Abstract
Context
Neurotensin is associated with cardiometabolic diseases but its role with mortality risk in humans is unknown.
Objective
This work aims to examine the prediction of proneurotensin (Pro-NT) with respect to total and cause-specific mortality in a middle-aged cohort.
Methods
In the population-based middle-aged cohort (n = 4632; mean age, 57 years) of the Malmö Diet and Cancer Study, Pro-NT was assessed and total as well as cause-specific mortality was studied. Main cause of death was based on the International Classification of Diseases.
Results
During a mean follow-up of 20 ± 3 years, 950 men and 956 women died. There was significantly increased mortality risk in individuals belonging to the highest quartile (Q) of Pro-NT (Q4, Pro-NT ≥ 149 pmol/L) compared with Qs 1 to 3 (Pro-NT < 149 pmol/L), hazard ratio (HR), 95% CI of 1.29 (1.17-1.42; P < .001). Data were adjusted for sex and age. No significant interaction was observed between Pro-NT and sex on mortality risk. Individuals within Q4 vs Qs 1 to 3 had an HR of 1.41 (95% CI, 1.18-1.68; P < .001) for death due to cardiovascular disease (n = 595/4632); 2.53 (95% CI, 1.37-4.67; P = .003), due to digestive tract disease (n = 42/4632), 1.62 (95% CI, 1.04-2.52; P = .032) due to mental and behavioral disease (n = 90/4632); and 1.91 (95% CI, 1.15-3.19; P = .013) due to unspecific causes (n = 64/4632). There was no significant relationship between Pro-NT and deaths due to cancer, infections, neurological, or other causes. Adjustment for cardiovascular risk factors only marginally changed these results.
Conclusion
The relationship between Pro-NT and total mortality risk was mainly driven by cardiovascular mortality, but high Pro-NT also predicts death from digestive, mental, and behavioral disease and deaths attributed to unspecific causes.
Keywords: cardiovascular diseases, digestive tract disease, mortality, obesity, proneurotensin
The obesity pandemic crisis and its associated risks for cardiometabolic diseases like type 2 diabetes, nonalcoholic fatty liver disease (NAFLD), and cardiovascular diseases (CVDs) are rising worldwide (1). Life expectancy is substantially lower in people with cardiometabolic diseases and therefore early identification of individuals at high risk of obesity and its complications is critically important (2). More than two-thirds of obesity-related deaths are due to CVD (1). This disease burden of developing CVD (due to the presence of one or more risk factors such as hypertension, diabetes, hyperlipidemia, or already established but subclinical disease) requires strategies for early detection and management plans.
Neurotensin (NT) is a 13–amino acid peptide that is released immediately after ingestion of fatty meals, and it facilitates lipid digestion and fat absorption in the small intestines as well as hepatic fat accumulation (3-5). Because the mature hormone NT is unstable and rapidly broken down in the blood stream in vivo (6), we instead measured proneurotensin (Pro-NT), which is a stabile precursor molecule of NT, produced in equimolar amounts relative to NT from the small intestines (7). It serves as a robust surrogate marker for the intestinal release of NT. NT produces a wide range of physiological and pharmacological effects by binding to specific NT receptors, 3 of which are well described (8). In fact, Pro-NT also has affinity to and stimulates NT-receptor-1 (8). Apart from the peripheral endocrine actions of NT, it is produced inside, and is an important neurotransmitter within, the central nervous system (CNS) (9), although Pro-NT measured in plasma is primarily assumed to stem from the small intestine (9). Specifically the knockout mice deficient in receptor subtypes NTSR1 (10), NTSR2 (11), and NTSR3 (12) have been studied to examine the mechanisms of NT-mediated central and peripheral responses. In addition to NT’s direct effect on cardiac physiology, it acts as a hormone in the gastrointestinal tract where it modulates gastrointestinal motility and pancreaticobiliary secretions (13). Elevated Pro-NT promotes fat accumulation in the liver, which presents a novel biomarker for the prognosis of NAFLD and its complication nonalcoholic steatohepatitis (14).
In the CNS, Pro-NT acts as a neuromodulator with the dopaminergic system and has endogenous antipsychotic effects. It even interacts with several other systems in the CNS, including serotonergic, glutamatergic, cholinergic, and GABAergic systems (15). Even elevated Pro-NT has shown sex-specific associations in, for example, women with cognitive impairment (16).
Pro-NT was found to be a predictor of incident CVD and even predicted type 2 diabetes and CVD mortality in population-based studies (17-19). Furthermore, associations of circulating Pro-NT with impaired renal function and its relation to all-cause mortality in patients with end-stage kidney disease was recently reported (20).
Thus, previous research implicates Pro-NT in the pathophysiology of several diseases and its associations with all-cause (ACM) and CVD mortality (19-21). However, studies supporting associations of Pro-NT with cause-specific mortality (CSM), other than CVD, are still unclear and require further investigations.
In contrast with previous research evidence suggesting an important role of Pro-NT in obesity and cardiometabolic diseases, the aim of this population-based, cohort study is to assess the association of Pro-NT with CSM. Moreover, given indications that the association between Pro-NT and poor outcomes might be sex specific (18, 19), we also examined interactions between Pro-NT and sex with respect to mortality risk.
Materials and Methods
Study Design and Settings
The Malmö Diet and Cancer Study (MDC) cohort is a population-based prospective cohort from Malmö, Sweden, with the aim of studying the epidemiology of cardiometabolic and cancer diseases. In total 28 449 participants including men (born 1923-1945) and women (born 1923-1950) underwent baseline examinations between 1991 and 1996. About 6103 randomly selected individuals for the study of carotid artery disease and CVD participated in the cardiovascular cohort (MDC-CC) between 1991 and 1994. As fasting plasma samples were available for the analysis of Pro-NT in 4632 participants of MDC-CC, the remaining 1471 participants were excluded from this study because of lack of fasting plasma samples.
Participants, Exposures, and Outcomes
Participants underwent physical examinations and responded to an extensive questionnaire about occupation, previous medical conditions, medications, and dietary and lifestyle habits. Current smoking was defined by self-reporting of any use within the last year. Diabetes was defined as fasting whole blood glucose level greater than or equal to 6.1 mmol/L (corresponding to a fasting plasma glucose value of ≥ 7.0 mmol/L or a self-reported physician’s diagnosis of diabetes or use of antidiabetic medication (22). Adding glycated hemoglobin A1c greater than 6.5% as criterium for diabetes resulted in only one additional case of diabetes. Blood pressure (mm Hg) was measured by using an oscillometric device twice after 5 minutes of rest in the supine position, and a mean figure calculated. Height was measured in centimeters to the nearest measured value whereas weight was measured in kilograms without shoes. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. All study participants provided written informed consent. The regional ethics committee of Lund University in Lund, Sweden, approved this study (Nos. LU 51-90 and Dnr 652/2005).
Blood samples drawn after an overnight fast were immediately stored at −80 °C. Fasting plasma Pro-NT levels were centrally quantified in a single laboratory (ICI Immunochemical Intelligence GmbH) with a high-sensitivity, one-step chemiluminometric sandwich immunoassay and coated-tube technique (SphingoTec GmbH). The lower limit of detection of Pro-NT precursor fragment was 1.9 pmol/L (7). All other analysis including fasting plasma glucose, total cholesterol and triglycerides levels in serum, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and very low-density lipoprotein were measured by standard laboratory methods in a certified laboratory using the Cobas Modular Analyzer Series (Roche). All analyses were based on standard and accredited procedures at the Department of Clinical Chemistry, Skane University Hospital.
Mortality End Points
The outcomes were ACM and CSM based on the International Classification of Diseases (ICD) codes Ninth or Tenth Revision (ICD-9; ICD-10) recorded as the underlying cause of death. During follow-up, information on ACM and CSM were retrieved by linking the individual’s 10-digit civil registration number with the Swedish National Cause of Death Register. Mortality was ascertained according to the registered underlying cause of death on the cause of death certificate in accordance with the ICD-9 or ICD-10 codes as follows: deaths due to CVD (ICD 9:390-459 or ICD 10: I 00-99), digestive tract diseases (ICD 9:520-579 or ICD 10: K 00-95), mental and behavioral disorders (ICD 9: 290-319 or ICD 10: F 01-99), symptoms and unspecific causes of death (ICD 9:780-789 or ICD 10: R 00-99), respiratory diseases (ICD 9:460-519 or ICD 10: J 00-99), genitourinary diseases (ICD 9: 580-629 or ICD 10: N 00-99), malignant cancers (ICD 9:140-239 or ICD 10: C 00-97), immunodeficiency and endocrine disorders (ICD 9:240-279 or ICD 10: D 80-89; E00-99), blood disorders (ICD 9:280-289 or ICD 10: D 50-D77), diseases in nerve system and ears (ICD 9:320-389 or ICD 10: G 00-99; H 00-99), infectious diseases (ICD 9: 001-139 or ICD 10: A 00-99; B 00-99), dermatological diseases (ICD 9: 680-709 or ICD 10: L 00-99), musculoskeletal diseases (ICD 9:710-739 or ICD 10: M 00-99), injuries and poison incidents (ICD 9:800-999 or ICD 10: U, V, W, X, Y 00-99). Participants were followed until emigration, death, or end of follow-up (December 31, 2018).
Statistical Analysis
The distribution of plasma Pro-NT concentration was skewed and therefore logarithmically transformed when analyzed as a continuous variable. Fasting Pro-NT concentrations were divided into population quartiles. Continuous clinical characteristics were compared across quartiles by using analysis of variance “linear trend” P values. Cox proportional hazards models were used to test the relationship of Pro-NT quartile and total mortality in models adjusted for age and sex, as well as in models adjusted for age, sex, BMI, systolic blood pressure (SBP), diabetes mellitus, use of antihypertensive medication, current smoking status, and LDL-C and HDL-C. When adding glycated hemoglobin A1c greater than 6.5% as a criterium for diabetes definition, only one additional individual had diabetes (n = 419 instead of n = 418) (22). Furthermore, the risk of ACM and CSM was compared between individuals belonging to Pro-NT Q4 (≥ 149 pmol/L) vs individuals belonging to Pro-NT Q1-3 (< 149 pmol/L) and Q4 vs Q1 in similar adjustment models as described earlier. We tested for interaction between Pro-NT and sex and analyzed data separately for men and women in relation to ACM. No significant multicollinearity was found between the covariates in the multivariate models. Survival and time to event analysis in ACM were visualized by the Kaplan-Meier method.
A 2-sided P value of less than .05 was considered statistically significant. SPSS software version 26.0 (IBM SPSS) was used for all statistical analyses and calculations of cohort data.
Results
The baseline characteristics of the study participants stratified by quartiles of Pro-NT are shown in Table 1. In the crude baseline analysis, Pro-NT in quartiles were significantly related to prevalence of diabetes, smoking, HDL-C, and female sex. Higher quartiles of Pro-NT contained greater proportions of participants with diabetes (P < .001) and current smokers (P = .007). The median (interquartile range) age of patients was 57.7 years (range, 51.7-63.7 years). Of 4632 patients (42.5% men, 57.5% women), 1906 (31.3%) died during a follow-up period of 20 ± 3 years.
Table 1.
Clinical characteristics of the study population stratified by quartile
| Pro-NT Quartile 1 | Pro-NT Quartile 2 | Pro-NT Quartile 3 | Pro-NT Quartile 4 | P for trend | |
|---|---|---|---|---|---|
| No. of participants | 1158 | 1158 | 1158 | 1158 | |
| Men, n (%) | 558 (48.2%) | 497 (42.9%) | 447 (38.6%) | 467 (40.3%) | < .001 |
| Age, mean (SD), y | 58.0 ± 6.00 | 57.7 ± 5.98 | 57.6 ± 5.96 | 57.7 ± 5.97 | .309 |
| SBP, mean (SD), mm Hg | 142.8 ± 19.7 | 141.6 ± 18.6 | 142.8 ± 19.7 | 142.2 ± 18.8 | .430 |
| Antihypertensive therapy, No. (%) | 188 (16.2%) | 197 (17%) | 180 (15.5%) | 224 (19.3%) | .112 |
| Diabetes mellitus, No. (%) | 77 (6.7%) | 100 8.7%) | 100 (8.7%) | 141 (12.3%) | < .001 |
| BMI, mean (SD) | 25.8 ± 3.84 | 25.7 ± 3.75 | 25.9 ± 4.01 | 26.0 ± 4.12 | .071 |
| LDL-C mean (SD), mmol/L | 4.16 ± 1.02 | 4.16 ± 0.96 | 4.21 ± 0.98 | 4.13 ± 0.98 | .741 |
| HDL-C mean (SD), mmol/L | 1.36 ± 0.36 | 1.39 ± 0.38 | 1.39 ± 0.37 | 1.39 ± 0.38 | .018 |
| Current smokers, n (%) | 298 (25.7%) | 278 (24.0%) | 290 (25.0%) | 354 (30.6%) | .007 |
Abbreviations: BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Pro-NT, proneurotensin; SBP, systolic blood pressure.
Proneurotensin and All-Cause Mortality
The cumulative incidence of ACM in quartiles of baseline fasting concentrations of plasma Pro-NT is shown in Table 2. Increased mortality risk with fasting plasma Pro-NT was found in quartile 4 vs quartile 1, analysis adjusted for sex and age HR 1.33 (95% CI, 1.17-1.42; P < .001). Mortality risk in quartiles 2 and 3 respectively did not significantly differ from ACM in quartile 1. Moreover, the increased ACM risk observed in quartile 4 vs quartile 1 remained significant in both sexes, and accordingly no significant interaction was observed between Pro-NT and sex on ACM risk.
Table 2.
Event rates and multivariate adjusted Cox proportional hazards models for baseline proneurotensin in relation to all-cause mortality (adjusted for age and sex)
| All-cause mortality | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P for trend |
|---|---|---|---|---|---|
| All participants | |||||
| No./events No. | 1158/458 | 1158/442 | 1158/470 | 1158/536 | |
| Pro-NT, median (range), pmol/L | 60.0 (3.00-75.8) | 89.0 (75.9-105) | 123 (105-149) | 190.4 (149-1150) | |
| HR (95% CI) | Ref (1.0) | 0.99 (0.87-1-12) | 1.10 (0.97-1.26) | 1.33 (1.17-1.50) | < .001 |
| Women | |||||
| No./events No. | 600/194 | 661/213 | 711/252 | 691/291 | |
| Pro-NT, median, pmol/L | 60.4 (3.00-75.8) | 89.1 (75.9-104.6) | 122.6 (104.7-148.5) | 192.0 (148.6-1154.5) | |
| HR (95% CI) | Ref (1.0) | 1.03 (0.85-1.25) | 1.11 (0.92-1.34) | 1.42 (1.19-1.70) | < .001 |
| Men | |||||
| No./events No. | 558/264 | 497/229 | 447/218 | 467/245 | |
| Pro-NT, median, pmol/L | 59.9 (0.00-75.8) | 89.6 (75.9-104.5) | 123.3 (104.8-148.3) | 188.4 (148.6-1057.4) | |
| HR (95% CI) | Ref (1.0) | 0.95 (0.80-1.14) | 1.10 (0.92-1.32) | 1.24 (1.04-1.48) | .014 |
Abbreviations: HR, hazard ratio; Pro-NT, proneurotensin; Ref, reference.
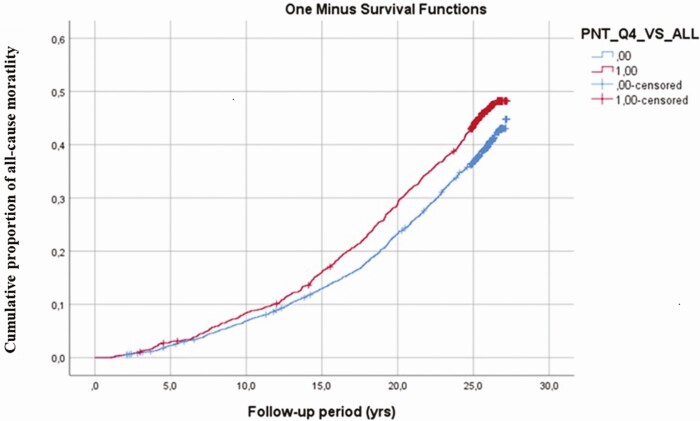
In line with the results of Cox regressions, Fig. 1 illustrates the Kaplan-Meier plots for ACM by Pro-NT quartiles during the follow-up. It shows that quartile 4 of Pro-NT deviates from the 3 lower quartiles.
Figure 1.
Kaplan- Meier plot shows cumulative proportion of all-cause mortality during follow-up in the fourth quartile vs quartiles 1 to 3 of baseline proneurotensin.
Subsequently, we compared individuals belonging to quartile 4 with those belonging to quartiles 1 to 3. Having Pro-NT greater than or equal to 149 pmol/L increased the relative risk of ACM, HR of 1.29 (95% CI, 1.17-1.43) (P < .001) in a model adjusted for age and sex, and 1.20 (95% CI, 1.08-1.33) (P = .001) after additional adjustment for cardiovascular risk factors (see Table 3).
Table 3.
Event rates and multivariate Cox proportional hazards models for baseline proneurotensin in relation to cause-specific mortality
| Age and sex adjusted | Fully adjusteda | |||||||
|---|---|---|---|---|---|---|---|---|
| All participants | Quartile 1-3 | Quartile 4 | P | All participants | Quartile 1-3 | Quartile 4 | P | |
| All-cause mortality | ||||||||
| No./events No. | 4632/1906 | 3474/1370 | 1158/536 | 4478/1823 | 3365/1316 | 1113/507 | ||
| HR (95% CI) | Ref 1.0 | 1.29 (1.17-1.43) | < .001 | 1.20 (1.08-1.33) | .001 | |||
| Cardiovascular diseases | ||||||||
| No./events No. | 4632/595 | 3474/419 | 1158/176 | 4478/562 | 3365/397 | 1113/165 | ||
| HR (95% CI) | 1.41 (1.18-1.68) | < .001 | 1.30 (1.08-1.56) | .005 | ||||
| Gastrointestinal diseases | ||||||||
| No./events No. | 4632/42 | 3474/24 | 1158/18 | 4478/42 | 3365/24 | 1113/18 | ||
| HR (95% CI) | 2.53 (1.37-4.67) | .003 | 2.37 (1.28-4.39) | .006 | ||||
| Mental and behavioral diseases | ||||||||
| No./events No. | 4632/90 | 3474/61 | 1158/29 | 4478/89 | 3365/61 | 1113/28 | ||
| HR (95% CI) | 1.62 (1.04-2.52) | .032 | 1.56 (0.99-2.34) | .057 | ||||
| Unspecific diseases | ||||||||
| No./events No. | 4632/64 | 3474/41 | 1158/23 | 4478/62 | 3365/40 | 1113/22 | ||
| HR (95% CI) | 1.91 (1.15-3.19) | .013 | 1.80 (1.07-3.04) | .028 | ||||
| Cancer | ||||||||
| No./events No. | 4632/683 | 3474/509 | 1158/174 | 4478/658 | 3365/493 | 1113/165 | ||
| HR (95% CI) | 1.10 (0.92-1.30) | .293 | ||||||
| Infectious diseases | ||||||||
| No./events No. | 4632/41 | 3474/30 | 1158/11 | 4478/35 | 3365/25 | 1113/10 | ||
| HR (95% CI) | 1.25 (0.62-2.50) | .531 | ||||||
| Endocrine diseases | ||||||||
| No./events No. | 4632/48 | 3474/34 | 1158/14 | 4478/45 | 3365/32 | 1113/13 | ||
| HR (95% CI) | 1.39 (0.75-2.59) | .300 | ||||||
| External causes | ||||||||
| No./events No. | 4632/58 | 3474/41 | 1158/17 | 4478/52 | 3365/39 | 1113/13 | ||
| HR (95% CI) | 1.35 (0.76-2.37) | .303 | ||||||
| Musculoskeletal diseases | ||||||||
| No./events No. | 4632/09 | 3474/6 | 1158/03 | 4478/09 | 3365/06 | 1113/03 | ||
| HR (95% CI) | 1.63 (0.41-6.56) | .487 | ||||||
| Urogenital diseases | ||||||||
| No./events No. | 4632/25 | 3474/20 | 1158/05 | 4478/24 | 3365/19 | 1113/05 | ||
| HR (95% CI) | 0.91 (0.34-2.43) | .854 | ||||||
| Respiratory diseases | ||||||||
| No./events No. | 4632/125 | 3474/92 | 1158/33 | 4478/122 | 3365/90 | 1113/32 | ||
| HR (95% CI) | 1.22 (0.82-1.1) | .331 | ||||||
| Neurological diseases | ||||||||
| No./events No. | 4632/107 | 3474/80 | 1158/27 | 4478/104 | 3365/77 | 1113/27 | ||
| HR (95% CI) | 1.11 (0.72-1.72) | .647 | ||||||
| Hematological diseases | ||||||||
| No./events No. | 4632/05 | 3474/04 | 1158/01 | 4478/05 | 3365/04 | 1113/01 | ||
| HR (95% CI) | 0.88 (0.99-7.88) | .91 | ||||||
Abbreviations: HR, hazard ratio; Pro-NT, proneurotensin; Ref, reference.
a Adjusted for age, sex, body mass index, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, diabetes, smoking, systolic blood pressure, and antihypertensive treatment.
Proneurotensin and Cause-specific Mortality
People in quartile 4 had an increased risk of CSM compared to people in quartiles 1 to 3 regarding mortality due to CVD, digestive diseases, psychiatry diseases, and deaths due to unspecific diseases, observed both in crude and multivariable adjusted models.
Of a total of 1906 deaths, a significant association of Pro-NT in quartile 4 vs quartiles 1 to 3 and CSM in the Cox regression model were observed when adjusted for age, sex, BMI, LDL-C, HDL-C, diabetes, smoking, SBP, and antihypertensive treatment. There were 595 reported deaths (31.2%) due to CVDs with an HR of 1.41 (95% CI, 1.18-1.68; P < .001); 42 reported deaths (2.20%) were due to digestive tract diseases with an HR of 2.53 (95% CI, 1.37-4.67; P = .003); 90 reported deaths (4.72%) were due to mental and behavioral diseases with an HR of 1.62 (95% CI, 1.04-2.52; P = .032); and 64 reported deaths (3.36%) had unspecific causes with an HR of 1.91 (95% CI, 1.15-3.19; P = .013). There was no significant relationship between Pro-NT levels and deaths from cancer, infections, neurological, or other causes as listed in Table 3. In testing Pro-NT as a continuous variable in relation to cause-specific survival in the multivariate Cox regression analysis after adjustment for age, sex, BMI, LDL-C, HDL-C, diabetes, smoking, SBP, and antihypertensive treatment, Pro-NT was a positive predictor of deaths due to CVD and gastrointestinal diseases but the results were not significant for deaths due to mental and behavioral diseases and unspecific diseases as listed in Supplementary Table 1 (23).
Calculation of HR by assessing Pro-NT distribution in quartile 4 vs quartile 1 in the same fully adjusted model showed that individuals in quartile 4 vs those in quartile 1 had a significantly increased risk for ACM, CVD mortality, gastrointestinal disease mortality, and unspecific disease mortality. No significant relationships were observed between Pro-NT and death due to mental and behavioral diseases as listed in Supplementary Table 2 (23).
After adding estimated glomerular filtration rate (according to Modification of Diet in Renal Disease) and the homeostasis model assessment of estimated insulin resistance (as a marker of insulin resistance) on top of all other covariates, the significant associations between Pro-NT and mortality outcomes remained significant, apart from deaths due to mental and behavioral diseases and unspecific diseases, the latter of which were borderline significant, as listed in Supplementary Table 3 (23).
The association between Pro-NT and cancer deaths (excluding cancers of unknown primary and hematological cancers) were insignificant, with an HR of 1.12 (95% CI, 0.93-1.95; P = .222) in an age- and sex-adjusted model, similar to the result of “all cancer mortality” as presented in Table 3.
Discussion
To the best of our knowledge, no previous studies have investigated the association of elevated plasma Pro-NT levels and CSM in prospective population-based cohorts. In this population-based cohort study with 20 years of follow-up, elevated plasma Pro-NT predicted ACM as well as CSM in CVD, gastrointestinal, psychiatric diseases, and deaths from unspecific causes. No specific association was observed with deaths caused by neoplasms, respiratory diseases, endocrine diseases, or external causes. Furthermore, sex-stratified analyses showed significant associations between Pro-NT and ACM in both sexes. No significant interaction was observed between sex and Pro-NT on ACM. This strongly suggests that the association between Pro-NT and mortality is the same both in men and women. Mature neurotensin hormone is unstable and rapidly breaks down in the circulation; however, a wide range of physiological and pharmacological effects of Pro-NT are seen by its affinity to specific NT receptor 1, which is used in this study (8).
Our results regarding the association of Pro-NT and cardiovascular mortality are in line with previous studies (19, 21). In contrast to a previous study from the MDC, in which follow-up time was substantially shorter and a sex-specific association with mortality and CVD mortality was observed (but significant only in women) (19), we here find robust associations in both sexes during a long-term follow-up.
The observed significance of elevated Pro-NT to predict gastrointestinal disease mortality in this study has not been reported previously. However, previous studies have shown that Pro-NT facilitates lipid digestion and fat absorption in the small intestines (4, 5) and a fatty meal is the most potent inducer of NT secretion, which results in decreased gastrointestinal motility and gastric acid secretions (24, 25). In our recent study, Pro-NT contributed to intestinal absorption of lipids into the bloodstream resulting in the rise of plasma triglycerides (3). Montén et al (26) observed a relationship between elevated postprandial Pro-NT levels and active celiac disease in a pediatric population, suggesting its proinflammatory role in the small intestines. Recently the role of Pro-NT and NAFLD and nonalcoholic steatohepatitis in obese adults was investigated, and a strong association between Pro-NT and NAFLD was found (14). Although the number of deaths due to gastrointestinal diseases was generally low, the association remained significant, strengthening our belief in a true relationship between elevated Pro-NT and mortality risk due to gastrointestinal diseases.
The observed association between Pro-NT and diseases caused by psychiatric diseases (mental and behavioral disorders) supports the previous study by Nicoli et al (16), who reported sex-specific cognitive impairment associated with higher levels of plasma Pro-NT in women. However, our study showed significant associations in all participants in crude and adjusted models, but sex-specific associations were not observed.
Elevated Pro-NT and its receptors have long been recognized and involved in various cancers ranging from tumor growth to metastatic spread (27), but no overall significant relationship was observed between Pro-NT and cancer mortality in this study. This was despite the proven effects of the neurotensin system on cancer cell proliferation, migration and invasion, as well as its antiapoptotic effects, resulting in growth stimulation of cancers (27). It could, however, be that different types of cancer may not share the same possible Pro-NT–dependent pathway. A similar lack of association was observed between Pro-NT levels and respiratory, endocrine, infectious, neurological, and urogenital diseases.
Strength and Limitations
This is a large, prospective cohort study showing comorbidity and mortality in relation to elevated plasma Pro-NT in a middle-aged population. Strengths of the study include a long-term follow-up of study individuals along with the use of standardized national registry-based diagnosis codes and information on potential confounders.
However, this study also has some potential limitations. The study population was dominated by Swedish-born, White Europeans, which limits the generalized application of this study to other ethnicities. Furthermore, the mortality is relatively low in this population sample with a mean age just younger than 60 years, giving a lower number of events in some of the major causes of death, especially in noncardiovascular and nonneoplasm groups. Thus, the power for statistical analysis is low for some of these categories. We also acknowledge a lack of data on hepatic function and were thus unable to adjust for this factor. Finally, there is always an uncertainty as to the cause of death as judged by the treating physician, so some caution in the interpretation is warranted.
Conclusion
Fasting Pro-NT was predictive of ACM and deaths due to CVDs, gastrointestinal diseases, mental and behavioral diseases, and diseases of unspecific causes. Furthermore, significant associations were observed between elevated levels of plasma Pro-NT and total mortality irrespective of sex. This suggests that Pro-NT may also be a useful biomarker for detecting individuals at higher risk of developing specific diseases and premature deaths and who may specifically benefit from preventive therapy.
Acknowledgments
All study individuals provided written informed consent to participate. The data do not include any individual personal data including individual details, images, or videos and hence consent for publication is not applicable to this study.
Glossary
Abbreviations
- ACM
all-cause mortality
- BMI
body mass index
- CNS
central nervous system
- CSM
cause-specific mortality
- CVD
cardiovascular disease
- HDL
high-density lipoprotein cholesterol
- HR
hazard ratio
- ICD
International Classification of Diseases
- LDL-C
low-density lipoprotein cholesterol
- MDC-CC
Malmö Diet and Cancer Study–cardiovascular cohort
- NAFLD
nonalcoholic fatty liver disease
- NT
neurotensin
- Pro-NT
proneurotensin
- SBP
systolic blood pressure.
Financial Support
This work was supported by research grants from the Knut and Alice Wallenberg Foundation, the Göran Gustafsson Foundation, the Swedish Heart Lung Foundation, the Swedish Research Council, the Novo Nordisk Foundation, Region Skåne, Skåne University Hospital, and the Swedish Foundation for Strategic Research (IRC) to O.M. The content of the manuscript is solely the responsibility of the authors.
Author Contributions
A.F. and O.M. conceived and conducted data analysis and all authors interpreted the results. A.F. and Z.B. drafted the manuscript with input from all the authors. All authors have read and approved the final version of submission.
Additional Information
Disclosures: J.S. is employed by Sphingotec GmbH, a company having patent rights to the proneurotensin assay and commercializing it. A.B. is chief executive officer of Sphingotec GmbH and holds shares in this company. The other authors declare that they have neither disclosures nor financial or nonfinancial competing interests.
Data Availability
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. GBD 2015 Obesity Collaborators; Afshin A, Forouzanfar MH, Reitsma MB, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1): 13-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Emerging Risk Factors Collaborators; Di Angelantonio E, Kaptoge S, Wormser D, et al. Association of cardiometabolic multimorbidity with mortality. JAMA. 2015;314(1):52-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fawad A, Fernandez C, Bergmann A, et al. Magnitude of rise in proneurotensin is related to amount of triglyceride appearance in blood after standardized oral intake of both saturated and unsaturated fat. Lipids Health Dis. 2020;19(1):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gui X, Carraway RE. Enhancement of jejunal absorption of conjugated bile acid by neurotensin in rats. Gastroenterology. 2001;120(1):151-160. [DOI] [PubMed] [Google Scholar]
- 5. Li J, Song J, Zaytseva YY, et al. An obligatory role for neurotensin in high-fat-diet-induced obesity. Nature. 2016;533(7603):411-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aronin N, Carraway RE, Ferris CF, Hammer RA, Leeman SE. The stability and metabolism of intravenously administered neurotensin in the rat. Peptides. 1982;3(4):637-642. [DOI] [PubMed] [Google Scholar]
- 7. Ernst A, Hellmich S, Bergmann A. Proneurotensin 1-117, a stable neurotensin precursor fragment identified in human circulation. Peptides. 2006;27(7):1787-1793. [DOI] [PubMed] [Google Scholar]
- 8. Friry C, Feliciangeli S, Richard F, Kitabgi P, Rovere C. Production of recombinant large proneurotensin/neuromedin N-derived peptides and characterization of their binding and biological activity. Biochem Biophys Res Commun. 2002;290(4): 1161-1168. [DOI] [PubMed] [Google Scholar]
- 9. Kinkead B, Dobner PR, Egnatashvili V, Murray T, Deitemeyer N, Nemeroff CB. Neurotensin-deficient mice have deficits in prepulse inhibition: restoration by clozapine but not haloperidol, olanzapine, or quetiapine. J Pharmacol Exp Ther. 2005;315(1):256-264. [DOI] [PubMed] [Google Scholar]
- 10. Pettibone DJ, Hess JF, Hey PJ, et al. The effects of deleting the mouse neurotensin receptor NTR1 on central and peripheral responses to neurotensin. J Pharmacol Exp Ther. 2002;300(1):305-313. [DOI] [PubMed] [Google Scholar]
- 11. Lafrance M, Roussy G, Belleville K, et al. Involvement of NTS2 receptors in stress-induced analgesia. Neuroscience. 2010;166(2):639-652. [DOI] [PubMed] [Google Scholar]
- 12. Ruan CS, Yang CR, Li JY, Luo HY, Bobrovskaya L, Zhou XF. Mice with Sort1 deficiency display normal cognition but elevated anxiety-like behavior. Exp Neurol. 2016;281:99-108. [DOI] [PubMed] [Google Scholar]
- 13. Zhao D, Pothoulakis C. Effects of NT on gastrointestinal motility and secretion, and role in intestinal inflammation. Peptides. 2006;27(10):2434-2444. [DOI] [PubMed] [Google Scholar]
- 14. Barchetta I, Cimini FA, Leonetti F, et al. Increased plasma proneurotensin levels identify NAFLD in adults with and without type 2 diabetes. J Clin Endocrinol Metab. 2018;103(6):2253-2260. [DOI] [PubMed] [Google Scholar]
- 15. Antonelli T, Fuxe K, Tomasini MC, et al. Neurotensin receptor mechanisms and its modulation of glutamate transmission in the brain: relevance for neurodegenerative diseases and their treatment. Prog Neurobiol. 2007;83(2):92-109. [DOI] [PubMed] [Google Scholar]
- 16. Nicoli CD, Howard VJ, Judd SE, Struck J, Manly JJ, Cushman M. Pro-neurotensin/neuromedin N and risk of cognitive impairment in a prospective study. J Alzheimers Dis. 2020;76(4):1403-1412. [DOI] [PubMed] [Google Scholar]
- 17. Januzzi JL Jr, Lyass A, Liu Y, et al. Circulating proneurotensin concentrations and cardiovascular disease events in the community: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2016;36(8):1692-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fawad A, Bergmann A, Struck J, Nilsson PM, Orho-Melander M, Melander O. Proneurotensin predicts cardiovascular disease in an elderly population. J Clin Endocrinol Metab. 2018;103(5):1940-1947. [DOI] [PubMed] [Google Scholar]
- 19. Melander O, Maisel AS, Almgren P, et al. Plasma proneurotensin and incidence of diabetes, cardiovascular disease, breast cancer, and mortality. JAMA. 2012;308(14):1469-1475. [DOI] [PubMed] [Google Scholar]
- 20. Tönjes A, Hoffmann A, Kralisch S, et al. Pro-neurotensin depends on renal function and is related to all-cause mortality in chronic kidney disease. Eur J Endocrinol. 2020;183(3):233-244. [DOI] [PubMed] [Google Scholar]
- 21. Wettersten N, Cushman M, Howard VJ, et al. Usefulness of proneurotensin to predict cardiovascular and all-cause mortality in a United States population (from the Reasons for Geographic and Racial Differences in Stroke Study). Am J Cardiol. 2018;122(1):26-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes–2018. Diabetes Care. 2018;41(Suppl 1):S13-S27. [DOI] [PubMed] [Google Scholar]
- 23.Fawad A, Bergmann A, Schulte J, et al. Supplementary data for “Plasma concentration of proneurotensin and prediction of cause-specific mortality in a middle-aged cohort during long-term follow-up.” Deposited August 11, 2021. https://www.ludc.lu.se/sites/ludc.lu.se/files/2021-08/Supplementary-Tables_Fawad_JCEM_2021.pdf [DOI] [PMC free article] [PubMed]
- 24. Spiller RC, Trotman IF, Higgins BE, et al. The ileal brake–inhibition of jejunal motility after ileal fat perfusion in man. Gut. 1984;25(4):365-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mogard MH, Maxwell V, Sytnik B, Walsh JH. Regulation of gastric acid secretion by neurotensin in man. Evidence against a hormonal role. J Clin Invest. 1987;80(4):1064-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Montén C, Torinsson Naluai Å, Agardh D. Role of proneurotensin as marker of paediatric coeliac disease. Clin Exp Immunol. 2016;186(3):387-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ouyang Q, Zhou J, Yang W, Cui H, Xu M, Yi L. Oncogenic role of neurotensin and neurotensin receptors in various cancers. Clin Exp Pharmacol Physiol. 2017;44(8):841-846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.