Abstract
Background:
Multiple reports associate the cardiac sodium channel gene (SCN5A) variants S1103Y and R1193Q with type 3 congenital long QT syndrome (LQTS) and drug-induced LQTS. These variants are, however, too common in ancestral populations to be highly arrhythmogenic at baseline: S1103Y allele frequency is 8.1% in Africans and R1193Q 6.1% in East Asians. R1193Q is known to increase late sodium current (INa-L) in cardiomyocytes derived from induced pluripotent stem cells (iPSC-CMs) but the role of these variants in modulating repolarization remains poorly-understood.
Methods:
We determined the effect of S1103Y on QT intervals among Africans in a large electronic health record. Using iPSC-CMs carrying naturally occurring or genome-edited variants, we studied action potential durations (APDs) at baseline and after challenge with the repolarizing potassium current (IKr) blocker dofetilide, and INa-L and IKr at baseline.
Results:
In 1479 African subjects with no confounding medications or diagnoses of heart disease, QT in S1103Y carriers was no different from that in non-carriers. Similarly, baseline APD was no different in cells expressing the Y allele (SY, YY cells) compared to isogenic cells with the reference allele (SS cells). However, INa-L was increased in SY and YY cells and the INa-L blocker GS967 shortened APD in SY/YY but not SS cells (p<0.001). IKr was increased almost 2-fold in SY/YY cells compared to SS cells (tail current: 0.66±0.1 vs 1.2±0.1 pA/pF, p<0.001). Dofetilide challenge prolonged APD at much lower concentrations in SY (4.1 nM [IQR 1.5–9.3], n=11) and YY (4.2 nM [1.7– 5.0], n=5) than in SS cells (249 nM [22.3–2905], n=14, p<0.001 and p<0.01, respectively) and elicited afterdepolarizations in 8/16 SY/YY cells but only in 1/14 SS cells. R1193Q cells similarly displayed no difference in baseline APD but increased IKr and increased dofetilide sensitivity.
Conclusions:
These common ancestry-specific variants do not affect baseline repolarization, despite generating increased INa-L. We propose that increased IKr serves to maintain normal repolarization but increases the risk of manifest QT prolongation with IKr block in variant carriers. Our findings further emphasize the need for inclusion of diverse populations in the study of adverse drug reactions.
Keywords: Drug-induced Torsades de Pointes, common variant, SCN5A, long QT syndrome, iPSC-CMs, late sodium current, IKr
Introduction
Both the congenital long QT syndrome (cLQTS) and the acquired form can present with the distinctive polymorphic ventricular tachycardia Torsades de Pointes (TdP), and a common cause of the acquired long QT syndrome is exposure to QT-prolonging drugs (diLQTS). A distinctive feature of this adverse drug reaction (ADR) is absence of marked QT interval prolongation at baseline, and this makes identification of patients at risk difficult. Multiple genetic studies in patients with diLQTS have identified rare pathogenic variants in the major cLQTS disease genes (KCNQ1, KCNH2, SCN5A) only in a minority of patients,1–5 and common variants in genes encoding potassium channel subunits KCNE1 and KCNE2 have also been implicated as modest effect size risk alleles.6 The most widely-accepted mechanism underlying diLQTS is the block of the cardiac potassium current IKr,7, 8 encoded by KCNH2 (formerly known as HERG). Recent studies have shown that prolonged exposure to some drugs with known diLQTS risk can also prolong repolarization by enhancing late sodium current (INa-L) in dog9, 10 and mouse cardiomyocytes,11 as in the SCN5A-linked form of cLQTS (LQT3).
The SCN5A variants S1103Y (dbSNP rs7626962) and R1193Q (rs41261344) have been repeatedly reported in cases of LQT3,12 diLQTS,13, 14 and sudden death,15–17 and we and others have reported that both display the hallmark LQT3 in vitro phenotype of increased INa-L in heterologous expression systems and in cardiomyocytes developed from induced pluripotent stem cells (iPSC-CMs).18–20 These variants, however, occur too commonly in some ancestral populations to be implicated as a cause of LQT3, a rare disease with a prevalence of ~1/2000.21 In the large genome aggregation database (gnomAD), the allele frequency for S1103Y subjects of African ancestry is 8.1%, and that for R1193Q in East Asian subjects is 6.1%. Homozygotes for both alleles have been reported in gnomAD and both alleles are rare in subjects of other ancestries (e.g., 0.03% for S1103Y and 0.1% for R1193Q in European ancestry subjects). The repeated associations between these variants and arrhythmia phenotypes suggest the hypothesis that these variants confer little or no discernable phenotype at baseline but can predispose to exaggerated repolarization prolongation with drug exposure. To test this hypothesis, we evaluated the impact of the S1103Y on QT interval at baseline in a large electronic health record (EHR) cohort. Further we have assessed the effect of the variants in iPSC-CMs derived from individuals with naturally occurring S1103Y, and isogenic controls generated by CRISPR/Cas9 editing of the variants to the reference allele for S1103Y and R1193Q. Our data not only support the working hypothesis, but also identify enhanced baseline IKr as the key mediator maintaining normal baseline repolarization in variant carriers. Our work thus defines a novel pharmacogenetic mechanism whereby common variants can predispose to a serious ADR and further emphasizes the need to include ancestrally diverse populations in studies of ADR incidence and mechanisms.
Materials and Methods
Data acquisition from electronic health records (EHRs) and experimental materials and methods are detailed in the Supplemental Materials. The study was approved by an institutional review committee and the subjects included in the study gave written informed consent.
Statistics
All data are expressed as mean±S.E. unless otherwise indicated. [Dof]Δ100ms values were non-normally distributed by Shapiro-Wilk W test but lognormally distributed by Kolmogorov D test and so were log-transformed for analysis. INa-L was neither normally distributed nor lognormally distributed so the Kruskal-Wallis test was employed. For normally distributed continuous variables, unpaired t-test or ANOVA was employed with Tukey’s test if an ANOVA found differences among groups. P<0.05 was considered as significant. Prism 5.0 (GraphPad Software) and JMP9.0 were used for analysis.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
S1103Y had no effect on baseline QTc
There were 8954 African American subjects with S1103Y genotypes identified in the DNA-linked biobank BioVU (Supplemental Table S1), including 1043 heterozygotes (SY) and 28 homozygotes (YY). There were 1479 subjects who met the inclusion criteria of absence of heart disease or exposure to a QT-prolonging drug. Figure 1 and Supplemental Table S2 show that while QTc intervals were longer in females than in males as expected, there was no effect of S1103Y on QTc interval.
Figure 1. QTc intervals in African American subjects.
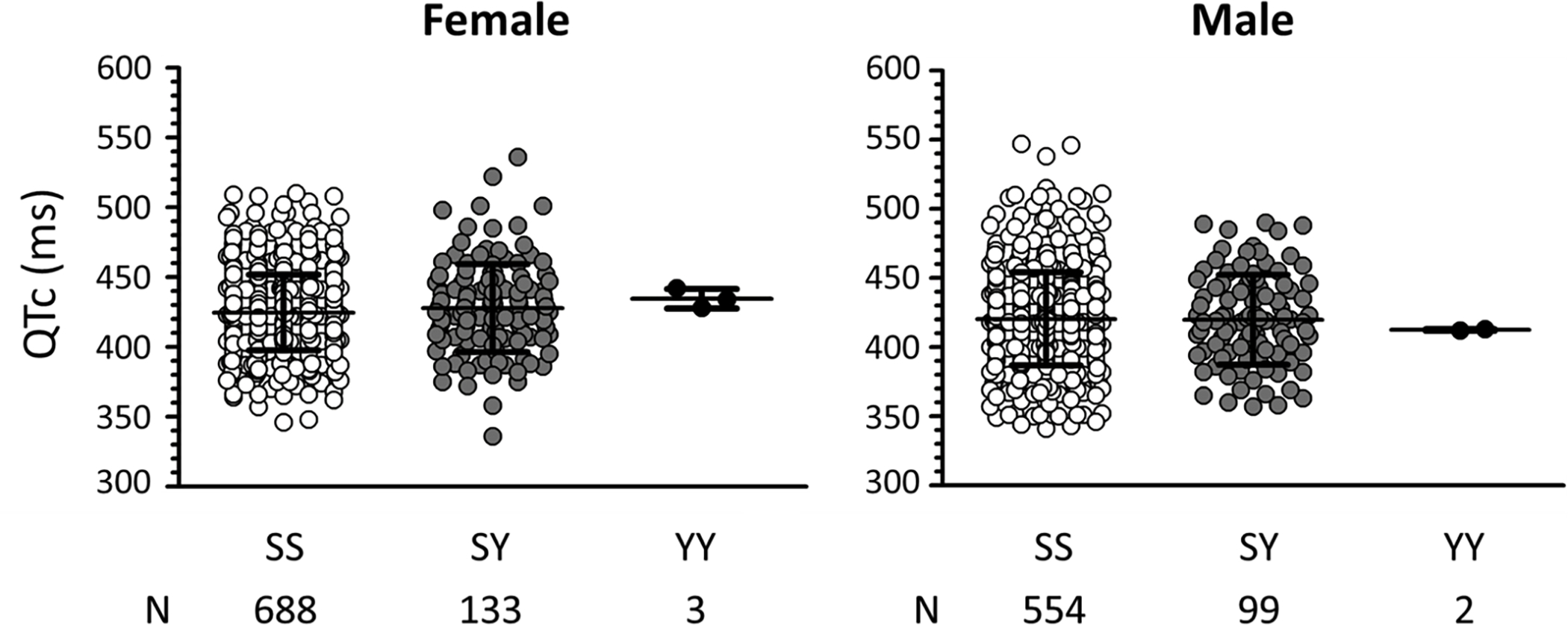
QTc intervals are plotted separately for females (left) and males (right). Open symbols denote intervals with the SS genotype, grey symbols with the SY genotype, and black symbols the YY genotype. N indicates the number of subjects included in the study. QTc intervals are presented in Supplemental Table S2.
S1103Y had no effect on baseline action potential duration (APD) in iPSC-CMs
To study S1103Y, we used five iPSC-CM lines (Table 1): (1) cells from a subject of European ancestry with the SS genotype (C1-SS); (2) C1-SS cells with the SY genotype introduced by CRISPR/Cas9 genome editing (C1-SY); (3) C1-SS cells edited to the YY genotype (C1-YY); (4) SY cells from an African American carrier subject (A1-SY) with normal QTc interval; and (5) A1-SY cells edited to the SS genotype (A1-SS). There were no differences in mean APD90 across the five lines (N=15–39 cells/line) at baseline (Figure 2A, Supplemental Table S3).
Table 1.
Cell lines.
| Line | Original SCN5A genotypes | Background | Introduced genotypes | Abbreviation |
|---|---|---|---|---|
| C1 | Wild-type | European | (Unedited) | C1-SS |
| S1103Y heterozygous | C1-SY | |||
| S1103Y homozygous | C1-YY | |||
| A1 | S1103Y heterozygous | African | Wild-type | A1-SS |
| (Unedited) | A1-SY | |||
| C2 | Wild-type | European | (Unedited) | C2-RR |
| R1193Q heterozygous | C2-RQ | |||
| R1193Q homozygous | C2-QQ |
Figure 2. Action potential duration and dofetilide sensitivity in iPSC-CMs with S1103Y variant.
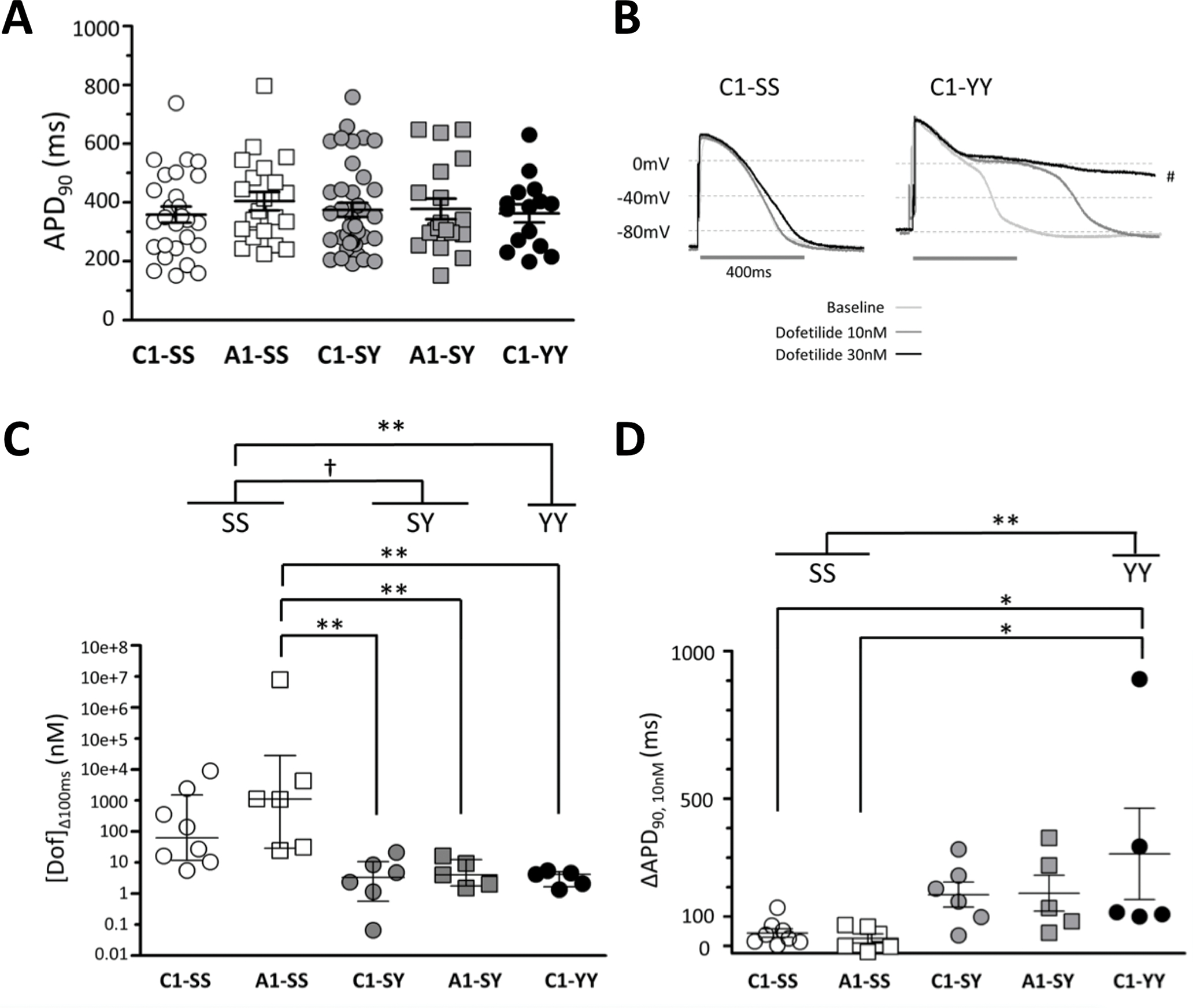
(A) Baseline action potential duration at 90% repolarization (APD90) was similar among C1-SS (n=27), A1-SS (n=21), C1-SY (n=39), A1-SY (n=19), and C1-YY (n=15) cells. (B) Representative traces during dofetilide perfusion in iPSC-CMs with the SS (C1-SS) and YY (C1-YY) genotype. Traces include APD90 at baseline, 10, and 30 nM dofetilide perfusion. The trace of C1-YY at 30 nM dofetilide was paced at 0.25 Hz due to extreme APD prolongation (#). (C) Dofetilide concentration that prolonged APD90 by 100 ms ([Dof]Δ100ms). (D) Absolute change in APD90 at 10 nM dofetilide. Open symbols denote data obtained from cells with the SS genotype, grey symbols from the SY genotype, and black symbols the YY genotype. Statistical comparisons were between lines, and between genotypes by combining same genotypes.*p<0.05, **p<0.01, †p<0.001.
S1103Y iPSC-CMs showed increased dofetilide sensitivity
While there was no effect of genotype on baseline APDs, exposure to serially-increasing concentrations of the potent IKr blocker dofetilide revealed strikingly differing sensitivities to the drug challenge (Figure 2B–C). Acute exposure to dofetilide (30 minutes) did not increase INa-L in iPSC-CMs with or without the S1103Y variant (Supplemental Figure S1). The median dofetilide concentration that prolonged APD90 by 100 ms ([Dof]Δ100ms) was 83.7 nM (interquartile range [IQR]: 11.9–1910, n=8) in C1-SS cells and 1121 nM (29.4–1.97e+6, n=6) in the A1-SS cells. By contrast, [Dof]Δ100ms was much lower, indicating greater drug sensitivity, in all cells with the Y allele: 3.5 nM (0.9–11.8, n=6) in C1-SY cells, 4.1 (1.8–12.9, n=5) in A1-SY cells, and 4.2 nM (1.7–5.0, n=5) in C1-YY cells (p<0.01: A1-SS vs A1-SY, A1-SS vs C1-SY, and A1-SS vs C1-YY). Analyzed by genotype, dofetilide sensitivity was greater in SY (4.1 [1.5–9.3], n=11) and YY cells (4.2 [1.7–5.0], n=5) than that in SS cells (249 [22.3–2905], n=14, p<0.001 and p<0.01, respectively) (Figure 2C).
In SS cells, very high concentrations of dofetilide were required to observe a 100 ms increase in APD90. Accordingly, we also compared absolute changes at a fixed low drug dose, 10 nM (below the IC50 for IKr block in guinea pig ventricular myocytes, 31.5 nM22). As shown in Figure 2D and Table 2, the change in APD90 in SS cells at 10 nM dofetilide was 43.6±14.7 ms (C1-SS: n=8) and 25.8±15.8 ms (A1-SS: n=6). By contrast, the change in APD90 at 10 nM dofetilide was greater in cells with the Y allele: 174.8±42.4 ms (n=6) in C1-SY, 179.5±60.8 ms (n=5) in A1-SY, and 313.0±154.5 ms (n=5) in C1-YY (p<0.05: C1-SS vs C1-YY, and A1-SS vs C1-YY). Analyzed by genotype, the change in APD90 at 10 nM dofetilide was greater in SY cells (176.9±34.1 ms, n=11) and YY cells (313.0±154.5 ms, n=5) than in SS cells (35.9±10.6 ms, n=14) (p<0.01, SS vs YY). Afterdepolarizations during dofetilide exposure were seen in 1/14 SS cells, 5/11 SY cells, and 3/5 YY cells (Table 2). Taken together, these data show that while there was no effect of genotype on baseline APD, SY and YY cells displayed enhanced sensitivity to arrhythmogenic drug-induced APD prolongation.
Table 2.
Dofetilide sensitivity by genotype in iPSC-CMs.
| SCN5A genotype | SS | SY | YY | ||
|---|---|---|---|---|---|
| Cell line | C1-SS | A1-SS | C1-SY | A1-SY | C1-YY |
|
[Dof]Δ100ms
(nM) Median [IQR] |
83.7 [11.9–1910] |
1121 [29.4–2e+6] |
3.5 [0.9–11.8] |
4.1 [1.8–12.9] |
4.2 [1.7–5.0] |
|
ΔAPD90
at 10nM dofetilide Mean±S.E. (ms) |
43.6±14.7 | 25.8±15.8 | 174.8±42.4 | 179.5±60.8 | 313.0±154.5 |
|
Afterdepolarizations, n/N
(Lowest concentration producing afterdepolarizations) |
1/8 (100 nM) | 0/6 | 3/6 (10 nM) | 2/5 (10 nM) | 3/5 (10nM) |
S1103Y produced increased INa-L in heterologous overexpression and in iPSC-CMs
HEK293T cells stably expressing Y1103-SCN5A showed increased INa-L (0.76±0.09% S.E. of peak INa, n=4) compared to cells expressing S1103-SCN5A (0.32±0.03% S.E. of peak INa, n=4) while peak INa was similar between S1103 and Y1103 cells (Supplemental Figure S2), as previously reported.14 Similarly, Figure 3A–C shows that while C1-SS iPSC-CMs displayed minimal INa-L (0.13±0.06% of peak INa, n=10), INa-L was markedly increased in cells with the Y allele: C1-SY: 0.32±0.05 % (n=12, p<0.05 vs. C1-SS) and C1-YY 2.22±0.33% (n=10, p<0.001 vs. C1-SS). Peak INa was not different among C1-SS, C1-SY, and C1-YY cells (242.2±37.2 [n=4 in one differentiation batch], 237.8±27.6 [n=5 in one differentiation batch], and 226.9±13.1 pA/pF [n=6 in one differentiation batch] at −30mV, respectively.).
Figure 3. Effect of S1103Y variant on late sodium current (INa-L) in iPSC-CMs.
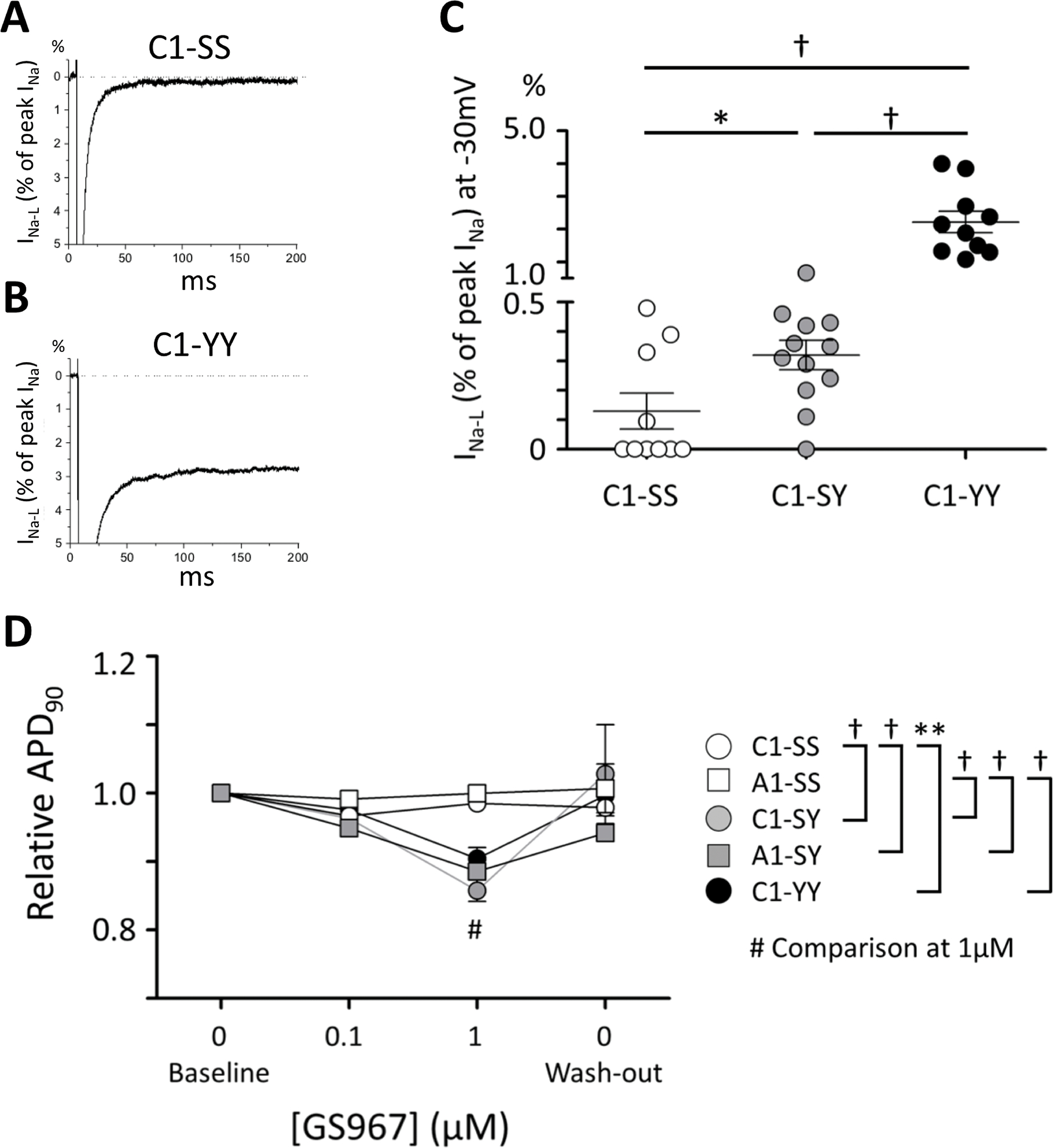
Representative traces of late sodium current (INa-L) in C1-SS (A) and C1-YY cells (B). INa-L is presented as the percentage of peak INa recorded at −30 mV. (C) Summary data of INa-L among C1-SS (n=10), C1-SY (n=12), and C1-YY (n=10) cells from three independent differentiation batches for each line. (D) APD90 relative to baseline in each line before perfusion with the INa-L blocker GS967 (baseline), at 0.1 μM and 1 μM GS967 perfusion, and after wash-out. *p<0.05, **p<0.01, †p<0.001.
Late sodium current inhibition shortens APD only in iPSC-CMs with the S1103Y variant
To demonstrate the physiologic importance of INa-L in cells expressing the Y1103 allele, we tested the effect of the specific late sodium current blocker GS967 on APD. GS967 is reported to block INa-L with an IC50 of 130 nM for ATX-II-induced INa-L but to have the no effect on IKr at 10 μM in rabbit isolated ventricular myocytes.23 In iPSC-CMs, GS967 had no effect on IKr at up to 10 μM (Supplemental Figure S3). When perfused during continuous AP recording, GS967 at 0.1 μM and 1 μM had no effect on APD90 in the SS cells (C1-SS and A1-SS) (Figure 3D). By contrast, GS967 significantly shortened APD90 in iPSC-CMs with the Y-allele (C1-SY vs C1-SS [p<0.001, n=3–4, at 1 μM], C1-YY vs C1-SS [p<0.01, n=4, at 1 μM], and A1-SS vs A1-SY [p<0.001, n=4, at 1 μM]), consistent with the idea that a late sodium current plays significant role during APD in iPSC-CMs with the Y1103 allele but not in the SS cells.
IKr increased in iPSC-CMs with S1103Y variant
The data thus indicate that iPSCs carrying the Y1103 allele display increased late current and corresponding APD shortening with GS967. Baseline APDs, however, were no different from cells with the S1103 allele, and the clinical (BioVU) data also indicate no effect on cardiac repolarization assessed as baseline QTc. This apparent paradox prompted us to examine IKr amplitude across these cell lines. As shown in Figure 4 and Supplemental Table S4, peak IKr tail current was almost twice as large in cells with the Y1103 allele (n=7–11/line) compared to the SS controls: peak IKr tail current density at +20 mV was 0.64±0.13 pA/pF in C1-SS and 0.69±0.16 in A1-SS cells, 1.04±0.16 in C1-SY, 1.16±0.13 in C1-YY (p<0.05 vs C1-SS between −30 and +20 mV), and 1.38±0.13 in A1-SY (p<0.01 vs A1-SS at −10, 0, +10, and +20 mV).
Figure 4. IKr in iPSC-CM.
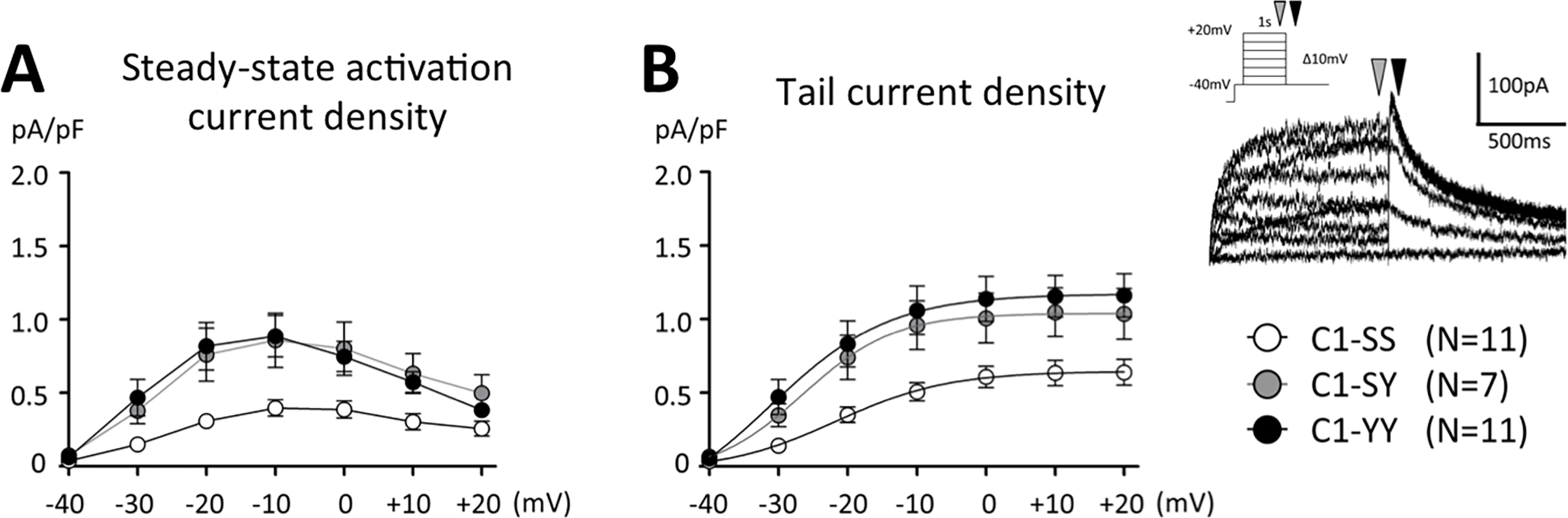
(A) Steady-state activation of IKr, assessed as an E-4031 sensitive current, was measured at 1 sec depolarizing pulse at 0.5 Hz (depicted as a grey arrow in the inset). (B) Peak tail current was measured at −40 mV (depicted as a black arrow in the inset). Summary data for all lines are presented in Supplemental Table S4.
R1193Q also had no effect on baseline action potential but increased dofetilide sensitivity
For these experiments, we used iPSCs with the reference genotype at amino acid position R1193 and introduced R1193Q to generate heterozygous and homozygous variant cell lines (Table 1). There was a minimal, statistically insignificant effect of R1193Q genotype on baseline APD90 (Figure 5A). Similarly, the R1193Q variant conferred increased dofetilide sensitivity: [Dof]Δ100ms in RR, RQ, and QQ cells were 164.1 nM [8.3–2.4e+5, n=6], 6.3 nM [4.0–42.6, n=6], and 13.1 nM [3.6–34.7, n=5], respectively (Figure 5B). APD90 increases caused by 10 nM dofetilide were also greater in RQ and QQ cells than in RR cells (Figure 5C). In addition, cells carrying the R1193Q variant displayed increased IKr (Supplemental Figure S4, Supplemental Table S5). A large BioVU-like resource of variant carriers was not available.
Figure 5. Baseline APD90 and dofetilide sensitivity in iPSC-CMs by R1193Q genotype.
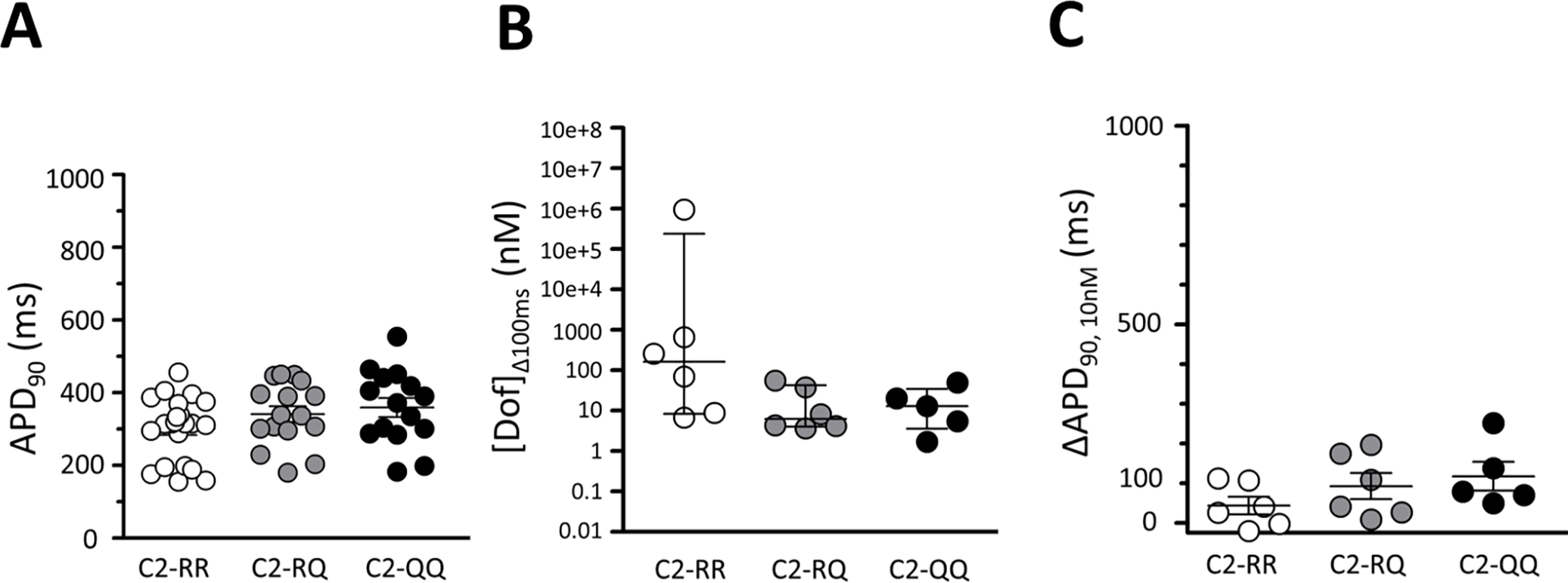
(A) APD90 among cells homozygous for the reference allele (C2-RR, n=23), heterozygous for R1193Q (C2-RQ, n=16), and homozygous for the R1193Q variant (C2-QQ, n=15). (B) The dofetilide concentration that prolonged APD90 by 100 ms ([Dof]Δ100ms) among C2-RR (n=6), C2-RQ (n=6), and C2-QQ (n=5). (C) Absolute change in APD90 at 10 nM dofetilide among C2-RR (n=6), C2-RQ (n=6), and C2-QQ (n=5).
KCNH2 transcripts were no different from controls in S1103Y cell lines
The increased IKr we observed could be due to transcriptional upregulation or post-transcriptional regulation (e.g., channel degradation). There was variability in normalized abundance of transcripts for KCNH2 (encoding the major IKr subunit) but no difference across cell lines (Supplemental Table S6). These data suggest that the increased IKr we observe reflects a post-transcriptional process.
Discussion
Most of the dominant Mendelian disease variants that strongly affect organismal fitness are not common in the population due to historical natural selection.24 An increasingly recognized exception is common pharmacogenetic variants that have not been subject to natural selection because most drug exposures are very new in human history.25 Most such variants described to date influence drug disposition, i.e., the amount of drug delivered to a target site of action, and pharmacogenetically-medicated ADRs thus arise from extremes of plasma drug concentrations.25 We describe here a novel mechanism, unrelated to variable plasma drug concentrations, whereby common genetic variants mediate susceptibility to drug-induced arrhythmias. The variants we have studied are remarkably common in African- and Asian-ancestry populations, and nearly absent in those of European ancestry that have been the focus of most pharmacogenetics research to date. This further emphasizes the need for ancestral diversity in studies of the genetic contribution to human traits like ADRs.
S1103Y and R1193Q: what is known
Small association studies and case reports have associated S1103Y with diverse arrhythmia phenotypes, including a susceptibility to arrhythmias and drug-induced arrhythmias in general,14 sudden arrhythmic death,15 sudden infant death syndrome (SIDS),16, 17, 26 atrial fibrillation,27 discharges of implantable cardioverter defibrillator (ICD) devices,28 and cLQTS in a European ancestry family.29 In the Jackson Heart Study (JHS), a large community-based cohort of >4000 African ancestry subjects, 13 independent SCN5A variants, including S1103Y, accounted for ~2% of the variability in ECG traits but did not identify significant risk allele for QT prolongation.30 Others reported that in the JHS, the Y allele was associated with marginally longer QTc intervals (3 ms) than the S allele, uncorrected for the presence of heart disease; however, hypokalemia, a common cause of acquired LQTS, markedly exaggerated that small difference, to 26 ms.13 A similar spectrum of phenotypes has been associated with R1193Q: cLQTS,12, 18 Brugada syndrome,18, 31, 32 progressive conduction system disease,12 ICD shocks,33 and SIDS.34 Previous work has shown that both variants generate increased INa-L in heterologous overexpression systems,14, 19, 20 and that both variants may further enhance increased INa-L when co-expressed with other more clearly pathogenic SCN5A variants.35, 36
What the present study adds
In the present study, we define a novel mechanism whereby these variants mediate susceptibility to a common serious ADR, drug-induced QT prolongation and resultant arrhythmias. In a large biobank, we were able to define a group of genotyped African American subjects in whom concomitant QT-prolonging drug therapy and evidence of underlying heart disease were absent: in this group, there was no effect of S1103Y on QTc interval, although the expected sex-specific effect on QTc was observed. We then used native and edited iPSC-CMs to show that APD, the in vitro correlate of QTc, was similarly not associated with S1103Y. We did find, however, that INa-L was enhanced in cells carrying the Y allele. The effect was physiologically important in iPSC-CMs since we observed that the specific INa-L blocker GS967 shortened APD in cells carrying the Y allele but not in cells without the Y allele. Surprisingly, despite the increase in INa-L, we did not observe an increase in APD. We resolved this apparent paradox by observing that IKr, a major repolarizing potassium current in heart, was markedly increased in cells carrying the Y allele. While this increase in IKr accounts for the unchanged APD across cell lines, it also renders these cells much more susceptible to action potential prolongation and arrhythmogenic afterdepolarizations as shown in Figure 6. While we did not have access to a large genotyped population of R1193Q carriers, the results of our iPSC-CM studies of this variant were similar to those with S1103Y.
Figure 6. Summary of findings.
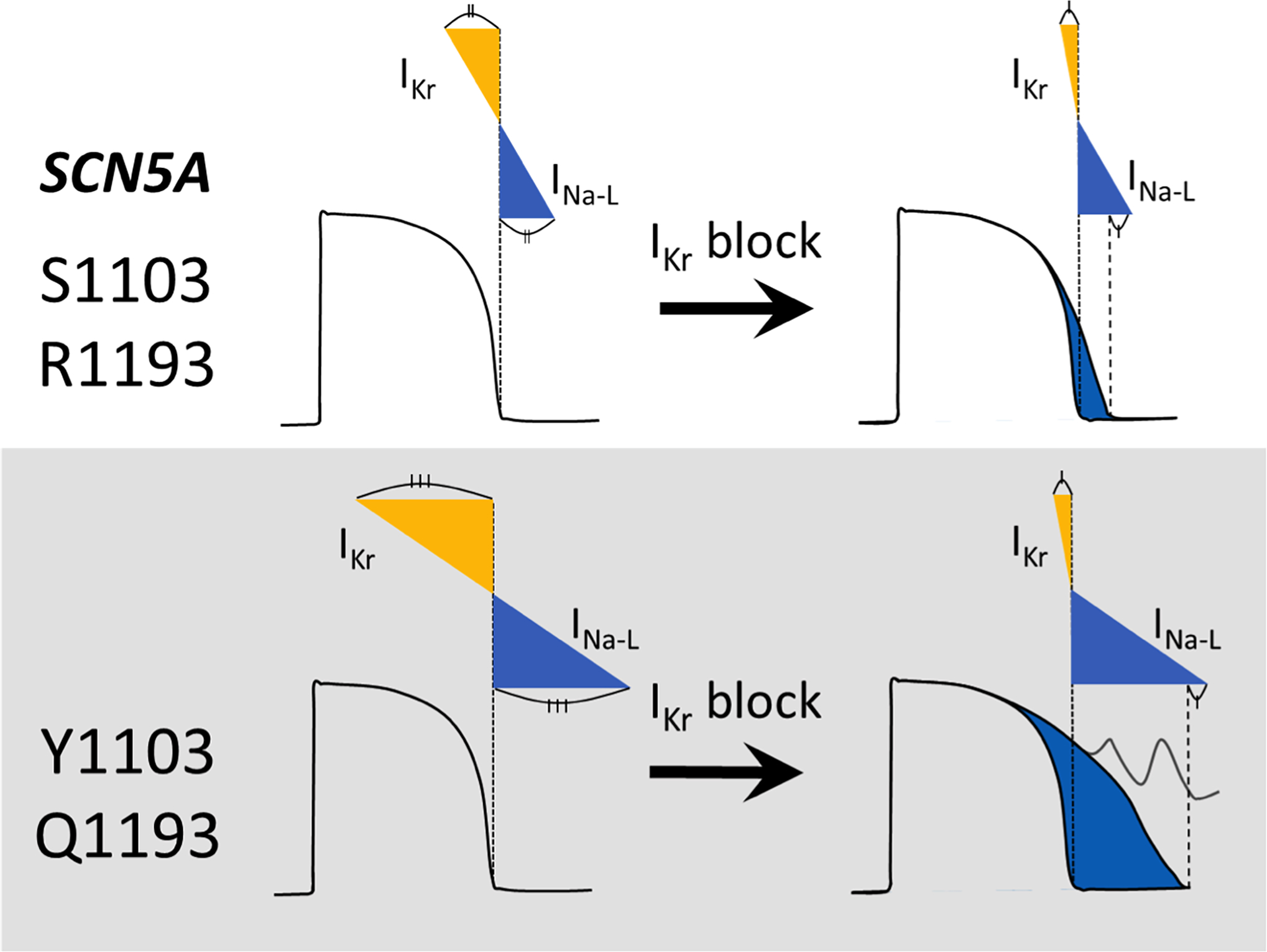
We find that while the common SCN5A variants S1103Y and R1193Q enhance pathogenic current INa-L, they also increase IKr. This “balanced repolarization” normalizes APD at baseline but manifests exaggerated APD prolongation and afterpolarizations when IKr is blocked.
Interaction between late sodium current and IKr
Recent reports have identified apparent co-regulation of INa-L and IKr similar to that observed here. Hegyi et al.37 found that INa-L and IKr are in “balance” in both rabbit and guinea pig cardiomyocytes: they reported substantial cell-to-cell variability in current amplitude but a strong correlation between the amplitudes of the two currents across cells. Eichel et al.38 showed that mRNA transcripts for SCN5A and KCNH2 were associated in a “microtranslatome” and that approximately half of KCNH2 translational complexes also included SCN5A transcripts. The role of the INa-L and of SCN5A variants in modulating this translational co-regulation has not been studied and the underlying mechanisms are as yet undefined. Our quantitative PCR data showing no difference in KCNH2 expression between lines suggests that the effect occurs after transcription.
Limitations
While we and others have suggested that INa-L and IKr are co-regulated, the underlying mechanisms and indeed the level at which this coregulation occurs remain undefined. Our data suggest substantial cell-cell variability in KCNH2 transcripts but overall no major effect of S1103Y genotype on KCNH2 transcripts, suggesting post-transcriptional regulation. We were not able to define a drug-free, heart disease-free R1193Q cohort to study genotype-dependence of the QTc interval, although our APD data would suggest there will be minimal baseline effect. There have not been large numbers of cases of diLQTS reported in genotyped subjects of African ancestry to date.
The role of late sodium current in drug-induced LQTS and clinical implications
Lu et al. have reported that chronic exposure to the anticancer agent nilotinib prolonged APD in canine ventricular myocytes in part by enhancing INa-L.9 We have previously shown that hours of exposure to dofetilide and other potent IKr blockers similarly increased INa-L in heterologous overexpression, iPSCs-CM, and mouse myocytes,11 reinforcing the idea that mechanisms beyond the widely-accepted idea of IKr block7, 8 can generate diLQTS. The enhanced INa-L may reflect inhibition of other molecular pathways (e.g., linked to receptor tyrosine kinases) as proposed previously.9 In the present study, iPSC-CMs were exposed to dofetilide only acutely and the APD-prolonging effect emerged in minutes. With chronic exposure to dofetilide or other potent IKr blockers, as is the case in clinical use, the additional effect of increased late INa-L may further exaggerate arrhythmia susceptibility in carriers of the Y1103 or Q1193 alleles.
An interesting discrepancy in our data is that while late current was much greater in YY than in SY cells, the increase in IKr was similar. While one outcome of these changes would be longer APDs in YY cells, we actually observed no difference in baseline APDs among SS, SY, and YY cells. We infer that the increase in IKr was sufficient to maintain normal APD in both heterozygote and homozygote cells. Further, a single variant allele did increase drug sensitivity, to both dofetilide and GS967.
As previously reported, the common D85N variant in the potassium channel subunit gene KCNE1 predisposes to diLQTS6 and it has been suggested that this variant be included in nascent efforts to inform genotype-guided therapy and minimize ADR risk using clinical decision support in EHRs.39 The minor allele frequency of the N allele varies from 0.1% (East Asian ancestry), 0.2% (African), 1.7% (European ancestry), to 2.5% (Ashkenazim), suggesting that its contribution to efforts to minimize ADRs may differ among ancestral populations. The present findings extend this concern to ancestrally diverse populations, and may assume special relevance with the suggested use of hydroxychloroquine during the initial phases of the COVID-19 pandemic, a disease that has disproportionately affected subjects of African ancestry.40
Supplementary Material
What is new?
Although the cardiac sodium channel variants S1103Y and R1193Q are common, occurring in 5–10% of specific ancestral populations, they have nevertheless been repeatedly associated with drug-induced torsades de pointes and other arrhythmias.
While both S1103Y and R1193Q generate increased late sodium current, baseline action potential durations in cardiomyocytes from induced pluripotent stem cells (iPSC-CMs) carrying these variants were unexpectedly normal.
The repolarizing potassium current IKr was markedly increased in iPSC-CMs with the variants, accounting for normal baseline APD, but with exposure to an IKr blocker they displayed exaggerated APD prolongation and afterdepolarizations.
What are the clinical implications?
Humans with ion channel variants displaying abnormal electrophysiology in heterologous expression systems may nevertheless have normal repolarization because of compensatory mechanisms detectable in cardiomyocytes.
Use of drugs that inhibit such compensatory mechanisms may expose variant carriers to increased arrhythmia risk.
These findings highlight the need to include ancestral diversity in genomic and pharmacogenomic studies.
Acknowledgments:
We thank Marcia A. Blair, Laura L. Short, and Lynn D. Hall for their cell culture contributions.
Source of Funding:
Electrocardiographic data at Vanderbilt University Medical Center were obtained using Vanderbilt’s Synthetic Derivative. The Synthetic Derivative resource is supported by CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences. This project was supported by NIH R01 HL149826 (D.M.R.), the Heart Rhythm Society Clinical Research Award in Honor of Mark Josephson and Hein Wellens (Y.W.), American Heart Association postdoctoral fellowship 830951 (Y.W.), NIH K99 HG010904 (A.M.G.), and American Heart Association Strategically Focused Research Network 18SFRN34110369 (G.E.D.).
Non-standard Abbreviations and Acronyms
- ADR
adverse drug response
- APD
action potential duration
- CRISPR/Cas9
Clustered Regularly Interspaced Short Palindromic Repeats/Cas9 enzyme
- EHR
electronic health record
- IKr
rapidly activating delayed rectifier potassium current
- INa-L
late sodium current
- iPSC-CMs
induced pluripotent stem cell-derived cardiomyocytes
- LQTS
long QT syndrome
- SCN5A
cardiac sodium channel gene
Footnotes
Disclosures: All authors report no conflict of interest.
References
- 1.Paulussen AD, Gilissen RA, Armstrong M, Doevendans PA, Verhasselt P, Smeets HJ, Schulze-Bahr E, Haverkamp W, Breithardt G, Cohen N, et al. Genetic variations of kcnq1, kcnh2, scn5a, kcne1, and kcne2 in drug-induced long qt syndrome patients. Journal of molecular medicine (Berlin, Germany). 2004;82:182–188 [DOI] [PubMed] [Google Scholar]
- 2.Weeke P, Mosley JD, Hanna D, Delaney JT, Shaffer C, Wells QS, Van Driest S, Karnes JH, Ingram C, Guo Y, et al. Exome sequencing implicates an increased burden of rare potassium channel variants in the risk of drug-induced long qt interval syndrome. Journal of the American College of Cardiology. 2014;63:1430–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adler A, Novelli V, Amin AS, Abiusi E, Care M, Nannenberg EA, Feilotter H, Amenta S, Mazza D, Bikker H, et al. An international, multicentered, evidence-based reappraisal of genes reported to cause congenital long qt syndrome. Circulation. 2020;141:418–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah RR. Drug-induced qt interval prolongation: Does ethnicity of the thorough qt study population matter? British journal of clinical pharmacology. 2013;75:347–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Itoh H, Crotti L, Aiba T, Spazzolini C, Denjoy I, Fressart V, Hayashi K, Nakajima T, Ohno S, Makiyama T, et al. The genetics underlying acquired long qt syndrome: Impact for genetic screening. European heart journal. 2016;37:1456–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaab S, Crawford DC, Sinner MF, Behr ER, Kannankeril PJ, Wilde AA, Bezzina CR, Schulze-Bahr E, Guicheney P, Bishopric NH, et al. A large candidate gene survey identifies the kcne1 d85n polymorphism as a possible modulator of drug-induced torsades de pointes. Circulation. Cardiovascular genetics. 2012;5:91–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yap YG, Camm AJ. Drug induced qt prolongation and torsades de pointes. Heart (British Cardiac Society). 2003;89:1363–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roden DM. Drug-induced prolongation of the qt interval. The New England journal of medicine. 2004;350:1013–1022 [DOI] [PubMed] [Google Scholar]
- 9.Lu Z, Wu CY, Jiang YP, Ballou LM, Clausen C, Cohen IS, Lin RZ. Suppression of phosphoinositide 3-kinase signaling and alteration of multiple ion currents in drug-induced long qt syndrome. Science translational medicine. 2012;4:131ra150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu Z, Jiang YP, Wu CY, Ballou LM, Liu S, Carpenter ES, Rosen MR, Cohen IS, Lin RZ. Increased persistent sodium current due to decreased pi3k signaling contributes to qt prolongation in the diabetic heart. Diabetes. 2013;62:4257–4265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang T, Chun YW, Stroud DM, Mosley JD, Knollmann BC, Hong C, Roden DM. Screening for acute ikr block is insufficient to detect torsades de pointes liability: Role of late sodium current. Circulation. 2014;130:224–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun A, Xu L, Wang S, Wang K, Huang W, Wang Y, Zou Y, Ge J. Scn5a r1193q polymorphism associated with progressive cardiac conduction defects and long qt syndrome in a chinese family. Journal of medical genetics. 2008;45:127–128 [DOI] [PubMed] [Google Scholar]
- 13.Akylbekova EL, Payne JP, Newton-Cheh C, May WL, Fox ER, Wilson JG, Sarpong DF, Taylor HA, Maher JF. Gene-environment interaction between scn5a-1103y and hypokalemia influences qt interval prolongation in african americans: The jackson heart study. American heart journal. 2014;167:116–122 e111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Splawski I, Timothy KW, Tateyama M, Clancy CE, Malhotra A, Beggs AH, Cappuccio FP, Sagnella GA, Kass RS, Keating MT. Variant of scn5a sodium channel implicated in risk of cardiac arrhythmia. Science (New York, N.Y.). 2002;297:1333–1336 [DOI] [PubMed] [Google Scholar]
- 15.Burke A, Creighton W, Mont E, Li L, Hogan S, Kutys R, Fowler D, Virmani R. Role of scn5a y1102 polymorphism in sudden cardiac death in blacks. Circulation. 2005;112:798–802 [DOI] [PubMed] [Google Scholar]
- 16.Van Norstrand DW, Tester DJ, Ackerman MJ. Overrepresentation of the proarrhythmic, sudden death predisposing sodium channel polymorphism s1103y in a population-based cohort of african-american sudden infant death syndrome. Heart rhythm : the official journal of the Heart Rhythm Society. 2008;5:712–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plant LD, Bowers PN, Liu Q, Morgan T, Zhang T, State MW, Chen W, Kittles RA, Goldstein SA. A common cardiac sodium channel variant associated with sudden infant death in african americans, scn5a s1103y. The Journal of clinical investigation. 2006;116:430–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang H, Zhao J, Barrane FZ, Champagne J, Chahine M. Nav1.5/r1193q polymorphism is associated with both long qt and brugada syndromes. The Canadian journal of cardiology. 2006;22:309–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroncke BM, Yang T, Roden DM. Multiple mechanisms underlie increased cardiac late sodium current. Heart rhythm : the official journal of the Heart Rhythm Society. 2019;16:1091–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Q The common scn5a mutation r1193q causes lqts-type electrophysiological alterations of the cardiac sodium channel. Journal of medical genetics. 2004;41:e66–e66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz PJ, Stramba-Badiale M, Crotti L, Pedrazzini M, Besana A, Bosi G, Gabbarini F, Goulene K, Insolia R, Mannarino S, et al. Prevalence of the congenital long-qt syndrome. Circulation. 2009;120:1761–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jurkiewicz NK, Sanguinetti MC. Rate-dependent prolongation of cardiac action potentials by a methanesulfonanilide class iii antiarrhythmic agent. Specific block of rapidly activating delayed rectifier k+ current by dofetilide. Circulation research. 1993;72:75–83 [DOI] [PubMed] [Google Scholar]
- 23.Belardinelli L, Liu G, Smith-Maxwell C, Wang WQ, El-Bizri N, Hirakawa R, Karpinski S, Li CH, Hu L, Li XJ, et al. A novel, potent, and selective inhibitor of cardiac late sodium current suppresses experimental arrhythmias. The Journal of pharmacology and experimental therapeutics. 2013;344:23–32 [DOI] [PubMed] [Google Scholar]
- 24.Whiffin N, Minikel E, Walsh R, O’Donnell-Luria AH, Karczewski K, Ing AY, Barton PJR, Funke B, Cook SA, MacArthur D, et al. Using high-resolution variant frequencies to empower clinical genome interpretation. Genetics in medicine : official journal of the American College of Medical Genetics. 2017;19:1151–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roden DM, McLeod HL, Relling MV, Williams MS, Mensah GA, Peterson JF, Van Driest SL. Pharmacogenomics. Lancet. 2019;394:521–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winkel BG, Yuan L, Olesen MS, Sadjadieh G, Wang Y, Risgaard B, Jabbari R, Haunsø S, Holst AG, Hollegaard MV, et al. The role of the sodium current complex in a nonreferred nationwide cohort of sudden infant death syndrome. Heart rhythm : the official journal of the Heart Rhythm Society. 2015;12:1241–1249 [DOI] [PubMed] [Google Scholar]
- 27.Ilkhanoff L, Arking DE, Lemaitre RN, Alonso A, Chen LY, Durda P, Hesselson SE, Kerr KF, Magnani JW, Marcus GM, et al. A common scn5a variant is associated with pr interval and atrial fibrillation among african americans. Journal of cardiovascular electrophysiology. 2014;25:1150–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun AY, Koontz JI, Shah SH, Piccini JP, Nilsson KR Jr., Craig D, Haynes C, Gregory SG, Hranitzky PM, Pitt GS. The s1103y cardiac sodium channel variant is associated with implantable cardioverter-defibrillator events in blacks with heart failure and reduced ejection fraction. Circulation. Cardiovascular genetics. 2011;4:163–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen S, Chung MK, Martin D, Rozich R, Tchou PJ, Wang Q. Snp s1103y in the cardiac sodium channel gene scn5a is associated with cardiac arrhythmias and sudden death in a white family. Journal of medical genetics. 2002;39:913–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeff JM, Brown-Gentry K, Buxbaum SG, Sarpong DF, Taylor HA, George AL Jr., Roden DM, Crawford DC. Scn5a variation is associated with electrocardiographic traits in the jackson heart study. Circulation. Cardiovascular genetics. 2011;4:139–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li L, Ruan Y, Liu N, Zhao Q, Zhang M, Li X, Zuo S, Le J, Wu K, Bai R, et al. “Pill-in-the-pocket” treatment of propafenone unmasks ecg brugada pattern in an atrial fibrillation patient with a common scn5a r1193q polymorphism. Frontiers in physiology. 2019;10:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu X, Liu W, Hu D, Sun Y, Li L, Li C. Patient with obstructive sleep apnea-hypopnea syndrome and scn5a mutation (r1193q polymorphism) associated with brugada type 2 electrocardiographic pattern. Journal of electrocardiology. 2009;42:250–253 [DOI] [PubMed] [Google Scholar]
- 33.Makarawate P, Chaosuwannakit N, Vannaprasaht S, Sahasthas D, Koo SH, Lee EJD, Tassaneeyakul W, Barajas-Martinez H, Hu D, Sawanyawisuth K. Scn5a genetic polymorphisms associated with increased defibrillator shocks in brugada syndrome. Journal of the American Heart Association. 2017;6:e005009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glengarry JM, Crawford J, Morrow PL, Stables SR, Love DR, Skinner JR. Long qt molecular autopsy in sudden infant death syndrome. Archives of disease in childhood. 2014;99:635–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan BH, Valdivia CR, Rok BA, Ye B, Ruwaldt KM, Tester DJ, Ackerman MJ, Makielski JC. Common human scn5a polymorphisms have altered electrophysiology when expressed in q1077 splice variants. Heart rhythm : the official journal of the Heart Rhythm Society. 2005;2:741–747 [DOI] [PubMed] [Google Scholar]
- 36.Niu DM, Hwang B, Hwang HW, Wang NH, Wu JY, Lee PC, Chien JC, Shieh RC, Chen YT. A common scn5a polymorphism attenuates a severe cardiac phenotype caused by a nonsense scn5a mutation in a chinese family with an inherited cardiac conduction defect. Journal of medical genetics. 2006;43:817–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hegyi B, Chen-Izu Y, Izu LT, Rajamani S, Belardinelli L, Bers DM, Bányász T. Balance between rapid delayed rectifier k(+) current and late na(+) current on ventricular repolarization: An effective antiarrhythmic target? Circulation. Arrhythmia and electrophysiology. 2020;13:e008130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eichel CA, Rios-Perez EB, Liu F, Jameson MB, Jones DK, Knickelbine JJ, Robertson GA. A microtranslatome coordinately regulates sodium and potassium currents in the human heart. eLife. 2019;8:e52654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giudicessi JR, Roden DM, Wilde AAM, Ackerman MJ. Classification and reporting of potentially proarrhythmic common genetic variation in long qt syndrome genetic testing. Circulation. 2018;137:619–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giudicessi JR, Roden DM, Wilde AAM, Ackerman MJ. Genetic susceptibility for covid-19-associated sudden cardiac death in african americans. Heart rhythm : the official journal of the Heart Rhythm Society. 2020;17:1487–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dumitrescu L, Ritchie MD, Brown-Gentry K, Pulley JM, Basford M, Denny JC, Oksenberg JR, Roden DM, Haines JL, Crawford DC. Assessing the accuracy of observer-reported ancestry in a biorepository linked to electronic medical records. Genetics in medicine : official journal of the American College of Medical Genetics. 2010;12:648–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ritchie MD, Denny JC, Zuvich RL, Crawford DC, Schildcrout JS, Bastarache L, Ramirez AH, Mosley JD, Pulley JM, Basford MA, et al. Genome- and phenome-wide analyses of cardiac conduction identifies markers of arrhythmia risk. Circulation. 2013;127:1377–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dowey SN, Huang X, Chou BK, Ye Z, Cheng L. Generation of integration-free human induced pluripotent stem cells from postnatal blood mononuclear cells by plasmid vector expression. Nature protocols. 2012;7:2013–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, Lan F, Diecke S, Huber B, Mordwinkin NM, et al. Chemically defined generation of human cardiomyocytes. Nature methods. 2014;11:855–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haeussler M, Schönig K, Eckert H, Eschstruth A, Mianné J, Renaud JB, Schneider-Maunoury S, Shkumatava A, Teboul L, Kent J, et al. Evaluation of off-target and on-target scoring algorithms and integration into the guide rna selection tool crispor. Genome biology. 2016;17:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the crispr-cas9 system. Nature protocols. 2013;8:2281–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glazer AM, Wada Y, Li B, Muhammad A, Kalash OR, O’Neill MJ, Shields T, Hall L, Short L, Blair MA, et al. High-throughput reclassification of scn5a variants. American journal of human genetics. 2020;107:111–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma J, Guo L, Fiene SJ, Anson BD, Thomson JA, Kamp TJ, Kolaja KL, Swanson BJ, January CT. High purity human-induced pluripotent stem cell-derived cardiomyocytes: Electrophysiological properties of action potentials and ionic currents. American journal of physiology. Heart and circulatory physiology. 2011;301:H2006–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsa E, Rajamohan D, Dick E, Young L, Mellor I, Staniforth A, Denning C. Drug evaluation in cardiomyocytes derived from human induced pluripotent stem cells carrying a long qt syndrome type 2 mutation. European heart journal. 2011;32:952–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


