Abstract
The major pulmonary complication of rheumatoid arthritis (RA) is interstitial lung disease (ILD), which causes significant morbidity and mortality and influences the natural course of disease. Recent advances in the management of arthritis have improved patient outcomes. However, exceptionally high medical needs still remain for effective therapies for the patients with ILD in RA. Better understanding of the shared and distinct pathophysiology of fibrotic diseases led to the development of novel antifibrotic agents such as nintedanib and pirfenidone. The further stratification analysis of the phase III INBUILD trial demonstrated beneficial effects of nintedanib in RA-ILD with a progressive phenotype by reducing the rate of decline in forced vital capacity (FVC) over 52 weeks by 60%. Pirfenidone is another antifibrotic agent currently under phase II clinical study (TRAIL1) aiming to evaluate its effects for RA-ILD. This review provides an overview of state-of-the-art pathogenesis and the current therapeutic options for RA-ILD, with a focus on antifibrotic strategies.
Keywords: antifibrotics, fibrosis, interstitial lung disease (ILD), nintedanib, pirfenidone, rheumatoid arthritis (RA), treatment
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease affecting 0.5–1% of the population worldwide.1,2 Pulmonary involvement is a common extra-articular manifestation of RA, affecting any of the lung compartments, and includes a wide spectrum of disorders, ranging from airways and pleural disease, bronchiectasis (BR), and nodules, parenchymal involvement as well as infection and drug toxicity. 3 Lung disease in RA can occur at any time point of natural history of RA, either predating the onset of articular symptoms or becoming clinically evident after joint manifestations occur. Risk factors for disease-related lung disease are incompletely understood, further complicating clinical decision making regarding detection and screening for this complication.
Interstitial lung disease (ILD) occurring in patients with RA is associated with significant morbidity and mortality and hence is a major factor affecting the natural course of disease.4–8 ILD is identified in up to 60% of patients with RA, with clinically significant disease reported in approximately 10%.7,9 The reported median survival in previous RA-ILD studies ranges from 3 to 10 years.6,10,11 The hazard rate ratios (HRRs) for death were 2 to 10 times increased for RA-ILD compared with non-ILD RA, irrespective of follow-up period. 4 Among risk factors for development of ILD in RA that have been identified in many but not all clinical studies are advanced age, male sex, a history of ever smoking, and seropositivity for rheumatoid factor (RF) or anti-citrullinated protein antibodies (ACPAs).3,9,10 High-resolution computed tomography (HRCT) pattern, a lower baseline % predicted forced vital capacity (FVC % predicted), and a 10% decline in FVC % predicted from baseline to any time during follow-up have been identified to be independently associated with an increased risk of death. 10
Usual interstitial pneumonia (UIP) is the predominant histological/radiological pattern of RA-ILD, reported in up to 66% of cases, 12 unlike systemic connective diseases such as systemic sclerosis (SSc), in which the most common pattern of ILD is non-specific interstitial pneumonia (NSIP). Notably, a predominance of NSIP has been reported in patients from China, suggesting differences in risk factors including genetics and exposures that may contribute to the development of ILD. 13
Despite the considerable heterogeneity of disease extent and course among patients with certain patterns of RA-ILD, the course of UIP has been generally associated with a worse outcome than NSIP.10,14 In western populations, the NSIP pattern accounts for approximately 23–33% of cases with RA-ILD. 15 Other patterns of ILD are also observed in patients with RA, including organizing pneumonia (OP), diffuse alveolar damage (DAD), lymphocytic interstitial pneumonia (LIP), and desquamative interstitial pneumonia (DIP), but are much less common, with reported incidences of 8%, <2%, <2%, and <1%, respectively, among patients with RA-ILD. 15
Predicting which patients will progress to clinically significant ILD has been a challenge for clinicians. It is known that ILD could be subclinical in 19–57% of patients, but it tends to progress up to 50% of the cases,9,16,17 so early detection and close follow-up are very important, especially in patients with any of the above-mentioned factors associated with increased risk such as age, male sex, smoking, and seropositivity, in order to potentially alter the disease course. More recent studies have found that patients with the Disease Activity Score-28 for Rheumatoid Arthritis (DAS 28) scores showing medium and high disease activity have a twofold increased risk of developing ILD compared with patients with low disease activity and this was independent from other risk factors,9,16–18 which may suggest that controlling systemic inflammation may have a positive effect in delaying the onset of ILD.
Several biomarkers have been studied in patients with RA-ILD, trying to find ways to enhance our ability to detect early ILD cases and predict who would progress. Serum levels of matrix metalloproteinase-7 (MMP-7), interferon-γ-inducible protein 10 (IP-10)/CXCL10, tumor markers such as CA126, and other proteins such as KL-16 and BAFF have been investigated; most of these markers are elevated in patients with RA who develop ILD, but their value as predictors of disease progression is still unclear.9,16,17,19–21
Other clinical parameters that have been found to be associated with severity of disease and likelihood of progression and mortality include disease severity at presentation and the rate of change of pulmonary physiology within the first 6 months of disease;9,16,17 the pattern of fibrosis on HRCT has been a bit more controversial; some studies have suggested that the UIP pattern is associated with worse prognosis;9,16,17 however, other studies found that the pattern on HRCT was not an independent predictor of survival, whereas baseline and changes over time of the pulmonary physiology were independent predictors of survival. 10
Although therapeutic strategies for treatment of the joint manifestations of RA have greatly improved in recent decades, the management of RA-associated ILD remains challenging. Importantly, mortality is still increased in RA, and cardiovascular disease and ILD are the primary contributors to premature deaths.22,23 Moreover, patients with fibrosing ILDs other than idiopathic pulmonary fibrosis (IPF) who have progressive lung disease despite management have a subsequent clinical course similar to patients with untreated IPF, with a high risk of further ILD progression and early mortality. 24 The emergence of antifibrotics as a novel therapeutic option for the treatment of RA-ILD with first positive results from phase III clinical trials such as INBUILD raises hope for improving the prognosis of RA-ILD. 25 In this review, we will mainly focus on the pathogenesis of fibrosis in RA-ILD. We will discuss the putative mechanisms of action and effects of antifibrotic therapeutic approaches, and review the current clinical evidence for the use of antifibrotics in RA-ILD.
Proposed pathogenesis of RA-ILD
Although the underlying pathogenesis of RA remains incompletely understood, it is widely accepted that both genetic and environmental factors contribute to the progression of ILD in patients with RA. Important in this disease process is tissue fibrosis. Generally, fibrosis is defined as an excessive deposition of connective tissue components, and can affect virtually every organ system, including lungs. 26 Repeated exposure to unknown noxia is posited to initiate chronic injury of airway and alveolar epithelial cells. In response to damage, aberrant and excessive tissue remodeling results in the destruction of the parenchyma and replacement by increasing deposits of connective tissue. The resultant loss of functional airway and alveoli due to fibrosis can lead to respiratory failure and mortality.
Susceptibility to fibrotic diseases
In RA-ILD, several susceptibility loci have been identified which may be implicated in the pathogenesis of RA-ILD. Coding region mutations in the genes TERT, RTEL1, PARN, and SFTPC lead to telomere shortening. Telomere length is commonly used as a surrogate marker of telomere function, with short telomeres indicating reduced telomere function. 27 Telomere-related gene mutations and Single Nucleotide Polymorphism (SNPs) are associated with organ fibrosis and especially with pulmonary fibrosis. 28 Telomere-related mutations account for up to 10% of sporadic IPF, 25% of familial IPF, and 10% of CTD (connective tissue disease)-ILD. 29
Another common susceptibility locus has been described within the Mucin 5B gene (MUC5B). The MUC5B promoter variant rs35705950, the strongest genetic risk factor for IPF, was also a strong risk factor for RA-ILD, especially among patients with evidence of a UIP pattern on imaging. 30 However, the MUC5B promoter variant does not associate with the development of RA itself. 31 Also, the MUC5B promoter variant has not been found to be associated with a risk of ILDs associated with SSc or autoimmune myositis.32,33 It was observed in at least 50% of patients with IPF and has been estimated to account for 30% of the genetic risk of developing IPF.34–37 Changes in gene of MUC5B may indicate the regeneration of damaged epithelium and impaired mucociliary function or mucus composition.38,39
Collectively, these findings suggest an at least partially shared genetic susceptibility between RA-ILD and IPF.
Autoimmune response
Environmental factors, infection, cigarette smoking, and host genetics, are known to influence predisposition to articular as well as pulmonary disease in RA. 15 Oral and airway dysbiosis, gastro-esophageal reflux disease (GERD), and senescence are increasingly believed to be potentially important in the development of lung disease in patients with RA.15,40,41 The reparative cascade also involves an early inflammatory response to these initiating triggers, which leads to leukocyte infiltration, activation, and accumulation in affected tissues. Lung tissues from individuals with RA-interstitial pneumonitis (IP) have substantially greater numbers of B cells and CD4+ T cells than lung tissues from individuals with idiopathic IP,42,43 implying that immune dysregulation might be more prevalent in RA-ILD than in idiopathic IP. The external trigger leads to the epitope exposure and breakdown of immune tolerance, resulting in the activation of T cell targeting self-peptides. Susceptible individuals positive for the HLA-DRB1 shared epitope develop lung protein citrullination in the setting of cigarette smoking.44,45 Protein citrullination leads to the production of ACPAs. 46 ACPAs have been observed in the sputum, but not in serum, in some individuals at risk of future RA, indicating that ACPAs are probably generated in the airways. In addition, ACPA of immunoglobulin A (IgA) isotype, primarily reflecting mucosal immunity, has been identified in individuals at risk for RA. 47 A non-specific ACPA response can occur in patients with BR, becoming citrulline-specific in patients who develop BR/RA. 48 ACPA titers are higher in patients with RA-ILD than in patients with non-RA-ILD, and elevated levels of ACPA were observed in patients with RA whose pulmonary function revealed greater restriction or impaired diffusion compared with RA patients without those conditions. 49 Notably, ILD may precede articular manifestations of RA in some cases. It is postulated that breakdown of immune tolerance might thus occur initially in the airways, and the resultant autoimmune response against citrullinated proteins occurring in the lungs and then secondarily spreading to the joints.50,51 This concept implicates crosstalk between joints and lungs in RA-ILD. However, further studies are required for confirmation.
Profibrotic signaling
The profibrotic mechanisms of ILD in the setting of RA have thus far been poorly characterized. However, work in other fibrotic disorders such as IPF and SSc-ILD identified a core set of profibrotic pathways shared across different fibrotic diseases of the lung. The core pathways are defined as pathways that are essential to lead from an initial stimulus to the development of fibrosis. These core pathways are thought to be conserved across different organs, diseases, and also individuals. So far, only a few pathways have been cross-validated across different organs to be functionally essential, thereby fulfilling the criteria for core pathways of fibrosis. 26 Two core pathways that are also relevant for the current antifibrotic therapies are transforming growth factor-beta (TGFβ) and platelet-derived growth factor (PDGF) signaling.
TGFβ signaling is widely recognized as a master regulator of physiological and pathological tissue repair responses. 52 Dysregulation of TGFβ1 and TGFβ2 activity has been linked to the pathogenesis of fibrotic diseases such as IPF 53 and SSc-ILD. 52 Activation of TGFβ signaling, for example, by fibroblast-specific overexpression of constitutively active TGFβ receptor type 1 (TGFβRI), is sufficient to induce a systemic fibrotic disease with progressive fibrosis in multiple tissues.52,54–56 Moreover, a number of preclinical studies have demonstrated that inhibition of TGFβ signaling exerts potent antifibrotic effects in various animal models across different organs. 57 In mice with bleomycin-induced pulmonary fibrosis, epithelial-specific deletion of TGFβRII resulted in an attenuated fibrotic response in the lung. 58
PDGF has four isoforms (A, B, C, and D), which form several dimeric proteins (AA, BB, AB, CC, and DD) that can promote fibrosis via their mitogenic and perhaps also chemoattractant properties, as well as in synergy with TGFβ. As platelet-derived growth factor receptor (PDGFR) is one of the primary targets of nintedanib, responsiveness to nintedanib in patients with IPF, 59 SSc-ILD, 60 and other fibrosing ILD, 25 including RA-ILD, provides strong indication that those pathways are also activated and contribute to disease progression in lung fibrosis.
Following initial stimulus, the progression of fibrosis is driven by myofibroblasts. Myofibroblasts are metabolically active fibroblasts that express contractile proteins, contract the tissue, and release abundant amounts of extracellular matrix. Although myofibroblasts are a heterogeneous population of cells that are derived from various cellular precursors, they are regulated by a shared set of core pathways, including TGFβ, PDGF, WNT, and hedgehog signaling, as well as several members of the family of nuclear receptors, such as PPARγ, 61 vitamin D receptor (VDR), 62 and nuclear receptor subfamily 4 group A member 1 (NR4A1). 55 Although these cells may initially differentiate in response to local inflammation, the chronic profibrotic milieu induces epigenetic modification in myofibroblasts, which renders them endogenously activated even in the absence of external stimuli, thereby inducing a self-amplifying loop of myofibroblast activation and tissue fibrosis.
Lessons from mouse models
Little is known about the mechanisms underlying the progression of RA-ILD, in part due to the lack of an appropriate mouse model. Interestingly, SKG mice, which are genetically prone to development of autoimmune arthritis, develop a pulmonary interstitial pneumonia that resembles human cellular and fibrotic NSIP, thus validating as a model of RA-ILD to some extent. 63 Based on this mouse model, the contribution of some subsets of immune cells to the development of ILD in RA has been revealed. Kwon et al. 64 demonstrated that interleukin (IL)-17A+GM-CSF+ neutrophils represented the major inflammatory cells in the lungs of curdlan-treated SKG mice. Sendo et al. found a unique cell population, CD11b+Gr-1dim tolerogenic dendritic cell (DC)-like cells, which is expanded in the lungs of SKG mice with ILD. Furthermore, adoptive transfer of CD11b+Gr-1dim tolerogenic DC-like cells significantly suppressed progression of ILD in SKG mice, implicating a potential role of unique suppressive myeloid cells, that were differentiated from monocytic MDSCs, in suppressing lung inflammation in this model. 65 Furthermore, they also demonstrated that tofacitinib could facilitate the expansion of MDSCs in the lung and ameliorate ILD severity in SKG mice. 66
Putative mechanisms of action and effects of antifibrotics
Recently, the tyrosine kinase inhibitor nintedanib has demonstrated efficacy and safety in IPF, 59 SSc-ILD, 60 and a broad range of other fibrosing ILDs with a progressive phenotype, 25 including RA-ILD. Data from clinical trials also implicate the antifibrotic efficiency of pirfenidone in IPF, 67 as well as in unclassifiable fibrosing ILDs with a progressive phenotype. 68 The comparable responsiveness to antifibrotics across ILD with distinct etiologies despite their heterogeneity supports the hypothesis that core pathways are activated across different disease entities to drive progression of fibrosis.
Nintedanib, formerly known by its development code BIBF 1120, is a small molecule tyrosine kinase inhibitor that targets profibrotic receptor tyrosine kinases involved in fibrosis such as PDGF receptor, fibroblast growth factor (FGF) receptor, vascular endothelial growth factor (VEGF) receptor, and TGFβ receptor, as well as kinases involved in inflammation (Src family kinases and colony-stimulating factor-1 receptor kinase).69,70 Nintedanib was designed to block kinase activity by occupying the intracellular ATP-binding pocket of specific tyrosine kinases. The binding mode was explored for FGFR-1 and VEGFR-2. Data from in vitro studies have shown that nintedanib interferes with processes active in fibrosis such as fibroblast proliferation, migration and differentiation, and the secretion of extracellular matrix (ECM). 71 As mentioned above, treatment with nintedanib has been demonstrated to ameliorate lung fibrosis in SKG mice. 66 In addition, nintedanib has shown consistent antifibrotic and anti-inflammatory activity therapeutically and preventively in three preclinical lung fibrosis mice models of bleomycin-induced lung fibrosis in mice and rats, and silica-induced lung fibrosis in mice.72–74
Pirfenidone is an orally active small molecule comprising a modified phenyl pyridine. Although the exact mode of actions remains incompletely understood, accumulating in vivo and in vitro evidence demonstrates that pirfenidone inhibits stress-activated kinases and modulates expression of several profibrotic growth factors and proinflammatory cytokines, including TGFβ, PDGF, stromal cell–derived factor/C-X-C ligand 12 (SDF-1a/CXCL12), tumor necrosis factor-α (TNFα), IL-1β, and fibrogenic T-helper type 2 cytokines IL-4 and IL-13. 75 Consistent with these findings, transcriptional profiling of lung homogenates and lung fibroblasts derived from patients with IPF treated with or without pirfenidone indicated that pirfenidone exerted beneficial effects via its action on multiple pathways in both lung fibroblasts and other pulmonary cells, through its ability to control extracellular matrix architecture and inflammatory reactions. 76 In consequence, pirfenidone reduces fibroblast proliferation and alveolar macrophage activation, and inhibits ECM deposition.77,78 Pirfenidone ameliorates bleomycin-induced pulmonary fibrosis in mice79–83 and has antifibrotic effects in other preclinical models, such as skin fibrosis, 84 intestinal fibrosis, 85 cardiac fibrosis, 86 and liver fibrosis. 87
Antifibrotic management of RA-ILD
To date, treatment recommendations for RA-ILD have largely been based on trial data derived from IPF or other CTD-related ILDs such as SSc-associated ILD. The latest American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) guidelines for the management of RA recommend a multidisciplinary approach for RA-related comorbidities, such as ILD.88,89 There is no convincing evidence that the application of immunomodulatory therapy in subclinical RA-ILD is consistently able to stabilize the lung disease or prevent progressive decline in pulmonary function. In asymptomatic patients with non-progressive ILD, a ‘wait and see’ approach is usually recommended, and overtreatment should be avoided. In patients with clinically apparent symptoms, treatment should generally be considered based on the clinical, functional, or radiologic deterioration and histopathologic patterns of patients. Numerous excellent reviews cover the topics of supportive care and provide recommendations for the use of immunosuppressive therapies in RA-ILD.3,12,15 Here, we particularly focus on the use of antifibrotics in the management of RA-ILD.
Challenges in clinical practice
The concept of ‘progressive fibrosing ILDs’ or ‘fibrosing ILDs with a progressive phenotype’ has been increasingly adopted in clinical trials and practice.25,68,90 Progression of fibrosing ILDs is reflected by an increase in fibrosis evident on a computed tomography scan, a decline in forced vital capacity (FVC) and gas exchange (i.e. diffusing capacity of the lungs for carbon monoxide, DLCO), worsening of symptoms and exercise capacity, deterioration in health-related quality of life, and higher mortality. 91 Acute deterioration in respiratory function accompanied by evidence of new abnormalities on imaging is recognized to be a reflection of disease progression. 92
While a universally or generally accepted clinical definition of progressive ILD is lacking, the definition used in the INBUILD trial has currency. Regardless of their underlying rheumatologic or pneumological diseases, all patients recruited into the INBUILD trial were required to meet at least one of the following criteria for progression of ILD within the 24 months before screening: a relative decline in FVC of at least 10% of the predicted value, a relative decline in the FVC of 5% to less than 10% of the predicted value, and worsening of respiratory symptoms or an increased extent of fibrosis on HRCT, or worsening of respiratory symptoms and an increased extent of fibrosis on HRCT. 92 Another definition of progressive ILD was applied in a phase II trial of pirfenidone in patients with unclassifiable progressive fibrosing ILD (NCT03099187). Progressive ILD in this trial is defined as either a more than 5% absolute decline in FVC % predicted or significant symptomatic worsening not due to other causes within the previous 6 months. 68 The diagnosis of progressive ILD relies largely on clinical, radiological, and pulmonary function test (PFT) findings. Moreover, radiation-free lung imaging modalities, like ultrasound, show initial promise as tools for ILD screening and follow-up; however, further studies on their validity and reproducibitlity across different centers may be required. 93
The overall prognosis of patients with pulmonary fibrosis is related to the progressive phenotype. In a retrospective analysis of data from 1132 patients with IPF who received placebo in the TOMORROW, 94 INPULSIS1/2, 59 CAPACITY, 67 and ASCEND 95 trials, patients who had an absolute FVC decline of 10–15% predicted had a 2.2-fold greater risk of mortality compared with those with an absolute FVC decline of 5%. 96 Moreover, having an acute exacerbation (AE) was associated with a hazard ratio (HR) for mortality of 10.3. 96 Another retrospective analysis combined the data of the patients using placebo from the INBUILD trial (n = 331) and the INPULSIS trial (n = 423). Results of this analysis demonstrated that a relative decline of FVC > 10% predicted was associated with an increased risk of death in the INBUILD trial (HR = 3.64) and the INPULSIS trial (HR = 3.95). 24 Therefore, thorough screening and regular follow-up by auscultation, lung function testing (FVC and DLCO), and imaging by HRCT are mandatory for patients with RA, especially for those who present with respiratory manifestations or have risk factors of the development of ILD.
AE of ILD is characterized by acute deterioration in respiratory status, with newly developed bilateral ground-glass opacities and/or consolidations on chest radiographs or CT scans, typically less than 1 month in duration. 97 Other alternative causes such as infection, left heart failure, pulmonary embolism, or other identifiable cause of lung injury need to be excluded. AEs can occur at any time during the disease course. RA-ILD has been reported to be the most common CTD-ILD associated with AE. 98 Approximately 20% of patients with RA-ILD develop an AE, with high mortality despite intense therapy.99,100 ILD diagnosis at an older age and the UIP pattern on HRCT are acknowledged risk factors for AE of ILD.99,100 Based on observations in IPF, it is proposed that epithelial injury or proliferation, coagulation abnormalities, and autoimmunity are contributing factors to AEs of lung disease. 101
Evidence from clinical trials
Nintedanib and pirfenidone have already been approved by the US Food and Drug Administration (FDA) and European Medicines Agency for the treatment of IPF. 102 Subsequently, nintedanib was also approved by FDA as the first antifibrotic drug for the treatment of SSc-ILD 103 and chronic fibrosing (scarring) ILD with a progressive phenotype independent of its underlying disease and pathology 104 in September 2019 and in March 2020, respectively.
The INBUILD study was a basket trial that assessed the efficacy and safety of nintedanib in patients with progressive ILD other than IPF independent of its underlying disease. The trials recruited a total of 663 patients, including 89 patients with RA-ILD. The patients who received nintedanib had a slower annual rate decline of FVC over a 52-week period in ILD than placebo (−80.8 ml/year with nintedanib and −187.8 ml/year with placebo), for a between-group difference of 107.0 ml/year (p < 0.001). 25 Of interest, the results were similar in patients with UIP-like fibrotic pattern or other radiological patterns. 25
Further subgroup analysis of data from the INBUILD trial evaluated 170 patients with autoimmune disease–related ILDs, of whom 89 had RA-ILD, 39 had SSc-ILD, 19 had MCTD mixed connective tissue disease (MCTD)-ILD, and 23 had other autoimmune disease–related ILDs.105,106 Compared with placebo, nintedanib reduced the rate of decline in FVC over 52 weeks in patients with progressive fibrosing autoimmune ILDs by 58%, a similar relative reduction observed in the overall population of the INBUILD trial (57%). In addition, the proportions of patients with absolute and relative declines in FVC % predicted of >5% and >10% over 52 weeks were lower in patients who received nintedanib than placebo. 107 Similarly, in the subgroup of RA-ILD, nintedanib reduced the rate of decline in FVC over 52 weeks by 60% (−79.0 ml/year with nintedanib and −196.9 ml/year with placebo), for a between-group difference of 117.9 ml/year (nominal p = 0.041). Interestingly, in autoimmune disease–related ILD, the efficacy of nintedanib versus placebo was numerically greater in patients with a UIP-like fibrotic pattern on HRCT than in those with other fibrotic patterns, but without statistical significance (difference 124.2 ml/year in UIP-like subgroup, 41.7 ml/year in non-UIP subgroup, p = 0.37). 105 Collectively, these data suggest that nintedanib slows the rate of decline in FVC in patients with progressive fibrosing autoimmune disease–related ILDs, including RA-ILD.
As disease-modifying anti-rheumatic drugs (DMARDs) and glucocorticoids are widely applied in the majority of the patients with RA, the potential effect of DMARDs and/or glucocorticoids on the efficacy of nintedanib in patients with progressive fibrosing autoimmune disease–related ILDs in the INBUILD trial was also retrospectively assessed. RA-ILD accounted for 52% of patients of autoimmune disease–related ILD in that study. The rate of FVC decline was slower in patients treated with nintedanib than placebo both in patients who were and were not taking DMARDs and/or glucocorticoids at baseline, indicating beneficial efficacy of nintedanib in these patients, regardless of background DMARD therapy and/or glucocorticoid use.105,108
Among patients with autoimmune disease–related ILDs, AE of ILD or death occurred in 10 patients (12.2%) in the nintedanib group and 18 (20.5%) in the placebo group (HR = 0.58, nominal p = 0.17) during the 52-week trial. The proportions of patients with progression of ILD or death were 40.2% in the nintedanib group and 53.4% in the placebo group (HR = 0.72; nominal p = 0.15). Deaths occurred in 9.8% of patients in the nintedanib group and 12.5% in the placebo group (HR = 0.80; nominal p = 0.62). Although not reaching statistical significance, nintedanib demonstrated beneficial effects in reducing the events of AE, progression of ILD, or death in autoimmune disease–related ILDs. However, further research is needed to confirm the effect of nintedanib on life-threatening events of autoimmune-related ILDs.
While analysis of data for nintedanib is favorable across autoimmune-related ILDs taken together, and for subgroups of patients with specific ILDs, the INBUILD trial was not designed or powered to show a benefit of nintedanib in the subgroup of patients with autoimmune disease–related ILDs.
Ongoing clinical trials of agents for RA-ILD
The TRAIL1 study was a phase II randomized, double-blind, placebo-controlled trial of pirfenidone aiming to investigate the efficiency and safety of pirfenidone in patients with RA-ILD (NCT02808871). 109 Only 123 patients were randomized of the intended 270; the study was stopped due to slow recruitment exacerbated by the COVID-19 pandemic. 110 Patients were administered pirfenidone 2403 mg/day as an add-on to existing treatment. The primary endpoint of the study was the incidence of the composite endpoint of decline in FVC % predicted of over 10% or death during the 52-week study period. The primary endpoint met by 11% on pirfenidone versus 15% on placebo [OR = 0.67 (0.22, 2.03), p = 0.48]. 110 Although pirfenidone was found to be safe and slowed decline of FVC over time in patients with RA-ILD, TRAIL1 was underpowered to detect a difference in the composite primary endpoint. This effect was more pronounced in those with a UIP pattern on baseline HRCT. No correlations between joint-disease activity and RA-ILD disease severity were noted.
The results of two multinational phase III trials, CAPACITY (004) 67 and ASCEND, 95 showed that pirfenidone significantly reduced the decline in FVC % predicted in the patients with IPF at week 72 and week 52, respectively. A recent phase II trial reported that the patients with progressive fibrosing unclassifiable ILD could benefit from pirfenidone treatment. 68 However, the small number of unclassifiable ILD patients with autoimmune features may limit further stratification analysis.
The list of potential molecular targets for the treatment of fibrosis is continuously growing. Several trials have been completed or are ongoing to assess the efficacy and safety of agents in the treatment of fibrosing ILDs, including RA-ILD (Table 1).
Table 1.
Clinical trials of antifibrotics for ILD, including RA-associated ILD (RA-ILD).
| Drug | Study name | Design and population | Phase | Clinical trial identifier | Status |
|---|---|---|---|---|---|
| Nintedanib | INBUILD | Efficacy and safety of nintedanib in patients with PF-ILD | III | NCT02999178 | Completed |
| Nintedanib | NA | A follow-up study investigating long-term treatment with nintedanib in patients with PF-ILD | III | NCT03820726 | Active, not recruiting |
| Nintedanib | NA | An expanded access program to provide nintedanib to patients of non-IPF-ILD who have no alternative treatment possibilities | – | NCT03843892 | Available |
| Pirfenidone | TRAIL1 | Safety, tolerability, and efficacy of pirfenidone in patients with RA-ILD | II | NCT02808871 | Enrollment closed |
| Pirfenidone | RELIEF | Efficacy and safety of pirfenidone for progressive, non-IPF lung fibrosis | II | EudraCT 2014-000861-32 DRKS00009822 |
Completed |
| Abatacept | APRIL | Safety of abatacept in RA-ILD; the investigators will perform a small clinical trial to assess the feasibility of performing a larger randomized controlled trial | – | NCT03084419 | Recruiting |
| Tofacitinib versus Methotrexate | PULMORA | Effects of tofacitinib versus methotrexate on clinical and molecular disease activity markers in joints and lungs in early RA | IV | NCT04311567; EudraCT 2019-004179-38 | Recruiting |
| Allogeneic bone marrow derived–mesenchymal stem cclls | NA | Safety of allogeneic bone marrow–derived mesenchymal stem cells for ILD in patients with connective tissue disease | I | NCT03929120 | Recruiting |
ILD, interstitial lung disease; NA, not available; PF, progressive fibrosing; RA, rheumatoid arthritis.
Management of safety issues
In clinical trials of the available antifibrotics, the most common adverse events have been gastrointestinal in nature. With nintedanib, diarrhea was the most common adverse event reported in 66.9% of patients on nintedanib compared with 23.9% in the placebo arms. 25 Abnormalities on liver-function testing were more common in the nintedanib group than in the placebo group (11.4% versus 3.6%). Other adverse events that were more frequent in the nintedanib group than in the placebo group include nausea (28.9% versus 9.4%), vomiting (18.4% versus 5.1%), abdominal pain (10.2% versus 2.4%), decreased appetite (14.5% versus 5.1%), and weight decrease (12.3% versus 3.3%). Elevations in liver enzymes showed a trend toward normalization after dose adjustment or discontinuation. 25 The safety profile of nintedanib in patients with autoimmune disease–related ILD was noted to be similar to that of patients without autoimmune disease–related ILD, 105 not significantly different in those patients who were taking concomitant glucocorticoids or DMARDs. 111
In patients taking with pirfenidone, gastrointestinal and skin-related events were the most commonly reported adverse events, but like nintedanib these were typically mild to moderate in severity. The percentages of patients with other adverse events (AEs) associated with pirfenidone (treatment-related photosensitivity, rash, weight decrease, and fatigue) were similar between the treatment groups (<10% difference). However, treatment-related gastrointestinal disorders were more frequent with pirfenidone than placebo (47% versus 26%). 58
Although adverse events are quite frequent, most are mild and often, but not always manageable with dose modification. 112 In total, 19.6% of patients on nintedanib and 15% of patients on pirfenidone discontinue treatment due to adverse events in daily practice, compared with placebo (10.3% and 4%, respectively).25,68 A 24-week, single-arm, open-label, phase IV study was performed to assess safety and tolerability of treatment with pirfenidone (1602–2403 mg/day) and nintedanib (200–300 mg/day) in patients with IPF. The results demonstrated that combined pirfenidone and nintedanib use for 24 weeks was tolerated by the majority of patients with IPF and associated with a similar pattern of treatment-emergent adverse events expected for either treatment alone. 113
Depending upon the individual clinical scenario, a stepwise management strategy is recommended to prevent and mitigate drug-related AEs and help maintain the drugs at an optimal dose for long-term treatment. Patient education is of critical importance, as is regular follow-up, timely reporting of AEs, sun exposure protection, and avoidance of unnecessary drugs or supplements. In case of drug-related AEs, dose titration and modification of the medication schedule or, if needed, drug discontinuation may be required.
Summary
ILD is a common manifestation of RA, which causes significant morbidity and mortality. Although there are significant advances in the management joint disease, the pharmaceutical options for the treatment of RA-ILD remain limited. The recently released data from the INBUILD trial provide evidence for the efficiency of antifibrotic agent nintedanib in patients with RA-ILD with a progressive phenotype. Other antifibrotic therapies such as pirfenidone are currently under investigation and may help to improve our management of RA-ILD.
Patients with RA with radiographic evidence of ILD, progressive respiratory symptoms such as cough and dyspnea, and worsening respiratory physiology despite traditional immunosuppressive therapy for RA-ILD such as rituximab, azathioprine, or mycophenolate mofetil may be candidates for antifibrotic therapy. 114 Monitoring of patients with RA-ILD on antifibrotics has not been yet established so we suggest to follow recommendations for patients with IPF that are being treated with these agents. While on nintedanib and pirfenidone, patients are typically monitored for hepatic toxicity with serum transaminases every month for the first 3 months and every 3 months after that. 60 Patients on pirfenidone may experience gastrointestinal symptoms such as nausea and also skin-related events especially photosensitivity. It is recommended for patients on pirfenidone to use sunscreen and avoid direct sun exposure. 95 Pulmonary function tests carried out every 3–6 months help assess progression of disease and response to therapy; the most important parameters would be the FVC and the DLCO since they are key predictors of mortality. The 6-minute walk testing (6MWT) performed every 6 months is also recommended to assess disease progression, with the caveat that patients with active joint disease or ambulatory difficulty, assessment may be difficult.
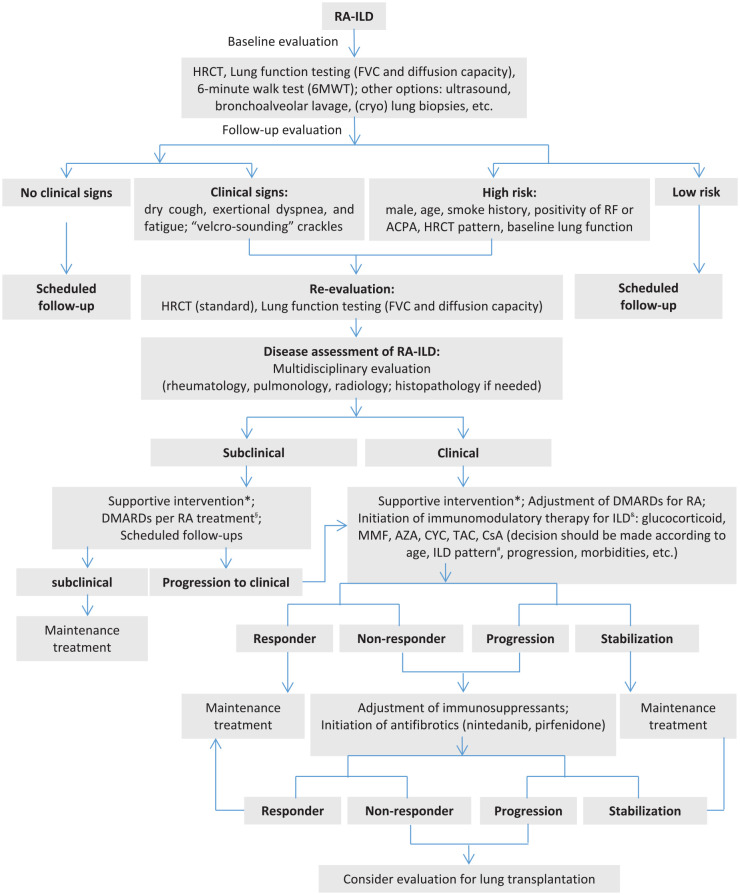
Despite this recent progress, the management of RA-ILD remains challenging. Here, we proposed a stepwise algorithm for the management of RA-ILD, mainly based on the individual experience in our centers and literature review (Figure 1). Open questions that require future studies include the following: (1) the efficacy of multimodal therapies combining antifibrotic agents and immunomodulatory agents, (2) potential differences in treatment strategy based on the ILD pattern, and (3) the timing of initiation and termination of antifibrotic therapy in RA-ILD.
Figure 1.
A stepwise algorithm for the management of RA-associated ILD (RA-ILD).
ACPA, anti-citrullinated protein antibody; AZA, azathioprine; CsA, cyclosporin A; CYC, cyclophosphamide; DMARDs, disease-modifying anti-rheumatic drugs; FVC, forced vital capacity; HRCT, high-resolution computed tomography; ILD, interstitial lung disease; MMF, mycophenolate mofetil; RA, rheumatoid arthritis; RF, rheumatoid factor; TAC, tacrolimus, UIP, usual interstitial pneumonia.
*Supportive interventions such as smoking cessation, pulmonary rehabilitation, oxygen therapy, preventive vaccination.
§DMARDs with potential pulmonary toxicity should be tightly monitored. &There is no clear evidence-based recommendations on the choice of immunomodulatory agent for the treatment of RA-ILD.
#Optimal therapy for RA-ILD with a UIP pattern is under debate.
Current and future research addressing the underlying pathophysiology of RA-ILD is essential for developing further therapeutic approaches for the treatment of RA-ILD. Studies evaluating the disease course, need for treatment, and standards for defining and assessing progressive lung disease in order to identify patients who may benefit from therapy are urgently needed in order to improve the often dismal prognosis of patients with progressive fibrosis.
Footnotes
Authors’ Note: Minrui Liang Department of Rheumatology, Huashan Hospital, Fudan University, Shanghai, China.
Author contributions: Minrui Liang: Conceptualization; Writing – original draft; Writing – review & editing.
Eric L. Matteson: Conceptualization; Supervision; Writing – review & editing.
Andy Abril: Conceptualization; Writing – review & editing.
Jörg H.W. Distler: Conceptualization; Resources; Supervision; Writing – original draft; Writing – review & editing.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Eric L. Matteson: consultant/advisory board, speaker (Boehringer Ingelheim); data safety monitoring board (Horizon); speaker (Novartis); editor, writer (UpToDate). Jörg H.W. Distler: consultancy relationships with Actelion, Active Biotech, Anamar, ARXX, Bayer Pharma, Boehringer Ingelheim, Celgene, Galapagos, GSK, Inventiva, Medac, Novartis, Pfizer, RuiYi, and UCB. Jörg H.W. Distler has received research funding from Anamar, Active Biotech, Array Biopharma, ARXX, aTyr, BMS, Bayer Pharma, Boehringer Ingelheim, Cantargia, Celgene, CSL Behring, Galapagos, GSK, Inventiva, Kiniksa, Sanofi-Aventis, RedX, and UCB. Jörg H.W. Distler is stock owner of 4D Science.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The current work was supported by a joint Sino-German Research project (82161138022).
ORCID iD: Eric L. Matteson  https://orcid.org/0000-0002-9866-0124
https://orcid.org/0000-0002-9866-0124
Contributor Information
Minrui Liang, Rheumatology and Clinical Immunology, Department of Internal Medicine 3, Friedrich-Alexander-University (FAU) Erlangen-Nürnberg, Erlangen, Germany.
Eric L. Matteson, Division of Rheumatology, Mayo Clinic College of Medicine and Science, Rochester, MN, USA.
Andy Abril, Division of Rheumatology, Mayo Clinic College of Medicine and Science, Jacksonville, FL, USA.
Jörg H.W. Distler, Rheumatology and Clinical Immunology, Department of Internal Medicine 3, Friedrich-Alexander-University (FAU) Erlangen-Nürnberg, Ulmenweg 18, 91054 Erlangen, Germany.
References
- 1. Chen Z, Bozec A, Ramming A, et al. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat Rev Rheumatol 2019; 15: 9–17. [DOI] [PubMed] [Google Scholar]
- 2. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet 2016; 388: 2023–2038. [DOI] [PubMed] [Google Scholar]
- 3. Farquhar H, Vassallo R, Edwards AL, et al. Pulmonary complications of rheumatoid arthritis. Semin Respir Crit Care Med 2019; 40: 194–207. [DOI] [PubMed] [Google Scholar]
- 4. Hyldgaard C, Hilberg O, Pedersen AB, et al. A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: comorbidity and mortality. Ann Rheum Dis 2017; 76: 1700–1706. [DOI] [PubMed] [Google Scholar]
- 5. Myasoedova E, Crowson CS, Turesson C, et al. Incidence of extraarticular rheumatoid arthritis in Olmsted County Minnesota, in 1995-2007 versus 1985-1994: a population-based study. J Rheumatol 2011; 38: 983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum 2010; 62: 1583–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Olson AL, Swigris JJ, Sprunger DB, et al. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med 2011; 183: 372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim EJ, Elicker BM, Maldonado F, et al. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2010; 35: 1322–1328. [DOI] [PubMed] [Google Scholar]
- 9. Gabbay E, Tarala R, Will R, et al. Interstitial lung disease in recent onset rheumatoid arthritis. Am J Respir Crit Care Med 1997; 156: 528–535. [DOI] [PubMed] [Google Scholar]
- 10. Solomon JJ, Chung JH, Cosgrove GP, et al. Predictors of mortality in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2016; 47: 588–596. [DOI] [PubMed] [Google Scholar]
- 11. Koduri G, Norton S, Young A, et al. Interstitial lung disease has a poor prognosis in rheumatoid arthritis: results from an inception cohort. Rheumatology 2010; 49: 1483–1489. [DOI] [PubMed] [Google Scholar]
- 12. Cassone G, Manfredi A, Vacchi C, et al. Treatment of rheumatoid arthritis-associated interstitial lung disease: lights and shadows. J Clin Med 2020; 9: 1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen J, Shi Y, Wang X, et al. Asymptomatic preclinical rheumatoid arthritis-associated interstitial lung disease. Clin Dev Immunol 2013; 2013: 406927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsuchiya Y, Takayanagi N, Sugiura H, et al. Lung diseases directly associated with rheumatoid arthritis and their relationship to outcome. Eur Respir J 2011; 37: 1411–1417. [DOI] [PubMed] [Google Scholar]
- 15. Wang D, Zhang J, Lau J, et al. Mechanisms of lung disease development in rheumatoid arthritis. Nat Rev Rheumatol 2019; 15: 581–596. [DOI] [PubMed] [Google Scholar]
- 16. Saag KG, Kolluri S, Koehnke RK, et al. Rheumatoid arthritis lung disease. Determinants of radiographic and physiologic abnormalities. Arthritis Rheum 1996; 39: 1711–1719. [DOI] [PubMed] [Google Scholar]
- 17. Zamora-Legoff JA, Krause ML, Crowson CS, et al. Progressive decline of lung function in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheumatol 2017; 69: 542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sparks JA, He X, Huang J, et al. Rheumatoid arthritis disease activity predicting incident clinically apparent rheumatoid arthritis-associated interstitial lung disease: a prospective cohort study. Arthritis Rheumatol 2019; 71: 1472–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen J, Doyle JL, Liu Y, et al. Biomarkers of rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheumatol 2015; 67: 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang T, Zheng XJ, Ji YL, et al. Tumour markers in rheumatoid arthritis-associated interstitial lung disease. Clin Exp Rheumatol 2016; 34: 587–591. [PubMed] [Google Scholar]
- 21. Oguz EO, Kucuksahin O, Turgay M, et al. Association of serum KL-6 levels with interstitial lung disease in patients with connective tissue disease: a cross-sectional study. Clin Rheumatol 2016; 35: 663–666. [DOI] [PubMed] [Google Scholar]
- 22. Young A, Koduri G, Batley M, et al. Mortality in rheumatoid arthritis. Increased in the early course of disease, in ischaemic heart disease and in pulmonary fibrosis. Rheumatology 2007; 46: 350–357. [DOI] [PubMed] [Google Scholar]
- 23. Sparks JA, Chang SC, Liao KP, et al. Rheumatoid arthritis and mortality among women during 36 years of prospective follow-up: results from the nurses’ health study. Arthritis Care Res 2016; 68: 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brown KK, Martinez FJ, Walsh SLF, et al. The natural history of progressive fibrosing interstitial lung diseases. Eur Respir J 2020; 55: 2000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Flaherty KR, Wells AU, Brown KK, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med 2019; 381: 1718–1727. [DOI] [PubMed] [Google Scholar]
- 26. Distler JHW, Györfi AH, Ramanujam M, et al. Shared and distinct mechanisms of fibrosis. Nat Rev Rheumatol 2019; 15: 705–730. [DOI] [PubMed] [Google Scholar]
- 27. Newton CA, Oldham JM, Ley B, et al. Telomere length and genetic variant associations with interstitial lung disease progression and survival. Eur Respir J 2019; 53: 180164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Juge PA, Borie R, Kannengiesser C, et al. Shared genetic predisposition in rheumatoid arthritis-interstitial lung disease and familial pulmonary fibrosis. Eur Respir J 2017; 49: 1602314. [DOI] [PubMed] [Google Scholar]
- 29. Hoffman TW, van Moorsel CHM, Borie R, et al. Pulmonary phenotypes associated with genetic variation in telomere-related genes. Curr Opin Pulm Med 2018; 24: 269–280. [DOI] [PubMed] [Google Scholar]
- 30. Juge PA, Soloman JJ, Garofoli R, et al. MUC5B promoter variant and rheumatoid arthritis with interstitial lung disease. N Engl J Med 2018; 379: 2209–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Okada Y, Wu D, Trynka G, et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature 2014; 506: 376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Borie R, Crestani B, Dieude P, et al. The MUC5B variant is associated with idiopathic pulmonary fibrosis but not with systemic sclerosis interstitial lung disease in the European Caucasian population. PLoS ONE 2013; 8: e70621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johnson C, Rosen P, Lloyd T, et al. Exploration of the MUC5B promoter variant and ILD risk in patients with autoimmune myositis. Respir Med 2017; 130: 52–54. [DOI] [PubMed] [Google Scholar]
- 34. Seibold MA, Wise AL, Speer MC, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med 2011; 364: 1503–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang Y, Noth I, Garcia JG, et al. A variant in the promoter of MUC5B and idiopathic pulmonary fibrosis. N Engl J Med 2011; 364: 1576–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fingerlin TE, Murphy E, Zhang W, et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet 2013; 45: 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Noth I, Zhang Y, Ma SF, et al. Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. Lancet Respir Med 2013; 1: 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Evans CM, Fingerlin TE, Schwarz MI, et al. Idiopathic pulmonary fibrosis: a genetic disease that involves mucociliary dysfunction of the peripheral airways. Physiol Rev 2016; 96: 1567–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Button B, Cai LH, Ehre C, et al. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 2012; 337: 937–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Scher JU, Joshua V, Artacho A, et al. The lung microbiota in early rheumatoid arthritis and autoimmunity. Microbiome 2016; 4: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mikuls TR, Payne JB, Deane KD, et al. Autoimmunity of the lung and oral mucosa in a multisystem inflammatory disease: the spark that lights the fire in rheumatoid arthritis? J Allergy Clin Immunol 2016; 137: 28–34. [DOI] [PubMed] [Google Scholar]
- 42. Turesson C, Matteson EL, Colby TV, et al. Increased CD4+ T cell infiltrates in rheumatoid arthritis-associated interstitial pneumonitis compared with idiopathic interstitial pneumonitis. Arthritis Rheum 2005; 52: 73–79. [DOI] [PubMed] [Google Scholar]
- 43. Atkins SR, Turesson C, Myers JL, et al. Morphologic and quantitative assessment of CD20+ B cell infiltrates in rheumatoid arthritis-associated nonspecific interstitial pneumonia and usual interstitial pneumonia. Arthritis Rheum 2006; 54: 635–641. [DOI] [PubMed] [Google Scholar]
- 44. Ishikawa Y, Ikari K, Hashimoto M, et al. Shared epitope defines distinct associations of cigarette smoking with levels of anticitrullinated protein antibody and rheumatoid factor. Ann Rheum Dis 2019; 78: 1480–1487. [DOI] [PubMed] [Google Scholar]
- 45. Scally SW, Petersen J, Law SC, et al. A molecular basis for the association of the HLA-DRB1 locus, citrullination, and rheumatoid arthritis. J Exp Med 2013; 210: 2569–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van Drongelen V, Ali WH, Holoshitz J. Uncovering a shared epitope-activated protein citrullination pathway. J Immunol 2020; 205: 579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Willis VC, Demoruelle MK, Derber LA, et al. Sputum autoantibodies in patients with established rheumatoid arthritis and subjects at risk of future clinically apparent disease. Arthritis Rheum 2013; 65: 2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Quirke AM, Perry E, Cartwright A, et al. Bronchiectasis is a model for chronic bacterial infection inducing autoimmunity in rheumatoid arthritis. Arthritis Rheumatol 2015; 67: 2335–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Giles JT, Danoff SK, Sokolove J, et al. Association of fine specificity and repertoire expansion of anticitrullinated peptide antibodies with rheumatoid arthritis associated interstitial lung disease. Ann Rheum Dis 2014; 73: 1487–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Paulin F, Doyle TJ, Fletcher EA, et al. Rheumatoid arthritis-associated interstitial lung disease and idiopathic pulmonary fibrosis: shared mechanistic and phenotypic traits suggest overlapping disease mechanisms. Rev Invest Clin 2015; 67: 280–286. [PMC free article] [PubMed] [Google Scholar]
- 51. Spagnolo P, Lee JS, Sverzellati N, et al. The lung in rheumatoid arthritis: focus on interstitial lung disease. Arthritis Rheumatol 2018; 70: 1544–1554. [DOI] [PubMed] [Google Scholar]
- 52. Györfi AH, Matei AE, Distler JHW. Targeting TGF-beta signaling for the treatment of fibrosis. Matrix Biol 2018; 68-69: 8–27. [DOI] [PubMed] [Google Scholar]
- 53. Guillotin D, Taylor AR, Plate M, et al. Transcriptome analysis of IPF fibroblastic foci identifies key pathways involved in fibrogenesis. Thorax 2021; 76: 73–82. [DOI] [PubMed] [Google Scholar]
- 54. Dees C, Chakraborty D, Distler JHW. Cellular and molecular mechanisms in fibrosis. Exp Dermatol 2021; 30: 121–131. [DOI] [PubMed] [Google Scholar]
- 55. Palumbo-Zerr K, Zerr P, Distler A, et al. Orphan nuclear receptor NR4A1 regulates transforming growth factor-beta signaling and fibrosis. Nat Med 2015; 21: 150–158. [DOI] [PubMed] [Google Scholar]
- 56. Pachera E, Assassi S, Salazar GA, et al. Long noncoding RNA H19X is a key mediator of TGF-beta-driven fibrosis. J Clin Invest 2020; 130: 4888–4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lafyatis R. Transforming growth factor beta – at the centre of systemic sclerosis. Nat Rev Rheumatol 2014; 10: 706–719. [DOI] [PubMed] [Google Scholar]
- 58. Li M, Krishnaveni MS, Li C, et al. Epithelium-specific deletion of TGF-beta receptor type II protects mice from bleomycin-induced pulmonary fibrosis. J Clin Invest 2011; 121: 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2071–2082. [DOI] [PubMed] [Google Scholar]
- 60. Distler O, Highland KB, Gahlemann M, et al. Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med 2019; 380: 2518–2528. [DOI] [PubMed] [Google Scholar]
- 61. Wei J, Zhu H, Komura K, et al. A synthetic PPAR-gamma agonist triterpenoid ameliorates experimental fibrosis: PPAR-gamma-independent suppression of fibrotic responses. Ann Rheum Dis 2014; 73: 446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zerr P, Vollath S, Zerr KP, et al. Vitamin D receptor regulates TGF-beta signalling in systemic sclerosis. Ann Rheum Dis 2015; 74: e20. [DOI] [PubMed] [Google Scholar]
- 63. Keith RC, Powers JL, Redente EF, et al. A novel model of rheumatoid arthritis-associated interstitial lung disease in SKG mice. Exp Lung Res 2012; 38: 55–66. [DOI] [PubMed] [Google Scholar]
- 64. Kwon OC, Lee E-J, Chang E-J, et al. IL-17A(+)GM-CSF(+) neutrophils are the major infiltrating cells in interstitial lung disease in an autoimmune arthritis model. Front Immunol 2018; 9: 1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sendo S, Saegusa J, Okano T, et al. CD11b+Gr-1(dim) tolerogenic dendritic cell-like cells are expanded in interstitial lung disease in SKG mice. Arthritis Rheumatol 2017; 69: 2314–2327. [DOI] [PubMed] [Google Scholar]
- 66. Sendo S, Saegusa J, Yamada H, et al. Tofacitinib facilitates the expansion of myeloid-derived suppressor cells and ameliorates interstitial lung disease in SKG mice. Arthritis Res Ther 2019; 21: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 2011; 377: 1760–1769. [DOI] [PubMed] [Google Scholar]
- 68. Maher TM, Corte TJ, Fischer A, et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med 2020; 8: 147–157. [DOI] [PubMed] [Google Scholar]
- 69. Hilberg F, Roth GJ, Krssak M, et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res 2008; 68: 4774–4782. [DOI] [PubMed] [Google Scholar]
- 70. Hilberg F, Tontsch-Grunt U, Baum A, et al. Triple angiokinase inhibitor nintedanib directly inhibits tumor cell growth and induces tumor shrinkage via blocking oncogenic receptor tyrosine kinases. J Pharmacol Exp Ther 2018; 364: 494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Epstein Shochet G, Wollin L, Shitrit D. Fibroblast-matrix interplay: nintedanib and pirfenidone modulate the effect of IPF fibroblast-conditioned matrix on normal fibroblast phenotype. Respirology 2018; 23: 756–763. [DOI] [PubMed] [Google Scholar]
- 72. Wollin L, Maillet I, Quesniaux V, et al. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J Pharmacol Exp Ther 2014; 349: 209–220. [DOI] [PubMed] [Google Scholar]
- 73. Huang J, Maier C, Zhang Y, et al. Nintedanib inhibits macrophage activation and ameliorates vascular and fibrotic manifestations in the Fra2 mouse model of systemic sclerosis. Ann Rheum Dis 2017; 76: 1941–1948. [DOI] [PubMed] [Google Scholar]
- 74. Huang J, Beyer C, Palumbo-Zerr K, et al. Nintedanib inhibits fibroblast activation and ameliorates fibrosis in preclinical models of systemic sclerosis. Ann Rheum Dis 2016; 75: 883–890. [DOI] [PubMed] [Google Scholar]
- 75. Gurujeyalakshmi G, Hollinger MA, Giri SN. Pirfenidone inhibits PDGF isoforms in bleomycin hamster model of lung fibrosis at the translational level. Am J Physiol 1999; 276: L311–L318. [DOI] [PubMed] [Google Scholar]
- 76. Kwapiszewska G, Gungl A, Wilhelm J, et al. Transcriptome profiling reveals the complexity of pirfenidone effects in idiopathic pulmonary fibrosis. Eur Respir J 2018; 52: 1800564. [DOI] [PubMed] [Google Scholar]
- 77. Schaefer CJ, Ruhrmund DW, Pan L, et al. Antifibrotic activities of pirfenidone in animal models. Eur Respir Rev 2011; 20: 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ruwanpura SM, Thomas BJ, Bardin PG. Pirfenidone: molecular mechanisms and potential clinical applications in lung disease. Am J Respir Cell Mol Biol 2020; 62: 413–422. [DOI] [PubMed] [Google Scholar]
- 79. Oku H, Shimizu T, Kawabata T, et al. Antifibrotic action of pirfenidone and prednisolone: different effects on pulmonary cytokines and growth factors in bleomycin-induced murine pulmonary fibrosis. Eur J Pharmacol 2008; 590: 400–408. [DOI] [PubMed] [Google Scholar]
- 80. Kakugawa T, Mukae H, Hayashi T, et al. Pirfenidone attenuates expression of HSP47 in murine bleomycin-induced pulmonary fibrosis. Eur Respir J 2004; 24: 57–65. [DOI] [PubMed] [Google Scholar]
- 81. Iyer SN, Wild JS, Schiedt MJ, et al. Dietary intake of pirfenidone ameliorates bleomycin-induced lung fibrosis in hamsters. J Lab Clin Med 1995; 125: 779–785. [PubMed] [Google Scholar]
- 82. Iyer SN, Margolin SB, Hyde DM, et al. Lung fibrosis is ameliorated by pirfenidone fed in diet after the second dose in a three-dose bleomycin-hamster model. Exp Lung Res 1998; 24: 119–132. [DOI] [PubMed] [Google Scholar]
- 83. Schelegle ES, Mansoor JK, Giri S. Pirfenidone attenuates bleomycin-induced changes in pulmonary functions in hamsters. Proc Soc Exp Biol Med 1997; 216: 392–397. [DOI] [PubMed] [Google Scholar]
- 84. Du J, Paz K, Flynn R, et al. Pirfenidone ameliorates murine chronic GVHD through inhibition of macrophage infiltration and TGF-beta production. Blood 2017; 129: 2570–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Meier R, Lutz C, Cosin-Roger J, et al. Decreased fibrogenesis after treatment with pirfenidone in a newly developed mouse model of intestinal fibrosis. Inflamm Bowel Dis 2016; 22: 569–582. [DOI] [PubMed] [Google Scholar]
- 86. Wang Y, Wu Y, Chen J, et al. Pirfenidone attenuates cardiac fibrosis in a mouse model of TAC-induced left ventricular remodeling by suppressing NLRP3 inflammasome formation. Cardiology 2013; 126: 1–11. [DOI] [PubMed] [Google Scholar]
- 87. Komiya C, Tanaka M, Tsuchiya K, et al. Antifibrotic effect of pirfenidone in a mouse model of human nonalcoholic steatohepatitis. Sci Rep 2017; 7: 44754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Smolen JS, Landewe RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020; 79: 685–699. [DOI] [PubMed] [Google Scholar]
- 89. Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017; 76: 960–977. [DOI] [PubMed] [Google Scholar]
- 90. Goldberg HJ. Understanding progressive fibrosing interstitial lung disease through therapeutic trials. N Engl J Med 2019; 381: 1775–1777. [DOI] [PubMed] [Google Scholar]
- 91. Spagnolo P, Distler O, Ryerson CJ, et al. Mechanisms of progressive fibrosis in connective tissue disease (CTD)-associated interstitial lung diseases (ILDs). Ann Rheum Dis 2021; 80: 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cottin V, Wollin L, Fischer A, et al. Fibrosing interstitial lung diseases: knowns and unknowns. Eur Respir Rev 2019; 28: 180100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang Y, Gargani L, Barskova T, et al. Usefulness of lung ultrasound B-lines in connective tissue disease-associated interstitial lung disease: a literature review. Arthritis Res Ther 2017; 19: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Richeldi L, Costabel U, Selman M, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med 2011; 365: 1079–1087. [DOI] [PubMed] [Google Scholar]
- 95. King TE, Jr, Bradford WZ, Fagan EA, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2083–2092. [DOI] [PubMed] [Google Scholar]
- 96. Paterniti MO, Bi Y, Rekic D, et al. Acute exacerbation and decline in forced vital capacity are associated with increased mortality in idiopathic pulmonary fibrosis. Ann Am Thorac Soc 2017; 14: 1395–1402. [DOI] [PubMed] [Google Scholar]
- 97. Kolb M, Bondue B, Pesci A, et al. Acute exacerbations of progressive-fibrosing interstitial lung diseases. Eur Respir Rev 2018; 27: 180071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Suda T, Kaida Y, Nakamura Y, et al. Acute exacerbation of interstitial pneumonia associated with collagen vascular diseases. Respir Med 2009; 103: 846–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Izuka S, Yamashita H, Iba A, et al. Acute exacerbation of rheumatoid arthritis-associated interstitial lung disease: clinical features and prognosis. Rheumatology 2021; 60: 2348–2354. [DOI] [PubMed] [Google Scholar]
- 100. Hozumi H, Nakamura Y, Johkoh T, et al. Acute exacerbation in rheumatoid arthritis-associated interstitial lung disease: a retrospective case control study. BMJ Open 2013; 3: e003132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An international working group report. Am J Respir Crit Care Med 2016; 194: 265–275. [DOI] [PubMed] [Google Scholar]
- 102. Antoniou KM, Wuyts W, Wijsenbeek M, et al. Medical therapy in idiopathic pulmonary fibrosis. Semin Respir Crit Care Med 2016; 37: 368–377. [DOI] [PubMed] [Google Scholar]
- 103. US Food and Drug Administration. FDA approves first treatment for patients with rare type of lung disease. Silver Spring, MD: FDA, 2019. [Google Scholar]
- 104. US Food and Drug Administration. FDA approves first treatment for group of progressive interstitial lung diseases. Silver Spring, MD: FDA, 2020. [Google Scholar]
- 105. Matteson E, Kelly C, Distler J, et al. Effect of nintedanib on progression of interstitial lung disease (ILD) in patients with autoimmune disease-related ILDs: further data from the INBUILD trial. Ann Rheum Dis 2020; 79: 76. [Google Scholar]
- 106. Wells AU, Flaherty KR, Brown KK, et al. Nintedanib in patients with progressive fibrosing interstitial lung diseases-subgroup analyses by interstitial lung disease diagnosis in the INBUILD trial: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Respir Med 2020; 8: 453–460. [DOI] [PubMed] [Google Scholar]
- 107. Matteson ELDO, Distler J, Kuwana M, et al. Reduced decline in forced vital capacity in patients with progressive fibrosing autoimmune disease-related interstitial lung diseases (ILDs) treated with nintedanib. Arthritis Rheum 2020; 71: 1048. [Google Scholar]
- 108. Aringer M, Pope J, Kelly C, et al. Efficacy and safety of nintedanib in patients with autoimmune disease-related interstitial lung disease treated with DMARDs and/or glucocorticoids at baseline. Ann Rheum Dis 2020; 79: 313–314. [Google Scholar]
- 109. Solomon JJ, Danoff SK, Goldberg HJ, et al. The design and rationale of the Trail1 trial: a randomized double-blind phase 2 clinical trial of pirfenidone in rheumatoid arthritis-associated interstitial lung disease. Adv Ther 2019; 36: 3279–3287. [DOI] [PubMed] [Google Scholar]
- 110. Solomon J, Woodhead F, Danoff S, et al. A randomized double-blinded placebo-controlled phase 2 study of safety tolerability and efficacy of pirfenidone in patients with rheumatoid arthritis interstitial lung disease (Abstract), 2021, https://acrabstracts.org/abstract/a-randomized-double-blinded-placebo-controlled-phase-2-study-of-safety-tolerability-and-efficacy-of-pirfenidone-in-patients-with-rheumatoid-arthritis [DOI] [PubMed]
- 111. Volkmann E, Castellvi I, Johnson S, et al. Nintedanib dose adjustments and adverse events in patients with progressive autoimmune disease-related interstitial lung diseases in the INBUILD trial. Ann Rheum Dis 2020; 79: 1019. [Google Scholar]
- 112. Noble PW, Albera C, Bradford WZ, et al. Pirfenidone for idiopathic pulmonary fibrosis: analysis of pooled data from three multinational phase 3 trials. Eur Respir J 2016; 47: 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Flaherty KR, Fell CD, Huggins JT, et al. Safety of nintedanib added to pirfenidone treatment for idiopathic pulmonary fibrosis. Eur Respir J 2018; 52: 1800230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. England BR, Hershberger D. Management issues in rheumatoid arthritis-associated interstitial lung disease. Curr Opin Rheumatol 2020; 32: 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]