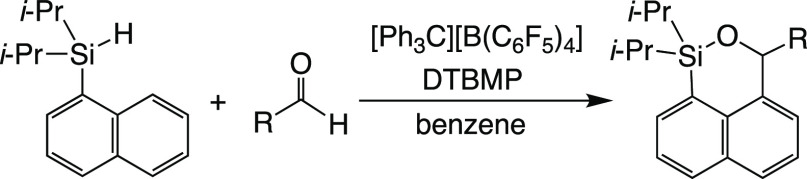
A strong Lewis acid silylium ion was utilized for dehydrogenative annulation between dialkyl(1-naphthyl)silanes 1 and aldehydes 2. Silane 1a was reacted with [Ph3C][B(C6F5)4] in the presence of 2,6-di-tert-butyl-4-methylpyridine and aldehydes 2 to afford the annulation product, 1-silabenzo[d,e]isochromanes 3, in moderate isolated yields. The annulation occurred only at the 8-position on the 1-naphthyl group. The silylium ion-promoted hydrosilylation proceeded competitively to afford silyl ethers 4 via the same intermediates, silylcarboxonium ions, in the dehydrogenative annulation. The ratio of 3 and 4 was affected by solvents and the electronic properties of aromatic aldehydes; for example, the use of less polar solvents and that of benzaldehydes with an electron-withdrawing group at the para-position predominantly yielded 3. This annulation reaction was applicable to aldehydes bearing a heteroaromatic group and aliphatic alkyl groups. Judging from these results, both the formation of silylcarboxonium ions by in situ-generated silylium ions and the electrophilic aromatic substitution are important for this annulation reaction.
Introduction
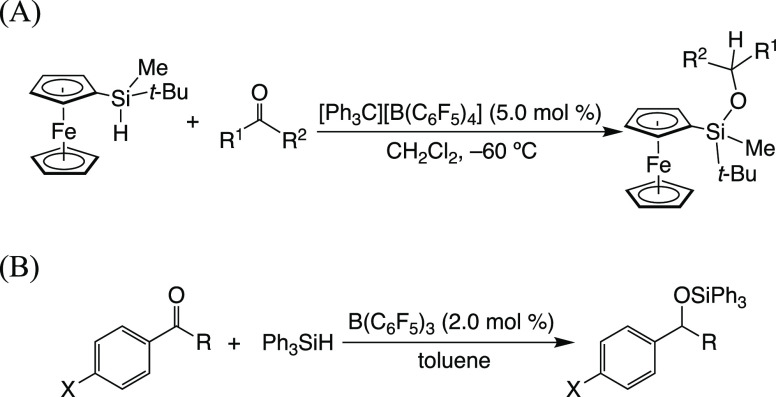
A silylium ion known as a Lewis acid has received much attention due to the activation of substrates in organic syntheses as reactants or catalysts.1 The vacant p orbital on the silylium ion can interact with unsaturated hydrocarbons as well as hetero-double-bonded compounds such as carbonyl compounds and imines.2 The high Lewis acidity generates active reaction intermediates, which undergo the desired reactions in both the stoichiometric and catalytic systems.3−7 Sakurai et al. achieved the reduction of ketones associated with silylcarboxonium ions formed by the reaction of trityl tetrakis[3,5-bis(trifluoromethyl)phenyl]borate with Et3SiH in the presence of ketones.8 The silylium ion generated by a catalytic amount of trityl cation functions as a chain carrier in the hydrosilylation of carbonyl compounds reported by Oestreich et al. (Scheme 1A).9 B(C6F5)3 is one of the most popular catalysts for hydrosilylation in the absence of transition metals, and Piers et al. established the effective hydrosilylation of aromatic aldehydes, ketones, and esters in the catalytic amount of B(C6F5)3 (Scheme 1B).10 The reaction mechanism was investigated in detail using silanes with a chiral silicon center where a Walden-type inversion occurred in the hydride transfer process of the silane to B(C6F5)3.11 The silylium ion is employed as a Lewis acid catalyst as well as a chain carrier, and Mukaiyama aldol reaction and Diels–Alder reaction are carried out.12,13 Understanding the behavior of a silylium ion toward carbonyl compounds allows the development of various reactions based on the silylium ion; however, an electrophilic substitution reaction of a generated silylcarboxonium ion species is limited in both stoichiometric and catalytic systems.
Scheme 1. Hydrosilylation of Carbonyl Compounds using Trityl Cation (A) and Borane as a Catalyst (B).
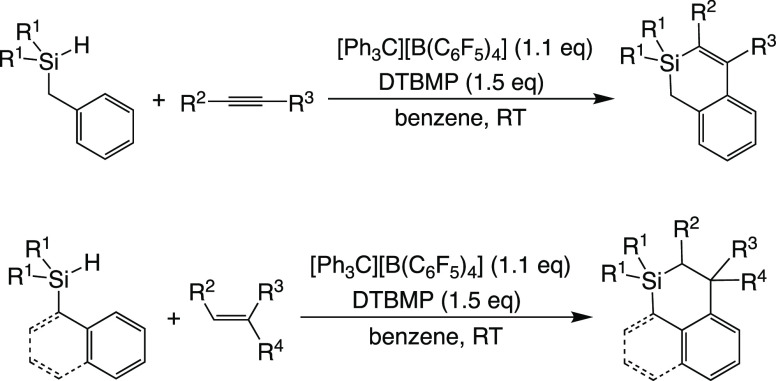
We were interested in the reactivity of tricoordinate heavier group 14 element cations and have studied the dehydrogenative annulation reactions between dialkylbenzylsilanes or dialkyl(1-naphthyl)silanes and unsaturated hydrocarbons through the formation of the corresponding silylalkenylium or silylalkylium ions, followed by their electrophilic substitution on the intramolecular aromatic ring (Scheme 2).14 In this paper, we investigate the reactions of dialkyl(1-naphthyl)silanes with aldehydes to obtain 1-silabenzo[d,e]isochromane derivatives and describe about the steric and electronic effects of the substituent groups on aromatic aldehydes and the reaction mechanism for dehydrogenative annulation.
Scheme 2. Silylium Ion-Promoted Dehydrogenative Annulation.
Results and Discussion
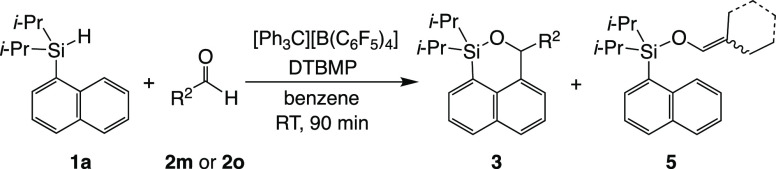
The dehydrogenative annulation via an in situ-generated silylium ion requires bases and solvents with poor coordinating properties. Among the bases explored in the annulation between diisopropyl(1-naphthyl)silane (1a) and benzaldehyde (2a) (Table 1, entries 1–4), only 2,6-di-tert-butyl-4-methylpyridine (DTBMP) afforded the desired cyclic product, that is, 1,1-diisopropyl-3-phenyl-1-silabenzo[d,e]isochromane (3aa), in 50% isolated yield, accompanied by benzyloxydiisopropyl(1-naphthyl)silane (4aa) as a hydrosilylation product in 10% yield.15 The bases other than DTBMP can bind to the silylium ion to some extent, while DTBMP has a sterically bulky substituent such as a tert-butyl group, resulting in the inhibition of the interaction with the silylium ion. The annulation occurred not at the 2-position on the naphthyl group but at the 8-position to give 3aa because of the higher reactivity of the latter position based on electron density. The hydrosilylation of carbonyl compounds initiated by the prepared or in situ-generated silylium ions has been reported previously and is a potential competing reaction for annulation.8,9 A change from benzene to CH2Cl2 as a solvent affected the product distribution to cause the ratio of 3aa to 4aa to be approximately 1:1 (Table 1, entry 6), while the yields and ratio in toluene were similar to those in benzene (Table 1, entries 2 and 5). In the aromatic solvents, more stable silylium–arene complexes are readily formed through hydride abstraction by Ph3C+, resulting in the rapid consumption of 1a. Therefore, hydride abstraction of the intermediary silylcarboxonium ion from 1a becomes slow because only a small amount of unreacted 1a remains. On the other hand, the hydride abstraction step is slow in CH2Cl2 because the silylium–CH2Cl2 complex is less stable, and consequently the residual starting material 1a can work as a hydride donor to give the hydrosilylation product. In addition, in CH2Cl2 which is a more polar solvent, silylcarboxonium borate exists as a solvent-separated ion pair rather than a contact ion pair. Its bimolecular reaction with 1a becomes more favored sterically and can compete with the intramolecular aromatic substitution reaction, which decreases the ratio of 3aa to 4aa.
Table 1. Dehydrogenative Annulation between Silanes 1 and Benzaldehyde (2a)a.
| yield (%)b |
|||||
|---|---|---|---|---|---|
| entry | base | solvent | R1 | 3 | 4 |
| 1 | 2,6-lutidine | benzene | i-Pr | c | c |
| 2 | DTBMP | benzene | i-Pr | 50 (3aa) | 10 (4aa) |
| 3 | K2CO3 | benzene | i-Pr | c | c |
| 4 | DBUd | benzene | i-Pr | e | e |
| 5 | DTBMP | toluene | i-Pr | 48 (3aa) | 8 (4aa) |
| 6 | DTBMP | CH2Cl2 | i-Pr | 28 (3aa) | 27 (4aa) |
| 7 | DTBMP | benzene | Me | c | c |
| 8 | DTBMP | benzene | Ph | 6 (3ca) | 31 (4ca) |
Reaction conditions: 1 (0.10 mmol), 2a (3.0 equiv), [Ph3C]-[B(C6F5)4] (1.1 equiv), and base (1.5 equiv).
Isolated yields based on 1.
Not obtained.
1,8-Diazabicyclo[5.4.0]-7-undecene.
No reaction.
The scope and limitations were studied in the dehydrogenative annulation reactions between silanes 1 and aldehydes 2. Upon using 1b with methyl groups, neither cyclic nor hydrosilylated products were obtained, but the reaction using 1c with phenyl groups gave the hydrosilylation product 4ca in 31% yield together with the annulation product 3ca (6% yield) (Table 2, entries 2 and 3). Aromatic groups on the silicon center are known to lead to the effective hydrosilylation of ketones relative to isopropyl groups.16 In the reaction using 1b, a complex of the generated silylium ion with an arene or a base seems stable and not reactive with an aldehyde. Therefore, we decided to use silane 1a as a substrate because 1a among 1a–c affords the annulation products in moderate yields. The introduction of electron-donating substituents such as MeO and Me groups to the para-position on the benzene ring of aldehyde decreased the sum of yields of 3 and 4 (Table 1, entries 7 and 8).
Table 2. Scope for Dehydrogenative Annulationa.
| entry | aldehydes 2 | yield (%)b |
|||
|---|---|---|---|---|---|
| R2 | 3 | 4 | |||
| 1 | Ph | 3aa | 50 (59) | 4aa | 10 (14) |
| 2 | 4-OMe-C6H4 | 3ab | 13 (21) | 4ab | 4 (7) |
| 3 | 4-Me-C6H4 | 3ac | 32 (38) | 4ac | 14 (20) |
| 4 | 4-Cl-C6H4 | 3ad | 50 (60) | 4ad | 13 (16) |
| 5 | 4-CN-C6H4 | 3ae | 63 (71) | 4ae | (2)cd |
| 6 | 4-NO2-C6H4 | 3af | 51 (73) | 4af | (3)cd |
| 7 | 2-Me-C6H4 | 3ag | 52 (57) | 4ag | 10 (12) |
| 8 | 3-Me-C6H4 | 3ah | 40 (46) | 4ah | 14 (18) |
| 9 | 1-naphthyl | 3ai | 53 (64) | 4ai | 23 (25) |
| 10 | 2-naphthyl | 3aj | 40 (47) | 4aj | 16 (24) |
| 11 | 3-thienyl | 3ak | 12 (17) | 4ak | (9)cd |
| 12 | n-Bu | 3al | 22 (24) | 4al | e |
| 13 | sec-Bu | 3am | 31 (34) | 4am | f |
| 14 | tert-Bu | 3an | 28 (30) | 4an | 15 (21) |
| 15 | cyclohexyl | 3ao | 26 (32) | 4ao | f |
Reaction conditions: 1a (0.10 mmol), 2 (3.0 equiv), [Ph3C]-[B(C6F5)4] (1.1 equiv), and DTBMP (1.5 equiv).
Isolated yields based on 1a. NMR yields based on the internal standard Me2Ph2Si in parentheses.
Not isolated.
Detection by the proton signals for NMR spectrum relative to the authentic sample. See Experimental Section in detail.
Not obtained.
Obtained silyl enol ether 5 instead of 4.
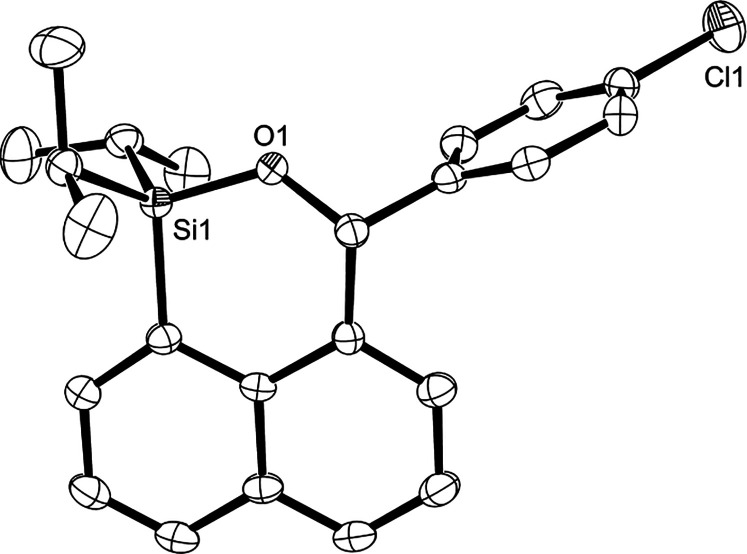
The use of benzaldehydes with the electron-withdrawing substituents (Cl, CN, and NO2) afforded 3 in the yields similar to that of benzaldehyde (Table 2, entries 4–6), and in the case of the cyano and nitro groups with a high Hammett constant, traces of hydrosilylation products (4ae and 4af) were observed in the 1H NMR spectra consistent with those synthesized by the alternative method (Scheme 3). The electronic substituent effect indicates that dehydrogenative annulation is associated with the electron density on the intermediary silylcarboxonium carbon accessible to the 8-position on the naphthalene, that is, the increased electronic deficiency of the silylcarboxonium carbon caused by the cyano or nitro groups accelerates both electrophilic aromatic substitution giving the annulation products 3 and hydride abstraction from 1a giving hydrosilylation products 4, but the former, which is an intramolecular reaction, is accelerated more than the latter intermolecular reaction. Product 3ad was obtained as single crystals suitable for X-ray diffraction analysis from Et2O solution. The molecular structure of 3ad revealed that the fused six-membered ring including a silicon atom involves the bond between the carbonyl carbon from p-chlorobenzaldehyde and the carbon at the 8-position on the 1-naphthyl group (Figure 1). The benzene ring is oriented perpendicular to naphthalene to prevent the steric repulsion between them. The introduction of a methyl group to the ortho- or meta-position in benzaldehyde increased the yield of desired products 3 relative to that to the para-position (Table 2, entries 3, 7, and 8), implying that the electron-donating property of the methyl group depends on its substituent position. As the CO plane of the silylcarboxonium ion formed from o-methylbenzaldehyde is out of the plane of the benzene ring due to steric hindrance to the methyl group, the silylcarboxonium carbon does not accept effective electron donation from the methyl group. Similar behavior is found in the lower pKa of o-methylbenzoic acid at 3.90 than that of p-methylbenzoic acid at 4.36.17
Scheme 3. Alternative Synthesis of Silyl Ethers 4 from a Silyl Chloride and Corresponding Alcohols.
Figure 1.
Crystal structure of 3ad, showing 50% probability of thermal ellipsoid. The hydrogen atoms are omitted for clarity.
For other aromatic aldehydes, 3ai and 3aj from regioisomers of naphthaldehydes were obtained in moderate yields (Table 2, entries 9 and 10). The use of 1-naphthaldehyde afforded the sum of isolated yields for 3ai and 4ai up to 76%. The silylcarboxonium ion surrounded by sterically crowded 1-naphthyl group prohibits undesired intermolecular reactions except for hydrosilylation. 3-Thiophenecarbaldehyde with a heteroaromatic ring gave the annulation product 3ak in 12% yield (Table 2, entry 11). The poor yield is attributed to the coordination of the thienyl group toward the generated silylium ion and/or the electron-donating property of the thienyl group to the silylcarboxonium carbon. Aliphatic aldehydes also afforded the annulation products 3al–ao in low yields, independent of the branched alkyl group (Table 2, entries 12–15). Interestingly, the diastereomer ratio of 3am that showed at 2.8:1 was attributed to the conformation preventing the steric repulsion between the silyloxy and the ethyl of sec-butyl group. Aldehydes with an α-proton did not give the corresponding hydrosilylation products 4 as a subproduct. The abstraction of α-proton accessible to DTBMP is sufficiently predicted, resulting in the isolation of the silyl enol ethers 5am (14%) and 5ao (8%) using 3-methylbutanal (2m) and cyclohexanecarbaldehyde (2o), respectively (Scheme 4).
Scheme 4. Dehydrogenative Annulation between 1a and Aldehydes with Secondary Alkyl Group.
The reaction mechanism for the dehydrogenative annulation between silanes 1 and aldehydes 2 is shown in Scheme 5. The hydride abstraction from silanes 1 by Ph3C+ forms the silylium–arene complex. Exchange of the arene to an aldehyde affords an intermediate with two resonance structures, that is, an adduct of silylium ion to the oxygen of the aldehyde and a silyloxycarbenium ion. An aldehyde with a more basic C=O moiety is expected to form the silylcarboxonium intermediate rapidly, and the following electrophilic aromatic substitution produces 3 and/or hydrosilylation products 4 in higher yields. However, the results for 4-substituted benzaldehydes indicate that the higher yields were obtained in the cases of electron-withdrawing substituents. The increase in basicity on the carbonyl oxygen accelerates the oligomerization where the excess aldehyde reacts with the silylcarboxonium ion, although it has an advantage for the coordination toward the silylium ion. Therefore, aldehydes with an electron-withdrawing group effectively undergo the annulation reaction because of the low basicity of the carbonyl oxygen. The intramolecular electrophilic aromatic substitution of the silylcarboxonium carbon occurs only at the 8-position on the 1-naphthyl group and then the proton is abstracted by DTBMP to give the annulation products 3. As a competing reaction with annulation, the silylcarboxonium carbon abstracts the hydride from silanes 1 to form the hydrosilylation products 4. The ratio of 3 to 4 is attributed to the hydride property of Si–H. The substitution of an aryl group on silicon generally facilitates hydride donation, and triarylsilanes in the B(C6F5)3 catalysis system undergo effective hydrosilylation of carbonyl compounds.16 In fact, the use of triarylsilane 1c predominantly gave the hydrosilylation product in contrast to that of 1a.
Scheme 5. Plausible Mechanism for Dehydrogenative Annulation and Hydrosilylation between 1 and Aldehydes 2.
Conclusions
We described the dehydrogenative annulation of dialkyl(1-naphthyl)silanes 1 with various aldehydes 2 via the silylcarboxonium ion intermediate. The in situ-generated silylium ions bound to various aldehydes underwent intramolecular electrophilic aromatic substitution at the 8-position of the 1-naphthyl group to form 1-silabenzo[d,e]isochromane derivatives 3, and no substitution at the 2-position was observed. The hydrosilylation of aldehydes competed with the annulation and afforded silyl ether derivatives 4. The intrinsic activity of the carbonyl moiety in aldehydes affects the ratio of 3 and 4, where benzaldehyde bearing an electron-withdrawing group at the para-position predominantly gave the annulation product with 1a. On the other hand, the use of CH2Cl2 as a solvent and aldehydes with an electron-donating substituent accelerates the hydrosilylation reaction. The activation of carbonyl moiety by a silylium ion, followed by the electrophilic aromatic substitution has been demonstrated as a synthetic method for 1-silabenzo[d,e]isochromanes.
Experimental Section
General Procedure
All experiments were carried out using standard vacuum line and Schlenk techniques under Ar atmosphere or in the drybox. All the reagents were of the highest grade available and were used without further purification. All solvents used for the syntheses were distilled according to the general procedure. Trityl tetrakis(pentafluorophenyl)borate18 and diisopropyl(1-naphthyl)silane (1a)14b were synthesized according to the previously reported method. 1H NMR and 13C NMR spectral measurements were performed on Bruker AV400M spectrometers. 1H and 13C chemical shifts are reported relative to the residual protonated solvent, respectively, according to the literature.19 High-resolution mass spectra were measured by a JEOL JMS-700N mass spectrometer operated by electron impact ionization (EI). Gel permeation liquid chromatography (GPLC) was performed by a Japan Analytical Industry LC-918 using chloroform as an eluent.
X-ray Crystallography
The crystal of 3ad was mounted on a glass fiber, and the diffraction data were collected at −100 °C on a Rigaku R-AXIS RAPID II large-area curved imaging plate detector using graphite monochromated Mo Kα radiation. The structure was solved by the direct method using SHELXT-2014/5.20 All nonhydrogen atoms were anisotropically refined by full-matrix least-squares calculation on F2 using SHELXL-2016/6.21 All hydrogen atoms were placed at geometrically calculated positions.
Preparation of Compounds
Dimethyl(1-naphthyl)silane (1b)
To 1-bromonaphthalene (0.30 g, 1.4 mmol) in Et2O (3 mL) was added 1.6 M t-BuLi pentane solution (1.8 mL, 2.9 mmol) at −80 °C, and the solution was warmed to room temperature. After chlorodimethylsilane (0.13 g, 1.4 mmol) was added to the reaction mixture at −80 °C, it was gradually warmed to room temperature and stirred for a day. The reaction was quenched by aq. NH4Cl, and the organic layer was extracted twice by Et2O. The combined organic layers dried over Na2SO4 were filtrated. The filtrate was concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (eluent: hexane/ethyl acetate = 20/1). Further purification was carried out by GPLC to obtain 1b (0.18 g, 67%) as an oil. 1H NMR (CDCl3, 400 MHz): δ 8.13 (d, J = 8.4 Hz, 1H, Ar), 7.90–7.86 (m, 2H, Ar), 7.74 (dd, J = 6.4 Hz, J = 1.2 Hz, 1H, Ar), 7.56–7.45 (m, 3H, Ar), 4.88 (sept, J = 4.0 Hz, 1H, SiH), 0.51 (d, J = 4.0 Hz, 6H, SiMe2). 13C NMR (CDCl3, 100 MHz): δ 137.1, 135.8, 133.8, 133.3, 130.1, 129.1, 127.8, 126.1, 125.7, 125.3, −3.1. HRMS (EI) m/z: [M]+ calcd for C12H14Si, 186.0865; found, 186.0865.
1-Naphthyldiphenylsilane (1c)
Silane 1c was synthesized similar to 1b, except for using chlorodiphenylsilane (0.31 g, 1.4 mmol) instead of chlorodimethylsilane, to obtain as a white powder (0.40 g, 89%). mp: 89–90 °C. 1H NMR (CDCl3, 400 MHz): δ 8.14 (d, J = 8.4 Hz, 1H, Ar), 7.99 (d, J = 8.0 Hz, 1H, Ar), 7.93 (d, J = 7.2 Hz, 1H, Ar), 7.70–7.64 (m, 5H, Ar), 7.55–7.39 (m, 9H, Ar), 5.98 (s, 1H, SiH). 13C NMR (CDCl3, 100 MHz): δ 137.5, 137.0, 136.1, 133.4, 133.3, 131.5, 130.9, 130.0, 129.0, 128.4, 128.2, 126.3, 125.9, 125.4. HRMS (EI) m/z: [M]+ calcd for C22H18Si, 310.1178; found, 310.1178.
Reaction Using Silanes 1 and Aldehydes 2
To [Ph3C][B(C6F5)4] (101 mg, 0.11 mmol), an aldehyde (0.30 mmol), and DTBMP (31 mg, 0.15 mmol) in benzene (2 mL) was slowly added a benzene solution (1 mL) of silanes 1 (0.10 mmol) at room temperature under Ar atmosphere, and the resulting solution was stirred for 90 min. The reaction mixture was quenched with H2O and then the organic layer was extracted with Et2O. The combined organic layers were dried over Na2SO4. After filtration, the filtrate was concentrated under reduced pressure to remove the volatiles. The crude product was purified by silica gel column chromatography (eluent: hexane/ethyl acetate = 10/1). Further purification was carried out by Preparative thin-layer chromatography and/or GPLC to obtain each of the products.
1,1-Diisopropyl-3-phenyl-1-silabenzo[d,e]isochromane (3aa)
3aa (17.2 mg, 50%) was obtained as a white powder accompanied by 4aa (3.6 mg, 10%) as a colorless oil from the reaction using 1a (24.1 mg, 99.4 μmol) and benzaldehyde (2a). mp: 51–53 °C. 1H NMR (CDCl3, 400 MHz): δ 7.95 (dd, J = 8.0 Hz, J = 1.2 Hz, 1H, Ar), 7.77 (d, J = 8.4 Hz, 1H, Ar), 7.68 (dd, J = 6.4 Hz, J = 1.2 Hz, 1H, Ar), 7.54 (dd, J = 8.0 Hz, J = 6.4 Hz, 1H, Ar), 7.39–7.29 (m, 6H, Ar), 6.84 (dt, J = 7.2 Hz, J = 1.2 Hz, 1H, Ar), 6.39 (s, 1H, OCHAr), 1.37 (sept, J = 7.2 Hz, 1H, CH(CH3)2), 1.18 (sept, J = 7.2 Hz, 1H, CH(CH3)2), 1.115 (d, J = 7.6 Hz, 3H, CH(CH3)2), 1.108 (d, J = 7.6 Hz, 3H, CH(CH3)2), 0.99 (d, J = 7.2 Hz, 3H, CH(CH3)2), 0.90 (d, J = 7.2 Hz, 3H, CH(CH3)2). 13C NMR (CDCl3, 100 MHz): δ 144.7, 138.7, 136.1, 133.3, 131.8, 130.3, 129.5, 128.44, 128.43, 128.3, 127.8, 125.6, 125.08, 125.00, 78.3, 18.8, 17.6, 17.4, 17.2, 14.1, 13.2. HRMS (EI) m/z: [M]+ calcd for C23H26OSi, 346.1753; found, 346.1753.
Benzyloxydiisopropyl(1-naphthyl)silane (4aa)
1H NMR (CDCl3, 400 MHz): δ 8.32 (d, J = 8.0 Hz, 1H, Ar), 7.89 (d, J = 8.0 Hz, 1H, Ar), 7.85 (dd, J = 8.0 Hz, J = 1.6 Hz, 1H, Ar), 7.79 (dd, J = 6.8 Hz, J = 1.2 Hz, 1H, Ar), 7.50–7.34 (m, 7H, Ar), 7.29 (d, J = 7.2 Hz, 1H, Ar), 4.93 (s, 2H, CH2Ph), 1.56 (sept, J = 7.6 Hz, 2H overlapped by H2O, CH(CH3)2), 1.16 (d, J = 7.6 Hz, 6H, CH(CH3)2), 1.09 (d, J = 7.6 Hz, 6H, CH(CH3)2). 13C NMR (CDCl3, 100 MHz): δ 141.3, 137.9, 135.2, 133.6, 133.0, 130.3, 129.0, 128.9, 128.4, 127.0, 126.2, 125.9, 125.5, 125.0, 65.7, 18.2, 18.0, 13.7. HRMS (EI) m/z: [M]+ calcd for C23H28OSi, 348.1909; found, 348.1903.
1,1,3-Triphenyl-1-silabenzo[d,e]isochromane (3ca)
3ca (2.5 mg, 6.1%) was obtained as a white powder accompanied by 4ca (12.7 mg, 31%) as a colorless oil from the reaction using 1c (30.6 mg, 98.6 μmol) and benzaldehyde (2a). mp: 176–178 °C. 1H NMR (CDCl3, 400 MHz): δ 7.99 (dd, J = 8.0 Hz, J = 1.2 Hz, 1H, Ar), 7.82–7.77 (m, 2H, Ar), 7.73–7.69 (m, 2H, Ar), 7.59–7.53 (m, 3H, Ar), 7.45–7.42 (m, 1H, Ar), 7.40–7.25 (m, 11H overlapped by residual protonated CDCl3, Ar), 6.90 (dt, J = 7.2 Hz, J = 1.6 Hz, 1H, Ar), 6.46 (s, 1H, OCHAr). 13C NMR (CDCl3, 100 MHz): δ 143.3, 138.7, 136.0, 135.6, 135.5, 134.4, 134.0, 133.52, 133.46, 131.0, 130.5, 130.4, 128.8, 128.5, 128.44, 128.41, 128.2, 128.0, 127.8, 125.9, 125.4, 125.3, 78.1. HRMS (EI) m/z: [M]+ calcd for C29H22OSi, 414.1440; found, 414.1426.
Benzyloxydiphenyl(1-naphthyl)silane (4ca)
1H NMR (CDCl3, 400 MHz): δ 8.21 (d, J = 8.4 Hz, 1H, Ar), 7.96 (d, J = 8.0 Hz, 1H, Ar), 7.88 (d, J = 8.8 Hz, 1H, Ar), 7.80 (dd, J = 6.8 Hz, J = 1.2 Hz, 1H, Ar), 7.69–7.66 (m, 4H, Ar), 7.49–7.42 (m, 4H, Ar), 7.40–7.29 (m, 9H, Ar), 7.27–7.22 (m, 1H, Ar), 4.95 (s, 2H, OCH2Ar). 13C NMR (CDCl3, 100 MHz): δ 140.7, 137.6, 137.0, 135.6, 134.7, 133.6, 132.1, 131.3, 130.2, 129.3, 128.9, 128.4, 128.1, 127.2, 126.6, 126.2, 125.8, 125.2, 66.0. HRMS (EI) m/z: [M]+ calcd for C29H24OSi, 416.1596; found, 416.1596.
3,3-Diisopropyl-1-phenyl-3-silaisochromane (3da)
3da (2.5 mg, 8.0%) was obtained as a colorless oil accompanied by 4da (13.6 mg, 44%) as a colorless oil from the reaction using 1d (20.3 mg, 98.4 μmol) and benzaldehyde (2a). 1H NMR (CDCl3, 400 MHz): δ 7.38–7.28 (m, 5H, Ar), 7.21–7.19 (m, 2H, Ar), 7.06–7.02 (m, 1H, Ar), 6.70 (d, J = 8.0 Hz, 1H, Ar), 5.94 (s, 1H, OCHAr), 2.15 (d, J = 15.6 Hz, 1H, SiCH2Ar), 2.04 (d, J = 15.2 Hz, 1H, SiCH2Ar), 1.05–1.01 (m, 1H, CH(CH3)2), 0.98–0.94 (m, 6H, CH(CH3)2), 0.91–0.83 (m, 7H, CH(CH3)2). 13C NMR (CDCl3, 100 MHz): δ 142.9, 141.3, 137.1, 130.8, 128.3, 128.0, 127.6, 127.5, 127.4, 124.8, 77.9, 17.6, 17.4, 17.3, 17.2, 14.6, 13.3, 13.2. HRMS (EI) m/z: [M]+ calcd for C20H26OSi, 310.1753; found, 310.1753.
Benzyl(benzyloxy)diisopropylsilane (4da)
1H NMR (CDCl3, 400 MHz): δ 7.36–7.29 (m, 4H, Ar), 7.27–7.24 (m, 1H overlapped by residual protonated CDCl3, Ar), 7.21–7.17 (m, 2H, Ar), 7.15–7.12 (m, 2H, Ar), 7.07 (tt, J = 7.2 Hz, J = 1.6 Hz, 1H, Ar), 4.75 (s, 2H, OCH2Ph), 2.30 (s, 2H, SiCH2Ph), 1.15–1.07 (m, 2H, CH(CH3)2), 1.05–1.01 (m, 12H, CH(CH3)2). 13C NMR (CDCl3, 100 MHz): δ 141.4, 139.5, 128.9, 128.4, 128.3, 127.0, 126.0, 124.3, 65.2, 20.8, 17.71, 17.68, 12.5. HRMS (EI) m/z: [M]+ calcd for C20H28OSi, 312.1909; found, 312.1909.
1,1-Diisopropyl-3-(4-methoxyphenyl)-1-silabenzo[d,e]isochromane (3ab)
3ab (4.7 mg, 13%) was obtained as a white powder accompanied by 4ab (1.7 mg, 4.5%) as a white powder from the reaction using 1a (24.2 mg, 99.8 μmol) and p-anisaldehyde (2b). mp: 125–126 °C. 1H NMR (CDCl3, 400 MHz): δ 7.94 (dd, J = 8.0 Hz, J = 1.2 Hz, 1H, Ar), 7.76 (d, J = 8.0 Hz, 1H, Ar), 7.66 (dd, J = 6.8 Hz, J = 1.6 Hz, 1H, Ar), 7.52 (dd, J = 8.4 Hz, J = 6.8 Hz, 1H, Ar), 7.31 (dd, J = 8.0 Hz, J = 6.8 Hz, 1H, Ar), 7.25–7.21 (m, 2H, Ar), 6.90–6.84 (m, 3H, Ar), 6.35 (s, 1H, OCHAr), 3.82 (s, 3H, OCH3), 1.35 (sept, J = 8.0 Hz, 1H, CH(CH3)2), 1.16 (sept, J = 7.6 Hz, 1H, CH(CH3)2), 1.095 (d, J = 7.6 Hz, 3H, CH(CH3)2), 1.087 (d, J = 7.2 Hz, 3H, CH(CH3)2), 0.98 (d, J = 7.2 Hz, 3H, CH(CH3)2), 0.89 (d, J = 7.6 Hz, 3H, CH(CH3)2). 13C NMR (CDCl3, 100 MHz): δ 159.2, 139.0, 137.2, 136.1, 133.3, 131.7, 130.3, 129.6, 128.2, 125.6, 125.1, 125.0, 113.8, 77.9, 55.4, 18.1, 17.6, 17.5, 17.2, 14.1, 13.2. HRMS (EI) m/z: [M]+ calcd for C24H28O2Si, 376.1859; found, 376.1858.
Diisopropyl((4-methoxyphenyl)methoxy)(1-naphthyl)silane (4ab)
mp: 76–77 °C. 1H NMR (CDCl3, 400 MHz): δ 8.32 (d, J = 8.4 Hz, 1H, Ar), 7.88 (d, J = 8.4 Hz, 1H, Ar), 7.85 (dd, J = 7.6 Hz, J = 1.6 Hz, 1H, Ar), 7.78 (dd, J = 6.8 Hz, J = 1.2 Hz, 1H, Ar), 7.49–7.38 (m, 3H, Ar), 7.32 (d, J = 8.4 Hz, 2H, Ar), 6.90 (d, J = 8.4 Hz, 2H, Ar), 4.85 (s, 2H, OCH2Ar), 3.82 (s, 3H, OCH3), 1.54 (sept, J = 7.6 Hz, 2H overlapped by H2O, CH(CH3)2), 1.14 (d, J = 7.2 Hz, 6H, CH(CH3)2), 1.08 (d, J = 7.6 Hz, 6H, CH(CH3)2). 13C NMR (CDCl3, 100 MHz): δ 158.9, 137.9, 135.7, 135.2, 133.6, 133.1, 130.3, 129.0, 128.9, 127.7, 125.9, 125.5, 125.0, 113.8, 65.5, 55.4, 18.2, 18.0, 13.7. HRMS (EI) m/z: [M]+ calcd for C24H30O2Si, 378.2015; found, 378.2015.
1,1-Diisopropyl-3-(4-methylphenyl)-1-silabenzo[d,e]isochromane (3ac)
3ac (11.5 mg, 32%) was obtained as a white powder accompanied by 4ac (5.0 mg, 14%) as a colorless oil from the reaction using 1a (24.2 mg, 99.8 μmol) and p-methylbenzaldehyde (2c). mp: 134–135 °C. 1H NMR (CDCl3, 400 MHz): δ 7.94 (dd, J = 8.0 Hz, J = 1.2 Hz, 1H, Ar), 7.76 (d, J = 8.0 Hz, 1H, Ar), 7.67 (dd, J = 6.8 Hz, J = 1.6 Hz, 1H, Ar), 7.53 (dd, J = 8.0 Hz, J = 6.8 Hz, 1H, Ar), 7.30 (dd, J = 8.0 Hz, J = 7.2 Hz, 1H, Ar), 7.22 (d, J = 8.0 Hz, 2H, Ar), 7.17 (d, J = 7.6 Hz, 2H, Ar), 6.85 (dt, J = 7.2 Hz, J = 1.2 Hz, 1H, Ar), 6.35 (s, 1H, OCHAr), 2.37 (s, 3H, ArCH3), 1.36 (sept, J = 7.6 Hz, 1H, CH(CH3)2), 1.18 (sept, J = 7.2 Hz, 1H, CH(CH3)2), 1.106 (d, J = 7.6 Hz, 3H, CH(CH3)2), 1.100 (d, J = 7.2 Hz, 3H, CH(CH3)2), 0.99 (d, J = 7.2 Hz, 3H, CH(CH3)2), 0.90 (d, J = 7.6 Hz, 3H, CH(CH3)2). 13C NMR (CDCl3, 100 MHz): δ 141.8, 138.9, 137.5, 136.1, 133.3, 131.7, 130.3, 129.6, 129.1, 128.4, 128.2, 125.6, 125.1, 125.0, 78.1, 21.4, 18.1, 17.6, 17.5, 17.3, 14.1, 13.2. HRMS (EI) m/z: [M]+ calcd for C24H28OSi, 360.1909; found, 360.1913.
Diisopropyl((4-methylphenyl)methoxy)(1-naphthyl)silane (4ac)
1H NMR (CDCl3, 400 MHz): δ 8.33 (d, J = 8.4 Hz, 1H, Ar), 7.89 (d, J = 8.4 Hz, 1H, Ar), 7.85 (dd, J = 7.6 Hz, J = 1.6 Hz, 1H, Ar), 7.78 (dd, J = 6.8 Hz, J = 1.2 Hz, 1H, Ar), 7.49–7.38 (m, 3H, Ar), 7.30 (d, J = 8.4 Hz, 2H, Ar), 7.17 (d, J = 7.2 Hz, 2H, Ar), 4.89 (s, 2H, OCH2Ar), 1.55 (sept, J = 7.6 Hz, 2H overlapped by H2O, CH(CH3)2), 1.15 (d, J = 7.2 Hz, 6H, CH(CH3)2), 1.08 (d, J = 7.6 Hz, 6H, CH(CH3)2). 13C NMR (CDCl3, 100 MHz): δ 138.3, 137.9, 136.6, 135.2, 133.6, 133.1, 130.2, 129.06, 129.02, 128.9, 126.3, 125.9, 125.5, 125.0, 65.7, 21.3, 18.2, 18.0, 13.7. HRMS (EI) m/z: [M]+ calcd for C24H30OSi, 362.2066; found, 362.2066.
3-(4-Chlorophenyl)-1,1-diisopropyl-1-silabenzo[d,e]isochromane (3ad)
3ad (19.1 mg, 50%) was obtained as a white powder accompanied by 4ad (5.1 mg, 13%) as a colorless oil from the reaction using 1a (24.3 mg, 0.100 mmol) and p-chlorobenzaldehyde (2d). mp: 144–146 °C. 1H NMR (CDCl3, 400 MHz): δ 7.95 (dd, J = 8.4 Hz, J = 1.6 Hz, 1H, Ar), 7.78 (d, J = 8.4 Hz, 1H, Ar), 7.67 (dd, J = 6.8 Hz, J = 1.6 Hz, 1H, Ar), 7.54 (dd, J = 8.4 Hz, J = 6.8 Hz, 1H, Ar), 7.35–7.29 (m, 3H, Ar), 7.28–7.25 (m, 2H, Ar), 6.81 (dt, J = 7.2 Hz, J = 1.2 Hz, 1H, Ar), 6.36 (s, 1H, OCHAr), 1.36 (sept, J = 7.6 Hz, 1H, CH(CH3)2), 1.18 (sept, J = 7.6 Hz, 1H, CH(CH3)2), 1.102 (d, J = 7.6 Hz, 3H, CH(CH3)2), 1.094 (d, J = 7.2 Hz, 3H, CH(CH3)2), 0.99 (d, J = 7.2 Hz, 3H, CH(CH3)2), 0.90 (d, J = 7.6 Hz, 3H, CH(CH3)2). 13C NMR (CDCl3, 100 MHz): δ 143.3, 138.1, 136.0, 133.6, 133.4, 131.9, 130.4, 129.8, 129.3, 128.6, 128.5, 125.5, 125.11, 125.05, 77.6, 18.0, 17.51, 17.46, 17.2, 14.1, 13.2. HRMS (EI) m/z: [M]+ calcd for C23H25ClOSi, 380.1363; found, 380.1364.
(4-Chlorophenylmethoxy)diisopropyl(1-naphthyl)silane (4ad)
1H NMR (CDCl3, 400 MHz): δ 8.27 (d, J = 8.4 Hz, 1H, Ar), 7.90 (d, J = 8.4 Hz, 1H, Ar), 7.85 (dd, J = 8.0 Hz, J = 1.6 Hz, 1H, Ar), 7.76 (dd, J = 6.8 Hz, J = 1.2 Hz, 1H, Ar), 7.50–7.37 (m, 3H, Ar), 7.33 (s, 4H, Ar), 4.87 (s, 2H, OCH2Ar), 1.55 (sept, J = 7.6 Hz, 2H overlapped by H2O, CH(CH3)2), 1.15 (d, J = 7.6 Hz, 6H, CH(CH3)2), 1.08 (d, J = 7.6 Hz, 6H, CH(CH3)2). 13C NMR (CDCl3, 100 MHz): δ 139.8, 137.9, 135.2, 133.6, 132.8, 130.4, 129.0, 128.8, 128.5, 127.5, 126.0, 125.6, 125.1, 65.1, 18.1, 18.0, 13.7. HRMS (EI) m/z: [M]+ calcd for C23H27ClOSi, 382.1520; found, 382.1519.
3-(4-Cyanophenyl)-1,1-diisopropyl-1-silabenzo[d,e]isochromane (3ae)
3ae (23.4 mg, 63%) was obtained as a white powder from the reaction using 1a (24.1 mg, 99.4 μmol) and 4-formylbenzonitrile (2e). Mp: 146–147 °C. 1H NMR (CDCl3, 400 MHz): δ 7.96 (dd, J = 8.4 Hz, J = 1.6 Hz, 1H, Ar), 7.80 (d, J = 8.0 Hz, 1H, Ar), 7.70–7.64 (m, 3H, Ar), 7.56 (dd, J = 8.0 Hz, J = 6.4 Hz, 1H, Ar), 7.46–7.43 (m, 2H, Ar), 7.32 (dd, J = 8.0 Hz, J = 7.2 Hz, 1H, Ar), 6.75 (dt, J = 7.2 Hz, J = 1.2 Hz, 1H, Ar), 6.42 (s, 1H, OCHAr), 1.37 (sept, J = 7.6 Hz, 1H, CH(CH3)2), 1.18 (sept, J = 7.6 Hz, 1H, CH(CH3)2), 1.098 (d, J = 7.6 Hz, 3H, CH(CH3)2), 1.090 (d, J = 7.6 Hz, 3H, CH(CH3)2), 0.99 (d, J = 7.2 Hz, 3H, CH(CH3)2), 0.89 (d, J = 7.6 Hz, 3H, CH(CH3)2). 13C NMR (CDCl3, 100 MHz): δ 150.0, 137.1, 135.8, 133.4, 132.3, 130.5, 129.1, 129.0, 128.8, 125.4, 125.3, 125.0, 119.0, 111.7, 77.6, 18.0, 17.5, 17.4, 17.2, 14.0, 13.1. HRMS (EI) m/z: [M]+ calcd for C24H25NOSi, 371.1705; found, 371.1705.
1,1-Diisopropyl-3-(4-nitrophenyl)-1-silabenzo[d,e]isochromane (3af)
3af (19.5 mg, 51%) was obtained as a white powder from the reaction using 1a (24.0 mg, 99.0 μmol) and 4-nitrobenzaldehyde (2f). mp: 130–132 °C. 1H NMR (CDCl3, 400 MHz): δ 8.25–8.21 (m, 2H, Ar), 7.96 (dd, J = 8.4 Hz, J = 1.6 Hz, 1H, Ar), 7.81 (d, J = 8.8 Hz, 1H, Ar), 7.69 (dd, J = 6.8 Hz, J = 1.6 Hz, 1H, Ar), 7.56 (dd, J = 8.0 Hz, J = 6.4 Hz, 1H, Ar), 7.53–7.49 (m, 2H, Ar), 7.32 (dd, J = 8.4 Hz, J = 7.2 Hz, 1H, Ar), 6.76 (dt, J = 7.2 Hz, J = 1.2 Hz, 1H, Ar), 6.47 (s, 1H, OCHAr), 1.38 (sept, J = 7.6 Hz, 1H, CH(CH3)2), 1.19 (sept, J = 7.6 Hz, 1H, CH(CH3)2), 1.103 (d, J = 7.6 Hz, 3H, CH(CH3)2), 1.095 (d, J = 7.2 Hz, 3H, CH(CH3)2), 0.99 (d, J = 7.6 Hz, 3H, CH(CH3)2), 0.90 (d, J = 7.2 Hz, 3H, CH(CH3)2). 13C NMR (CDCl3, 100 MHz): δ 151.8, 147.6, 137.0, 135.8, 133.5, 132.1, 130.5, 129.3, 128.91, 128.87, 125.36, 125.33, 125.0, 123.8, 77.3, 17.98, 17.46, 17.2, 14.0, 13.1. HRMS (EI) m/z: [M]+ calcd for C23H25NO3Si, 391.1604; found, 391.1603.
1,1-Diisopropyl-3-(2-methylphenyl)-1-silabenzo[d,e]isochromane (3ag)
3ag (18.5 mg, 52%) was obtained as a colorless oil accompanied by 4ag (3.6 mg, 10%) as a white powder from the reaction using 1a (24.0 mg, 99.0 μmol) and o-methylbenzaldehyde (2g). 1H NMR (CDCl3, 400 MHz): δ 7.95 (dd, J = 8.4 Hz, J = 1.6 Hz, 1H, Ar), 7.77 (d, J = 8.0 Hz, 1H, Ar), 7.69 (dd, J = 6.4 Hz, J = 1.2 Hz, 1H, Ar), 7.55 (dd, J = 8.0 Hz, J = 6.4 Hz, 1H, Ar), 7.29 (dd, J = 8.4 Hz, J = 7.2 Hz, 1H, Ar), 7.26–7.24 (m, 2H, Ar), 7.19–7.16 (m, 2H, Ar), 6.76 (dt, J = 7.2 Hz, J = 1.2 Hz, 1H, Ar), 6.56 (s, 1H, OCHAr), 2.38 (s, 3H, ArCH3), 1.41 (sept, J = 7.2 Hz, 1H, CH(CH3)2), 1.19 (sept, J = 7.6 Hz, 1H, CH(CH3)2), 1.165 (d, J = 7.2 Hz, 3H, CH(CH3)2), 1.158 (d, J = 7.6 Hz, 3H, CH(CH3)2), 1.00 (d, J = 7.6 Hz, 3H, CH(CH3)2), 0.90 (d, J = 7.6 Hz, 3H, CH(CH3)2). 13C NMR (CDCl3, 100 MHz): δ 142.2, 138.3, 137.0, 136.4, 133.4, 131.8, 130.7, 130.3, 129.8, 129.0, 128.3, 127.8, 126.2, 125.2, 125.0, 124.7, 19.8, 18.3, 17.7, 17.6, 17.3, 14.2, 13.2. HRMS (EI) m/z: [M]+ calcd for C24H28OSi, 360.1909; found, 360.1912.
Diisopropyl((2-methylphenyl)methoxy)(1-naphthyl)silane (4ag)
mp: 44–46 °C. 1H NMR (CDCl3, 400 MHz): δ 8.32 (d, J = 8.4 Hz, 1H, Ar), 7.90 (d, J = 8.4 Hz, 1H, Ar), 7.85 (dd, J = 8.0 Hz, J = 1.6 Hz, 1H, Ar), 7.79 (dd, J = 6.8 Hz, J = 1.2 Hz, 1H, Ar), 7.60 (d, J = 8.0 Hz, 1H, Ar), 7.50–7.37 (m, 3H, Ar), 7.28–7.23 (m, 1H overlapped by residual protonated CDCl3, Ar), 7.21 (dt, J = 7.6 Hz, J = 1.6 Hz, 1H, Ar), 7.15 (d, J = 6.8 Hz, 1H, Ar), 4.90 (s, 2H, OCH2Ar), 2.24 (s, 3H, ArCH3), 1.57 (sept, J = 7.6 Hz, 2H overlapped by H2O, CH(CH3)2), 1.17 (d, J = 7.2 Hz, 6H, CH(CH3)2), 1.10 (d, J = 7.2 Hz, 6H, CH(CH3)2). 13C NMR (CDCl3, 100 MHz): δ 139.1, 138.0, 135.14, 135.11, 133.6, 133.1, 130.3, 129.9, 129.0, 128.9, 127.0, 126.6, 126.0, 125.9, 125.5, 125.1, 64.0, 18.8, 18.2, 18.1, 13.8. HRMS (EI) m/z: [M]+ calcd for C24H30OSi, 362.2066; found, 362.2066.
1,1-Diisopropyl-3-(3-methylphenyl)-1-silabenzo[d,e]isochromane (3ah)
3ah (14.4 mg, 40%) was obtained as a white powder accompanied by 4ah (5.0 mg, 14%) as a white powder from the reaction using 1a (24.3 mg, 0.100 mmol) and m-methylbenzaldehyde (2h). mp: 39–40 °C. 1H NMR (CDCl3, 400 MHz): δ 7.94 (dd, J = 8.4 Hz, J = 1.2 Hz, 1H, Ar), 7.76 (d, J = 8.0 Hz, 1H, Ar), 7.68 (dd, J = 6.8 Hz, J = 1.6 Hz, 1H, Ar), 7.53 (dd, J = 8.4 Hz, J = 6.8 Hz, 1H, Ar), 7.30 (dd, J = 8.4 Hz, J = 7.2 Hz, 1H, Ar), 7.25 (t, J = 7.6 Hz, 1H, Ar), 7.21 (s, 1H, Ar), 7.14 (d, J = 7.2 Hz, 1H, Ar), 7.10 (d, J = 7.2 Hz, 1H, Ar), 6.83 (dt, J = 7.2 Hz, J = 1.2 Hz, 1H, Ar), 6.35 (s, 1H, OCHAr), 2.36 (s, 3H, ArCH3), 1.37 (sept, J = 7.6 Hz, 1H, CH(CH3)2), 1.19 (sept, J = 7.6 Hz, 1H, CH(CH3)2), 1.120 (d, J = 7.6 Hz, 3H, CH(CH3)2), 1.116 (d, J = 7.2 Hz, 3H, CH(CH3)2), 0.99 (d, J = 7.6 Hz, 3H, CH(CH3)2), 0.92 (d, J = 7.2 Hz, 3H, CH(CH3)2). 13C NMR (CDCl3, 100 MHz): δ 144.6, 138.9, 138.0, 136.1, 133.3, 131.7, 130.3, 129.5, 129.2, 128.6, 128.3, 128.2, 125.6, 125.5, 125.1, 125.0, 78.3, 21.6, 18.1, 17.6, 17.5, 17.3, 14.2, 13.2. HRMS (EI) m/z: [M]+ calcd for C24H28OSi, 360.1909; found, 360.1909.
Diisopropyl((3-methylphenyl)methoxy)(1-naphthyl)silane (4ah)
mp: 39–40 °C. 1H NMR (CDCl3, 400 MHz): δ 8.34 (d, J = 8.4 Hz, 1H, Ar), 7.89 (d, J = 8.0 Hz, 1H, Ar), 7.85 (dd, J = 8.0 Hz, J = 1.6 Hz, 1H, Ar), 7.79 (dd, J = 7.2 Hz, J = 1.6 Hz, 1H, Ar), 7.50–7.38 (m, 3H, Ar), 7.28–7.21 (m, 3H overlapped by residual protonated CDCl3, Ar), 7.09 (d, J = 7.6 Hz, 1H, Ar), 4.90 (s, 2H, OCH2Ar), 2.37 (s, 3H, ArCH3), 1.56 (sept, J = 7.2 Hz, 2H overlapped by H2O, CH(CH3)2), 1.16 (d, J = 7.6 Hz, 6H, CH(CH3)2), 1.09 (d, J = 7.6 Hz, 6H, CH(CH3)2). 13C NMR (CDCl3, 100 MHz): δ 141.2, 137.9, 135.2, 133.6, 133.1, 130.3, 129.1, 128.9, 128.3, 127.8, 127.0, 125.9, 125.5, 125.0, 123.3, 65.8, 21.6, 18.1, 18.0, 13.7. HRMS (EI) m/z: [M]+ calcd for C24H30OSi, 362.2066; found, 362.2067.
1,1-Diisopropyl-3-(1-naphthyl)-1-silabenzo[d,e]isochromane (3ai)
3ai (20.8 mg, 53%) was obtained as a colorless oil accompanied by 4ai (9.1 mg, 23%) as a colorless oil from the reaction using 1a (24.0 mg, 99.0 μmol) and 1-naphthaldehyde (2i). 1H NMR (C6D6, 373 K, 400 MHz): δ 8.42–8.38 (m, 1H, Ar), 7.77 (d, J = 8.0 Hz, 1H, Ar), 7.70–7.56 (m, 4H, Ar), 7.37 (dd, J = 8.0 Hz, J = 6.4 Hz, 1H, Ar), 7.28–7.24 (m, 2H, Ar), 7.13–7.09 (m, 3H, Ar), 7.01 (t, J = 7.6 Hz, 1H, Ar), 6.87 (d, J = 7.2 Hz, 1H, Ar), 1.33 (sept, J = 7.6 Hz, 1H, CH(CH3)2), 1.16 (d, J = 7.2 Hz, 3H, CH(CH3)2), 1.12 (d, J = 7.2 Hz, 3H, CH(CH3)2), 1.04 (sept, J = 7.2 Hz, 1H, CH(CH3)2), 0.95 (d, J = 6.8 Hz, 3H, CH(CH3)2), 0.82 (d, J = 7.2 Hz, 3H, CH(CH3)2). 13C NMR (C6D6, 373 K, 100 MHz): δ 140.8, 138.8, 137.1, 135.0, 134.2, 132.8, 132.1, 130.7, 130.5, 128.92, 128.89, 128.6, 127.0, 126.4, 126.1, 126.0, 125.8, 125.5, 125.4, 125.2, 18.2, 17.6, 17.3, 14.5, 13.8. HRMS (EI) m/z: [M]+ calcd for C27H28OSi, 396.1909; found, 396.1909.
Diisopropyl((1-naphthyl)methoxy)(1-naphthyl)silane (4ai)
1H NMR (CDCl3, 400 MHz): δ 8.35 (d, J = 8.4 Hz, 1H, Ar), 7.97–7.94 (m, 1H, Ar), 7.91–7.79 (m, 5H), 7.75 (dd, J = 6.8 Hz, J = 1.2 Hz, 1H, Ar), 7.53–7.42 (m, 5H, Ar), 7.38–7.33 (m, 1H, Ar), 5.40 (s, 2H, OCH2Ar), 1.62 (sept, J = 7.6 Hz, 2H, CH(CH3)2), 1.19 (d, J = 7.6 Hz, 6H, CH(CH3)2), 1.13 (d, J = 7.6 Hz, 6H, CH(CH3)2). 13C NMR (CDCl3, 100 MHz): δ 138.0, 136.5, 135.2, 133.64, 133.61, 133.1, 130.8, 130.3, 129.0, 128.9, 128.7, 127.7, 125.99, 125.96, 125.70, 125.67, 125.5, 125.1, 124.0, 123.4, 64.2, 18.2, 18.1, 13.8. HRMS (EI) m/z: [M]+ calcd for C27H30OSi, 398.2066; found, 398.2066.
1,1-Diisopropyl-3-(2-naphthyl)-1-silabenzo[d,e]isochromane (3aj)
3aj (15.8 mg, 40%) was obtained as a white powder accompanied by 4aj (6.5 mg, 16%) as a colorless oil from the reaction using 1a (24.1 mg, 99.4 μmol) and 2-naphthaldehyde (2j). mp: 150–152 °C. 1H NMR (CDCl3, 400 MHz): δ 7.96 (dd, J = 8.4 Hz, J = 1.2 Hz, 1H, Ar), 7.87–7.80 (m, 3H, Ar), 7.78 (d, J = 8.0 Hz, 1H, Ar), 7.75 (s, 1H, Ar), 7.70 (dd, J = 7.4 Hz, J = 1.2 Hz, 1H, Ar), 7.58–7.53 (m, 2H, Ar), 7.51–7.46 (m, 2H, Ar), 7.28 (dd, J = 8.4 Hz, J = 7.6 Hz, 1H overlapped by residual protonated CDCl3, Ar), 6.84 (dt, J = 7.6 Hz, J = 1.6 Hz, 1H, Ar), 6.42 (s, 1H, OCHAr), 1.41 (sept, J = 7.6 Hz, 1H, CH(CH3)2), 1.22 (sept, J = 7.6 Hz, 1H, CH(CH3)2), 1.15 (d, J = 7.6 Hz, 6H, CH(CH3)2), 1.01 (d, J = 7.2 Hz, 3H, CH(CH3)2), 0.94 (d, J = 7.6 Hz, 3H, CH(CH3)2). 13C NMR (CDCl3, 100 MHz): δ 142.0, 138.6, 136.1, 133.4, 133.25, 133.24, 131.8, 130.4, 129.4, 128.5, 128.3, 128.2, 127.9, 127.2, 126.5, 126.2, 126.1, 125.8, 125.1, 125.0, 78.5, 18.1, 17.59, 17.52, 17.3, 14.2, 13.2. HRMS (EI) m/z: [M]+ calcd for C27H28OSi, 396.1909; found, 396.1909.
Diisopropyl((2-naphthyl)methoxy)(1-naphthyl)silane (4aj)
1H NMR (CDCl3, 400 MHz): δ 8.37 (d, J = 8.4 Hz, 1H, Ar), 7.92–7.80 (m, 7H, Ar), 7.52–7.43 (m, 5H, Ar), 7.41–7.36 (m, 1H, Ar), 5.09 (s, 2H, OCH2Ar), 1.60 (sept, J = 7.6 Hz, 2H, CH(CH3)2), 1.19 (d, J = 7.6 Hz, 6H, CH(CH3)2), 1.12 (d, J = 7.6 Hz, 6H, CH(CH3)2). 13C NMR (CDCl3, 100 MHz): δ 138.8, 137.9, 135.2, 133.62, 133.57, 133.0, 132.9, 130.3, 129.0, 128.9, 128.1, 128.0, 127.8, 126.1, 126.0, 125.7, 125.6, 125.1, 124.8, 124.6, 66.0, 18.2, 18.1, 13.8. HRMS (EI) m/z: [M]+ calcd for C27H30OSi, 398.2066; found, 398.2066.
1,1-Diisopropyl-3-thiophenyl-1-silabenzo[d,e]isochromane (3ak)
3ak (4.2 mg, 12%) was obtained as a white powder from the reaction using 1a (23.9 mg, 98.6 μmol) and 3-thiophenecarbaldehyde (2k). mp: 50–51 °C. 1H NMR (CDCl3, 400 MHz): δ 7.94 (dd, J = 8.0 Hz, J = 1.2 Hz, 1H, Ar), 7.79 (d, J = 8.0 Hz, 1H, Ar), 7.63 (dd, J = 6.4 Hz, J = 1.2 Hz, 1H, Ar), 7.52 (dd, J = 8.0 Hz, J = 6.4 Hz, 1H, Ar), 7.38 (dd, J = 8.0 Hz, J = 7.2 Hz, 1H, Ar), 7.27 (dd, J = 4.8 Hz, J = 2.8 Hz, 1H, Ar), 7.12 (dd, J = 4.8 Hz, J = 1.2 Hz, 1H, Ar), 7.07 (dt, J = 7.2 Hz, J = 1.2 Hz, 1H, Ar), 6.79 (dt, J = 2.8 Hz, J = 1.2 Hz, 1H, Ar), 6.49 (s, 1H, OCHAr), 1.30 (sept, J = 7.2 Hz, 1H, CH(CH3)2), 1.07 (sept, J = 7.2 Hz, 1H, CH(CH3)2), 1.04 (d, J = 7.6 Hz, 3H, CH(CH3)2), 1.01 (d, J = 7.6 Hz, 3H, CH(CH3)2), 0.98 (d, J = 6.8 Hz, 3H, CH(CH3)2), 0.83 (d, J = 7.2 Hz, 3H, CH(CH3)2). 13C NMR (CDCl3, 100 MHz): δ 137.8, 135.7, 133.4, 131.7, 130.2, 129.4, 128.4, 128.0, 125.9, 125.1, 125.0, 123.1, 74.3, 17.9, 17.5, 17.3, 17.1, 13.9, 13.3. HRMS (EI) m/z: [M]+ Calcd for C21H24OSSi, 352.1317; found, 352.1313.
3-n-Butyl-1,1-diisopropyl-1-silabenzo[d,e]isochromane (3al)
3al (7.2 mg, 22%) was obtained as a colorless oil from the reaction using 1a (24.0 mg, 99.0 μmol) and pentanal (2l). 1H NMR (CDCl3, 400 MHz): δ 7.89 (dd, J = 8.4 Hz, J = 1.6 Hz, 1H, Ar), 7.73 (d, J = 8.0 Hz, 1H, Ar), 7.59 (dd, J = 6.8 Hz, J = 1.6 Hz, 1H, Ar), 7.48 (dd, J = 8.4 Hz, J = 6.8 Hz, 1H, Ar), 7.41 (dd, J = 8.4 Hz, J = 7.2 Hz, 1H, Ar), 7.26–7.23 (m, 1H overlapped by residual protonated CDCl3, Ar), 5.29 (dd, J = 8.8 Hz, J = 4.4 Hz, 1H, OCHAr), 1.88–1.73 (m, 2H, n-Bu), 1.62–1.58 (m, 1H overlapped by H2O, n-Bu), 1.48–1.16 (m, 5H, n-Bu and i-Pr), 1.19 (d, J = 7.2 Hz, 3H, CH(CH3)2), 1.13 (d, J = 7.2 Hz, 3H, CH(CH3)2), 0.92 (d, J = 7.6 Hz, 3H, CH(CH3)2), 0.90 (t, J = 7.2 Hz, 3H, n-Bu), 0.84 (d, J = 7.2 Hz, 3H, CH(CH3)2). 13C NMR (CDCl3, 100 MHz): δ 140.0, 135.2, 133.4, 131.4, 130.1, 129.0, 127.6, 125.1, 124.9, 123.3, 76.8, 41.4, 28.6, 22.7, 17.8, 17.6, 17.31, 17.27, 14.3, 13.9, 13.5. HRMS (EI) m/z: [M]+ calcd for C21H30OSi, 326.2066; found, 326.2064.
3-sec-Butyl-1,1-diisopropyl-1-silabenzo[d,e]isochromane (3am)
3am (10.2 mg, 31%) was obtained with d.r. = 2.8:1 as a colorless oil accompanied by 5am (4.7 mg, 14%) with d.r. = 1.6:1 as a colorless oil from the reaction using 1a (24.2 mg, 99.8 μmol) and 3-methylbutanal (2m). HRMS (EI) m/z: [M]+ calcd for C21H30OSi, 326.2066; found, 326.2066. Major diastereomer: 1H NMR (CDCl3, 400 MHz): δ 7.89 (dd, J = 8.0 Hz, J = 1.6 Hz, 1H, Ar), 7.73 (d, J = 8.0 Hz, 1H, Ar), 7.58 (dd, J = 6.8 Hz, J = 1.6 Hz, 1H, Ar), 7.49–7.39 (m, 2H, Ar), 7.29 (dt, J = 7.2 Hz, J = 1.2 Hz, 1H, Ar), 5.34 (d, J = 3.6 Hz, 1H, OCHAr), 2.04–1.98 (m, 1H, sec-Bu), 1.62–1.51 (m, 1H overlapped by H2O, sec-Bu), 1.45–1.25 (m, 3H, sec-Bu and i-Pr), 1.15 (d, J = 7.6 Hz, 3H, i-Pr), 1.10 (d, J = 7.2 Hz, 3H, i-Pr), 0.97 (t, J = 7.2 Hz, 3H, sec-Bu), 0.94 (d, J = 7.6 Hz, 3H, i-Pr), 0.91 (d, J = 7.6 Hz, 3H, i-Pr), 0.82 (d, J = 6.4 Hz, 3H, sec-Bu). 13C NMR (CDCl3, 100 MHz): δ 138.8, 136.6, 133.42, 131.2, 130.2, 129.49, 127.6, 125.0, 124.7, 123.3, 78.1, 42.6, 27.2, 17.93, 17.88, 17.43, 17.39, 14.3, 13.6, 13.1, 12.3. Minor diastereomer: 1H NMR (CDCl3, 400 MHz): δ 7.89 (dd, J = 8.0 Hz, J = 1.6 Hz, 1H, Ar), 7.73 (d, J = 8.0 Hz, 1H, Ar), 7.58 (dd, J = 6.8 Hz, J = 1.6 Hz, 1H, Ar), 7.49–7.39 (m, 2H, Ar), 7.26–7.23 (m, 1H overlapped by residual protonated CDCl3, Ar), 5.12 (d, J = 5.2 Hz, 1H, OCHAr), 1.92–1.85 (m, 1H, sec-Bu), 1.62–1.51 (m, 1H overlapped by H2O, sec-Bu), 1.40–1.18 (m, 3H, sec-Bu and i-Pr), 1.21 (d, J = 7.2 Hz, 3H, i-Pr), 1.14 (d, J = 7.2 Hz, 3H, i-Pr), 0.89 (d, J = 7.2 Hz, 3H, i-Pr), 0.85 (t, J = 7.6 Hz, 3H, sec-Bu), 0.85 (d, J = 6.8 Hz, 3H, sec-Bu), 0.80 (d, J = 7.6 Hz, 3H, i-Pr). 13C NMR (CDCl3, 100 MHz): δ 138.4, 136.2, 133.38, 131.3, 130.1, 129.40, 127.7, 124.85, 124.79, 123.8, 80.9, 43.1, 23.8, 17.9, 17.7, 17.43, 17.33, 16.8, 14.2, 13.3, 11.9.
1-(Diisopropyl(1-naphthyl)silyloxy)-3-methyl-1-butene (5am)
HRMS (EI) m/z: [M]+ calcd for C21H30OSi, 326.2066; found, 326.2065. Major diastereomer: 1H NMR (CDCl3, 400 MHz): δ 8.21–8.18 (m, 1H, Ar), 7.89 (d, J = 8.4 Hz, 1H, Ar), 7.86–7.83 (m, 1H, Ar), 7.79–7.75 (m, 1H, Ar), 7.50–7.44 (m, 3H, Ar), 6.24–6.22 (m, 1H, C=CH), 1.89 (q, J = 7.2 Hz, 2H, CH2CH3), 1.75 (d, J = 1.2 Hz, 3H, C=CCH3), 1.56–1.47 (m, 2H, i-Pr), 1.132 (d, J = 7.2 Hz, 6H, i-Pr), 1.041 (d, J = 7.2 Hz, 6H, i-Pr), 0.95 (t, J = 7.6 Hz, 3H, CH2CH3). 13C NMR (CDCl3, 100 MHz): δ 137.7, 135.13, 134.0, 133.6, 132.8, 130.4, 129.0, 128.9, 125.89, 125.5, 125.0, 118.9, 27.0, 17.91, 17.7, 13.82, 13.4, 12.8. Minor diastereomer: 1H NMR (CDCl3, 400 MHz): δ 8.21–8.18 (m, 1H, Ar), 7.89 (d, J = 8.4 Hz, 1H, Ar), 7.86–7.83 (m, 1H, Ar), 7.79–7.75 (m, 1H, Ar), 7.50–7.44 (m, 3H, Ar), 6.17–6.16 (m, 1H, C=CH), 2.27 (q, J = 7.6 Hz, 2H, CH2CH3), 1.56–1.47 (m, 5H, C=CCH3 and i-Pr), 1.127 (d, J = 7.2 Hz, 6H, i-Pr), 1.08–1.02 (m, 9H, i-Pr and CH2CH3). 13C NMR (CDCl3, 100 MHz): δ 137.7, 135.11, 133.6, 133.5, 132.8, 130.4, 129.0, 128.9, 125.91, 125.5, 125.0, 118.5, 22.0, 17.89, 17.7, 16.7, 13.77, 12.3.
3-tert-Butyl-1,1-diisopropyl-1-silabenzo[d,e]isochromane (3an)
3an (9.2 mg, 28%) was obtained as a colorless oil accompanied by 4an (5.0 mg, 15%) as a colorless oil from the reaction using 1a (24.2 mg, 99.8 μmol) and 2,2-dimethylpropanal (2n). 1H NMR (CDCl3, 400 MHz): δ 7.85 (dd, J = 8.4 Hz, J = 1.6 Hz, 1H, Ar), 7.73 (dd, J = 8.0 Hz, J = 1.2 Hz, 1H, Ar), 7.57 (dd, J = 6.4 Hz, J = 1.2 Hz, 1H, Ar), 7.44 (dd, J = 8.0 Hz, J = 6.4 Hz, 1H, Ar), 7.38 (dd, J = 8.0 Hz, J = 7.2 Hz, 1H, Ar), 6.76 (dt, J = 7.2 Hz, J = 1.2 Hz, 1H, Ar), 5.06 (s, 1H, OCHAr), 1.51 (sept, J = 7.6 Hz, 1H, CH(CH3)2), 1.37 (d, J = 7.6 Hz, 3H, CH(CH3)2), 1.33 (d, J = 7.6 Hz, 3H, CH(CH3)2), 1.07 (sept, J = 7.6 Hz, 1H, CH(CH3)2), 0.79 (s, 9H, t-Bu), 0.66 (d, J = 7.2 Hz, 3H, CH(CH3)2), 0.57 (d, J = 7.2 Hz, 3H, CH(CH3)2). 13C NMR (CDCl3, 100 MHz): δ 136.8, 135.9, 132.8, 130.9, 129.7, 129.6, 127.8, 125.7, 124.6, 124.0, 86.0, 38.8, 27.0, 18.4, 18.0, 17.44, 17.40, 14.5, 13.7. HRMS (EI) m/z: [M]+ calcd for C21H30OSi, 326.2066; found, 326.2066.
Diisopropyl(2,2-dimethylpropoxy)(1-naphthyl)silane (4an)
1H NMR (CDCl3, 400 MHz): δ 8.38–8.34 (m, 1H, Ar), 7.88–7.82 (m, 2H, Ar), 7.76 (dd, J = 6.8 Hz, J = 1.2 Hz, 1H, Ar), 7.49–7.44 (m, 3H, Ar), 3.48 (s, 2H, OCH2t-Bu), 1.49 (sept, J = 7.6 Hz, 2H, CH(CH3)2), 1.13 (d, J = 7.2 Hz, 6H, CH(CH3)2), 1.06 (d, J = 7.2 Hz, 6H, CH(CH3)2), 0.99 (s, 9H, t-Bu). 13C NMR (CDCl3, 100 MHz): δ 138.0, 134.9, 133.9, 133.5, 130.0, 129.4, 128.8, 125.6, 125.4, 125.0, 74.1, 33.4, 26.6, 18.2, 18.0, 13.6. HRMS (EI) m/z: [M]+ calcd for C21H32OSi, 328.2222; found, 328.2222.
3-Cyclohexyl-1,1-diisopropyl-1-silabenzo[d,e]isochromane (3ao)
3ao (9.0 mg, 26%) was obtained as a colorless oil accompanied by 5ao (2.7 mg, 7.7%) as a colorless oil from the reaction using 1a (23.9 mg, 98.6 μmol) and cyclohexanecarbaldehyde (2o). 1H NMR (CDCl3, 400 MHz): δ 7.88 (dd, J = 8.4 Hz, J = 1.6 Hz, 1H, Ar), 7.73 (d, J = 8.0 Hz, 1H, Ar), 7.58 (dd, J = 6.8 Hz, J = 1.6 Hz, 1H, Ar), 7.48 (dd, J = 8.0 Hz, J = 6.4 Hz, 1H, Ar), 7.40 (dd, J = 8.0 Hz, J = 6.8 Hz, 1H, Ar), 7.22 (dt, J = 7.2 Hz, J = 1.2 Hz, 1H, Ar), 5.00 (d, J = 6.0 Hz, 1H, OCHAr), 1.91–1.87 (m, 1H, Cy), 1.72–1.60 (m, 4H, Cy), 1.36 (sept, J = 7.6 Hz, 1H, CH(CH3)2), 1.26–1.11 (m, 13H, Cy and i-Pr), 0.87 (d, J = 7.6 Hz, 3H, CH(CH3)2), 0.74 (d, J = 7.2 Hz, 3H, CH(CH3)2). 13C NMR (CDCl3, 100 MHz): δ 137.8, 136.0, 133.3, 131.3, 130.1, 129.3, 127.7, 124.82, 124.76, 124.1, 81.3, 46.3, 30.8, 28.3, 26.63, 26.58, 26.3, 18.0, 17.6, 17.4, 17.3, 14.1, 13.3. HRMS (EI) m/z: [M]+ calcd for C23H32OSi, 352.2222; found, 352.2222.
(Cyclohexylidenemethoxy)(diisopropyl) (1-naphthyl)silane (5ao)
1H NMR (CDCl3, 400 MHz): δ 8.21–8.18 (m, 1H, Ar), 7.89 (d, J = 8.4 Hz, 1H, Ar), 7.86–7.83 (m, 1H, Ar), 7.77 (dd, J = 7.2 Hz, J = 1.6 Hz, 1H, Ar), 7.50–7.45 (m, 3H, Ar), 6.19–6.17 (m, 1H, C=CH), 2.38–2.34 (m, 2H, Cy), 1.93–1.89 (m, 2H, Cy), 1.57–1.44 (m, 8H overlapped by H2O, Cy and i-Pr), 1.13 (d, J = 7.6 Hz, 6H, CH(CH3)2), 1.04 (d, J = 7.6 Hz, 6H, CH(CH3)2). 13C NMR (CDCl3, 100 MHz): δ 137.7, 135.2, 133.6, 132.7, 131.5, 130.4, 129.1, 128.9, 125.9, 125.5, 125.0, 121.1, 30.7, 28.6, 27.22, 27.17, 25.7, 17.9, 17.7, 13.7. HRMS (EI) m/z: [M]+ calcd for C23H32OSi, 352.2222; found, 352.2222.
Alternative Synthesis of 4ae, 4af, and 4ak
To 1-bromonaphthalene (0.50 g, 2.4 mmol) in Et2O (5 mL) was added 1.6 M t-BuLi pentane solution (3.0 mL, 4.8 mmol) at −80 °C, and the solution was warmed to room temperature. After dichlorodiisopropylsilane (0.44 g, 2.4 mmol) was added to the reaction mixture at −80 °C, it was gradually warmed to room temperature and stirred for a day. After filtration, volatiles were removed from the filtrate under reduced pressure to obtain chlorodiisopropyl(1-naphthyl)silane which was used for the following reactions without further purification. To chlorodiisopropyl(1-naphthyl)silane (0.20 mmol) in CH2Cl2 (3 mL) was added an alcohol (0.30 mmol), triethylamine (40 μL, 0.29 mmol), and 4-dimethylaminopyridine (DMAP, 2.4 mg, 20 μmol), and the solution was stirred overnight at room temperature. The reaction mixture was quenched with 1 M HCl and then the organic layer was extracted with CH2Cl2. The combined organic layers were dried over Na2SO4. After filtration, the filtrate was concentrated under reduced pressure to remove the volatiles. The crude product was purified by silica gel column chromatography (eluent: hexane/ethyl acetate = 10/1). Further purification was carried out by GPLC to obtain each of the products.
((4-Cyanophenyl)methoxy)diisopropyl(1-naphthyl)silane (4ae)
4ae (35.8 mg, 50%) was obtained as a colorless oil from the reaction using chlorodiisopropyl(1-naphthyl)silane (52.8 mg, 0.191 mmol) and 4-hydroxymethylbenzonitrile. 1H NMR (CDCl3, 400 MHz): δ 8.23 (d, J = 8.4 Hz, 1H, Ar), 7.91 (d, J = 8.0 Hz, 1H, Ar), 7.86 (dd, J = 8.0 Hz, J = 1.2 Hz, 1H, Ar), 7.76 (dd, J = 6.8 Hz, J = 1.6 Hz, 1H, Ar), 7.66 (d, J = 8.4 Hz, 2H, Ar), 7.53–7.44 (m, 4H, Ar), 7.38 (ddd, J = 8.1 Hz, J = 6.8 Hz, J = 1.6 Hz, 1H, Ar), 4.95 (s, 2H, OCH2Ar), 1.58 (sept, J = 7.6 Hz, 2H overlapped by H2O, CH(CH3)2), 1.17 (d, J = 7.6 Hz, 6H, CH(CH3)2), 1.10 (d, J = 7.6 Hz, 6H, CH(CH3)2). 13C NMR (CDCl3, 100 MHz): δ 146.8, 137.8, 135.2, 133.6, 132.4, 132.3, 130.6, 129.1, 128.6, 126.5, 126.1, 125.6, 125.1, 119.2, 110.8, 65.1, 18.1, 18.0, 13.7. HRMS (EI) m/z: [M]+ calcd for C24H27NOSi, 373.1862; found, 373.1861.
Diisopropyl(1-naphthyl)((4-nitrophenyl)methoxy)silane (4af)
4af (44.8 mg, 62%) was obtained as a colorless oil from the reaction using chlorodiisopropyl(1-naphthyl)silane (54.9 mg, 0.198 mmol) and (4-nitrophenyl)methanol. 1H NMR (CDCl3, 400 MHz): δ 8.25–8.21 (m, 3H, Ar), 7.92 (d, J = 8.0 Hz, 1H, Ar), 7.87 (dd, J = 8.0 Hz, J = 1.6 Hz, 1H, Ar), 7.77 (dd, J = 7.2 Hz, J = 1.6 Hz, 1H, Ar), 7.57 (d, J = 8.8 Hz, 2H, Ar), 7.51–7.44 (m, 2H, Ar), 7.38 (ddd, J = 8.4 Hz, J = 6.4 Hz, J = 1.2 Hz, 1H, Ar), 4.99 (s, 2H, OCH2Ar), 1.60 (sept, J = 7.6 Hz, 2H overlapped by H2O, CH(CH3)2), 1.18 (d, J = 7.6 Hz, 6H, CH(CH3)2), 1.11 (d, J = 7.2 Hz, 6H, CH(CH3)2). 13C NMR (CDCl3, 100 MHz): δ 148.8, 147.2, 137.8, 135.2, 133.6, 132.3, 130.6, 129.1, 128.5, 126.5, 126.1, 125.7, 125.1, 123.7, 65.0, 18.1, 18.0, 13.7. HRMS (EI) m/z: [M]+ calcd for C23H27NO3Si, 393.1760; found, 393.1761.
Diisopropyl(1-naphthyl)((3-thienyl)methoxy)silane (4ak)
4ak (18.4 mg, 27%) was obtained as a colorless oil from the reaction using chlorodiisopropyl(1-naphthyl)silane (53.1 mg, 0.192 mmol) and 3-thienylmethanol. 1H NMR (CDCl3, 400 MHz): δ 8.33 (d, J = 8.0 Hz, 1H, Ar), 7.90 (d, J = 8.4 Hz, 1H, Ar), 7.86 (dd, J = 7.6 Hz, J = 2.0 Hz, 1H, Ar), 7.79 (dd, J = 7.2 Hz, J = 1.6 Hz, 1H, Ar), 7.50–7.40 (m, 3H, Ar), 7.31 (dd, J = 5.2 Hz, J = 2.8 Hz, 1H, Ar), 7.26–7.24 (m, 1H, Ar), 7.09 (dd, J = 5.2 Hz, J = 1.6 Hz, 1H, Ar), 4.92 (d, J = 1.2 Hz, 2H, OCH2Ar), 1.55 (sept, J = 7.6 Hz, 2H overlapped by H2O, CH(CH3)2), 1.16 (d, J = 7.2 Hz, 6H, CH(CH3)2), 1.09 (d, J = 7.6 Hz, 6H, CH(CH3)2). 13C NMR (CDCl3, 100 MHz): δ 142.7, 137.9, 135.2, 133.6, 133.0, 130.3, 129.0, 128.9, 126.4, 125.9, 125.8, 125.5, 125.0, 120.8, 62.4, 18.1, 18.0, 13.7. HRMS (EI) m/z: [M]+ calcd for C21H26OSSi, 354.1474; found, 354.1473.
Acknowledgments
We thank Center for Industry, University, and Government Cooperation in Nagasaki University for measurements of high-resolution mass spectroscopy. This work was performed under the Cooperative Research Program of “Network Joint Research Center for Materials and Devices” (H.A.). This work was financially supported by a Grant-in-Aid for Scientific Research (no. 18K02983, H.A.) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c06228.
Crystallographic data for 3ad and NMR spectra of 1, 3, 4, and 5 (PDF)
Accession Codes
CCDC 2112414 contains the supplementary crystallographic data for compound 3ad in this paper. The data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif.
The authors declare no competing financial interest.
Supplementary Material
References
- a Lambert J. B.; Kania L.; Zhang S. Modern Approaches to Silylium Cations in Condensed Phase. Chem. Rev. 1995, 95, 1191–1201. 10.1021/cr00037a003. [DOI] [Google Scholar]; b Reed C. A. The Silylium Ion Problem, R3Si+. Bridging Organic and Inorganic Chemistry. Acc. Chem. Res. 1998, 31, 325–332. 10.1021/ar960132q. [DOI] [Google Scholar]; c Klare H. F. T.; Albers L.; Süsse L.; Keess S.; Müller T.; Oestreich M. Silylium Ions: From Elusive Reactive Intermediates to Potent Catalysts. Chem. Rev. 2021, 121, 5889–5985. 10.1021/acs.chemrev.0c00855. [DOI] [PubMed] [Google Scholar]
- a Lambert J. B.; Zhang S.; Stern C. L.; Huffman J. C. Crystal Structure of a Silyl Cation with No Coordination to Anion and Distant Coordination to Solvent. Science 1993, 260, 1917–1918. 10.1126/science.260.5116.1917. [DOI] [PubMed] [Google Scholar]; b Ibad M. F.; Langer P.; Schulz A.; Villinger A. Silylium-Arene Adducts: An Experimental and Theoretical Study. J. Am. Chem. Soc. 2011, 133, 21016–21027. 10.1021/ja209693a. [DOI] [PubMed] [Google Scholar]; c Xie Z.; Bau R.; Benesi A.; Reed C. A. The Silylium Ion (R3Si+) Problem: Effect of Alkyl Substituents R. Organometallics 1995, 14, 3933–3941. 10.1021/om00008a045. [DOI] [Google Scholar]; d Müller T.; Juhasz M.; Reed C. A. The X-ray Structure of a Vinyl Cation. Angew. Chem., Int. Ed. 2004, 43, 1543–1546. 10.1002/anie.200352986. [DOI] [PubMed] [Google Scholar]; e Omann L.; Qu Z. W.; Irran E.; Klare H. F. T.; Grimme S.; Oestreich M. Electrophilic Formylation of Arenes by Silylium Ion Mediated Activation of Carbon Monoxide. Angew. Chem., Int. Ed. 2018, 57, 8301–8305. 10.1002/anie.201803181. [DOI] [PubMed] [Google Scholar]
- a Schäfer A.; Saak W.; Haase D.; Müller T. Silyl Cation Mediated Conversion of CO2 into Benzoic Acid, Formic Acid, and Methanol. Angew. Chem., Int. Ed. 2012, 51, 2981–2984. 10.1002/anie.201107958. [DOI] [PubMed] [Google Scholar]; b Konno M.; Chiba M.; Nemoto K.; Hattori T. Electrophilic Aromatic Substitution of Arenes with CO2 Mediated by R3SiB(C6F5)4. Chem. Lett. 2012, 41, 913–914. 10.1246/cl.2012.913. [DOI] [Google Scholar]; c von Wolff N.; Lefèvre G.; Berthet J.-C.; Thuéry P.; Cantat T. Implications of CO2 Activation by Frustrated Lewis Pairs in the Catalytic Hydroboration of CO2: A View Using N/Si+ Frustrated Lewis Pairs. ACS Catal. 2016, 6, 4526–4535. 10.1021/acscatal.6b00421. [DOI] [Google Scholar]
- a Roy A.; Bonetti V.; Wang G.; Wu Q.; Klare H. F. T.; Oestreich M. Silylium-Ion-Promoted Ring-Opening Hydrosilylation and Disilylation of Unactivated Cyclopropanes. Org. Lett. 2020, 22, 1213–1216. 10.1021/acs.orglett.0c00173. [DOI] [PubMed] [Google Scholar]; b He T.; Wang G.; Bonetti V.; Klare H. F. T.; Oestreich M. Silylium-Ion-Promoted (5+1) Cycloaddition of Aryl-Substituted Vinylcyclopropanes and Hydrosilanes Involving Aryl Migration. Angew. Chem., Int. Ed. 2020, 59, 12186–12191. 10.1002/anie.202004320. [DOI] [PMC free article] [PubMed] [Google Scholar]; c He T.; Wang G.; Long P.-W.; Kemper S.; Irran E.; Klare H. F. T.; Oestreich M. Intramolecular Friedel-Crafts alkylation with a silylium-ion-activated cyclopropyl group: formation of tricyclic ring systems from benzyl-substituted vinylcyclopropanes and hydrosilanes. Chem. Sci. 2021, 12, 569–575. 10.1039/d0sc05553k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müther K.; Mohr J.; Oestreich M. Silylium Ion Promoted Reduction of Imines with Hydrosilanes. Organometallics 2013, 32, 6643–6646. 10.1021/om4002796. [DOI] [Google Scholar]
- a Furukawa S.; Kobayashi J.; Kawashima T. Development of a Sila-Friedel–Crafts Reaction and Its Application to the Synthesis of Dibenzosilole Derivatives. J. Am. Chem. Soc. 2009, 131, 14192–14193. 10.1021/ja906566r. [DOI] [PubMed] [Google Scholar]; b Chen Q.-A.; Klare H. F. T.; Oestreich M. Brønsted Acid-Promoted Formation of Stabilized Silylium Ions for Catalytic Friedel-Crafts C-H Silylation. J. Am. Chem. Soc. 2016, 138, 7868–7871. 10.1021/jacs.6b04878. [DOI] [PubMed] [Google Scholar]
- a Douvris C.; Ozerov O. V. Hydrodefluorination of Perfluoroalkyl Groups Using Silylium-Carborane Catalysts. Science 2008, 321, 1188–1190. 10.1126/science.1159979. [DOI] [PubMed] [Google Scholar]; b Douvris C.; Nagaraja C. M.; Chen C.-H.; Foxman B. M.; Ozerov O. V. Hydrodefluorination and Other Hydrodehalogenation of Aliphatic Carbon–Halogen Bonds Using Silylium Catalysis. J. Am. Chem. Soc. 2010, 132, 4946–4953. 10.1021/ja100605m. [DOI] [PubMed] [Google Scholar]; c Allemann O.; Duttwyler S.; Romanato P.; Baldridge K. K.; Siegel J. S. Proton-Catalyzed, Silane-Fueled Friedel-Crafts Coupling of Fluoroarenes. Science 2011, 332, 574–577. 10.1126/science.1202432. [DOI] [PubMed] [Google Scholar]; d Klare H. F. T. Catalytic C-H Arylation of Unactivated C-H Bonds by Silylium Ion-Promoted C(sp2)-F Bond Activation. ACS Catal. 2017, 7, 6999–7002. 10.1021/acscatal.7b02658. [DOI] [Google Scholar]
- Kira M.; Hino T.; Sakurai H. Siloxycarbenium Tetrakis[3,5-bis(trifluoromethyl)phenyl]borates and Their Role in Reactions of Ketones with Nucleophiles. Chem. Lett. 1992, 21, 555–558. 10.1246/cl.1992.555. [DOI] [Google Scholar]
- Müther K.; Oestreich M. Self-Regeneration of a Silylium Ion Catalyst in Carbonyl Reduction. Chem. Commun. 2011, 47, 334–336. 10.1039/c0cc02139c. [DOI] [PubMed] [Google Scholar]
- a Parks D. J.; Piers W. E. Tris(pentafluorophenyl)boron-Catalyzed Hydrosilation of Aromatic Aldehydes, Ketones, and Esters. J. Am. Chem. Soc. 1996, 118, 9440–9441. 10.1021/ja961536g. [DOI] [Google Scholar]; b Parks D. J.; Blackwell J. M.; Piers W. E. Studies on the Mechanism of B(C6F5)3-Catalyzed Hydrosilation of Carbonyl Functions. J. Org. Chem. 2000, 65, 3090–3098. 10.1021/jo991828a. [DOI] [PubMed] [Google Scholar]
- Rendler S.; Oestreich M. Conclusive Evidence for an SN2-Si Mechanism in the B(C6F5)3-Catalyzed Hydrosilylation of Carbonyl Compounds: Implications for the Related Hydrogenation. Angew. Chem., Int. Ed. 2008, 47, 5997–6000. 10.1002/anie.200801675. [DOI] [PubMed] [Google Scholar]
- a Johannsen M.; Jørgensen K. A.; Helmchen G. Synthesis and Application of the First Chiral and Highly Lewis Acidic Silyl Cationic Catalyst. J. Am. Chem. Soc. 1998, 120, 7637–7638. 10.1021/ja981021k. [DOI] [Google Scholar]; b Hara K.; Akiyama R.; Sawamura M. Strong Counteranion Effects on the Catalytic Activity of Cationic Silicon Lewis Acids in Mukaiyama Aldol and Diels–Alder Reactions. Org. Lett. 2005, 7, 5621–5623. 10.1021/ol052206g. [DOI] [PubMed] [Google Scholar]; c Klare H. F. T.; Bergander K.; Oestreich M. Taming the Silylium Ion for Low-Temperature Diels-Alder Reactions. Angew. Chem., Int. Ed. 2009, 48, 9077–9079. 10.1002/anie.200904520. [DOI] [PubMed] [Google Scholar]; d Schmidt R. K.; Müther K.; Mück-Lichtenfeld C.; Grimme S.; Oestreich M. Silylium Ion-Catalyzed Challenging Diels-Alder Reactions: The Danger of Hidden Proton Catalysis with Strong Lewis Acids. J. Am. Chem. Soc. 2012, 134, 4421–4428. 10.1021/ja211856m. [DOI] [PubMed] [Google Scholar]; e Sakaguchi Y.; Iwade Y.; Sekikawa T.; Minami T.; Hatanaka Y. Chiral silicon Lewis acids having a pentacoordinate stereogenic silicon center: 29Si NMR studies and application to asymmetric Diels-Alder reactions. Chem. Commun. 2013, 49, 11173–11175. 10.1039/c3cc46501b. [DOI] [PubMed] [Google Scholar]; f Gatzenmeier T.; Turberg M.; Yepes D.; Xie Y.; Neese F.; Bistoni G.; List B. Scalable and Highly Diastereo- and Enantioselective Catalytic Diels-Alder Reaction of α,β-Unsaturated Methyl Esters. J. Am. Chem. Soc. 2018, 140, 12671–12676. 10.1021/jacs.8b07092. [DOI] [PubMed] [Google Scholar]
- a Tap A.; Blond A.; Wakchaure V. N.; List B. Chiral Allenes via Alkynylogous Mukaiyama Aldol Reaction. Angew. Chem., Int. Ed. 2016, 55, 8962–8965. 10.1002/anie.201603649. [DOI] [PubMed] [Google Scholar]; b Bae H. Y.; Höfler D.; Kaib P. S. J.; Kasaplar P.; De C. K.; Döhring A.; Lee S.; Kaupmees K.; Leito I.; List B. Approaching sub-ppm-level asymmetric organocatalysis of a highly challenging and scalable carbon-carbon bond forming reaction. Nat. Chem. 2018, 10, 888–894. 10.1038/s41557-018-0065-0. [DOI] [PubMed] [Google Scholar]
- a Arii H.; Kurihara T.; Mochida K.; Kawashima T. Silylium Ion-promoted Dehydrogenative Cyclization: Synthesis of Silicon-containing Compounds Derived from Alkynes. Chem. Commun. 2014, 50, 6649–6652. 10.1039/c4cc01648c. [DOI] [PubMed] [Google Scholar]; b Arii H.; Yano Y.; Nakabayashi K.; Yamaguchi S.; Yamamura M.; Mochida K.; Kawashima T. Regioselective and Stereospecific Dehydrogenative Annulation Utilizing Silylium Ion-Activated Alkenes. J. Org. Chem. 2016, 81, 6314–6319. 10.1021/acs.joc.6b00793. [DOI] [PubMed] [Google Scholar]
- Using benzyldiisopropylsilane (1d), which we used often in the previous papers,14 instead of 1a and benzaldehyde (2a), the corresponding annulation product, 3,3-diisopropyl-1-phenyl-3-silaisochromane (3da), was obtained in 8.0% isolated yield accompanied with the hydrosilylation product, benzyl(benzyloxy)diisopropylsilane (4da), in 44% yield.
- Rubin M.; Schwier T.; Gevorgyan V. Highly Efficient B(C6F5)3-Catalyzed Hydrosilylation of Olefins. J. Org. Chem. 2002, 67, 1936–1940. 10.1021/jo016279z. [DOI] [PubMed] [Google Scholar]
- Habibi-Yangjeh A.; Danandeh-Jenagharad M.; Nooshyar M. Prediction Acidity Constant of Various Benzoic Acids and Phenols in Water Using Linear and Nonlinear QSPR Models. Bull. Korean Chem. Soc. 2005, 26, 2007–2016. 10.5012/bkcs.2005.26.12.2007. [DOI] [Google Scholar]
- Ihara E.; Young V. G. Jr.; Jordan R. F. Cationic Aluminum Alkyl Complexes Incorporating Aminotroponiminate Ligands. J. Am. Chem. Soc. 1998, 120, 8277–8278. 10.1021/ja9817444. [DOI] [PubMed] [Google Scholar]
- Fulmer G. R.; Miller A. J. M.; Sherden N. H.; Gottlieb H. E.; Nudelman A.; Stoltz B. M.; Bercaw J. E.; Goldberg K. I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. 10.1021/om100106e. [DOI] [Google Scholar]
- Sheldrick G. M. SHELXT- Integrated space-group and crystal-structure determination. Acta Crystallogr., Sect. A: Found. Adv. 2015, 71, 3–8. 10.1107/s2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick G. M. Crystal structure refinement with SHELXL. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71, 3–8. 10.1107/s2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.