Abstract
Context
Bile acids (BA) are known for their role in intestinal lipid absorption and can also play a role as signaling molecules to control energy metabolism. Prior evidence suggests that alterations in circulating BA levels and in the pool of circulating BA are linked to an increased risk of obesity and a higher incidence of type 2 diabetes in middle-aged adults.
Objective
We aimed to investigate the association between plasma levels of BA with cardiometabolic risk factors in a cohort of well-phenotyped, relatively healthy young adults.
Methods
Body composition, brown adipose tissue, serum classical cardiometabolic risk factors, and a set of 8 plasma BA (including glyco-conjugated forms) in 136 young adults (age 22.1 ± 2.2 years, 67% women) were measured.
Results
Plasma levels of chenodeoxycholic acid (CDCA) and glycoursodeoxycholic acid (GUDCA) were higher in men than in women, although these differences disappeared after adjusting for body fat percentage. Furthermore, cholic acid (CA), CDCA, deoxycholic acid (DCA), and glycodeoxycholic acid (GDCA) levels were positively, yet weakly associated, with lean body mass (LBM) levels, while GDCA and glycolithocholic acid (GLCA) levels were negatively associated with 18F-fluorodeoxyglucose uptake by brown adipose tissue. Interestingly, glycocholic acid (GCA), glycochenodeoxycholic acid (GCDCA), and GUDCA were positively associated with glucose and insulin serum levels, HOMA index, low-density lipoprotein cholesterol, tumor necrosis factor alpha, interleukin (IL)-2, and IL-8 levels, but negatively associated with high-density lipoprotein cholesterol, ApoA1, and adiponectin levels, yet these significant correlations partially disappeared after the inclusion of LBM as a confounder.
Conclusion
Our findings indicate that plasma levels of BA might be sex dependent and are associated with cardiometabolic and inflammatory risk factors in young and relatively healthy adults.
Keywords: biomarkers, cardiometabolic risk, brown adipose tissue, dyslipidemia, insulin resistance
Obesity is a major health problem that increases the risk of cardiometabolic disorders such as dyslipidemia, insulin resistance, hypertension, and diseases such as type 2 diabetes (T2D) and cardiovascular diseases (CVD) (1). Even though CVD usually manifests clinically at middle-age, its onset takes place in the first decades of life (2). In fact, the incidence of CVD is worryingly increasing among the young population (2). Thus, early detection of alterations in the cardiometabolic profile of young individuals may allow the identification of high-risk individuals and treat them adequately (3). For this reason, the discovery and implementation of novel predictive biomarkers may help in detecting the onset of (cardio)metabolic diseases, enabling early prevention and intervention strategies (4).
Bile acids (BA) are synthesized in hepatocytes from cholesterol by enzymes of the cytochrome P450 family and can subsequently be conjugated with glycine (~75%) or taurine (~25%) to form primary BA that are secreted into the bile (5). Due to their emulsifying properties, BA support the intestinal absorption of dietary lipids and fat-soluble vitamins during the digestion process (6). Primary BA can be absorbed by enterocytes present in the ileum to be transported via the portal vein to hepatocytes (7). Alternatively, primary BA can be metabolized by gut microbiota to form secondary BA (8), which can be absorbed in the colon (7). The process in which primary and secondary BA are reabsorbed to again reach the liver is termed enterohepatic circulation. Primary and secondary BA also reach the systemic circulation (9) from where they can reach peripheral tissues and organs and exert signaling functions to regulate glucose and lipid metabolism (10, 11) predominantly mediated by nuclear and G protein-coupled receptors (GPCRs) (12).
Interestingly, several studies have shown significant differences in BA amount between individuals, in particular, higher circulating BA levels in obese than in lean females (13), and between the sexes, namely, higher circulating BA levels in males than in females (13, 14). Several observational studies have shown that higher levels and alterations in the pool of circulating BA are linked to an increased risk of obesity and a higher incidence of T2D (15). On the contrary, it has been reported that lower serum levels of total BA were associated with higher presence and severity of coronary artery disease and myocardial infarction (16). Bearing that in mind, a recent meta-analysis concluded that circulating BA levels are not associated with adiposity but with higher fecal BA excretion (17). Some studies have shown a link between BA and altered glucose metabolism, with levels of some circulating BA being positively associated with insulin resistance (18, 19). This observational evidence has led to interventions targeting BA metabolism through the use of BA sequestrants (20). Crucially, some of the improvements in glucose metabolism observed after bariatric surgery in T2D individuals might be related to changes in the pool of circulating BA (21). Despite these findings, the relationship between plasma BA with cardiometabolic risk factors in young adults, a population where the incidence of CVD is rapidly increasing, remains to be elucidated.
In the present study, we aimed to investigate the association between plasma levels of BA and cardiometabolic risk factors in a well-phenotyped cohort of relatively healthy young adults.
Methods
Study Population
This study was performed using baseline measurements from the ACTIBATE study that primarily aimed at investigating the effect of exercise on brown adipose tissue (BAT) activity (ClinicalTrials.gov ID: NCT02365129) (22). The study included 136 young healthy adults (45 male and 91 female participants), as shown in Table 1. All participants were recruited via advertisements in electronic media and leaflets, and all gave their written informed before enrollment. The inclusion criteria were: age from 18 to 25 years; being sedentary, that is, less than 20 minutes of moderate or vigorous physical activity on < 3 days/week (self-reported); not smoking; stable body weight over the past 3 months (changes < 3 kg); not presenting any cardiometabolic disease (eg, hypertension or diabetes); not taking any medication that might affect cardiovascular function; having no history of cancer among first-degree relatives. The study protocol and design were approved by the Human Research Ethics Committee of the University of Granada (n°924) and the Servicio Andaluz de Salud, in adherence with the Declaration of Helsinki (2013).
Table 1.
Age, anthropometry, body composition and cardiometabolic profile of the subjects included in the study (n = 136)
| All (n) | Mean | SD | Men (n) | Mean | SD | Women (n) | Mean | SD | P value (sex) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | 136 | 22.1 | 2.2 | 45 | 22.3 | 2.3 | 91 | 21.9 | 2.2 | 0.319 |
| ANTHROPOMETRY AND BODY COMPOSITION | ||||||||||
| Waist circumference, cm | 130 | 81.0 | 13.8 | 43 | 89.9 | 15.2 | 87 | 76.5 | 10.5 | <0.001 |
| BMI, kg/m2 | 136 | 24.9 | 4.6 | 45 | 26.8 | 5.5 | 91 | 23.9 | 3.7 | 0.002 |
| LBM, kg | 136 | 41.8 | 9.7 | 45 | 52.8 | 7.2 | 91 | 36.3 | 5.0 | <0.001 |
| LMI, kg/m2 | 136 | 14.7 | 2.4 | 45 | 17.2 | 2.1 | 91 | 13.5 | 1.4 | <0.001 |
| FMI, kg/m2 | 136 | 8.8 | 3.0 | 45 | 8.1 | 3.6 | 91 | 9.1 | 2.7 | 0.094 |
| Body fat mass, kg | 136 | 24.7 | 8.8 | 45 | 24.8 | 11.0 | 91 | 24.6 | 7.5 | 0.884 |
| Body fat percentage, % | 136 | 35.5 | 7.6 | 45 | 29.7 | 7.6 | 91 | 38.3 | 5.9 | <0.001 |
| VAT mass, g | 136 | 336 | 174 | 45 | 418 | 176 | 91 | 296 | 159 | <0.001 |
| CLINICAL PARAMETERS | ||||||||||
| Glucose, mg/dL | 132 | 87.6 | 6.6 | 43 | 88.9 | 7.4 | 89 | 87.0 | 6.1 | 0.134 |
| Insulin, μIU/mL | 132 | 8.3 | 4.9 | 43 | 9.1 | 6.4 | 89 | 8.0 | 4.0 | 0.638 |
| Total cholesterol, mg/dL | 132 | 165 | 32 | 43 | 160 | 31 | 89 | 168 | 33 | 0.183 |
| HDL-C, mg/dL | 132 | 53 | 11 | 43 | 46 | 7 | 89 | 56 | 11 | <0.001 |
| LDL-C, mg/dL | 132 | 96 | 25 | 43 | 97 | 26 | 89 | 96 | 25 | 0.990 |
| Triglycerides, mg/dL | 132 | 83 | 45 | 43 | 88 | 47 | 89 | 80 | 43 | 0.313 |
| HOMA index | 132 | 1.8 | 1.2 | 43 | 2.1 | 1.6 | 89 | 1.7 | 1.0 | 0.562 |
| C-reactive protein, mg/L | 132 | 2.4 | 3.4 | 43 | 2.1 | 2.3 | 89 | 2.5 | 3.8 | 0.937 |
| Systolic pressure, mm Hg | 134 | 117 | 12 | 44 | 125 | 11 | 90 | 113 | 10 | <0.001 |
| Diastolic pressure, mm Hg | 134 | 70.9 | 7.6 | 44 | 72.2 | 9.2 | 90 | 70.3 | 6.7 | 0.185 |
Data are presented as mean and SD. P values are obtained from analyses of the variance after log10 transformation of all blood parameters.
Abbreviations: ATP III, National Cholesterol Education Program Adult Treatment Panel III; BMI, body mass index; FMI, fat mass index; HDL-C, high-density lipoprotein cholesterol; HOMA, homeostatic model assessment; LDL-C, low-density lipoprotein cholesterol; LMI, lean mass index; VAT, visceral adipose tissue.
Cardiometabolic Risk Factors
Anthropometry and body composition
Waist circumference was measured in the minimum perimeter with a measuring tape (at 1-mm precision), at the end of a normal breath expiration, with the arms relaxed on both sides of the body. When the minimum perimeter could not be detected, such as in people who were overweight or obese, the measurements were taken just 2 cm above the umbilicus, following a horizontal plane. Body weight and height were measured using a SECA model 799 electronic column scale and a stadiometer (SECA, Hamburg, Germany). Lean body mass (LBM), body fat mass, and visceral adipose tissue were determined with a Hologic Discovery Wi dual-energy x-ray absorptiometer (DXA) (Hologic, Marlborough, MA, USA). Body mass index (BMI), lean mass index (LMI), and fat mass index (FMI) were calculated by dividing body weight, LBM, and body fat mass (in kg) by the square of the height (in cm), respectively. Body fat percentage was calculated as the body fat mass divided by the total body mass and multiplied by 100.
Brown adipose tissue assessment
Activation of BAT was assessed after a personalized cooling protocol for each participant, carried out on 2 independent days and extensively described elsewhere (23). Briefly, participants were first exposed for 30 minutes in a warm room to allow for acclimation, before the transfer to a mild-cold room. Participants then wore a cooling vest (Polar Products Inc., Stow, OH, USA) and the water temperature was slightly decreased until they reached a shivering state. After 48 to 72 hours, the participants went to the hospital where they were exposed to the same cooling protocol for 2 hours at ~4 ºC above their shivering threshold. After 1 hour of cold exposure, a bolus of ~185 MBq of 18F-fluorodeoxyglucose (18F-FDG) was injected, and a positron emission tomography/computed tomography (PET/CT) scan (Siemens Biograph 16 PET/CT, Siemens Healthcare, Erlangen, Germany) was performed 2 hours later. The 18F-FDG-PET/CT scans were analyzed using the open-source Beth Israel plugin for the FIJI program (24). The determination of BAT volume and 18F-FDG uptake was based on an individualized standardized uptake value (SUV) of [1.2/ (LBM/body mass)] (25), with a Hounsfield Unit range from −190 to −10. The BAT volume was determined as the number of pixels in this range with an SUV value above the SUV threshold. The BAT activity was determined as the mean SUV (SUVmean), that is, the mean quantity of 18F-FDG in the above same pixels, and the peak SUV (SUVpeak), that is, the mean of the 3 highest 18F-FDG uptake values in 3 pixels within a volume of <1 cm3. BAT radiodensity was calculated as a proxy of the fat content (26). We also quantified the 18F-FDG uptake in the descending aorta (as reference tissue), subcutaneous white adipose tissue in the dorsocervical and tricipital areas, as well as in several skeletal muscles (paracervical, sternocleidomastoid, scalene, longus colli, trapezius, parathoracic, supraspinatus, subscapular, deltoid, pectoralis major, and triceps braquis muscles at both the left and right sides of the body). Lastly, we grouped different skeletal muscles for these analyses as previously described (27).
Blood parameters
Blood samples were collected from the antecubital vein at baseline after at least 10 hours of fasting in the morning (8:00 to 9:00 am) (22). Samples were collected in Vacutainer Tubes and immediately centrifuged to obtain serum (Vacutainer SST II Advance tubes) and plasma (Vacutainer Hemogard tubes, containing potassium salt of EDTA as an anticoagulant), of which aliquots were stored at −80 °C until analyses. Serum samples were used for the assessment of blood cardiovascular risk factors (except for leptin and adiponectin, which were determined in plasma), while plasma samples were used for BA quantitation. A detailed description of all the parameter determinations is listed in the Supplementary Information (28).
Determination of Plasma Bile Acid Levels
Primary BA, namely, cholic acid (CA), chenodeoxycholic acid (CDCA), glycocholic acid (GCA), and glycochenodeoxycholic acid (GCDCA), as well as secondary BA, specifically, glycodeoxycholic acid (GDCA), deoxycholic acid (DCA), glycolithocholic acid (GLCA), and glycoursodeoxycholic acid (GUDCA), were assessed in plasma samples using a validated liquid chromatography–tandem mass spectrometry (LC-MS/MS) method. Supplementary Table 1 (28) lists the targeted BA. Full description of BA determinations is listed in Supplementary Information (28).
Statistical Analysis
Categorical and continuous variables were used to describe the clinical characteristics of the study participants. Since plasma BA levels, serum cardiometabolic, and inflammatory risk factors did not follow a normal distribution, we log10-transformed the variables to achieve normal distribution. We subsequently evaluated whether plasma BA levels were different between men and women using analyses of variance (ANOVA). Since body composition parameters differed between men and women, we next investigated whether observed sex differences in plasma BA levels persisted after adjusting for body composition parameters using analyses of covariance (ANCOVA). Since the association between plasma BA levels and cardiometabolic risk factors followed a similar direction in men and women separately (no statistical interaction, Supplementary Figure 1) (28), these analyses were also performed including both sexes together (all P > 0.05). Pearson correlations between plasma BA levels with cardiometabolic and inflammatory risk factors were performed using R software (R Studio, Boston, MA, USA). Multiple linear regressions were conducted to determine the association of plasma BA levels with cardiometabolic risk factors after adjusting for LBM and body fat percentage. BA correlation plots were built using the R package “corrplot”. Supplementary Figures 1 and 2 (28) were built using the GraphPad Prism version 8.0.0 for Windows (GraphPad Software, San Diego, California, USA). ANOVAs, ANCOVAs, and chi-square tests were performed with SPSS (v. 22.0, IBM SPSS Statistics, IBM), with a level of significance set at P < 0.05.
Results
The characteristics of the participants are shown in Table 1. Compared with women (n = 91), men (n = 45) had lower body fat percentage (−23%), lower serum HDL-C (−18%), and higher SBP (+11%) (all P < 0.001, Table 1). We did not find significant differences in the other cardiometabolic parameters (all P ≥ 0.05, Table 1).
Plasma Levels of Chenodeoxycholic Acid and Glycoursodeoxycholic Acid Are Higher in Men Than in Women
Plasma BA levels were similar between men and women (Table 2), except for CDCA and GUDCA, which were higher in men (+40% and +31%, respectively). Based on the body composition differences observed between sexes (Table 1) and to explore to what extent the different body components could be contributing to plasma levels of BA, we repeated these analyses including the BMI, LMI, and body fat mass as confounders, and the differences for CDCA and GUDCA persisted (data not shown). Intriguingly, the differences in plasma CDCA and GUDCA levels between men and women only disappeared when adjusting for body fat percentage (P = 0.119 and P = 0.141, respectively, Table 2).
Table 2.
Comparison of plasma levels of primary and secondary bile acids between men and women (n = 133)
| Men (n=43) | Women (n=90) | P | P 1 | ||||
|---|---|---|---|---|---|---|---|
| Bile Acid | Mean | SD | Mean | SD | |||
| PRIMARY (CA) | CA | 36.5 | 61.7 | 23.5 | 40.8 | 0.131 | 0.255 |
| GCA | 2.8 | 3.3 | 2.0 | 1.9 | 0.136 | 0.230 | |
| SECONDARY (CA) | DCA | 21.7 | 18.2 | 16.9 | 19.2 | 0.075 | 0.221 |
| GDCA | 3.0 | 2.4 | 2.0 | 2.1 | 0.067 | 0.288 | |
| PRIMARY (CDCA) | CDCA | 0.7 | 0.9 | 0.5 | 0.7 | 0.039 | 0.119 |
| GCDCA | 7.3 | 5.4 | 5.4 | 3.8 | 0.072 | 0.303 | |
| SECONDARY (CDCA) | GLCA | 5.1 | 4.6 | 4.6 | 4.3 | 0.374 | 0.976 |
| GUDCA | 21.3 | 17.1 | 16.2 | 20.3 | 0.010 | 0.141 |
Data are presented as mean and SD. P values are derived from analyses of variance (ANOVA). P1 values are derived from the analyses of covariance adjusting for body fat percentage. Plasma bile acids levels were log10 transformed.
Abbreviations: CA, cholic acid; GCA, glycocholic acid; CDCA, chenodeoxycholic acid; glycochenodeoxycholic acid. DCA, deoxycholic acid; GDCA, glycodeoxycholic acid, GLCA, glycolithocholic acid; GUDCA, glycoursodeoxycholic acid.
Plasma Levels of Bile Acids Are Positively Associated With the Amount of Lean Body Mass and Negatively Associated With Brown Adipose Tissue Levels
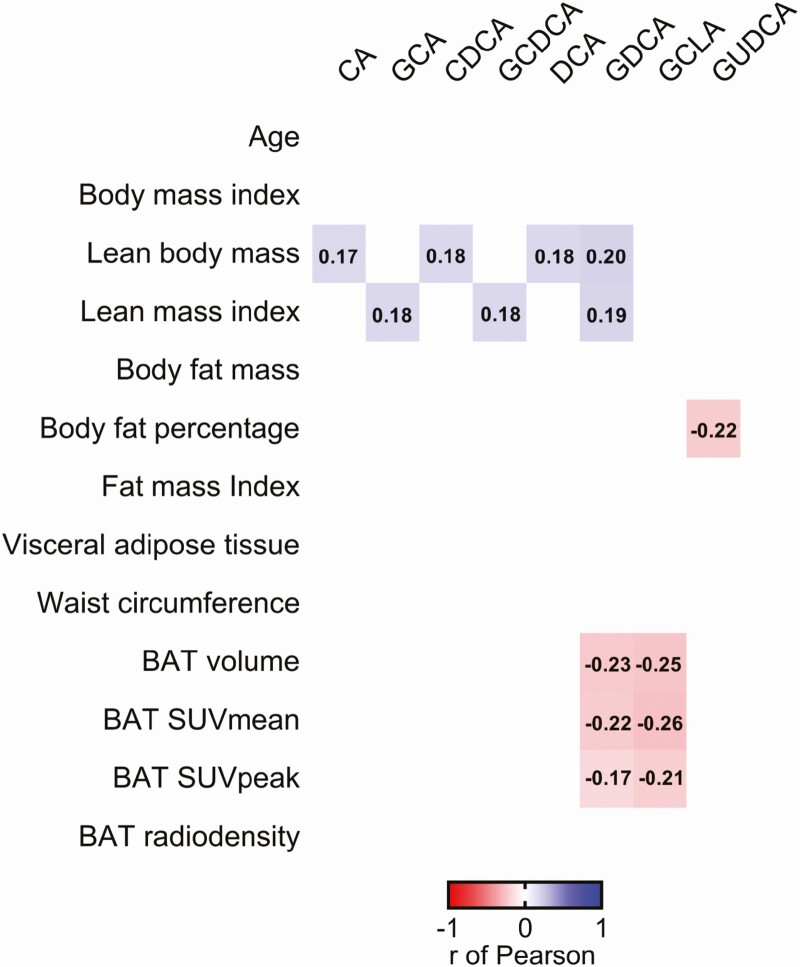
We found that plasma levels of CA, GCA, CDCA, GCDCA, DCA, and GDCA were positively correlated with LBM and LMI (0.17 ≤ r ≤ 0.20; P ≤ 0.049, Fig. 1), whereas only plasma levels of GUDCA were negatively correlated with body fat percentage (r = −0.22; P = 0.011, Fig. 1). It has been shown that a single dose of CDCA (15 mg/kg) increases human BAT 18F-FDG uptake (29), a thermogenic tissue associated with improved cardiometabolic health (30). In the present study, plasma levels of CDCA were not related to any BAT-related outcomes (all P ≥ 0.05, Fig. 1), while plasma levels of GDCA and GLCA were negatively correlated with BAT volume (r = −0.23; P = 0.009 and r = −0.25; P = 0.003, respectively), BAT SUV mean (r = −0.22; P = 0.012 and r = −0.26; P = 0.003, respectively) and BAT SUV peak (r = −0.17; P = 0.048 and r = −0.21; P = 0.017, respectively), but not with BAT radiodensity (all P ≥ 0.05; Fig. 1). We studied the correlation between plasma levels of BA with 18F-FDG uptake by other tissues (Supplementary Figure 2) (28). No associations were found between plasma BA levels and 18F-FDG uptake by skeletal muscle, subcutaneous adipose tissue at the dorsocervical or tricipital areas, or descending aorta as reference tissue (Supplementary Figure 2) (28), suggesting that plasma levels of GDCA and GLCA are solely related to BAT 18F-FDG uptake.
Figure 1.
Correlations between plasma levels of bile acids with body composition and brown adipose tissue parameters in young adults (n = 133). Every colored box represents a significant correlation coefficient (all P < 0.05), whereas invisible (white) boxes represent nonsignificant correlations. Values within the boxes express the r of Pearson coefficient. Bile acids concentration values were log10 transformed. Abbreviations: BAT, brown adipose tissue; CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; GCA, glycocholic acid; GCDCA, glycochenodeoxycholic acid; GDCA, glycodeoxycholic acid; GLCA, glycolithocholic acid; GUDCA, glycoursodeoxycholic acid; SUV, standardized uptake value.
The Association of Plasma Levels of Bile Acids With Cardiometabolic and Inflammatory Risk Factors Partially Disappear When Adjusting for Lean Body Mass
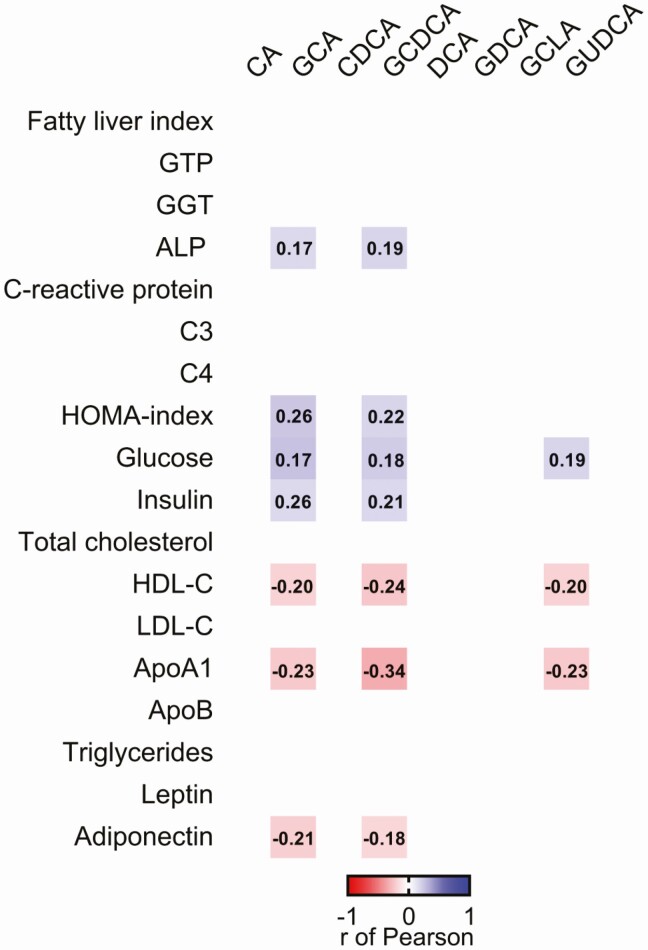
We found that plasma levels of GCA and GCDCA were positively correlated with the concentration of serum alkaline phosphatase, glucose, and insulin, as well as the HOMA index (0.17 ≤ r ≤ 0.26; P < 0.05, Fig. 2), while only plasma levels of GUDCA were positively correlated with serum glucose levels (r = 0.19; P = 0.031, Fig. 2). On the other hand, plasma levels of GCA, GCDCA, and GUDCA were negatively correlated with the serum HDL-C and ApoA1 levels (−0.34 ≤ r ≤ −0.20; P < 0.05, Fig. 2). Furthermore, plasma levels of GCA and GCDCA were negatively correlated with plasma levels of adiponectin (r = −0.21; P = 0.018 and r = −0.18; P = 0.045, respectively, Fig. 2). Additionally, we also found that plasma levels of GCA were positively correlated with plasma levels of the pro-inflammatory cytokines interleukin (IL)-8 and tumor necrosis factor alpha (r = 0.23; P = 0.015 and r = 0.21; P = 0.027), whereas the plasma levels of GDCA were positively related with IL-2 and IL-8 (r = 0.22; P = 0.025 and r = 0.21; P = 0.048) (Figure S3) (28). Since circulating BA are weakly correlated with LBM in this cohort (Fig. 1), we performed a sensitivity analysis, which showed that most of the significant correlations between plasma BA levels and cardiometabolic risk factors disappeared after adjusting for LBM (Table S2) (28), while all significant correlations remained when body fat percentage was included in the model. The correlations between plasma levels of BA and the inflammatory risk factors persisted after adjusting for LBM and body fat percentage (data not shown)
Figure 2.
Correlations between plasma levels of bile acids concentrations with cardiometabolic risk factors in young adults (n = 133). Every colored box represents a significant correlation coefficient (all P < 0.05), whereas invisible (white) boxes represent nonsignificant correlations. Values within the boxes represent the r of Pearson coefficient. All blood parameters were log10 transformed. Abbreviations: ALP, alkaline phosphatase; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; GCA, glycocholic acid; GCDCA glycochenodeoxycholic acid; GDCA, glycodeoxycholic acid; GGT, gamma-glutamyl transferase; GLCA, glycolithocholic acid; GTP, phosphoglycerate kinase; GUDCA, glycoursodeoxycholic acid; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Discussion
Here, we aimed to investigate whether plasma levels of BA were related to cardiometabolic risk factors in a well-phenotyped cohort of relatively healthy young adults. The findings indicate that CDCA and GUDCA levels were higher in men than in women, but these differences disappeared after adjusting for body fat percentage. We also observed that plasma BA levels were not related to adiposity; however, CA, CDCA, DCA, and GDCA levels were positively related to LBM. Furthermore, levels of GDCA and GLCA were negatively related to 18F-FDG-uptake by BAT. Interestingly, plasma levels of GCA, GCDCA, and GUDCA were weakly related to adverse cardiometabolic and inflammatory profiles, albeit these associations partially disappeared when LBM was included as a confounder in the model.
Plasma levels of CDCA and GUDCA were higher in men than in women, which is in concurrence with other studies (13, 14). A study that included individuals with a wide age range (20-70 years) showed that men had higher serum concentrations of CA, CDCA, UDCA, and GUDCA than women (13), while another study in older adults (53 ± 15 years) found that serum CA and CDCA levels were higher in men than women (14). In our study, the differences in plasma levels of CDCA and GUDCA disappeared when adjusting for body fat percentage. However, none of the aforementioned studies considered the differences in body fat composition between men and women in their analyses. Several possible hypotheses have been proposed to explain the higher BA levels in men compared to women, such as differences in (i) sex hormone levels (eg, estrogen and progesterone); (ii) medication (eg, statins); (iii) total cholesterol levels; or (iv) body fat distribution (13). Of special interest are the differences in levels of estrogen and progesterone in women, as their circulating levels exhibit significant variations during the premenopausal, menopause, and postmenopausal stages (31). Since the group of women included in the aforementioned studies significantly differed in menopause stages (13, 14), our results might be not comparable to cohorts with women within different menopause stages. Future studies should include estrogen and progesterone as potential confounders in their analyses, as well as other factors that can have an impact on them (eg, the use of contraceptive pills (32)). Furthermore, it is well known that the body fat distribution between men and women is different. Women generally have a larger body fat percentage as they are more likely to accumulate fat subcutaneously and on their lower extremities than men (33). Ideally, to study whether plasma levels of BA are different between men and women, both groups should be matched in terms of body fat composition, which was not the case (Table 1) in our study. Therefore, we adjusted the analysis for body composition outcomes (ie, BMI, LBM, and body fat percentage), which showed that body fat percentage could be partially explaining the differences observed in plasma levels of CDCA and GUDCA between men and women.
We observed that plasma levels of BA were not related to adiposity levels (Fig. 1). Accordingly, a recent meta-analysis, including 42 studies in humans, concluded that there are no significant differences in serum or plasma BA levels between obese and lean individuals. This meta-analysis study demonstrated that BA excretion in the feces was higher in obese individuals, suggesting that BA removal and/or production was higher upon obesity conditions (17). Therefore, future studies should include the measurement of BA both in blood and feces to allow the estimation of BA removal.
In this study, we found that plasma levels of CDCA and CA were not related to BAT, whereas plasma levels of GDCA and GLCA were negatively correlated with BAT. Preclinical studies have shown a link between BA and BAT activation, but findings were controversial (29, 34-36). Injection of CA in mice increased energy expenditure via the BA receptor Takeda G-protein receptor 5 (TGR5) present in BAT and skeletal muscle (34). Another study showed that the cardiometabolic benefits of the injection of CA were independent of BAT activation in diet-induced obese mice (35). Alternatively, mice fed with CDCA showed an increase in the uncoupling protein 1 (UCP1) gene expression levels in BAT, the molecular hallmark of BAT activity (36). A human study showed that the injection of CDCA (15 mg/kg) for 2 days increased BAT 18F-FDG uptake and energy expenditure in women (29), suggesting that CDCA injection enhances human cardiometabolic health via BAT activation. However, most of the studies that investigated the effect of BA on BAT metabolism used oral administration of BA (34-36), making the comparison between those findings and the findings of the current study impossible. Whether there exists a link between circulating CA, CDCA, GDCA, and GLCA and BAT activity at baseline conditions (which probably will differ from the response to a BA acute administration) remains to be further explored in humans.
Our results reveal that plasma levels of BA (GCA and GCDCA) are related to an adverse cardiometabolic and inflammatory profiles. However, when LBM was included as a confounder, many of these associations disappeared. Plasma levels of CA, DCA, and CDCA have been reported to be positively associated with homeostatic model assessment (HOMA) index and insulin levels in T2D patients (18). Similar to our study, plasma GCA, GDCA, and GUDCA levels were positively associated with HOMA index in a cohort of healthy adults (19). Additionally, it is known that BA can act as pro-inflammatory molecules when they are dysregulated (37) and can drive the expression of pro-inflammatory genes in hepatocytes (38), although this phenomenon should be further investigated. Curiously, none of these studies investigated whether those significant correlations were independent of body composition parameters. Preclinical studies have shown that BA can modulate insulin secretion, glucose homeostasis, and immune response via activation of the TGR5 and the farnesoid X receptor (FXR) (34, 39), supporting that the activation of these receptors by BA may be a possible therapy for combating cardiometabolic diseases. Additionally, our results suggest that the relationship of GCA and GCDCA with an adverse cardiometabolic profile could be driven by LBM. Since skeletal muscle represents approximately 40% of the total body mass in humans and is linked to lower insulin resistance (40) and expresses both FXR and TGR5 receptors (41), further research is needed to understand the physiological relevance of this association between plasma levels of BA and LBM.
Limitations
Our study suffers from an inherent limitation of a cross-sectional design and, thus, no causality can be established. The study population included young and relatively healthy adults; therefore, these findings may not be extrapolatable to even younger, older, or unhealthy people. We did not measure estrogen or progesterone levels in women, nor did we register whether they were taking contraception pills. Moreover, we only analyzed glycine-conjugated BA but not taurine-conjugated BA. Additionally, the 18F-FDG-PET/CT scan was static (a limitation in the estimation of cold-induced BAT metabolic activity (42)), and while 18F-FDG uptake is the most commonly used method, it also suffers from limitations in the assessment of BAT metabolic activity (43).
Conclusion
Our study reveals that plasma levels of BA might be sex dependent. Moreover, the findings indicate that plasma levels of BA are associated with cardiometabolic and inflammatory risk factors in young and relatively healthy adults. Further prospective studies are needed to confirm these results and to unveil the putative role of circulating BA in the onset and development of cardiometabolic diseases.
Acknowledgments
The authors would like to thank all the participants of this study for their time and effort.
Glossary
Abbreviations
- ANCOVA
analysis of covariance
- ANOVA
analysis of variance
- BA
bile acids
- BAT
brown adipose tissue
- BMI
body mass index
- CA
cholic acid
- CDCA
chenodeoxycholic acid
- CVD
cardiovascular disease
- DCA
deoxycholic acid
- 18F-FDG
18F-fluorodeoxyglucose
- FMI
fat mass index
- FXR
farnesoid X receptor
- GCA
glycocholic acid
- GCDCA
glycochenodeoxycholic acid
- GDCA
glycodeoxycholic acid
- GLCA
glycolithocholic acid
- GUDCA
glycoursodeoxycholic acid
- HDL-C
high-density lipoprotein cholesterol
- IL
interleukin
- LBM
lean body mass
- LMI
lean mass index
- PET/CT
positron emission tomography/computed tomography
- SUV
standardized uptake value
- T2D
type 2 diabetes
- TGR5
Takeda G-protein receptor 5
Financial Support
The study was supported by the Spanish Ministry of Economy and Competitiveness via Retos de la Sociedad (DEP2016-79512-R to J.R.R.) and European Regional Development Funds (ERDF), the Spanish Ministry of Education (FPU 16/02828), the University of Granada Plan Propio de Investigación 2016-Excellence actions: Unit of Excellence on Exercise and Health (UCEES), the Junta de Andalucía, Consejería de Conocimiento, Investigación y Universidades (ERDF: ref. SOMM17/6107/UGR), The Netherlands CardioVascular Research Initiative “the Dutch Heart Foundation, Dutch Federation of University Medical Centers, the Netherlands Organisation for Health Research and Development and the Royal Netherlands Academy of Sciences” (CVON2017-20 GENIUS-2) to P.C.N.R., and the Chinese Scholarship Council (CSC; No. 201707060012 to X.D., No. 201607060017 to W.Y.). B.M.T. is supported by an individual postdoctoral grant from the Fundación Alfonso Martin Escudero.
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
All supplementary information and extended methods are located in a digital research materials repository listed in “References.”
References
- 1. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288-298. [DOI] [PubMed] [Google Scholar]
- 2. Andersson C, Vasan RS. Epidemiology of cardiovascular disease in young individuals. Nat Rev Cardiol. 2018;15(4): 230-240. [DOI] [PubMed] [Google Scholar]
- 3. Wang J, Tan GJ, Han LN, Bai YY, He M, Liu HB. Novel biomarkers for cardiovascular risk prediction. J Geriatr Cardiol. 2017;14(2):135-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Perlis RH. Translating biomarkers to clinical practice. Mol Psychiatry. 2011;16(11):1076-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ðanić M, Stanimirov B, Pavlović N, et al. Pharmacological applications of bile acids and their derivatives in the treatment of metabolic syndrome. Front Pharmacol. 2018;9:1382. doi:10.3389/fphar.2018.01382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89(1):147-191. [DOI] [PubMed] [Google Scholar]
- 7. Dawson PA, Karpen SJ. Intestinal transport and metabolism of bile acids. J Lipid Res. 2015;56(6):1085-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24(1):41-50. [DOI] [PubMed] [Google Scholar]
- 9. van Berge-Henegouwen GP, Hofmann AF. Systemic spill-over of bile acids. Eur J Clin Invest. 1983;13(6):433-437. [DOI] [PubMed] [Google Scholar]
- 10. Ahmad TR, Haeusler RA. Bile acids in glucose metabolism and insulin signalling — mechanisms and research needs. Nat. Rev. Endocrinol. 2019;15(12):701-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jacinto S, Fang S. Essential roles of bile acid receptors FXR and TGR5 as metabolic regulators. Anim Cells Syst (Seoul). 2014;18(6):359-364. [Google Scholar]
- 12. Chiang JY. Bile acid metabolism and signaling. Compr Physiol. 2013;3(3):1191-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xie G, Wang Y, Wang X, et al. Profiling of serum bile acids in a healthy Chinese population using UPLC-MS/MS. J Proteome Res. 2015;14(2):850-859. [DOI] [PubMed] [Google Scholar]
- 14. Trottier J, Caron P, Straka RJ, Barbier O. Profile of serum bile acids in noncholestatic volunteers: gender-related differences in response to fenofibrate. Clin Pharmacol Ther. 2011;90(2):279-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chávez-Talavera O, Tailleux A, Lefebvre P, Staels B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology. 2017;152(7):1679-1694.e3. [DOI] [PubMed] [Google Scholar]
- 16. Li W, Shu S, Cheng L, et al. Fasting serum total bile acid level is associated with coronary artery disease, myocardial infarction and severity of coronary lesions. Atherosclerosis. 2020;292:193-200. [DOI] [PubMed] [Google Scholar]
- 17. So SSY, Yeung CHC, Schooling CM, El-Nezami H. Targeting bile acid metabolism in obesity reduction: a systematic review and meta-analysis. Obes Rev. 2020;21(7):e13017. [DOI] [PubMed] [Google Scholar]
- 18. Cariou B, Chetiveaux M, Zaïr Y, et al. Fasting plasma chenodeoxycholic acid and cholic acid concentrations are inversely correlated with insulin sensitivity in adults. Nutr Metab (Lond). 2011;8(1):48. doi:10.1186/1743-7075-8-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ginos BNR, Navarro SL, Schwarz Y, et al. Circulating bile acids in healthy adults respond differently to a dietary pattern characterized by whole grains, legumes and fruits and vegetables compared to a diet high in refined grains and added sugars: a randomized, controlled, crossover feeding study. Metabolism. 2018;83:197-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Molinaro A, Wahlström A, Marschall HU. Role of bile acids in metabolic control. Trends Endocrinol Metab. 2018;29(1):31-41. [DOI] [PubMed] [Google Scholar]
- 21. González-regueiro JA, Moreno-castañeda L, Uribe M, Chávez-tapia NC. The role of bile acids in glucose metabolism and their relation with diabetes. Ann Hepatol. 2019;16:S15-S20. [DOI] [PubMed] [Google Scholar]
- 22. Sanchez-delgado G, Martinez-tellez B, Olza J, et al. Activating brown adipose tissue through exercise (ACTIBATE) in young adults: rationale, design and methodology. Contemp. Clin. Trials. 2015;45:416-425. [DOI] [PubMed] [Google Scholar]
- 23. Martinez-Tellez B, Sanchez-Delgado G, Garcia-Rivero Y, et al. A new personalized cooling protocol to activate brown adipose tissue in young adults. Front. Physiol. 2017;8(NOV):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 2012;9(7):676-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen KY, Cypess AM, Laughlin MR, et al. Brown Adipose Reporting Criteria in Imaging STudies (BARCIST 1.0): recommendations for Standardized FDG-PET/CT experiments in humans. Cell Metab. 2016;24(2):210-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. U Din M, Raiko J, Saari T, et al. Human brown fat radiodensity indicates underlying tissue composition and systemic metabolic health. J Clin Endocrinol Metab 2017;102(7):2258-2267. [DOI] [PubMed] [Google Scholar]
- 27. Blondin DP, Labbé SM, Phoenix S, et al. Contributions of white and brown adipose tissues and skeletal muscles to acute cold-induced metabolic responses in healthy men. J Physiol. 2015;593(3):701-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Osuna-Prieto FJ, Rubio-Lopez J, Di X, et al. Data from: Plasma levels of bile acids are related to cardiometabolic risk factors in young adults. Figshare. Deposited October 4, 2021. 10.6084/m9.figshare.16732945.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Broeders EPM, Nascimento EBM, Havekes B, et al. The bile acid chenodeoxycholic acid increases human brown adipose tissue activity. Cell Metab. 2015;22(3):418-426. [DOI] [PubMed] [Google Scholar]
- 30. Becher T, Palanisamy S, Kramer DJ, et al. Brown adipose tissue is associated with cardiometabolic health. Nat Med. 2021;27(1):58-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Santoro N, Randolph JF Jr. Reproductive hormones and the menopause transition. Obstet Gynecol Clin North Am. 2011;38(3):455-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berg EG. The chemistry of the pill. ACS Cent. Sci. 2015;1(1):5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Power ML, Schulkin J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: possible evolutionary origins. Br J Nutr. 2008;99(5):931-940. [DOI] [PubMed] [Google Scholar]
- 34. Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484-489. [DOI] [PubMed] [Google Scholar]
- 35. Fromme T, Hüttinger K, Maurer S, et al. Bile acid supplementation decreases body mass gain in C57BL/6J but not 129S6/SvEvTac mice without increasing energy expenditure. Sci Rep. 2019;9(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Teodoro JS, Zouhar P, Flachs P, et al. Enhancement of brown fat thermogenesis using chenodeoxycholic acid in mice. Int J Obes (Lond). 2014;38(8):1027-1034. [DOI] [PubMed] [Google Scholar]
- 37. Chen ML, Takeda K, Sundrud MS. Emerging roles of bile acids in mucosal immunity and inflammation. Mucosal Immunol. 2019;12(4):851-861. [DOI] [PubMed] [Google Scholar]
- 38. Allen K, Jaeschke H, Copple BL. Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am J Pathol. 2011;178(1):175-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ma H, Patti ME. Bile acids, obesity, and the metabolic syndrome. Best Pract Res Clin Gastroenterol. 2014;28(4):573-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2011;96(9):2898-2903. [DOI] [PubMed] [Google Scholar]
- 41. Sasaki T, Kuboyama A, Mita M, et al. The exercise-inducible bile acid receptor Tgr5 improves skeletal muscle function in mice. J Biol Chem. 2018;293(26):10322-10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schilperoort M, Hoeke G, Kooijman S, Rensen PC. Relevance of lipid metabolism for brown fat visualization and quantification. Curr Opin Lipidol. 2016;27(3):242-248. [DOI] [PubMed] [Google Scholar]
- 43. Carpentier AC, Blondin DP, Virtanen KA, Richard D, Haman F, Turcotte ÉE. Brown adipose tissue energy metabolism in humans. Front Endocrinol (Lausanne). 2018;9:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All supplementary information and extended methods are located in a digital research materials repository listed in “References.”