Abstract
A healthy 23-year-old man with fever and a tender mass in his right anterior neck was found to have a branchial cleft cyst infected with Bordetella bronchiseptica. Initial testing suggested a Brucella species, but further laboratory testing identified the organism definitively. B. bronchiseptica infection in healthy adults is an unusual event.
CASE REPORT
A previously healthy 23-year-old man was admitted to our hospital with a fever of 39°C (102°F) and a tender mass in his right anterior neck. The patient initially noted a small, painless swelling in his right neck 10 days prior to presentation. Over the ensuing week, the mass progressively enlarged, becoming increasingly erythematous and tender, and the patient noted daily spiking fevers. An immigrant from rural Mexico, he had been living in the United States for 6 months, working on a farm in central Pennsylvania as a mushroom collector. He reported no sick contacts. There were numerous goats, dogs, and cats on the farm, but he denied having been bitten or scratched by any of them. He denied having consumed unpasteurized dairy products.
Physical examination revealed a young, thin male in no acute distress. He was normotensive, with a temperature of 39.1°C (102.2°F) on admission. The right anterior neck mass measured 2 by 4 cm and was firm, tender, and nonmobile. The skin overlying the mass was warm and erythematous. No draining sinus or fistula was present, and he had no associated lymphadenopathy or rashes. The remainder of his exam was unremarkable.
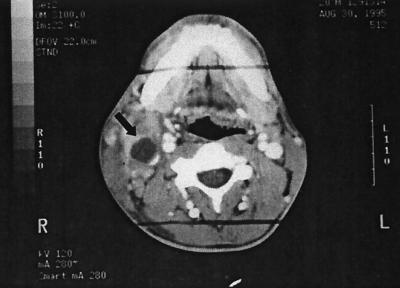
The patient had an elevated white blood cell count of 13,200/μl, with 78% polymorphonuclear leukocytes. Tests of liver function were normal, and 2 sets of blood cultures showed no growth. A rapid plasma reagin test was negative, and tests for human immunodeficiency virus were nonreactive. The patient's chest radiograph was normal. A computed tomographic scan of the neck showed a 2.5- by 4.5-cm multilocular, necrotic mass in the right neck with associated induration of the adjacent soft tissues and deviation of the trachea (Fig. 1). No lymphadenopathy was present.
FIG. 1.
Computed tomographic scan of the neck showing a 2.5- by 4.5-cm multilocular, necrotic mass (arrow) in the right neck with associated induration of the adjacent soft tissues and deviation of the trachea. No associated lymphadenopathy was noted.
Fine-needle aspiration of the mass was performed, the material was stained with Papanicolaou stain, and numerous neutrophils and squamous epithelial cells were evident. The large number of squamous epithelial cells in the aspirate confirmed the presence of a branchial cleft cyst. Acid-fast bacilli were not seen by Kinyoun staining of the specimen. Gram staining of the aspirate showed leukocytes with intracellular, pleomorphic, gram-negative coccobacilli. Based on this Gram stain and the patient's history of animal exposures, a presumptive diagnosis of brucellosis was made. The patient was subsequently begun on intravenous doxycycline and rifampin.
A sample of the aspirate was inoculated onto blood, chocolate, and brucella agar plates and incubated at 37°C in an atmosphere of 5 to 10% carbon dioxide for 5 days. Multiple translucent, spheroidal colonies approximately 2 to 3 mm in diameter were isolated. The isolate was positive in tests for catalase, oxidase, and urea hydrolysis, suggesting that the isolate was a Brucella species. A subculture of the isolate was sent to the Special Bacteriology Reference Laboratory at the Centers for Disease Control and Prevention for definitive identification. Testing was performed using the standard protocols of the Special Bacteriology Reference Laboratory (21), and the organism was identified as Bordetella bronchiseptica. The isolate grew well on tryptone glucose extract agar at 25 and 35°C but not at 42°C. Good growth was also obtained in nutrient broth with 0 and 6% NaCl and on MacConkey and salmonella-shigella agars but not on cetrimide agar. Positive results were obtained for oxidase, catalase, urease, nitrate reduction, and alkalinization of citrate and litmus milk. Negative results were obtained for indole production; hydrolysis of esculin and gelatin; alkalinization of acetamide, serine, and tartrate; nitrite reduction; and acid production from carbohydrates, including d-glucose, d-xylose, d-mannitol, lactose, sucrose, and maltose. No acid or H2S was produced in triple-sugar iron agar, although a small amount of H2S was detected with lead acetate paper. The organism was motile and peritrichous. We subsequently tested the isolate with the API 20E system (Analytab Products, Plainview, N.Y.), and it was also identified as B. bronchiseptica (API biotype 021000451). Susceptibility testing by the Vitek antimicrobic system (Biomerieux, Marcy l'Etoile, France) showed the microorganism to be susceptible to doxycycline, gentamicin, piperacillin, and tetracycline; intermediate to ceftazidime; and resistant to rifampin.
Because of persistent fever and pain in the right neck, aspiration of the branchial cleft cyst was repeated 4 days after the initial procedure and 3 days into antimicrobial therapy. Purulent material was again obtained, but the specimen was not sent for culture. Gradual defervescence occurred, and the mass resolved. The patient underwent surgical excision of the branchial cleft cyst after completion of 6 weeks of oral doxycycline. No other symptoms were reported on follow-up visits.
Branchial cleft cysts are epithelium-lined cavities that represent a persistence of the primitive branchial apparatus (13). These congenital neck lesions appear most often as a soft, round mass located along the anterior border of the sternocleidomastoid muscle. They can become inflamed and develop as abscesses typically after upper respiratory tract or odontogenic infections (13).
B. bronchiseptica has been recognized as a respiratory tract pathogen in many domestic and wild animals, but evidence also suggests that it may occasionally be encountered as a commensal of the human respiratory tract and, rarely, as a pathogen in human disease (20). B. bronchiseptica is a pleomorphic, non-spore-forming, gram-negative coccobacillus (10). It is an obligate aerobe that grows readily on simple nutritive media after 48 h of incubation at 35°C (10). It is positive in tests for catalase, oxidase, urease, nitrate reduction, and citrate utilization. It is negative in tests for indole, tyrosine hydrolysis, and acid production by either oxidation or fermentation of glucose and maltose. The organism is capable of growth on salmonella-shigella agar but not on potassium tellurite medium. B. bronchiseptica is motile and peritrichous (20).
B. bronchiseptica is pathogenic in mammalian species and produces an endotoxin that is similar in chemical composition and physiological effects to those of other gram-negative microorganisms (12). The bacterium can adhere to respiratory epithelial cells by using fimbriae and filamentous hemagglutinins. It invades respiratory epithelial cells and alveolar macrophages and diminishes the overall bactericidal ability of these cells by producing the enzyme adenylate cyclase (12). These virulence factors enable the organism to successfully colonize the respiratory tract.
Over 60 cases of B. bronchispetica infection have been reported in humans since the organism's identification in 1911. These occurred primarily in patients with underlying cellular or humoral immunodeficiency (1, 17, 20). Conditions such as hematologic malignancy (9), organ transplantation (1, 4), and chronic alcoholism (8) have all been implicated in B. bronchiseptica infection. Its role as an opportunistic pathogen in patients with AIDS has been increasingly noted (5–7, 19). A history of exposure to animals is often present but is not always identifiable (15). Clinical B. bronchiseptica infection in healthy adults is an unusual event, and very few cases occur in immunocompetent hosts (17).
The respiratory tract is the most common site for B. bronchiseptica infection in humans, and clinical manifestations include sinusitis, tracheobronchitis, whooping cough-like illness, and pneumonia (4). There are no definitive radiographic features associated with B. bronchiseptica pulmonary infections, but diffuse infiltrates (4), interstitial pneumonia (5, 6), and lobar pneumonia (1, 6, 9, 19) are the most frequent findings. Cavitary lesions are rarely identified (6, 7). Extrapulmonary clinical infection is uncommon, and only bacteremia (1, 14), endocarditis (16), peritonitis (2), and meningitis (3) have been described. No cases of branchial cleft cyst infection with B. bronchiseptica have been reported to date. Direct invasion of our patient's branchial cleft cyst likely occurred following colonization of his respiratory tract with the microorganism. The source of his infection was likely derived from the close contact he had with the animals residing on the farm where he worked.
The optimal therapy for B. bronchiseptica infection has not been clearly established (19). In vitro studies of the organism's antimicrobial susceptibilities have involved small numbers of isolates and limited numbers of antibiotics (18). From recent in vitro antimicrobial susceptibility studies, the most effective agents against B. bronchiseptica appear to be the aminoglycosides, antipseudomonal penicillins, tetracyclines, and chloramphenicol (17, 20). However, despite the good in vitro activities of these antibiotics, the clinical responses to them have often been disappointing (4, 18). This suggests that in vitro testing may not be well suited for studying B. bronchiseptica.
A longer duration of therapy has been recommended for a definitive cure (11), and this approach was undertaken for our patient. No studies have been performed to elucidate the precise length of antibiotic treatment. Several authors have reported complete eradication of infection with 2 weeks of therapy (5, 15, 19). Neutropenic or other immunocompromised patients may require prolonged antibiotic therapy of 4 or more weeks to attain complete clinical recovery (9).
This case represents the first report of an infected branchial cleft cyst due to B. bronchiseptica in an immunocompetent patient, expanding the spectrum of clinical disease that may be seen with this organism. Since our patient had no evidence of immunodeficiency, this case provides further evidence that human infection with B. bronchiseptica is not always associated with severe underlying disease.
Acknowledgments
We thank Robbin Weyant from the Special Bacteriology Reference Laboratory for helpful suggestions with the manuscript.
REFERENCES
- 1.Bauwens J E, Spach D H, Schacker T W, Mustafa M M, Bowden R A. Bordetella bronchiseptica pneumonia and bacteremia following bone marrow transplantation. J Clin Microbiol. 1992;30:2474–2475. doi: 10.1128/jcm.30.9.2474-2475.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrd L H, Anama L, Gutkin M, Chmel H. Bordetella bronchiseptica peritonitis associated with continuous ambulatory peritoneal dialysis. J Clin Microbiol. 1981;14:232–233. doi: 10.1128/jcm.14.2.232-233.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang K C, Zakheim R M, Cho C T, Montgomery J C. Posttraumatic purulent meningitis due to Bordetella bronchiseptica. J Pediatr. 1975;86:639–640. doi: 10.1016/s0022-3476(75)80178-8. [DOI] [PubMed] [Google Scholar]
- 4.Choy K W, Wulffrat N M, Wolfs T F W, Geelen S P M, Kraaijeveld C A, Fleer A. Bordetella bronchiseptica respiratory infection in a child after bone marrow transplantation. Pediatr Infect Dis J. 1999;18:481–482. doi: 10.1097/00006454-199905000-00022. [DOI] [PubMed] [Google Scholar]
- 5.Decker G R, Lavelle J P, Kumar P N, Pierce P F. Pneumonia due to Bordetella bronchiseptica in a patient with AIDS. Rev Infect Dis. 1991;13:1250–1251. doi: 10.1093/clinids/13.6.1250. [DOI] [PubMed] [Google Scholar]
- 6.Dworkin M S, Sullivan P S, Buskin S E, Harrington R D, Ollife J, MacArthur R D, Lopez C E. Bordetella bronchiseptica infection in human immunodeficiency virus-infected patients. Clin Infect Dis. 1999;29:1095–1099. doi: 10.1086/514761. [DOI] [PubMed] [Google Scholar]
- 7.Garcia San Miguel L, Quereda C, Martinez M, Martin-Davila P, Cobo J, Guerrero A. Bordetella bronchiseptica cavitary pneumonia in a patient with AIDS. Eur J Clin Microbiol Infect Dis. 1998;17:675–676. doi: 10.1007/BF01708357. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh H K, Tranter J. Bordetella bronchicanis (bronchiseptica) infection in man: review and a case report. J Clin Pathol. 1979;32:546–548. doi: 10.1136/jcp.32.6.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez L, Grazziutti M, Sumoza D, Beran M, Rolston K. Bacterial pneumonia due to Bordetella bronchiseptica in a patient with acute leukemia. Clin Infect Dis. 1998;26:1002–1003. doi: 10.1086/517630. [DOI] [PubMed] [Google Scholar]
- 10.Goodnow R A. Biology of Bordetella bronchiseptica. Microbiol Rev. 1980;44:722–738. doi: 10.1128/mr.44.4.722-738.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gueirard P, Weber C, Le Coustumier A, Guiso N. Human Bordetella bronchiseptica infection related to contact with infected animals: persistence of bacteria in host. J Clin Microbiol. 1995;33:2002–2006. doi: 10.1128/jcm.33.8.2002-2006.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harvill E T, Preston A, Cotter P A, Allen A G, Maskell D J, Miller J F. Multiple roles for Bordetella lipopolysaccharide molecules during respiratory tract infection. Infect Immun. 2000;68:6720–6728. doi: 10.1128/iai.68.12.6720-6728.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang R Y, Damrose E J, Alavi S, Maceri D R, Shapiro N L. Third branchial cleft anomaly presenting as a retropharyngeal abscess. Int J Pediatr Otorhinolaryngol. 2000;54:167–172. doi: 10.1016/s0165-5876(00)00355-4. [DOI] [PubMed] [Google Scholar]
- 14.Katzenstein D A, Ciofalo L, Jordan M C. Bordetella bronchiseptica bacteremia. West J Med. 1984;140:96–98. [PMC free article] [PubMed] [Google Scholar]
- 15.Papasian C J, Downs N J, Talley R L, Romberger D J, Hodges G R. Bordetella bronchiseptica bronchitis. J Clin Microbiol. 1987;25:575–577. doi: 10.1128/jcm.25.3.575-577.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinnott J T, Blazejowski C, Bazzini M D. Bordetella bronchiseptica endocarditis: a tale of a boy and his dog. Clin Microbiol Newsl. 1989;14:111–112. [Google Scholar]
- 17.Tamion F, Girault C, Chevron V, Pestel M, Bonmarchand G. Bordetella bronchiseptica pneumonia with shock in an immunocompetent patient. Scand J Infect Dis. 1996;28:197–198. doi: 10.3109/00365549609049077. [DOI] [PubMed] [Google Scholar]
- 18.Winters J L, O'Connor W L, Broughton R A, Noonan J A. Bordetella bronchispetica pneumonia in a patient with Down syndrome: a case report and review. Pediatrics. 1992;89:1262–1265. [PubMed] [Google Scholar]
- 19.Woodard D R, Cone L A, Fostvedt K. Bordetella bronchiseptica infection in patients with AIDS. Clin Infect Dis. 1995;20:193–194. doi: 10.1093/clinids/20.1.193. [DOI] [PubMed] [Google Scholar]
- 20.Woolfrey B F, Moody J A. Human infections associated with Bordetella bronchiseptica. Clin Microbiol Rev. 1991;4:243–255. doi: 10.1128/cmr.4.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weyant R S, Moss C W, Weaver R E, Hollis D G, Jordan J G, Cook E C, Daneshvar M I. Identification of unusual pathogenic gram-negative aerobic and facultatively anaerobic bacteria. 2nd ed. Baltimore, Md: Williams and Wilkins; 1996. [Google Scholar]