Summary
Background
Tracheal intubation is common in the care of critically ill adults and is frequently complicated by hypotension, cardiac arrest, or death. We aimed to evaluate administration of an intravenous fluid bolus to prevent cardiovascular collapse during intubation of critically ill adults.
Methods
We did a pragmatic, multicentre, unblinded, randomised trial in nine sites (eight ICUs and one emergency department) around the USA. Critically ill adults (≥18 years) undergoing tracheal intubation were randomly assigned (1:1, block sizes of 2, 4, and 6, stratified by study site) to either an intravenous infusion of 500 mL of crystalloid solution or no fluid bolus. The primary outcome, assessed in the intention-to-treat population, was cardiovascular collapse, defined as a new systolic blood pressure <65 mm Hg; new or increased vasopressor receipt between induction and 2 min after tracheal intubation; or cardiac arrest or death within 1 h of tracheal intubation. Adverse events were assessed in the as-treated population. This trial, which is now complete, is registered with ClinicalTrials.gov, number NCT03026777.
Findings
Patients were enrolled from Feb 6, 2017, to Jan 9, 2018, when the data and safety monitoring board stopped the trial on the basis of futility. By trial termination, 337 (63%) of 537 screened adults had been randomly assigned. Cardiovascular collapse occurred in 33 (20%) of 168 patients in the fluid bolus group compared with 31 (18%) of 169 patients in the no fluid bolus group (absolute difference 1·3% [95% CI −7·1% to 9·7%]; p=0·76). The individual components of the cardiovascular collapse composite outcome did not differ between groups (new systolic blood pressure <65 mm Hg 11 [7%] in the bolus group vs ten [6%] in the no-bolus group, new or increased vasopressor 32 [19%] vs 31 [18%], cardiac arrest within 1 h seven [4%] vs two [1%], death within 1 h of intubation two [1%] vs one [1%]). In-hospital mortality was not significantly different in the fluid bolus group (48 [29%]) compared with no fluid bolus (59 [35%]).
Interpretation
Administration of an intravenous fluid bolus did not decrease the overall incidence of cardiovascular collapse during tracheal intubation of critically ill adults compared with no fluid bolus in this trial.
Introduction
Millions of critically ill adults undergo tracheal intubation each year.1,2 As many as one in four critically ill adults undergoing tracheal intubation have cardiovascular collapse—defined as shock, cardiac arrest, or death during or immediately following the procedure.3,4 Peri-intubation cardiovascular collapse is associated with a significant increase in the risk of mortality.5,6
The administration of an intravenous fluid bolus beginning before induction of anaesthesia has been proposed as a way to prevent cardiovascular collapse during tracheal intubation in the intensive care unit (ICU).3,4,7–9 In one observational study,3 implementation of a ten-item pre-intubation checklist, which included pre-induction fluid bolus administration in patients without cardiogenic pulmonary oedema, was associated with a decreased incidence of cardiovascular collapse. No randomised trials have examined the effect of fluid bolus administration on outcomes of tracheal intubation. In addition to the hypothesised beneficial effects on peri-intubation haemodynamics, fluid bolus administration among critically ill adults might incur immediate10,11 and delayed risks.12–14 In clinical practice, approximately half of critically ill adults undergoing tracheal intubation receive fluid bolus administration in North America and Europe.3,15,16
In this pragmatic, multicentre, randomised trial, we aimed to test the hypothesis that the administration of an intravenous fluid bolus would reduce the incidence of cardiovascular collapse compared with no fluid bolus.
Methods
Study design and participants
The Preventing cardiovascular collaPse with Administration of fluid REsuscitation before tracheal intubation (PrePARE) trial was a pragmatic, multicentre, unblinded, randomised trial comparing administration of a fluid bolus beginning before induction with no fluid bolus administration during tracheal intubation of critically ill adults. At seven of the nine study sites, co-enrolment could occur in an independent randomised trial comparing prophylactic bag-mask ventilation (BMV) with no prophylactic ventilation during tracheal intubation (PreVent Trial), the results of which have been previously reported.17 Patients enrolled in PrePARE and not co-enrolled in PreVent were excluded from PreVent on the basis of the bedside clinician’s evaluation of the PreVent exclusion criteria. The protocol was approved at all sites by either a local or central Institutional Review Board with a waiver of informed consent. The statistical analysis plan was published online before completion of enrolment, and the trial protocol has also been published at this site.
Eligible critically ill patients (aged ≥18 years) undergoing tracheal intubation in the nine participating study sites were enrolled.
Study sites comprised six medical ICUs, one trauma ICU, one neurological ICU, and one emergency department at tertiary-care medical centres across the USA (appendix p 9). Patients were excluded if awake intubation was planned, if intubation was required too immediately to permit randomisation, if treating clinicians felt administration of a fluid bolus was required or contraindicated for the optimal care of the patient, or if patients were prisoners or pregnant.
Randomisation and masking
Patients were randomly assigned in a 1:1 ratio to fluid bolus or no fluid bolus administration in permuted blocks of two, four, and six, stratified by study site. A study investigator (MWS) generated the allocation sequence using Sealed Envelope randomisation after which group assignment was concealed in opaque envelopes at each study site until after the decision had been made by the treating team to enrol a patient in the trial. Owing to the nature of the intervention, patients, clinicians, and study staff were aware of study group assignment after randomisation.
Procedures
For patients assigned to the fluid bolus group, the treating team initiated intravenous administration of 500 mL of crystalloid solution before induction of anaesthesia.
The study protocol recommended the fluid bolus be placed above the level of the intravenous access, infused by both gravity and bag pressure, and infused to completion of 500 mL through induction and laryngoscopy (appendix p 3).
For patients randomly assigned to the no fluid bolus group, the study protocol recommended against the administration of any new crystalloid solutions between enrolment and 2 min after completion of tracheal intubation. Intravenous fluid administration initiated as a part of clinical care before enrolment was continued in either study group.
With the exception of patients also enrolled in the PreVent trial of bag mask ventilation,17 all aspects of the intubation procedure were at the discretion of the clinical team.
Periprocedural endpoints were collected by independent observers (ICU providers trained in the definitions of each outcome) who were present in the patient’s room but did not participate in the procedure. To confirm the accuracy of the data collected by the independent observers, the primary investigators concurrently assessed the same endpoints in a non-random convenience sample of study intubations.
Outcomes
The composite primary outcome of cardiovascular collapse consisted of the following components: systolic blood pressure newly less than 65 mm Hg between induction and 2 min after tracheal intubation; new or increased vasopressor use between induction and 2 min after tracheal intubation; cardiac arrest within 1 h of tracheal intubation; or death within 1 h of tracheal intubation.3,4,18 Secondary outcomes included each individual component of the composite primary outcome; any additional fluids given to either group, started between induction and 2 min after tracheal intubation; lowest systolic blood pressure between induction and 2 min after tracheal intubation; change in systolic blood pressure from induction to lowest systolic blood pressure; number of laryngoscopy attempts required for intubation; number of ventilator-free days; number of ICU-free days; and in-hospital mortality over a 28 day follow-up period. A full list of outcomes is provided in the appendix (p 6). Safety outcomes were lowest oxygen saturation, highest fraction of inspired oxygen, and highest positive end-expiratory pressure in the 24 h after intubation; cumulative diuretic dose (in furosemide equivalents) on the day of enrolment and from enrolment to 3 days after enrolment; and cumulative intravenous fluid administration from enrolment to 3 days after enrolment.
Statistical analysis
On the basis of previous research,3 we anticipated a 15% incidence of cardiovascular collapse in the fluid bolus group and 25% in the no fluid bolus group. To detect this relative risk reduction of 40%, we planned to enrol a total of 500 patients to provide 80% statistical power with a two-sided alpha level of 0·05. A single, planned interim analysis was done by the Data and Safety Monitoring Board (DSMB) with complete data from the first 250 patients using prespecified stopping rules for efficacy, safety, and futility (appendix p 7).
The primary analysis was an unadjusted, intention-to-treat comparison of the proportions of patients in each study group who had the primary outcome by means of a χ2 test. Prespecified secondary analyses included evaluation for heterogeneity of treatment effect by baseline co-variates, such as random assignment to BMV for patients co-enrolled in the PreVent trial, by means of formal tests of interaction in a logistic regression model. The complete prespecified statistical analysis plan is available in the appendix (p 5). Analyses were done by means of IBM SPSS Statistics (version 23.0) or Stata (version 15.1). This trial is registered with ClinicalTrials.gov, number NCT03026777.
Role of the funding source
The funders had no role in conception, design, or conduct of the study; collection, management, analysis, interpretation, or presentation of the data; or preparation, review, or approval of the manuscript. DRJ, JDC, MWS, and TWR had access to the raw data; the corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
Eligible patients were enrolled from Feb 6, 2017, through to Jan 9, 2018, when the DSMB stopped the trial on the basis of prespecified futility stopping criteria at the single, planned interim analysis of complete data from the first 250 patients enrolled. Details of the interim stopping analyses can be found in the appendix (p 7).
Of 537 critically ill adults intubated at the nine sites between beginning enrolment and notification of trial termination by the DSMB, 511 met the inclusion criteria and 337 (63%) were enrolled and randomly assigned to either the fluid bolus group (n=168) or the no fluid bolus group (n=169; figure 1; Table 1; appendix p 11).
Figure 1:
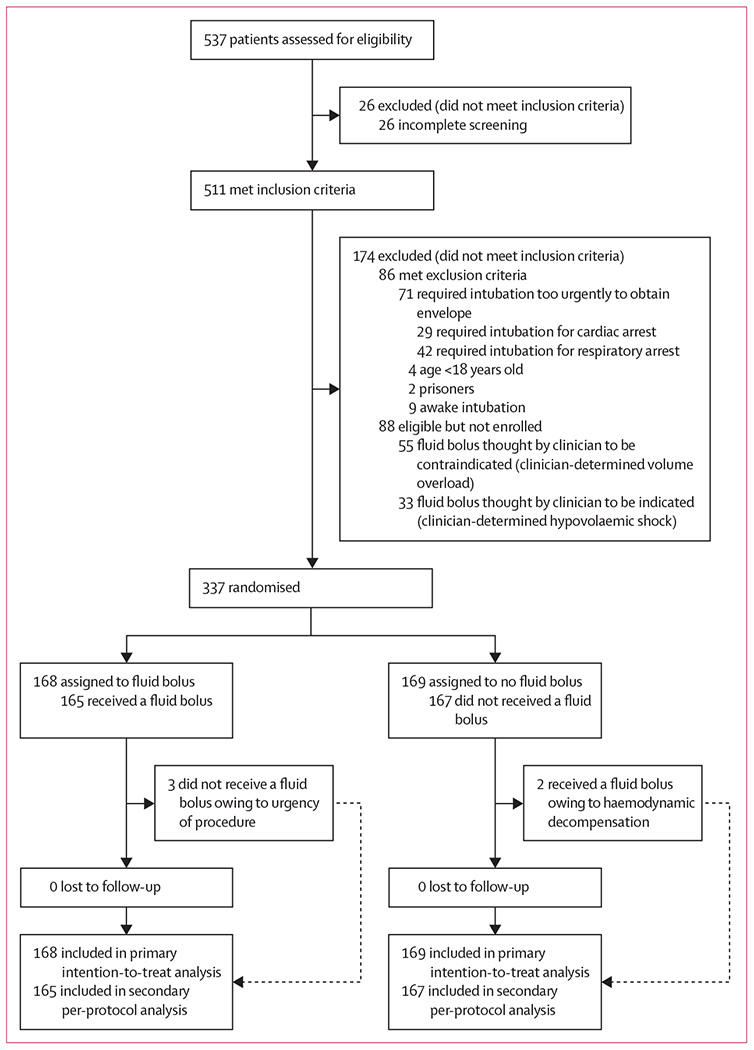
Trial profile
Table 1:
Baseline characteristics
| Fluid bolus (n=168) | No fluid bolus (n=169) | |
|---|---|---|
| Age, years | 61 (47–70) | 58 (46–68) |
|
| ||
| Sex | ||
| Female | 81 (48%) | 73 (43%) |
| Male | 87 (52%) | 96 (57%) |
|
| ||
| White | 115 (68%) | 121 (72%) |
|
| ||
| Acute physiology and chronic health evaluation II score | 21 (14–27) | 20 (16–27) |
|
| ||
| Body-mass index, kg/m2 | 27 (23–32) | 26 (22–32) |
|
| ||
| Active diagnoses since intensive care unit admission | ||
| Septic shock | 39 (23%) | 33 (20%) |
| Cardiogenic shock | 4 (2%) | 4 (2%) |
| Haemorrhagic shock | 4 (2%) | 4 (2%) |
| Pneumonia | 66 (39%) | 64 (38%) |
| Altered mental status | 69 (41%) | 72 (43%) |
| Gastrointestinal blood loss | 21 (12%) | 26 (15%) |
| Acute renal failure | 62 (37%) | 62 (37%) |
| Status epilepticus | 7 (4%) | 5 (3%) |
| Stroke | 10 (6%) | 10 (6%) |
| Acute coronary syndrome | 10 (6%) | 10 (6%) |
| Chronic obstructive pulmonary disease exacerbation | 7 (4%) | 4 (2%) |
|
| ||
| Indication for intubation | ||
| Hypoxic respiratory failure | 85 (51%) | 69 (41%) |
| Altered mental status | 47 (28%) | 52 (31%) |
| Facilitate another procedure | 16 (10%) | 25 (15%) |
| Hypercarbic respiratory failure | 16 (10%) | 15 (9%) |
| Impending airway collapse | 4 (2%) | 7 (4%) |
| Status epilepticus | 3 (2%) | 1 (1%) |
| Haemodynamic instability | 4 (2%) | 2 (1%) |
|
| ||
| Vasopressor receipt in 6 h before intubation | 28 (17%) | 28 (17%) |
|
| ||
| Baseline left ventricular ejection fraction (n=224) | 55% (55–60) | 55% (55–60) |
|
| ||
| Non-invasive ventilation receipt in 6 h before intubation | 44 (26%) | 37 (22%) |
|
| ||
| Lowest systolic blood pressure in 6 h before intubation, mm Hg | 103 (91–124) | 104 (89–125) |
Data given as median (IQR) or number (%) of patients.
Post-randomisation procedural characteristics did not differ between groups, including in choice or dose of sedative and neuromuscular blocking procedural medications (appendix p 12).
Among 168 patients assigned to the fluid bolus group, 165 patients (98%) received the full 500 mL fluid bolus and three patients (2%) did not receive a fluid bolus. Among 169 patients assigned to the no fluid bolus group, two patients (1%) received a fluid bolus and 167 patients (99%) did not receive a fluid bolus (figure 1). To assess for the separation between study groups regarding the volume of fluid delivered before induction of anaesthesia, we used a convenience sample of 38 patients (11%) in which the volume of fluid infused before induction was directly observed by a study investigator. The median volume of crystalloid infused before induction was 200 mL (IQR 200–325; mean 262 mL, SD [119]) in the fluid bolus group and 0 mL (IQR 0–0, p<0·0001) in the no fluid bolus group. In the same convenience sample, the primary outcome was also observed by a study investigator. Agreement regarding a systolic blood pressure of less than 65 mm Hg, need for new or increased vasopressors, or cardiac arrest or death within 1 h of intubation was 100% between the independent observer and the study investigator.
The primary outcome of cardiovascular collapse occurred in 33 (20%) of 168 patients in the fluid bolus group compared with 31 (18%) of 169 patients in the no fluid bolus group (absolute difference 1·3% [95% CI −7·1 to 9·7], p=0·76; figure 2).
Figure 2: Cardiovascular collapse in the fluid bolus vs no fluid bolus groups.
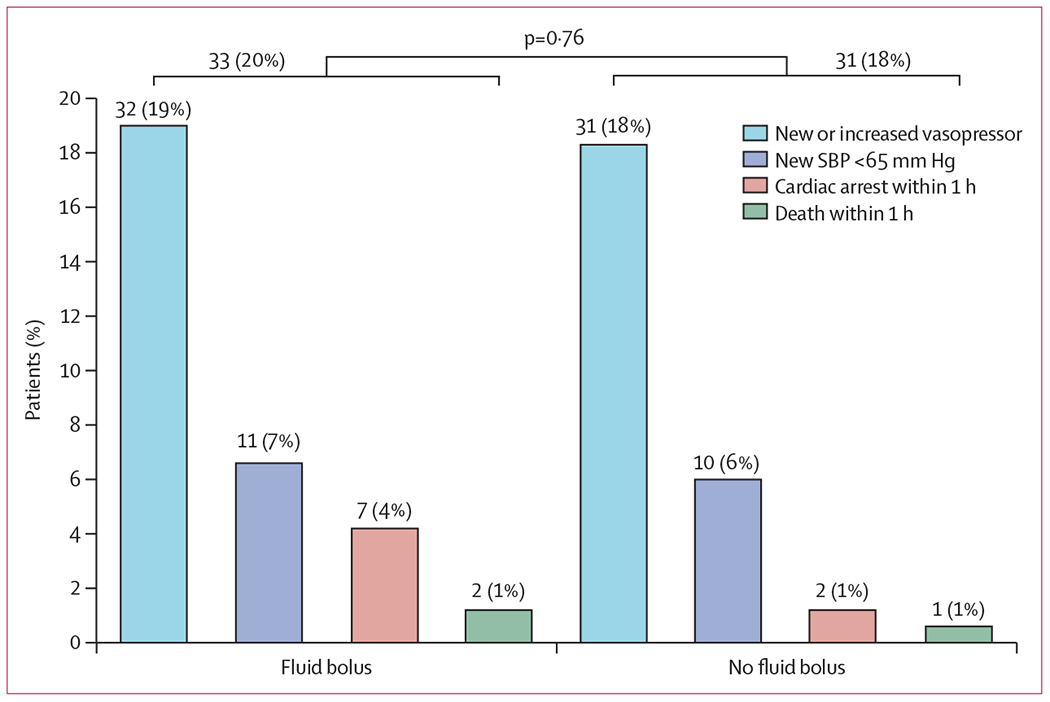
Horizontal bars represent the overall incidence of the primary outcome in each group. The p value represents the test for a difference between groups in the overall incidence of the primary outcome. Number (%) of patients is given above each bar. SBP=systolic blood pressure.
The incidence of each component of the composite outcome did not differ significantly between groups (table 2). Results were similar in prespecified analyses adjusting for age, severity of illness, receipt of vasopressors before enrolment, and lowest systolic blood pressure before enrolment (appendix p 13). In a prespecified, per-protocol analysis of the 332 patients who received the intervention to which they were assigned, the incidence of cardiovascular collapse did not differ between groups (appendix p 17).
Table 2:
Clinical outcomes for the fluid bolus vs no fluid bolus groups
| Fluid bolus (n=168) | No fluid bolus (n=169) | p value | Absolute difference (95% CI)* | Mean difference (95% CI)* | |
|---|---|---|---|---|---|
| Primary outcome | |||||
|
| |||||
| Cardiovascular collapse | 33 (20%) | 31 (18%) | 0·76 | 1·3% (−7·1 to 9·7) | NA |
|
| |||||
| Components of the primary outcome | |||||
|
| |||||
| Death within 1 h of intubation | 2 (1%) | 1 (1%) | 0·55 | 0·6%(−1·4 to 2·6) | NA |
| Cardiac arrest within 1 h of intubation | 7 (4%) | 2 (1%) | 0·8 | 3·0% (−0·5 to 6·4) | NA |
| New systolic blood pressure <65 mm Hg between induction and 2 min after intubation | 11 (7%) | 10 (6%) | 0·80 | 0·7% (−4·5 to 5·9) | NA |
| New or increased vasopressor between induction and 2 min after intubation | 32 (19%) | 31 (18%) | 0·86 | 0·7% (−76 to 9·0) | NA |
|
| |||||
| Exploratory periprocedural outcomes | |||||
|
| |||||
| Alternate composite outcome† | 39 (23%) | 42 (25%) | 0·72 | −1·6% (−10·8 to 7·5) | NA |
| New or worsening shock in 1 h after intubation | 53 (32%) | 47 (28%) | 0·45 | 3·8%(−6·0 to 13·6) | NA |
| New systolic blood pressure <90 mm Hg between induction and 2 min after intubation | 34 (20%) | 34 (20%) | 0·95 | 0·2% (−8·4 to 8·9) | NA |
| Lowest systolic blood pressure between induction and 2 min after intubation, mm Hg | 119 (95 to 140) | 119 (94 to 141) | 0·85 | NA | 1·1 (−6·4 to 8·5) |
| Change in systolic blood pressure from induction to 2 min after intubation, mm Hg | −9 (−26 to 0) | −6 (−26 to 0) | 0·93 | NA | −0·2 (−6·1 to 5·7) |
| Lowest arterial oxygen saturation between induction and 2 min after intubation, % | 94% (84 to 99) | 95% (82 to 99) | 0·95 | NA | −0·6 (−3·4 to 2·2) |
| Arterial oxygen saturation <90% | 56 (33%) | 58 (34%) | 0·78 | −1·4% (−117 to 8·8) | NA |
| Arterial oxygen saturation <80% | 28 (17%) | 33 (20%) | 0·46 | −3·1% (−11·5 to 5·2) | NA |
| Desaturation >3% between induction and 2 min after intubation | 72 (43%) | 72 (43%) | 0·96 | −0·3% (−10·9 to 10·4) | NA |
| Change in arterial oxygen saturation from induction to 2 min after intubation, % | −2% (−9 to 0) | −2% (−11 to 0) | 0·90 | NA | −0·9 (−3·5 to 17) |
|
| |||||
| Exploratory clinical outcomes | |||||
|
| |||||
| Ventilator-free days | 20 (0 to 25) | 19 (0 to 25) | 0·55 | NA | −0·7 (−3·7 to 1·7) |
| ICU-free days | 16 (0 to 24) | 14 (0 to 23) | 0·49 | NA | −0·6 (−2·9 to 1·6) |
| In-hospital mortality | 48 (29%) | 59 (35%) | 0·21 | −6·3% (−16·2 to 3·5) | NA |
Data given as median (IQR) or number (%) of patients. p value is based on the Mann-Whitney U test or χ2 test.
NA=not applicable.
Differences between categorical variables are displayed as absolute difference and differences between continuous variables are displayed as mean differences.
New systolic blood pressure <90 mm Hg, new or increased vasopressors, cardiac arrest within 1 h, death within 1 h. All exploratory outcomes we re pre-planned secondary outcomes specified in the statistical analysis plan.
Administration of a fluid bolus did not significantly affect any of the prespecified secondary outcomes (table 2; appendix p 14) or safety outcomes (table 3). In the overall study population regardless of randomisation assignment, occurrence of cardiovascular collapse was significantly associated with decreased ICU-free days, ventilator-free days, and survival (table 4).
Table 3:
Safety outcomes
| Fluid bolus (n=168) | No fluid bolus (n=169) | p value | Mean difference (95% CI) | |
|---|---|---|---|---|
| Lowest arterial oxygen saturation in 6–24 h after intubation, % | 95% (92 to 97) | 95% (92 to 97) | 0·93 | 0·3 (−1·5 to 2·0) |
| Highest fraction of inspired oxygen in 6–24 h after intubation | 0·5 (0·4 to 0·7) | 0·5 (0·4 to 0·67) | 0·73 | −0·0 (−0·1 to 0·0) |
| Highest positive end–expiratory pressure in 6–24 h after intubation, cm H2O | 5 (5 to 8) | 5 (5 to 8) | 0·36 | −0·3 (−0·9 to 0·4) |
| Cumulative diuretic dose in the 24 h after intubation, mg in furosemide equivalents | 0 (0 to 60) | 0 (0 to 0) | 0·74 | 0·0 (−11·7 to 11·7) |
| Cumulative diuretic dose from intubation to 72 h after intubation, mg in furosemide equivalents | 0 (0 to 60) | 0 (0 to 57) | 0·78 | 23·4 (−24·5 to 71·3) |
| Cumulative intravenous fluid administration from intubation to 72 h after intubation, mL | 2061 (955 to 4411) | 2036 (628 to 4317) | 0·68 | 441 (−346 to 1229) |
Data given as median (IQR) or number (%) of patients. p value is based on the Mann-Whitney U test or χ2 test.
Table 4:
Clinical outcomes in all patients enrolled with and without cardiovascular collapse
| Cardiovascular collapse (n=64) | No cardiovascular collapse (n=273) | p value | Absolute difference (95% CI) | Mean difference (95% CI) | |
|---|---|---|---|---|---|
| ICU-free days | 6(0–21) | 17 (0–24) | 0·26 | NA | 3·1 (0·2 to 6·0) |
| Ventilator-free days | 2(2–23) | 21 (0–26) | 0·0090 | NA | 3·6 (0·4 to 6·8) |
| In-hospital mortality | 29 (45%) | 78 (29%) | 0·010 | 167 (3·4 to 30·0) | NA |
Data given as median (IQR) or number (%) of patients. p value is based on the Mann-Whitney U test or χ2 test.
NA=not applicable.
Analyses of heterogeneity of treatment effect are presented in figure 3 and the appendix p 16. The use of non-invasive positive pressure ventilation for pre-oxygenation (pinteraction=0·032) and BMV between induction and laryngoscopy (pinteraction=0·0080) significantly modified the effect of fluid bolus administration on cardiovascular collapse. For patients receiving positive pressure ventilation, either by non-invasive ventilation before induction or BMV after induction, fluid bolus administration appeared to decrease the incidence of cardiovascular collapse compared with no fluid bolus (figure 3). For patients not receiving positive pressure ventilation, administration of a fluid bolus appeared to increase the incidence of cardiovascular collapse.
Figure 3: Risk of cardiovascular collapse by subgroup for patients receiving fluid bolus administration vs no fluid bolus administration.
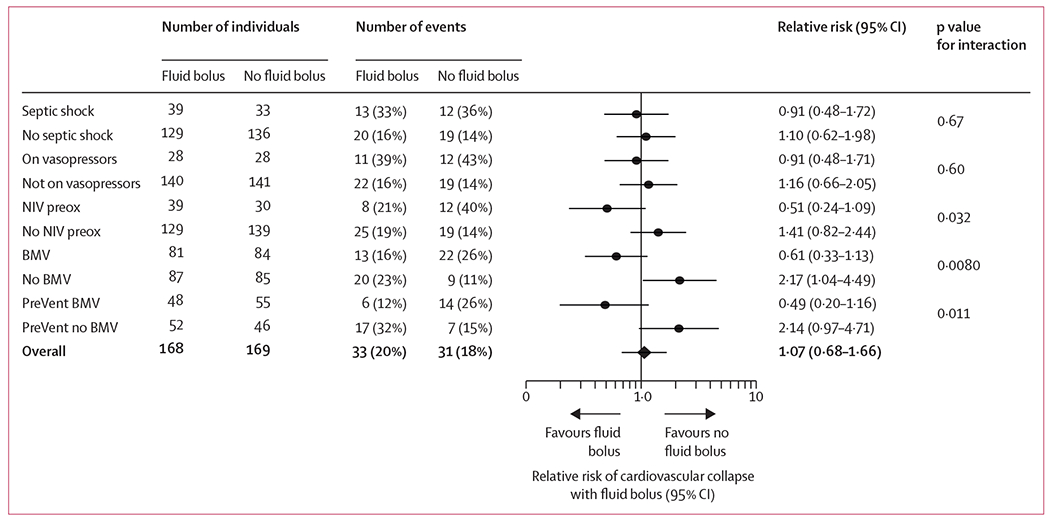
On vasopressors refers to patients who were receiving vasopressor infusions any time in the 6 h before enrolment. NIV preox=non-invasive positive pressure ventilation for pre-oxygenation. BMV=bag-mask ventilation to ventilate or oxygenate the patient during the tracheal intubation procedure in all patients enrolled in the trial. PreVent BMV=randomisation assignments in the 201 patients who were co-enrolled in a separate randomised trial of prophylactic vs no prophylactic bag-mask ventilation.
Among the 201 patients co-enrolled in a randomised trial comparing prophylactic BMV between induction and laryngoscopy with no ventilation, fewer patients randomly assigned to BMV appeared to have cardiovascular collapse when also randomised to fluid bolus administration, whereas patients randomly assigned to no BMV appeared to have cardiovascular collapse when randomly assigned to no fluid bolus administration (pinteraction=0·011; figure 3).
Discussion
During tracheal intubation of critically ill adults, the risk of cardiovascular collapse might be increased owing to hypovolaemia, impaired systemic vascular resistance, receipt of sedative medications, and reduced venous return from positive pressure ventilation—all of which are potentially amenable to prevention by administration of an intravenous fluid bolus.19–24 Our multicentre randomised trial, however, found that administration of a fluid bolus did not affect the overall incidence of cardiovascular collapse during intubation of critically ill adults, compared with no fluid bolus administration.
There are several potential explanations for these findings. First, the recommended and commonly used7,16 volume of 500 mL of crystalloid might have been inadequate to influence patient haemodynamics during intubation. Second, the timing of fluid bolus administration beginning before induction and infusing through induction and laryngoscopy might have produced different results than if the full bolus had been administered before induction. Third, administration of a fluid bolus might simply not improve haemodynamics for all patients undergoing intubation in the ICU. Published data suggest that in the days after ICU admission most patients do not show an increase in cardiac output in response to administration of a fluid bolus.25
Fourth, administration of a fluid bolus might have had differential effects for patients with different peri-intubation physiology. Specifically, we found that among patients receiving positive pressure ventilation before induction, either via non-invasive ventilation for pre-oxygenation or bag-mask ventilation between induction and laryngoscopy, administration of a fluid bolus appeared to decrease the risk of cardiovascular collapse. By contrast, among patients not receiving positive pressure ventilation, including those receiving oxygenation with a high-flow nasal cannula, administration of a fluid bolus appeared to increase the risk of cardiovascular collapse.
Fluid bolus administration might attenuate the decrease in venous return associated with pre-intubation positive pressure ventilation among patients receiving non-invasive ventilation for pre-oxygenation or BMV between induction and laryngoscopy. The only previous study3 to evaluate fluid bolus administration during intubation in the ICU was a before-and-after study of a ten-item pre-intubation checklist, in which cardiovascular collapse occurred less often in the intervention group. In this study, all patients in the intervention group received both non-invasive positive pressure ventilation for pre-oxygenation and a fluid bolus. These findings are consistent with the effect of fluid bolus administration on cardiovascular collapse observed among the subgroup of patients receiving pre-intubation positive pressure ventilation in our trial.
For patients in our trial not receiving positive pressure ventilation, fluid bolus administration appeared to increase the risk of cardiovascular collapse. Several studies have reported a decrease in blood pressure and cardiac output with fluid bolus administration, especially with rapid infusion of the bolus10,26,27 as was used in the current trial and during general anesthesia.11 Potential mechanisms by which fluid bolus administration might cause cardiovascular collapse include dilution of endogenous catecholamines,26 stimulation of atrial natriuretic peptide release, and damage to the glycocalyx.10,27 Alternatively, the absence of positive pressure ventilation might cause de-recruitment of the lung and hypoxaemia, which is associated with increased pulmonary vascular resistance.28–30 Fluid bolus administration during this time of increased pulmonary vascular resistance might cause transient pressure overload of the right ventricle and decrease in cardiac output.31 Poor outcomes in hypoxaemic patients receiving fluid boluses have previously been described.32
The current trial has several strengths. The multicentre, randomised design and pragmatic nature of the intervention improve generalisability. Protocol compliance was high, with only five patients (1%) receiving the non-assigned therapy. The composite outcome of cardiovascular collapse has been used in other studies of intubation3,18 and was strongly associated with patient-centred outcomes in the current study.
The current trial also has limitations. Absence of blinding could have influenced the non-protocolised use of vasopressors or differences in co-interventions; however, we found no significant differences between groups regarding laryngoscope selection, medication selection or doses, or operator experience. The incidence of the primary outcome was lower than previous studies, possibly owing to 17% of patients screened being excluded for a physician-established volume status precluding random assignment, which limits the power of this trial to make inferences about the absence of an effect of a fluid bolus on the incidence of cardiovascular collapse. The trial was stopped early by the DSMB at the planned interim analysis for futility of the intervention among all enrolled patients. This lower-than-planned sample size limits power to exclude an effect of a fluid bolus in patient subgroups with non-significant interactions. The possibility of positive-pressure ventilation modifying the effect of a fluid bolus on cardiovascular collapse should be considered hypothesis-generating as this subgroup analysis could also be a result of type I error. This multicentre trial was done in eight ICUs and one emergency department with varied patient populations. Although this heterogeneity might increase the external validity of the results, it might also limit the ability to discern the effect of a fluid bolus on cardiovascular collapse in a more homogenous critically ill population. The volume of intravenous fluids that patients received before enrolment was not recorded, therefore it is unknown if this covariate was balanced between groups. Finally, the only protocol requirement was to begin the fluid bolus at any time before the administration of procedural medications, a practice described in past observational studies.3,15,16 It is not known whether the results of the trial would have differed if the trial had required the entire volume of the fluid bolus to be infused before induction.
Our findings do not support routine administration of a fluid bolus before induction among patients not receiving positive pressure ventilation. Given the beneficial effects of BMV during intubation seen in the PreVent trial,17 future research should examine the hypothesis-generating effect modification seen in the current trial regarding whether fluid bolus administration prevents cardiovascular collapse among patients receiving pre-intubation positive pressure ventilation.
Supplementary Material
Research in context.
Evidence before this study
Tracheal intubation is common in the care of critically ill patients. As many as 25% of critically ill adults have cardiovascular collapse during the intubation procedure. Cardiovascular collapse during intubation is attributed to three potential mechanisms, all of which might respond to increasing preload via intravenous fluid administration: hypotension associated with common induction medications, increased venous capacitance from decreased circulating catecholamines, and decreased venous return secondary to positive pressure applied to the thoracic cavity. Administration of an intravenous fluid bolus to prevent cardiovascular collapse has been studied indirectly in one observational study of a pre-intubation checklist, which included both pre-induction fluid bolus and positive pressure ventilation. Use of the checklist was associated with an 11% absolute reduction in the incidence of cardiovascular collapse. The PrePARE trial, the results of which are reported here, was a pragmatic, multicentre, unblinded, randomised, controlled trial in nine sites across the USA in which critically ill adults undergoing tracheal intubation were randomly assigned 1:1 to an intravenous infusion of 500 mL of crystalloid solution beginning before induction versus no fluid bolus. Trial design began with a search of PubMed and ClinicalTrials.gov from inception to Nov 14, 2016. This search was repeated before the interim analysis done by the Data Safety and Monitoring Board. Evidence from the PreVent trial was not published prior to the start of the PrePARE trial. PreVent and PrePARE started simultaneously and PrePARE finished prior to PreVent finishing.
Added value of this study
The PrePARE trial is, to our knowledge, the only randomised trial examining the effect of intravenous fluid bolus administration to prevent cardiovascular collapse among critically ill adults undergoing tracheal intubation. Fluid bolus administration did not decrease the overall incidence of cardiovascular collapse compared with no fluid bolus administration. Positive pressure ventilation modified the effect of fluid bolus administration on cardiovascular collapse. Fluid bolus administration appeared to decrease the risk of cardiovascular collapse among patients receiving pre-intubation positive pressure ventilation and appeared to increase the risk of cardiovascular collapse among patients not receiving pre-intubation positive pressure ventilation.
Implications of all the available evidence
Among all critically ill adults undergoing tracheal intubation, the existing evidence does not support administration of a fluid bolus beginning before induction to prevent the common complication of cardiovascular collapse. The results of the PrePARE and PreVent trials together suggest that an intubation strategy involving fluid bolus administration without pre-intubation positive pressure ventilation should not be routine care. Whether fluid bolus administration prevents cardiovascular collapse among patients who receive positive pressure ventilation before intubation should be the focus of future research.
Acknowledgments
The authors thank the patients, nurses, respiratory therapists, residents, fellows, advanced practice providers, and attending physicians of the participating intensive care units.
Funding
US National Institutes of Health.
Declaration of interests
TWR reported serving on an advisory board for Avisa Pharma, as a Data and Safety Monitoring Board member for Takeda, and Director of Medical Affairs for Cumberland Pharmaceuticals, during the conduct of the study. All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Investigators doing this study were supported by a National Heart, Lung, and Blood Institute (NHLBI) T32 award (HL087738 to JDC and HL10534T-07 to DWR). MWS was supported in part by the National Heart, Lung, and Blood Institute (K23HL143053). Data collection used the Research Electronic Data Capture (REDCap) tool developed and maintained with Vanderbilt Institute for Clinical and Translational Research grant support (UL1 TR000445 from NCATS/NIH). All remaining authors declare no competing interests.
Footnotes
Data sharing
Following publication and upon reasonable request, a completely de-identified dataset and data dictionary with individual participant data may be provided by the authors. Request to share data from the PrePARE trial should be sent, along with a brief research proposal, to the corresponding author. The dataset will be provided to researchers whose proposed use of the data has been approved by the steering committee and an Institutional Review Board.
See Online for appendix
For the statistical analysis plan see https://rocket.app.vumc.org/index.php?doc_id=20364
References
- 1.Semler MW, Self WH, Wanderer JP, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med 2018; 378:829–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frat J-P, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 2015; 372:2185–96. [DOI] [PubMed] [Google Scholar]
- 3.Jaber S, Jung B, Corne P, et al. An intervention to decrease complications related to endotracheal intubation in the intensive care unit: a prospective, multiple-center study. Intensive Care Med 2010; 36: 248–55. [DOI] [PubMed] [Google Scholar]
- 4.Umobong EU, Mayo PH. Critical care airway management. Crit Care Clin 2018; 34: 313–24. [DOI] [PubMed] [Google Scholar]
- 5.Green RS, Turgeon AF, McIntyre LA, et al. Postintubation hypotension in intensive care unit patients: a multicenter cohort study. J Crit Care 2015; 30:1055–60. [DOI] [PubMed] [Google Scholar]
- 6.Heffner AC, Swords DS, Nussbaum ML, Kline JA, Jones AE. Predictors of the complication of postintubation hypotension during emergency airway management. J Crit Care 2012; 27: 587–93. [DOI] [PubMed] [Google Scholar]
- 7.Myatra SN, Ahmed SM, Kundra P, et al. The all India difficult airway association 2016 guidelines for tracheal intubation in the intensive care unit. Indian J Anaesth 2016; 60: 922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reynolds SF, Heffner J. Airway management of the critically ill patient: rapid-sequence intubation. Chest 2005; 127:1397–412. [DOI] [PubMed] [Google Scholar]
- 9.Sherren P, Tricklebank S, Glover G. Development of a standard operating procedure and checklist for rapid sequence induction in the critically ill. Scand J Trauma Resusc Emerg Med 2014; 22:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ukor IF, Hilton AK, Bailey MJ, Bellomo R. The haemodynamic effects of bolus versus slower infusion of intravenous crystalloid in healthy volunteers. J Crit Care 2017; 41: 254–59. [DOI] [PubMed] [Google Scholar]
- 11.Norberg A, Hahn RG, Li H, et al. Population volume kinetics predicts retention of 0.9% saline infused in awake and isoflurane-anesthetized volunteers. Anesthesiology 2007; 107:24–32. [DOI] [PubMed] [Google Scholar]
- 12.Marik PE, Linde-Zwirble WT, Bittner EA, Sahatjian J, Hansell D. Fluid administration in severe sepsis and septic shock, patterns and outcomes: an analysis of a large national database. Intensive Care Med 2017; 43: 625–32. [DOI] [PubMed] [Google Scholar]
- 13.Sakr Y, Rubatto Birri PN, Kotfis K, et al. Higher fluid balance increases the risk of death from sepsis: results from a large international audit. Crit Care Med 2017; 45: 386–94. [DOI] [PubMed] [Google Scholar]
- 14.Boyd JH, Forbes J, Nakada T-A, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med 2011; 39:259–65. [DOI] [PubMed] [Google Scholar]
- 15.De Jong A, Molinari N, Terzi N, et al. Early identification of patients at risk for difficult intubation in the intensive care unit: development and validation of the MACOCHA score in a multicenter cohort study. Am J Respir Crit Care Med 2013; 187:832–39. [DOI] [PubMed] [Google Scholar]
- 16.Green RS, Fergusson DA, Turgeon AF, et al. Resuscitation prior to emergency endotracheal intubation: results of a national survey. West J Emerg Med 2016; 17: 542–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casey JD, Janz DR, Russell DW, et al. Bag-mask ventilation during tracheal intubation of critically ill adults. N Engl J Med 2019; 380:811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lascarrou JB, Boisramé-Helms J, Bailly A, et al. Video laryngoscopy vs direct laryngoscopy on successful first-pass orotracheal intubation among ICU patients: a randomized clinical trial. JAMA 2017; published online Jan 24. DOI: 10.1001/jama.2016.20603. [DOI] [PubMed] [Google Scholar]
- 19.Muzi M, Berens RA, Kampine JP, Ebert TJ. Venodilation contributes to propofol-mediated hypotension in humans. Anesth Analg 1992; 74:877–83. [DOI] [PubMed] [Google Scholar]
- 20.Shah SB, Chowdhury I, Bhargava AK, Sabbharwal B. Comparison of hemodynamic effects of intravenous etomidate versus propofol during induction and intubation using entropy guided hypnosis levels. J Anaesthesiol Clin Pharmacol 2015; 31:180–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reich DL, Hossain S, Krol M, et al. Predictors of hypotension after induction of general anesthesia. Anesth Analg 2005; 101: 622–28. [DOI] [PubMed] [Google Scholar]
- 22.Perbet S, De Jong A, Delmas J, et al. Incidence of and risk factors for severe cardiovascular collapse after endotracheal intubation in the ICU: a multi center observational study. Crit Care 2015; 19:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heffner AC, Swords DS, Neale MN, Jones AE. Incidence and factors associated with cardiac arrest complicating emergency airway management. Resuscitation 2013; 84:1500–04. [DOI] [PubMed] [Google Scholar]
- 24.Shafi S, Gentilello L. Pre-hospital endotracheal intubation and positive pressure ventilation is associated with hypotension and decreased survival in hypovolemic trauma patients: an analysis of the National Trauma Data Bank. J Trauma 2005; 59:1140–45. [DOI] [PubMed] [Google Scholar]
- 25.Lammi MR, Aiello B, Burg GT, et al. Response to Fluid Boluses in the Fluid and Catheter Treatment Trial. Chest 2015; published online May 28. DOI: 10.1378/chest.15-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balasaraswathi K, Glisson SN, El-Etr AA, Azad C. Effect of priming volume on serum catecholamines during cardiopulmonary bypass. Can Anaesth Soc J 1980; 27:135–39. [DOI] [PubMed] [Google Scholar]
- 27.Byrne L, Obonyo NG, Diab SD, et al. Unintended consequences; fluid resuscitation worsens shock in an ovine model of endotoxemia. Am J Respir Crit Care Med 2018; published online Oct 15. DOI. 10.1164/rccm.201801-00640C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whittenberger JL, McGregor M, Berglund E, Borst HG. Influence of state of inflation of the lung on pulmonary vascular resistance. J Appl Physiol 1960; 15:878–82. [DOI] [PubMed] [Google Scholar]
- 29.Duggan M, McCaul CL, McNamara PJ, Engelberts D, Ackerley C, Kavanagh BP. Atelectasis causes vascular leak and lethal right ventricular failure in uninjured rat lungs. Am J Respir Crit Care Med 2003; 167:1633–40. [DOI] [PubMed] [Google Scholar]
- 30.Denault A, Deschamps A, Tardif J-C, Lambert J, Perrault L. Pulmonary hypertension in cardiac surgery. Curr Cardiol Rev 2010; 6:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Müller-Strahl G, Hemker J, Zimmer H-G. Comparison between left and right heart function in the isolated biventricular working rat heart. Exp Clin Cardiol 2002; 7:7–19. [PMC free article] [PubMed] [Google Scholar]
- 32.Andrews B, Muchemwa L, Kelly P, Lakhi S, Heimburger DC, Bernard GR. Simplified severe sepsis protocol: a randomized controlled trial of modified early goal-directed therapy in Zambia. Crit Care Med 2014; 42:2315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


