Abstract

Hydrochloric acid leaching has been widely used in the recovery process of vanadium due to its efficient selectivity. It was necessary to further separate vanadium from hydrochloric acid leaching solution. Four extractants of P204, P507, Cyanex272, and N1923 were compared for extraction of vanadium from a simulated hydrochloric acid solution, and it is concluded that N1923 was an effective extractant suitable for the extraction and separation of V (V) in the medium. The single-stage extraction efficiency of vanadium reached more than 90% with a pH value of 2.0, extraction time of 5 min, and XN1923 of 0.2 at 30 °C. The functional group characteristics of the extraction complex were analyzed by means of an extraction slope method, FT-IR, and 1H NMR to judge the extraction mechanism of vanadium with N1923 as an extractant. The extraction of V (V) by using N1923 was in the coordination form of a molar ratio of 2:1, and the extraction process was an endothermic reaction. The N–H vibrational absorption peak in the −NH2 group still appeared in the loaded N1923, in which the chemical shift of 1H in the primary amine and secondary carbon still existed. This technology was a more efficient process for extraction of vanadium from hydrochloric acid solution.
1. Introduction
Vanadium is a transition refractory metal, and the melting point can reach 1890 °C. Furthermore, it is not easy to oxidize it in air, and it is quite stable in alkali, sulfuric acid, and hydrochloric acid.1 Therefore, vanadium is usually widely used in metallurgy, aerospace, chemical catalysts, batteries, and other industries in the form of ferrovanadium, vanadium compounds, and metal vanadium.2,3 Among them, one of the application direction of a vanadium battery is as an excellent green environmental protection battery with strong development momentum, in which no harmful substances are produced in the process of manufacturing, use, and waste. The vanadium battery has a special battery structure and can discharge deeply with high current density, in which the advantages of rapid charging, high specific energy, low price, and wide application fields are achieved.4
It is well-known that vanadium reserves are mainly produced from vanadium titanomagnetite.5 In addition, vanadium resources are also partially contained in phosphorite ore, siltstone, bauxite, carbonaceous crude oil, coal, and shale.6−8 At present, the main extraction method of vanadium is the process of pretreatment, leaching, separation, and enrichment according to the physical, chemical, and mineral composition characteristics of raw materials, in which sulfuric acid leaching is widely used because of its high vanadium recovery.9,10 However, a large number of metal impurities in the raw materials with vanadium could also be dissolved and exist in the sulfuric acid leaching solution due to the poor selectivity of the sulfuric acid leaching process, which results in difficulties in the subsequent separation and purification of vanadium from the leaching solution.11 Therefore, many scholars have proposed that the selective leaching of vanadium could be realized by leaching with hydrochloric acid from vanadium titanomagnetite, bauxite, red mud, vanadium waste catalyst, and other raw materials.12−15 For example, the leaching efficiency of vanadium and iron was more than 80%, while the leaching efficiency of titanium was less than 5% from vanadium titanomagnetite by acid leaching with hydrochloric acid of an appropriate concentration, which could realize the effective separation of titanium and vanadium.16
The acid leaching solution containing vanadium was obtained by sulfuric acid leaching or hydrochloric acid leaching, which could also contain a certain amount of metal impurity ions needing to be separated and purified.1,17,18 At present, the solvent extraction process was a usual mature method for the separation and purification of vanadium from acidic solution.19−21 There are many studies on the extraction and separation of vanadium from the sulfuric acid leaching solution system, in which extractants such as P204, P507, Cyanex272, TOMAC, TOMAC, Aliquat 336, and other ionic liquids were used for the extraction and separation of vanadium from iron, aluminum, and other impurity ions, in which the extraction mechanism was also reasonably analyzed.22−25 However, the separation methods and efficient extractaction of vanadium were less determined from the hydrochloric acid solution, and the extraction mechanism between vanadium and extractant was less deeply analyzed.
Therefore, the extraction effects of P204, P507, Cyanex272, and N1923 on vanadium from hydrochloric acid solution were comparatively studied in this paper. The effects of extraction parameters on the extraction efficiency of vanadium and the separation effect from impurity ions were investigated. The morphology of the extraction complex by using N1923 extractant was analyzed by a slope method. The extraction mechanism between N1923 and vanadium was verified by using FT-IR and 1H NMR. The countercurrent extraction was carried out and determined with a McCable-Thiele diagram. The research may provide a new method and idea for the separation and purification of vanadium from hydrochloric acid solution medium.
2. Results and Discussion
2.1. Effects of Extractants and pH Value
The effects of the extractants and pH value on the extraction efficiency of vanadium were investigated under the conditions of an extractant concentration (Xextractant) of 0.2, extraction time of 5 min, extraction temperature of 30 °C, and O/A of 1, and the results are shown in Figure 1.
Figure 1.

Effects of extractants and pH value.
It can be seen from Figure 1 that the pH value and the extractants have significant effects on the extraction efficiency of vanadium. The extractants of P507 and Cyanex272 reached maximum vanadium extraction efficiencies of 72 and 64% respectively, at a pH value of 1.5, while a maximum vanadium extraction efficiency of 50% was obtained by using P204 at a pH value of 2.0. The result was similar to the previous research results on the extractant effect, in which P507 and Cyanex272 were more suitable than P204 for recovering vanadium at low pH.26 However, the extraction efficiency of vanadium showed a decreasing trend by using these three extractants, as the pH value of the solution continued to increase to more than 2.0. It indicates that the functional groups of the extractants had no obvious effect on the vanadium in the solution. These three extractants belonged to cationic extractants, and the ionic morphology of vanadium may be changed closely in the solution.27 However, the extraction performance of N1923 was obviously different from those of P507, Cyanex272, and P204. The extraction efficiency of vanadium increased significantly from 5 to 92% by using N1923 with the pH value of the solution increasing from 1.0 to the range of 2.0–2.5. In addition, although the extraction efficiency of vanadium decreased with the further increase of the pH value, it still remained more than 83%. The extraction of vanadium was significantly higher than those of the three extractants. It is related to the ionic form of vanadium and the extraction behavior of N1923.28 According to the experimental results, it can be found that different pH values could have a decisive impact on the extraction effects of the four extractants. The concentration of pentavalent vanadium and the pH value of the solution will have an impact on the ion morphology of vanadium.29 The result of pentavalent vanadium morphology is shown in Figure 2.
Figure 2.

Effect of concentration and pH value on pentavalent vanadium morphology.
Figure 2 shows that the dissolved vanadium concentration had a certain impact on the existing form of vanadium. Generally, when the vanadium concentration was lower (log C of −2, namely a vanadium concentration of 509 mg/L), the existing form was simpler (VO2+, H2VO4–, HVO42–, VO43–). The more complex forms of vanadium (H2V10O284–, H2V6O172–, H3V2O7–, etc.) existed with the increase of vanadium concentration ((log C of −1 to 0, namely a vanadium concentration of 5.09 to 50.9 g/L), in which the precipitated solid of V2O5 could even appear. The influence of the pH value on the existing form of vanadium was mainly reflected in the complex valence state with a log Cv less than −2. V(V) mainly existed in the form of VO2+ at a pH value of the solution of less than 2. The vanadium mainly existed in the form of H2VO4– with a pH value of 9 to 11. VO43– was the main form at a pH value of greater than 11. In general, VO2+ was the main form of pentavalent vanadium in a strongly acidic solution medium, which was also the object of extraction and separation. The structural diagram of pentavalent vanadium in the solution is shown in Figure 3.
Figure 3.
Microstructure schematic diagram of V(V) in solution.
Figure 3 shows the structural formula and 3D structural diagram of pentavalent vanadium in the solution, in which VO2+ existed in the form of two V=O double bonds, and V was positively charged due to an electron deficiency. VO43–, HVO42–, and H2VO4– existed in the form of one V=O double bond and three V–O single bonds, in which the vanadium was at saturation. VO43– existed as a −3 valence due to the three V–O single bonds not combined with H. HVO42– existed as a −2 valence due to the two V–O single bonds not combined with H. H2VO4– existed as a −1 valence due to the one V–O single bond not combined with H. When the structure of the vanadium oxide complex was complex (HV6O173– and H2V6O172–), the six vanadium atoms were connected by six V–O single bonds, in which the V on both sides contained two V=O double bonds. The middle four vanadium atoms were connected by one V=O double bond and one V–O single bond, respectively. HV6O173– existed as a −3 valence due to the existence of V–OH in the four V–O single bonds. And H2V6O172– existed as a −2 valence due to the existence of two V–OH groups in the four V–O single bonds. When the vanadium oxide complex was more complex (H2V10O284– and HV10O285–), its structure was connected in the form of a single ring. The two vanadium atoms on both sides were respectively connected with two V=O double bonds and one V–O single bond, in which the O in the V–O single bond was also connected with one V and the eight vanadium atoms were connected in a single ring in the form of V–O single bonds. And then, the eight vanadium atoms still contained one V=O and one V–O single bond. At this time, the oxygen atom connected with the six vanadium atoms in the middle was negatively charged due to multiple electrons. H2V10O284– was an overall −4 valence due to two O combined with the H of V–OH, and HV10O285– was an overall −5 valence due to one O combined with the H of V–OH. Therefore, the charged atom will be the center of the reaction during the extraction reaction between various extractants and vanadium (such as V in VO2+ and O in various vanadium-containing anions). The structure and 3D diagram of the four extractants are shown in Figure 4.
Figure 4.
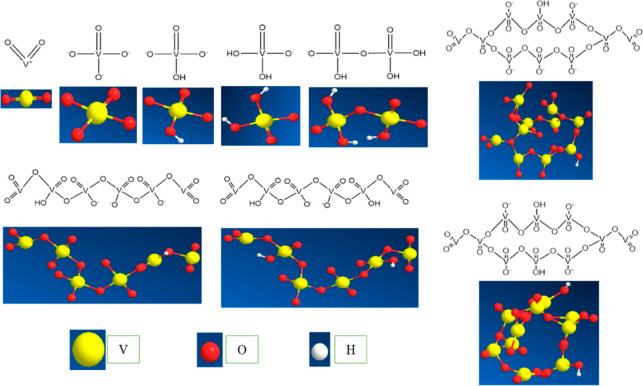
Structure and 3D diagram of extractants.
P204 is fully called di(2-ethylhexyl)phosphate, in which the P atom was covalent with three bonds of P=O and P–OH. And the other two P–O single bonds were formed, in which O was connected with alkyl (R). The vanadium-containing cations could be selectively exchanged through H+ in its hydroxyl group. P507 is fully called 2-ethylhexyl phosphate mono-2-ethylhexyl ester. P507 had one less ester oxygen atom than P204, and its acidity was weaker than P204. Therefore, P507 usually had a higher separation coefficient for vanadium, but its back-extraction acidity was higher. Cyanex272 is fully known as di(2,4,4-trimethylamyl)phosphinic acid, which had one ester oxygen atom less than P507. N1923 is an extractant with a primary amine and secondary carbon. The R group connected to the secondary carbon was an alkyl group with 19–23 carbon numbers, in which the extraction functional group was −NH2 and the secondary carbon was connected to it.
2.2. Selective Separation Effect of Extractants
N1923 and P507 show a good extraction effect of vanadium according to the extraction experiment results of extractant types. P507 could better extract vanadium at a pH value of 1.5 (72% showed in Figure 1), while N1923 shows a high extraction efficiency of vanadium at pH values of 2.0–2.5 (92% shown in Figure 1).
Therefore, the effect of different concentrations of Fe(III) ions on the extraction efficiency of vanadium was investigated in order to further verify the selective effect of the two extractants on vanadium and impurity ions. The results are shown in Figure 5.
Figure 5.

Effect of iron impurity ions on vanadium extraction.
It can be seen from Figure 5 that the extraction efficiency of vanadium decreased and the extraction efficiency of iron increased by the two extractants with the addition of iron ions in the solution. It should be noted that the vanadium extraction efficiency decreased from 72 to 65% by using P507 extractant with an iron ion concentration of 0.5 g/L, in which the iron extraction efficiency reached nearly 40%. It indicates that P507 has a poor selectivity for vanadium extraction in the solution. The extraction efficiencies of vanadium and iron were the same, both about 50% with a concentration of impurity iron ions of 1 g/L. The iron extraction efficiency was even greater than the vanadium extraction efficiency with the continued increase of the iron ion concentration. It is related to the extraction characteristics of P507 on metal cations, in which the higher the valence of the cations, the better the extraction effect of the metals.29
The extraction efficiency of vanadium was always higher than the extraction efficiency of iron by using N1923 extractant with the increase of iron ion concentration. The recovery of iron was less than 10% by using N1923, while the extraction efficiency of vanadium remained more than 65% especially with an iron concentration of less than 1 g/L. The selective extraction process of vanadium could be realized by using N1923. It also illustrates that the extraction mechanism of N1923 was not the cation exchange.30 Furthermore, the extraction effect of N1923 could also not be simply judged by the valence state of metal ions.
2.3. Effect of N1923 Concentration (XN1923)
N1923 not only had an obvious extraction effect of vanadium but also can realize the effective selective separation of vanadium and iron. It was an effective vanadium extractant in hydrochloric acid medium. The effect of XN1923 on vanadium extraction was investigated in order to analyze the extraction form, and the results are shown in Figure 6.
Figure 6.

Effect of XN1923 on vanadium extraction.
Figure 6 shows that the extraction and recovery of vanadium were increased with the increase of XN1923, which is consistent with the extraction law of most extractants.22 The vanadium extraction efficiency just exceeded 40% with an XN1923 of 5%. However, the vanadium extraction efficiency reached more than 90% with an XN1923 of 20%. The vanadium extraction rate did not increase significantly with a sustained increase of XN1923. The change trend of DV was also closely related to the concentration of XN1923. DV also showed an increasing trend with the increase of XN1923. The relationship curve of log DV to log[RNH2] was analyzed by the slope method in order to analyze the extraction mechanism of vanadium by using N1923.
The main form of vanadium was VO2+ in the hydrochloric acid solution.31 The extraction thermodynamics of vanadium was investigated by using N1923. The extraction reaction can be expressed as follows.
| 1 |
The extraction equilibrium constant K was calculated as follows.
| 2 |
| 3 |
where (RNH2) is N1923, and the finished expression was as follows.
| 4 |
| 5 |
The D values were tested under the condition of different concentrations of N1923 at a pH of 2.0 in 25 °C. The relationship of log DV to log[RNH2] was expressed, and the result is shown in Figure 7.
Figure 7.

Relationship curve between log D and log[N1923]
Figure 7 shows that the linear fit was very good, and the correlation coefficient (R2) was 0.9912 in the equation of y = 2.0048x + 2.4289. Therefore, the reaction model was established as eq 6.
| 6 |
The extraction process of vanadium was in the coordination form by using N1923 according to Figure 7 and eq 6, in which the complexation was in the form of a molar ratio of 2:1. There was no ion exchange in this process, which would also be verified in the subsequent extraction mechanism analysis with FT-IR and 1H NMR.
2.4. Effect of Temperature
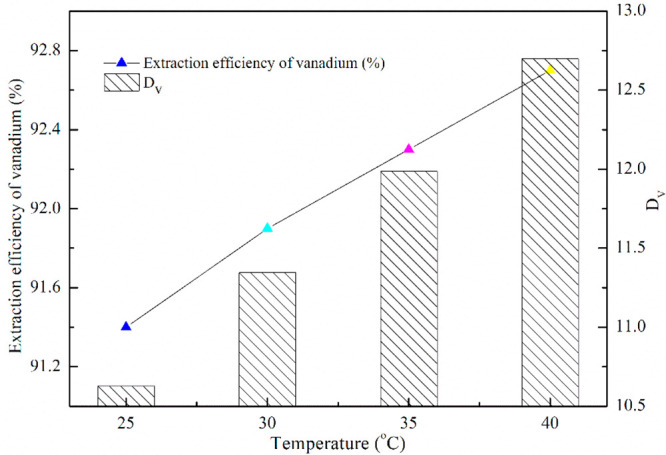
The extraction reaction belonging to endothermic reaction or exothermic reaction could be judged by the extraction temperature so as to reasonably select the extraction parameters. The effects of different extraction temperatures (25, 30, 35, 40 °C) on the vanadium extraction efficiency were investigated, and the results are shown in Figure 8.
Figure 8.
Effect of extraction temperature.
Figure 8 shows that the extraction temperature has little effect on the vanadium extraction. The vanadium extraction efficiency shows a slight increasing trend with an increase of extraction temperature. Eq 7 could be obtained under the conditions of a constant pH value as well as concentration of the extractant and vanadium.32
| 7 |
The enthalpy change (ΔH) was regarded as a certain value when the reaction temperature does not change significantly. Therefore, eq 8 was obtained as follows.
| 8 |
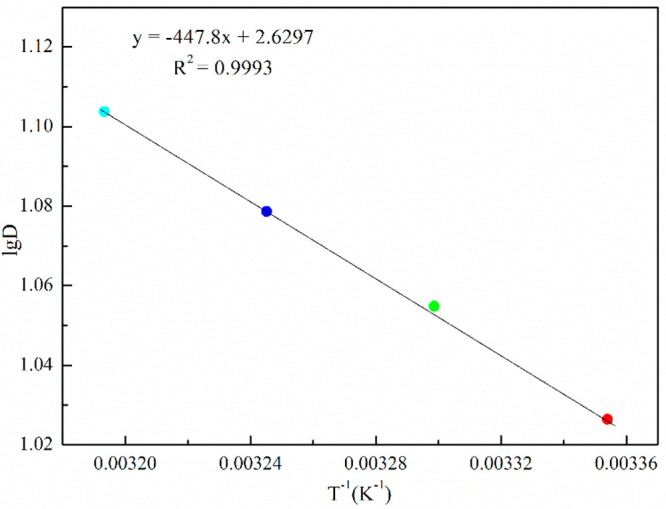
The plot log D to 1/T was investigated, and the result is shown in Figure 9.
Figure 9.
Relationship between log D and temperature.
Figure 9 shows that the curve had a good fitting degree with the correlation coefficient (R2) of 0.9993, in which ΔH was calculated to be 8.57 kJ/mol according to the slope. The result of ΔH > 0 indicates that the extraction reaction of vanadium was endothermic, in which an appropriate increase of the extraction temperature was beneficial to the extraction of vanadium.
2.5. Verification of Extraction Mechanism
N1923 and loaded N1923 were comparatively analyzed by FT-IR (see Figure 10) and 1H NMR (see Figure 11) to further verify the extraction process between N1923 and vanadium.
Figure 10.
FT-IR of N1923 and loaded N1923.
Figure 11.

1H NMR of N1923 and loaded N1923.
It can be seen from Figure 10 that the symmetric stretching vibration peak of 3300 cm–1 came from N–H in the free RNH2, in which the in-plane bending vibration peak of 1620 cm–1 from N–H existed. The vibration of N–H in the −NH2 group still existed after the formation of the extraction complex, but the positions of absorption peaks moved to 3280 and 1610 cm–1, respectively. It indicates that the N atom in RNH2 was combined with VO2+ in the form of a coordination bond, which could weaken the strength of the N–H bond, reduce the bond force constant, and result in the vibration frequency of the bond to move toward a low wavenumber.33 In addition, it can be found that the vibration peak intensity of 750 cm–1 from loaded-vanadium N1923 also significantly fluctuated, which was the stretching vibration peak intensity range of C–Cl.34 It also indicates that the Cl– existed in the form of an anion outside the extraction complex in the process of extracting vanadium from a hydrochloric acid medium.
As can be seen from Figure 11, the chemical shift of 1H on the −NH2 in N1923 was 1.21 ppm, and the chemical shift 1H on the secondary carbon connected to the N atom was 2.74 ppm. When the VO2(RNH2)2Cl existed in the loaded N1923, its chemical shifts moved to 4.08 and 3.82 ppm, respectively. It further proves that RNH2 was combined with VO2Cl in the form of a coordination bond. Therefore, the density of the electron cloud around the oxygen atom in VO2+ and the Cl ion decreased and resulted in the smaller shielding effect of the hydrogen atom connected with it. Finally, the chemical shift of 1H on a primary amine and secondary carbon moved to the low field direction.
To sum up, the extraction mechanism of vanadium by using N1923 in hydrochloric acid solution medium can be summarized in Figure 12.
Figure 12.
Schematic diagram of extraction mechanism of vanadium by using N1923.
The vanadium mostly existed in the form of VO2+ at a pH value of less than 2 in the medium of a hydrochloric acid solution. The N atom of −NH2 in N1923 was negatively charged due to the lone electron pair, which can be coordinated with the positively charged V in VO2+ in the form of a molar ratio of 2:1. The secondary carbon was partially positively charged in N1923 by migrating the electron cloud of a secondary C atom to the connected N atom due to the greater electronegativity of N than that of C. Therefore, the positively charged secondary C atom could attract Cl– from the hydrochloric acid solution for coordination, in which the extraction complex of [RNH2–VO2+–NH2R]Cl was formed. Finally, the [RNH2–VO2+–NH2R]Cl was electrically neutral in hydrochloric acid solution and insoluble in the water phase for separation.35
2.6. Effect of Metal Impurity Ions
In order to investigate the effect of metal impurity ions on the extraction of vanadium by using N1923, certain amounts of ferrous chloride, aluminum chloride, calcium chloride, sodium chloride, and potassium chloride were added to 100 mL of simulated solution of 300 mg/L vanadium, in which the concentration of iron, aluminum, calcium, sodium, and potassium ions in the solution was controlled at 500 mg/L. The effects of various metal ions under different pH values were investigated with an O/A of 1:1, an XN1923 of 0.2, and an extraction time of 5 min at room temperature. The results are shown in Figure 13.
Figure 13.

Effect of metal impurity ions.
It can be seen from Figure 13 that the iron, aluminum, calcium, sodium, and potassium ions had no obvious effect on the extraction of vanadium by using N1923 under the conditions. At pH values of 1–3, the extraction efficiency of sodium and potassium was less than 3%, in which the synergistic extraction effect with vanadium was not obvious. The extraction efficiency of the calcium ion was lower than 5%, while a certain synergistic effect between aluminum and iron existed. The extraction efficiency of aluminum and iron was close to 10% especially at a pH value of greater than 2, in which the extraction efficiency of vanadium could reach 87% at a pH value of 2. The extraction efficiency of vanadium remained more than 75% with the increase of the pH value, which can realize the selective extraction and separation of vanadium by using N1923 from iron, aluminum, calcium, sodium, and potassium in the hydrochloric acid solution medium.
2.7. Multistage Countercurrent Extraction
The multistage countercurrent extraction process of vanadium was studied to determine the extraction order with different O/A ratios (1:1–1:10) under the conditions of an XN1923 of 0.2, a pH value of 2.0, and an extraction time of 5 min. The McCable-Thiele diagram of vanadium extraction is shown in Figure 14.
Figure 14.
McCable-Thiele diagram of vanadium extraction by using N1923.
Figure 14 shows that the theoretical extraction stage of vanadium was four by using N1923 under the condition of an O/A of 1:4. The simulated solution containing multiple impurity ions was carried out with four-stage countercurrent extraction, and the analysis results of chemical composition from raffinate are shown in Table 1.
Table 1. Chemical Composition of Extraction Tail Liquid.
| element | V | Al | Fe | Ca | Na | K |
|---|---|---|---|---|---|---|
| concentration (mg/L) | 2 | 462 | 454 | 487 | 496 | 494 |
It can be seen from Table 1 that the extraction efficiency of vanadium could reach 99% after a four-stage countercurrent extraction, in which the extraction efficiency of aluminum and iron was 7.6 and 9.2%, respectively. The extraction efficiencies of other impurity ions such as calcium, sodium, and potassium were less than 5%. It indicates that the effective separation of vanadium and impurity ions in hydrochloric acid solution medium was realized by using N1923.
In short, compared with the previous studies with extractants such as P204, P507, and Cyanex272, N1923 extractant could effectively separate and extract vanadium from the hydrochloric acid medium. The extractants of P204, P507, and Cyanex272 belong to organic phosphoric acid extractants, which had better extraction ability for VO2+ than VO2+ with a strong complexation ability for iron.36 N1923 extractant is characterized by a high separation coefficient and high selectivity between vanadium and impurity ions. In addition, the N1923 extractant belongs to a secondary carbon primary amine extractant, which was cheap and easy to obtain. N1923 extractant had low solubility in water, which was nontoxic and harmless. The loaded N1923 was very easy for back-extraction. Therefore, N1923 has a very high recycling efficiency, and it can be reused and sustainable.37
3. Conclusion
N1923 was a suitable extractant for separating and recovering vanadium from hydrochloric acid solution compared with P204, P507, and cyanexe272. The single-stage extraction efficiency of vanadium was more than 90% under the conditions of a solution pH value of 2.0, an extraction time of 5 min, and an XN1923 of 0.2 at 30 °C. The slope method determines that the extraction of V (V) was in the coordination form of a molar ratio of 2:1 with N1923 in the hydrochloric acid medium. The extraction process belonged to an endothermic reaction. The results of FT-IR and 1H NMR show that the vibrational absorption peak of N–H in the −NH2 group still appeared in the loaded N1923, which was only offset. Furthermore, the chemical shift of 1H in primary amine and secondary carbon still existed. It indicates that the exchange extraction between functional groups and vanadium-containing ions did not occur in the process, in which the coordinated extraction of [RNH2–VO2+–NH2R]Cl was formed. The extraction efficiency of vanadium was up to 99%, and the extraction efficiency of aluminum and iron was less than 10% with a four-stage countercurrent extraction according to the McCable-Thiele diagram. The extraction efficiency of calcium, potassium, and sodium was still less than 5%, which proves that the efficient selective extraction of vanadium was also obtained by using N1923.
4. Experiments
4.1. Experimental Materials
The extractants used in the experiment mainly included di-(2-ethylhexyl) phosphoric acid (namely P204, C16H35O4P, molecular weight of 322), 2-ethylhexyl phosphate mono 2-ethylhexyl ester (namely P507, C16H35O3P, molecular weight of 306), bis(2,4,4-trimethylamyl) phosphinic acid (namely Cyanex272, C16H35O2P, molecular weight of 290), and secondary carbon primary amine extractant (namely N1923, C19NH41, molecular weight of 283) with analytical purity. The reagents used in the test were of analytical purity, including vanadium pentoxide (V2O5), hydrochloric acid (HCl), ferrous chloride (FeCl2), aluminum chloride (AlCl3), calcium chloride (CaCl2), sodium chloride (NaCl), potassium chloride (KCl), and sodium hydroxide (NaOH). The distilled water was fully used in the test as the dissolvent.
4.2. Experimental Process
Simulated solution preparation: A certain amount of vanadium pentoxide was weighed and dissolved into the hydrochloric acid solution of 2 mol/L according to the vanadium concentration requirement of 300 mg/L in the experimental process. The simulated hydrochloride solution containing pentavalent vanadium was obtained by completely stirring and dissolving for standby. Then, certain amounts of ferric chloride, aluminum chloride, calcium chloride, sodium chloride, and potassium chloride were added to the simulated hydrochloride solution to dissolve it fully according to the requirements of the type and concentration of impurity ions in the impurity ion influence experiments.
Organic phase preparation: Certain volumes of P204, P507, Cyanex272 and N1923 was individually taken and mixed with some sulfonated kerosene according to the concentration and proportion requirements of extractants, in which the organic phase was stirred evenly for standby.
The simulated solution was adjusted to the appropriate pH value by using 1 mol/L sodium hydroxide solution or 1 mol/L hydrochloric acid solution. Then, the adjusted solution of 50 mL was mixed the different organic phases of 50 mL into a 500 mL conical flask, respectively. And then, the conical flask was placed in a temperature-controlled oscillator and vibrated for 6 min with an oscillation intensity of 300 rpm. The mixed solution was put into a separating funnel of 250 mL and stood for 10 min, and then, the extraction tail liquid was discharged, and the volume was measured. The concentration of vanadium (or other metals) in the simulated solution and extraction tail liquid was detected and analyzed, respectively. The calculation method of the extraction efficiency of vanadium (or other metals) and distribution ratio are shown in eqs 9 and 10.
| 9 |
| 10 |
where a is the extraction efficiency of vanadium (or other metals) (%), V is the volume of the simulated solution (mL), C is the concentration of vanadium (or other metals) in simulated solution (mg/L), V1 is the volume of extraction tail liquid (mL), C1 is the concentration of vanadium (or other metals) in extraction tail liquid (mg/L), C2 is the concentration of vanadium in the organic phase (mg/L), and D is the distribution ratio of vanadium.
4.3. Isothermal Extraction Experiment
The simulated solution of 50 mL and organic phase of 50 mL were tested each time, in which the volume concentration of organic phase (XN1923) was controlled as 0.05, 0.1, 0.15, 0.2, and 0.25 respectively. The extraction experiment was carried out under the condition of a temperature of 30 °C and pH value of 2.0, and then, the extraction tail liquid was collected. Different distribution ratios were obtained by collecting the extraction tail liquid and analyzing the concentration of vanadium in the simulated solution and adsorption tail liquid. Finally, the relationship between XN1923 and Dv was investigated, and linear fitting was carried out.
4.4. Extraction Thermodynamics Experiment
The extractant volatilized seriously, which could affect the operability of the experiment while the extractant temperature was higher than 40 °C. Therefore, the extraction thermodynamic tests were carried out under the condition of a reaction temperature less than 40 °C. The organic phase with an XN1923 of 0.2 was collected and mixed evenly with the simulated solution with an O/A of 1:1 under the condition of a pH value of 2.0. And then, the extraction experiments were carried out at 25, 30, 35, and 40 °C, individually, in which the extraction tail liquid was collected, respectively. Finally, the relationship between temperature and Dv was investigated, and linear fitting was carried out by obtaining different distribution ratios with the analysis of the concentration of vanadium in the simulated solution and adsorption tail liquid.
4.5. Detection and Analysis
The concentrations of vanadium (other metals) in simulated solution and extraction tail liquid were analyzed by using inductively coupled atomic emission spectrometry (ICP-AES). The functional groups of extractant and the loaded organic phase were analyzed with infrared spectroscopy (FT-IR). The structural characteristics of extractant and loaded organic phase were analyzed by using nuclear magnetic resonance hydrogen spectroscopy (1H NMR).
Acknowledgments
The research is financially supported by the National Natural Science Foundation of China (51904097 and 51804103), the training program for young backbone teachers in Colleges and universities of Henan Province (2019GGJS056), the Open Foundation of State Environmental Protection Key Laboratory of Mineral Metallurgical Resources Utilization and Pollution Control (HB202106), the Scientific and Technological Project of Henan Province (202102310548), and the Program for Innovative Research Team in the University of Henan Province (21IRTSTHN006).
The authors declare no competing financial interest.
References
- Li M.; Zhang B. G.; Zou S. Q.; Liu Q. S.; Yang M. Highly selective adsorption of vanadium (V) by nano-hydrous zirconium oxide-modified anion exchange resin. Journal of Hazardous Materials. 2020, 384, 121386. 10.1016/j.jhazmat.2019.121386. [DOI] [PubMed] [Google Scholar]
- Peng X. F.; Zhang Y.; Fan B. Q.; Zheng S. L.; Wang X. J.; Zhang Y.; Li P.; Liu F. Q. Complexation separation for vanadium and chromium by dithiocarbamate and its application in treatment of chromium-vanadium-bearing slag. Transactions of Nonferrous Metals Society of China. 2019, 29, 2400–2410. 10.1016/S1003-6326(19)65146-0. [DOI] [Google Scholar]
- Fan Y.; Wang X.; Wang M. Separation and recovery of chromium and vanadium from vanadium-containing chromate solution by ion exchange. Hydrometallurgy. 2013, 136, 31–35. 10.1016/j.hydromet.2013.03.008. [DOI] [Google Scholar]
- Wang S. H.; Guo Y. F.; Zheng F. Q.; Chen F.; Yang L. Z.; Jiang T.; Qiu G. Z. Behavior of vanadium during reduction and smelting of vanadium titanomagnetite metallized pellets. Transactions of Nonferrous Metals Society of China 2020, 30, 1687–1696. 10.1016/S1003-6326(20)65330-4. [DOI] [Google Scholar]
- Dong Y.; Lin H.; Liu Y.; Zhao Y. Blank roasting and bioleaching of stone coal for vanadium recycling. Journal of Cleaner Production. 2020, 243, 118625. 10.1016/j.jclepro.2019.118625. [DOI] [Google Scholar]
- Wang G.; Diao J.; Liu L.; Li M.; Li H.; Li G.; Xie B. Highly efficient utilization of hazardous vanadium extraction tailings containing high chromium concentrations by carbothermic reduction. Journal of Cleaner Production. 2019, 237, 117832. 10.1016/j.jclepro.2019.117832. [DOI] [Google Scholar]
- Yang Z. C.; Zhao B. Review on the extraction of vanadium from V-bearing solid wastes. Guangzhou Chemical Industry. 2015, 43, 39–40. [Google Scholar]
- Chen B.; Bao S. X.; Zhang Y. M. Synergetic strengthening mechanism of ultrasound combined with calcium fluoride towards vanadium extraction from low-grade vanadium-bearing shale. International Journal of Mining Science and Technology. 2021, 31, 1095–1106. 10.1016/j.ijmst.2021.07.008. [DOI] [Google Scholar]
- Chen B.; Bao S.; Zhang Y.; Li S. A high-efficiency and sustainable leaching process of vanadium from shale in sulfuric acid systems enhanced by ultrasound. Separation and Purification Technology. 2020, 240, 116624. 10.1016/j.seppur.2020.116624. [DOI] [Google Scholar]
- Xue N. N.; Zhang Y. M.; Liu T.; Zheng Q. S. Efficient separation of black shale-hosted vanadium induced by formation of kalunite-jarosite solid solution in two-stage pressurized acid leaching coupled with lixivium recycling. Separation and Purification Technology. 2021, 269, 118762. 10.1016/j.seppur.2021.118762. [DOI] [Google Scholar]
- Mazurek K.; Białowicz K.; Trypuc M. Recovery of vanadium, potassium and iron from a spent catalyst using urea solution. Hydrometallurgy. 2010, 103, 19–24. 10.1016/j.hydromet.2010.02.008. [DOI] [Google Scholar]
- Liu M.; Wu J. L.; Yang F.; Huang W. X. Containing vanadium in vanadium bauxite efficient leaching experiments. Shandong Chemical Industry. 2015, 44, 112–115. [Google Scholar]
- Wang K. Q.; Li S. H. Experimental study on the vanadium recovery by hydrochloric acid leaching of alumina red mud. Rare Metals and Cemented Carbides. 2012, 40, 5–8. [Google Scholar]
- Wu E. H.; Hou J.; Li J.; Huang P. Experimental study on separation of iron and titanium from vanadium-titanium iron concentrate by hydrochloric acid leaching at atmospheric pressure. Iron Steel Vanadium Titanium. 2017, 38, 8–12. [Google Scholar]
- Xiao W. H.; Zhao H. X.; Song N.; Chen D. S.; Liu Y. H.; Wang L. N.; Qi T. Leaching of Fe, V and Ti from pre-reduced vanadium-bearing titanomagnetite bulk concentrate with HCl and H2SO4. Chinese Journal of Process Engineering. 2016, 16, 737–743. [Google Scholar]
- Wu E. H.; Hou J.; Li J.; Huang P.; Xu Z.; Liu Q. S.; Zhao L.; Yan X. Selective leaching of iron and titanium dioxide from vanadium-titanium magnetite ore using hydrochloric acid at atmospheric pressure. Hydrometallurgy of China. 2018, 37, 442–446. [Google Scholar]
- Li M.; Zhang B. G.; Zou S. Q.; Liu Q. S.; Yang M. Highly selective adsorption of vanadium(V) by nano-hydrous zirconium oxide-modified anion exchange resin. Journal of Hazardous Materials. 2020, 384, 121386. 10.1016/j.jhazmat.2019.121386. [DOI] [PubMed] [Google Scholar]
- Tian X.; Bao S. X.; Zhang Y. M. Selective adsorption mechanism of resin-activated carbon composite electrode for capacitive deionization. Colloids and Surfaces: A Physicochemical and Engineering Aspects. 2021, 610, 125935. 10.1016/j.colsurfa.2020.125935. [DOI] [Google Scholar]
- Gomes H. I.; Jones A.; Rogerson M.; Greenway G. M.; Lisbona D. F.; Burke I. T.; Mayes W. M. Removal and recovery of vanadium from alkaline steel slag leachates with anion exchange resins. Journal of Environmental Management. 2017, 187, 384–392. 10.1016/j.jenvman.2016.10.063. [DOI] [PubMed] [Google Scholar]
- Ju J. R.; Feng Y. L.; Li H. R.; Liu S. L.; Xu C. L. Separation and recovery of V, Ti, Fe and Ca from acidic wastewater and vanadium-bearing steel slag based on a collaborative utilization process. Separation and Purification Technology. 2021, 276, 119335. 10.1016/j.seppur.2021.119335. [DOI] [Google Scholar]
- Ying Z.; Chen M.; Wu G.; Li J.; Liu J.; Wei Q.; Ren X. Separation and recovery vanadium (V) and chromium (VI) using amide extractants based on the steric hindrance effect. Journal of Environmental Chemical Engineering. 2021, 9, 105939. 10.1016/j.jece.2021.105939. [DOI] [Google Scholar]
- Wen J.; Ning P.; Cao H.; Liu F.; Zhang Y. Modeling of liquid–liquid extraction of vanadium with primary amine N1923 in H2SO4 medium. Hydrometallurgy. 2018, 177, 57–65. 10.1016/j.hydromet.2018.02.013. [DOI] [Google Scholar]
- Li D.; Chen D. S.; Zhang G. Z.; Zhao H. X.; Qi T.; Wang W. J.; Wang L. N.; Liu Y. H. Separation of vanadium(IV) from Iron(II) in hydrochloric acid solution by solvent extraction with P507. Chinese Journal of Process Engineering. 2017, 17, 1182–1187. [Google Scholar]
- Liu Z.; Huang J.; Zhang Y.; Liu T.; Hu P.; Liu H.; Luo D. Separation and recovery of vanadium and aluminum from oxalic acid leachate of shale by solvent extraction with Aliquat 336. Separation and Purification Technology. 2020, 249, 116867. 10.1016/j.seppur.2020.116867. [DOI] [Google Scholar]
- Luo D. S.; Huang J.; Zhang Y. M.; Liu H.; Hu P. C. Efficient and environment-friendly vanadium(V) extraction from vanadium shale leachate using tri-n-octylmethylammonium chloride. Separation and Purification Technology. 2020, 237, 116482. 10.1016/j.seppur.2019.116482. [DOI] [Google Scholar]
- Zhang L.; Lv P.; He Y.; Li S.; Chen K.; Yin S. Purification of chlorine-containing wastewater using solvent extraction. Journal of Cleaner Production. 2020, 273, 122863. 10.1016/j.jclepro.2020.122863. [DOI] [Google Scholar]
- Zheng Q. S.; Zhang Y. M.; Xue N. N. Migration and coordination of vanadium separating from black shale involved by fluoride. Separation and Purification Technology. 2021, 266, 118552. 10.1016/j.seppur.2021.118552. [DOI] [Google Scholar]
- Zhu X. B.; Li W.; Zhang Q.; Zhang C. X.; Chen L. J. Separation characteristics of vanadium from leach liquor of red mud by ion exchange with different resins. Hydrometallurgy. 2018, 176, 42–48. 10.1016/j.hydromet.2018.01.009. [DOI] [Google Scholar]
- Zhu X. B.; Li W.; Tang S.; Zeng M. J.; Bai P. Y.; Chen L. J. Selective recovery of vanadium and scandium by ion exchange with D201 and solvent extraction using P507 from hydrochloric acid leaching solution of red mud. Chemosphere. 2017, 175, 365–372. 10.1016/j.chemosphere.2017.02.083. [DOI] [PubMed] [Google Scholar]
- Zou D.; Li H.; Chen J.; Li D. Recovery of scandium from spent sulfuric acid solution in titanium dioxide production using synergistic solvent extraction with d2ehpa and primary amine N1923. Hydrometallurgy. 2020, 197, 105463. 10.1016/j.hydromet.2020.105463. [DOI] [Google Scholar]
- Li C. C.; Yang L. F.; Wang Y.; Li H. Y.; Wang D. S.; Liu Y. Z.; Zhou X. Z.; Wang X. F.; Liu M. B.; Li Y. X. Extraction separation of rare earth and aluminum from ion-adsorbed rare earth leaching solution by primary amine N1923. Journal of the Chinese Society of Rare Earths. 2019, 37, 351–359. [Google Scholar]
- Jing X.; Wu Z.; Du J.; Wang X.; Jing X.; Ning P. Kinetics of V(V) extraction in V(V)-SO42--(Na+, H+)-primary amine N1923-sulfonated kerosene system using single drop technique. Separation and Purification Technology. 2019, 215, 473–479. 10.1016/j.seppur.2019.01.037. [DOI] [Google Scholar]
- Guo G. L.; Luo M. B.; Xu J. J. Recent progress of primary amine N1923 research. Fujian Analysis and testing. 2009, 18, 31–35. [Google Scholar]
- Cheng D. P.; Xia S. J. Study on the mechanism of mercury (II) absorption by primary amine N1923 extraction resin. Environmental Science. 1996, 17, 12–15. [Google Scholar]
- Li Y. M.; Zhao S. Y.; Wang L. Y.. Organic Chemistry; Science Press: Beijing, 2014; pp: 276–299. [Google Scholar]
- LI X.-b.; WEI C.; WU J.; LI C.-x.; LI M.-t.; DENG Z.-g.; XU H.-s. Thermodynamics and mechanism of vanadium(IV) extraction from sulphate medium with D2EHPA, EHEHPA and CYANEX 272 in kerosene. Transactions of Nonferrous Metals Society of China 2012, 22, 461–466. 10.1016/S1003-6326(11)61199-0. [DOI] [Google Scholar]
- Zhang Y.; Zhang T. A. Extraction of vanadium from the leaching solution from converter vanadium slag without roasting using N1923. Nonferrous Metals Science and Engineering 2015, 6, 14–19. [Google Scholar]