“But when old age was pressing hard on him, with all its might, and he couldn’t move his limbs, much less lift them up, then in her thûmos she thought up this plan, a very good one indeed: she put him in her chamber, and she closed the shining doors over him.’*
Homeric Hymn to Aphrodite, 235ff
Historically, the aging process was first encountered in the writings of the ancient Greek hymns, which were probably written in the seventh century B.C.1 In one Homeric script, Tithonus was the prince of Troy and the lover of Eos, the Goddess of the Dawn. Eos asked Zeus to make Tithonus immortal, but she forgot to ask for the gift of “eternal youth”.1 While Tithonus lived forever, he had to experience the intricacies of old age and became weakened and debilitated. He later begged for the end of his life.
Even though the concept of aging was initially introduced by geriatricians, interventional cardiologists deserve the credit for applying systematic age-related risk assessments in clinical cardiovascular practice. The seminal Placement of Aortic Transcatheter Valves (PARTNER) trial demonstrated that transcatheter aortic valve replacement (TAVR) was a lifesaving treatment in older adults (average age ~ 83 years) with severe symptomatic aortic valve stenosis and prohibitive surgical risk. A significant proportion of PARTNER patients were felt to be inoperable due to the presence of geriatric syndromes, which are defined as age-related clinical conditions that do not fit in one disease category.2 Typically, geriatric syndromes have a detrimental influence on the presentation and progression of cardiovascular disease, such that the diagnosis, prognosis, and management are worsened by the presence of these conditions. The PARTNER trial accelerated the integration of geriatric concepts into the practice of interventional cardiology and that in turn led to the implementation of geriatric assessment in other cardiovascular populations of older adults. Of these conditions, physical frailty is a commonly encountered age-related risk particularly among older adults with severe symptomatic aortic valve stenosis.3
Despite methodological and conceptual debates, frailty has remained an abstract construct in the mind of most clinicians, who have relied on the “eyeball test” to determine frailty in practice. The Oxford Dictionary defines the word frailty as “weakness and poor health”. It was not until 2001, that a more concise and scientific definition of physical frailty syndrome was established by Fried et al. as “a clinical syndrome of increased vulnerability resulting from age associated decline in reserve and function across multiple physiologic systems, such that the ability to cope with everyday acute stress is compromised”.4 It uses the presence of at least three of five components to establish the diagnosis of frailty, which includes shrinking, weakness, poor endurance and energy, slowness, and low physical activity level.
The spectrum of frailty ranges from robust (not-frail), to pre-frail, and physically frail.4 The pre-frail state increases the risk of progression to frailty and frailty increases the risk of disability, a state that is distinct from frailty. Depending on the instrument used, the prevalence of frailty ranges from 4.0% to 59.1% among community-dwelling older adults and the prevalence of pre-frailty from 18.7% to 53.1%5, but the highest estimates are observed among older patients with severe symptomatic aortic valve disease.6 There is a strong bidirectional association between cardiovascular disease and frailty with a stepwise response seen from robust to frail. Pre-frailty and frailty are independently associated with a higher risk of developing major adverse cardiovascular outcomes and frailty can predict adverse geriatric outcomes including physical and cognitive decline, impaired mobility, and inability to perform activities of daily living (ADL).7, 8
In this issue, Arnold et al.9 examined whether frailty exerts a differential treatment effects on mortality and health status in older adults treated with TAVR or surgical aortic valve replacement (SAVR). To accomplish their goal, they evaluated the population enrolled in the PARTNER 2A and PARTNER 3 trial that randomized TAVR vs. SAVR in patients at intermediate and low surgical risk, in addition to the SAPIEN 3 Intermediate Risk Registry (n=3,025). Frailty was assessed using a 4-domain scale including weak grip strength, slow gait speed, serum albumin, and ability to perform ADL independently. Two thirds of patients in the study population were pre-frail (n=2,041) and only 6.1% had physical frailty (n=185). Frail patients were older, more likely to be women, had higher comorbidities, and lower Kansas City Cardiomyopathy Questionnaire (KCCQ) Overall Summary score. The authors found that frailty was associated with increased risk of mortality and worse health status, but there was no interaction between frailty and treatment modality (SAVR or TAVR) on death or quality of life at 2 years.
The study findings are insightful, highlighting the efficacy and safety of transcatheter and surgical therapies even among frail patients with low and intermediate surgical risk, and represent an important advancement in the field. While these findings are quite intriguing, one would expect that a less invasive approach (TAVR) would result in more favorable outcomes in frail patients than a more invasive treatment (SAVR). Multiple reasons may explain these findings. First, the study population had low to intermediate surgical risk, with a low prevalence of frailty syndrome, therefore it is possible that the statistical power was insufficient to demonstrate differential treatment effects. Second, continuous variables included in the frailty instrument were categorized with a consequent loss of granularity. Third, there is a chance that the multivariable model did not entirely adjust for the risk imbalances between the TAVR and SAVR groups. Finally, all variables included in the instrument were treated as if they had the same weight in the determination of frailty status. The differential contribution of anemia, weakness, immobility, or loss of independence on the severity of frailty is unknown.
There is a lack of a standardized measurement tool for frailty in patients with cardiovascular disease. Numerous instruments to measure frailty are currently available in practice. These tools measure different phenotypes of patients living with frailty. For example, a frail patient identified by the Fried or Fried+ may be a quite different than a patient identified by Rockwood, Short Physical Performance Battery, or the Essential Frailty Toolset. The present study emphasizes the need to standardize definitions and measurements of frailty among cardiovascular patients enrolled in clinical trials similar to other important metrics that influence health outcomes, such as bleeding. Standardization can facilitate comparisons of frailty across studies, standardize the approach to frailty syndrome during cardiovascular illness, and inform clinical decision making.
In the Frailty-AVR study6, the prevalence of frailty using the most cited instrument, the Fried phenotype, was 25% among SAVR patients and 49% among TAVR patients in contrast to a frailty prevalence of 6.1% in the present study, which suggests that the study population had fewer geriatric and age-related complexities. Whether frailty acts as an effect measure modifier in the association between treatment and outcomes is yet to be determined among high-risk patients with higher burden of geriatric syndromes (e.g., physical, or cognitive decline), or higher complexity of coexisting cardiovascular disease. In addition, frailty can influence other geriatric outcomes including cognitive decline, dementia, loss of independence, and immobility. Such outcomes are also important in addition to mortality and KCCQ, moving the needle beyond disease-centered approach towards a more holistic approach to management. The influence of frailty on these geriatric outcomes in older patients with severe aortic valve disease need to be considered as more octogenarians and nonagenarians are treated with TAVR.
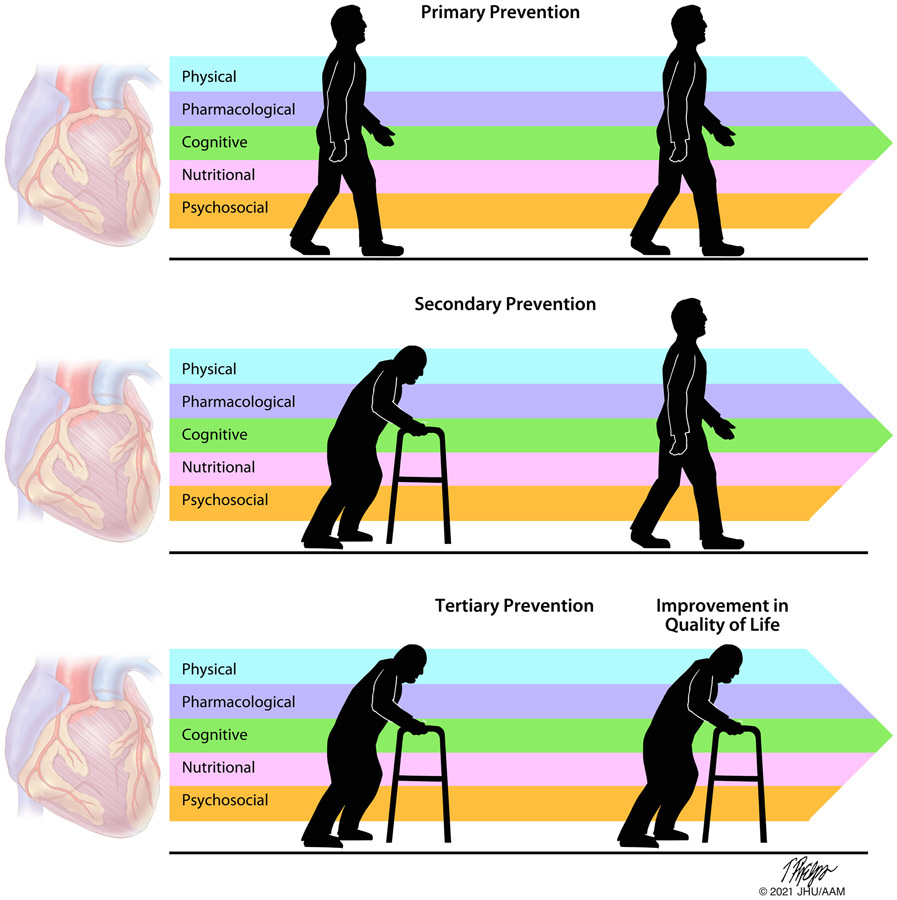
What is the next step? Frailty is a marker of poor prognosis for cardiovascular and geriatric outcomes across different populations including severe aortic valve disease, ischemic heart disease, or peripheral vascular disease. Interventional cardiologists are tasked to care for older adults, enroll them in trials to investigate efficacy and safety of new therapies, and aim to prevent complications during cardiovascular interventions in practice. Efforts to integrate the concept of frailty in cardiovascular practice are essential, but early interventions aimed at prevention or reversal of frailty are needed to improve overall health in the older adult populations. Transitional tailored progressive cardiac rehabilitation programs post TAVR may have an important role in the older adult population as part of comprehensive care to improve long-term outcomes including reversal of frailty (Figure 1).10 Several trials are currently investigating the influence of a combination of therapies including physical resistance exercises, pharmacologic, nutritional, cognitive, and psychosocial interventions to prevent or treat frailty in older patients with cardiovascular disease.
Figure 1.
Interventions aimed to prevent or reverse frailty in patients with valvular heart disease. Physical, pharmacological, cognitive, nutritional, and psychosocial interventions or a combination thereof have the potential to prevent the onset of frailty (primary prevention), reverse frailty (secondary prevention), or improve the quality of life in older patients with preexisting frailty (tertiary prevention).
Arnold and colleagues9 should be commended for emphasizing that frail and pre-frail patients with severe aortic stenosis still benefit from aortic valve replacement and that frailty should not be used to prevent older patients from receiving lifesaving therapies. Their study findings emphasize that frailty is a marker of poor prognosis and that better treatment options for frailty are needed to improve overall outcomes and prevent disability. Because concerted and coordinated efforts to prevent and reverse frailty are urgently needed to improve outcomes among older adults undergoing cardiovascular interventions, it is time that we all speak the same language. The adoption of a standardized definition of frailty and the use of a universal instrument across the spectrum of patients with cardiovascular disease through a “Frailty Academic Research Consortium” are imperative.
ACKNOWLEDEMENT:
The authors would like to acknowledge Professor Tim Phelps for his assistance with medical illustration.
SOURCES OF FUNDING:
Dr. Damluji receives research funding from the Pepper Scholars Program of the Johns Hopkins University Claude D. Pepper Older Americans Independence Center funded by the National Institute on Aging P30-AG021334 and mentored patient-oriented research career development award from the National Heart, Lung, and Blood Institute K23-HL153771-01.
Footnotes
DISCLOSURES: The authors declare that they have no relevant interests.
Modified; a thûmos Greek word indicating a human desire for recognition
REFERENCES
- 1.Ferrucci L, Mahallati A and Simonsick EM. Frailty and the foolishness of Eos. J Gerontol A Biol Sci Med Sci. 2006;61:260–1. [DOI] [PubMed] [Google Scholar]
- 2.Damluji AA, Forman DE, van Diepen S, Alexander KP, Page RL 2nd, Hummel SL, Menon V, Katz JN, Albert NM, Afgilsalo J, et al. Older Adults in the Cardiac Intensive Care Unit: Factoring Geriatric Syndromes in the Management, Prognosis, and Process of Care: A Scientific Statement From the American Heart Association. Circulation. 2019:Cir0000000000000741. [DOI] [PubMed] [Google Scholar]
- 3.Veronese N, Cereda E, Stubbs B, Solmi M, Luchini C, Manzato E, Sergi G, Manu P, Harris T, Fontana L, et al. Risk of cardiovascular disease morbidity and mortality in frail and pre-frail older adults: Results from a meta-analysis and exploratory meta-regression analysis. Ageing Res Rev. 2017;35:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. [DOI] [PubMed] [Google Scholar]
- 5.Collard RM, Boter H, Schoevers RA and Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60:1487–92. [DOI] [PubMed] [Google Scholar]
- 6.Afilalo J, Lauck S, Kim DH, Lefèvre T, Piazza N, Lachapelle K, Martucci G, Lamy A, Labinaz M, Peterson MD, et al. Frailty in Older Adults Undergoing Aortic Valve Replacement: The FRAILTY-AVR Study. J Am Coll Cardiol. 2017;70:689–700. [DOI] [PubMed] [Google Scholar]
- 7.Damluji AA, Chung S-E, Xue Q-L, Hasan RK, Moscucci M, Forman DE, Bandeen-Roche K, Batchelor W, Walston JD, Resar JR, et al. Frailty and cardiovascular outcomes in the National Health and Aging Trends Study. Eur Heart J. 2021;42:3856–3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damluji AA, Chung S-E, Xue Q-L, Hasan RK, Walston JD, Forman DE, Bandeen-Roche K, Moscucci M, Batchelor W, Resar JR, et al. Physical Frailty Phenotype and the Development of Geriatric Syndromes in Older Adults with Coronary Heart Disease. Am J Med. 2021;134:662–671.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnold SV ZY, Leon MB, Sathananthan J, Alu M, Thourani VH, Smith CR, Mack MJ, Cohen DJ. The Impact of Frailty and Pre-Frailty on Outcomes of Transcatheter or Surgical Aortic Valve Replacement. Circ Cardiovasc Interv. 2022;15. [DOI] [PubMed] [Google Scholar]
- 10.Kitzman DW, Whellan DJ, Duncan P, Pastva AM, Mentz RJ, Reeves GR, Nelson MB, Chen H, Upadhya B, Reed SD, et al. Physical Rehabilitation for Older Patients Hospitalized for Heart Failure. N Engl J Med. 2021;385:203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]