Abstract
Background
Lenvatinib is a multikinase inhibitor approved to treat radioiodine-refractory differentiated thyroid cancer (RR-DTC) at a starting dose of 24 mg/day. This study explored, in a double-blinded fashion, whether a starting dose of 18 mg/day would provide comparable efficacy with reduced toxicity.
Methods
Patients with RR-DTC were randomized to lenvatinib 24 mg/day or 18 mg/day. The primary efficacy endpoint was objective response rate as of week 24 (ORRwk24); the odds ratio noninferiority margin was 0.4. The primary safety endpoint was frequency of grade ≥3 treatment-emergent adverse events (TEAEs) as of week 24. Tumors were assessed using RECIST v1.1. TEAEs were monitored and recorded.
Results
The ORRwk24 was 57.3% (95% CI 46.1, 68.5) in the lenvatinib 24-mg arm and 40.3% (95% CI 29.3, 51.2) in the lenvatinib 18-mg arm, with an odds ratio (18/24 mg) of 0.50 (95% CI 0.26, 0.96). As of week 24, the rates of TEAEs grade ≥3 were 61.3% in the lenvatinib 24-mg arm and 57.1% in the lenvatinib 18-mg arm, a difference of −4.2% (95% CI −19.8, 11.4).
Conclusion
A starting dose of lenvatinib 18 mg/day did not demonstrate noninferiority compared to a starting dose of 24 mg/day as assessed by ORRwk24 in patients with RR-DTC. The results represent a clinically meaningful difference in ORRwk24. The safety profile was comparable, with no clinically relevant difference between arms. These results support the continued use of the approved starting dose of lenvatinib 24 mg/day in patients with RR-DTC and adjusting the dose as necessary.
Keywords: lenvatinib, RR-DTC, tyrosine kinase inhibitor, starting dose
Thyroid cancer is estimated to have a worldwide incidence of approximately 567 000 cases (1), and differentiated thyroid cancer (DTC) makes up about 95% of these cases (2). DTC is typically associated with a good prognosis, and approximately 85% of patients are cured following some combination of surgery, radioiodine therapy, and thyroid-stimulating hormone suppression (3-6). However, the remaining patients with radioiodine refractory (RR)-DTC have a 5-year survival rate of as low as 10% (4).
Inhibition of tumor cell growth pathways through the use of tyrosine kinase inhibitors has led to their application in the treatment of RR-DTC (2). Specifically, sorafenib was approved for the treatment of RR-DTC based on the pivotal DECISION trial, where median progression-free survival (PFS) was 5.8 months in patients treated with placebo vs 10.8 months in patients treated with sorafenib; the objective response rate (ORR) for sorafenib was 12.2% (7). Lenvatinib, a multikinase inhibitor (8-11), was approved for the treatment of patients with locally recurrent or metastatic progressive RR-DTC based on the global Phase 3 SELECT study, in which median PFS was 3.6 months in patients treated with placebo vs 18.3 months in patients treated with lenvatinib; the ORR for lenvatinib was 64.8% (12,13). However, 82.4% of patients in the lenvatinib arm had a dose interruption, and 67.8% of patients had a dose reduction due to an adverse event (AE), leading to a mean lenvatinib dose of 17.2 mg/day (12). AEs led to the discontinuation of lenvatinib in 14.2% of patients.
Since the approval of lenvatinib, the authors have noted that some physicians prefer starting patients at a lower dose out of concern that AEs will be encountered (14,15). Moreover, regulatory concerns arose regarding whether a lower dose of lenvatinib would provide comparable efficacy but improved safety relative to the approved 24-mg/day starting dose in patients with RR-DTC. Therefore, this study sought to assess the safety and efficacy of a starting dose of lenvatinib 18 mg/day (LEN18) vs lenvatinib 24 mg/day (LEN24). To control for provider and patient bias, the assigned lenvatinib dose was given in a double-blinded fashion.
Methods
Study Design
This multicenter, randomized, double-blind, Phase 2 study compared the safety and efficacy of LEN18 with LEN24 in 28-day cycles in patients with RR-DTC (NCT02702388). The study was initially designed with 3 lenvatinib dosing arms, utilizing starting doses of 14 mg/day, 20 mg/day, and 24 mg/day. However, this was subsequently revised to a 2-arm study design based on the results of a population pharmacokinetics/pharmacodynamics modeling analysis, which was conducted with 7 dosing regimens, some of which included uptitration to 24 mg (16). The modeling analysis used simulated tumor-size profiles to determine that LEN24 predicted a derived ORR as of 24 weeks (ORRwk24) of 50.0%, while LEN18 could potentially provide an ORRwk24 of 41.5% with an improved safety profile (ie, a reduction in the number of patients who required a dose reduction due to an AE). These data indicated that the 14-mg/day starting dose was unlikely to provide comparable efficacy to, and the 20 mg/day starting dose would not be distinguishable from, LEN24 (16), and therefore the study design was revised (as of February 13, 2017). The 41 patients who had been randomly assigned to those dose groups were unblinded shortly after enrollment, transitioned off the study, and are not included in this analysis.
Randomization and masking were performed centrally by an interactive voice and web response system. Patients were randomly assigned 1:1 to the LEN24 arm or the LEN18 arm and were stratified by age (≤65 years or >65 years) and Eastern Cooperative Oncology Group performance status (ECOG PS; 0 vs 1 or 2). Dosing was blinded so that all patients received their dose as a combination of 4 capsules irrespective of their dose. Randomization data were kept strictly confidential, filed securely by an appropriate group with the sponsor, and accessible only to authorized persons (eg, Eisai Global Safety) until the time of unblinding, per standard operating procedure. Patients who experienced toxicities underwent dose interruptions and dose adjustments based on the grade of the toxicity. Dose reductions were performed in a blinded fashion per physician direction.
Patients received treatment, in a double-blinded fashion, until disease progression, development of unacceptable toxicity, request to discontinue, withdrawal of consent, or loss to follow-up. Upon disease progression, patients were followed for survival and PFS after the next line of treatment until data cutoff for the primary analysis, which occurred when the final patient enrolled had completed the week-24 tumor assessment.
The randomization phase of this study consisted of the treatment period and the follow-up period. During the treatment period, patients received lenvatinib orally at a starting dose of 24 mg/day or 18 mg/day. Dose modification for AEs for all patients followed the study protocol. Dose-reduction steps for patients in the LEN24 arm were 20 mg, 14 mg, 10 mg, and 8 mg per day. In the LEN18 arm, dose reductions were performed to 14 mg, 10 mg, 8 mg, and 4 mg per day. The follow-up period began immediately after the off-treatment visit and continued until patient death, withdrawal of consent, or the data cutoff for the primary analysis. Patients were followed every 12 weeks (±1 week) during the follow-up period and monitored for survival, PFS after the next line of treatment, and all anticancer treatments received.
Patients
Eligible patients were ≥18 years of age, had an ECOG PS of ≤2, 1 or no prior vascular endothelial growth factor (VEGF)/VEGF receptor–targeted therapy, adequate organ function, and a histologically or cytologically confirmed diagnosis of RR-DTC, with both evidence of disease progression within 13 months before providing informed consent and measurable disease assessed by Response Evaluation Criteria In Solid Tumors version 1.1 (RECIST v1.1) confirmed by central radiographic review. A complete list of the inclusion and exclusion criteria can be found in the trial listing on www.clinicaltrials.gov (NCT02702388).
Written informed consent was provided by all patients before undergoing any study-specific procedures. These practices were designed to ensure adherence to Good Clinical Practice Guidelines and the principles of the World Medical Association Declaration of Helsinki.
Study Endpoints
The primary efficacy endpoint was ORRwk24, and the primary safety endpoint was treatment-emergent AEs (TEAEs) of grade ≥3 in the first 24 weeks after randomization. ORRwk24 was selected as the primary endpoint because it better predicts PFS and allows a sample size and study duration with a defined cutoff not constrained by accumulation of events. SELECT data showed that pronounced tumor responses to lenvatinib in patients with RR-DTC typically occur within 8 weeks (17). Specifically, the median time to first objective response was 2.0 months (95% CI 1.9, 3.5), and a rapid initial decline in tumor size (median decrease of 25%) was seen by the time of the first radiological tumor assessment at 8 weeks. Key secondary endpoints of this study included PFS, safety, and tolerability. Exploratory endpoints included overall survival (OS). Tumor responses were assessed by investigator per RECIST v1.1. All AEs were recorded and were reported using Common Terminology Criteria for Adverse Events (v4.03) grades.
Statistics
Efficacy analyses were conducted using the full analysis set, which included all randomly assigned patients. The noninferiority analysis comparing ORRwk24 between the LEN24 and LEN18 arms was performed with a 1-sided alpha of 0.025, based on the calculated odds ratio of ORRwk24 response (18 mg vs 24 mg) along with its 95% CI using the Cochran-Mantel-Haenszel method, stratified by the randomization stratification factors. The test was performed per the 95% CI using the noninferiority margin of 0.4. Overall ORR was analyzed as a sensitivity analysis according to the same approach for ORRwk24. The odds ratio and 95% CIs between treatment groups were further analyzed in forest plots by subgroups defined by the randomization stratification factors: age (≤65 vs >65 years) and ECOG PS (0 vs 1 or 2). Additional subgroups included sex, race, prior VEGF-targeted therapy (0 vs 1), region, histology, baseline thyroid-stimulating hormone level, baseline weight group (≤60 vs >60 kg), prior anticancer radiotherapy (yes vs no), and prior anticancer medication (yes vs no).
A best overall response of stable disease was required to be at least 7 weeks following randomization. Durable stable disease was defined as the duration of stable disease ≥23 weeks after randomization. Disease control rate (defined as stable disease + complete response + partial response), and clinical benefit rate (defined as complete response + partial response + durable stable disease), and the corresponding 2-sided 95% CIs were calculated by treatment group. Treatment differences (percentage-point difference) for the LEN24 vs LEN18 arms were summarized along with the corresponding 95% CIs based on the normal approximation.
Duration of response was defined for responders as the time from the date of first documented response until date of documented progression or death in the absence of disease progression, with the end of response coinciding with the date of progression or death from any cause used for the PFS endpoint. Duration of response was censored at 28 days after treatment end date. The time to first objective response was defined as the time from randomization to the first documentation of a response of partial response or complete response. Medians of duration of response and time to response were summarized using Kaplan-Meier product-limit estimates for each treatment group and were presented with 2-sided 95% CIs.
PFS was defined as the time from the date of randomization to the date of first documentation of disease progression or date of death, whichever occurred first. Patients were censored 28 days after treatment end date. The stratified log-rank test, using ECOG PS and age group as strata, was used to compare differences in PFS as assessed by investigator between the LEN24 and LEN18 arms. The hazard ratios and 95% CIs for the treatment comparisons were estimated by stratified Cox regression including treatment as a factor and ECOG PS and age group as strata. The Kaplan-Meier product-limit estimate for each treatment group was reported and plotted over time. OS was analyzed in a similar manner to PFS.
All safety analyses were performed on the safety analysis set, which included all patients who were randomly assigned and received at least 1 dose of study drug. For the analysis of the primary safety endpoint, the frequency (number and percentage) of TEAEs with Common Terminology Criteria for Adverse Events (v4.03) grade ≥3 were summarized by treatment group; the difference in the percentage between the treatment arms was presented with a 95% CI using asymptotic normal approximation. AEs and serious AEs, laboratory test results, other safety assessments, and their changes from baseline were summarized using descriptive statistics. Time to treatment discontinuation because of an AE, number of dose reductions, and time to first dose reduction were summarized.
Results
Patient Disposition and Baseline Characteristics
Patient baseline characteristics are shown in Table 1. Of the 152 patients, 75 were randomly assigned to the LEN24 arm, and 77 were randomly assigned to the LEN18 arm. The overall median age was 65.5 years (range 21-92 years). ECOG PS was generally similar between the arms, whereas more patients in the LEN18 arm (n = 25, 32.5%) had received prior VEGF-targeted therapy vs patients in the LEN24 arm (n = 14, 18.7%).
Table 1.
Demographic and other baseline characteristics
| Parameter | Lenvatinib starting dose/day | |
|---|---|---|
| 24 mg (n = 75) | 18 mg (n = 77) | |
| Median age, years | 65.0 | 66.0 |
| Range | (36-92) | (21-89) |
| Sex, male, n (%) | 41 (54.7) | 37 (48.1) |
| ECOG performance status, n (%) | ||
| 0 | 44 (58.7) | 45 (58.4) |
| 1 | 31 (41.3) | 29 (37.7) |
| 2 | 0 | 3 (3.9) |
| TSH ≤ 0.5 (µIU/mL), n (%) | 69 (92.0) | 71 (92.2) |
| Geographic region, n (%) | ||
| Europe | 15 (20.0) | 27 (35.1) |
| North America | 36 (48.0) | 33 (42.9) |
| Othera | 24 (32.0) | 17 (22.1) |
| DTC subtype, n (%) | ||
| Papillary | 63 (84.0) | 58 (75.3) |
| Follicular | 12 (16.0) | 19 (24.7) |
| Locally advanced DTC, n (%) | 1 (1.3) | 0 |
| Metastatic DTC, n (%) | 74 (98.7) | 77 (100) |
| Prior VEGF-targeted therapies, n (%) | ||
| 0 | 61 (81.3) | 52 (67.5) |
| 1 | 14 (18.7) | 25 (32.5) |
| Sorafenibb | 11 (14.7) | 13 (16.9) |
| Pazopanibb | 0 | 7 (9.1) |
| Cabozantinibb | 2 (2.7) | 2 (2.6) |
| Vandetanibb | 1 (1.3) | 1 (1.3) |
| Prior therapy, n (%) | ||
| Anticancer medicationsc | 21 (28.0) | 28 (36.4) |
| Radiotherapy | 22 (29.3) | 35 (45.5) |
| Radioiodine therapyd | 74 (98.7) | 75 (97.4) |
| Antithyroid cancer surgerye | 75 (100) | 76 (98.7) |
Abbreviations: 131I, radioiodine; DTC, differentiated thyroid cancer; ECOG, Eastern Cooperative Oncology Group; RECIST, Response Evaluation Criteria in Solid Tumors; TSH, thyroid-stimulating hormone; VEGF, vascular endothelial growth factor.
aIncludes patients from Republic of Korea (n = 20) and Russian Federation (n = 21). Australia (n = 5) is included in North America.
bVEGF-targeted therapies administered to ≥2 patients. Patients could be included in more than 1 category.
cIncludes but is not limited to VEGF-targeted therapy and cytotoxic chemotherapy. It does not include prior radioiodine therapy.
dThere were 3 patients in the study who apparently did not receive prior radioiodine therapy. In each of these patients, there was no uptake on 131I scan, but there was disease progression by RECIST v1.1. These patients met the inclusion criterion “Subjects must be 131I-refractory/resistant as defined by at least 1 of the following: (a) one or more measurable lesions that does/do not demonstrate iodine uptake on any radioiodine scan.”
eIncludes thyroid adenoma removal, thyroid cystectomy, thyroid nodule removal, thyroid operation, and thyroidectomy.
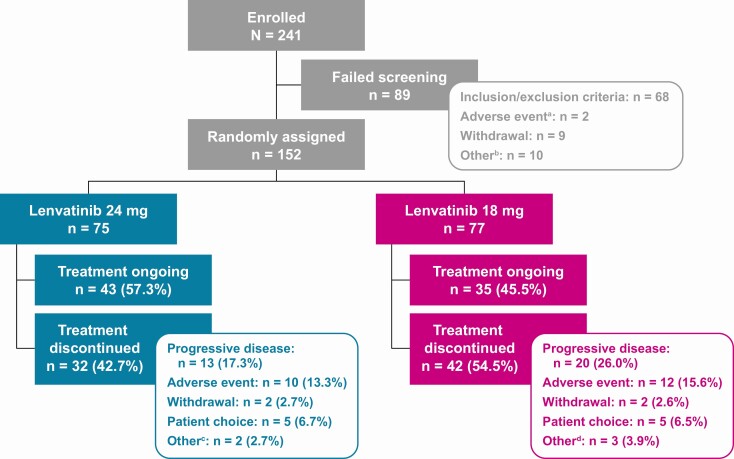
At the data cutoff date (December 12, 2019), 43 patients (57.3%) in the LEN24 arm and 35 patients (45.5%) in the LEN18 arm had treatment ongoing; 13 patients (17.3%) in the LEN24 arm and 20 patients (26.0%) in the LEN18 arm had discontinued the study drug because of disease progression (Fig. 1). Equal numbers of patients in both arms discontinued treatment due to withdrawal of consent (n = 2, each arm) or patient choice (n = 5, each arm) (Fig. 1).
Figure 1.
Patient enrollment, randomization, and treatment. aOf the 2 patients who failed screening due to an adverse event, both had serious adverse events requiring hospitalization (dyspnea and increasing cancer bone pain; pathologic femoral shaft fracture and a traumatic radius fracture). bOther reasons for failing screening were exceeding the screening window (n = 9) and patient decision (n = 1). cOther reasons for treatment discontinuation were clinical disease progression (n = 2). dOther reasons for treatment discontinuation were clinical disease progression (n = 1), sponsor decision (n = 1), and prohibited anticancer treatment (n = 1).
Efficacy
As of week 24, patients in the LEN24 arm had an ORR of 57.3% (95% CI 46.1, 68.5) compared to an ORR of 40.3% (95% CI 29.3, 51.2) in the LEN18 arm (Table 2). The difference between treatment arms, using the LEN24 arm as the control, was −17.1% (95% CI −32.7, −1.4) with an odds ratio of 0.50 (95% CI 0.26, 0.96). The lower limit of the CI of the odds ratio was lower than the predefined noninferiority margin of 0.4; therefore, noninferiority of LEN18 compared to LEN24 was not met. Overall ORR was similar to ORRwk24 for both groups (Table 2).
Table 2.
Summary of tumor responses as assessed by investigator using RECIST v1.1
| Tumor responses | Lenvatinib starting dose/day | |
|---|---|---|
| 24 mg (n = 75) | 18 mg (n = 77) | |
| Week 24 | ||
| Best overall response, % (n) | ||
| CR | 0 | 0 |
| PR | 57.3 (43) | 40.3 (31) |
| SDa | 36.0 (27) | 46.8 (36) |
| PD | 2.7 (2) | 5.2 (4) |
| Not evaluable | 4.0 (3) | 7.8 (6) |
| Objective response rate, CR + PR, % (n) [95% CI] | 57.3 (43) [46.1, 68.5] | 40.3 (31) [29.3, 51.2] |
| Difference (18 mg − 24 mg), % (95% CI) | −17.1 (−32.7, −1.4) | |
| Odds ratio (18 mg/24 mg) (95% CI) | 0.50 (0.26, 0.96) | |
| Tumor responses, overall | ||
| Best overall response, % (n) | ||
| CR | 0 | 0 |
| PR | 64.0 (48) | 46.8 (36) |
| SDa | 29.3 (22) | 40.3 (31) |
| Durable SDb | 20.0 (15) | 27.3 (21) |
| PD | 2.7 (2) | 5.2 (4) |
| Not evaluable | 4.0 (3) | 7.8 (6) |
| Objective response rate (CR + PR), % (n) [95% CI] | 64.0 (48) [53.1, 74.9] | 46.8 (36) [35.6, 57.9] |
| Difference (18 mg − 24 mg), % (95% CI) | −17.2 (−32.8, −1.7) | |
| Odds ratio (18 mg/24 mg) (95% CI) | 0.50 (0.26, 0.95) | |
| Clinical benefit rate (CR + PR + durable SD), % (n) [95% CI] | 84.0 (63) [75.7, 92.3] | 74.0 (57) [64.2, 83.8] |
| Disease control rate (CR + PR + SD), % (n) [95% CI] | 93.3 (70) [87.7, 99.0] | 87.0 (67) [79.5, 94.5] |
| Time to first objective response, months, median (95% CI) | 3.7 (2.0, 3.9) | 5.8 (3.8, 18.3) |
| Duration of response,c months, median (95% CI) | NE (18.4, NE) | 20.8 (15.1, NE) |
Abbreviations: CR, complete response; NE, not estimable; PD, progressive disease; PR, partial response; RECIST v1.1, Response Evaluation Criteria In Solid Tumors version 1.1; SD, stable disease.
aStable disease is defined as 7 or more weeks after randomization.
bDurable SD is defined as SD for ≥23 weeks.
cAmong patients who had an objective response: lenvatinib 24-mg arm n = 48, lenvatinib 18-mg arm n = 36.
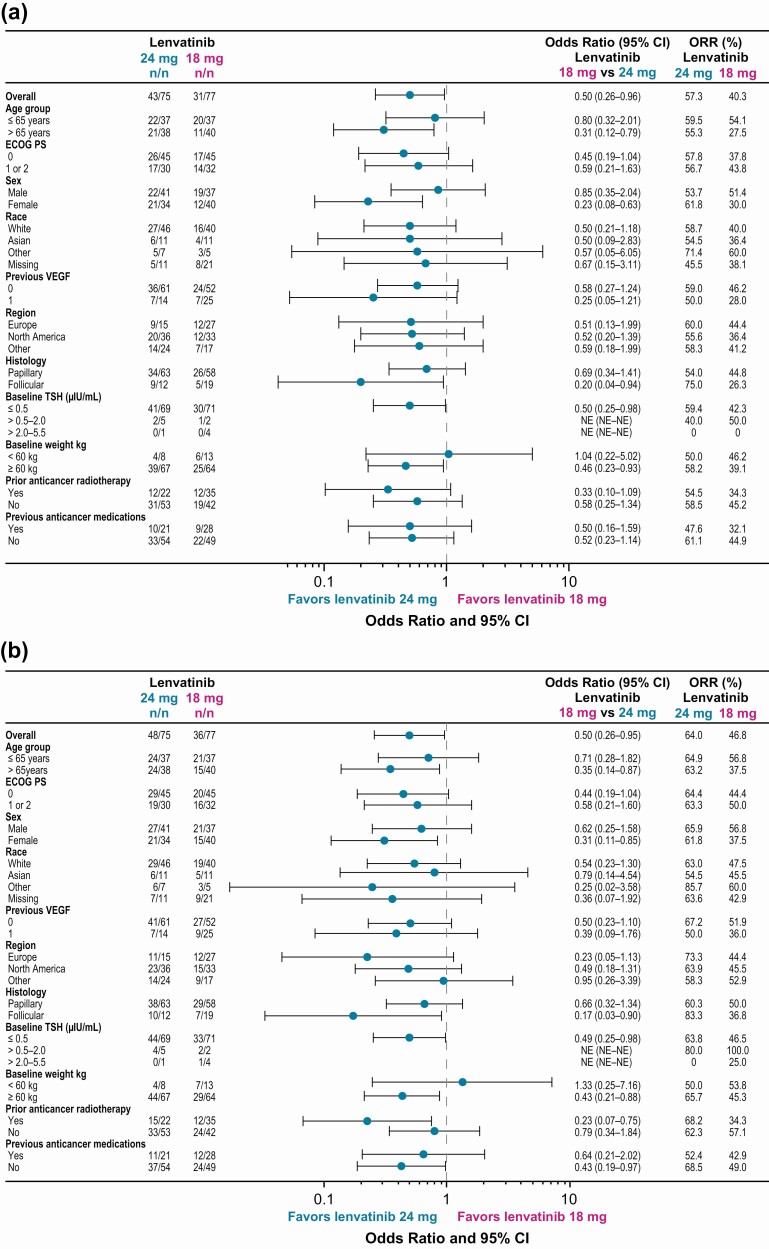
Among patients with an objective response, median duration of response was not reached [95% CI 18.4, not estimable (NE)] in the LEN24 arm (n = 48) and 20.8 months (95% CI 15.1, NE) in the LEN18 arm (n = 36). The disease control rate was 93.3% in the LEN24 arm and 87.0% in the LEN18 arm. ORRwk24 and overall ORR according to baseline characteristics are shown in Figure 2. Subgroup analyses of ORR favored (numerically) the LEN24 arm regardless of exposure to prior VEGF-targeted therapy as of week 24 (Fig. 2A) and overall (Fig. 2B).
Figure 2.
Forest plot of objective response rate by baseline characteristics (investigator assessment per RECIST v1.1) as of week 24 (A) and overall (B). Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; NE, not estimable; ORR, objective response rate; RECIST v1.1, Response Evaluation Criteria In Solid Tumors version 1.1; TSH, thyroid-stimulating hormone; VEGF, vascular endothelial growth factor.
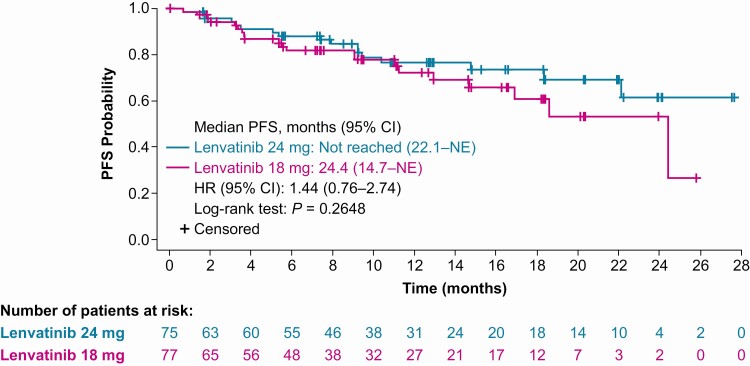
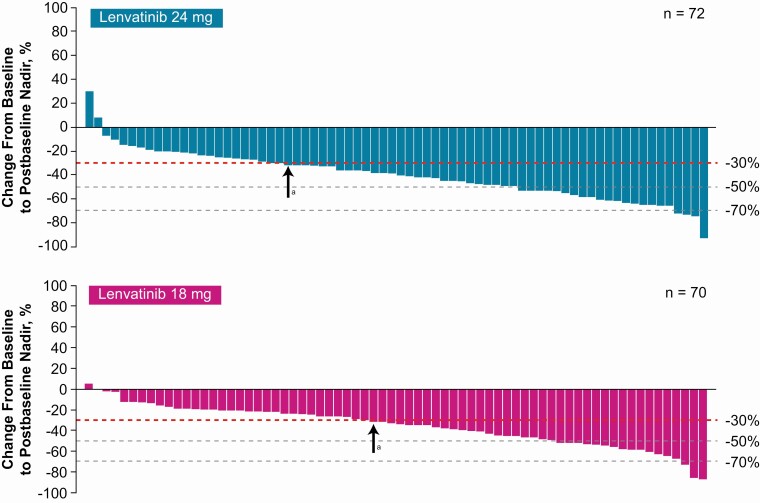
Median PFS was not reached in the LEN24 arm (95% CI 22.1, NE) and was 24.4 months in the LEN18 arm (95% CI 14.7, NE) (Fig. 3). Median duration of follow-up for PFS was 12.8 months (95% CI 10.8, 15.3) in the LEN24 arm and 11.2 months (95% CI 7.5, 14.6) in the LEN18 arm. Most patients experienced a decrease in size of target lesions (Fig. 4). Median OS was not reached in either treatment arm. At 12 months, the OS rate was 90.0% (95% CI 80.0, 95.1) in the LEN24 arm and 86.5% (95% CI 75.3, 92.8) in the LEN18 arm. Median duration of follow-up for OS was 15.5 months (95% CI 13.0, 19.4) in the LEN24 arm and 14.6 months (95% CI 12.2, 17.6) in the LEN18 arm.
Figure 3.
Kaplan-Meier plot of PFS as assessed by investigator using RECIST v1.1. Abbreviations: HR, hazard ratio; NE, not estimable; PFS, progression-free survival; RECIST v1.1, Response Evaluation Criteria In Solid Tumors version 1.1.
Figure 4.
Percentage changes in the sums of diameters of target lesions from baseline to postbaseline nadir (by investigator using RECIST v1.1). Abbreviation: RECIST v1.1, Response Evaluation Criteria In Solid Tumors version 1.1. aPatients to the right of the arrow achieved at least a 30% reduction of target lesions.
Safety
The primary safety endpoint demonstrated that, as of week 24, incidences of grade ≥3 severity TEAEs were similar between arms. There was a decreased incidence of grade ≥3 TEAEs of 4.2% (95% CI −19.8, 11.4) in the LEN18 arm (n = 44, 57.1%) compared to patients in the LEN24 arm (n = 46, 61.3%) (Table 3). The most common TEAEs of grade ≥3 as of week 24 were (LEN24; LEN18) hypertension (n = 19, 25.3%; n = 15, 19.5%), proteinuria (n = 5, 6.7%; n = 4, 5.2%), and asthenia (n = 2, 2.7%; n = 4, 5.2%) (Table 3). The most common overall TEAEs are listed in Table 4.
Table 3.
Summary of TEAEs
| Parameter | Lenvatinib starting dose/day | |
|---|---|---|
| 24 mg (n = 75) | 18 mg (n = 77) | |
| TEAEs as of week 24 | ||
| Patients with grade ≥3 severity TEAEs as of week 24, % (n) | 61.3 (46) | 57.1 (44) |
| Difference [18 mg − 24 mg], % (95% CI) | −4.2 (−19.8, 11.4) | |
| Most common grade ≥3 TEAEs (≥2%) as of week 24, % (n) | ||
| Hypertension | 25.3 (19) | 19.5 (15) |
| Proteinuria | 6.7 (5) | 5.2 (4) |
| Asthenia | 2.7 (2) | 5.2 (4) |
| Diarrhea | 2.7 (2) | 2.6 (2) |
| Hyponatremia | 1.3 (1) | 3.9 (3) |
| Increased lipase | 2.7 (2) | 2.6 (2) |
| Myalgia | 1.3 (1) | 3.9 (3) |
| Stomatitis | 2.7 (2) | 2.6 (2) |
| Vomiting | 2.7 (2) | 2.6 (2) |
| TEAEs overall, % (n) | ||
| Patients with any TEAEs | 100 (75) | 97.4 (75) |
| Patients with TEAE worst grade of | ||
| 2 (intolerable) | 13.3 (10) | 13.0 (10) |
| 3 | 65.3 (49) | 59.7 (46) |
| 4 | 2.7 (2) | 7.8 (6) |
| 5 | 8.0 (6) | 3.9 (3) |
| ≥3 | 76.0 (57) | 71.4 (55) |
| Patients with serious TEAEs | 33.3 (25) | 40.3 (31) |
| Fatal | 8.0 (6) | 3.9 (3) |
| Nonfatal | 30.7 (23) | 39.0 (30) |
| Patients with TEAEs leading to | ||
| Dose discontinuation | 14.7 (11) | 16.9 (13) |
| Dose reduction | 69.3 (52) | 59.7 (46) |
| Dose interruption | 64.0 (48) | 66.2 (51) |
| Dose reduction or interruption | 82.7 (62) | 80.5 (62) |
| Patients with any treatment-related TEAEs | 98.7 (74) | 93.5 (72) |
| Patients with related TEAEs of grade ≥ 3 | 68.0 (51) | 57.1 (44) |
| Patients with related TEAEs leading to | ||
| Dose discontinuation | 9.3 (7) | 13.0 (10) |
| Dose reduction | 69.3 (52) | 58.4 (45) |
| Dose interruption | 60.0 (45) | 55.8 (43) |
| Dose reduction or interruption | 80.0 (60) | 72.7 (56) |
Abbreviation: TEAE, treatment-emergent adverse event.
Table 4.
Most common TEAEs (≥25%) overall
| Preferred term, % (n) | Lenvatinib starting dose | |
|---|---|---|
| 24 mg (n = 75) | 18 mg (n = 77) | |
| Hypertension | 57.3 (43) | 51.9 (40) |
| Diarrhea | 56.0 (42) | 51.9 (40) |
| Weight decreased | 36.0 (27) | 42.9 (33) |
| Fatigue | 40.0 (30) | 35.1 (27) |
| Nausea | 40.0 (30) | 35.1 (27) |
| Proteinuria | 44.0 (33) | 31.2 (24) |
| Arthralgia | 38.7 (29) | 26.0 (20) |
| Palmar-plantar erythrodysesthesia syndrome | 34.7 (26) | 28.6 (22) |
| Decreased appetite | 34.7 (26) | 27.3 (21) |
| Asthenia | 21.3 (16) | 28.6 (22) |
| Stomatitis | 21.3 (16) | 28.6 (22) |
Abbreviation: TEAE, treatment-emergent adverse event.
Among patients in the LEN24 and LEN18 arms, TEAEs led to dose interruption in 48 (64.0%) and 51 (66.2%) patients, respectively; dose reduction in 52 (69.3%) and 46 (59.7%) patients, respectively; and treatment discontinuation in 11 (14.7%) and 13 (16.9%) patients, respectively. The median time to first dose reduction (among all patients including censors) was 15.3 weeks (95% CI 12.1, 20.1) in the LEN24 arm and 24.1 weeks (95% CI 11.1, 35.9) in the LEN18 arm. Treatment-related TEAEs that led to dose modifications are shown in Table 3.
Overall, 9/152 (5.9%) patients experienced a fatal TEAE; 6/75 (8.0%) patients in the LEN24 arm and 3/77 (3.9%) patients in the LEN18 arm (Table 3). Of these, 1 TEAE was considered possibly related to study treatment by the investigator (sudden death of unknown cause in the LEN24 arm). The other fatalities were ascribed to disease progression and TEAEs deemed not related to lenvatinib treatment (subcutaneous emphysema, septic shock, and malignant pleural effusion).
Treatment Exposure
The median overall daily dose intensity per patient was 18.7 mg/day for patients in the LEN24 arm and 15.4 mg/day for patients in the LEN18 arm. The number of patient-months for patients in the LEN24 and LEN18 arms were 928.2 and 792.9, respectively. The maximum durations of dose interruptions were generally similar between the dosing arms (Table 5).
Table 5.
Dose interruption details
| Parameter, % (n) | Lenvatinib starting dose/day | |
|---|---|---|
| 24 mg (n = 75) | 18 mg (n = 77) | |
| Number of dose interruptions | ||
| 1 | 13.3 (10) | 24.7 (19) |
| 2 | 16.0 (12) | 16.9 (13) |
| 3 | 10.7 (8) | 7.8 (6) |
| ≥4 | 34.7 (26) | 24.7 (19) |
| Maximum interruption duration in days | ||
| 1 | 4.0 (3) | 2.6 (2) |
| 2-3 | 4.0 (3) | 6.5 (5) |
| 4-7 | 10.7 (8) | 15.6 (12) |
| 8-14 | 25.3 (19) | 20.8 (16) |
| 15-28 | 22.7 (17) | 14.3 (11) |
| >28 | 8.0 (6) | 14.3 (11) |
Values are based on drug exposure data.
Discussion
Lenvatinib has previously demonstrated efficacy in the treatment of patients with RR-DTC (12) and is approved for locally recurrent or metastatic progressive RR-DTC (13,18,19). However, AEs are commonly seen and frequently lead to dose modifications or discontinuations (20). For this reason, it has been proposed that a lower starting dose of lenvatinib might result in reduced toxicity in patients with RR-DTC without compromising efficacy. Hence, this study compared, in a double-blinded fashion, LEN18 with the approved LEN24 in patients with RR-DTC to see whether the lower starting dose was able to maintain the same efficacy while conferring less toxicity.
The study did not demonstrate noninferiority of LEN18 compared to the approved LEN24 for RR-DTC (odds ratio 0.5; 95% CI 0.26, 0.96). The 17% difference in ORRwk24 and overall ORR suggests that the LEN24 arm provides a clinically relevant difference compared to the LEN18 arm. When baseline characteristic subgroup analyses were conducted, ORRwk24 and overall ORR numerically favored the LEN24 arm over the LEN18 arm in every group assessed, with the exception of baseline bodyweight <60 kg (odds ratio as of week 24: 1.04; 95% CI 0.22, 5.02). Results of the subgroup analyses should be interpreted with an appropriate level of caution due to small sample sizes and small numbers of events in each subgroup category. Further, although this analysis was not powered to assess PFS and the study design included a cutoff leading to censoring for PFS when the last enrolled patient had their week 24 assessments, PFS for the LEN24 arm was numerically higher than the LEN18 arm to a clinically relevant magnitude (Fig. 3). As such, LEN18 did not demonstrate comparable efficacy to LEN24, and therefore a lower starting dose of lenvatinib may compromise treatment efficacy.
The primary safety endpoint demonstrated that the incidences of grade ≥3 severity TEAEs up to week 24 were similar in the LEN24 and LEN18 arms (61.3% vs 57.1%, respectively; a difference of 4.2%); moreover, the LEN18 arm did not have a better safety profile overall. As expected, the median time to first dose reduction in all censored patients was shorter in the LEN24 arm (15.3 weeks) compared to the LEN18 arm (24.1 weeks). Overall, the safety profile in this analysis was comparable between arms and consistent with the known safety profile of lenvatinib monotherapy (12,21). There were no unexpected toxicities, and most TEAEs were managed with dose modifications and supportive therapy.
Although caution should be used in comparing clinical trials, results from the LEN24 arm of this study are consistent with those from the global Phase 3 SELECT (12). In SELECT, the ORR as confirmed by independent radiologic review using RECIST v1.1 was 64.8% in patients who received LEN24. This rate is similar to the overall ORR (64.0%), as assessed by investigators using RECIST v1.1, seen in patients in the LEN24 arm of this study.
Similarly, the safety profiles were comparable between the LEN24 arm in the current study and SELECT. Specifically, most patients in both studies experienced a treatment-related AE (LEN24 arm of this study, 98.7%; SELECT, 97.3%), whereas somewhat fewer experienced a treatment-related TEAE of grade ≥3 severity in this study (LEN24 arm, 68.0%; SELECT, 75.9%). Notably, as of week 24 in this analysis in the LEN24 arm, the grade ≥3 TEAE of hypertension occurred in 25.3% of patients, compared to the 41.8% incidence of the grade ≥3 treatment-related AE of hypertension in SELECT. Lenvatinib discontinuations for the primary reason of an AE occurred at similar rates between the 2 studies (LEN24 arm, 13.3%; SELECT, 14.2%). Additionally, lenvatinib dose reduction rates due to TEAEs (LEN24 arm, 69.3%; SELECT, 67.8%) were also comparable between the studies, whereas the rates of lenvatinib interruption due to TEAEs (LEN24 arm, 64.0%; SELECT, 82.4%) were somewhat higher within SELECT. Improvements in treatment-related TEAEs of grade ≥3 severity (especially hypertension) and dose interruption rates (due to TEAEs) seen in the current study are most likely because of the increased experience of clinicians with lenvatinib in the 5 years since its approval and their improved ability to anticipate TEAEs (ie, changes in blood pressure) and manage toxicity earlier. The double-blinded nature of this study ensured that physicians were able to evaluate, grade, and treat the toxicities for patients in both treatment arms without bias. Moreover, patients were not biased to the type or severity of toxicities they experienced. This key aspect of this study deserves to be emphasized because lack of bias in TEAE assessment adds weight to the findings.
A limitation of this study is that it was not powered for PFS or OS because of sample size limitations and because all follow-up ended when the last patient enrolled had reached week 24. Despite these limitations, a trend was seen for improved PFS in the LEN24 arm compared to the LEN18 arm. This study was also limited because patients generally had good performance status and were in better overall health than some patients with RR-DTC seen in clinical practice. For optimal management, patients should be monitored closely and frequently after initiation of treatment.
Additionally, although patients in this study were not stratified by prior VEGF-targeted therapy and there were more patients who had received prior VEGF-targeted therapy in the LEN18 arm (32.5%) compared to the LEN24 arm (18.7%), a subgroup analysis indicated that LEN24 may result in improved ORR irrespective of prior VEGF-targeted treatment, although this did not reach statistical significance (Fig. 2).
Determining the correct starting dose for cancer treatment is challenging because of the balance between maximizing efficacy while minimizing toxicity. Historically in oncology, higher dose intensity is thought to be associated with better outcomes. With development of newer targeted treatments and immunotherapies where maximum tolerated doses may greatly exceed complete inhibition of biologic targets, it has been suggested that dosing should be continually evaluated throughout drug development and that doses lower than the maximum tolerated dose should be considered (22). Across oncology, use of objective nonbiased data has been relied on as the cornerstone of optimal therapy for cancer patients. Because patients seen in clinical practice do not need to meet inclusion or exclusion criteria required of patients included in clinical trials, patients in practice often begin treatment with differences in baseline health. Application of the clinical data always relies on physician discretion to compare the patient characteristics against the population studied in a clinical trial and make adjustments where appropriate. While patients may have preferences for particular anticancer medications or doses, these are not commonly taken into consideration in oncology practices. Clinicians need to make dosing decisions based on the health and needs of their patients. However, because the choice of starting dose is subject to prescriber bias, clear, unbiased data are needed. Thus, our goal in conducting this study in a randomized, blinded fashion was to remove both physician and patient bias from the choice of starting dose of lenvatinib. The results of this study indicate that starting patients at 24 mg/day, with dose reductions as early and as frequently as necessary, is important for optimizing lenvatinib treatment. Specifically, the findings from this study support the use of the approved starting dose of lenvatinib 24 mg/day in patients with RR-DTC, with dose adjustments as tolerated, to obtain maximum clinical benefit.
Acknowledgments
Financial Support: This study was funded by Eisai Inc., Woodcliff Lake, NJ, USA, and by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Medical writing support was provided by Heather A. Mitchell, PhD, Oxford PharmaGenesis Inc., Newtown, PA, USA, and was funded by Eisai Inc., Woodcliff Lake, NJ, USA, and also Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Author Contributions: Study design: T.A.B., C.D., and M.S.B. Data acquisition: M.H.T., A.G., B.K., C.D.L.F., I.R., M.K.K., Y.J.P., B.H., Y.P., M.S.B., and S.L. Data analysis and interpretation: all authors. Data verification: R.X. and T.A.B. Drafting the manuscript, technical review and/or revision, review of the final draft, and approval to submit: all authors.
Clinical Trial Information: ClinicalTrials.gov identifier: NCT02702388.
Previous Presentation: These data were presented in part at the European Society for Medical Oncology Asia Virtual Congress, November 20-22, 2020 (Abstract no. 426P).
Additional Information
Disclosures: M.S.B.: University of Pennsylvania School of Medicine has received grant money for the conduct of this trial. M.S.B. has received honoraria and consulting fees from Eisai, Bayer, Lilly, Loxo, and Blueprint Medicines. Y.P.: Nothing to disclose. B.K.: Research funding (all funding to institution): Eisai, Merck, Bristol Myers Squibb, Xencor, and Eli Lilly & Co. C.D.L.F.: Honoraria: Eisai, Roche, Servier, Amgen, Bayer, Pierre Fabre Oncologie, and Bristol Myers Squibb; nonfinancial support: Roche, Servier, Amgen, Bayer, Pierre Fabre Oncologie, and Bristol Myers Squibb. B.G.M.H.: Advisory board member for Eisai, MSD, Bristol Myers Squibb, Roche, AZ, Pfizer, Boehringer Ingelheim, and Takeda. A.G.G.: Eisai and Blueprint Medicine advisory board participant. Y.J.P.: Nothing to declare. I.R.: Honoraria: Eisai, Bristol Myers Squibb, and Merck Serono. M.K.K.: Research funding (institution): Eisai, Exelixis, Ipsen; honoraria or consulting fees to M.K.K.: Eisai, Bayer, and Lilly. S.L.: Research funding (institution) from Novartis and Sanofi Genzyme; advisory board member for Eisai, Bayer, and Lilly. T.A.B.: Former employee (and current consultant) of Eisai Inc. C.D.: Employee of Eisai Inc. R.X.: Employee of Eisai Inc. M.H.T.: Consulting/advisory board member (honoraria paid): Bristol Myers Squibb, Eisai Inc, Novartis, Bayer, Sanofi/Genzyme, Array Biopharma, LOXO Oncology, Blueprint Medicines, and Arqule; speakers’ bureau (honoraria paid): Bristol Myers Squibb and Eisai Inc; research funding (all funding to institution): Bristol Myers Squibb, Merck Sharp & Dohme Corp, Pharmacyclics, AstraZeneca, Eisai, Incyte, EMD Serono, Novartis, Seattle Genetics, AbbVie, Genentech, Eli Lilly, Roche, Acerta Pharma, Genzyme Corporation, and Pfizer.
Data Availability
The source data for this article are considered both commercially confidential and HIPAA confidential; therefore, the data holders (Eisai) do not intend to publicly post or share the source data. Eisai may, however, consider requests on a case-by-case basis if contacted by an individual researcher (access will be permitted after signature of a data-access agreement).
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. [DOI] [PubMed] [Google Scholar]
- 2. Cabanillas ME, Habra MA. Lenvatinib: role in thyroid cancer and other solid tumors. Cancer Treat Rev. 2016;42:47-55. [DOI] [PubMed] [Google Scholar]
- 3. Eustatia-Rutten CF, Corssmit EP, Biermasz NR, Pereira AM, Romijn JA, Smit JW. Survival and death causes in differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2006;91(1):313-319. [DOI] [PubMed] [Google Scholar]
- 4. Araque KA, Gubbi S, Klubo-Gwiezdzinska J. Updates on the management of thyroid cancer. Horm Metab Res. 2020;52(8):562-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fleeman N, Houten R, Chaplin M, et al. A systematic review of lenvatinib and sorafenib for treating progressive, locally advanced or metastatic, differentiated thyroid cancer after treatment with radioactive iodine. BMC Cancer. 2019;19(1):1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Busaidy NL, Cabanillas ME. Differentiated thyroid cancer: management of patients with radioiodine nonresponsive disease. J Thyroid Res. 2012;2012:618985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brose MS, Nutting CM, Jarzab B, et al. ; DECISION investigators . Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, Phase 3 trial. Lancet. 2014;384(9940):319-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matsui J, Funahashi Y, Uenaka T, Watanabe T, Tsuruoka A, Asada M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res. 2008;14(17):5459-5465. [DOI] [PubMed] [Google Scholar]
- 9. Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122(3):664-671. [DOI] [PubMed] [Google Scholar]
- 10. Tohyama O, Matsui J, Kodama K, et al. Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014;2014:638747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yamamoto Y, Matsui J, Matsushima T, et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell. 2014;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372(7):621-630. [DOI] [PubMed] [Google Scholar]
- 13. Lenvima (lenvatinib) [prescribing information]. Eisai Inc; 2020. [Google Scholar]
- 14. Locati LD, Piovesan A, Durante C, et al. Real-world efficacy and safety of lenvatinib: data from a compassionate use in the treatment of radioactive iodine-refractory differentiated thyroid cancer patients in Italy. Eur J Cancer. 2019;118:35-40. [DOI] [PubMed] [Google Scholar]
- 15. Kim SY, Kim SM, Chang H, et al. Safety of tyrosine kinase inhibitors in patients with differentiated thyroid cancer: real-world use of lenvatinib and sorafenib in Korea. Front Endocrinol. 2019;10:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hayato S, Shumaker R, Ferry J, Binder T, Dutcus CE, Hussein Z. Exposure-response analysis and simulation of lenvatinib safety and efficacy in patients with radioiodine-refractory differentiated thyroid cancer. Cancer Chemother Pharmacol. 2018;82(6):971-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Robinson B, Schlumberger M, Wirth LJ, et al. Characterization of tumor size changes over time from the Phase 3 study of lenvatinib in thyroid cancer. J Clin Endocrinol Metab. 2016;101(11):4103-4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. NCCN Thyroid Carcinoma Panel. Thyroid carcinoma. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Version 1. 2021. Accessed June 2, 2021. https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf
- 19. Filetti S, Durante C, Hartl D, et al. ; ESMO Guidelines Committee. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(12):1856-1883. [DOI] [PubMed] [Google Scholar]
- 20. Hao Z, Wang P. Lenvatinib in management of solid tumors. Oncologist. 2020;25(2):e302-e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163-1173. [DOI] [PubMed] [Google Scholar]
- 22. Bullock JM, Rahman A, Liu Q. Lessons learned: dose selection of small molecule-targeted oncology drugs. Clin Cancer Res. 2016;22(11):2630-2638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The source data for this article are considered both commercially confidential and HIPAA confidential; therefore, the data holders (Eisai) do not intend to publicly post or share the source data. Eisai may, however, consider requests on a case-by-case basis if contacted by an individual researcher (access will be permitted after signature of a data-access agreement).