Abstract
Context
Although polycystic ovary syndrome (PCOS) is the most common endocrinopathy affecting women of reproductive age, risk factors that may cause the syndrome are poorly understood. Based on epidemiologic studies, PCOS is thought to cause several adverse outcomes such as cardiovascular disease; however, the common presence of comorbidities such as obesity may be responsible for such associations, rather than PCOS in and of itself. To overcome the limitations of observational studies, investigators have employed Mendelian randomization (MR), which uses genetic variants to interrogate causality between exposures and outcomes.
Evidence Acquisition
To clarify causes and consequences of PCOS, this review will describe MR studies involving PCOS, both as an exposure and as an outcome. The literature was searched using the terms “Mendelian randomization,” “polycystic ovary syndrome,” “polycystic ovarian syndrome,” and “PCOS” (to May 2021).
Evidence Synthesis
MR studies have suggested that obesity, testosterone levels, fasting insulin, serum sex hormone-binding globulin concentrations, menopause timing, male-pattern balding, and depression may play a causal role in PCOS. In turn, PCOS may increase the risk of estrogen receptor–positive breast cancer, decrease the risk of endometrioid ovarian cancer, and have no direct causal effect on type 2 diabetes, coronary heart disease, or stroke.
Conclusions
The accumulation of genome-wide association studies in PCOS has enabled multiple MR analyses identifying factors that may cause PCOS or be caused by PCOS. This knowledge will be critical to future development of measures to prevent PCOS in girls at risk as well as prevent complications in those who have PCOS.
Keywords: polycystic ovary syndrome, Mendelian randomization, genome-wide association study
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy in women of reproductive age. Thus, a clear understanding of risk factors for PCOS (causes) and adverse outcomes of PCOS (consequences) is critical to improving the care of women with this condition. While there is little debate surrounding the negative effects of PCOS on skin and the reproductive system, it has also been associated with several nonreproductive conditions. Studies have linked PCOS to a myriad of co-morbidities, including endometrial cancer, obesity, type 2 diabetes (T2D), depression, anxiety, nonalcoholic fatty liver disease, sleep apnea, and eating disorders, to name a few (1).
A limitation of observational epidemiologic studies is that they can associate 2 conditions but do not provide information on causation. An apparent link between PCOS and a particular comorbidity may be mediated by confounding factors. These relationships must be disentangled to allow accurate counseling and treatment of women with PCOS. With the advent of large-scale genome-wide association studies (GWAS), investigators have been able to use genetic variants to deduce causality between exposures and putative outcomes. This technique has been widely applied in cardiometabolic disease (2). As an example of how Mendelian randomization (MR) can inform on effective treatment, MR found evidence that higher low density lipoprotein cholesterol (LDL-C), but not lower high-density lipoprotein cholesterol (HDL-C), was causal for coronary heart disease (CHD) (3,4). Thus, treatment to prevent CHD is focused on LDL-C lowering, while many interventions aimed at raising HDL-C have been ineffective (5,6).
MR studies depend on the availability of robust genetic variants for the conditions under investigation. With the recent publication of the largest GWAS for PCOS (7), investigators have taken advantage of this opportunity to conduct MR studies examining potential causes and consequences of PCOS. Not only did this international GWAS meta-analysis yield 14 robust loci for PCOS, summary data on all variants were made publicly available, greatly facilitating the ability of others to conduct MR analyses.
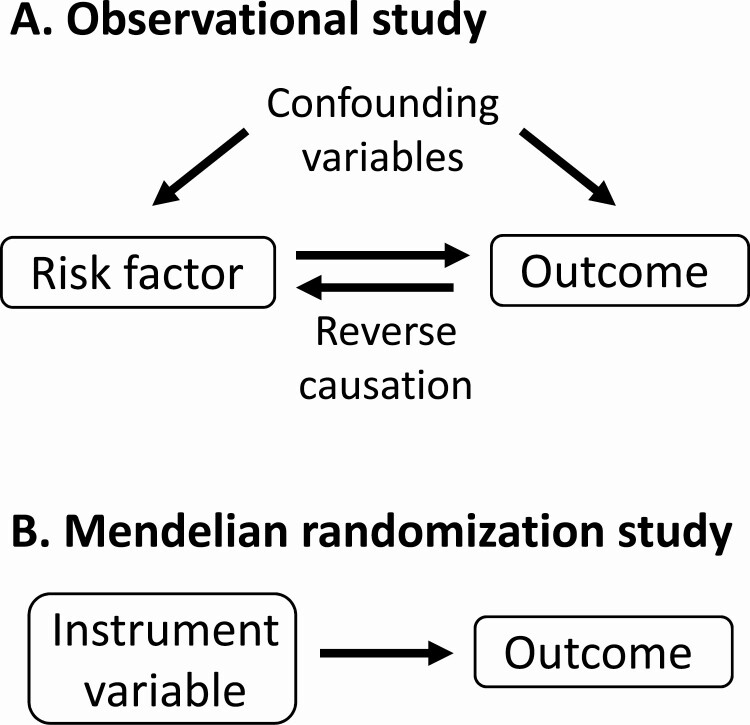
MR is a novel genetic analytical approach that exploits genetic variants associated with an exposure as instrumental variables to assess the causality of the exposure on clinical outcomes of interest (8). MR provides more robust causal inferences than conventional observational studies because genetic variants are randomly assigned from parents to offspring at conception, overcoming the problems of confounding factors and reverse causation that are commonly present in observational studies (Fig. 1). MR relies on 3 assumptions: (1) instrument variables are reliably associated with the risk factor of interest; (2) genetic variants are not associated with confounders of the risk factor-outcome association; and (3) genetic variants affect the outcome only through the risk factor. The latter 2 assumptions are collectively known as independence from pleiotropy. Pleiotropy refers to a genetic variant (or variants in linkage disequilibrium) being associated with multiple traits. Pleiotropic variants can influence the outcome through pathways other than the risk factor. MR studies may be biased if genetic variants are pleiotropic. The presence of substantial pleiotropy can be assessed by conducting sensitivity analyses using techniques such as MR-Egger and MR-PRESSO (9,10).
Figure 1.
Advantage of Mendelian randomization over observational studies. (A) Observational studies can demonstrate association between a risk factor and a possible outcome but provide limited information on causation because the relationship may be mediated by confounding variables or reverse causation. (B) In Mendelian randomization, the risk factor is replaced by a genetic instrument variable that strongly represents the risk factor, avoiding the effect of confounding variables or reverse causation. This allows a more robust analysis of possible causation.
One-sample MR is performed using 1 data set, in which genetic variants, exposure, and outcome are measured in the same participants. In 2-sample MR, the associations of the genetic variants with the risk factor and with the outcome are estimated in 2 different study samples, which allows MR to be conducted using summary level data from independent cohorts that collectively have many exposures and outcomes measured. In a 1-sample MR analysis with weak instrument variables, the causal estimate is biased in the direction of the confounded observational association between the risk factor and outcome, whereas in a 2-sample analysis, the causal estimate is less biased and any bias due to weak instruments is in the direction of the null. Thus, the bias in 2-sample MR is less serious as it is conservative leading to lower false-positive rates (11).
In this review, we will summarize the MR studies involving PCOS published to date. Several studies examined PCOS as the outcome (Table 1) while others examined PCOS as the exposure (Table 2) Some of the studies address long-standing fundamental questions in the field [eg, relationship of PCOS to cardiovascular disease (CVD) and cancer] while others involving more esoteric traits have been done based on conveniently available data sets. Studies were identified by a search of PubMed (through May 2021) using search terms “Mendelian randomization,” “polycystic ovary syndrome,” “polycystic ovarian syndrome,” and “PCOS.”
Table 1.
Mendelian randomization studies of various exposures with PCOS as the outcome
| Exposure | Year | Authors | Population of exposure | Population of outcome | SNPs, n | Effect | P-value |
|---|---|---|---|---|---|---|---|
| BMI | 2015 | Day et al | 249 796 Europeans | 6368 cases and 88 558 controls, European | 32 | OR: 1.90 (1.55, 2.34) | 2.5E-09 |
| BMI | 2018 | Day et al | 339 224 individuals (Europeans and non-Europeans) | 10 074 cases and 103 164 controls, European | NR | Beta: 0.72 (0.072) | 1.56E-23 |
| BMI | 2019 | Brower et al | 339 224 individuals (Europeans and non-Europeans) | 750 PCOS cases and 1567 BMI-matched controls, European | 92 | OR: 4.89 (1.46, 16.32) | NR |
| BMI | 2020 | Zhao et al | up to 173 430 East Asians | 4386 cases and 8017 controls, East Asians | 78 | OR: 2.21 (1.54, 3.17) | 1.77E-05 |
| BMI | 2020 | Ahn et al | 9953 Korean women | 946 PCOS cases and 976 controls, Korean | 3 | Beta: −0.124 (0.153) | 0.42 |
| Favorable adiposity | 2021 | Martin et al | UKBB, 451 099 Europeans | FinnGen + Day et al PCOS GWAS, 10 536 cases and 340 236 controls | 36 | OR: 0.51 (0.21, 1.23) | 0.13 |
| Unfavorable adiposity | 2021 | Martin et al | UKBB, 451 099 Europeans | FinnGen + Day et al PCOS GWAS, 10 536 cases and 340 236 controls | 38 | OR: 7.13 (3.66, 13.90) | 8.0E-09 |
| Testosterone | 2020 | Ruth et al | UKBB, 188 507 individuals | 4890 cases and 20 405 controls, European | 133 | OR: 1.55 (1.32, 1.82) | 1.9E-07 |
| SHBG | 2020 | Ruth et al | UKBB, 189 473 individuals | 4890 cases and 20 405 controls, European | 214 | OR: 0.69 (0.58, 0.81) | 1.9E-05 |
| SHBG | 2015 | Day et al | 28 837 Europeans | 6368 cases and 88 558 controls, European | 20 | OR: 0.86 (0.78, 0.93) | 5.4E-04 |
| AMH | 2020 | Verdiesen et al | 7049 premenopausal European women | 4890 cases and 20 405 controls, European | 4 | OR: 1.29 (0.85, 1.95) | 0.23 |
| DHEAS | 2015 | Day et al | 14 846 Europeans | 5184 cases and 82 759 controls, European | 8 | OR: 1.11 (0.99, 1.23) | 0.06 |
| Age at menopause | 2015 | Day et al | 92 371 Europeans | 6368 cases and 88 558 controls, European | 18 | OR: 1.60 (1.35, 1.91) | 1.5E-08 |
| Menopause timing | 2018 | Day et al | 92 371 Europeans | 10 074 cases and 103 164 controls, European | NR | Beta: 0.1 (0.022) | 1.31E-05 |
| Age at menarche | 2015 | Day et al | Up to 182 416 European women | 5184 cases and 82 759 controls, European | 113 | OR: 0.91 (0.79, 1.06) | 0.23 |
| Insulin resistance | 2015 | Day et al | Up to 18 565 Europeans | 6368 cases and 88 558 controls, European | 10 | OR: 1.11 (1.05, 1.19) | 5.6E-04 |
| Fasting insulin | 2018 | Day et al | 85 573 nondiabetic Europeans | 10 074 cases and 103 164 controls, European | NR | Beta: 0.03 (0.007) | 1.73E-05 |
| Insulin secretion | 2015 | Day et al | Up to 18 565 Europeans | 5184 cases and 82 759 controls, European | 23 | Higher risk | 0.19 |
| HDL cholesterol | 2015 | Day et al | >100 000 Europeans | 5184 cases and 82 759 controls, European | 71 | OR: 0.37 (0.13, 1.11) | 0.08 |
| LDL cholesterol | 2015 | Day et al | >100 000 Europeans | 5184 cases and 82 759 controls, European | 58 | OR: 1.04 (0.94, 1.16) | 0.43 |
| Total cholesterol | 2015 | Day et al | >100 000 Europeans | 5184 cases and 82 759 controls, European | 74 | OR: 0.98 (0.88, 1.09) | 0.71 |
| Triglycerides | 2015 | Day et al | >100 000 Europeans | 5184 cases and 82 759 controls, European | 40 | OR: 1.03 (0.90, 1.18) | 0.65 |
| Diastolic BP | 2015 | Day et al | 200 000 Europeans | 5184 cases and 82 759 controls, European | 26 | OR: 1.01 (0.99, 1.03) | 0.24 |
| Systolic BP | 2015 | Day et al | 200 000 Europeans | 5184 cases and 82 759 controls, European | 25 | OR: 1.05 (0.82, 1.34) | 0.68 |
| Male pattern balding | 2018 | Day et al | 52 874 white British men from UKBB | 10 074 cases and 103 164 controls, European | NR | Beta: 0.05 (0.017) | 0.0034 |
| Depression | 2018 | Day et al | 135 458 cases and 344 901 controls, European | 10 074 cases and 103 164 controls, European | NR | Beta: 0.77 (0.213) | 0.00029 |
| Birth weight | 2015 | Day et al | up to 69 308 Europeans | 5184 cases and 82 759 controls, European | 6 | Higher risk | 0.22 |
| Adult height | 2015 | Day et al | 183 727 Europeans | 5184 cases and 82 759 controls, European | 178 | Lower risk | 0.51 |
| Education years | 2020 | Adams et al | 293 723 Europeans | UKBB, 571 cases and 462 362 controls | 68 | OR: 1.00 (1.00, 1.00) | 0.28 |
| Periodontitis | 2021 | Wu et al | 12 289 cases and 22 326 controls, European | 4890 cases and 20 405 controls, European | 7 | OR: 1.17 (0.91, 1.49) | 0.21 |
| Beta-hydroxyisovalerate | 2020 | Sun et al | 7824 adult individuals, European | 4890 cases and 20 405 controls, European | 25 | OR: 2.84 (1.20, 6.76) | 0.0179 |
| 3-Dehydrocarnitine | 2020 | Sun et al | 7824 adult individuals, European | 4890 cases and 20 405 controls, European | 27 | OR: 6.72 (2.22, 20.32) | 0.0007 |
| Dihomolinolenate (20:3n3 or n6) | 2020 | Sun et al | 7824 adult individuals, European | 4890 cases and 20 405 controls, European | 25 | OR: 3.22 (1.21, 8.59) | 0.0194 |
| 1-Arachidonoylglycerophosphoethanolamine | 2020 | Sun et al | 7824 adult individuals, European | 4890 cases and 20 405 controls, European | 29 | OR: 2.97 (1.20, 7.36) | 0.0187 |
| 2-Linoleoylglycerophosphocholine | 2020 | Sun et al | 7824 adult individuals, European | 4890 cases and 20 405 controls, European | 22 | OR: 2.71 (1.05, 7.01) | 0.0402 |
| Hexanoylcarnitine | 2020 | Sun et al | 7824 adult individuals, European | 4890 cases and 20 405 controls, European | 23 | OR: 2.65 (1.35, 5.19) | 0.0045 |
| Hexadecanedioate | 2020 | Sun et al | 7824 adult individuals, European | 4890 cases and 20 405 controls, European | 26 | OR: 1.79 (1.11, 2.88) | 0.0161 |
| Epiandrosterone sulfate | 2020 | Sun et al | 7824 adult individuals, European | 4890 cases and 20 405 controls, European | 15 | OR: 1.54 (1.08, 2.21) | 0.0186 |
| 2-Tetradecenoyl carnitine | 2020 | Sun et al | 7824 adult individuals, European | 4890 cases and 20 405 controls, European | 19 | OR: 0.52 (0.30, 0.90) | 0.0193 |
| 7-Alpha-hydroxy-3-oxo-4-cholestenoate (7-Hoca) | 2020 | Sun et al | 7824 adult individuals, European | 4890 cases and 20 405 controls, European | 16 | OR: 0.16 (0.04, 0.68) | 0.0132 |
| N2,N2-Dimethylguanosine | 2020 | Sun et al | 7824 adult individuals, European | 4890 cases and 20 405 controls, European | 64 | OR: 0.39 (0.18, 0.85) | 0.0186 |
| Glycylvaline | 2020 | Sun et al | 7824 adult individuals, European | 4890 cases and 20 405 controls, European | 7 | OR: 2.11 (1.14, 3.89) | 0.017 |
| 4-Hydroxyhippurate | 2020 | Sun et al | 7824 adult individuals, European | 4890 cases and 20 405 controls, European | 11 | OR: 2.95 (1.24, 7.05) | 0.0148 |
Effect data are given as beta (SE) or odds ratio (95% CI).
Abbreviations: AMH, anti-Müllerian hormone; BMI, body mass index; BP, blood pressure; DHEAS, dehydroepiandrosterone sulfate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NR, not reported; OR, odds ratio; PCOS, polycystic ovary syndrome; SHBG, sex hormone-binding globulin; SNPs, SNPs, single nucleotide polymorphisms; UKBB, UK Biobank.
Table 2.
Mendelian randomization studies of various outcomes where PCOS is the exposure
| Outcome | Year | Authors | Population of exposure | Population of outcome | SNPs, n | Effect | P-value |
|---|---|---|---|---|---|---|---|
| BMI | 2019 | Brower et al | 10 074 cases and 103 164 controls | 2685 white individuals | 16 | Beta: 0.003 (−0.008, 0.013) | NR |
| BMI | 2020 | Zhao et al | 4386 cases and 8017 controls, East Asians | up to 173 430 East Asians | 11 | OR: 0.998 (0.998, 1.009) | 0.77 |
| T2D in East Asians (all) | 2021 | Zhu et al | 14 562 cases and 17 266 controls, East Asians | AGEN consortium, 77 418 cases and 356 122 controls, East Asian | 13 | OR: 0.98 (0.96, 1.01) | 0.13 |
| T2D in East Asians (female) | 2021 | Zhu et al | 14 562 cases and 17 266 controls, East Asians | AGEN consortium, 27 370 cases and 135 055 controls, East Asian | 13 | OR: 0.98 (0.95, 1.02) | 0.33 |
| T2D in East Asians (male) | 2021 | Zhu et al | 14 562 cases and 17 266 controls, East Asians | AGEN consortium, 28 027 cases and 89 312 controls, East Asian | 13 | OR: 0.99 (0.95, 1.02) | 0.45 |
| T2D in Europeans (all) | 2021 | Zhu et al | 10 074 cases and 103 164 controls | DIAMANTE consortium, 74 124 cases and 824 006 controls | 14 | OR: 0.97 (0.92, 1.01) | 0.16 |
| T2D in Europeans (female) | 2021 | Zhu et al | 10 074 cases and 103 164 controls | DIAMANTE consortium, 30 053 cases and 434 336 controls | 14 | OR: 0.95 (0.88, 1.02) | 0.16 |
| T2D in Europeans (male) | 2021 | Zhu et al | 10 074 cases and 103 164 controls | DIAMANTE consortium, 41 846 cases and 383 767 controls | 14 | OR: 0.98 (0.93, 1.03) | 0.42 |
| CHD | 2021 | Zhu et al | 10 074 cases and 103 164 controls | UKBB + CARDIoGRAMplusC4D, 122 733 cases and 424 528 controls, mostly European | 14 | OR: 1.00 (0.96, 1.04) | 0.88 |
| Any stroke | 2021 | Zhu et al | 10 074 cases and 103 164 controls | MEGASTROKE consortium, 40 585 cases and 406 111 controls | 14 | OR: 0.98 (0.93, 1.02) | 0.33 |
| Any ischemic stroke | 2021 | Zhu et al | 10 074 cases and 103 164 controls | MEGASTROKE consortium, 34 217 cases and 406 111 controls | 14 | OR: 0.98 (0.93, 1.03) | 0.40 |
| Large artery stroke | 2021 | Zhu et al | 10 074 cases and 103 164 controls | MEGASTROKE consortium, 4373 cases and 406 111 controls | 14 | OR: 0.88 (0.78, 1.00) | 0.06 |
| Cardioembolic stroke | 2021 | Zhu et al | 10 074 cases and 103 164 controls | MEGASTROKE consortium, 7193 cases and 406 111 controls | 14 | OR: 0.92 (0.83, 1.02) | 0.10 |
| Small vessel stroke | 2021 | Zhu et al | 10 074 cases and 103 164 controls | MEGASTROKE consortium, 5386 cases and 406 111 controls | 14 | OR: 1.10 (0.95, 1.27) | 0.21 |
| Borderline ovarian cancer | 2019 | Harris et al | 10 074 cases and 103 164 controls | OCAC, 3103 cases and 40 941 controls | 14 | OR: 1.08 (0.94, 1.25) | 0.27 |
| Serous ovarian cancer (borderline) | 2019 | Harris et al | 10 074 cases and 103 164 controls | OCAC, 1954 cases and 40 941 controls | 14 | OR: 1.06 (0.89, 1.27) | 0.52 |
| Mucinous ovarian cancer (borderline) | 2019 | Harris et al | 10 074 cases and 103 164 controls | OCAC, 1149 cases and 40 941 controls | 14 | OR: 1.13 (0.90, 1.41) | 0.29 |
| Invasive ovarian cancer | 2019 | Harris et al | 10 074 cases and 103 164 controls | OCAC, 22 406 cases and 40 941 controls | 14 | OR: 0.92 (0.85, 0.99) | 0.03 |
| Low-grade serous ovarian cancer (invasive) | 2019 | Harris et al | 10 074 cases and 103 164 controls | OCAC, 1 012 cases and 40 941 controls | 14 | OR: 1.09 (0.83, 1.43) | 0.53 |
| High-grade serous ovarian cancer (invasive) | 2019 | Harris et al | 10 074 cases and 103 164 controls | OCAC, 13 037 cases and 40 941 controls | 14 | OR: 0.91 (0.82, 0.998) | 0.046 |
| Mucinous ovarian cancer (invasive) | 2019 | Harris et al | 10 074 cases and 103 164 controls | OCAC, 1417 cases and 40 941 controls | 14 | OR: 1.13 (0.92, 1.38) | 0.25 |
| Endometrioid ovarian cancer (invasive) | 2019 | Harris et al | 10 074 cases and 103 164 controls | OCAC, 2810 cases and 40 941 controls | 14 | OR: 0.77 (0.65, 0.92) | 0.003 |
| Clear cell ovarian cancer (invasive) | 2019 | Harris et al | 10 074 cases and 103 164 controls | OCAC, 1366 cases and 40 941 controls | 14 | OR: 0.90 (0.73, 1.10) | 0.30 |
| Overall breast cancer | 2020 | Wu et al | 10 074 cases and 103 164 controls | BCAC, 122 977 cases and 105 974 controls | 13 | OR: 1.08 (1.05, 1.12) | 7.98E-07 |
| ER + breast cancer | 2020 | Wu et al | 10 074 cases and 103 164 controls | BCAC, 69 501 cases and 105 974 controls | 13 | OR: 1.10 (1.06, 1.14) | 3.78E-07 |
| ER- breast cancer | 2020 | Wu et al | 10 074 cases and 103 164 controls | BCAC, 21 468 cases and 105 974 controls | 13 | OR: 1.01 (0.95, 1.07) | 0.675 |
| Luminal A-like | 2021 | Zhu et al | 10 074 cases and 103 164 controls | NR | 14 | OR: 1.10 (1.06, 1.15) | 1.53E-06 |
| Luminal B/HER2-negative-like | 2021 | Zhu et al | 10 074 cases and 103 164 controls | NR | 14 | OR: 1.10 (1.02, 1.19) | 0.014 |
| Luminal B-like | 2021 | Zhu et al | 10 074 cases and 103 164 controls | NR | 14 | OR: 1.19 (1.06, 1.33) | 0.003 |
| HER2-enriched-like | 2021 | Zhu et al | 10 074 cases and 103 164 controls | NR | 14 | OR: 0.98 (0.85, 1.12) | 0.732 |
| Triple-negative | 2021 | Zhu et al | 10 074 cases and 103 164 controls | NR | 14 | OR: 1.02 (0.94-1.10) | 0.612 |
| FEV1 | 2020 | van der Plaat et al | 10 074 cases and 103 164 controls | 159 511 white nonasthmatic women of the UKBB | 19 | −2.10mL (−10.1, 5.93) | 0.608 |
| FVC | 2020 | van der Plaat et al | 10 074 cases and 103 164 controls | 159 511 white nonasthmatic women of the UKBB | 19 | −11.2mL (−21.7, −0.63) | 0.038 |
| FEV1/FVC | 2020 | van der Plaat et al | 10 074 cases and 103 164 controls | 159 511 white nonasthmatic women of the UKBB | 19 | 0.16% (0.00, 0.31) | 0.05 |
| Airflow obstruction | 2020 | van der Plaat et al | 10 074 cases and 103 164 controls | 159 511 white nonasthmatic women of the UKBB | 19 | OR: 0.96 (0.88, 1.04) | 0.311 |
| Spirometric restriction | 2020 | van der Plaat et al | 10 074 cases and 103 164 controls | 159 511 white nonasthmatic women of the UKBB | 19 | OR: 1.07 (0.99, 1.15) | 0.086 |
| Periodontitis | 2021 | Wu et al | 10 074 cases and 103 164 controls | 12 289 cases and 22 326 controls, European | 13 | OR: 0.97 (0.88, 1.06) | 0.50 |
| Offspring birth weight | 2021 | Wu et al | 10 074 cases and 103 164 controls | Early Growth Genetics Consortium and UKBB (n = 257 734) | 13 | Beta: −0.013 (0.012) | 0.26 |
Cohorts are of European ancestry if not stated. Effect data are given as beta (95% CI) [first row], beta (SE) [last row], the reported effect [van der Plaat et al], or odds ratio (95% CI).
Abbreviations: AGEN, Asian Genetic Epidemiology Network; BCAC, Breast Cancer Association Consortium; BMI, body mass index; CARDIoGRAMplusC4D, Coronary ARtery DIsease Genome wide Replication and Meta-analysis (CARDIoGRAM) plus the Coronary Artery Disease (C4D) Genetics consortium; CHD, coronary heart disease; DIAMANTE: DIAbetes Meta-ANalysis of Trans-Ethnic association studies; ER, estrogen receptor; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; NR, not reported; OCAC, Ovarian Cancer Association Consortium; OR, odds ratio; PCOS, polycystic ovary syndrome; SNPs, single nucleotide polymorphisms; T2D, type 2 diabetes; UKBB: UK Biobank.
PCOS as Outcome
Studies to identify causal risk factors for PCOS hold promise for future modalities of disease prevention, when such risk factors are modifiable. For example, given the apparent causal effect of obesity on PCOS described next, measures to achieve weight loss may prevent the development of PCOS in girls at risk.
Obesity
Obesity is commonly present in women with PCOS and is associated with a more severe phenotype of the syndrome (12). Whether obesity is a cause or consequence of PCOS has been a matter of debate. MR has allowed this question to be addressed. In 2015, Day et al performed a large-scale GWAS for PCOS in 7229 cases and 181 645 controls of European ancestry and included MR analyses to explore risk factors in PCOS etiology (13). Genetically predicted higher body mass index (BMI) was associated with higher PCOS risk [odds ratio (OR) = 1.90 per + 1 SD; 95% CI = 1.55, 2.34; P = 2.5 × 10−9]. Subsequently, in 2018, Day et al reported a GWAS meta-analysis of 10 074 PCOS cases and 103 164 controls of European ancestry (7). Their MR analyses also suggested a causal role in PCOS pathogenesis for BMI (beta = 0.72; SE = 0.072; P = 1.56 × 10−23).
In 2 bidirectional MR studies in European and East Asian cohorts, similar findings were observed in that increased BMI was causal for PCOS (14, 15). These studies examined the reverse question and found that PCOS does not have a causal effect to increase BMI. However, in a GWAS and MR study conducted by Ahn et al in Korean women (9953 females for obesity traits and 946 cases and 976 controls for PCOS), 4 single nucleotide polymorphisms (SNPs) showed evidence of a highly significant association (P < 1 × 10−6) with BMI, and use of 3 out of these 4 SNPs as instruments (1 was excluded due to its association with confounding factors) found no significant association between genetically defined obesity and PCOS (16). Of note, this study derived SNP-BMI estimates from a relatively small sample size whereas the other MR studies derived SNP-BMI estimates from summary data from very large GWAS, providing strong instruments. Thus, the balance of evidence strongly supports increased BMI as a causal factor for PCOS.
Some obese individuals do not manifest any obesity-related metabolic conditions or diseases, whereas some normal weight individuals develop diseases like T2D, heart disease, and hypertension (17,18). Genetic studies have identified favorable adiposity alleles associated with higher adiposity but a favorable metabolic profile (lower risk of T2D, hypertension, and heart disease) (19,20). In 1 example of such a study, Martin et al performed a multivariate GWAS using body fat percentage and metabolic biomarkers from UK Biobank (UKBB) and identified 2 distinct clusters of variants associated with higher adiposity: 1 with a favorable metabolic profile [favorable adiposity (FA)] and the other with an unfavorable metabolic profile (unfavorable adiposity) (21). Their MR studies provided evidence for a risk-increasing effect of unfavorable adiposity (OR = 7.13 per + 1 SD; 95% CI = 3.66, 13.90; P = 8 × 10-9) and a suggestive protective effect of FA (OR = 0.51 per + 1 SD; 95% CI = 0.21, 1.23; P = 0.13) on PCOS. This supports the concept that different patterns of adiposity may increase or decrease the risk of metabolic disease, possibly related to the location of fat depots (FA was associated with lower visceral fat).
Hormone Levels
One of the central features of PCOS is elevated blood testosterone level. Testosterone has been correlated with many health conditions and testosterone supplements are commonly used for positive effects on sexual function, bone health, and body composition. However, its effects on disease outcomes are unknown. Ruth et al performed a large GWAS and identified genetic determinants of testosterone levels and related sex hormone traits in 425 097 UKBB participants (22). MR analyses using these genetic variants demonstrated that a genetically determined 1 SD higher total testosterone (OR = 1.26; 95% CI 1.13, 1.41; P = 4.1 × 10−5) or bioavailable testosterone (OR = 1.66; 95% CI 1.42, 1.95; P = 3.2 × 10−9) increased the risk of PCOS. Of interest, increased testosterone increased risk of T2D in women (bioavailable testosterone: OR = 1.47; 95% CI = 1.28, 1.69; P = 2.4 × 10−7) but decreased it in men (total testosterone; OR = 0.85; 95% CI = 0.77, 0.95; P = 2.4 × 10−3).
Ruth et al also reported that a genetically determined 1 SD higher sex hormone-binding globulin (SHBG) decreased the risk of T2D (OR = 0.44; 95% CI = 0.35, 0.54; P = 6.5 × 10−13) and PCOS (OR = 0.66; 95% CI = 0.57, 0.77; P = 3.6 × 10−7) in women as well as the risk of T2D (OR = 0.73; 95% CI = 0.6, 0.9; P = 2.7 × 10−3) in men (22). Day et al in 2015 also reported a causal role of lower serum SHBG (OR = 0.86 per + 1 SD; 95% CI = 0.78, 0.93; P = 5.4 × 10−4) in PCOS etiology while the effect of dehydroepiandrosterone sulfate did not reach statistical significance (13).
Higher anti-Müllerian hormone (AMH) concentrations are often present in women with PCOS. It has been hypothesized that AMH may be involved in the pathogenesis of PCOS by causing anovulation due to its inhibitory influence on the actions of follicle-stimulating hormone that normally promotes follicular development from the small antral stage to ovulation (23). Verdiesen et al conducted a GWAS meta-analysis including data from 7049 premenopausal women of European ancestry and identified four loci associated with AMH levels at genome-wide significance (P < 5 × 10−8) (24). Exploratory MR analyses did not support a causal effect of circulating AMH on breast cancer (OR = 1.00; 95% CI = 0.74, 1.36; P = 0.98) or PCOS (OR = 1.29; 95% CI = 0.85, 1.95; P = 0.23). However, the authors stated that the MR results should be interpreted with caution because they could not extensively explore how valid the genetic instruments were, and weak instruments may have biased estimates toward the null.
Miscellaneous Risk Factors
In 2015, Day et al reported causal roles in PCOS etiology for higher insulin resistance (OR = 1.11 per + 1 SD; 95% CI = 1.05, 1.19; P = 5.6 × 10−4) and later menopause (OR = 1.60 per + 1 SD; 95% CI = 1.35, 1.91; P = 1.5 × 10−8), while they found no evidence for causal effects of birth weight, adult height, age at menarche, insulin secretion, blood pressure, or lipid fractions on PCOS (13). The 10-SNP genetic instrument that they used to represent insulin resistance was derived from a study (25) where among 19 SNPs associated with fasting insulin at genome-wide significance, these 10 were at least nominally (P < 0.05) associated with a dyslipidemic profile (lower HDL-C and higher triglycerides), suggestive of a role in insulin resistance. Subsequently, in 2018, Day et al reported that fasting insulin (beta = 0.03; SE = 0.007; P = 1.73 × 10−5), later menopause timing (beta = 0.1; SE = 0.022; P = 1.31 × 10−5), depression (beta = 0.77; SE = 0.213; P = 2.9 × 10−4), and male-pattern balding (beta = 0.05; SE = 0.017; P = 0.003) appear to play a causal role in PCOS (7).
Within the past decade, advances in metabolomics have led to several reports identifying circulating molecules that are correlated with the occurrence of PCOS (26-28). However, whether altered metabolite levels are cause or consequence of PCOS is uncertain, limiting the ability to target them therapeutically. Sun et al conducted a 2-sample MR analysis to estimate the causal effects of genetically determined metabolite levels on the risk of PCOS (29). They obtained summary level data on 486 metabolites from a GWAS comprising 7824 adult individuals from 2 European population studies (30). Genetic associations with PCOS were obtained from the most recent large GWAS meta-analysis for PCOS (7). With the use of genetic variants as proxies, 24 metabolites were identified to have causal effects on PCOS, among which 13 were known metabolites. The 13 known metabolites included 9 lipids, a xenobiotic, a peptide, an amino acid, and a nucleotide. The most significant chemical compound with predicted causal effects on PCOS was 3-dehydrocarnitine. A genetically determined 1 SD higher 3-dehydrocarnitine level increased the risk of PCOS (OR = 6.72; 95% CI = 2.22, 20.32; P = 0.0007). Additional metabolites with either risk increasing or risk decreasing effects on PCOS are listed in Table 1. The biological significance of these associations remains to be elucidated.
Adams et al demonstrated that while more years of schooling was protective against development of T2D (OR = 0.39; 95% CI = 0.26, 0.58; P = 3.89 × 10−6), it had no effect on risk for PCOS (31).
PCOS as Exposure
A clear understanding of the true complications of PCOS is essential to appropriately counsel affected women in regard to prevention of comorbidities, especially because women with PCOS often have other features (eg, obesity, insulin resistance) that may cause complications, rather than PCOS in and of itself.
Cardiometabolic Outcomes
PCOS has been associated with T2D and CVD; however, whether these associations are causal is uncertain. This is a question of great clinical significance given the morbidity associated with T2D and CVD. We conducted a 2-sample MR study to investigate the causal effect of PCOS on T2D, CHD, and stroke (32). The summary GWAS data for T2D SNPs in Europeans were obtained from the DIAbetes Meta-ANalysis of Trans-Ethnic association studies (DIAMANTE) consortium, including 74 124 case and 824 006 control subjects of European ancestry. The summary data on T2D in East Asians were retrieved from the Asian Genetic Epidemiology Network (AGEN) consortium with 77 418 case and 356 122 control subjects. SNP-CHD associations were acquired from the CHD GWAS meta-analysis of the UKBB with the Coronary ARtery DIsease Genome wide Replication and Meta-analysis (CARDIoGRAM) plus the Coronary Artery Disease (C4D) Genetics (CARDIoGRAMplusC4D) consortium, which included 34 541 CHD cases and 261 984 controls from UKBB and 88 192 cases and 162 544 controls from CARDIoGRAMplusC4D, of whom ∼90% were of European origin. The summary statistics on stroke and stroke subtypes were from the MEGASTROKE consortium which included 40 585 cases and 406 111 controls of European ancestry. T2D in Asians and T2D in Europeans were both analyzed sex-combined and sex-stratified. Stroke was analyzed as any stroke as well as 4 stroke subtypes (ischemic, large artery, cardioembolic, small vessel). We demonstrated that genetically predicted PCOS was not associated with risk of T2D, CHD, or any stroke traits. The same results were observed in sensitivity analyses that excluded from the instrument variable SNPs associated with BMI, waist-to-hip ratio adjusted for BMI, and testosterone. This suggests that PCOS in and of itself does not increase the risk of these outcomes. Other common features of PCOS (obesity, elevated testosterone, low sex hormone binding globulin levels) likely explain the epidemiologic associations between PCOS and cardiometabolic diseases, given that prior MR studies have causally linked these features to diabetes and/or CHD (22,33,34).
Cancer
Harris et al (35). performed an MR study to examine the association between PCOS and ovarian cancer overall and by histotype, using summary data from a GWAS of ovarian cancer in Europeans within the Ovarian Cancer Association Consortium (22 406 with invasive disease, 3103 with borderline disease and 40 941 controls) (36). They found an inverse association between genetically predicted PCOS and invasive ovarian cancer risk (OR = 0.92; 95% CI = 0.85, 0.99; P = 0.03), with the most robust protective association observed for the endometrioid histotype (OR = 0.77; 95% CI = 0.65, 0.92; P = 0.003). Similar results regarding the endometrioid histotype were obtained in an MR study by Yarmolinsky et al, which also used the summary statistics from Ovarian Cancer Association Consortium (37).
Wu et al (38) conducted a 2-sample MR study using summary statistics from the Breast Cancer Association Consortium (39), with a total of 122 977 breast cancer cases and 105 974 controls of European ancestry. They demonstrated that genetically predicted PCOS was associated with an increased risk of breast cancer overall (OR = 1.08; 95% CI = 1.05, 1.12; P = 7.98 × 10−7) and estrogen receptor (ER)-positive breast cancer (OR = 1.10; 95% CI = 1.06, 1.14; P = 3.78 × 10−7), whereas no causal association was observed for ER-negative breast cancer. A subsequent study using the same data reported similar results (40).
In our MR study, using summary statistics on breast cancer subtypes from Breast Cancer Association Consortium comprising 133 384 breast cancer cases and 113 789 controls of European ancestry (41), we found that genetically predicted PCOS increased the risk of luminal A-like (ER+ and/or PR+, HER2−, grades 1 and 2; OR = 1.10; 95% CI = 1.06, 1.15; P = 1.53 × 10−6), luminal B/human epidermal growth factor receptor 2 (HER2)-negative-like (ER + and/or PR+, HER2−, grade 3; OR = 1.10; 95% CI = 1.02, 1.19; P = 0.014), and luminal B-like (ER + and/or PR+, HER2+; OR = 1.19; 95% CI = 1.06, 1.33; P = 0.003) subtypes but did not increase the risk of HER2-enriched-like (ER− and PR−, HER2+) and triple-negative (ER−, PR−, HER2−) subtypes (42). Our findings are consistent with the 2 previously mentioned studies wherein genetically predicted PCOS was associated with ER-positive rather than ER-negative breast cancer, since luminal A-like, luminal B/HER2-negative-like, and luminal B-like subtypes are all ER-positive and/or progesterone receptor-positive. These studies reveal a previously unrecognized (43) risk of breast cancer in PCOS; however, it is reassuring that PCOS does not increase the risk of breast cancer subtypes that have the worst prognosis (HER2-enriched-like and triple-negative).
Miscellaneous Outcomes
Evidence is limited regarding the effect of PCOS on lung function. Oligomenorrhea, a cardinal symptom of PCOS, has been associated with lower forced vital capacity (FVC) (44). Van der Plaat et al applied 2-sample MR to evaluate the causal effect of PCOS on 5 lung function outcomes, including forced expiratory volume in 1 s (FEV1), FVC, FEV1/FVC, airflow obstruction (FEV1/FVC < lower limit of normal and spirometric restriction (FVC < lower limit of normal) (45). The SNP-lung function effect estimates were obtained from UKBB data from 159 511 white nonasthmatic women (40-71 years old). Genetically predicted PCOS was associated with lower FVC (−11.2 mL; 95% CI = −21.7, −0.63), P = 0.038) and marginally with higher FEV1/FVC (0.16%; 95% CI = 0.00, 0.31, P = 0.050) and higher risk of spirometric restriction (OR = 1.07; 95% CI = 0.99, 1.15; P = 0.086). However, no effect was found for FEV1 and airflow obstruction. The authors stated that the mechanisms underlying the detrimental effect of PCOS on FVC are unclear, but it is possible that insulin resistance and hyperinsulinemia may play a role because they are commonly present in PCOS and are also associated with lower FVC (46,47).
As a condition characterized by chronic inflammation, periodontitis has been linked to metabolic disorders such as diabetes mellitus, female hormonal alterations, obesity, and PCOS (48,49). Wu et al performed a bidirectional MR study in European ancestry cohorts, evaluating the causal relationships between PCOS and periodontitis (12 289 cases of periodontitis and 22 326 controls). Genetically determined PCOS was not causally associated with risk of periodontitis (OR = 0.97; 95% CI = 0.88, 1.06; P = 0.50). Similarly, in the reverse MR (4890 cases of PCOS and 20 405 controls), genetic liability to periodontitis was not causally associated with risk of PCOS (OR 1.17; 95% CI = 0.91, 1.49; P = 0.21) per 1-unit increase in the log-OR of periodontitis (50). In another MR study, Wu et al found that genetically predicted higher risk of PCOS did not cause a decrease in offspring birth weight in Europeans (51).
Limitations of MR in PCOS
A limitation of any MR study is the inability to be absolutely certain that pleiotropy does not exist among the SNPs used as instrument variables, even if sensitivity analyses suggest that pleiotropy is not present. It is generally not feasible to assess the association of the instrument SNPs with all potential confounding variables to identify any SNPs with pleiotropy because some confounders are unknown. There are some caveats related to MR studies specifically in regard to PCOS. As relatively few GWAS (compared to other common diseases such as T2D) have been performed in PCOS, the number of SNPs representing PCOS is fairly low, possibly limiting power (though formal analysis using F statistics suggests that the 14 European SNPs do form a strong instrument) (7). Another issue that may affect the instrument SNPs is referral bias. As cases for GWAS studies come largely from centers specializing in PCOS, the SNPs identified may not well represent milder forms of PCOS for which patients infrequently seek care or are not referred. Another concern relates to imprecision in making the diagnosis of PCOS, especially given the lack of a single robust pathognomonic criterion for the diagnosis. Imprecision in diagnosis is a minor issue in specialized centers that carefully phenotype patients and rule out disorders that may mimic PCOS (eg, hyperprolactinemia, hypothalamic amenorrhea). However, this may not have been done in all cohorts included in large PCOS meta-analyses. Furthermore, the largest GWAS for PCOS included a substantial number of cases where PCOS was defined by self-report (7). While the observation that the same SNPs were identified by GWAS regardless mode of diagnosis provides some reassurance, the possibility that some of the self-reported cases may have had other etiologies remains a concern. Finally, to date the various subtypes of PCOS (phenotype A, B, C, D) (52) have been analyzed together in GWAS. Future large GWAS specifically dissecting these subtypes will provide opportunities for subtype-specific MR studies.
Conclusion
MR represents an elegant example of how GWAS findings can illuminate biology even before a deep understanding of how the specific variants influence disease risk. Although free of reverse causation and confounding that affect observational studies, MR does not definitively prove or refute causation between an exposure and an outcome, largely because it is difficult to absolutely rule out pleiotropy. To mitigate this, many MR studies perform sensitivity analyses, including application of techniques such as MR-Egger to detect pleiotropy and/or excluding instrument SNPs with known associations with potential confounders. When repeated MR studies in independent cohorts yield similar results, a strong case can be made for causality (or lack thereof).
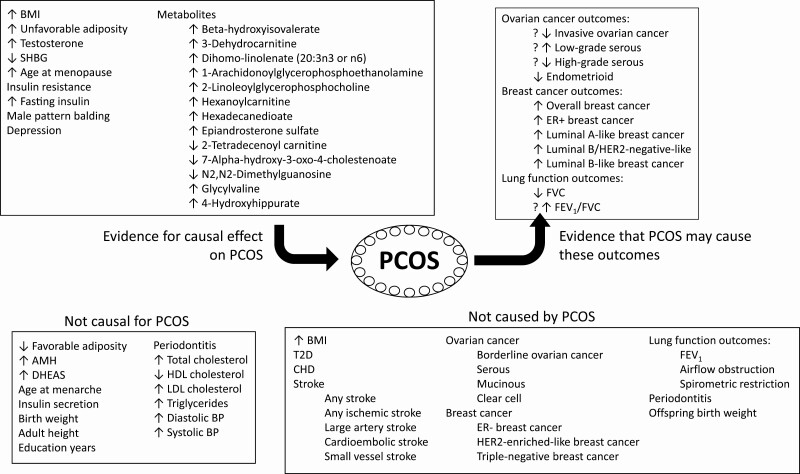
MR studies have been widely used to elucidate the causal relationships between PCOS and other traits and diseases. As summarized in Figure 2, MR has suggested that obesity, testosterone levels, fasting insulin, serum SHBG concentrations, menopause timing, male-pattern balding, and depression appear to play a causal role in PCOS etiology. Furthermore, PCOS has been found to be causally associated with an increased risk of breast cancer (specifically ER + breast cancer), a decreased risk of endometrioid ovarian cancer, and a detrimental effect on FVC (1 of the lung function outcomes). However, genetically predicted PCOS was not causally associated with T2D, CHD, stroke, periodontitis, or offspring birth weight. The potentially most clinically impactful results are those assessing the effect (or lack thereof) of PCOS on T2D, CHD, breast cancer, and ovarian cancer, where the genetic effects were not appreciated by prior observational studies. Given the advantages of MR over observational studies, accumulating GWAS results from large consortia that measure different traits will enable investigators to further explore the risk factors and the outcomes of PCOS, especially where existing studies yielded borderline or inconsistent results. The anticipated continued vigorous rate of publication of MR studies in PCOS will help us understand the development of PCOS and better counsel our patients, with the ultimate goal of preventing PCOS in women at risk and preventing complications in those who already have PCOS.
Figure 2.
Overview of Mendelian randomization studies involving polycystic ovary syndrome. Mendelian randomization studies of polycystic ovary syndrome as the outcome or as the exposure are summarized, with significant results at the top of the figure. Results of borderline significance are denoted with a question mark. Abbreviations: AMH, anti-Mullerian hormone; BMI, body mass index; CHD, coronary heart disease; DHEAS, dehydroepiandrosterone sulfate; ER, estrogen receptor; FEV1, forced expiratory volume in 1 second; FVC forced vital capacity; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SHBG, sex hormone-binding globulin; T2D, type 2 diabetes.
Financial Support
This work was supported in part by the National Center for Advancing Translational Sciences, CTSI grant UL1TR000124, and the National Institute of Diabetes and Digestive and Kidney Disease Diabetes Research Center (DRC) grant P30-DK063491 to the Southern California Diabetes Research Center. M.O.G. was supported by the Eris M. Field Chair in Diabetes Research.
Additional Information
Disclosures: The authors have nothing to declare.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
References
- 1. Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7(4):219-231. [DOI] [PubMed] [Google Scholar]
- 2. Frayling TM, Stoneman CE. Mendelian randomisation in type 2 diabetes and coronary artery disease. Curr Opin Genet Dev. 2018;50:111-120. [DOI] [PubMed] [Google Scholar]
- 3. Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380(9841):572-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Linsel-Nitschke P, Götz A, Erdmann J, et al. ; Wellcome Trust Case Control Consortium (WTCCC); Cardiogenics Consortium. Lifelong reduction of LDL-cholesterol related to a common variant in the LDL-receptor gene decreases the risk of coronary artery disease-a Mendelian Randomisation study. PLoS One. 2008;3(8):e2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Landray MJ, Haynes R, Hopewell JC, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371(3):203-212. [DOI] [PubMed] [Google Scholar]
- 6. Barter PJ, Caulfield M, Eriksson M, et al. ; ILLUMINATE Investigators. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357(21):2109-2122. [DOI] [PubMed] [Google Scholar]
- 7. Day F, Karaderi T, Jones MR, et al. ; 23andMe Research Team. Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet. 2018;14(12):e1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smith GD, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32(1):1-22. [DOI] [PubMed] [Google Scholar]
- 9. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burgess S, Davies NM, Thompson SG. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol. 2016;40(7):597-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lim SS, Norman RJ, Davies MJ, Moran LJ. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14(2):95-109. [DOI] [PubMed] [Google Scholar]
- 13. Day FR, Hinds DA, Tung JY, et al. Causal mechanisms and balancing selection inferred from genetic associations with polycystic ovary syndrome. Nat Commun. 2015;6:8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brower MA, Hai Y, Jones MR, et al. Bidirectional Mendelian randomization to explore the causal relationships between body mass index and polycystic ovary syndrome. Hum Reprod. 2019;34(1):127-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao YL, Xu YP, Wang XM, et al. Body mass index and polycystic ovary syndrome: a 2-sample bidirectional Mendelian randomization study. J Clin Endocr Metab. 2020;105(6):1778-1784. [DOI] [PubMed] [Google Scholar]
- 16. Ahn Y, Lee H, Cho YS. Identification of genetic variants for female obesity and evaluation of the causal role of genetically defined obesity in polycystic ovarian syndrome. Diabetes Metab Syndr Obes. 2020;13:4311-4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stefan N, Kantartzis K, Machann J, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168(15):1609-1616. [DOI] [PubMed] [Google Scholar]
- 18. Ruderman NB, Schneider SH, Berchtold P. The “metabolically-obese,” normal-weight individual. Am J Clin Nutr. 1981;34(8):1617-1621. [DOI] [PubMed] [Google Scholar]
- 19. Ji Y, Yiorkas AM, Frau F, et al. Genome-wide and abdominal MRI data provide evidence that a genetically determined favorable adiposity phenotype is characterized by lower ectopic liver fat and lower risk of type 2 diabetes, heart disease, and hypertension. Diabetes. 2019;68(1):207-219. [DOI] [PubMed] [Google Scholar]
- 20. Yaghootkar H, Lotta LA, Tyrrell J, et al. Genetic evidence for a link between favorable adiposity and lower risk of type 2 diabetes, hypertension, and heart disease. Diabetes. 2016;65(8):2448-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martin S, Cule M, Basty N, et al. Genetic evidence for different adiposity phenotypes and their opposing influences on ectopic fat and risk of cardiometabolic disease. Diabetes. 2021;70(8):1843-1856. [DOI] [PubMed] [Google Scholar]
- 22. Ruth KS, Day FR, Tyrrell J, et al. ; Endometrial Cancer Association Consortium. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med. 2020;26(2):252-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Homburg R, Crawford G. The role of AMH in anovulation associated with PCOS: a hypothesis. Hum Reprod. 2014;29(6):1117-1121. [DOI] [PubMed] [Google Scholar]
- 24. Verdiesen RM, van der Schouw YT, van Gils CH, et al. Genome-wide association study meta-analysis identifies three novel loci for circulating anti-Mullerian hormone levels in women. Posted November 2, 2020. medRxiv. doi: 10.1101/2020.10.29.20221390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scott RA, Fall T, Pasko D, et al. ; RISC study group; EPIC-InterAct consortium. Common genetic variants highlight the role of insulin resistance and body fat distribution in type 2 diabetes, independent of obesity. Diabetes. 2014;63(12):4378-4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhao Y, Fu L, Li R, et al. Metabolic profiles characterizing different phenotypes of polycystic ovary syndrome: plasma metabolomics analysis. BMC Med. 2012;10:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chang AY, Lalia AZ, Jenkins GD, et al. Combining a nontargeted and targeted metabolomics approach to identify metabolic pathways significantly altered in polycystic ovary syndrome. Metabolism. 2017;71:52-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang Z, Hong Y, Chen M, et al. Serum metabolomics reveals metabolic profiling for women with hyperandrogenism and insulin resistance in polycystic ovary syndrome. Metabolomics. 2020;16(2):20. [DOI] [PubMed] [Google Scholar]
- 29. Sun S, Jiao M, Han C, et al. Causal effects of genetically determined metabolites on risk of polycystic ovary syndrome: a Mendelian randomization study. Front Endocrinol. 2020;11:621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shin SY, Fauman EB, Petersen AK, et al. ; Multiple Tissue Human Expression Resource (MuTHER) Consortium. An atlas of genetic influences on human blood metabolites. Nat Genet. 2014;46(6):543-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adams CD, Boutwell BB. Can increasing years of schooling reduce type 2 diabetes (T2D)?: evidence from a Mendelian randomization of T2D and 10 of its risk factors. Sci Rep. 2020;10(1):12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhu T, Cui J, Goodarzi MO. Polycystic ovary syndrome and risk of type 2 diabetes, coronary heart disease, and stroke. Diabetes. 2021;70(2):627-637. [DOI] [PubMed] [Google Scholar]
- 33. Riaz H, Khan MS, Siddiqi TJ, et al. Association between obesity and cardiovascular outcomes: a systematic review and meta-analysis of mendelian randomization studies. JAMA Netw Open. 2018;1(7):e183788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Perry JR, Weedon MN, Langenberg C, et al. ; MAGIC. Genetic evidence that raised sex hormone binding globulin (SHBG) levels reduce the risk of type 2 diabetes. Hum Mol Genet. 2010;19(3):535-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Harris HR, Cushing-Haugen KL, Webb PM, et al. ; Australian Ovarian Cancer Study Group. Association between genetically predicted polycystic ovary syndrome and ovarian cancer: a Mendelian randomization study. Int J Epidemiol. 2019;48(3):822-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Phelan CM, Kuchenbaecker KB, Tyrer JP, et al. ; AOCS Study Group; EMBRACE Study; GEMO Study Collaborators; HEBON Study; KConFab Investigators; OPAL Study Group. Identification of 12 new susceptibility loci for different histotypes of epithelial ovarian cancer. Nat Genet. 2017;49(5):680-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yarmolinsky J, Relton CL, Lophatananon A, et al. Appraising the role of previously reported risk factors in epithelial ovarian cancer risk: a Mendelian randomization analysis. PLoS Med. 2019;16(8):e1002893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu PF, Li RZ, Zhang W, Hu HY, Wang W, Lin Y. Polycystic ovary syndrome is causally associated with estrogen receptor-positive instead of estrogen receptor-negative breast cancer: a Mendelian randomization study. Am J Obstet Gynecol. 2020;223(4):583-585. [DOI] [PubMed] [Google Scholar]
- 39. Michailidou K, Lindström S, Dennis J, et al. ; NBCS Collaborators; ABCTB Investigators; ConFab/AOCS Investigators. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017;551(7678):92-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wen Y, Wu X, Peng H, et al. Breast cancer risk in patients with polycystic ovary syndrome: a Mendelian randomization analysis. Breast Cancer Res Treat. 2021;185(3):799-806. [DOI] [PubMed] [Google Scholar]
- 41. Zhang H, Ahearn TU, Lecarpentier J, et al. ; kConFab Investigators; ABCTB Investigators; EMBRACE Study; GEMO Study Collaborators. Genome-wide association study identifies 32 novel breast cancer susceptibility loci from overall and subtype-specific analyses. Nat Genet. 2020;52(6):572-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhu T, Cui J, Goodarzi MO. Polycystic ovary syndrome and breast cancer subtypes: a Mendelian randomization study. Am J Obstet Gynecol. 2021;225(1):99-101. [DOI] [PubMed] [Google Scholar]
- 43. Harris HR, Terry KL. Polycystic ovary syndrome and risk of endometrial, ovarian, and breast cancer: a systematic review. Fertil Res Pract. 2016;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Real FG, Svanes C, Omenaas ER, et al. Menstrual irregularity and asthma and lung function. J Allergy Clin Immunol. 2007;120(3):557-564. [DOI] [PubMed] [Google Scholar]
- 45. van der Plaat DA, Minelli C, Jarvis DL, Garcia-Aymerich J, Leynaert B, Gómez-Real F. Polycystic ovary syndrome and lung function: a Mendelian randomization study. Am J Obstet Gynecol. 2020;223(3):455-457. [DOI] [PubMed] [Google Scholar]
- 46. Shorakae S, Boyle J, Teede H. Polycystic ovary syndrome: a common hormonal condition with major metabolic sequelae that physicians should know about. Intern Med J. 2014;44(8):720-726. [DOI] [PubMed] [Google Scholar]
- 47. Lawlor DA, Ebrahim S, Smith GD. Associations of measures of lung function with insulin resistance and Type 2 diabetes: findings from the British Women’s Heart and Health Study. Diabetologia. 2004;47(2):195-203. [DOI] [PubMed] [Google Scholar]
- 48. Machado V, Escalda C, Proenca L, Mendes JJ, Botelho J. Is there a bidirectional association between polycystic ovarian syndrome and periodontitis? A systematic review and meta-analysis. J Clin Med. 2020;9(6):1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Linden GJ, Lyons A, Scannapieco FA. Periodontal systemic associations: review of the evidence. J Periodontol. 2013;84(4 suppl):S8-S19. [DOI] [PubMed] [Google Scholar]
- 50. Wu PF, Zhang XH, Zhou P, et al. Assessment of bidirectional relationships between polycystic ovary syndrome and periodontitis: Insights from a Mendelian randomization analysis. Front Genet. 2021;12:664101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wu PF, Li RZ, Zhang W, Su ZZ, Lin YH. Polycystic ovary syndrome is not associated with offspring birth weight: a mendelian randomization study. Biomed Environ Sci. 2021;34(2):170-174. [DOI] [PubMed] [Google Scholar]
- 52. Dewailly D, Catteau-Jonard S, Reyss AC, Leroy M, Pigny P. Oligoanovulation with polycystic ovaries but not overt hyperandrogenism. J Clin Endocrinol Metab. 2006;91(10):3922-3927. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.