Abstract
In this randomized controlled pilot trial, we compared three-dimensional (3D)-printed made-to-measure splints to conventional custom-made thermoplastic splints. In a clinical setting, we evaluated their general applicability and possible benefits for immobilization in hand surgical patients. We included 20 patients with an indication for immobilization of at least 4 weeks, regardless of the splint design. Patient comfort and satisfaction were assessed with questionnaires at splint fitting, as well as 2 and 4–6 weeks later. The 3D splints were designed and printed in-house with polylactic acid from a 3D surface scan. Our data suggest that 3D-printed splinting is feasible, and patient satisfaction ratings were similar for 3D-printed and thermoplastic splints. The 3D splint production process needs to be optimized and other materials need to be tested before routine implementation is possible or more patients can be enrolled in further studies. Validated quality assessment tools for current splinting are lacking, and further investigation is necessary.
Keywords: Hand surgery, 3D printing, Occupational therapy, Splint quality, Additive manufacturing
1. Introduction
Operative and conservative treatment of trauma and degenerative diseases of the hand relies on immobilization of the affected structures. Important features of immobilization devices include accurate fit of the impaired hand and preservation of the non-affected hand functions.
One of the fast evolving technologies with interest in medical fields is the three-dimensional (3D) technology. 3D scanning systems and 3D printers allow the fabrication of 3D physical objects with applications in multiple medical disciplines such as orthopedics, spinal surgery, maxillofacial surgery, or neurosurgery.
The fabrication of orthoses and splints is a potentially easy application in 3D printing. Several studies and case reports have proposed different design algorithms for 3D splints and assistive devices for hand surgery[1-10]. Others have demonstrated the possibility of fabricating 3D-printed splints with similar mechanical properties to those of fiberglass casts[11,12]. In healthy volunteers, the comfort and satisfaction of 3D-printed short arm splints were rated as being superior to that of fiberglass casts[13]. Comparison of 3D-printed splints that have perforated designs to circular plaster casts seems unfair, yet we found only one study that compared 3D printed with thermoplastic splints. It showed a greater potential of the 3D-printed splints to reduce spasticity and swelling and to improve motor function over 6 weeks of treatment in hemiparetic patients[14].
Few other studies have tested 3D-printed hand splints in a clinical setting. Two independent pilot trials reported good feasibility and high patient satisfaction of adults and children treated for fractures of the radius with wrist splints made of 3D-printed polypropylene, polyamide, or thermoplastic-modified acrylonitrile butadiene styrene[15,16]. However, we were unable to find suitable validated questionnaires for quality control assessments of custom-made temporary immobilizing hand splints, despite the importance of patient-reported outcome measurements designed for this purpose. Only the Client Satisfaction with Device part of the Orthotics and Prosthetics Users’ Survey includes some questions on patient satisfaction with their prosthesis or orthosis[17].
Personalized made-to-measure splints, based on a 3D surface scan, could provide clinically equivalent fit and comfort. We hypothesized that higher patient satisfaction would lead to better wearing compliance, fewer complications such as pressure sores, and faster rehabilitation. To test our hypothesis, we aimed, as a first step in this pilot study, to evaluate the general feasibility and possible benefits of splint production by 3D printing in a clinical setting. To the best of our knowledge, this is the first randomized clinical trial to test 3D-printed splints in a wide range of hand pathologies and to compare them to widely used standard thermoplastic splints.
2. Methods
We conducted a randomized controlled pilot trial at the hand surgical clinic of our university hospital. Here, we used plaster casts and ready-made braces for provisional acute immobilization. Later, occupational therapists fabricate custom-made thermoplastic splints for the injured hand as a more beneficial and pathology-tailored solution. In our experience, however, these splints often need readjustment due to unsatisfactory fit, especially subsequent to heavy initial swelling.
Patients were eligible to participate in our study if they had an indication for immobilization for at least 4 weeks after trauma, underwent post-operative rehabilitation, or experienced chronic pain. Eligible participants had to be at least 18 years old and able to provide written informed consent. We made no exclusions because of certain pathological condition or splint design to achieve the widest possible range of patients and to “imitate” future use of 3D splints. Patients were, therefore, excluded only if they had tissue loss, an external fixation, documented hypersensitivity reactions to polylactic acid (PLA), drug or alcohol abuse, or inability to follow the procedures of the study because of other medical or cognitive conditions. In the case of a second indication for immobilization during the study period, patients were not enrolled a 2nd time. All participants were randomized through envelope draw to two equally sized groups: An interventional 3D group and a control group with standard thermoplastic splints. Blinding was not feasible.
All patients initially received a provisional immobilization device. Between days 2 and 5 after study inclusion, patients presented to occupational therapy for the handover and adjustment (3D group) or direct production (control group) of their individualized splint. Treatment algorithms of pathological condition and splint design were defined by clinical standards regardless of the study group allocation.
Quality was assessed at 3 time points during regular occupational therapy sessions: Directly after splint adjustment (Questionnaire 1 in Appendix), 2 weeks after splint adjustment (Questionnaire 2 in Appendix), and 4–6 weeks after splint adjustment at the end of the patient’s study participation (Questionnaire 3 in Appendix). Figure 1 shows a summary of the study timeline.
Figure 1.
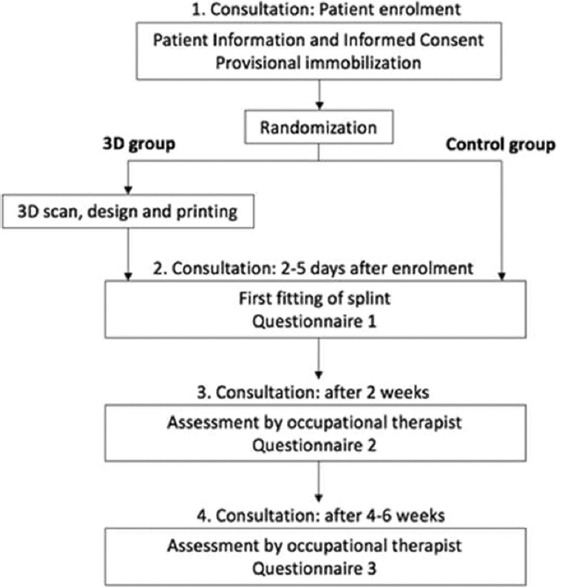
Summary of the study timeline.
2.1. Ethics statement
This study was approved by the Ethikkommission Nordwest- und Zentralschweiz (EKNZ- 2019-00351). Written informed consent was obtained from the patients for their anonymized information to be published in this article.
2.2. Device production
All patients of the control group were treated with a custom-made splint from thermoplastic material, fabricated by an experienced occupational therapist (Figure 2). For patients of the 3D group, splint production occurred between study inclusion and the first occupational therapy appointment. Therefore, a 3D surface scan of the patient’s affected hand was obtained at an additional meeting. The splint was then designed by the first author after basic training in 3-matic®, in accordance with the recommendations of an experienced occupational therapist. The 3D splint design was kept as similar as possible to that for traditional splints made of thermoplastic material, without the addition of local reinforcements or further meshes, to achieve a reliable comparison of the two devices. The virtual computer-aided design process comprised smoothing of surfaces, filling in holes in the acquired 3D mesh, and defining an offset of 1 mm over the skin for comfort. Bony prominences were respected by manually increasing local offset. The material thickness was set to 3 mm, which is comparable to that of thermoplastic material. The splints were printed in our in-house 3D print laboratory (3dprintlab@usb.ch) from PLA on a MakerBot Replicator+ (Figure 3). We chose to use fused deposition modeling (FDM) because of its low price and widespread availability. PLA is a bio-based, biocompatible, biodegradable, and non-toxic polymer, already widely used for orthopedic and dental applications[18] and approved by authorities for medical use on skin. After printing, each splint was post-processed manually by removal of support structures from the printing process and smoothing of the entire splint surface. Table 1 summarizes the splint production properties. During the first occupational therapy session, straps were added and, in the case of an unsatisfying fit, the 3D splint was directly adjusted to the patient’s hand by the occupational therapist using wet and/or dry heat to deform the splint (Figure 4).
Figure 2.
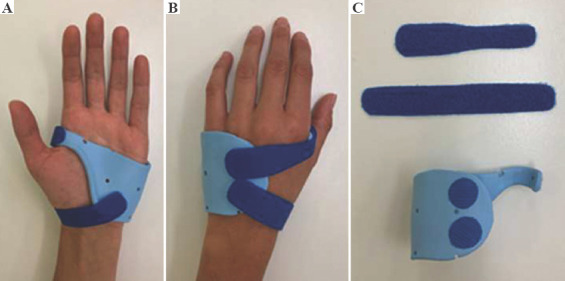
Traditional thermoplastic metacarpal brace for a metacarpal fracture. (A) Palmar view. (B) Dorsal view. (C) Material of the custom-made splint.
Figure 3.
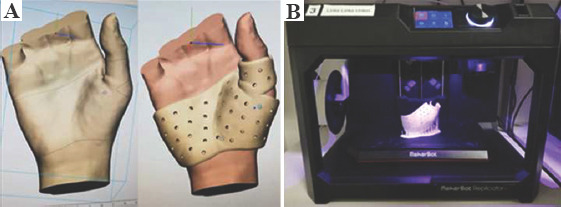
3D design of a metacarpophalangeal joint immobilizing splint for an ulnar collateral ligament tear. (A) Virtual computer-aided design. (B) In-house 3D print laboratory.
Table 1.
3D splint properties
| Surface scanner | Vectra M5 Scanner (Canfield Scientific Inc., Parsippany, NJ, USA) |
|---|---|
| 3D design software | 3-matic® (Materialise, Leuven, Belgium) |
| 3D printer | MakerBot Replicator+ (MakerBot Industries, Brooklyn, NY, USA) |
| Printing material | Polylactic acid |
| Printing technique | Fused deposition modeling |
| Printing pattern | Linear |
| Layer thickness | 0.2 mm |
| Post-processing | Manual |
| Favorable mechanical properties of printed splints | Rigid, lightweight, with limited thermoplasticity (adjustability), biodegradable |
Patient characteristics
Figure 4.
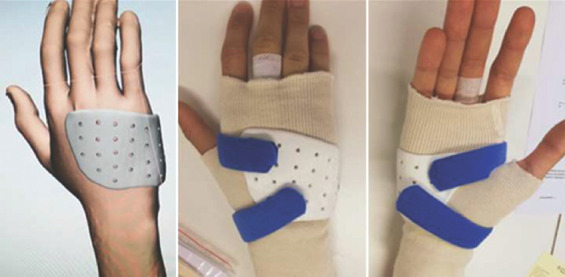
3D-printed metacarpal brace for a metacarpal fracture.
2.3. Data acquisition and statistical analysis
To assess comfort and satisfaction, we asked patients to complete questionnaires concerning their wearing experience, general problems while wearing the splint, and satisfaction with different aspects of the splint. The questionnaires were specifically designed for this study, as no validated tools were available (Appendix).
In addition to these questionnaires, the treating occupational therapists completed an assessment in which they were asked to rate their satisfaction with the splint for both groups, to record adjustments made, and to collect data on the incidence and nature of complications. Production times for both splint types were documented.
Data analysis was mainly qualitative because of the planned small number of participants in this feasibility trial. Statistical analysis included descriptive statistics and calculation of P-values with the Mann–Whitney U test. Data for dropouts were not included.
3. Results
Within a 6-month period in 10/2019 and 04/2020, 24 of 32 screened patients were enrolled in this trial (10 in control group and 14 in 3D group). Eight patients did not match the inclusion criteria. Four patients had to be excluded during follow-up and were replaced, including a patient from the 3D group who needed a change of splint design; for organizational reasons, the occupational therapist made the new splint from thermoplastic material. Another patient was excluded because of communication obstacles that became apparent only during the response to questionnaires.
Two patients went on vacation, which led to wearing incompliance in a patient and missing data for the other. The remaining 20 patients completed all three questionnaires. Of these, two patients had a missing second assessment from the occupational therapist because they did not present to the respective consultation but assured the study team by phone that everything was fine. One patient missed the third assessment because he did not bring the splint to the occupational therapy visit. Patient characteristics are summarized in Table 2.
Table 2.
Patient characteristics
| Characteristic | 3D group | Control group |
|---|---|---|
| Sample size (n) | 10 | 10 |
| Male/female ratio | 7/3 | 8/2 |
| Age at inclusion in years, mean (range) | 41.2 (23 – 64) | 34.1 (19 – 62) |
| Etiology of pathology | ||
| Traumatic | 9 | 9 |
| Degenerative | 1 | 1 |
| Leading pathological condition | ||
| Fracture | 8 | 6 |
| Ligament injury | 1 | 2 |
| Tendinous injury | - | 1 |
| Osteoarthritis | 1 | 1 |
| Treatment strategy | ||
| Conservative | 6 | 7 |
| Surgical | 4 | 3 |
| Splint type | ||
| MC brace | 7 | 2 |
| MC brace including the wrist | 1 | 1 |
| Thumb MCPJ splint | 1 | 1 |
| Thumb MCPJ splint including the wrist | 1 | 1 |
| MCPJ extension block splint | - | 1 |
| Controlled active motion splint | - | 1 |
| Wrist immobilizing radius splint | - | 1 |
| PIPJ extension block splint | - | 1 |
| Mallet finger splint (stack) | - | 1 |
MC, metacarpophalangeal; MCPJ, metacarpophalangeal joint; PIPJ, proximal interphalangeal joint.
The mean wearing time of the definitive splint was 35.1 days until the end of follow-up (32.9 days in the 3D group and 37.3 days in the control group). Analysis of the questionnaires did not reveal any clear differences between the two groups in terms of patient satisfaction and comfort. In both groups, all patients initially scored high for splint fit, adjustment process, and satisfaction with the weight of the splint. Overall satisfaction scores at the end of their wearing period ranged between 5 and 10 with only small differences between groups (Table 3 for detailed numbers). One patient in the 3D group reported strong pain while wearing the splint; however, she could not differentiate whether the pain was caused by her fibromyalgia or the splint.
Table 3.
Patient satisfaction
| Assessment item | 3D group | Control group |
|---|---|---|
| Splint fit (Questionnaire 1) | 9.15 (1.11) | 9.2 (0.79) |
| Adjustment process (Questionnaire 1) | 9.5 (0.97) | 9.6 (0.7) |
| Satisfaction with weight of splint (Questionnaire 1) | 9.6 (0.7) | 9.2 (1.03) |
| Overall satisfaction (Questionnaire 3) | 8.4 (1.65) | 9.2 (1.32) |
Numbers represent mean (standard deviation).
In two patients of the 3D group, partial breakage of a 3D splint (Figure 5) occurred after 26 days and 37 days of wearing time, which did not lead to any treatment complications. A third 3D splint showed fine fissures at the 2-week follow-up, which could be closed by the occupational therapist after heating the splint with hot air.
Figure 5.

Partial breakage of a 3D wrist immobilizing splint.
Adjustment rates were similar for 3D and thermoplastic splints, with a mean (standard deviation) of 1.44 (1.24) adjustments for 3D splints and of 1.0 (1.15) adjustment for thermoplastic splints over the whole course of follow-up. Occupational therapists’ overall reported satisfaction with splints was good or very good in all cases, except in the cases of splint breakage.
We observed a huge variation in production time, as shown in Table 4. The mean duration of the first occupational therapy consultation was similar in the control group (39.5 min) to that in the 3D group (30.5 min). However, while thermoplastic splints were entirely fabricated within that time, the complete production process of 3D splints took a mean of 179.5 min, excluding printing time.
Table 4.
Production time
| 3D group | Control group | |
|---|---|---|
| Scan + wound check | 22 (10–40) | N/A |
| Design | 99.5 (35–240) | N/A |
| Printing | 337.7 (175–842) | N/A |
| Post-processing | 27.5 (15–60) | N/A |
| First consultation | 30.5 (15–60) | 39.5 (30–60) |
| Total (printing excluded) | 179.5 (85–390) | 39.5 (30–60) |
| Total (printing included) | 517.2 (274–1097) | 39.5 (30–60) |
All times are given in minutes as mean (range). N/A, not applicable.
4. Discussion
The aim of this trial was to evaluate the feasibility of the use of 3D-printed made-to-measure splints in a clinical hand surgical setting and to compare them to standard custom-made thermoplastic splints by an occupational therapist. For this trial, the 3D splint designs were similar to current thermoplastic designs, and the potential benefits of the new method were not explored.
Our results suggest that it is generally feasible to produce different hand splint designs by 3D scanning and printing with satisfactory comfort for the patient. Several limitations need to be addressed, however, before 3D-printed hand splints can be used regularly instead of the current thermoplastic splints.
First, our setting for the interventional 3D group was not optimized compared with that for the well-established in-house control group with conventional splints. Additional visits that included the patient, a member of the study group, and an occupational therapist for hand positioning because of restricted scanner access were necessary. This partially accounts for the greater production time for 3D splints. For more production efficiency in future studies with larger patient series, the scanner would preferably be located in the occupational therapist’s treatment room. Nonetheless, the chosen scanner provided good-quality 3D surface scans with a single photoshoot, which was extremely helpful for patients with pain or tremor, or for whom keeping a certain hand or finger in a desired position was difficult. The 3D surface scans provided static mesh images, whereas the estimation of soft-tissue thickness over bony prominences was difficult. The accuracy of splint fit, therefore, depended on the experience and anatomical knowledge of the splint designer. In our pilot study, only the first author designed the splints. The learning curve remained steep despite previous instruction by the software provider and the help of occupational therapists. Designing time varied enormously, depending on splint type and scan quality, but an improvement in efficiency was clearly noticeable. For future trials, we propose addressing these issues by developing algorithms that prepare the 3D data and produce a first virtual splint design that needs small adjustments. This will potentially ease the design process so that occupational therapists could help not only with hand positioning in the scanner but also with the computer-aided splint design.
Second, for the printing of splints, FDM was proposed as a potentially applicable technique[9]. We chose this technique because of its wide availability, relatively quick production time, and cost-effectiveness, also for low-and-middle-income countries. However, there was substantial time loss in post-processing the prints. Furthermore, the FDM method revealed limitations in material stability by splint breakage, occurring after heavier use of the splints. This could be improved with printing methods that do not rely on layering, such as laser sintering. The method needs to be balanced against higher printing costs and further increases in production time as a result of the need for periodic cooling. Regarding the material, PLA is environmentally sustainable and has the advantage of possible splint adjustment after printing because of its thermoformability. Throughout our study, the likelihood of splint adjustments was similar in the two groups. In our opinion, material used for hand splints should, therefore, possess a minimum of formability to allow comfortable wearing and removal of the splint, as well as modifications to accommodate swelling and bony prominences. As many different printing methods and materials are currently under investigation, we are looking forward to further tests. We would prefer a moldable, adjustable, and lightweight yet rigid and environmentally sustainable material that could be printed within a few hours, as opposed to a ready-made splint off the shelf.
Third, although we gained important insights into issues that need improvement, our results do not confirm the hypothesized differences in patient comfort and satisfaction. This may be partly due to the small sample size, which is the major limitation of our study, as it was designed to accommodate a variety of hand surgical patients and different splint designs. We are aware that this leads to a selection bias in a small study group, as illustrated by the uneven distribution of splint designs between the two groups. On the other hand, patients seemed to be already highly satisfied with the custom-made thermoplastic splints from our occupational therapists. The previously mentioned studies, which showed superior patient satisfaction with 3D splints, drew their conclusions from a comparison of 3D-printed splints to fiberglass casts rather than thermoplastic splints. We believe that in comparison with a rigid cast that cannot be taken off, a removable lightweight splint model would always be favored. In our study, we minimized the design bias. Exploration of the full potential of individualized design would probably have led to larger differences between the two groups; at the same time, it would have reduced their comparability. We would need to perform a larger trial with a high number of participants to detect or rule out potential significant differences between 3D and thermoplastic splints in our setting.
Fourth, we are aware that the innovative character of 3D-printed splints and their so-called coolness factor could have led to a response bias in our study. The nature of the intervention, however, makes blinding of participants impossible. A further risk of bias lies in the monitored answering of questionnaires during therapy sessions. To determine and compare satisfaction and wearing comfort, we had to use non-validated questionnaires, as, unfortunately, no suitable assessment tool was found in literature for temporary splinting. Therefore, our questionnaires are the only available critical assessment tool of splint quality that we are aware of, and no comparison to other groups was feasible. Our simple questionnaires may not be able to satisfactorily detect small differences in patient comfort and satisfaction, as we found generally high acceptance rates. Refinement of these questionnaires is, therefore, necessary for further studies.
5. Conclusions
Splint adjustment seems to be inevitable, even for 3D-printed made-to-measure splints. In our opinion, the cost-effectiveness of 3D-printed splints can be superior to current standards only if multiple adjustments can be prevented by introducing other materials and/or innovative designs. This needs to be confirmed by a medicoeconomic analysis in a larger patient cohort. We do, however, emphasize that clinical studies are crucial for assessing the quality of current daily splinting routines with thermoplastic splints. They are a necessary starting point for comparison to new splinting methods in the future.
Acknowledgments
The authors wish to thank Dr. Dr. med. B. Benitez, who facilitated access to the 3D scanner; Dr. Ph. Brantner and Dr. Dr. F. Thieringer of the in-house 3D Print Laboratory for their support and Barb Every for proofreading.
The authors received no financial support for the research, authorship, and/or publication of this article.
Appendix
Appendix
Questionnaire 1 for patients
Patient ID:
The following questionnaire will ask various questions. Please answer these truthfully and completely. You will be asked about the properties of your splint as well as the manufacturing process. You should assign a grade from 1 to 10 for each question (1= minimum, 10 = maximum). Feel free to contact us if you have any questions or difficulties. Thank you very much!
A. How do you rate the general fit of your new splint?

B. How much pain do you have with the new splint?

C. How heavy is the new splint?

D. Is the splint aesthetically pleasing?

E. How was the adjustment process overall?

__________________________________________________________________________________________
Place and date: Patient signature:
_________________________________________________________________________________________
Assessment 1 (by investigator)
Patient ID:
A. Satisfied with fit? yes / no
a. If no, why not? ______________________
B. Adjustments made? yes / no
a. If yes, which? ______________________
C. Any change of procedure? yes / no
a. If yes, which? ______________________
D. Next appointment:______________________ at Occupational therapy/Consultation with doctor
_______________________________________________________________________________________
Place and date: Investigator signature:
Questionnaire 2 for patients
Patient ID:
The following questionnaire will ask various questions. Please answer these truthfully and completely. Please judge the proprieties of the splints on a scale 1-10 (1=minimum, 10= maximum) and explain more specifically where needed. Please relate your answers to the time interval from the last questionnaire. Feel free to contact us if you have any questions or difficulties. Thank you very much!
A. How do you rate the general fit of your splint?

B. How much pain do you have with the splint?

C. How heavy is the splint?

a. Did you have neck pain due to splint wearing? O never O sometimes O often O always
D. How easy do you find putting the splint on and off?

E. Is the splint aesthetically pleasing?

F. Is the splint causing pain?

a. If yes, where?
_____________________________________________
G. Is the splint itchy?

a. If yes, where?
_____________________________________________
H. Do you sweat under the splint?

I. Is the splint smelly?

J. How easy is it to clean the splint?

a. How do you clean the splint?
K. Have skin changes occurred under the splint? O Yes O No
a. If yes: How? O Rash
O Pressure point
O Open wound
O ______________________
Where? ___________________________________
When? ____________________________________
Did you have to change the splint? O Yes O No
L. Are there mechanical or material issues of the splint? O Yes O No
a. If yes: What? O Splint broke
O Cracks
O The splint was deformed
O ______________________
Where? ___________________________________
When? ____________________________________
Did you therefore have to take the splint off ?
O Yes O No
M. How many times was your splint adjusted? O not once
O ___ times
a. If yes: Why? __________________________
N. How many times was your splint replaced? O never
O ___ times
a. If yes: Why? _____________________________________________________________________________________________________________________________________________
Place and date: Patient signature:
______________________________________________________________________________________
Assessment 2 (by investigator)
Patient ID:
A. Satisfied with fit? yes / no
a. If no, why not? ______________________
B. Adjustments made? yes / no
a. If yes, which? ______________________
C. Any adverse events? yes / no
a. If yes, which? ______________________
D. Any change of procedure? yes / no
a. If yes, which? ______________________
E. Next appointment:______________________ at Occupational therapy / Consultation with doctor
______________________________________________________________________________________
Place and date: Investigator signature:
Questionnaire 3 for patients
Patient ID:
The following questionnaire will ask various questions. Please answer these truthfully and completely. Please judge the proprieties of the splints on a scale 1-10 (1=minimum, 10= maximum) and explain more specifically where needed. Please relate your answers to the time interval from the last questionnaire. Feel free to contact us if you have any questions or problems. Thank you very much!
A. How do you rate the general fit of your new splint?

B. How much pain do you have with the splint?

C. How heavy is the splint?

a. Did you have neck pain due to splint wearing? O never O sometimes O often O always
D. How easy do you find putting the splint on and off?

E. Is the splint aesthetically pleasing?

F. Is the splint causing pain?

a. If yes, where? ______________________________
G. Is the splint itchy?

a. If yes, where? _____________________________________________________
H. Do you sweat under the splint?

I. Is the splint smelly?

J. How easy is it to clean the splint?

K. How do you clean the splint? ____________________________________________________
L. Have skin changes occurred under the splint?
O Yes O No
a. If yes: How? O Rash
O Pressure point
O Open wound
O ______________________
Where? __________________________________________________________
When?
Did you have to change the splint because of it?
O Yes O No
M. Are there mechanical or material issues of the splint? O Yes O No
a. If yes: What? O Splint broke
O Cracks
O The splint was deformed
O ______________________
Where? ___________________________________
When? __________________________________________________________
Did you therefore have to take the splint off ? O Yes O No
N. How many times did your splint had to be adjusted? O not once
O ___ times
a. If yes: Why? ___________________________________________________________
O. How many times did your splint had to be replaced? O never
O ___ times
a. If yes: Why? _________________________________________________
P. What final grade would you give to your splint over the entire duration of the therapy?
__________________________________________________________________________________________
Place and date: Patient signature:
_______________________________________________________________________________________
Assessment 3 (by investigator)
Patient ID:
A. Satisfied with fit? yes / no
a. If no, why not? ______________________
B. Any adverse events? yes / no
a. If yes, which? ______________________
C. End of therapy? yes / no
a. If no, why not? ______________________
D. Splint returned? yes / no
E. General satisfaction with splint performance over the whole treatment period:
O bad O ok O good O very good
_______________________________________________________________________________________
Place and date: Investigator signature:
Publisher’s note
Whioce Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Conflict of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
LW, CF, LS, and AK conceived and planned the study. LW, CF, and LS conducted the experiments/questionnaires, and LW performed the statistical analyses. All authors were involved in the preparation of this manuscript. LW and AK wrote the manuscript. DJS and AK clinically supervised the study and revised the manuscript. All authors read and approved the final manuscript.
References
- 1.de Souza MA, Schmitz C, Marega Pinhel M, et al. Proposal of Custom Made Wrist Orthoses Based on 3D Modelling and 3D Printing. Annu Int Conf IEEE Eng Med Biol Soc. 2017;2017:3789–92. doi: 10.1109/EMBC.2017.8037682. https://doi.org/10.1109/EMBC.2017.8037682. [DOI] [PubMed] [Google Scholar]
- 2.Baronio G, Harran S, Signoroni A. A Critical Analysis of a Hand Orthosis Reverse Engineering and 3d Printing Process. Appl Bionics Biomech. 2016;2016:8347478. doi: 10.1155/2016/8347478. https://doi.org/10.1155/2016/8347478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaya F, Pedro PS, Silva JL, et al. Design of an Orthopedic Product by Using Additive Manufacturing Technology:The Arm Splint. J Med Syst. 2018;42:54. doi: 10.1007/s10916-018-0909-6. https://doi.org/10.1007/s10916-018-0909-6. [DOI] [PubMed] [Google Scholar]
- 4.Chu CH, Wang IJ, Sun JR, et al. Customized Designs of Short Thumb Orthoses Using 3D Hand Parametric Models. Assist Technol. 2020;7:1–8. doi: 10.1080/10400435.2019.1709917. https://doi.org/10.1080/10400435.2019.1709917. [DOI] [PubMed] [Google Scholar]
- 5.Lee KH, Kim DK, Cha YH, et al. Personalized Assistive Device Manufactured by 3D Modelling and Printing Techniques. Disabil Rehabil Assist Technol. 2019;14:526–31. doi: 10.1080/17483107.2018.1494217. https://doi.org/10.1080/17483107.2018.1494217. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Tanaka H. Rapid Customization System for 3D-Printed Splint Using Programmable Modeling Technique-a Practical Approach. 3D Print Med. 2018;4:5. doi: 10.1186/s41205-018-0027-6. https://doi.org/10.1186/s41205-018-0027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nam HS, Seo CH, Joo SY, et al. The Application of three-Dimensional Printed Finger Splints for Post Hand Burn Patients:A Case Series Investigation. Ann Rehabil Med. 2018;42:634–8. doi: 10.5535/arm.2018.42.4.634. https://doi.org/10.5535/arm.2018.42.4.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paterson A, Bibb R, Campbell RI. Evaluation of a Digitised Splinting Approach with Multiple-Material Functionality Using Additive Manufacturing Technologies. In: Bourell D, Crawford RH, Seepersad CC, et al., editors. Proceedings of the 23rd Annual International Solid Freeform Fabrication Symposium-An Additive Manufacturing Conference. University of Texas at Austin; Austin: 2012. pp. 656–72. [Google Scholar]
- 9.Paterson A, Bibb R, Campbell RI, et al. Comparing Additive Manufacturing Technologies for Customised Wrist Splints. Rapid Prototyp J. 2015;21:230–43. [Google Scholar]
- 10.Sari MI, Sahin I, Gokce H, et al. Ring Orthosis Design and Production by Rapid Prototyping Approach. J Hand Ther. 2020;33:170–3. doi: 10.1016/j.jht.2019.02.003. https://doi.org/10.1016/j.jht.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Cazon A, Kelly S, Paterson AM, et al. Analysis and Comparison of Wrist Splint Designs Using the Finite Element Method:Multi-Material Three-Dimensional Printing Compared to Typical Existing Practice with Thermoplastics. Proc Inst Mech Eng H. 2017;231:881–97. doi: 10.1177/0954411917718221. https://doi.org/10.1177/0954411917718221. [DOI] [PubMed] [Google Scholar]
- 12.Hoogervorst P, Knox R, Tanaka K, et al. A Biomechanical Comparison of Fiberglass Casts and 3-Dimensional-Printed, Open-Latticed, Ventilated Casts. Hand (NY) 2019;16:842–9. doi: 10.1177/1558944719831341. https://doi.org/10.1177/1558944719831341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham J, Wang M, Frizzell K, et al. Conventional vs 3-Dimensional Printed Cast Wear Comfort. Hand (NY) 2018;15:388–92. doi: 10.1177/1558944718795291. https://doi.org/10.1177/1558944718795291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng Y, Liu G, Yu L, et al. Effects of a 3D-Printed Orthosis Compared to a Low-Temperature Thermoplastic Plate Orthosis on Wrist Flexor Spasticity in Chronic Hemiparetic Stroke Patients: A Randomized Controlled Trial. Clin Rehabil. 2020;34:194–204. doi: 10.1177/0269215519885174. https://doi.org/10.1177/0269215519885174. [DOI] [PubMed] [Google Scholar]
- 15.Chen YJ, Lin H, Zhang X, et al. Application of 3D-Printed and Patient-specific Cast for the Treatment of Distal Radius Fractures:Initial Experience. 3D Print Med. 2017;3:11. doi: 10.1186/s41205-017-0019-y. https://doi.org/10.1186/s41205-017-0019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guida P, Casaburi A, Busiello T, et al. An Alternative to Plaster Cast Treatment in a Pediatric Trauma Center using the CAD/CAM Technology to Manufacture Customized Three-Dimensional-Printed Orthoses in a Totally Hospital Context:A Feasibility Study. J Pediatr Orthop B. 2019;28:248–55. doi: 10.1097/BPB.0000000000000589. https://doi.org/10.1097/BPB.0000000000000589. [DOI] [PubMed] [Google Scholar]
- 17.Heinemann AW, Bode RK, O'Reilly C. Development and measurement properties of the Orthotics and Prosthetics Users'Survey (OPUS):A Comprehensive Set of Clinical Outcome Instruments. Prosthet Orthot Int. 2003;27:191–206. doi: 10.1080/03093640308726682. https://doi.org/10.1080/0309364030∖682. [DOI] [PubMed] [Google Scholar]
- 18.Nofar M, Sacligil D, Carreau PJ, et al. Poly (Lactic Acid) Blends:Processing, Properties and Applications. Int J Biol Macromol. 2019;125:307–60. doi: 10.1016/j.ijbiomac.2018.12.002. https://doi.org/10.1016/j.ijbiomac.2018.12.002. [DOI] [PubMed] [Google Scholar]


