Abstract
Histone acetylation, catalyzed by histone acetyltransferases, has emerged as a promising therapeutic strategy in Alzheimer's disease (AD). By longitudinally characterizing spatial memory at 3, 6, and 9 mo of age, we show that acute activation and inhibition of the histone acetyltransferase PCAF remediated memory impairments in 3xTG-AD mice in an age-related bidirectional manner. At 3 and 6 mo of age, PCAF activation ameliorated memory deficits. At 9 mo of age, PCAF activation had no effect on spatial memory, whereas PCAF inhibition improved memory deficits in females. This work reveals a complex potential therapeutic role for PCAF in AD, initially benefitting memory but becoming detrimental as the disease progresses.
Alzheimer's disease (AD) is a debilitating neurodegenerative disorder characterized by progressive cognitive impairment related to brain pathology. Despite significant advances in the understanding of the molecular mechanisms that underlie AD, current therapies are minimally effective (Casey et al. 2010; Ibrahim and Gabr 2019). Epigenetic modifications act above the level of the DNA sequence to regulate gene expression in response to environmental stimuli, eliciting dynamic changes that are critical for forming memories and maintaining the proper balance of proteins that produce and degrade molecules that cause pathology in neurodegenerative diseases such as AD (Selvi et al. 2010; Sweatt 2013). Therefore, epigenetic therapies may be effective in treating both memory decline and neuropathology in AD.
Histone acetylation, one of the most extensively studied epigenetic modifications, generally promotes gene expression via the addition of acetyl groups, by histone acetyltransferases (HATs), to lysine residues on histone tails around which DNA is wrapped, thereby promoting access of transcriptional machinery to the DNA (Kuo and Allis 1998). Histone deacetylation, catalyzed by histone deacetylases (HDACs), removes acetyl groups from histone proteins and induces gene repression (De Ruijter et al. 2003). Both HAT activation and HDAC inhibition increase histone acetylation, which regulates the expression of genes that support many facets of learning and memory (Gräff and Tsai 2013; Peixoto and Abel 2013).
Studies examining the therapeutic effects of increasing histone acetylation in AD have predominantly focused on HDAC inhibition. In transgenic mouse models of AD, global and subtype-specific HDAC inhibition have been shown to attenuate behavioral deficits in contextual fear conditioning (Fischer et al. 2007; Francis et al. 2009; Kilgore et al. 2010; Cuadrado-Tejedor et al. 2015, 2017), Morris water maze (MWM) (Ricobaraza et al. 2009; Gräff et al. 2012; Govindarajan et al. 2013; Sung et al. 2013; Cuadrado-Tejedor et al. 2015, 2017), exploratory behavior (Govindarajan et al. 2011), and nesting behavior (Zhang and Schluesener 2013), as well as restore levels of histone acetylation (Fischer et al. 2007; Francis et al. 2009; Ricobaraza et al. 2009, 2012; Govindarajan et al. 2011; Gräff et al. 2012) and decrease amyloid and tau pathology (Sung et al. 2013; Zhang and Schluesener 2013). Although HDAC inhibition appears to have a robust effect on memory enhancement (memory impairments were attenuated even in 15-mo-old mice with very advanced stages of AD pathology) (Govindarajan et al. 2011), there are several limitations associated with using HDAC inhibitors to treat cognitive impairment. The mechanism of action of many HDAC inhibitors is poorly understood, broad-spectrum HDAC inhibition can be toxic, few selective HDAC inhibitors are commercially available, memory-enhancing effects of HDACs can be dependent on proper HAT function (Vecsey et al. 2007; Chen et al. 2010), and the primary function of HDACs is to remove acetyl groups added by HATs (Day and Sweatt 2011). This suggests that modulation of specific HATs could be a promising alternative therapeutic approach.
Although selective HAT activators are commercially available for prominent HATs (e.g., CREB-binding protein [CBP], E1A-binding protein [p300], and p300/CBP-associated factor [PCAF]) and increasing lysine acetylation has positive effects on cognition, very few studies have evaluated the therapeutic potential of HATs in preclinical AD models. One study demonstrated that increased CBP expression attenuated MWM impairments in 6-mo-old 3xTG mice (Caccamo et al. 2010). Similarly, Chatterjee et al. (2018) demonstrated that CSP-TTK21, a CBP and p300 activator, restores long-term depression (LTD), dendritic spine density, and MWM performance in 8-mo-old THY-Tau22 transgenic mice. These results demonstrate that increasing the activity of the HATs CBP and p300 positively regulates cognition in mouse models of AD and therefore could be an effective therapeutic strategy.
Interestingly, PCAF may function atypically in AD. While PCAF activation enhances memory in normal rodents (Wei et al. 2012; Mitchnick 2018), in Aβ-treated rodents, PCAF inhibition or KO attenuates AD-like cognitive deficits (Duclot et al. 2010b; Park et al. 2013, 2015), suggesting that PCAF activity may actually be detrimental when disease is present. Indeed, the PCAF inhibitor, C-30-27, decreased Aβ-induced inflammation and cell death in cells (Park et al. 2013) and inhibited nonhistone acetylation of NF-κB in Aβ-treated rats, improving MWM performance (Park et al. 2015). Similarly, Duclot et al. (2010b) demonstrated that PCAF KO mice were protected against Aβ-induced toxicity and cognitive impairment following intraventricular Aβ treatment. These studies suggest that HAT activity and acetylation patterns in AD are likely complex and memory deficits may not always be ameliorated by simply activating HATs.
We hypothesize that PCAF functions bidirectionally in AD, initially benefitting memory in young/cognitively unimpaired mice but is detrimental as neuropathology/cognitive impairments become more severe. We explored the therapeutic potential of PCAF activation and inhibition on hippocampus-dependent spatial memory in male and female wild-type (Wt; B6129SF2/J) and triple transgenic [3xTG; B6;129 Psen1tm1Mpm Tg(APPSwe,tauP301L)1Lfa/Mmjax] mice. Spatial memory was longitudinally tested at 3, 6, and 9 mo of age in the same group of mice. These timepoints were chosen based on our previous work (Beraldo et al. 2019). We show that PCAF bidirectionally regulates spatial memory in 3xTG mice. At 3 and 6 mo of age, PCAF activation attenuated memory deficits, but by 9 mo of age, this approach was no longer effective and PCAF inhibition, which impaired memory in wild-type (Wt) mice, attenuated the long-term spatial memory deficit in female 3xTG mice.
To mimic systemic drug administration that is used therapeutically, we pharmacologically manipulated PCAF using the activator SPV106 and inhibitor embelin. Mice were habituated to the injection procedure with two injections of physiological saline prior to the start of each behavioral experiment. SPV106 (Sbardella et al. 2008; Milite et al. 2011), or vehicle (1% DMSO) treatments were administered 3 d presample at 25 mg/kg intraperitoneally (i.p.). Six days were left between SPV106 injections in a within-subjects design. This dosing regimen was based on our pilot data and findings from another group demonstrating behavioral enhancements following systemic SPV106 administration in healthy rodents 3-d after administration (Wei et al. 2012; Mitchnick et al. 2016). Embelin (2,5-dihydroxy-3-undecyl-2,5-cyclohexadiene-1,4-dione; Abcam) is a noncompetitive PCAF antagonist. Acute embelin or vehicle (5% DMSO + 5%Tween 20) treatments were administered immediately postsample (Mitchnick et al. 2016) at 10 mg/kg i.p. At least 3 d were left between embelin injections in a within-subjects design. Twelve days were left between SPV106 and embelin experiments at 9 mo of age to allow for ample drug washout.
We used the object location (OL) (Fig. 1) task to assess hippocampus-dependent spatial memory because the hippocampus (HPC) is a brain region severely affected by neuropathology at relatively early stages of AD, AD patients frequently suffer from impairments in various aspects of spatial processing including spatial navigation and spatial memory (e.g., Toledo-Morrell and Dickerson 2000; DeIpolyi et al. 2007; Moodley et al. 2015), and the one-trial nature of this task does not require extensive training, aversive stimuli, or reward; therefore, memory can be evaluated in a manner similar to daily human interaction with objects (Ennaceur and Delacour 1988; Dere et al. 2007). In the sample phase, mice explored two identical objects for 10 min. Then there was a 5-min or 3-h retention delay to assess short- and long-term memory, respectively. At the end of the retention delay, mice underwent a 3-min choice phase, in which one object from the sample phase was moved to an adjacent corner of the arena. Objects had no apparent biological significance to mice and were distinct in size (5–15 cm tall), material (glass, metal, and plastic), and color and were previously found to produce no obvious preference biases in mice. The order of object pairs and the side of the apparatus (left or right) where the novel object was placed during the choice phase were counterbalanced. Prior to each behavioral trial, objects were wiped with 50% ethanol (to eliminate olfactory cues), and the testing apparatus was wiped with dry paper towel. Performance was determined by calculating a discrimination ratio [DR = (relocated object exploration − familiar object exploration)/(total object exploration)]. A DR significantly greater than the sample DR indicates novelty preference, from which we infer intact spatial memory. Split plot analysis of variance (ANOVA) was used to analyze DRs with retention delay and drug as within-subjects factors and sex and genotype as between-subjects factors. We report partial eta squared (ηp2) as an index of effect size. Paired samples t-tests were used as a complementary analysis to compare sample and choice DRs, as a significant increase in the DR from sample (when objects are equally novel) to choice is indicative of intact memory. Sample DRs are reported in Supplemental Tables S1 and S2. Outliers (>2 SD ± mean) were excluded from analyses (see Supplemental Tables S3, S4). All statistical analyses were conducted with a significance level of α = 0.05 using IBM SPSS statistics. Where appropriate, the Bonferroni correction was applied. To account for confounding genotype and/or drug effects on memory performance, we also examined exploratory behavior and the correlation between total exploration (sample or choice) and task performance (choice DR) (see Supplemental Tables S5–S8).
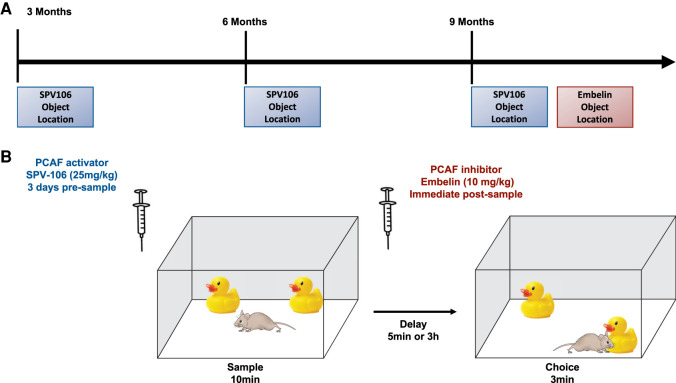
Figure 1.
(A) Schematic representation of the longitudinal experimental time line. Behavioral testing was conducted at 3, 6, and 9 mo of age in the same group of mice. (B) Schematic representation of the object location (OL) task. Object location was run in an open-field arena (45 × 45 × 30 cm). Prior to behavioral testing all mice were extensively handled and habituated to an empty testing apparatus for 10 min on two consecutive days. In the sample phase, the mice explored two identical objects. Following a variable retention delay, in the choice phase, the mice explored copies of the sample objects. One object remained in the same position as the sample phase, while the other object was positioned in a novel location. Memory is inferred by preferential exploration of the object in the novel location during the choice phase. Objects had no apparent biological significance to mice and were distinct in size (5–15 cm tall), material (glass, metal, and plastic), and color. The order of object pairs and the side of the apparatus (left or right) where the novel object was placed during the choice phase were counterbalanced. SPV106 or vehicle (1% DMSO) was administered systemically 3 d presample at 25 mg/kg i.p. Acute embelin or vehicle (5% DMSO + 5%Tween 20) treatments were administered immediately postsample (Mitchnick et al. 2016) at 20 mg/kg.
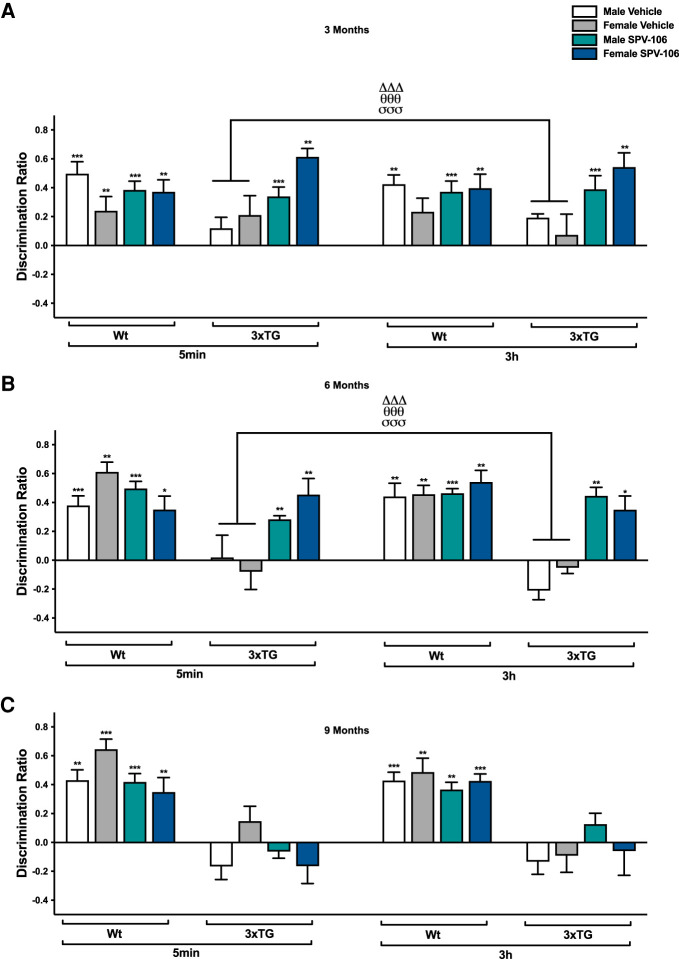
Administration of the PCAF activator SPV106 ameliorated OL deficits in 3-mo-old 3xTG mice (genotype × sex interaction: F(1,43) = 4.626, P = 0.037, η2p = 0.097; genotype × drug: F(1,43) = 13.929, P = 0.001, η2p = 0.245; sex × drug: F(1,43) = 8.264, P = 0.005, η2p = 0.167; and main effect of drug: F(1,43) = 20.764, P < 0.001, η2p = 0.326) (Fig. 2A). Post hoc t-tests revealed a significant difference between vehicle and SPV106-treated 3xTG mice (t(45) = −5.522, P < 0.001) and between vehicle-treated 3xTG mice and Wt mice (vehicle: t(92) = 2.977, P = 0.032; SPV106: t(92) = 3.672, P < 0.001). Complementary analysis with paired sample t-tests between sample and choice discrimination ratios (DRs) indicated intact spatial memory in all Wt mice (vehicle-treated males, 5 min: t(11) = −6.584, P < 0.001; SPV106-treated males, 5 min: t(11) = −6.197, P < 0.001; vehicle-treated males, 3 h: t(11) = −4.567, P = 0.001; SPV106-treated males, 3 h: t(11) = −7.113, P < 0.001; vehicle-treated females, 5 min: t(11) = −3.692, P = 0.004; SPV106-treated females, 5 min: t(11) = −3.283, P = 0.007; SPV106-treated females, 3 h: t(11) = −4.458, P = 0.001), except vehicle-treated Wt females at 3 h. Vehicle-treated 3xTG mice were impaired, and pretreatment with SPV106 ameliorated these impairments (males, 5 min: t(11) = −6.197, P < 0.001; males, 3 h: t(11) = −5.814, P < 0.001; females, 5 min: t(11) = −4.068, P = 0.002; females, 3 h: t(11) = −4.020, P = 0.002).
Figure 2.
Age-dependent amelioration of spatial memory by PCAF activation (SPV106 at 25 mg/kg) in 3xTG mice. (A) At 3 mo of age, vehicle-treated 3xTG mice were delay-independently impaired on OL; treatment with SPV106 ameliorated this impairment. N = 12 Wt male, N = 12 Wt female, N = 12 3xTG male, N = 12 3xTG female. (B) At 6 mo of age, vehicle-treated 3xTG mice were delay-independently impaired on OL; treatment with SPV106 continued to ameliorate this impairment. N = 12 Wt male, N = 10 Wt female, N = 11 3xTG male, N = 11 3xTG female. (C) At 9 mo of age, vehicle-treated 3xTG mice were delay-independently impaired on OL, but treatment with SPV106 failed to attenuate this impairment. N = 12 Wt male, N = 10 Wt female, N = 11 3xTG male, N = 11 3xTG female. Data are mean ± SEM. (*) P < 0.05, (**) P < 0.01, (***) P < 0.001, indicates significant differences between sample and choice DR. (ΔΔΔ) P < 0.001 significantly different from vehicle-treated Wt mice, (θθθ) P < 0.001 significantly different from SPV106-treated Wt mice, (σσσ) P < 0.001 significantly different from SPV106-treated 3xTG mice.
Similarly, at 6 mo old, SPV106 ameliorated OL deficits in 3xTG mice (genotype × drug: F(1,43) = 35.906, P < 0.001, η2p = 0.455; genotype: F(1,43) = 51.508, P < 0.001, η2p = 0.545; and main effect of drug: F(1,43) = 33.185, P < 0.001, η2p = 0.436) (Fig. 2B). Post hoc t-tests indicated a significant difference between vehicle and SPV106-treated 3xTG mice (t(45) = −6.747, P < 0.001) and between Wt and vehicle-treated 3xTG mice and Wt mice (vehicle: t(92) = 8.631, P < 0.001; SPV106: t(92) = 8.707, P < 0.001). Complementary paired samples t-tests between sample and choice DRs suggested intact spatial memory in Wt mice (vehicle-treated males, 5 min: t(11) = −6.824, P < 0.001; SPV106-treated males, 5 min: t(11) = −8.338, P < 0.001; vehicle-treated males, 3 h: t(11) = −3.953, P = 0.002; SPV106-treated males, 3 h: t(11) = −12.295, P < 0.001; vehicle-treated females, 5 min: t(11) = −8.058, P < 0.001; SPV106-treated females, 5 min: t(11) = −2.443, P = 0.033; vehicle-treated females, 3 h: t(11) = −3.178, P = 0.009; SPV106-treated females, 3 h: t(11) = −4.423, P = 0.001), as well as SPV106-treated 3xTG mice (males, 5 min: t(11) = −3.501, P = 0.005; males, 3 h: t(11) = −3.595, P = 0.004; females, 5 min: t(11) = −4.836, P = 0.001; females, 3 h: t(11) = −2.622, P = 0.025).
At 9 mo old, SPV106 failed to attenuate OL deficits in 3xTG mice (drug × sex: F(1,40) = 6.154, P = 0.017, η2p = 0.133; and main effect of genotype: F(1,40) = 99.230, P < 0.001, η2p = 0.713) (Fig. 2C). Likewise, paired samples t-tests between sample and choice DRs indicated intact spatial memory in Wt mice (vehicle-treated males, 5 min: t(11) = −4.303, P = 0.001; SPV106-treated males, 5 min: t(11) = −7.066, P < 0.001; vehicle-treated males, 3 h: t(11) = −5.440, P < 0.001; SPV106-treated males, 3 h: t(11) = −4.236, P = 0.001; vehicle-treated females, 5 min: t(9) = −9.508, P < 0.001; SPV106-treated females, 5 min: t(9) = −3.618, P = 0.006; vehicle-treated females, 3 h: t(9) = −3.249, P = 0.010; SPV106-treated females, t(9) = −6.215, P < 0.001) but not 3xTG mice.
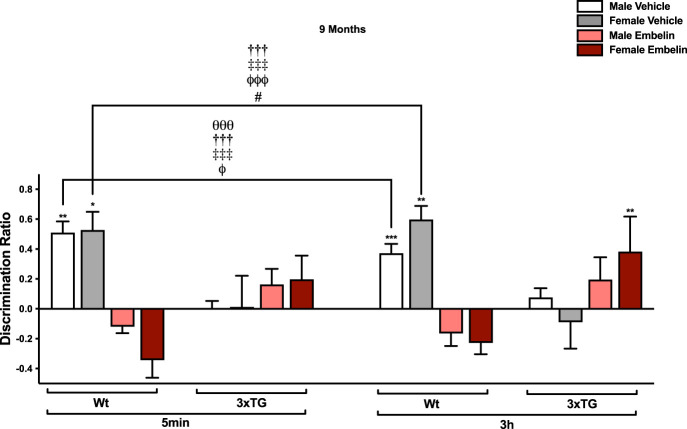
We subsequently evaluated the effects of acute PCAF inhibition on OL, in the same group of mice, after SPV106 failed to ameliorate spatial memory deficits. At this age, PCAF inhibition using embelin, impaired memory in Wt mice but attenuated spatial memory deficits in aged female 3xTG mice (genotype × sex × drug: F(1,38) = 6.330, P = 0.016, η2p = 0.143; genotype × drug: F(1,38) = 110.642, P < 0.001, η2p = 0.744; and main effect of drug: F(1,38) = 28.441, P < 0.001, η2p = 0.428) (Fig. 3). Post hoc t-tests revealed significant differences between vehicle-treated Wt males and embelin-treated Wt mice (male: t(21) = 8.442, P < 0.001; female: t(40) = 1.074, P < 0.001), as well as vehicle-treated 3xTG mice (males: t(40) = 6.572, P < 0.001; females: t(28.822) = 3.863, P = 0.014). In addition, there were also significant differences between vehicle-treated Wt females and embelin-treated Wt mice (males: t(40) = −9.520, P < 0.001; females t(19) = 11.730, P < 0.001), vehicle-treated 3xTG mice (males: t(29.873) = −7.766, P < 0.001, females: t(31.274) = 4.700, P < 0.001), and embelin-treated 3xTG males (t(38) = −3.688, P = 0.014). Complementary paired sample t-tests between sample and choice DR suggest intact spatial memory in vehicle-treated Wt mice (males, 5 min: t(10) = −4.958, P = 0.001; males, 3 h: t(10) = −5.137, P < 0.001; females, 5 min: t(9) = −2.819, P = 0.020; females, 3 h: t(9) = −4.239, P = 0.002) and embelin-treated 3xTG females at the 3 h delay (t(10) = −2.803, P = 0.019).
Figure 3.
Regulation of spatial memory by PCAF inhibition (embelin 10 mg/kg). At 9 mo of age, embelin impaired Wt mice at both delays. Vehicle-treated 3xTG mice were impaired at the 5 min and 3 h delay; embelin may attenuate this impairment, particularly in 3xTG females at 3 h. N = 11 Wt male, 10 Wt female, 10 3xTG male, 11 3xTG female. N = 11 Wt male, 10 Wt female, 10 3xTG male, 11 3xTG female. Data are mean ± SEM. (*) P < 0.05, (**) P < 0.01, (***) P < 0.001 indicates significant differences between sample and choice DR. (θθθ) P < 0.001 significantly different from embelin-treated Wt males, (†††) P < 0.001 significantly different from embelin-treated Wt females, (‡‡‡) P < 0.001 significantly different from vehicle-treated 3xTG males, (φ) P < 0.01, (φφφ) P < 0.001 significantly different from vehicle-treated 3xTG females, (#) P < 0.05 significantly different from embelin-treated 3xTG males.
By longitudinally evaluating the effects of PCAF activation on OL memory, these experiments demonstrate severe impairments in short- and long-term OL memory in 3xTG mice and suggest that PCAF activation can ameliorate spatial memory deficits, but likely in an age-dependent manner. These findings suggest a complex role of PCAF in AD-relevant cognition, by which PCAF is initially beneficial for OL memory but may become detrimental, possibly interacting with the ongoing development of AD pathology. Our findings help to clarify the discrepant results demonstrating that while PCAF activation enhances memory in normal rodents (Maurice et al. 2008; Duclot et al. 2010a; Wei et al. 2012; Mitchnick et al. 2016; Mitchnick 2018), in Aβ-treated rodents PCAF inhibition or KO attenuates AD-like cognitive deficits (Duclot et al. 2010b; Park et al. 2013, 2015).
Th effects of PCAF activation and inhibition on spatial memory are likely mediated by alterations in the acetylation of both histone and nonhistone proteins. The beneficial effects of PCAF activation on long-term memory reported here are likely linked to increases in histone acetylation. Indeed, SPV106 has been shown to increase global levels of H3 and H4 acetylation (Sbardella et al. 2008; Milite et al. 2011), which can correlate with transcriptional activity and long-term memory enhancement (e.g., Levenson et al. 2004; Pokholok et al. 2005; Bousiges et al. 2010; Peleg et al. 2010; Chatterjee et al. 2018). It is also possible that PCAF-induced acetylation of nonhistone proteins facilitates OL memory, especially given the enhancing effects of SPV106 on short-term OL memory (5 min). Previous work from our group and others has demonstrated that PCAF, unlike other HATs, is necessary for short-term memory (Maurice et al. 2008; Duclot et al. 2010a; Mitchnick et al. 2016). Given the relatively short retention delays (<20 min) used in these experiments, it is more likely that acetylation of cytosolic proteins that initiate nongenomic effects (e.g., intracellular signaling cascades, protein stability, or protein degradation) supports short-term memory. Indeed, Mitchnick (2018) show that PCAF interacts with estrogen receptor α to facilitate short-term object memory. By 9 mo of age, SPV106 failed to attenuate spatial memory deficits, suggesting that the memory promoting effects of PCAF activation are insufficient to overcome OL impairments induced by AD neuropathology at this age. It is also possible that PCAF activation contributes to AD pathology. Indeed, histone acetylation and PCAF activity have been shown to regulate genes upstream of Aβ degradation (e.g., APP, β-site APP cleavage enzyme [BACE] 1, PS1, and somatostatin) and inflammation (e.g., interleukin [IL]-1β, IL-6, and tumor necrosis factor-α [TNF-α]) (Ito et al. 2000; Duclot et al. 2010b; Guo et al. 2011). Furthermore, PCAF activity has been shown to promote the nonhistone acetylation of NF-κB, which has been linked to increased inflammation and levels of Aβ (Park et al. 2013, 2015). At this advanced age, the beneficial effect of embelin treatment in 3xTG females could result from inhibition of these pathological cascades.
It is not clear why the OL impairments were not attenuated in aged 3xTG males. Perhaps there is some protective effect of estrogens in females; indeed, coadministration of an estrogen receptor antagonist has been shown to block the memory-enhancing effects of SPV106 (Mitchnick et al. 2016). The lack of remediation by embelin in aged males may also suggest that OL impairments are worse in 3xTG males, which is in agreement with our previous findings, using a similar object task, demonstrating that 3xTG males had more severe object memory impairments when the spatial nature of the task was increased (Creighton et al. 2019). In addition to this major sex difference, we also observed more subtle differences in the magnitude of the discrimination ratio between male and female mice. For example, at 3 mo of age, Wt males performed better than Wt females. This difference was not observed at later ages. Sex-specific effects are particularly relevant in AD since females often exhibit more severe pathological change and cognitive deficits (Rocca et al. 1986; Ruitenberg et al. 2001). Similar sex differences in pathology, longevity, and cognition are seen in several transgenic mouse models of AD, including 3xTG mice (Carroll et al. 2010; Clinton et al. 2007; Creighton et al. 2019; Hirata-Fukae et al. 2008; Rae and Brown 2015; Mendell et al. 2020).
These experiments demonstrate a significant amelioration of cognitive deficits in 3xTG mice, even at advanced ages, following either acute activation or inhibition of PCAF, providing additional support for the use of epigenetic therapies in neurodegenerative diseases like AD. It remains to be seen whether other HATs and other epigenetic factors also function bidirectionally in AD. Although many studies have shown that epigenetic therapies strictly attenuate cognitive deficits in transgenic AD mouse models, these studies have not used a systematic longitudinal design. In conclusion, the complex role of PCAF throughout the progression of AD suggests that greater mechanistic insight into the interactions between HATs, AD pathology, and cognition is required for the success of future epigenetic therapies.
Supplementary Material
Acknowledgments
This work was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (400176) to B.D.W.
Footnotes
[Supplemental material is available for this article.]
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.053536.121.
References
- Beraldo FH, Palmer D, Memar S, Wasserman DI, Lee WJ V, Liang S, Creighton SD, Kolisnyk B, Cowan MF, Mels J, et al. 2019. MouseBytes, an open-access high-throughput pipeline and database for rodent touchscreen-based cognitive assessment. Elife 8: e49630. 10.7554/eLife.49630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousiges O, De Vasconcelos AP, Neidl R, Cosquer B, Herbeaux K, Panteleeva I, Loeffler J-P, Cassel J-C, Boutillier A-L. 2010. Spatial memory consolidation is associated with induction of several lysine-acetyltransferase (histone acetyltransferase) expression levels and H2B/H4 acetylation-dependent transcriptional events in the rat hippocampus. Neuropsychopharmacology 35: 2521–2537. 10.1038/npp.2010.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A, Maldonado MA, Bokov AF, Majumder S, Oddo S. 2010. CBP gene transfer increases BDNF levels and ameliorates learning and memory deficits in a mouse model of Alzheimer's disease. Proc Natl Acad Sci 107: 22687–22692. 10.1073/pnas.1012851108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JC, Rosario ER, Kreimer S, Villamgna A, Gentzschein E, Stanczyk FZ, Pike CJ. 2010. Sex differences in β-amyloid accumulation in 3xTG mice: role of neonatal sex steroid hormone exposure. Brain Res 1366: 233–245. 10.1016/j.brainres.2010.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey DA, Antimisiaris D, O'Brien J. 2010. Drugs for Alzheimer's disease: are they effective? P T 35: 208–211. [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Cassel R, Schneider-Anthony A, Merienne K, Cosquer B, Tzeplaeff L, Halder Sinha S, Kumar M, Chaturbedy P, Eswaramoorthy M, et al. 2018. Reinstating plasticity and memory in a tauopathy mouse model with an acetyltransferase activator. EMBO Mol Med 10: e8587. 10.15252/emmm.201708587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Zou X, Watanabe H, Van Deursen JM, Shen J. 2010. CREB binding protein is required for both short-term and long-term memory formation. J Neurosci 30: 13066–13077. 10.1523/JNEUROSCI.2378-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton LK, Billings LM, Green KN, Caccamo A, Ngo J, Oddo S, McGaugh JL, LaFerla FM. 2007. Age-dependent sexual dimorphism in cognition and stress response in the 3xTG-AD mice. Neurobiol Dis 28: 76–82. 10.1016/j.nbd.2007.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton SD, Mendell AL, Palmer D, Kalisch BE, MacLusky NJ, Prado VF, Prado MAM, Winters BD. 2019. Dissociable cognitive impairments in two strains of transgenic Alzheimer's disease mice revealed by a battery of object-based tests. Sci Rep 9: 57. 10.1038/s41598-018-37312-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado-Tejedor M, Garcia-Barroso C, Sanzhez-Arias J, Mederos S, Rabal O, Ugarte A, Franco R, Pascual-Lucas M, Segura V, Perea G, et al. 2015. Concomitant histone deacetylase and phosphodiesterase 5 inhibition synergistically prevents the disruption in synaptic plasticity and it reverses cognitive impairment in a mouse model of Alzheimer's disease. Clin Epigenetics 7: 1–11. 10.1186/s13148-015-0142-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado-Tejedor M, Garcia-Barroso C, Sanchez-Arias JA, Rabal O, Perez-Gonzalez M, Mederos S, Ugarte A, Franco R, Segura V, Perea G, et al. 2017. A first-in-class small-molecule that acts as a dual inhibitor of HDAC and PDE5, and that rescues hippocampal synaptic impairment in Alzheimer's disease mice. Neuropsychopharmacology 42: 524–539. 10.1038/npp.2016.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Sweatt JD. 2011. Epigenetic treatments for cognitive impairments. Neuropsychopharmacology 37: 247–260. 10.1038/npp.2011.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeIpolyi AR, Rankin KP, Mucke L, Miller BL, Gorno-Tempini ML. 2007. Spatial cognition and the human navigation network in AD and MCI. Neurology 69: 986–997. 10.1212/01.wnl.0000271376.19515.c6 [DOI] [PubMed] [Google Scholar]
- Dere E, Huston JP, De Souza Silva MA. 2007. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev 31: 673–704. 10.1016/j.neubiorev.2007.01.005 [DOI] [PubMed] [Google Scholar]
- De Ruijter A, Van Gennip A, Caron H, Kemp S, Van Kuilenburg A. 2003. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem 749: 737–749. 10.1042/bj20021321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclot F, Jacquet C, Gongora C, Maurice T. 2010a. Alteration of working memory but not in anxiety or stress response in p300/CBP associated factor (PCAF) histone acetylase knockout mice bred on a C57BL/6 background. Neurosci Lett 475: 179–183. 10.1016/j.neulet.2010.03.077 [DOI] [PubMed] [Google Scholar]
- Duclot F, Meffre J, Jacquet C, Gongora C, Maurice T. 2010b. Mice knock out for the histone acetyltransferase p300/CREB binding protein-associated factor develop a resistance to amyloid toxicity. Neuroscience 167: 850–863. 10.1016/j.neuroscience.2010.02.055 [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. 1988. A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav Brain Res 31: 47–59. 10.1016/0166-4328(88)90157-X [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Wang X, Dobbin MM, Tsai L. 2007. Recovery of learning and memory is associated with chromatin remodelling. Nature 447: 178–182. 10.1038/nature05772 [DOI] [PubMed] [Google Scholar]
- Francis YI, Fà M, Ashraf H, Zhang H, Staniszewski A, Latchman DS, Arancio O. 2009. Dysregulation of histone acetylation in the APP/PS1 mouse model of Alzheimer's disease. J Alzheimer's Dis 18: 131–139. 10.3233/JAD-2009-1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan N, Agis-Balboa RC, Walter J, Sananbenesi F, Fischer A. 2011. Sodium butyrate improves memory function in an Alzheimer's disease mouse model when administered at an advanced stage of disease progression. J Alzheimer's Dis 26: 187–197. 10.3233/JAD-2011-110080 [DOI] [PubMed] [Google Scholar]
- Govindarajan N, Rao P, Burkhardt S, Sananbenesi F, Schlüter OM, Bradke F, Lu J, Fischer A. 2013. Reducing HDAC6 ameliorates cognitive deficits in a mouse model for Alzheimer's disease. EMBO Mol Med 5: 52–63. 10.1002/emmm.201201923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräff J, Tsai LH. 2013. Histone acetylation: molecular mnemonics on the chromatin. Nat Rev Neurosci 14: 97–111. 10.1038/nrn3427 [DOI] [PubMed] [Google Scholar]
- Gräff J, Rei D, Guan J-S, Wang W-Y, Seo J, Hennig KM, Nieland TJF, Fass DM, Kao PF, Kahn M, et al. 2012. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature 483: 222–226. 10.1038/nature10849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JU, Ma DK, Mo H, Ball MP, Jang M-H, Bonaguidi MA, Balazer JA, Eaves HL, Xie B, Ford E, et al. 2011. Neuronal activity modifies the DNA methylation landscape in the adult brain. Nat Neurosci 14: 1345–1351. 10.1038/nn.2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata-Fukae C, Li H, Hoe H, Gray AJ, Minami SS, Hamada K, Niikura T, Hua F, Tsukagoshi-Nagai H, Horikoshi-Sakuraba Y, et al. 2008. Females exhibit more extensive amyloid, but not tau, pathology in an Alzheimer transgenic model. Brain Res 1216: 92–103. 10.1016/j.brainres.2008.03.079 [DOI] [PubMed] [Google Scholar]
- Ibrahim MM, Gabr MT. 2019. Multitarget therapeutic strategies for Alzheimer's disease. Neural Regen Res 14: 437–440. 10.4103/1673-5374.245463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Barnes PJ, Adcock IM. 2000. Glucocorticoid receptor recruitment of histone deacetylase 2 inhibits interleukin-1beta-induced histone H4 acetylation on lysines 8 and 12. Mol Cell Biol 20: 6891–6903. 10.1128/MCB.20.18.6891-6903.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgore M, Miller CA, Fass DM, Hennig KM, Haggarty SJ, Sweatt JD, Rumbaugh G. 2010. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer's disease. Neuropsychopharmacology 35: 870–880. 10.1038/npp.2009.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M, Allis CD. 1998. Roles of histone acetyltransferases and deacetylases in gene regulation. Bioessays 20: 615–626. [DOI] [PubMed] [Google Scholar]
- Levenson JM, O'Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. 2004. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem 279: 40545–40559. 10.1074/jbc.M402229200 [DOI] [PubMed] [Google Scholar]
- Maurice T, Duclot F, Meunier J, Naert G, Givalois L, Meffre J, Célérier A, Jacquet C, Copois V, Mechti N, et al. 2008. Altered memory capacities and response to stress in p300/CBP-associated factor (PCAF) histone acetylase knockout mice. Neuropsychopharmacology 33: 1584–1602. 10.1038/sj.npp.1301551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell AL, Creighton SD, Wilson HA, Jardine KH, Isaacs L, Winters BD, MacLusky NJ. 2020. Inhibition of 5α reductase impairs cognitive performance, alters dendritic morphology and increases tau phosphorylation in the hippocampus of male 3xTg-AD mice. Neuroscience 429: 185–202. 10.1016/j.neuroscience.2020.01.011 [DOI] [PubMed] [Google Scholar]
- Milite C, Castellano S, Benedetti R, Tosco A, Ciliberti C, Vicidomini C, Boully L, Franci G, Altucci L, Mai A, et al. 2011. Modulation of the activity of histone acetyltransferases by long chain alkylidenemalonates (LoCAMs). Bioorganic Med Chem 19: 3690–3701. 10.1016/j.bmc.2011.01.013 [DOI] [PubMed] [Google Scholar]
- Mitchnick KA. 2018. The dissociable involvement of epigenetic mechanisms in hippocampus- and perirhinal cortex-mediated object memory in male rats. PhD thesis. The University of Guelph, Guelph, ON, Canada. [Google Scholar]
- Mitchnick KA, Creighton SD, Cloke JM, Wolter M, Zaika O, Christen B, Van TM, Kalisch BE, Winters BD. 2016. Dissociable roles for histone acetyltransferase p300 and PCAF in hippocampus and perirhinal cortex-mediated object memory. Genes Brain Behav 15: 542–557. 10.1111/gbb.12303 [DOI] [PubMed] [Google Scholar]
- Moodley K, Minati L, Contarino V, Prioni S, Wood R, Cooper R, D'Incerti L, Tagliavini F, Chan D. 2015. Diagnostic differentiation of mild cognitive impairment due to Alzheimer's disease using a hippocampus-dependent test of spatial memory. Hippocampus 25: 939–951. 10.1002/hipo.22417 [DOI] [PubMed] [Google Scholar]
- Park SY, Lee YH, Seong AR, Lee J, Jun W, Yoon HG. 2013. Selective inhibition of PCAF suppresses microglial-mediated β-amyloid neurotoxicity. Int J Mol Med 32: 469–475. 10.3892/ijmm.2013.1407 [DOI] [PubMed] [Google Scholar]
- Park SY, Kim MJ, Kim YJ, Lee YH, Bae D, Kim S, Na Y, Yoon HG. 2015. Selective PCAF inhibitor ameliorates cognitive and behavioral deficits by suppressing NF-KB-mediated neuroinflammation induced by AB in a model of Alzheimer's disease. Int J Mol Med 35: 1109–1118. 10.3892/ijmm.2015.2099 [DOI] [PubMed] [Google Scholar]
- Peixoto L, Abel T. 2013. The role of histone acetylation in memory formation and cognitive impairments. Neuropsychopharmacology 38: 62–76. 10.1038/npp.2012.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, Cota P, Wittnam JL, Gogol-Doering A, Opitz L, et al. 2010. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science 328: 753–756. 10.1126/science.1186088 [DOI] [PubMed] [Google Scholar]
- Pokholok DK, Harbison CT, Levine S, Cole M, Hannett NM, Tong IL, Bell GW, Walker K, Rolfe PA, Herbolsheimer E, et al. 2005. Genome-wide map of nucleosome acetylation and methylation in yeast. Cell 122: 517–527. 10.1016/j.cell.2005.06.026 [DOI] [PubMed] [Google Scholar]
- Rae EA, Brown RE. 2015. The problem of genotype and sex differences in life expectancy in transgenic AD mice. Neurosci Biobehav Rev 57: 238–251. 10.1016/j.neubiorev.2015.09.002 [DOI] [PubMed] [Google Scholar]
- Ricobaraza A, Cuadrado-Tejedor M, Pérez-Mediavilla A, Frechilla D, Del Río J, García-Osta A. 2009. Phenylbutyrate ameliorates cognitive deficit and reduces tau pathology in an Alzheimer's disease mouse model. Neuropsychopharmacology 34: 1721–1732. 10.1038/npp.2008.229 [DOI] [PubMed] [Google Scholar]
- Ricobaraza A, Cuadrado-tejedor M, Marco S, Perez-Otano I, Garcia-Osta A. 2012. Phenylbutyrate rescues dendritic spine loss associated with memory deficits in a mouse model of Alzheimer disease. Hippocampus 22: 1040–1050. 10.1002/hipo.20883 [DOI] [PubMed] [Google Scholar]
- Rocca WA, Amaducci LA, Schoenberg BS. 1986. Epidemiology of clinically diagnosed Alzheimer's disease. Ann Neurol 19: 415–424. 10.1002/ana.410190502 [DOI] [PubMed] [Google Scholar]
- Ruitenberg A, Ott A, van Swieten JC, Hofman A, Breteler MMB. 2001. Incidence of dementia: does gender make a difference? Neurobiol Aging 22: 575–580. 10.1016/s0197-4580(01)00231-7 [DOI] [PubMed] [Google Scholar]
- Sbardella G, Castellano S, Vicidomini C, Rotili D, Nebbioso A, Miceli M, Altucci L, Mai A. 2008. Identification of long chain alkylidenemalonates as novel small molecule modulators of histone acetyltransferases. Bioorganic Med Chem Lett 18: 2788–2792. 10.1016/j.bmcl.2008.04.017 [DOI] [PubMed] [Google Scholar]
- Selvi BR, Cassel JC, Kundu TK, Boutillier AL. 2010. Tuning acetylation levels with HAT activators: therapeutic strategy in neurodegenerative diseases. Biochim Biophys Acta Gene Regul Mech 1799: 840–853. 10.1016/j.bbagrm.2010.08.012 [DOI] [PubMed] [Google Scholar]
- Sung YM, Lee T, Yoon H, DiBattista AM, Song JM, Sohn Y, Moffat EI, Turner RS, Jung M, Kim J, et al. 2013. Mercaptoacetamide-based class II HDAC inhibitor lowers Aβ levels and improves learning and memory in a mouse model of Alzheimer's disease. Exp Neurol 239: 192–201. 10.1016/j.expneurol.2012.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. 2013. The emerging field of neuroepigenetics. Neuron 80: 624–632. 10.1016/j.neuron.2013.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Morrell L, Dickerson B. 2000. Hemispheric differences in hippocampal volume predict verbal and spatial memory performance in patients with Alzheimer's disease. Hippocampus 142: 136–142. [DOI] [PubMed] [Google Scholar]
- Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, Cabrera SM, McDonough CB, Brindle PK, Abel T, et al. 2007. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci 27: 6128–6140. 10.1523/JNEUROSCI.0296-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Coelho CM, Li X, Marek R, Yan S, Anderson S, Meyers D, Mukherjee C, Sbardella G, Castellano S, et al. 2012. p300/CBP-associated factor selectively regulates the extinction of conditioned fear. J Neurosci 32: 11930–11941. 10.1523/JNEUROSCI.0178-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Schluesener HJ. 2013. Oral administration of histone deacetylase inhibitor MS-275 ameliorates neuroinflammation and cerebral amyloidosis and improves behavior in a mouse model. J Neuropathol Exp Neurol 72: 178–185. 10.1097/NEN.0b013e318283114a [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.