Abstract
Context
X-linked hypophosphatemia (XLH) is an inherited skeletal disorder that can lead to lifelong deleterious musculoskeletal and functional consequences. Although often perceived as a childhood condition, children and adults both experience the negative effects of XLH. Adolescents and young adults (AYAs) benefit from effective health care transition (HCT) preparation to support the transfer from pediatric- to adult-focused care. Whereas transition timelines, milestones, and educational tools exist for some chronic conditions, they do not meet the unique needs of patients with XLH.
Evidence Acquisition
To produce the first expert recommendations on HCT preparation for AYAs with XLH developed by clinical care investigators and transition experts, a formal literature search was conducted and discussed in an advisory board meeting in July 2020. A modified Delphi method was used to refine expert opinion and facilitate a consensus position.
Evidence Synthesis
We identified the need for psychosocial and access-related resources for disease education, genetic counseling, family planning, and AYA emancipation from caregiver-directed care. Additionally, we recognized that it is necessary to facilitate communication with patients through channels familiar and accessible to AYAs and teach patients to advocate for their health care/access to specialists.
Conclusion
Clear HCT preparation guidelines and treatment-related goals are defined. Individualized timelines and practical strategies for HCT preparation are proposed to optimize health outcomes resulting from continuous clinical care throughout the patient lifecycle. We provide an expert consensus statement describing a tailored HCT preparation program specifically for AYAs with XLH to aid in the effective transfer from pediatric- to adult-focused health care.
Keywords: health care transition, X-linked hypophosphatemia, metabolic bone disorders, expert opinion consensus, adolescent care, transition preparation
X-linked hypophosphatemia (XLH) is a rare hereditary and progressive skeletal disorder. XLH arises from mutations in the PHEX gene that result in excess circulatory fibroblast growth factor 23 and urinary phosphate wasting (1). Physical and functional effects of XLH are identifiable in childhood and, when untreated, disease-related symptoms and complications continue to progress during adulthood (1, 2).
Despite the chronic and progressive nature of disease manifestations, XLH is often perceived as a “childhood-only” disease. Clinical symptoms of XLH may persist or reemerge later in life, but standard clinical practice has been to discontinue conventional therapy once skeletal growth is completed. In adulthood, therapy is restarted based on symptoms and this often leads to gaps in care. Such practice can lead to misconceptions regarding the ongoing need for XLH patient care beyond childhood. The lack of recognition of XLH symptoms during adulthood delays appropriate treatment. Further, disease nomenclature used in the medical literature often focuses on the pediatric aspects of rickets (ie, X-linked hypophosphatemic rickets) before the closure of the epiphyseal plates and neglect lifelong manifestations of XLH after growth plates fuse (3, 4). At worst, a constellation of nomenclature and perception challenges, along with potential health care continuity barriers, contributes to loss to follow-up of adolescent and young adult (AYA) patients with XLH, creating missed opportunities to improve health outcomes.
Health care transition (HCT) is defined as the “process of moving from a child to an adult model of health care with or without a transfer to a new clinician” (5). HCT programs aim to reduce patient loss to follow-up and improve the quality of care through structured guidance provided to AYAs and their caregivers (6). As seen with other common or rare lifelong conditions of pediatric onset, loss to follow-up can be minimized by appropriate strategies and practice standards specifically addressing HCT preparation. For patients with XLH, a tailored HCT preparation approach that addresses specific disease manifestations is required to meet the unique needs of patients with this rare condition. Avoidance of loss to follow-up in patients with XLH may be associated with positive clinical outcomes, such as prevention of progressive osteomalacia (bone pain, pseudofractures, fractures), and management of dental abnormalities (7) or other medical complications during adulthood. Continued clinical care relationships may help prevent misdiagnoses of musculoskeletal pain as being caused by etiologies other than those common to XLH, and ensure timely and appropriate management.
The importance of successful HCT from pediatric to adult care in XLH is recognized in several regional consensus publications (3, 8, 9); however, few publications describe how HCT preparation should be achieved. The 2 objectives of this manuscript are to review the literature and to provide the first expert consensus statement on HCT preparation for AYAs with XLH from pediatric- to adult-focused health care developed by clinical care investigators and transition experts. Effective HCT of AYAs to adult-focused care is a challenge for patients worldwide, and we strive to address issues relevant to all regions of the world. In some sections, recommendations may be specific to care in the United States, reflecting the experience of the authors.
X-linked Hypophosphatemia Epidemiology, Clinical Manifestations, and Disease Impact
XLH is a rare condition occurring in approximately 1 in 20 000 to 25 000 people (data from Denmark, Norway, and Japan) (10). Diagnosis is based on signs of rickets and/or osteomalacia in the presence of hypophosphatemia and renal phosphate wasting, along with the appropriate inheritance pattern, though spontaneous mutations are common. As part of diagnosis, molecular genetic analysis for PHEX mutations (vs other genetic causes) and fibroblast growth factor 23 level measurement are advisable, to confirm the diagnosis and effectively treat hypophosphatemia (3). Musculoskeletal manifestations of XLH in childhood include rickets, bone deformities, and short stature. Animal models indicate normophosphatemia in utero (11), and manifestations of XLH are not typically evident at birth (11). Studies demonstrate that phosphate excretion is significantly lower in neonates than in 3- and 6-month-old infants, possibly explaining the typical presentation of XLH after age 6 months (12). Rickets, lower limb bowing, and slow growth rate typically appear in the first 2 years of life, especially after weight bearing begins, whereas other manifestations may become apparent later (1, 13).
The disease burden of XLH can have a considerable effect on individuals during childhood, including lower health-related quality of life (HRQoL) (14). The effect of XLH disease or management may influence a young person’s HRQoL related to sports participation and activity, as well as sleep, play, and school (14). During adulthood, bone and joint pain, osteomalacia-related fractures or pseudofractures, early osteoarthrosis, enthesopathy, muscle weakness, hearing loss, and/or severe dental anomalies are common (1). Therefore, adults with XLH face ongoing and progressive functional limitations such as stiffness and pain as they age, which influences the experience of and decisions around work, travel, physical and mental health, and social and family life (2). Although there is no available evidence supporting a beneficial effect on, or delayed progression of, osteoarthritis or enthesopathy with medical therapy in patients with XLH, patient transition that includes follow-up of these manifestations is very important. Involvement of an XLH specialist may help to optimize appropriate supportive and surgical management.
Patients with XLH face a changing disease burden as they age. A recent qualitative study evaluated perceived XLH disease burden and mapped patient response statements to categories such as treatment, psychologic, education, movement, health care, and employment (15). Treatment of XLH in children is focused on managing growth and limb deformities (with the burden related to medical and surgical treatments as well as to the underlying disease itself). Management of XLH during adulthood addresses acute and chronic complications of osteomalacia, osteoarthritis, enthesopathy, musculoskeletal pain, and dysmobility. During adulthood, interventions are aimed at alleviating the cumulative burden of the long-term and newly arising complications (15). In adolescents, the effect of XLH contains aspects of the disease burden felt in childhood and adulthood. The disease burden in adolescents relates to rickets, psychological and treatment issues, as well as securing health care and effective patient education. Of these, more psychologic effects of XLH tend to emerge during the adolescent years, potentially leading to worse mental well-being in some individuals (15). For example, AYAs with XLH become increasingly aware of the future implications of XLH, including the likelihood of passing XLH on to their own children, as well as more immediate effects such as limitations to participation in physical activities with peers (14). Currently available XLH-specific tools include the TRxANSITION Index and STARx questionnaires, which do not specifically address severe psychological or psychosocial problems requiring psychologist referral. It is suggested that clinicians incorporate a standard adolescent questionnaire into their practice to assess for mental health issues. Available options include the pediatric Patient-Reported Outcomes Measurement Information System (PROMIS) and then transitioning to an adult PROMIS PRO as is appropriate for the individual patient. Clinicians may also consider screening pediatric patients using the PedDepSx (Pediatric Depressive Symptoms), and adult patients with the Patient Health Questionnaire–9. There is a good linkage between PROMIS, Patient Health Questionnaire–9, and other validated screening tools (16, 17). Finally, an online survey developed by UCLA (the Revised Child Anxiety and Depression Survey [RCADS], validated in children and adolescents; available at https://www.childfirst.ucla.edu/resources/) has been used with success at some centers.
Taken together, these emergent psychological and psychosocial effects, alongside the preexisting childhood burden, can result in poorer mental well-being in the adolescent age group than in either adults or children (15). Indeed, survey results found the potential for the perceived burden of XLH to be especially complex during adolescence, which occurs during the same time that patients must receive HCT preparation from pediatric to adult-centered care (15). Patient registries are needed to further describe the overall disease burden and effect of XLH in greater detail (13, 18). Longitudinal studies are currently ongoing to characterize the natural history of XLH and long-term effectiveness of interventions both in pediatric and adult settings (10, 19).
Current XLH Recommendations and Support Gaps
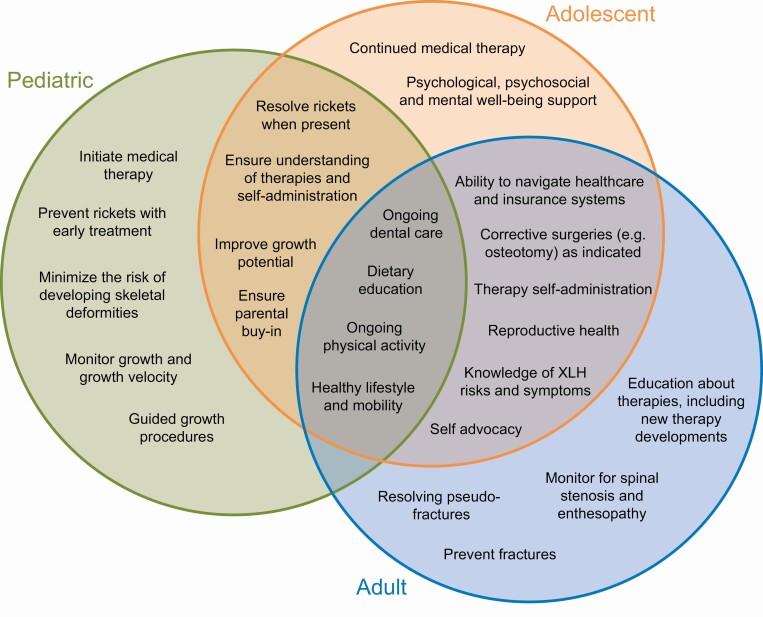
XLH is a multisystem disorder requiring patient care to be delivered via an integrated team of specialized health care professionals (HCPs) to minimize long-term sequelae and optimize HRQoL (20). Clinical practice recommendations for the diagnosis and management of XLH advocate that all patients, irrespective of age, should be evaluated regularly by a multidisciplinary team organized and led by an expert in rare metabolic bone disorders (3). Because the clinical, biochemical, and radiological complications of XLH vary widely between patients, monitoring and any treatment should be individualized on the basis of a patient’s clinical manifestations, medical history, and stage of development. Further, ongoing monitoring is indicated in patients without current medical treatment to enable timely and safe reinitiation of therapy when it becomes necessary. High-level management goals of XLH and key differences in management goals at the pediatric-, adolescent-, and adult-focused levels of care are shown in Fig. 1.
Figure 1.
X-linked hypophosphatemia management goals for pediatric, adolescent, and adult patients (3, 20).
In terms of delivering care, optimal workflows include experts in metabolic bone disorders (commonly endocrinologists, nephrologists, geneticists) liaising with the patient’s local health care providers (internist, general practitioner, pediatrician, and advanced practice providers [nurse practitioners; physicians assistants]), radiologists, orthopedic surgeons, physical therapists, rheumatologists, and dentists. Depending on the patient’s needs, neurosurgeons, otolaryngologists, ophthalmologists, audiologists, orthodontists, dieticians, rehabilitation specialists, pain management specialists, genetic counselors, occupational therapists, and social workers or psychologists may also be involved in the patient’s care.
Currently available clinical practice recommendations for the management of XLH include some guidance on transition of care (callout box) (3); however, no specific recommendations have been developed to address this process directly. Current XLH recommendations do not specifically define HCT support or describe what successful transition of care requires in XLH, and fail to provide a framework on how to design, implement, or use HCT programs for XLH or offer guidance on how programs can support AYAs or their families in the transition from pediatric- to adult-centered care.
Callout Box. Details related to health care transition to adult-focused care of adolescent and young adult (AYA) patients, adapted from Haffner D et al http://creativecommons.org/licenses/by/4.0/ (3)
Genetic counseling should be offered to patients with X-linked hypophosphatemia (XLH), especially at the transition from pediatric- to adult-centered care and to families planning pregnancies, or to any who are already pregnant
Patients should have at least twice-yearly dental examinations after tooth eruption, orthodontic evaluation around age 12 years, and an extended dental evaluation with transition to adult-centered care
Before transfer to adult-centered services, AYAs should undergo a full skeletal clinical and radiographic assessment to enable definition of any residual deformity and facilitate appropriate follow-up arrangements
Literature Search Strategy
We assembled a panel of pediatric complex care specialists and endocrinologists/nephrologists/metabolic bone disorders subspecialists in pediatric- and adult-centered care experienced in the management of XLH and other metabolic bone disorders to develop a structured HCT program for AYAs with XLH. A formal literature search was conducted to identify evidence gaps and refine the scope of this consensus statement. PubMed was searched in an iterative narrative manner using the following search terms: “transition of care,” “los(s)(t) to follow up,” “XLH transition,” “XLH adolescent,” and “XLH cost effectiveness” in June 2020. Outputs from the “transition of care” and “los(s)(t) to follow up” search terms were inspected to identify articles related to rare diseases, rare metabolic bone disorders, and other endocrine or other diseases. The abstracts of search results were assessed for relevance. All articles directly related to XLH transition of care and cost-effectiveness were included. Articles from non-XLH patient populations were included based on the determination of experts that relevant lessons could be applied or modified to be pertinent to an XLH context. The bibliographies of selected citations were searched for further references of interest. Experts also provided additional references for inclusion based on their clinical experience working with patients with XLH. Panel discussions from a transition of care advisory board including XLH specialists took place in July 2020. A modified Delphi method was used to refine expert opinion and facilitate a consensus position. Panel members completed surveys addressing different aspects of pediatric and adult XLH care. Each survey was followed by a conference call, during which results were presented and statements discussed. Expert opinion was used to adapt available HCT preparation paradigms for specific use with patients with XLH.
Our approach was guided by existing models for research/clinical care in other childhood-onset chronic conditions that use validated tools to measure transition readiness. After reviewing available HCT program models (6, 21-28), it is our view that existing models do not address all the unique needs associated with a rare musculoskeletal disease such as XLH. Our proposed transition program aims to address the comorbidities and highly specific needs of AYAs with XLH. Recommended tools and resources focus on how to better facilitate communication between pediatric and adult-centered HCPs and their interdisciplinary teams.
Existing Adolescent and Young Adult Health Care Transition Programs: Experience to Date
Challenges to Continuous Care for Adolescent and Young Adult Patients
Changing from one medical provider to another during the AYA years may be particularly problematic to continuity of care (6), especially in the United States, where gaps in health care insurance may occur during the young adult years, once patients are no longer covered by their caregivers’ insurance and patients may experience difficulty securing insurance independently for a variety of reasons. Lack of insurance limits access both to medical providers and to medical therapies.
Even among patients with adequate insurance, conversion rates from pediatric to adult care can be discouraging. A 2002 consensus statement from the American Academy of Pediatrics, the American Academy of Family Physicians, and the American College of Physicians underscored the importance of a planned HCT for adolescents with special health care needs (29). A 2018 update, however, reported that the vast majority of US youths receiving long-term care (> 80%) were not receiving adequate preparation to meet the national HCT performance targets (5). Furthermore, a prior 2005 to 2006 survey of AYAs with special health care needs in the United States found that only 42% of AYAs had discussed shifting to an adult care provider (30), and this statistic remained similar 10 years later (41%) (31), leaving significant room for improvement.
Adolescent and Young Adult Health Care Transition in Principle
The ideal HCT involves uninterrupted, developmentally appropriate preparation that starts in the pediatric age and continues in the adult-focused setting. The process of HCT can be divided into 3 stages: (1) setting the stage: initiation of HCT preparation and transition readiness assessment; (2) moving forward: ongoing provision of HCT services; and (3) reaching the goal: transfer to adult-focused providers (32). The International and Interdisciplinary HCT Research Consortium developed a model for clinical and research HCT services, identifying the patient, family/social support, environment, and health care system as the 4 domains influencing transition outcomes (33). Another model developed in a cancer population is the Social-ecological Model of AYA Readiness for Transition (SMART) (34).
Health Care Transition Program Resources, Costs, and Barriers
Lack of supportive resources for adolescents with chronic conditions often results both in decreased access to care and impaired health and function, likely leading to increased subsequent medical costs (35). Negative effects attributed to missing HCT interventions have included medical complications, higher emergency department and hospital use, and higher overall costs of care (5).
There is a need to link transition efforts to posttransfer outcomes to evaluate the efficacy and cost savings of transition intervention services. Obtaining this information is challenging because of difficulties tracking AYAs across institutions, lack of established posttransfer markers of success within some chronic illnesses, lack of standardization between studies, and limited reimbursement for transition-related services (36-38). Although data on the health economic impact of structured transition interventions are scarce, positive outcomes associated with structured transition programs have been reported (39). For example, quantitative evidence supporting the cost-savings effect of multidisciplinary transition care in renal transplant recipients has been shown in a Canadian study. After integration of a transition clinic within the British Columbia Children’s Hospital, the average per-patient cost of care decreased from CAD $17 127 to 38 909 to CAD $11 380 to 34 312 ($US equivalent in 2021 is $US 13 764 to 31 269 and 9145 to 27 574). Patient outcomes and patient survival also improved among those who received support within the transition clinic (40).
In the US fee-for-service setting, there are few managed care reimbursement codes that support pediatric-to-adult transitional support (41). Consequently, gaps in HCP payment structures and care may leave AYAs and their families to navigate the transition process without access to these critical services (41), or may leave pediatric centers liable for out-of-pocket costs. Transition of care may be driven by individual champions of care coordination (such as physicians, nurses, and/or social workers) who provide extra support without compensation for time, leading to underrecognition of costs. A general challenge in tracking costs is that upfront costs of such a program are borne by an entity that will no longer be responsible for the patient. In other words, the investment in transition often comes from the pediatric side, a side that does not see the financial benefit that ideally emerges later during adolescent and adult care. Development of reimbursement programs following value-based payment models would be supported by the availability of transition-related managed care codes (41). Institutional infrastructure to support uninsured and out-of-network patients, as well as dedicated care coordinators to help navigate the transition and identify providers knowledgeable in XLH, may improve transition outcomes.
Health Care Transition Program Outcomes
Despite the challenges outlined earlier, certain HCT programs that support diseases other than XLH have been successful in helping patients to adhere to care during transition to adult care providers. Among non-XLH patients, findings from available randomized controlled trials suggest structured transition programs may positively affect patients’ knowledge of their condition, self-efficacy, and/or confidence (42). A systematic review evaluating 43 studies of patients with various chronic medical conditions found that structured transition interventions often resulted in positive outcomes, most notably related to adherence to care and use of ambulatory care in the adult setting (39).
On the other hand, challenges remain even when well-described transition guidance exists. For example, the American Diabetes Association has published expert consensus guidelines on HCT for emerging adults with type 1 diabetes in the United States (21). Evidence suggests that transition preparation can be variable and patients still experience gaps in obtaining adult diabetes care (21, 22). A focus group study on transition of care in diabetes found a need for gradual transfer of ownership of disease management from parent to child and the need for better communication between pediatric and adult services during the transition process (23). A type 1 diabetes transition review determined 5 recommendations for effective receivership within adult care, including effective communication between pediatric/adult HCPs, objective assessment of patient/provider knowledge, patient/adult provider relationship, support for psychosocial needs of AYAs, and support for AYA engagement through a team-based approach (24).
X-linked Hypophosphatemia Transition Within the Rare Disease Model
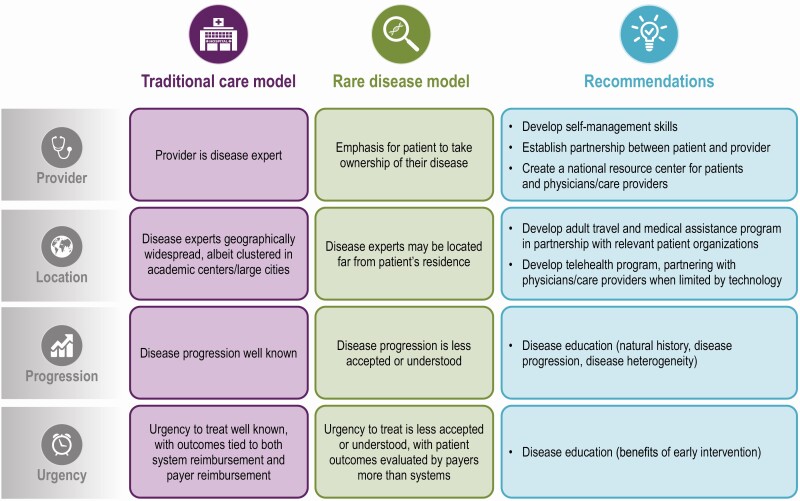
Traditional care models for common diseases have limited generalizability to rare diseases, especially rare diseases that are genetic in nature wherein the HCT experience of caregivers can influence the expectations of adolescents. The approach taken toward preparation and execution of XLH-specific HCT programs must consider the challenges specific to rare diseases (Fig. 2). For example, in rare diseases there can be limited access to disease experts, which can result in limited knowledge of disease progression among patients and HCPs (43). Patient advocacy groups for XLH, such as the XLH Network, and other rare and genetic diseases (eg, the National Organization for Rare Disorders or the Genetic and Rare Diseases Information Center), may provide patients disease knowledge. Indeed, the XLH Network patient advocacy organization developed a toolkit on transition from pediatric to adult care for patients and their caregivers available at https://www.xlhlink.com (44), as well as a “Voice of the Patient” report for physicians about the diagnosis and treatment of XLH (45).
Figure 2.
Care models of traditional and rare diseases (43).
In the rare-disease setting, care models emphasize the need to (1) improve patient access to disease experts; (2) implement telehealth when possible to enhance patient–clinician–researcher collaboration; and (3) integrate care with the latest research (43). The Six Core Elements of a transition of care model are preparing a transition policy, tracking and monitoring the transition process, assessing the patient’s transition readiness, developing a full transition plan, initial transfer into the adult facility, and transfer confirmation with ongoing care conducted by the adult clinician (5).
Health Care Transition Experience Within Rare Diseases
HCT models from pediatric- to adult-focused services have been described in other lifelong rare diseases, notably hemophilia (6) and sickle cell disease (25). Both are briefly described here.
In a hemophilia-specific HCT program at the University of Munich, the transition process typically begins during puberty and can span into early adulthood; however, the exact timing of transfer is tailored to the individual and some patients continue treatment at the pediatric center into their late 20s. Multiple transitional appointments at the adult-focused center are attended both by the pediatrician and adult physician, along with a social worker and clinical psychologist (6). Implementation of this program has allowed the responsible HCPs to identify several concepts, including the value of providing a patient a social worker who specializes in the transition process, the need to prepare patients early, and the benefit of keeping regular appointments both with the pediatrician and adult physician in attendance (6).
The SICKLE program (25) highlights similar priorities, including the need both for the pediatric and adult HCPs to be present during transitional appointments, the value of including a transition navigator, and the benefit of beginning transition preparation as early as possible (25). The SICKLE recommendations also note that patients should be empowered to self-manage symptoms such as pain, and that AYAs should ideally be supported by caregivers and HCPs to develop an understanding of their condition (25). Family input was also identified as a critical component in supporting AYAs to become the key communicator during conversations between clinician, patient, and caregiver (27).
Key Applications of Existing Rare-Disease Transition Programs to X-linked Hypophosphatemia
As in XLH, patients with phenylketonuria (PKU) also have loss to follow-up for many reasons, including personal choice, treatment costs and/or lack of insurance coverage for medical formula or foods, and unavailability of adult PKU clinics (28). Some studies have estimated that more than half of patients with PKU drop out from medical care during adulthood (28). Reengagement strategies, aimed at patients with PKU who had no clinical contact for 2 consecutive years, were refined to 6 best practice recommendations: (1) ensure patients are aware of the current treatment guidelines; (2) communicate to patients any new treatment and diet options as they become available; (3) consider the neuropsychological and neurocognitive aspects of the disease; (4) prioritize motivated lost to follow-up patients; (5) explore new approaches of outreach; and (6) formalize approaches to track or identify patients (28). In this study, access to new pharmacological agents, research insights, types of formula or medical nutrition, and options for treatment were identified as motivating factors for lost to follow-up patients to return to the clinic (28). Recommendations emphasize the importance of clear communication to patients; surveys of patients with PKU found that communication materials that were motivational in tone achieved greater engagement (28). Other tactical approaches include adding AYA-only hours to pediatric clinics and communicating to AYAs via social media or text messages (28), although compliance with local privacy and data confidentiality regulations (eg, HIPAA; Health Insurance Portability and Accountability Act) must be considered. Indeed, technology-based interventions such as texting adolescents with chronic disease was shown to improve performance of disease management tasks, health-related self-efficacy, and patient-initiated communications (46).
As recommended within the rare-disease model of care, and also within the COVID-19 pandemic environment, integration of telemedicine (an audiovisual interaction between patient and HCP facilitated by computers, mobile devices, or telephones) may enhance the care of patients with XLH by improving access to geographically distant experts and support the HCT. Telemedicine has the potential to eliminate geographical barriers, is economical (time and financial), and requires little or no travel or work absence (47). Telemedicine appointments may enable the reintegration of lost to follow-up patients with rare diseases into the clinical system (28). In the context of COVID-19, there has been a widespread trend to integrate telemedicine into practice (48).
Two surveys on the effect of COVID-19 on HCPs and patients with inherited rare metabolic disorders (eg, amino acid– and organic acid–related disorders, lysosomal storage disorders) found that patients experienced extensive disruption of care because of COVID-19–related restrictions, with most appointments and treatments canceled, reduced, or postponed. However, almost all HCPs (90%) were able to substitute face-to-face visits with telemedicine appointments (49). A majority (92%) of patients with rare diseases using telehealth during the COVID-19 pandemic found the experience positive (50). In the United States, certain regulatory barriers were temporarily removed in the COVID-19 pandemic environment to support telemedical approaches among people who can no longer receive in-person care, and rates of use are rising (51). However, several complex legal and insurance barriers still exist that vary from state to state and by health insurance policies. State-specific licensing and malpractice liability rules may also provide impediments to implementation. For example, US HCPs may need to be licensed within the state of the patient’s residence to legally provide telehealth services.
Although the use of telemedicine and virtual care has increased rapidly during the COVID-19 pandemic, there is a need to educate physicians and patients alike on how they can best use telemedicine and virtual care (52). Indeed, telemedicine approaches can be associated with delays or decreased patient/provider adherence to completing laboratory testing, radiographs, and other tests. Additionally, different age and socioeconomic groups may have differing levels of access to and fluency within the digital telehealth setting. For example, a recent study of patients with diabetes revealed that socioeconomic barriers can make telemedicine approaches less accessible to certain individuals (53).
Finally, there is a general need to train students of all health disciplines and residents about chronic-disease HCT. A survey of internal medicine and pediatric residents indicated that most of the internal medicine residents felt underprepared to transition youth with childhood-onset illnesses to adult-oriented care (54). Integrating HCT training can prepare health care providers for their patients, including those with rare diseases. For example, at the Vanderbilt University Medical Center and Indiana University School of Medicine, adult endocrine fellows rotate through the pediatric endocrinology clinic as part of the learning curriculum. Physicians must be skilled to ascertain whether patients themselves are ready to be managed within an adult care environment. Fortunately, assessments to determine each individual’s readiness for transition of care from pediatric- to adult-focused care are available, and include evaluations of patient knowledge of the disease, self-efficacy in terms of communicating with health care providers, and the ability to manage symptoms without caregiver support (25, 26). Several publications in the United States and other countries have provided evidence for disease-neutral tools such as the provider-administered/verified TRxANSITION Index (55–58) and the self-administered STARx Questionnaire (56, 57, 59-62) in pediatric and adult-focused settings both for AYAs and their caregivers (63, 64).
Expert Opinion: A Health Care Transition Program in X-linked Hypophosphatemia
This section outlines recommendations for supporting HCT for patients with XLH. Three areas of competency are defined: (1) patient foundational knowledge of their disease and the health care acquisition process; (2) information transfer and timelines; and (3) supportive behaviors to drive engagement (see Table 1; Figs. 3 and 4). Actions required for successful HCT to a multidisciplinary team do not need to be executed exhaustively in all cases for the transition to be successful. Some of the specific detail proposed here is tailored to the US health care environment; however, many concepts have broad relevance to XLH communities within different health care contexts.
Table 1.
Expert recommendations for transition of care in X-linked hypophosphatemia
| Foundational knowledge |
| The foundations for successful HCT for patients with XLH should be built earlier than adolescence, ideally at the time patients can begin to understand their own diagnosis |
| • The main categories of knowledge competencies include those related to the XLH disease understanding (HCPs and patients/families) and working effectively within the health care acquisition environment (HCPs) |
| • Disease education, genetic counseling, and guidance on family planning should be started early and provided frequently to encourage patients to advocate for their health care |
| • Core disease area knowledge requirements for HCPs (in pediatric and adult practice) and patients/families: |
| – HCPs and patients recognize that XLH is a lifelong progressive disease |
| – HCPs and patients recognize genetic etiology, understand recurrence risks, variability of expression, and benefits of therapies |
| • XLH is inherited in families (but can also come from spontaneous new mutation) and arises from mutations in PHEX |
| • Patients may experience bone pain, broken bones, or pseudofractures (may be referred to as “stress fractures”), joint stiffness, joint pain, difficulty with range of motion, or with walking, muscle weakness, calcifications, weakness |
| • The aim of therapeutic management is to prevent/reduce deleterious bone and other manifestations and improve/preserve QoL |
| • Treatment options for XLH exist, including: |
| • FGF23 monoclonal antibodies provide a targeted treatment option for patients with XLH |
| • Active forms of vitamin D (calcitriol) plus phosphate salts |
| • These medical treatment options require careful laboratory monitoring for safety and efficacy |
| – HCPs recognize signs and symptoms of XLH. There may be a role for pediatric providers to educate receiving adult-focused providers |
| • Key parameters for assessment include laboratory (serum calcium, intact parathyroid hormone, creatinine, alkaline phosphatase, fasting serum phosphorus, urine calcium, urine creatinine, 25 OH vitamin D), anthropometric (height, weight, intermalleolar distance, intercondylar distance), radiologic (X-rays of lower extremities and wrists, bone age assessment, assessment for fractures, pseudofractures, or enthesopathy), other (blood pressure, renal ultrasonography, orthopedic, craniofacial, neurologic, dental, hearing, physical therapy, QoL, genetic counseling). Frequency of assessment is based on patient age or clinical indications |
| – Patients ideally are familiar with other family members who may be affected |
| – HCPs understand/support health care literacy of patient and family |
| • The patient/family may have low health care literacy, which means patient/family may need additional support and education to help navigate their health |
| • The patient/family may have high health care literacy and know more about their disease than their HCP, especially the primary care doctor, and it is important to recognize that and not ignore their concerns |
| • Core health care acquisition environment (US focus) knowledge requirements for HCPs and patients include: |
| – HCPs understand the disorder and are motivated to care for the patient using current approaches to management, including routine surveillance |
| – HCPs must manage insurance company agents with little knowledge of XLH, and communicate prioritizing expert care over within-network care |
| – Patients understand challenges and necessity of obtaining insurance coverage for cost of therapy/monitoring/testing/surgeries, etc and the importance of not having lapses in coverage |
| Information transfer and timelines |
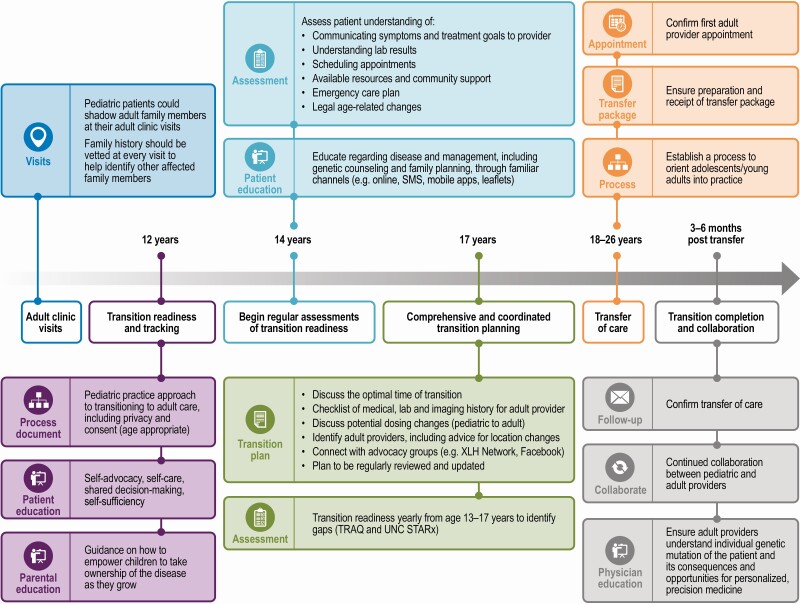
| • Transition readiness tracking can begin when patients with XLH are approximately age 12 years, and ramp up over time until care transfer, at approximately age 18 to 26 years (Fig. 3) |
| • Patient self-direction is emphasized at age 14 years (eg, patient knows how to communicate symptoms to a provider; patient understands laboratory test results; patient begins involvement in booking appointments) and should be supported through the final year(s) of pediatric care (eg, through discussions of the optimal time of transfer; skill of finding an adult-focused provider; how medical records and treatment goals will be forwarded to adult care provider) |
| • Pediatric-focused HCPs should follow up with a patient to confirm transfer of care, and pediatric and adult care providers should maintain a continued collaboration through the early posttransition phase of care |
| • A proposed clinical transition portfolio should be developed by the pediatric provider to share with the adult care provider, transitioning patient, and family (Fig. 4). In addition, a portfolio of supporting materials (eg, disease-specific education, checklists, provider information, insurance information) underpins optimization of outcomes as adolescents/young adults with XLH transfer from pediatric- to adult-focused health care |
| Supportive behaviors to drive engagement |
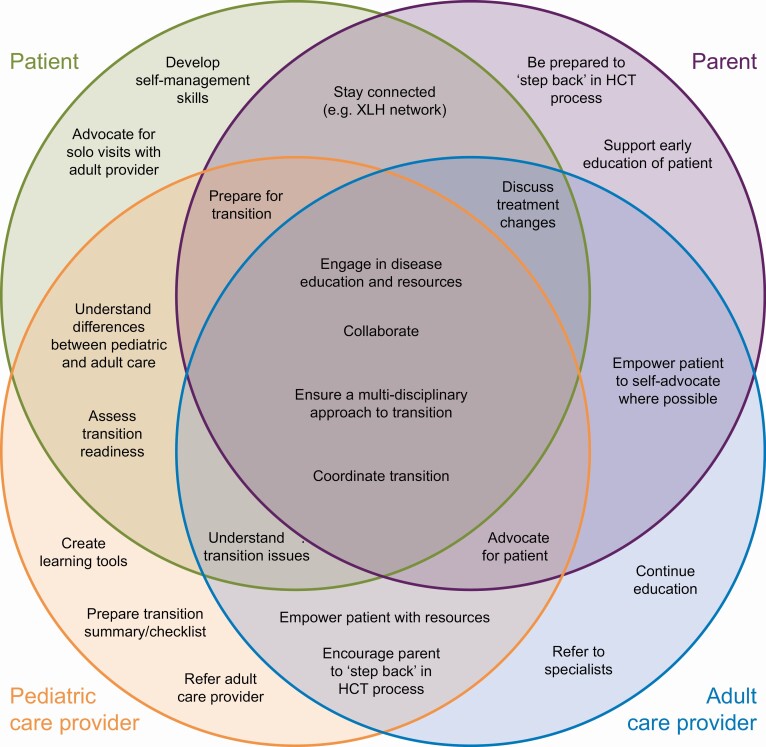
| • Clear HCT preparation guidelines and treatment-related goals should be defined for all stakeholders. Describe the roles and responsibilities of patients, parents, pediatric-focused HCP, and adult-focused HCP in driving toward a successful HCT (Fig. 2) |
| • Unique elements of XLH clinical care that should be specifically captured as part of an XLH transition (eg, gene testing results, discussions as to continuing medical therapy; timing and performance of full orthopedic clinical and radiographic assessment; physical therapy surveillance evaluations and treatment; last radiograph and renal ultrasound information; symptoms the patient should know could be due to XLH; and reasons to seek care, including bone pain, hearing loss) |
| • Channels that are familiar to and accepted by adolescents/young adults (eg, online portals, text messages) are recommended for communication and education |
| • Psychosocial and access-related resources for adolescents/young adults enabling emancipation from parental involvement are required, including guidance for parents on how to empower their child to take ownership of their disease (develop self-management skills) as they grow and mature |
Abbreviations: FGF23, fibroblast growth factor 23; HCP, health care professional; HCT, health care transition; QoL, quality of life; XLH, X-linked hypophosphatemia.
Figure 3.
Supportive behaviors to drive health care transition program engagement. HCT, health care transition; XLH, X-linked hypophosphatemia.
Figure 4.
Timelines of transfer. TRAQ, Transition Readiness Assessment Questionnaire; UNC-STARx, University of North Carolina transition readiness questionnaire.
We identified the need for psychosocial and educational resources for AYA emancipation from caregiver involvement, including a guidance document for caregivers on how to empower their child to take ownership of their XLH as they grow. In addition, it is helpful to provide disease education, genetic counseling, and guidance on family planning, to teach patients to advocate for their own health care (as access to specialists may be limited by geographical location), and to facilitate communication/education to patients through channels familiar to and accepted by AYAs (eg, online portals or text messages). Specific timelines of transfer, which can be individualized, are essential. Clear HCT preparation guidelines and treatment-related goals are defined for all stakeholders. A portfolio of supporting materials underpins the optimization of outcomes as AYAs with XLH and rare metabolic bone disorders transfer from pediatric- to adult-focused health care (see Table 1; Fig. 5).
Figure 5.
Patients with XLH: transition passport. 5TSTS, five-times sit to stand test; 6MWD, 6-minute walking distance; BIN, bank identification number; DXA, dual-energy x-ray absorptiometry; GARD, Genetic and Rare Diseases Information Center; NORD, National Organization for Rare Disorders; TUG, timed up and go test; XLH, X-linked hypophosphatemia.
In addition to offering practical solutions related to structuring successful transition of care, we also recognize that an additional challenge is to address the burden on the pediatric provider required to collect the recommended information and provide it to the adult clinician, because most medical record systems are not able to collate the required information efficiently. Mechanisms to decrease the burden on the pediatric provider are likely to enhance the amount of information available. Early communication between pediatric- and adult-focused providers may also help avoid redundancy in testing.
Case Study
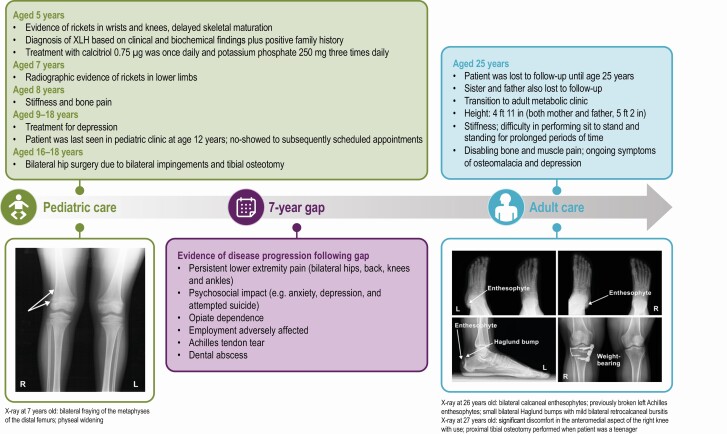
Fig. 6 illustrates a case study of a suboptimal transition, highlighting potential consequences exacerbated by delay between pediatric and adult care.
Figure 6.
Suboptimal transition from adolescent to adult care for a patient with X-linked hypophosphatemia (XLH).
Conclusion
XLH is a rare, chronic multisystem disease that, despite being labeled as a childhood disease, progresses over time throughout adulthood with worsening musculoskeletal signs and symptoms. Lifelong multidisciplinary care of patients with XLH is needed, involving physicians, physiotherapists, surgeons, psychologists, dentists, and social workers. By taking inspiration from HCT initiatives in other diseases, and tailoring care delivery models to the unique needs of patients with XLH, more AYAs can avoid loss to follow-up during the transition to adult-oriented care, leading to improved health care outcomes throughout life. Based on expert consensus of XLH and transition specialists, a program herein has been developed to aid HCT preparation for patients with XLH. The development of HCT tools and resources specific to XLH may apply to other rare metabolic bone disorders to promote continuous, effective clinical care. Adopting these processes should promote better outcomes for AYAs with these conditions.
Acknowledgments
We thank our patients and their families for guiding us to provide optimal care. We also thank Helene Wellington, AMICULUM, who provided medical writing support funded by Ultragenyx Pharmaceutical in partnership with Kyowa Kirin International plc in accordance with Good Publication Practice guidelines. The work submitted for publication is original and has not been published other than as an abstract or preprint in any language or format and has not been submitted elsewhere for print or electronic publication consideration. The authors affirm that each person listed as an author participated in a substantive manner, in accordance with ICMJE authorship guidelines, and is prepared to take public responsibility for it.
Financial Support: Medical writing support was funded by Ultragenyx Pharmaceutical in partnership with Kyowa Kirin International plc in accordance with Good Publication Practice guidelines.
Glossary
Abbreviations
- AYAs
adolescents and young adults
- HCP
health care professional
- HCT
health care transition
- HRQoL
health-related quality of life
- PKU
phenylketonuria
- PROMIS
pediatric Patient-Reported Outcomes Measurement Information System
- XLH
X-linked hypophosphatemia
Additional Information
Disclosures: K.D. is a DMP investigator and consultant for Ultragenyx Pharmaceutical Inc; R.D. serves on the scientific advisory board and/or as a consultant for Amgen, Ultragenyx Pharmaceutical Inc, and Radius Health; J.S. has received research funding and serves on the advisory board for Ultragenyx Pharmaceutical Inc; E.A.I. has received research funding and serves on advisory boards for Ultragenyx Pharmaceutical Inc; G.S.G. is an advisory group consultant for Ultragenyx Pharmaceutical Inc; J.D.M. serves as an advisory group consultant for Ultragenyx Pharmaceutical Inc and Ultragenyx Pharmaceutical Inc Speaker Bureau. G.P. is a consultant and speaker for Ultragenyx Pharmaceutical Inc; A.I.H. is an employee of Ultragenyx Pharmaceutical Inc; P.R. is an employee of Ultragenyx Pharmaceutical Inc; and M.D.F. is a consultant for ProKidney.
Data Availability
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.
References
- 1. Lecoq AL, Brandi ML, Linglart A, Kamenický P. Management of X-linked hypophosphatemia in adults. Metabolism. 2020;103S:154049. [DOI] [PubMed] [Google Scholar]
- 2. Hughes M, Macica C, Meriano C, Doyle M. Giving credence to the experience of X-linked hypophosphatemia in adulthood: an interprofessional mixed-methods study. J Patient Cent Res Rev. 2020;7(2):176-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haffner D, Emma F, Eastwood DM, et al. Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia. Nat Rev Nephrol. 2019;15(7):435-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dahir K, Roberts MS, Krolczyk S, Simmons JH. X-linked hypophosphatemia: a new era in management. J Endocr Soc. 2020;4(12):bvaa151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. White PH, Cooley WC; Transitions Clinical Report Authoring Group; American Academy of Pediatrics; American Academy Of Family Physicians; American College of Physicians . Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2018;142(5):e20182587. [DOI] [PubMed] [Google Scholar]
- 6. Bidlingmaier C, Olivieri M, Schilling FH, Kurnik K, Pekrul I. Health care transition of adolescents and young adults with haemophilia: the situation in Germany and the Munich experience. Hamostaseologie. 2020;40(1):97-104. [DOI] [PubMed] [Google Scholar]
- 7. Connor J, Olear EA, Insogna KL, et al. Conventional therapy in adults with X-linked hypophosphatemia: effects on enthesopathy and dental disease. J Clin Endocrinol Metab. 2015;100(10):3625-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laurent MR, De Schepper J, Trouet D, et al. Consensus recommendations for the diagnosis and management of X-linked hypophosphatemia in Belgium. Front Endocrinol (Lausanne). 2021;12:641543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Al Juraibah F, Al Amiri E, Al Dubayee M, et al. Diagnosis and management of X-linked hypophosphatemia in children and adolescent in the Gulf Cooperation Council countries. Arch Osteoporos. 2021;16(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Padidela R, Nilsson O, Makitie O, et al. The international X-linked hypophosphataemia (XLH) registry (NCT03193476): rationale for and description of an international, observational study. Orphanet J Rare Dis. 2020;15(1):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ohata Y, Yamazaki M, Kawai M, et al. Elevated fibroblast growth factor 23 exerts its effects on placenta and regulates vitamin D metabolism in pregnancy of Hyp mice. J Bone Miner Res. 2014;29(7):1627-1638. [DOI] [PubMed] [Google Scholar]
- 12. Bistarakis L, Voskaki I, Lambadaridis J, Sereti H, Sbyrakis S. Renal handling of phosphate in the first six months of life. Arch Dis Child. 1986;61(7):677-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beck-Nielsen SS, Mughal Z, Haffner D, et al. FGF23 and its role in X-linked hypophosphatemia-related morbidity. Orphanet J Rare Dis. 2019;14(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Skrinar A, Dvorak-Ewell M, Evins A, et al. The lifelong impact of X-linked hypophosphatemia: results from a burden of disease survey. J Endocr Soc. 2019;3(7):1321-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferizović N, Marshall J, Williams AE, et al. Exploring the burden of X-linked hypophosphataemia: an opportunistic qualitative study of patient statements generated during a technology appraisal. Adv Ther. 2020;37(2):770-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaat AJ, Kallen MA, Nowinski CJ, Sterling SA, Westbrook SR, Peters JT. PROMIS pediatric depressive symptoms as a harmonized score metric. J Pediatr Psychol. 2020;45(3):271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kroenke K, Stump TE, Chen CX, et al. Responsiveness of PROMIS and Patient Health Questionnaire (PHQ) Depression Scales in three clinical trials. Health Qual Life Outcomes. 2021;19(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seefried L, Smyth M, Keen R, Harvengt P. Burden of disease associated with X-linked hypophosphataemia in adults: a systematic literature review. Osteoporos Int. 2021;32(1):7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ultragenyx Pharmaceutical Inc. X-linked hypophosphatemia disease monitoring program. ClinicalTrials.gov Identifier: NCT03651505. 2021. Accessed August 2021. https://clinicaltrials.gov/ct2/show/NCT03651505
- 20. Raimann A, Mindler GT, Kocijan R, et al. Multidisciplinary patient care in X-linked hypophosphatemic rickets: one challenge, many perspectives. Wien Med Wochenschr. 2020;170(5-6):116-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peters A, Laffel L; American Diabetes Association Transitions Working Group . Diabetes care for emerging adults: recommendations for transition from pediatric to adult diabetes care systems: a position statement of the American Diabetes Association, with representation by the American College of Osteopathic Family Physicians, the American Academy of Pediatrics, the American Association of Clinical Endocrinologists, the American Osteopathic Association, the Centers for Disease Control and Prevention, Children with Diabetes, The Endocrine Society, the International Society for Pediatric and Adolescent Diabetes, Juvenile Diabetes Research Foundation International, the National Diabetes Education Program, and the Pediatric Endocrine Society (formerly Lawson Wilkins Pediatric Endocrine Society). Diabetes Care. 2011;34(11):2477-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garvey KC, Finkelstein JA, Laffel LM, Ochoa V, Wolfsdorf JI, Rhodes ET. Transition experiences and health care utilization among young adults with type 1 diabetes. Patient Prefer Adherence. 2013;7:761-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang JS, Gottschalk M, Pian M, Dillon L, Barajas D, Bartholomew LK. Transition to adult care: systematic assessment of adolescents with chronic illnesses and their medical teams. J Pediatr. 2011;159(6):994-998.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iyengar J, Thomas IH, Soleimanpour SA. Transition from pediatric to adult care in emerging adults with type 1 diabetes: a blueprint for effective receivership. Clin Diabetes Endocrinol. 2019;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Inusa BPD, Stewart CE, Mathurin-Charles S, et al. Paediatric to adult transition care for patients with sickle cell disease: a global perspective. Lancet Haematol. 2020;7(4):e329-e341. [DOI] [PubMed] [Google Scholar]
- 26. Sickle Cell Society. Sickle Cell Disease in Childhood: Standards and Recommendations for Clinical Care. 3rd ed. Public Health England; 2019. [Google Scholar]
- 27. Porter JS, Graff JC, Lopez AD, Hankins JS. Transition from pediatric to adult care in sickle cell disease: perspectives on the family role. J Pediatr Nurs. 2014;29(2):158-167. [DOI] [PubMed] [Google Scholar]
- 28. Beazer J, Breck J, Eggerding C, Gordon P, Hacker S, Thompson A; PKU Lost to Follow-Up Recommendations Group . Strategies to engage lost to follow-up patients with phenylketonuria in the United States: best practice recommendations. Mol Genet Metab Rep. 2020;23:100571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. American Academy of Pediatrics, American Academy of Family Physicians, American College of Physicians–American Society of Internal Medicine. A consensus statement on health care transitions for young adults with special health care needs. Pediatrics. 2002;110(6 Pt 2):1304-1306. [PubMed] [Google Scholar]
- 30. Lotstein DS, Ghandour R, Cash A, McGuire E, Strickland B, Newacheck P. Planning for health care transitions: results from the 2005-2006 National Survey of Children With Special Health Care Needs. Pediatrics. 2009;123(1):e145-e152. [DOI] [PubMed] [Google Scholar]
- 31. Lebrun-Harris L, McManus M, Ilango S, et al. Transition planning among US youth with and without special health care needs. Pediatrics. 2018;142:e20180194. [DOI] [PubMed] [Google Scholar]
- 32. Mahan JD, Betz CL, Okumura MJ, Ferris ME. Self-management and transition to adult health care in adolescents and young adults: a team process. Pediatr Rev. 2017;38(7):305-319. [DOI] [PubMed] [Google Scholar]
- 33. Betz CL, Ferris ME, Woodward JF, Okumura MJ, Jan S, Wood DL. The health care transition research consortium health care transition model: a framework for research and practice. J Pediatr Rehabil Med. 2014;7(1):3-15. [DOI] [PubMed] [Google Scholar]
- 34. Schwartz LA, Tuchman LK, Hobbie WL, Ginsberg JP. A social-ecological model of readiness for transition to adult-oriented care for adolescents and young adults with chronic health conditions. Child Care Health Dev. 2011;37(6):883-895. [DOI] [PubMed] [Google Scholar]
- 35. Buschur EO, Glick B, Kamboj MK. Transition of care for patients with type 1 diabetes mellitus from pediatric to adult health care systems. Transl Pediatr. 2017;6(4):373-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gray WN, Schaefer MR, Resmini-Rawlinson A, Wagoner ST. Barriers to transition from pediatric to adult care: a systematic review. J Pediatr Psychol. 2018;43(5):488-502. [DOI] [PubMed] [Google Scholar]
- 37. Coyne B, Hallowell SC, Thompson M. Measurable outcomes after transfer from pediatric to adult providers in youth with chronic illness. J Adolesc Health. 2017;60(1):3-16. [DOI] [PubMed] [Google Scholar]
- 38. Nabbout R, Arzimanoglou A, Chin RFM, Grinspan Z, Speechley K, Camfield P. The evaluation and costs of transition programs for youth with epilepsy. Epilepsy Behav. 2019;93:133-137. [DOI] [PubMed] [Google Scholar]
- 39. Gabriel P, McManus M, Rogers K, White P. Outcome evidence for structured pediatric to adult health care transition interventions: a systematic review. J Pediatr. 2017;188:263-269.e15. [DOI] [PubMed] [Google Scholar]
- 40. Prestidge C, Romann A, Djurdjev O, Matsuda-Abedini M. Utility and cost of a renal transplant transition clinic. Pediatr Nephrol. 2012;27(2):295-302. [DOI] [PubMed] [Google Scholar]
- 41. McManus M, White P, Schmidt A, et al. Health care gap affects 20% of United States population: transition from pediatric to adult health care. Health Policy Open. 2020;1:100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Campbell F, Biggs K, Aldiss SK, et al. Transition of care for adolescents from paediatric services to adult health services. Cochrane Database Syst Rev. 2016;4:CD009794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Augustine EF, Dorsey ER, Saltonstall PL. The care continuum: an evolving model for care and research in rare diseases. Pediatrics. 2017;140(3):e20170108. [DOI] [PubMed] [Google Scholar]
- 44. Ultragenyx Pharmaceutical. XLH transitions toolkit. 2020. Accessed August 2021. http://www.xlhnetwork.org/application/files/1916/0311/3210/XLH_TRANSITIONS_TOOLKIT.pdf
- 45. XLH Network. Voice of the patient report. 2019. Accessed August 2021. http://www.xlhnetwork.org/application/files/5515/9317/2550/VOP_Report.pdf
- 46. Huang JS, Terrones L, Tompane T, et al. Preparing adolescents with chronic disease for transition to adult care: a technology program. Pediatrics. 2014;133(6):e1639-e1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Blue R, Yang AI, Zhou C, et al. Telemedicine in the era of coronavirus disease 2019 (COVID-19): a neurosurgical perspective. World Neurosurg. 2020;139:549-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Contreras CM, Metzger GA, Beane JD, Dedhia PH, Ejaz A, Pawlik TM. Telemedicine: patient-provider clinical engagement during the COVID-19 pandemic and beyond. J Gastrointest Surg. 2020;24(7):1692-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lampe C, Dionisi-Vici C, Bellettato CM, et al. ; MetabERN Collaboration Group . The impact of COVID-19 on rare metabolic patients and healthcare providers: results from two MetabERN surveys. Orphanet J Rare Dis. 2020;15(1):341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. NORD Rare Insights. Ensuring access to telehealth for rare diseases. Accessed August 2021. https://rarediseases.org/wp-content/uploads/2020/10/NRD-2098-RareInsights-Telehealth-Report-1.pdf
- 51. Royce TJ, Sanoff HK, Rewari A. Telemedicine for cancer care in the time of COVID-19. JAMA Oncol. 2020;6(11):1698-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bokolo A Jr. Use of telemedicine and virtual care for remote treatment in response to COVID-19 pandemic. J Med Syst. 2020;44(7):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tilden DR, Datye KA, Moore DJ, French B, Jaser SS. The rapid transition to telemedicine and its effect on access to care for patients with type 1 diabetes during the COVID-19 pandemic. Diabetes Care. 2021;44(6):1447-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Patel MS, O’Hare K. Residency training in transition of youth with childhood-onset chronic disease. Pediatrics. 2010;126(Suppl 3):S190-S193. [DOI] [PubMed] [Google Scholar]
- 55. Zhong Y, Gilleskie DB, van Tilburg MAL, et al. Longitudinal self-management and/or transition readiness per the TRxANSITION index among patients with chronic conditions in pediatric or adult care settings. J Pediatr. 2018;203:361-370.e1. [DOI] [PubMed] [Google Scholar]
- 56. Zhong Y, Patel N, Ferris M, Rak E. Health literacy, nutrition knowledge, and health care transition readiness in youth with chronic kidney disease or hypertension: a cross-sectional study. J Child Health Care. 2020;24(2):246-259. [DOI] [PubMed] [Google Scholar]
- 57. Johnson MA, Javalkar K, van Tilburg M, Haberman C, Rak E, Ferris ME. The relationship of transition readiness, self-efficacy, and adherence to preferred health learning method by youths with chronic conditions. J Pediatr Nurs. 2015;30(5):e83-e90. [DOI] [PubMed] [Google Scholar]
- 58. Javalkar K, Johnson M, Kshirsagar AV, Ocegueda S, Detwiler RK, Ferris M. Ecological factors predict transition readiness/self-management in youth with chronic conditions. J Adolesc Health. 2016;58(1):40-46. [DOI] [PubMed] [Google Scholar]
- 59. Arvanitis M, Hart LC, DeWalt DA, et al. Transition readiness not associated with measures of health in youth with IBD. Inflamm Bowel Dis. 2021;27(1):49-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Richards J, Nazareth M, van Tilburg MAL, et al. Engagement in household chores in youth with chronic conditions: health care transition implications. OTJR (Thorofare N J). 2021;41(1):6-14. [DOI] [PubMed] [Google Scholar]
- 61. Ferris M, Cohen S, Haberman C, et al. Self-management and transition readiness assessment: development, reliability, and factor structure of the STARx questionnaire. J Pediatr Nurs. 2015;30(5):691-699. [DOI] [PubMed] [Google Scholar]
- 62. Cohen SE, Hooper SR, Javalkar K, et al. Self-management and transition readiness assessment: concurrent, predictive and discriminant validation of the STARx questionnaire. J Pediatr Nurs. 2015;30(5):668-676. [DOI] [PubMed] [Google Scholar]
- 63. Hart LC, Díaz-González de Ferris M, Nazareth M, et al. Evaluation of the TRxANSITION index-parent version for assessment of readiness to transition to adult care among youth with chronic conditions. J Pediatr Nurs. 2021;58:1-8. [DOI] [PubMed] [Google Scholar]
- 64. Nazareth M, Hart L, Ferris M, Rak E, Hooper S, van Tilburg MAL. A parental report of youth transition readiness: the parent STARx questionnaire (STARx-P) and re-evaluation of the STARx child report. J Pediatr Nurs. 2018;38:122-126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.