Abstract
Purpose
In X-linked hypophosphatemia (XLH), excess fibroblast growth factor-23 causes hypophosphatemia and low calcitriol, leading to musculoskeletal disease with clinical consequences. XLH treatment options include conventional oral phosphate with active vitamin D, or monotherapy with burosumab, a monoclonal antibody approved to treat children and adults with XLH. We have previously reported outcomes up to 64 weeks, and here we report safety and efficacy follow-up results up to 160 weeks from an open-label, multicenter, randomized, dose-finding trial of burosumab for 5- to 12-year-old children with XLH.
Methods
After 1 week of conventional therapy washout, patients were randomized 1:1 to burosumab every 2 weeks (Q2W) or every 4 weeks (Q4W) for 64 weeks, with dosing titrated based on fasting serum phosphorus levels between baseline and week 16. From week 66 to week 160, all patients received Q2W burosumab.
Results
Twenty-six children were randomized initially into each Q2W and Q4W group and all completed treatment to week 160. In 41 children with open distal femoral and proximal tibial growth plates (from both treatment groups), total Rickets Severity Score significantly decreased by 0.9 ± 0.1 (least squares mean ± SE; P < 0.0001) from baseline to week 160. Fasting serum phosphorus increases were sustained by burosumab therapy throughout the study, with an overall population mean (SD) of 3.35 (0.39) mg/dL, within the pediatric normal range (3.2-6.1 mg/dL) at week 160 (mean change from baseline P < 0.0001). Most adverse events were mild to moderate in severity.
Main conclusions
In children with XLH, burosumab administration for 160 weeks improved phosphate homeostasis and rickets and was well-tolerated. Long-term safety was consistent with the reported safety profile of burosumab.
Clinicaltrials.gov
Keywords: hypophosphatemia, calcitriol, phosphate, rickets, growth, osteomalacia
X-linked hypophosphatemia (XLH), initially described in 1937, features impaired phosphate homeostasis and is the most common heritable form of rickets/osteomalacia (1-3). XLH is caused by loss-of-function mutations in the phosphate regulating endopeptidase homolog X-linked (PHEX) gene, leading to increased circulating levels of fibroblast growth factor-23 (FGF-23). Increased FGF-23 activity impairs renal phosphate reabsorption and decreases synthesis of 1,25-dihydroxyvitamin D, abbreviated 1,25(OH)2D (also known as calcitriol), resulting in chronic hypophosphatemia. In affected children, musculoskeletal manifestations include rickets, lower limb deformity, impaired growth, and bone and joint pain. Other complications include craniosynostosis, dental abscesses, and limited physical functioning (4-7).
Beginning in the 1980s, Glorieux and colleagues showed that treatment with oral phosphate and calcitriol improved rickets in patients with XLH, establishing a treatment standard for decades (8). Although this approach ameliorates some skeletal abnormalities, responses to therapy and improved outcomes depend on the severity of rickets and the skill of the treating physician. Treatment with oral phosphate and active vitamin D also requires multiple daily treatment doses, and a lack of patient adherence can be problematic. Furthermore, this therapy can induce nephrocalcinosis and hyperparathyroidism and requires frequent monitoring and dose adjustments to assess and ensure safety and efficacy (3, 9, 10).
Burosumab, a fully human IgG1 monoclonal antibody to FGF23, was approved by various international regulatory authorities beginning in 2018 to treat XLH and tumor-induced osteomalacia in children and adults (11). Previously, we reported that in 2 phase 2 open-label trials in children with XLH 1 to 4 years old (NCT02750618) and 5 to 12 years old (NCT02163577), administration of burosumab for 64 weeks improved phosphate homeostasis, rickets severity, lower limb deformity, growth, and physical functioning (12, 13). Additionally, we conducted a randomized, active-controlled, phase 3, open-label trial in children 1 to 12 years old (NCT02915705) with XLH and a total Thacher Rickets Severity Score (RSS) of ≥ 2.0. This study showed that 64 weeks of burosumab treatment significantly improved phosphate homeostasis, rickets, lower limb deformity, growth, and mobility compared with administration of oral phosphate and active vitamin D (14). Here, we report the end-of-study results from the phase 2, open-label trial (NCT02163577) in children 5 to 12 years old with XLH who were treated with burosumab for >3 years, the longest treatment duration reported to date in this patient population (13, 15).
Materials and Methods
Study UX023-CL201 began as an open-label, multicenter, randomized, dose-finding trial of the efficacy and safety of burosumab in 52 children 5 to 12 years old with XLH; details of the study design were previously published (13). Briefly, eligible children with evidence of rickets were Tanner stage 2 or less based on breast or testicular development, and were required to discontinue oral phosphate supplements and active vitamin D metabolites or analogues during the screening period.
Children were randomized to receive burosumab injections subcutaneously either every 2 weeks (Q2W) or every 4 weeks (Q4W) for the first 64 weeks. During an initial 16-week dose-escalation period, the dose of burosumab was increased according to the following: 0.1 mg/kg, 0.2 mg/kg, and 0.3 mg/kg for the Q2W regimen; and 0.2 mg/kg, 0.4 mg/kg, and 0.6 mg/kg for the Q4W regimen; and was further titrated with the goal to identify a burosumab dose that maintained fasting serum phosphorus concentrations in the target range of 3.5 to 5.0 mg/dL (1.13-1.62 mmol/L).
With biochemical evidence that serum phosphorus concentrations were more stable over time with the Q2W regimen, children in the Q4W group transitioned to the Q2W regimen at week 64 and were administered 60% of their established Q4W total dose (rounded to the nearest 10 mg) biweekly (Q4W→Q2W) through week 160. Children in the Q2W regimen continued to receive the Q2W regimen (Q2W→Q2W) through week 160. The dose level was restricted to ≤ 2.0 mg/kg for either regimen. Dose adjustments continued until the target low-normal phosphorus level was reached or no further increase in serum phosphorus was observed after dose escalation, provided there were no safety concerns.
The primary endpoint was change from baseline to week 40 and to week 64 in total RSS using an expert reader blinded to treatment and time sequence of the radiographs, as previously reported (13). Radiographic Global Impression of Change (RGI-C) was used to assess rachitic changes of the physeal regions of each wrist and knee from baseline to week 40 and to week 64. Using similar methodology, we used the RGI-C score to assess specifically the overall deformities of the lower extremities (eg, bowing, knock-knees) observed on standing long leg radiographs through a global (total) score (16). By definition, because the GI-C methods evaluate changes over time, there is no score applied to the baseline radiograph. For each of these scores, GI-C was determined as the mean score from 3 GI-C trained, independent radiologists blinded to treatment (13). For the current analysis at week 160, it was determined that by the end of the study, the distal femoral and/or proximal tibial growth plates appeared closed on anteroposterior radiographs in 11 girls. Accordingly, these children were excluded from the group rickets analysis.
The following additional key parameters were prospectively planned to be assessed through week 160: serum phosphorus, renal tubular maximum reabsorption rate of phosphate per glomerular filtration rate (TmP/GFR), serum 1,25(OH)2D, alkaline phosphatase (ALP), lower limb deformity observed on standing long leg anteroposterior x-rays evaluated using the qualitative RGI-C scoring system, standing height z-score, growth velocity (both centimeters/year and z-score), the 6-Minute Walk Test (6MWT) (17), and the Pediatric Orthopaedic Society of North America—Pediatrics Outcomes Data Collection Instrument (POSNA-PODCI) (18).
TmP/GFR is a clinically relevant index of renal phosphate handling and is decreased in patients with XLH because of the direct effects of FGF-23 to suppress renal phosphate reabsorption (19). The normal TmP/GFR reference range is age-specific (20).
Statistical Analysis
Clinical outcomes are presented as mean, least squares (LS) mean, or LS mean change from baseline with SD, SE, or 95% CIs. LS mean, SE, 95% CIs, and 2-sided P values are from the generalized estimating equation (GEE) model. For serum phosphorus, 1,25(OH)2D, and ALP, the GEE model includes the change from baseline as the dependent variable, visit as the categorical variable, and adjusts for baseline measurement with exchangeable covariance structure.
For the RSS and RGI-C, the GEE model includes the change from baseline in RSS as the dependent variable, visit as a factor, and age and RSS at baseline as covariates with exchangeable covariance structure. For standing height z-scores, the GEE model includes change from baseline as the dependent variable, visit and gender as factors, and age and standing height z-scores at baseline as covariates with exchangeable covariance structure. For the safety analysis, all children received at least 160 weeks of treatment, and no child exceeded 216 weeks of treatment.
Results
Baseline Demographics and Disposition
As reported, all 52 enrolled children completed the 64-week treatment period, 26 in both the Q2W and Q4W groups, and the long-term extension period with Q2W dosing for an additional 96 weeks (Fig. 1) for a minimum of 160 weeks of treatment (13). There were no discontinuations from study or treatment. Baseline characteristics were consistent with children with XLH, as reported previously (13).
Figure 1.
UX023-CL201 CONSORT diagram.
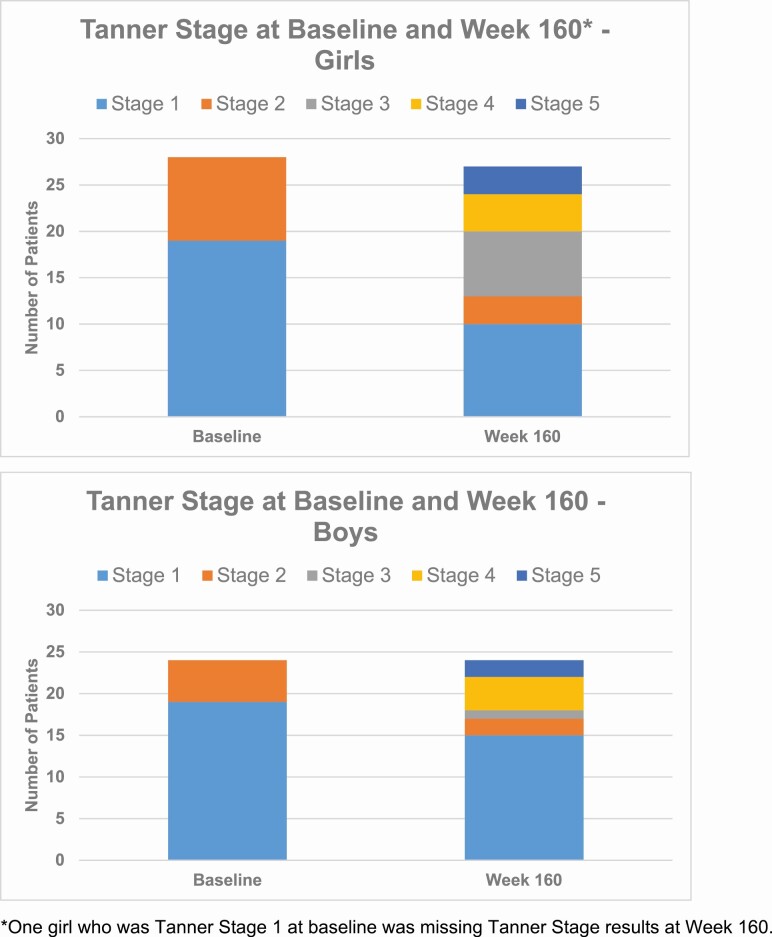
At baseline, all children were either Tanner stage 1 or 2. By week 160, 5 were Tanner stage 5. The distribution of Tanner staging at baseline and week 160 by patient sex is shown in Fig. 2.
Figure 2.
Tanner stage at baseline and week 160.
Pharmacodynamic Assessments
Burosumab-induced increases in serum phosphorus levels were sustained through week 160. When children switched from Q4W to Q2W burosumab dosing at week 66, mean serum phosphorus was maintained at or above the predefined pediatric lower limit of normal (3.2 mg/dL [1.0 mmol/L; Fig. 3A]). The overall population mean (SD) serum phosphorus concentration at week 160 was 3.35 (0.39) mg/dL, reflecting a 46% increase from baseline (mean change from baseline P < 0.0001). Nearly all (96%, 50/52) children achieved a normal serum phosphorus level (3.2-6.1 mg/dL [1.0-2.0 mmol/L]) well before week 160. The 2 who did not achieve a normal serum phosphorus level by week 160 nevertheless showed improvements in rickets severity as assessed by change from baseline in RSS scores and in the RGI-C (Table 1).
Figure 3.
(A) Sustained improvement in serum phosphorus with burosumab—note that data from week 0 to week 64 were previously reported (13). (B) Sustained improvement in TmP/GFR with burosumab—note that data from week 0 to week 64 were previously reported (13). (C) Sustained improvement in serum 1,25(OH)2D with burosumab—note that data from week 0 to week 64 were previously reported (13).
Table 1.
Global RGI-C scores and change from baseline in RSS for 2 children who did not achieve normal serum phosphorusa by week 160
| Global RGI-C score | RSS change from baseline | Serum phosphorus, mg/dL | |
|---|---|---|---|
| Child 1 | |||
| Baseline | 2.0 | ||
| Week 40 | 2.0 | -2.0 | 2.8 |
| Week 64 | 2.0 | -1.5 | 3.1 |
| Week 88 | 1.7 | -0.5 | 2.8 |
| Week 160 | 2.0 | -1.5 | 2.8 |
| Child 2 | |||
| Baseline | 2.0 | ||
| Week 40 | 1.7 | -1.5 | 2.4 |
| Week 64 | 1.7 | -1.0 | 2.4 |
| Week 88 | 2.0 | -1.0 | 2.9 |
| Week 160 | 2.0 | -1.5 | 2.5 |
a Serum phosphorus normal reference range: 3.2 to 6.1 mg/dL (1.0-2.0 mmol/L). Increase in RGI-C score indicates improvement. Decrease in RSS score indicates improvement.
Abbreviations: RGI-C, Radiographic Global Impression of Change; RSS, Rickets Severity Score.
TmP/GFR increased in all children, with an overall population mean (SD) of 3.45 mg/dL (0.56) at week 160, reflecting a 69% increase from baseline (mean change from baseline P < 0.0001). Notably, 92% of children (48 of 52) also achieved TmP/GFR values within the normal range (20) (3.42-4.40 mg/dL [1.10-1.42 mmol/L]) during the study. The change from Q4W to Q2W burosumab dosing resulted in more stable TmP/GFR values from week 64 to the end of the treatment extension period at week 160 (Fig. 3B).
Serum 1,25(OH)2D concentrations increased significantly from baseline (P < 0.05) for both dose groups at each study visit up to week 64 and overall up to week 160 (Fig. 3C). Overall population mean (SD) serum 1,25(OH)2D level at week 160 was 60 (18) pg/mL (0.14 nmol/L), reflecting a 79% increase from baseline (mean change from baseline P < 0.0001). During the treatment extension period when all children received burosumab Q2W, serum 1,25(OH)2D levels were steadily sustained above the baseline.
Skeletal Assessments
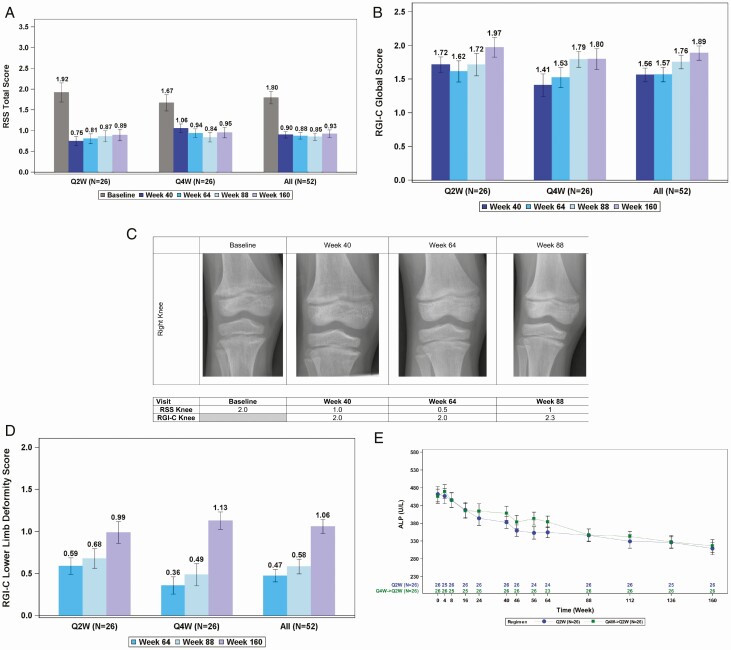
Total RSS, which decreased significantly from baseline at week 40 and week 64, remained decreased at subsequent assessments in the 41 children (79% from both treatment arms) with open distal femur and proximal tibia growth plates, with an LS mean ± SE change from baseline to week 160 of -0.9 ± 0.1, P < 0.0001 (Fig. 4A).
Figure 4.
(A) RSS total score decreased from baseline indicating improved rickets. (B) RGI-C global score increased indicating improved rickets. (C) Sustained healing of rickets in an 8-year-old boy. (D) RGI-C lower limb deformity score increased indicating improvement throughout the study. (E) Sustained improvement in serum alkaline phosphatase (ALP) with burosumab—note that data from week 0 to week 64 were previously reported (13).
RGI-C global score LS mean ± SE increased throughout the study and was +1.57 ± 0.1 at week 64, +1.75 ± 0.1 at week 88, and +1.89 ± 0.1 at week 160 (all P < 0.0001; Fig. 4B). In addition, substantial healing of rickets, as determined by an RGI-C global score ≥ +2, was observed in 23 of 41 (56%) children at week 160. Example radiographs from an 8-year-old boy demonstrate improvement in rickets at the knee over the course of the study (Fig. 4C).
RGI-C lower limb deformity score improved significantly in all 52 children at week 64, LS mean ± SE of +0.5 ± 0.1, as reported previously (13). RGI-C lower limb deformity score improved further to +0.58 ± 0.1 at week 88 and to +1.05 ± 0.1 at week 160 (all P < 0.0001; Fig. 4D).
By week 64, burosumab was associated with a significant decrease in ALP activity, a rickets severity marker, from an overall study population mean (SD) of 459 (105) U/L at baseline to below the age-dependent upper limit of normal (297-385 U/L) for both the Q2W and Q4W dose regimens. Reductions in ALP continued after week 64 when all children received the Q2W regimen, with an overall study population mean (SD) of 312 (89) at week 160 (mean change from baseline P < 0.0001; Fig. 4E); 75% of patients (39 of 52) had ALP values ≤ 385 U/L at week 160.
Growth, Mobility, and Patient-reported Outcomes
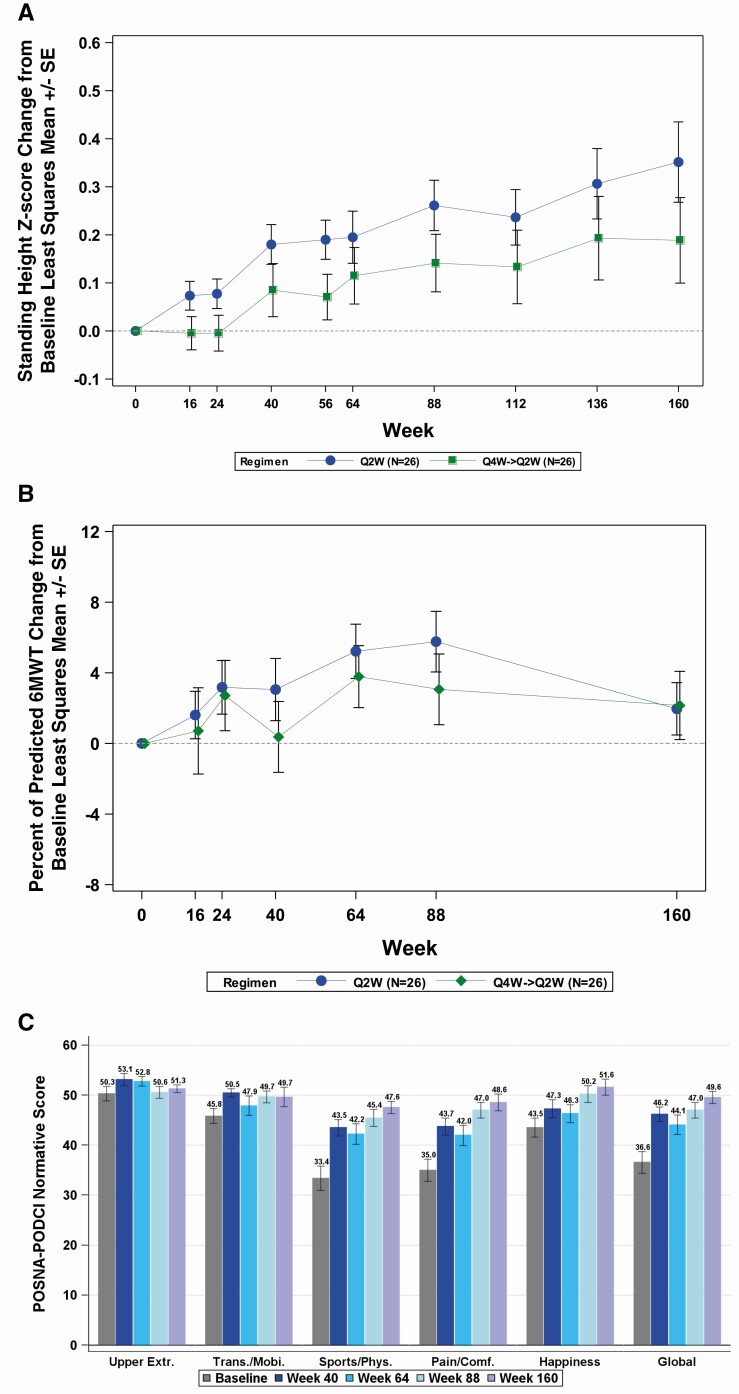
Mean (SD) standing height z-score and growth velocity (centimeters/year) gradually increased during the study (Fig. 5A). In the Q2W→Q2W group, mean (SD) standing height z-score was -1.72 (1.03) at baseline, -1.54 (1.13) at week 64, and -1.38 (1.06) at week 160, reflecting an LS mean ± SE change at week 64 of 0.20 ± 0.05 (P = 0.0003) and at week 160 of 0.35 ± 0.08 (P < 0.0001). Mean (SD) growth velocity was 5.45 (1.17) cm/year at baseline, 6.18 (1.49) cm/year at week 64, and 6.12 (1.91) cm/year from week 112 to week 160, reflecting a mean (SD) change of 0.73 (1.40) cm/year from baseline to week 64 and 0.67 (2.32) cm/year from week 112 to week 160. Mean (SD) growth velocity z-score was -0.58 (1.46) at baseline, -0.04 (1.58) at week 64, and 0.83 (2.46) from week 112 to week 160, reflecting an LS mean ± SE change at week 160 of 1.35 ± 0.59 (P = 0.028).
Figure 5.
(A) Change in standing height z-score from baseline. (B) Change in percent of predicted 6MWT from baseline (least square mean ± SE). (C) POSNA-PODCI normative score (mean ± SE).
In the Q4W→Q2W group, mean (SD) standing height z-score was -2.05 (0.96) at baseline, -1.92 (0.84) at week 64, and -1.85 (0.85) at week 160, reflecting an LS mean ± SE change at week 64 of 0.11 ± 0.06 (P = 0.05) and at week 160 of 0.19 ± 0.09 (P < 0.05). Mean (SD) growth velocity was 5.24 (1.40) cm/year at baseline, 5.61 (1.22) cm/year at week 64, and 5.78 (2.45) cm/year from week 112 to week 160, reflecting a mean (SD) change of 0.37 (2.16) cm/year from baseline to week 64 and 0.54 (3.16) cm/year from week 112 to week 160. Mean (SD) growth velocity z-score was -0.79 (1.44) at baseline, -0.45 (1.64) at week 64, and -0.32 (2.46) from week 112 to week 160, reflecting an LS mean ± SE change at week 160 of 0.49 ± 0.60 (P = 0.421).
For the 6MWT, the baseline mean (± SD) percentage of predicted distance walked was 80.4% (± 14.1). The maximum LS mean (± SE) change from baseline in the percentage predicted (for age and sex) distance walked in the 6MWT was observed at week 88 in the Q2W→Q2W group (6% [± 2]; P = 0.001) and at week 64 in the Q4W→Q2W group (3% [± 2]; P = 0.031; Fig. 5B). The observed mean (± SE) percent of predicted (for age and sex) distance walked in the 6MWT was 85.6% (1.8) at week 88 in the Q2W→Q2W group and 84.7% (2.7) at week 64 in the Q4W→Q2W group.
At baseline, overall population (mean [SD]) scores for the POSNA-PODCI Upper Extremity (50.3 [10.4]), Transfer/Mobility (45.8 [10.6]), and Happiness (43.5 [13.6]) domains were within the normal range (normative healthy reference population mean [± SD]: 50 [± 10]). These domains improved throughout the study, with an LS mean ± SE change from baseline to week 160 of 0.9 ± 0.8 (P = 0.26), 3.7 ± 1.7 (P = 0.03), and 7.8 ± 1.4 (P < 0.0001), respectively (Fig. 5C).
The POSNA-PODCI Sports/Physical Functioning, Pain/Comfort, and Global Functioning domains significantly improved from below the normative healthy reference population range at baseline (mean [SD]: 33.4 [17.4], 35.0 [15.9], and 36.6 [15.5], respectively) to within the normal range at all postbaseline visits. The LS mean ± SE score change from baseline to week 160 in the Sports/Physical Functioning, Pain/Comfort, and Global Functioning domains were 13.2 ± 1.4 (P < 0.0001), 12.7 ± 1.6 (P < 0.0001), and 11.7 ± 1.3 (P < 0.0001), respectively.
Safety
All children experienced ≥ 1 adverse event (AE; Table 2). Most AEs were mild (grade 1) to moderate (grade 2) in severity, and the most frequent (incidence ≥ 10%) AEs considered related to treatment were: injection site reaction (46%), injection site erythema (37%), injection site bruising (12%), injection site pruritus (12%), injection site swelling (12%), injection site pain (10%), pain in extremity (10%), and vitamin D deficiency (10%).
Table 2.
Adverse events
| Incident | Weeks 0-65 | Weeks 66-160 | Weeks 0-160 |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| TEAEs | 52 (100) | 52 (100) | 52 (100) |
| TEAEs related to burosumab | 35 (67) | 29 (56) | 38 (73) |
| TEAEs occurring in > 20% of children from weeks 0 through 160 | |||
| Headache | 27 (52) | 30 (58) | 39 (75) |
| Cough | 24 (46) | 27 (52) | 36 (69) |
| Vomiting | 20 (39) | 20 (39) | 29 (56) |
| Arthralgia | 21 (40) | 20 (39) | 28 (54) |
| Nasopharyngitis | 21 (40) | 18 (35) | 28 (54) |
| Pain in extremity | 21 (40) | 16 (31) | 27 (52) |
| Injection site reaction | 19 (37) | 13 (25) | 26 (50) |
| Oropharyngeal pain | 10 (19) | 23 (44) | 26 (50) |
| Pyrexia | 16 (31) | 20 (39) | 25 (48) |
| Upper respiratory tract infection | 18 (35) | 11 (21) | 25 (48) |
| Injection site erythema | 12 (23) | 20 (39) | 23 (44) |
| Rhinorrhea | 9 (17) | 17 (33) | 22 (42) |
| Nasal congestion | 8 (15) | 17 (33) | 20 (39) |
| Abdominal pain upper | 10 (19) | 14 (27) | 18 (35) |
| Diarrhea | 11 (21) | 15 (29) | 18 (35) |
| Ear pain | 6 (12) | 14 (27) | 18 (35) |
| Nausea | 10 (19) | 10 (19) | 17 (33) |
| Seasonal allergy | 13 (25) | 9 (17) | 17 (33) |
| Toothache | 6 (12) | 12 (23) | 17 (33) |
| Rash | 13 (25) | 2 (4) | 15 (29) |
| Sneezing | 2 (4) | 11 (21) | 12 (23) |
| Myalgia | 8 (15) | 5 (10) | 11 (21) |
| Vitamin D decreased | 7 (13) | 5 (10) | 11 (21) |
| Serious AEs | 1 (2) | 0 (0) | 1 (2) |
| AEs leading to discontinuation | 0 (0) | 0 (0) | 0 (0) |
| AEs leading to death | 0 (0) | 0 (0) | 0 (0) |
Abbreviations: AEs, adverse events; TEAE, treatment-emergent adverse event.
Of the most frequent AEs that occurred in >20% of children from weeks 0 to 160, most occurred with similar frequencies during the original treatment period (weeks 0-65) and the treatment extension period (weeks 66-160; Table 2). The AE with the largest decrease from the original treatment period to the treatment extension period was rash, with a decrease of 21% (25% to 4%). Other AEs that decreased >10% from the original treatment period to the treatment extension period were upper respiratory tract infection (-14%; 35% to 21%), and injection site reaction (-12%; 37% to 25%). The AE with the largest increase from the original treatment period to the treatment extension period was oropharyngeal pain, with an increase of 25% (19% to 44%). Other AEs that increased >10% from the original treatment period to the treatment extension period were nasal congestion (+18%; 15% to 33%), sneezing (+17%; 4% to 21%), rhinorrhea (+16%; 17% to 33%), injection site erythema (+16%; 23% to 39%), ear pain (+15%; 12% to 27%), and toothache (+11%; 12% to 23%.
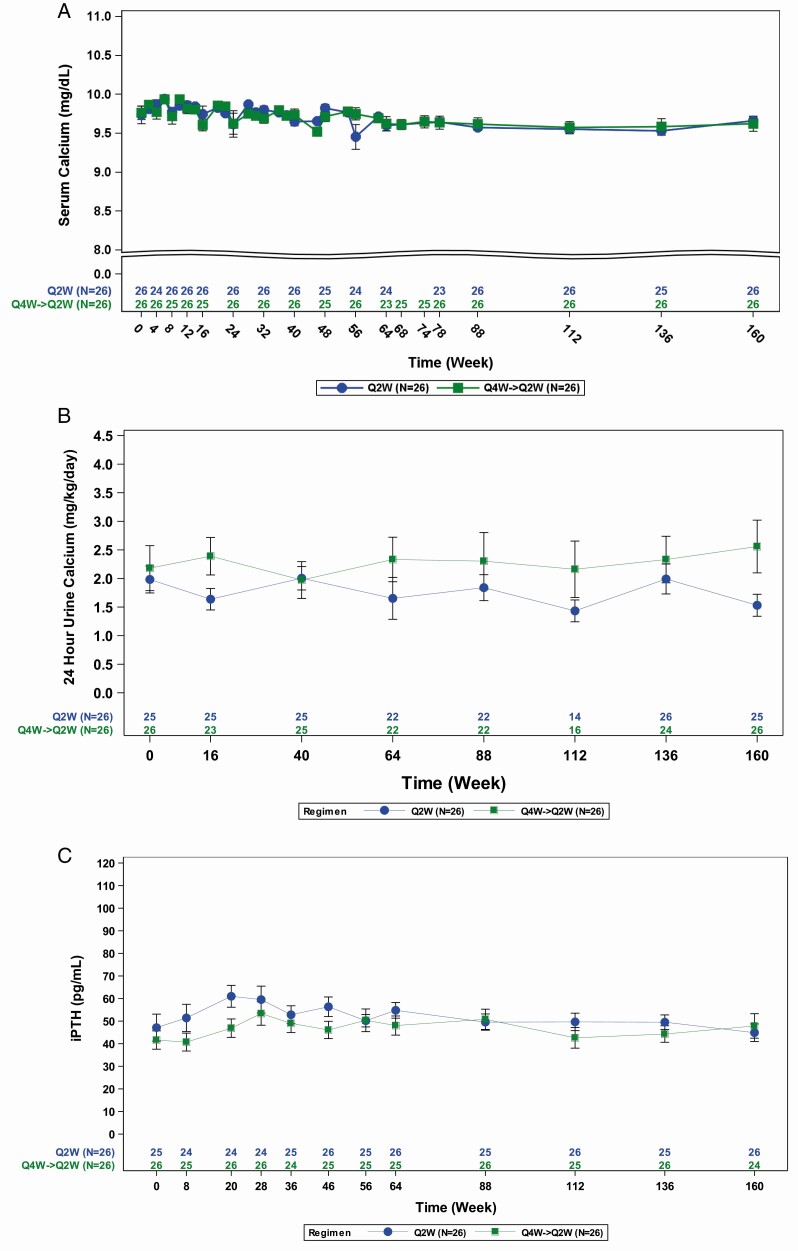
One child had 2 AEs classified as serious because of brief hospitalizations (fever/muscle pain at week 48 and headache at week 182); each resolved within 1 week and neither were considered related to burosumab. No child discontinued therapy, showed hyperphosphatemia, or had clinically meaningful increases in serum calcium concentration (Fig. 6A), urine calcium excretion (Fig. 6B), or circulating PTH (Fig. 6C). At week 160, the change from baseline in nephrocalcinosis score was 0 in 39 children, +1 in 9 children, +2 in 1 child, and -1 in 3 children.
Figure 6.
(A) No clinically meaningful changes in serum calcium. (B) No clinically meaningful changes in 24-hour urine calcium excretion. (C) No clinically meaningful changes in serum PTH (iPTH).
Forty of 46 children tested negative for antidrug antibodies at baseline and remained negative throughout the study. Six children tested positive for antidrug antibodies at baseline. Of these 6 children, 2 were negative at all postbaseline assessments, 1 was transiently positive at 2 postbaseline assessments, and 3 were positive at all or most postbaseline assessments. There was a minimal increase in antibody titer for these children (maximum 1:16).
Three of the 6 children who were positive for antidrug antibodies were also positive for neutralizing anti-burosumab antibodies. One child was positive for neutralizing antibodies at weeks 88, 136, and 160, and end of study; this child had intermittent injection site reactions throughout the study, but achieved a normal serum phosphorus level at week 40, which was maintained through the end of study; this child also had improvements in RSS scores over the course of the study. The second child was positive at week 112 only; this child had 1 injection site reaction during the study and had intermittent low serum phosphorus levels throughout the study, with stable RSS scores over the course of the study. The third child was positive at weeks 16, 56, and 112; this child also had intermittent injection site reactions throughout the study, but had generally increasing serum phosphorus levels near or above the lower limit of normal throughout the study, with improvements in RSS scores over the course of the study.
Discussion
Sustained burosumab treatment of children with XLH for 160 weeks improved phosphorus metabolism, rickets, leg deformities, mobility, and growth, and decreased their pain scores. Improved rickets was first demonstrated radiographically after 40 weeks of burosumab therapy and was maintained through 160 weeks of treatment. Improvements in lower limb deformity continued throughout the 160-week treatment period. Importantly, the radiograph readers for RSS and RGI-C assessments were blinded to time and treatment status, providing objectivity similar to that of a trial blinded to therapeutic regimens. Burosumab treatment was well-tolerated. Most AEs were mild to moderate in severity, and as expected in a pediatric XLH population receiving a subcutaneously injected therapeutic.
Limitations of this study included that radiographic features of rickets normally become less evident as the growth plates progress toward closure, which could account for some of the improvements in RSS and RGI-C scores observed in some of the older children. Consequently, RSS and RGI-C assessments could not be performed in the 11 girls with apparent growth plate closure. Similarly, the long duration of our study may have contributed to the observed decreases in ALP at week 160 because serum ALP levels are age and gender dependent, correlating with the timing of growth plate closure and growth velocity deceleration as children reach the end of puberty; however, the observed decreases in ALP early in the study were most likely attributable to the therapeutic effects of burosumab on bone turnover. Skeletal maturity was assessed by the RSS reader using anteroposterior radiographs of the lower extremity, a reading that is difficult to correlate with standard assessments of bone age using hand–wrist radiographs. However, the observed robust treatment effect of burosumab was consistent with that demonstrated in later burosumab trials. For a conservative interpretation, children with apparently closed growth plates were excluded from the rickets assessments, and RSS and RGI-C significantly improved in those with open growth plates.
When administered for 160 weeks to children and adolescents with XLH, burosumab Q2W maintained serum phosphate, urinary phosphate reabsorption, and endogenous calcitriol synthesis. However, normal serum phosphorus levels and TmP/GFR were not achieved in all patients. Throughout the study, in 2 patients (4%), serum phosphorus increased but not to a level in the normal range, and in 4 patients (8%), TmP/GFR increased but not to a level in the normal range. Nevertheless, their rickets improved, suggesting normalization of serum phosphorus concentration may not be necessary to see benefits of burosumab on skeletal health.
Our data show that sustained burosumab administration benefitted children with XLH as they transitioned through puberty and growth plates began to fuse. To date, none of the burosumab clinical trials have assessed dose adjustments in adolescent XLH patients that transition to adulthood. The prescribing information indicates that the dosing interval is Q2W until 18 years old and Q4W after age 18 years, as per the design of the burosumab clinical trials (21). The optimal timing for conversion from Q2W dosing to Q4W dosing remains uncertain. Attainment of final height may be an appropriate time for this transition. The XLH Data Monitoring Program is an ongoing, long-term study evaluating real-world experience with burosumab (NCT03651505) that may provide insight into the optimal treatment transition from pediatrics to adulthood.
Although burosumab was effective in improving phosphorus metabolism, improvement in some features of XLH may take longer than 160 weeks to be fully realized; consequently, the long-term XLH Data Monitoring Program will explore such effects. For example, standing height z-score improved significantly at 160 weeks compared with baseline but the changes were modest. Such small improvements in height z-scores may be the result of normally decreasing growth velocities that occur between age 2 years and age 8 to 10 years in girls and age 9 to 11 years in boys. Importantly, improvements associated with burosumab administration continued at week 160, suggesting that early XLH diagnosis and treatment can improve outcomes.
In conclusion, sustained burosumab therapy for more than 3 years in children with XLH resulted in clinically meaningful improvements across several clinical outcomes and demonstrated an acceptable benefit-risk profile.
Acknowledgments
Meng Mao (M.M.) and Chao-Yin Chen (C.-Y.C.) were employed at Ultragenyx Pharmaceutical at the time that the work included in this manuscript was completed, and the authors acknowledge their contributions to this study. M.M. is now an employee of Ascendis Pharmaceuticals and C.-Y.C. is now an employee of 89bio. James Ziobro of Ultragenyx Pharmaceutical Inc. provided medical writing assistance in the preparation of this manuscript.
Glossary
Abbreviations
- 1,25(OH)2D
1,25-dihydroxyvitamin D
- 6MWT
6-Minute Walk Test
- AE
adverse event
- ALP
alkaline phosphatase
- FGF-23
fibroblast growth factor-23
- GEE
generalized estimating equation
- LS
least squares
- POSNA-PODCI
Pediatric Orthopaedic Society of North America—Pediatrics Outcomes Data Collection Instrument
- Q2W
every 2 weeks
- Q4W
every 4 weeks
- RGI-C
Radiographic Global Impression of Change
- RSS
Rickets Severity Score
- TmP/GFR
tubular maximum reabsorption rate of phosphate per glomerular filtration rate
- XLH
X-linked hypophosphatemia
Additional Information
Disclosures: A.L.: Research investigator, consultant, and travel support from Ultragenyx Pharmaceutical Inc. E.A.I.: Research funding from Ultragenyx Pharmaceutical Inc.; advisory board for Ultragenyx Pharmaceutical Inc. M.P.W.: Research grant support, honoraria, and travel support from Ultragenyx Pharmaceutical Inc. and Alexion Pharmaceuticals Inc. A.A.P.: Speakers’ honoraria and research support paid to his institution from Ultragenyx Pharmaceutical Inc. W.H.: Research investigator, consultant, and travel support from Ultragenyx Pharmaceutical Inc. A.M.B.: Research investigator, consultant, and travel support from Ultragenyx Pharmaceutical Inc. R.P.: Research investigator for Ultragenyx Pharmaceutical Inc.; consultant for Ultragenyx Pharmaceutical Inc. and Alexion Pharmaceuticals Inc. W.v.H.: Research investigator and travel support from Ultragenyx Pharmaceutical Inc. and Alexion Pharmaceuticals Inc. G.S.G.: Research investigator and consultant for Ultragenyx Pharmaceutical Inc. A.C., A.S., and M.S.R.: Employees and stockholders of Ultragenyx Pharmaceutical Inc. T.O.C.: Research Investigator, advisory board member, and consultant for Ultragenyx Pharmaceutical Inc.; advisory board member for Inozyme; consultant for Inozyme, Regeneron, and Kyowa Kirin.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Albright F, Butler AM, Bloomberg E. Rickets resistant to vitamin D therapy. Am J Dis Child. 1937;54(3):529-547. [Google Scholar]
- 2. Francis F, Hennig S, Meitinger T, et al. A gene (PEX) with homologies to endopeptidases is mutated in patients with X–linked hypophosphatemic rickets. Nat Genet. 1995;11(2):130-136. [DOI] [PubMed] [Google Scholar]
- 3. Carpenter TO, Imel EA, Holm IA, Jan de Beur SM, Insogna KL. A clinician’s guide to X-linked hypophosphatemia. J Bone Miner Res. 2011;26(7):1381-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cagnoli M, Richter R, Böhm P, Knye K, Empting S, Mohnike K. Spontaneous growth and effect of early therapy with calcitriol and phosphate in X-linked hypophosphatemic rickets. Pediatr Endocrinol Rev. 2017;15(Suppl 1):119-122. [DOI] [PubMed] [Google Scholar]
- 5. Linglart A, Dvorak-Ewell M, Marshall A, et al. Impaired mobility and pain significantly impact the quality of life of children with X-linked hypophosphatemia. Bone Abstracts. 2015;4:198. [Google Scholar]
- 6. McWhorter AG, Seale NS. Prevalence of dental abscess in a population of children with vitamin D-resistant rickets. Pediatr Dent. 1991;13(2):91-96. [PubMed] [Google Scholar]
- 7. Vakharia JD, Matlock K, Taylor HO, Backeljauw PF, Topor LS. Craniosynostosis as the presenting feature of X-linked hypophosphatemic rickets. Pediatrics. 2018;141(Suppl 5):S515-S519. [DOI] [PubMed] [Google Scholar]
- 8. Glorieux FH, Marie PJ, Pettifor JM, Delvin EE. Bone response to phosphate salts, ergocalciferol, and calcitriol in hypophosphatemic vitamin D-resistant rickets. N Engl J Med. 1980;303(18):1023-1031. [DOI] [PubMed] [Google Scholar]
- 9. Rafaelsen S, Johansson S, Ræder H, Bjerknes R. Hereditary hypophosphatemia in Norway: a retrospective population-based study of genotypes, phenotypes, and treatment complications. Eur J Endocrinol. 2016;174(2):125-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Linglart A, Biosse-Duplan M, Briot K, et al. Therapeutic management of hypophosphatemic rickets from infancy to adulthood. Endocr Connect. 2014;3(1):R13-R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lamb YN. Burosumab: first global approval. Drugs. 2018;78(6):707-714. [DOI] [PubMed] [Google Scholar]
- 12. Whyte MP, Carpenter TO, Gottesman GS, et al. Efficacy and safety of burosumab in children aged 1-4 years with X-linked hypophosphataemia: a multicentre, open-label, phase 2 trial. Lancet Diabetes Endocrinol. 2019;7(3):189-199. [DOI] [PubMed] [Google Scholar]
- 13. Carpenter TO, Whyte MP, Imel EA, et al. Burosumab therapy in children with X-linked hypophosphatemia. N Engl J Med. 2018;378(21):1987-1998. [DOI] [PubMed] [Google Scholar]
- 14. Imel EA, Glorieux FH, Whyte MP, et al. Burosumab versus conventional therapy in children with X-linked hypophosphataemia: a randomised, active-controlled, open-label, phase 3 trial. Lancet. 2019;393(10189):2416-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Whyte MP, Imel E, Portale AA, et al. Burosumab, a Fully Human Anti-FGF23 Monoclonal Antibody, for X-Linked Hypophosphatemia (XLH): Results from Two Phase 2 Trials in Affected Children 1-12 Years Old 2018. Chicago, IL: Endocrine Society Annual Meeting (ENDO; ). [Google Scholar]
- 16. Whyte MP, Fujita KP, Moseley S, Thompson DD, McAlister WH. Validation of a novel scoring system for changes in skeletal manifestations of hypophosphatasia in newborns, infants, and children: the radiographic global impression of change scale. J Bone Miner Res. 2018;33(5):868-874. [DOI] [PubMed] [Google Scholar]
- 17. Geiger R, Strasak A, Treml B, et al. Six-minute walk test in children and adolescents. J Pediatr. 2007;150(4):395-9, 399.e1. [DOI] [PubMed] [Google Scholar]
- 18. Daltroy LH, Liang MH, Fossel AH, Goldberg MJ. The POSNA pediatric musculoskeletal functional health questionnaire: report on reliability, validity, and sensitivity to change. Pediatric Outcomes Instrument Development Group. Pediatric Orthopaedic Society of North America. J Pediatr Orthop. 1998;18(5):561-571. [DOI] [PubMed] [Google Scholar]
- 19. Imel EA, Zhang X, Ruppe MD, et al. Prolonged correction of serum phosphorus in adults with X-linked hypophosphatemia using monthly doses of KRN23. J Clin Endocrinol Metab. 2015;100(7):2565-2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kruse K, Kracht U, Göpfert G. Renal threshold phosphate concentration (TmPO4/GFR). Arch Dis Child. 1982;57(3):217-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burosumab (CRYSVITA®) prescribing information. Ultragenyx Pharmaceutical Inc. 06/2020. https://www.ultragenyx.com/wp-content/uploads/2021/08/Crysvita_Full_Prescribing_Information.pdf. Accessed October 20, 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.