Abstract
Context
The apparent increased incidence of congenital hypothyroidism (CH) is partly due to increased detection of transient disease.
Objective
This work aims to identify predictors of transient CH (T-CH) and establish a predictive tool for its earlier differentiation from permanent CH (P-CH).
Methods
A retrospective cohort study was conducted of patients diagnosed with CH from 2006 to 2015 through Newborn Screening Ontario (NSO).
Results
Of 469 cases, 360 (76.8%) were diagnosed with P-CH vs 109 (23.2%) with T-CH. Doses of levothyroxine predicting T-CH were less than 3.9 μg/kg at age 6 months, less than 3.0 μg/kg at ages 1 and 2 years, and less than 2.5 μg/kg at age 3 years. Descriptive statistics and multivariable logistic modeling demonstrated several diverging key measures between patients with T-CH vs P-CH, with optimal stratification at age 1 year. Thyroid imaging was the strongest predictor (P < .001). Excluding imaging, significant predictors in the first year of life included thyroxine dose/kg (P < .001-.002), increase in thyrotropin (TSH) above the reference interval during treatment (P = .002), screening TSH (P = .03), and a history of maternal thyroid disease (P = .02). Based on the 1-year model without imaging, a risk score was developed to identify children with T-CH who may benefit from an earlier trial off therapy, to reduce excess medicalization and health care costs.
Conclusion
A levothyroxine dose of less than 3 μg/kg at ages 1 and 2 years and less than 2.5 μg/kg at age 3 years can be predictive of T-CH. A novel risk score was developed that can be clinically applied to predict the likelihood of a successful trial off therapy for a given patient at age 1 year.
Keywords: newborn screening, transient, congenital hypothyroidism, risk score
Congenital hypothyroidism (CH) is a common pediatric condition characterized by a deficit or lack of production of thyroid hormone with the potential to cause permanent neurocognitive sequelae if not diagnosed and treated in a timely manner. This makes it an ideal condition for newborn screening (NBS) programs, with the first program starting in Quebec, Canada, in 1974 (1). CH screening in Ontario began in 1978 (2). Since then, the reported incidence of CH has increased appreciably from 1 in 6500 to 1 in 3000 (2-6) and several authors attribute this, at least in part, to the increased detection of transient CH (T-CH) (5, 6). T-CH can be caused by a variety of factors including iodine deficiency or excess, maternal TSH receptor–blocking antibodies or antithyroid drugs, neonatal factors such as intrauterine growth restriction or critical illness, and mild forms of dyshormonogenesis (7). For those with T-CH, there is eventual restoration of normal thyroid function, usually within a few months postnatally (7). Presently, clear guidelines on the identification and management of T-CH are lacking (5, 8-10). As a result, management has historically paralleled permanent CH (P-CH) with thyroxine treatment until age 3 years to ensure optimal growth and neurodevelopment (8, 9, 11).
Notably, case reports of patients with T-CH who discontinued treatment before age 3 years without medical supervision have not demonstrated any neurodevelopmental effects (12, 13). The consequences of delayed differentiation for those with T-CH include unnecessary medicalization, frequent blood work, daily levothyroxine supplementation, extensive follow-up, and increased use of medical resources, all which could be spared if not truly required (8, 9, 12, 13). Several studies have identified potential predictive factors for T-CH vs P-CH, including sex, gestational age (GA), birth weight (BW), prematurity, family history, screening thyrotropin level (TSH), diagnostic TSH and free thyroxine (T4), and levothyroxine dose requirements (14-24). Based on these data, in December 2020, the European Society of Paediatric Endocrinology (ESPE) CH consensus guidelines recommended a possible reevaluation, including a trial off levothyroxine therapy before age 3 years for those more likely to have transient disease, such as those with a gland in situ (GIS), negative first-degree family history of CH, and levothyroxine dose requirements of less than 3 μg/kg/day at age 6 months (10). The practice of early trial off levothyroxine therapy is still sometimes met with apprehension, given the potential neurodevelopmental consequences of a “failed” trial off, as well as the lack of robust predictors of T-CH. These concerns include, but are not limited to, variation in definition of levothyroxine dose associated with T-CH, smaller study sample sizes, and inclusion of specific populations, such as prematurity, and geographic considerations. Therefore, the objective of our study is to further evaluate potential predictors for the earlier differentiation of T-CH and to establish a predictive tool identifying the likelihood of T-CH to be used by clinicians who provide care to this patient population.
Materials and Methods
Study Design
A retrospective cohort study was performed on all newborns who screened positive for CH (dried-blood spot screening TSH ≥ 17 mIU/L) in Ontario, Canada, April 1, 2006 to September 1, 2015. Inclusion criteria included a confirmed diagnosis of CH (patients started on levothyroxine treatment at the discretion of their local endocrinologist based on confirmatory serum TSH and free T4) and follow-up through 1 of the 5 NSO treatment centers (tertiary care pediatric endocrinology clinics) for a minimum of 3 years. Participants were excluded from analysis if they were transferred to another center, lost to follow-up, had incomplete medical records before age 3 years, had transient hyperthyrotropinemia resolving within the first month of life, or were diagnosed with a co-morbid condition known to impact thyroid function (such as trisomy 21). Neonatal screening was carried out measuring TSH on dried-blood spot samples collected 24 to 72 hours after birth and analyzed using the Perkin-Elmer autoDELFIA immunoassay. Data collected consisted of diagnostic and long-term follow-up information from age 6 months to 3 years including sex, GA, BW, screening TSH, age at screening sample collection, diagnostic TSH and free T4 (with their respective reference intervals [RIs]), diagnostic thyroid imaging (if available), age at initiation of levothyroxine therapy, maternal thyroid disease during pregnancy, maternal thyroid medications during pregnancy, family history of thyroid disease (either congenital or acquired in a first- or second-degree relative), and genetic testing relevant to the thyroid. Follow-up occurred approximately every 3 to 4 months by a pediatric endocrinologist at 1 of the 5 NSO treatment centers. Follow-up information collected from the chart included thyroid function tests and their local RI, and levothyroxine dose per kilogram as documented during visits that occurred every 3 to 4 months by a pediatric endocrinologist at 1 of the 5 NSO treatment centers. Children aged 3 years or older underwent a trial off therapy unless permanent CH was previously diagnosed (see definitions described later).
This study was approved by the CHEO Research Ethics Board and Clinical Trials Ontario.
Definitions
Patients were defined as having P-CH if 1) their TSH rose above 8 mIU/L within 6 months of discontinuation of levothyroxine therapy AND the patient was restarted on levothyroxine treatment following the trial off therapy, OR 2) if they were diagnosed with P-CH without a trial off therapy at the discretion of their local endocrinologist with evidence of an ectopic or athyreotic gland on imaging, an increase in TSH above 10 mIU/L during levothyroxine treatment after age 6 months, and/or increasing thyroxine requirements over time to 50 μg/day or greater.
Patients were defined as having T-CH if their TSH and free T4 levels remained within the RI of the local diagnostic laboratory or less than 8 mIU/L for a minimum of 6 months after discontinuation of levothyroxine replacement therapy AND subsequently remained off levothyroxine treatment at time of discharge from their endocrinologist.
Patients were classified as indeterminate CH if 1) they did not have a trial off therapy after age 3 years but maintained a euthyroid state throughout treatment (TSH and free T4 levels always within the RI of the local diagnostic laboratory after age 6 months) AND did not have increasing thyroxine dose requirements over time, OR 2) had a trial off therapy with an increase in their TSH above the RI of the local diagnostic laboratory but less than 6 mIU/L with a normal free T4 AND restarted on levothyroxine treatment within 6 months of discontinuation of levothyroxine therapy. Owing to the diagnostic uncertainty of the indeterminate group, these patients were excluded from the final analysis.
Patients were defined as having a GIS if they underwent a nuclear medicine scan and/or an ultrasound demonstrating a GIS.
Statistical Analysis
Statistical analyses were performed using R software (25). For comparison of clinical parameters between T-CH and P-CH, continuous variables were expressed as the median interquartile range (IQR) and were analyzed via the Wilcoxon test, while categorical values were expressed using frequency (percentage) and analyzed via Pearson chi-square test. To identify the optimum cutoff values of continuous biochemical parameters including screening TSH and levothyroxine dose per kilogram, the Youden index (JMax) was calculated, representing ([sensitivity + specificity] – 1) from a receiver operating characteristic (ROC) curve, using T-CH as the dependent variable. To identify predictive factors associated with T-CH, multivariable logistic regression modelling was used with T-CH as the dependent variable. Modeling was completed for all patients at birth, at age 6 months, and at 1 year (± imaging), as well for patients with a GIS only at these time points. For analysis of predictors at birth, BW, GA, sex, known maternal thyroid disease, screening TSH, diagnostic TSH and free T4, and imaging were used as independent variables. For analysis of predictors at age 6 months, the aforementioned variables plus the levothyroxine dose per kilogram at age 6 months were used as test variables. For analysis of predictors at age 1 year, the aforementioned variables plus the levothyroxine dose per kilogram at age 1 year and a TSH increase above the RI between ages 6 months to 1 year were used as test variables. Discrimination performance of the models were assessed using the concordance statistic. A risk score was developed based on the predictors with P values of less than .1 in the 1-year multivariable logistic regression model without imaging. P values, odds ratios (OR), and their corresponding 95% CIs were calculated, with P values less than or equal to .05 considered statistically significant. The ORs compare the 75th to the 25th percentiles for continuous variables, and present vs absent for discrete variables.
Results
Patients
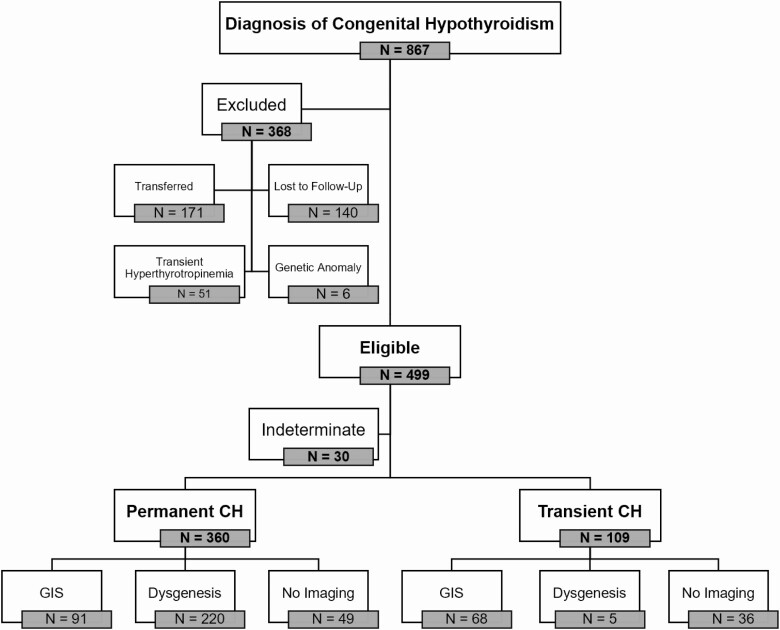
During the study period, 1921 newborns screened positive for CH, with 867 diagnosed with CH and started on levothyroxine; 499 met the inclusion criteria, of whom 30 were classified as indeterminate, leaving 469 (94%) eligible individuals included for analysis (Fig. 1). The clinical and biochemical characteristics of participants and subgroups are described in Table 1. The overall sex ratio was 61.4% in favor of girls with a greater discordance in those with dysgenesis than GIS (70.5% vs 53.4% female). The median GA was 39 weeks, with 33 (7.0%) born prematurely (< 37 weeks), and the median BW was 3.34 kg. Of the 469 participants, 384 (81.9%) had imaging performed, either at diagnosis or before age 3 years.
Figure 1.
Patient population and outcome. Of 1.36 million newborns screened, 867 were diagnosed with congenital hypothyroidism (CH) and started on treatment. Overall, 499 met inclusion criteria; 30 were classified as indeterminate, leaving 469 (94%) of eligible individuals included for analysis. At age 3 years, 360 of the 469 (76.8%) met the criteria for permanent CH and 109 (23.2%) were successfully trialed off levothyroxine and diagnosed with transient CH. Dysgenesis, ectopic thyroid or athyreosis on imaging; GIS, gland in situ on imaging; indeterminate CH, inconclusive or no trial off therapy at age 3 years.
Table 1.
Clinical characteristics of individuals with permanent and transient congenital hypothyroidism
| Variable | Permanent CH (n = 360) | n | Transient CH (n = 109) | n | P |
|---|---|---|---|---|---|
| Sex, % | 357 | 109 | |||
| Female | 228 (63.9) | 58 (53.2) | |||
| Male | 129 (36.1) | 51 (46.8) | |||
| Gestational age at birth, wk a | 40.0 (38.0-41.0) | 349 | 39.0 (38.0-40.0) | 109 | .032 |
| Birth weight, kg a | 3.3 (3.0-3.7) | 358 | 3.3 (2.9-3.6) | 109 | .187 |
| Maternal thyroid disease, % | 44 (12.4) | 354 | 27 (24.8) | 109 | < .002 |
| Screening TSH, mIU/L a | 118.9 (39.1-222.1) | 360 | 29.7 (19.8-61.0) | 109 | < .001 |
| Diagnostic TSH a | 100.0 (32.5-300.0) | 355 | 18.4 (12.0-74.6) | 109 | < .001 |
| Diagnostic FT4 value a | 11.2 (6.1-17.3) | 343 | 17.4 (12.7-21.9) | 108 | < .001 |
| Imaging results, % | 311 | 73 | |||
| GIS | 91 (29.3) | 68 (93.2) | |||
| Apparent athyreosis | 77 (24.8) | 5 (6.8) | |||
| Ectopic | 143 (46.0) | 0 (0.0) | |||
| TSH > RI after 6 mo, % | 328 (91.6) | 358 | 20 (18.3) | 109 | < .001 |
| Free T4 < RI after 6 mo, % | 11 (3.1) | 359 | 0 (0.0) | 109 | .064 |
| LT4 dose, μg/kg/d a | |||||
| 6 mo | 4.3 (3.4-5.4) | 354 | 3.3 (2.7-3.7) | 106 | < .001 |
| 1 y | 4.0 (3.2-4.8) | 350 | 2.7 (2.1-3.1) | 107 | < .001 |
| 2 y | 3.8 (3.2-4.5) | 352 | 2.1 (1.6-2.5) | 109 | < .001 |
| 3 y | 3.7 (3.1-4.3) | 337 | 2.0 (1.8-2.4) | 36 | < .001 |
| Maximum LT4 dose, μg/kg/d a | 4.8 (4.0, 5.7) | 360 | 3.3 (2.7-3.7) | 109 | < .001 |
| Increasing LT4 dose requirements, % | 332 (92.2) | 360 | 18 (16.5) | 109 | < .001 |
| LT4 dose ≥ 50 μg/d, % | 275 (76.4) | 360 | 2 (1.8) | 109 | < .001 |
P values from Wilcoxon test (if continuous) or Pearson chi-square test (if categorial).
Abbreviations: GIS, gland in situ; LT4, levothyroxine; RI, reference interval; T4, thyroxine; TSH, thyrotropin.
a Median interquartile range, % = frequency.
Permanent vs Transient Congenital Hypothyroidism
At age 3 years, 360 of 469 individuals (76.8%) met the criteria for P-CH, and 109 (23.2%) were successfully trialed off levothyroxine and diagnosed with T-CH. Overall, 91.6% (328 individuals) with P-CH vs 18.3% (20 individuals) with T-CH had a documented elevated TSH above the RI beyond age 6 months, 92.2% (332 individuals) vs 16.5% (18 individuals) required increasing levothyroxine doses over time, and 25.3% (91 individuals) vs 62.4% (68 individuals) had a GIS found on imaging. All patients with an ectopic gland (N = 143) had permanent disease. For those with apparent athyreosis (N = 82), 94.0% (77 individuals) had P-CH, and the remaining 6% with T-CH are presumed to have had maternal blocking antibodies present in the neonatal period.
At the time of diagnosis, significant differences between P-CH and T-CH included median screening TSH (118.9 vs 29.7 mIU/L, P < .001), median diagnostic TSH (100.0 vs 18.4 mIU/L, P < .001), diagnostic free T4 (11.2 vs 17.4 pmol/L, P < .001), and a positive maternal history of thyroid disease (10.7% vs 23.9%, P < .001; see Table 1). There were no significant differences in BW or GA. Analysis using ROC curves for screening TSH demonstrated an optimized screening TSH cutoff of less than 75 mIU/L to distinguish between T-CH and P-CH, with a sensitivity of 79.8% (0.71-0.86) and specificity of 61.1% (0.56-0.66).
There were significant differences in the mean levothyroxine dose per kilogram between P-CH and T-CH at all time points (see Table 1). Evaluating absolute doses of levothyroxine, the positive predictive value for T-CH for patients on a given dose at each time point is shown in Table 2.
Table 2.
Positive predictive values for transient congenital hypothyroidism based on daily levothyroxine dose and age
| Daily levothyroxine dose, μg | Age | |||
|---|---|---|---|---|
| 6-mo PPV, % | 1-y PPV, % | 2-y PPV, % | 3-y PPV, % | |
| < 25 | 58 | 73 | 90 | 100 |
| 25-< 37.5 | 38 | 54 | 74 | 70 |
| 37.5 | 10 | 8 | 19 | 20 |
| ≥ 37.5 | 9 | 6 | 5 | 4 |
Abbreviation: PPV, positive predictive value for transient congenital hypothyroidism.
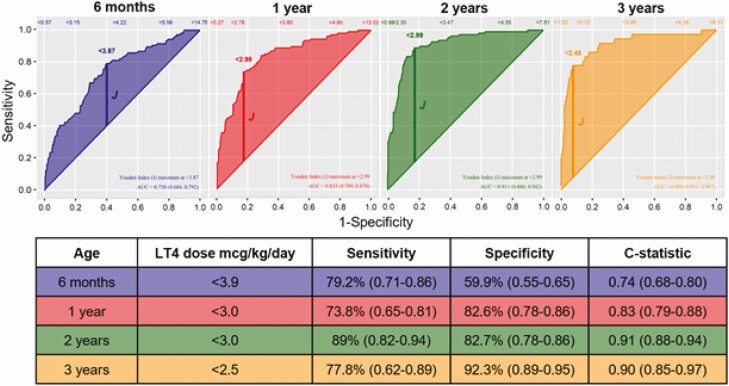
Analysis using ROC curves for levothyroxine dose over time are highlighted in Fig. 2. The levothyroxine doses per kilogram with the best combination of sensitivity and specificity for differentiating between T-CH and P-CH were 3.9 μg/kg at age 6 months, 3.0 μg/kg at both ages 1 and 2 years, and 2.5 μg/kg at age 3 years. The ROC curves for levothyroxine dose over time were also performed on GIS-only patients, revealing a lower levothyroxine dose cutoff at age 6 months (< 2.9 μg/kg), albeit with a significantly decreased sensitivity (42%). The dose cutoffs at ages 1, 2, and 3 years were comparable at less than 3.0 μg/kg (sensitivity 71%, specificity 66%), less than 2.7 μg/kg (sensitivity 78%, specificity 74%), and less than 2.5 μg/kg (sensitivity 69%, specificity 78%). Levothyroxine dose cut points for highest specificity and sensitivity are outlined in Table 3.
Figure 2.
Receiver operating characteristic curve of various thresholds of levothyroxine for predicting transient congenital hypothyroidism (T-CH). Optimal thresholds in favor of T-CH according to age and shown in the table. Levothyroxine (LT4) dose of less than 3 μg/kg at ages 1 and 2 years and less than 2.5 μg/kg at age 3 years is in favor of T-CH.
Table 3.
Daily levothyroxine dose thresholds for prediction of transient congenital hypothyroidism between ages 6 months and 3 years
| Age | Optimal cut point dose, μg/kg/d | Dose for 100% sensitivity for T-CH, μg//kg/d | Dose for 100% specificity for T-CH, μg//kg/d |
|---|---|---|---|
| 6 mo | < 3.9 | < 6.5 | < 0.4 |
| 1 y | < 3.0 | < 6.0 | < 0.1 |
| 2 y | < 3.0 | < 5.2 | < 1.1 |
| 3 y | < 2.5 | < 4.9 | < 1.5 |
Abbreviation: T-CH, transient congenital hypothyroidism.
Predictors of Transient Congenital Hypothyroidism
Multivariable logistic regression was completed at the time of diagnosis, at age 6 months, and at age 1 year to identify early predictors of T-CH. Recognizing that imaging is not always available, 3 models were performed: All patients with known imaging results (N = 404), all patients (not factoring in imaging results) (N = 469), and only those with known GIS (N = 159).
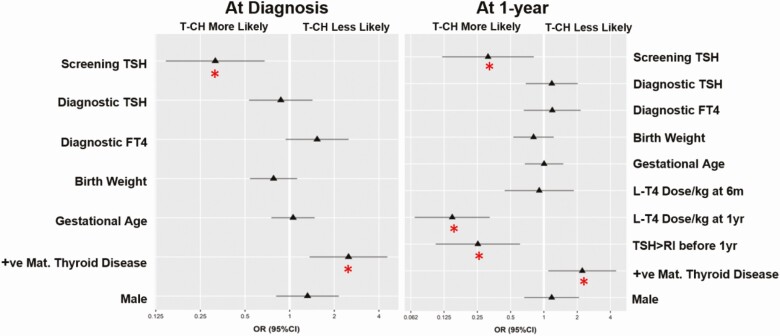
Modeling at the time of diagnosis with imaging (c = 0.84, P < .001) demonstrated the presence of thyroid dysgenesis (ectopic or athyreotic gland) to be such a strong predictor against T-CH (P < .001) that no other potential predictors reached significance; OR of 0.22 (0.06-0.49) and 0.07 (0.03-0.16) for an athyreotic or ectopic gland, respectively. When imaging results were not factored in (c = 0.76, P < .001), screening TSH (P = .003) and history of maternal thyroid disease were the next best predictors of T-CH (P = .003) with an OR of 0.32 (0.15-0.68) and an OR of 2.49 (1.36-4.56), respectively (Fig. 3). Notably, when looking at the GIS-only patients (c = 0.57, P = .795), there were no strong independent predictors of T-CH.
Figure 3.
Forest plots of odds ratios (95% CI) for predictors of transient congenital hypothyroidism (T-CH) for all patients at diagnosis and at age 1 year. Red asterisk represents predictors that were statistically significant (P < .05). The odds ratios compare the 75th to the 25th percentiles for continuous variables, absence of maternal thyroid disease, and (male) sex.
At age 6 months, in the model not factoring in imaging results (c = 0.81, P < .001), significant predictive factors included screening TSH (P = .005), OR of 0.31 (0.14-0.70); history of maternal thyroid disease (P = .001), OR of 2.95 (1.54-5.65); and levothyroxine dose per kilogram (P ≤ .001), OR of 0.28 (0.17-0.45). For patients with GIS (c = 0.635, P = .18), levothyroxine dose per kilogram is the only strong independent predictive factor (P = .01), OR of 0.47 (0.26-0.84).
At age 1 year, significant predictive measures in the model not factoring in imaging results (c = 0.87, P < .001; see Fig. 3) included screening TSH (P = .017), OR of 0.31 (0.12-0.81); history of maternal thyroid disease (P = .027), OR of 2.23 (1.10-4.52); thyroxine dose per kilogram at age 1 year (P ≤ .001), OR of 0.15 (0.07-0.32); and TSH above the upper limit of RI from age 6 months to 1 year (P = .002), OR of 0.25 (0.11-0.61). Notably, the levothyroxine dose per kilogram at age 6 months is no longer a strong predictive factor (P = .79) in the 1-year model. In those with a GIS (c = 0.74, P = .004), TSH above the upper limit of normal between age 6 months to 1 year and levothyroxine dose per kilogram at age 1 year remained strong independent predictors (P = .045), OR of 0.31 (0.10-0.97) and (P = .005), OR of 0.280 (0.11-0.69), respectively; however, maternal thyroid disease and screening TSH were no longer predictive.
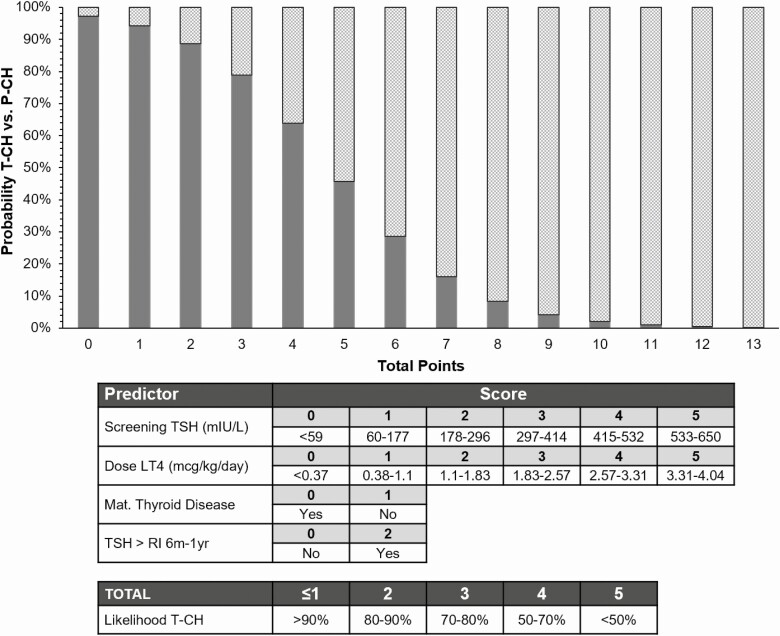
A risk score was generated from the data to predict the likelihood of T-CH at age 1 year for all individuals, assuming that no imaging was performed (Fig. 4). A score of 4 or lower indicates a greater than 50% chance the patient has T-CH, with a score of 2 or lower increasing this likelihood to greater than 80%.
Figure 4.
Risk score of probability of transient congenital hypothyroidism (T-CH) (solid) vs permanent congenital hypothyroidism (P-CH) (checkered) based on predictors with P less than .1 from multivariable logistic regression of patients aged 1 year (imaging excluded). LT4, levothyroxine; RI, reference interval; TSH, thyrotropin.
Discussion
Overall, our study showed that approximately 1 in 4 patients diagnosed with CH have transient disease, highlighting the need for earlier diagnostic reevaluation. This is comparable to the prevalence of T-CH cited in other studies (12%-53%), recognizing that our study was not exclusive to those with a GIS (14, 16-18, 21, 22). The prevalence of T-CH increases to approximately 1 in 2 patients when looking only at patients with a GIS, which is also consistent with previous reports analyzing eutopic patients only (38%-53%) (15, 20, 23, 24).
At the time of diagnosis, the most prominent differences between those with P-CH and T-CH included thyroid dysgenesis, screening TSH, diagnostic TSH and free T4, and history of maternal thyroid disease. Patients with T-CH demonstrated significantly lower screening and diagnostic TSH values and higher diagnostic free T4 levels, which is consistent with previous reports (18, 21). However, there was significant overlap and these values were not able to reliably distinguish P-CH and T-CH at the time of diagnosis. When thyroid imaging was excluded as a predictor at the time of diagnosis, screening TSH became a significant independent predictor for T-CH with a cut point of less than 75 mIU/L. We did not find an association of T-CH with lower GA or prematurity in our study population.
In our study, 81.9% of patients had thyroid imaging as part of their diagnostic workup. The presence of thyroid dysgenesis was the strongest reliable predictor for P-CH at the time of diagnosis. The presence of an ectopic or absent gland has a positive predictive value of 97.8% for permanent CH, which can give patients diagnostic clarity in this instance. The small number of patients (n = 5) with apparent athyreosis and T-CH reported is thought to most likely be due to the presence of maternal blocking antibodies. If imaging shows a GIS, no additional factors were able to reliably distinguish which of these patients was likely to have T-CH vs P-CH.
To maximize the generalizability of our scoring system, we excluded imaging from our model. The clinical benefit of imaging is debated and is not routinely performed in many centers. Arguments against routine imaging include lack of availability, radiation exposure, false-negative results in the presence of maternal blocking antibodies, and increased costs with lack of influence on immediate disease management (26-28). Therefore, the question is whether there are other indicators that could be used as a surrogate for imaging. Oron et al (18) reported a screening TSH cut point of greater than 63.5 as the only parameter to distinguish between dysgenesis and GIS at diagnosis. In our study, this cut point was greater than 75 mIU/L for the prediction of both dysgenesis and P-CH. Therefore, screening TSH may be helpful to distinguish between patients with GIS and dysgenesis. In keeping with other studies, knowing that a patient has a GIS at the time of diagnosis did not help to predict the likelihood of T-CH (18). Additional testing such as thyroglobulin levels or the presence of maternal blocking antibodies may further aid in the differentiation of P-CH and T-CH in the absence of thyroid imaging in the future.
From age 6 months to 3 years, infants in the T-CH group consistently received lower levothyroxine doses per kilogram during the entirety of the treatment period in comparison to those with P-CH, which is consistent with previous reports (15-23, 29). We report similar levothyroxine dose cutoffs predicting T-CH as less than 3.9 μg/kg at age 6 months, less than 3.0 μg/kg at ages 1 and 2 years, and less than 2.5 μg/kg at age 3 years. On average, the cutoff values reported in our study cohort are on the higher end of the spectrum in comparison to others (especially at age 6 months); however, our study sample is much larger and is not exclusive to patients with a GIS, and thus may be overall more representative. For example, one study that analyzed 17 patients with T-CH reported a levothyroxine dose cutoff of more than 2.2 μg/kg at age 6 months in those with GIS to be predictive of P-CH; however, when the researchers expanded their population to include all patients, this cutoff became greater than 4.4 μg/kg (18). In our study, when analyzing only patients with a GIS, our cutoff decreases to less than 2.9 μg/kg at age 6 months to be predictive of T-CH. A clinically practical threshold we identified is that more than 90% of children with an absolute dose of levothyroxine greater than or equal to 37.5 μg after age 6 months and nearly all children with an absolute dose of 50 μg or greater at any point have permanent disease. Those maintained on a dose of 25 μg or less at any age have a high likelihood of transient disease. This can help inform families of the likelihood of permanent disease early on, even without imaging.
Overall, the strongest and greatest number of independent predictors for T-CH (all patients, not factoring in imaging) appeared at the 1-year mark. We therefore chose this multivariable logistic regression model and its associated significant predictive factors (P < .1) to develop our risk score predicting the likelihood of T-CH. While there were statistically significant predictors noted at age 6 months, there were several factors noted within our data set suggesting that developing a risk score based on this time point was inferior. For example, the levothyroxine dose per kilogram at age 6 months was no longer an independent predictor when incorporated into the 1-year multivariable logistic regression model. Moreover, analyses of the descriptive characteristics of those with T-CH vs P-CH demonstrated an overlap in the IQRs for the levothyroxine dose per kilogram at age 6 months, but by age 1 year, this overlap of the IQRs disappears. Additionally, the sensitivities and specificities of the thyroxine dose per kilogram cutoff were stronger and more consistent at age 1 year than at age 6 months. This is important to highlight because the current EPSE guidelines suggest a possible early trial off therapy at age 6 months if the levothyroxine dose is less than 3 μg/kg for patients with a GIS (10). Applying the current ESPE guidelines to our data set, only 35 of our 469 participants were on these low doses; 27 (77.1%) were diagnosed with T-CH and 8 (22.9%) diagnosed with P-CH. Moreover, a 3-μg/kg cutoff captures only 24.8% of our participants diagnosed with transient disease. The levothyroxine dose cut point at age 6 months in our data set was higher at 3.9 μg/kg/day. A possible explanation for this observation in our study population includes targeting lower TSH or higher free T4 levels while on treatment. Altogether, our data suggest that waiting until age 1 year may result in a greater likelihood of a successful early trial off therapy for patients with T-CH, especially if imaging is not available.
There are several limitations to our study, including but not limited to its retrospective nature and variation in management both over time and between centers. Examples of this variation include changing guidelines with respect to TSH targets while on treatment, decreasing use of diagnostic thyroid imaging, lack of standardization of TSH and free T4 assays due to the real-world nature of follow-up in a broad geographic area, and variations in clinical practice regarding a trial off therapy and thresholds to restart levothyroxine. We attempted to address some of these limitations by applying strict criteria to classify those with T-CH and P-CH, and categorizing follow-up and diagnostic TSH and free T4 values as normal or abnormal to accommodate differences in RIs across centers. Of note, screening TSH was universally completed at NSO and therefore does not have this limitation. Our risk score applies to primary TSH screening with samples collected beyond age 24 hours. Moreover, practice variation is important for practitioners to consider when assessing the generalizability of our results to their respective practices, including variation in screening methodology, prescribing practices, and product availability.
Our study is one of the largest to date evaluating predictors of transient CH, and applies to all patients, not just those with a known GIS. It is the first to develop a predictive risk score for T-CH, to aid clinicians in facilitating an earlier trial off levothyroxine, especially given the current trend to exclude imaging in diagnostic protocols. Additional strengths of our study include that it is multicenter, allowing for increased generalizability, and analyzed TSH and free T4 levels during treatment, which has not often been completed in other studies, especially noting that an increase in TSH above the RI from age 6 months to 1 year proved to be a strong predictive factor for permanent disease.
Next steps include the prospective evaluation of the TSH and levothyroxine dose cut points, as well as our predictive risk score. Following this, it will be imperative to study both the short- and long-term outcomes of an early trial off levothyroxine. Moreover, analysis of the level of satisfaction and the cost savings from the health care, clinician, patient, and family perspectives with an early trial off therapy would be valuable when analyzing future changes to the diagnosis and management of CH.
Conclusion
Based on the results of this study, approximately 1 in 4 children treated for congenital hypothyroidism have transient disease and likely can be trialed off levothyroxine therapy earlier than current recommendations. At the time of diagnosis, in the absence of known thyroid dysgenesis, there are no clear predictors of T-CH; therefore, the decision regarding delayed treatment initiation and monitoring of thyroid function should be based on current guidelines, with the option of conservative monitoring if the TSH is less than 20 with normal free T4 levels (8). Our study identified the screening TSH, a history of maternal thyroid disease, the levothyroxine dose per kilogram (per 1-μg/kg increase), and an increase in TSH above the RI during treatment from age 6 months to 1 year as key predictors of T-CH. Moreover, a levothyroxine dose of less than 3 μg/kg at ages 1 and 2 years and less than 2.5 μg/kg at age 3 years can be predictive of T-CH. Applying our risk score, those with 3 points or less have a greater than 75% chance of having transient disease at age 1 year and can likely undergo a supervised trial off therapy, thereby helping clinicians feel more confident in initiating an earlier trial off in these patients.
Acknowledgments
Financial Support: The authors received no financial support for the research, authorship, and/or publication of this article.
Glossary
Abbreviations
- BW
birth weight
- CH
congenital hypothyroidism
- ESPE
European Society of Paediatric Endocrinology
- GA
gestational age
- GIS
gland in situ
- IQR
interquartile range
- NBS
newborn screening
- NSO
Newborn Screening Ontario
- OR
odds ratio
- P-CH
permanent congenital hypothyroidism
- RI
reference interval
- ROC
receiver operating characteristic
- T4
thyroxine
- T-CH
transient congenital hypothyroidism
- TSH
thyrotropin
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Dussault JH. The anecdotal history of screening for congenital hypothyroidism. J Clin Endocrinol Metab. 1999;84(12):4332-4334. [DOI] [PubMed] [Google Scholar]
- 2. Newborn Screening Ontario. Newborn Screening Manual. 2nd ed. 2018. https://www.newbornscreening.on.ca/en/publications/newborn-screening-manual [Google Scholar]
- 3. Grosse SD, Van Vliet G. Prevention of intellectual disability through screening for congenital hypothyroidism: how much and at what level? Arch Dis Child. 2011;96(4):374-379. [DOI] [PubMed] [Google Scholar]
- 4. Goldenberg AJ, Comeau AM, Grosse SD, et al. Evaluating harms in the assessment of net benefit: a framework for newborn screening condition review. Matern Child Health J. 2016;20(3):693-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mitchell ML, Hsu HW, Sahai I; Massachusetts Pediatric Endocrine Work Group . The increased incidence of congenital hypothyroidism: fact or fancy? Clin Endocrinol (Oxf). 2011;75(6):806-810. [DOI] [PubMed] [Google Scholar]
- 6. Parks JS, Lin M, Grosse SD, et al. The impact of transient hypothyroidism on the increasing rate of congenital hypothyroidism in the United States. Pediatrics. 2010;125(Suppl 2):S54-S63. [DOI] [PubMed] [Google Scholar]
- 7. Lawrence SE, von Oettingen JE, Deladoëy J.. Fetal and Postnatal Disorders of Thyroid Function. Maternal-Fetal and Neonatal Endocrinology. Elsevier; 2020:735-754. [Google Scholar]
- 8. Saleh DS, Lawrence S, Geraghty MT, et al. Prediction of congenital hypothyroidism based on initial screening thyroid-stimulating-hormone. BMC Pediatr. 2016;16:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lain S, Trumpff C, Grosse SD, Olivieri A, Van Vliet G. Are lower TSH cutoffs in neonatal screening for congenital hypothyroidism warranted? Eur J Endocrinol. 2017;177(5):D1-D12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Trotsenburg P, Stoupa A, Léger J, et al. Congenital hypothyroidism: a 2020-2021 consensus guidelines update—an ENDO-European Reference Network initiative endorsed by the European Society for Pediatric Endocrinology and the European Society for Endocrinology. Thyroid. 2021;31(3):387-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Léger J, Olivieri A, Donaldson M, et al. ; ESPE-PES-SLEP-JSPE-APEG-APPES-ISPAE; Congenital Hypothyroidism Consensus Conference Group . European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. Horm Res Paediatr. 2014;81(2):80-103. [DOI] [PubMed] [Google Scholar]
- 12. Kemper AR, Ouyang L, Grosse SD. Discontinuation of thyroid hormone treatment among children in the United States with congenital hypothyroidism: findings from health insurance claims data. BMC Pediatr. 2010;10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Korzeniewski SJ, Grigorescu V, Kleyn M, et al. Transient hypothyroidism at 3-year follow-up among cases of congenital hypothyroidism detected by newborn screening. J Pediatr. 2013;162(1):177-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fu C, Luo S, Li Y, et al. The incidence of congenital hypothyroidism (CH) in Guangxi, China and the predictors of permanent and transient CH. Endocr Connect. 2017;6(8):926-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cho MS, Cho GS, Park SH, Jung MH, Suh BK, Koh DG. Earlier re-evaluation may be possible in pediatric patients with eutopic congenital hypothyroidism requiring lower L-thyroxine doses. Ann Pediatr Endocrinol Metab. 2014;19(3):141-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Itonaga T, Higuchi S, Shimura K, et al. Levothyroxine dosage as predictor of permanent and transient congenital hypothyroidism: a multicenter retrospective study in Japan. Horm Res Paediatr. 2019;92(1):45-51. [DOI] [PubMed] [Google Scholar]
- 17. Messina MF, Aversa T, Salzano G, et al. Early discrimination between transient and permanent congenital hypothyroidism in children with eutopic gland. Horm Res Paediatr. 2015;84(3):159-164. [DOI] [PubMed] [Google Scholar]
- 18. Oron T, Lazar L, Ben-Yishai S, et al. Permanent vs transient congenital hypothyroidism: assessment of predictive variables. J Clin Endocrinol Metab. 2018;103(12):4428-4436. [DOI] [PubMed] [Google Scholar]
- 19. Park ES, Yoon JY. Factors associated with permanent hypothyroidism in infants with congenital hypothyroidism. BMC Pediatr. 2019;19(1):453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saba C, Guilmin-Crepon S, Zénaty D, et al. Early determinants of thyroid function outcomes in children with congenital hypothyroidism and a normally located thyroid gland: a regional cohort study. Thyroid. 2018;28(8):959-967. [DOI] [PubMed] [Google Scholar]
- 21. Zdraveska N, Zdravkovska M, Anastasovska V, Sukarova-Angelovska E, Kocova M. Diagnostic re-evaluation of congenital hypothyroidism in Macedonia: predictors for transient or permanent hypothyroidism. Endocr Connect. 2018;7(2):278-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kang MJ, Chung HR, Oh YJ, Shim YS, Yang S, Hwang IT. Three-year follow-up of children with abnormal newborn screening results for congenital hypothyroidism. Pediatr Neonatol. 2017;58(5):442-448. [DOI] [PubMed] [Google Scholar]
- 23. Higuchi S, Hasegawa Y. Levothyroxine dosages less than 2.4 μg/kg/day at 1 year and 1.3 μg/kg/day at 3 years of age may predict transient congenital hypothyroidism. Clin Pediatr Endocrinol. 2019;28(4):127-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rabbiosi S, Vigone MC, Cortinovis F, et al. Congenital hypothyroidism with eutopic thyroid gland: analysis of clinical and biochemical features at diagnosis and after re-evaluation. J Clin Endocrinol Metab. 2013;98(4):1395-1402. [DOI] [PubMed] [Google Scholar]
- 25. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2021. [Google Scholar]
- 26. Goldis M, Waldman L, Marginean O, Rosenberg HK, Rapaport R. Thyroid imaging in infants. Endocrinol Metab Clin North Am. 2016;45(2):255-266. [DOI] [PubMed] [Google Scholar]
- 27. Chang YW, Lee DH, Hong YH, Hong HS, Choi DL, Seo DY. Congenital hypothyroidism: analysis of discordant US and scintigraphic findings. Radiology. 2011;258(3):872-879. [DOI] [PubMed] [Google Scholar]
- 28. Livett T, LaFranchi S. Imaging in congenital hypothyroidism. Curr Opin Pediatr. 2019;31(4):555-561. [DOI] [PubMed] [Google Scholar]
- 29. Hong SY, Chung HR, Lee SY, Shin CH, Yang SW. Factors distinguishing between transient and permanent hypothyroidism in patients diagnosed as congenital hypothyroidism by newborn screening. J Korean Soc Pediatr Endocrinol 2005;10(2):154-160. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.