Abstract
Background and Purpose:
Recent evidence suggests that young women (18–45 years) may be at higher risk of ischemic strokes than men of the same age. The goal of this systematic review is to reconcile and synthesize existing evidence of sex differences among young adults with ischemic strokes.
Methods:
We searched PubMed from January 2008 to July 2021 for relevant articles and reviews and consulted their references. We included original studies that (1) were population-based, (2) reported stroke incidence by sex or sex specific incidence rate ratios of young adults ≤45 years. We excluded studies that (1) omitted measurements of error for incidence rates or incidence rate ratios, (2) omitted age adjustment, and (3) were not in English. Statistical synthesis was performed to estimate sex difference by age group (≤35, 35–45 and ≤45) and stroke type.
Results:
We found 19 studies that reported on sex-specific stroke incidence among young adults, including three that reported on overlapping data. Nine studies did not find a statistically significant sex difference among young adults ≤45 years. Three studies found higher rates of ischemic stroke among men among young adults ≥ 30 to 35 years. Four studies found more women with ischemic strokes among young adults ≤35 years. Overall, in young adults ≤35 years, the estimated effect size favored more ischemic strokes in women (IRR 1.44[1.18–1.76], I2=82%) and a non-significant sex difference in young adults 35–45 years (IRR 1.08[0.85–1.38], I2=95%).
Conclusion:
Overall, there were 44% more women ≤35 years with ischemic strokes than men. This gap narrows in young adults, 35 to 45 years, and there is conflicting evidence whether more men or women have ischemic strokes in the 35 to 45 age group.
MESH Terms: Young Adult, Women, Incidence, Ischemic Stroke
Subject Terms: Ischemic Stroke, Women
Introduction:
The American Heart Association’s 2021 Heart Disease and Stroke Statistics Update notes that “age-specific incidence rates [of stroke] are substantially lower in females than males in younger and middle-aged groups”1 Historically, stroke epidemiologists believed men have a higher incidence of strokes in every age group until the very elderly.2, 3 A systematic review by Appelros et al. in 2009, looking at sex differences in stroke epidemiology, found that among young adults age 35 to 44 years, there were 49% more men than women with incident strokes (including ischemic strokes, intracranial hemorrhages (ICH), and strokes of undetermined causes), and did not find any sex difference among adults younger than age 35 years.4 However, this study, which was focused on stroke incidence in all adult age groups, only included a small number of cases in young adults under the age of 45 years. Since that review, more recent evidence focused on the young adult age group has reported that there are more young women (age 18 to 45) with ischemic strokes compared to young men, suggesting that young women may be disproportionately at risk compared to their male counterparts.5, 6 A better understanding of these sex differences is important to be able to implement strategies to more effectively prevent and treat strokes in this age group. The goal of this review is to reconcile and synthesize the updated evidence to better understand sex differences in young adults with ischemic strokes.
Methods:
The authors declare that all supporting data are available within the article and its online supplementary files. Institutional review was exempted because this study relied only on previously published data.
We used methods previously described by Appelros et al. to search PubMed using the algorithms (incidence[ti] OR epidemiology[ti] OR prevalence[ti]) AND (cerebrovascular[ti] OR stroke[ti]); subtype[ti] AND (cerebrovascular[ti] OR stroke[ti]) from January 2008 until July 2021.4 Articles prior to 2008 were included in the systematic review by Appelros et al. We examined both original studies and reviews on sex differences in stroke or young adult strokes. We reviewed these manuscripts’ citations to extract additional relevant articles. We included original studies that (1) were population-based, (2) reported first-ever stroke incidence by sex or sex specific incidence rate ratios (IRR) of adults younger than age 45 years. Since ischemic strokes comprise approximately 87% of all stroke types1, we included studies that did not differentiate between stroke types, as the majority of these cases were ischemic strokes. We excluded studies that (1) omitted any measurements of error for incidence rates or IRR, (2) omitted age adjustments, and (3) were not written in English. One reviewer (MHL) screened and reviewed full text as well as online supplements for eligibility. Any studies with missing or ambiguous data or concern for high risk of bias were discussed with a second reviewer (SNP or SS) until consensus was reached, and studies with high risk of bias were not included.
Study information was extracted by one reviewer (MHL) and checked for accuracy by a second reviewer (SS). Information extracted included study characteristics (authors, publication year, country), sex-specific stroke incidence by age group, characteristics of the study population, study design (retrospective or prospective), methods of stroke ascertainment (i.e. administrative claims or clinical data), and the method of calculating incidence (i.e. Poisson model, adjustments for age and other variables) to assess study quality and potential sources of bias (Supplemental Table T1).
Results from each study were synthesized into IRR of women/men. Studies which did not adjust for age were excluded because crude incidence rates of stroke in women and men may not be comparable if they have different age distributions even within each age-group. To obtain the IRR for each study, the female and male crude incidence rates have to be standardized to the age structure of the total (female and male) population or another standardized population. One study was excluded for not meeting this criteria.7
We calculated combined IRR by age group (≤35 years, 35–45 years, and ≤45 years) and by ischemic stroke versus all stroke types. Studies that had qualifying IRR within each age group were included. Sensitivity analysis was performed to include data prior to 2008 from Appelros et al.4 The analysis was performed on all stroke types in age ≤ 35 years, 35–45 years, and ≤ 45 years, because there was no differentiation of ischemic strokes. Analyses for an overall effect were performed with random effects models on the log of the ratio estimates, weighted according to the variability of the estimates. The variability of the estimates was calculated from the 95% confidence intervals. The combined ratios were estimated, along with 95% confidence intervals, and p values testing the null hypothesis of the IRR equaling one. Statistical significance was determined as p<0.05. The study heterogeneity was measured with the I2 statistic and forest plots were generated. The meta-analyses were performed in STATA MP 15.1 using the “metan”.
Results:
Our search criteria yielded 1951 articles, of which 212 were relevant based on title review and underwent abstract and full text review. We found 34 articles that contained stroke incidence, including those extracted from citations of relevant articles. Finally, 19 articles satisfied the inclusion and exclusion criteria, including 3 articles that reported on overlapping data (Table 1, Supplemental Figure F1).5, 6, 8–24 Of the 16 unique studies, including a combined total of 69,793 young adults with stroke (33,775 women and 36,018 men calculated based on IRR), 9 did not find a statistically significant sex difference among adults younger than age 45 years. Six of the 9 studies identified fewer than 100 cases of stroke in adults younger than age 45 years, including 4 studies that identified fewer than 50 cases of stroke. Of these 9 studies, all but one were prospective studies, one was a door-to-door survey, and all performed case ascertainment based on clinical data (Supplemental Table T1).
Table 1:
Study Characteristics and Incidence Rate Ratios of Women to Men in Young Adults
| Study, Year | Location | Ages | Stroke Types, n of strokes | Years | Incidence Rate Ratio (women:men), 95% CI, n of strokes | Additional Citations | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Akyea, 20219 | United Kingdom | ≥ 18 | |||||||||
| IS, ICH, Udet | 1998–2017 | ||||||||||
| Bejot, 202111 | Dijon, France | 18–55 | Giroud,201717* | ||||||||
| IS, ICH, Undet, SAH* | 1987–2012* | ||||||||||
| IS | 1985–2017 | ||||||||||
| 1985–2003 | |||||||||||
| 2003–2017 | |||||||||||
| Vyas, 202122 | Ontario, Canada | ≥ 18 | |||||||||
| IS, TIA, ICH, SAH | 2003 – 2018 | ||||||||||
| IS | |||||||||||
| Leppert, 20206 | United States | ≥ 15 | |||||||||
| IS | 2010 – 2014 | ||||||||||
| Madsen, 202018 | GCNKSS, United States | ≥ 20 | |||||||||
| IS, ICH, SAH | 1993/1994 | ||||||||||
| 1999 | |||||||||||
| 2005 | |||||||||||
| 2010 | |||||||||||
| 2015 | |||||||||||
| Barra, 201910 | Norway | 15–54 | |||||||||
| IS, ICH, SAH, Undet | 2010–2015 | ||||||||||
| IS | 2010–2015 | ||||||||||
| Ekker, 20195 | Netherlands | 18–50 | Vaartjes,200821 | ||||||||
| IS, ICH, Udet | 1998–2010 | ||||||||||
| IS | |||||||||||
| Aked, 20188 | Lund, Sweden | ≥15 | |||||||||
| IS, ICH, SAH, Undet | 2001–2002 | ||||||||||
| 2015–2016 | |||||||||||
| Correia, 201714 | Porto, Portugal | all | |||||||||
| IS, ICH, SAH, Udet | 1998–2000 | n=22 | |||||||||
| Wang, 201724 | China | ≥ 20 | |||||||||
| IS, ICH, SAH, Udet | 2013 | ||||||||||
| Cabral, 201612 | Joinville, Brazil | all | Cabral,200913 | ||||||||
| IS, ICH, SAH | 1995 | n=44 | |||||||||
| 2012–2013 | n=68 | ||||||||||
| Newbury, 201619 | SEARCH, Australia | ||||||||||
| IS, ICH, SAH | 2009–2011 | ||||||||||
| Corso, 201215 | Aosta, Italy | all | |||||||||
| IS | 2008–2011 | ||||||||||
| Diaz-Guzman, 201216 | IBERICTUS, Spain | > 17 | |||||||||
| IS, ICH, TIA, Udet | 2006 | ||||||||||
| Palm, 201020 | Rhein, Germany | all | |||||||||
| IS, ICH, SAH | 2006–2007 | ||||||||||
| Walker, 201023 | Tanzania | all | |||||||||
| IS, ICH, SAH | 2003–2006 | ||||||||||
Yellow highlight-non-statistically significant sex differences, red highlight-higher incidence in women, blue highlight-higher incidence in men. IS-Ischemic Strokes; ICH-Intracranial Hemorrhage; SAH-Subarachnoid Hemorrhage; TIA-Transient Ischemic Attack, Udet-undetermined cause; IRR-Incidence Rate Ratio; GCKNSS-Greater Cincinnati Northern Kentucky Stroke Study; SEARCH-Stroke Epidemiology in an Australian Rural Cohort; IBERICTUS-Stroke Project of the Spanish Cerebrovascular Disease Study.
Data from Giroud et al17
Three studies found a sex difference with higher incidence amongst men, which was present only in the older age groups. One study from Norway found 41% more men with ischemic strokes in adults age 35 to 44 years (IRR [women/men] 0.71 [0.64:0.79]).10 Another study of strokes (including ischemic, ICH, and undetermined) in the United Kingdom, found 18% more men among adults age 30 to 34 years (IRR 0.85[0.76–0.96]), which increased to 45% more men in the 40 to 44 years age group (IRR 0.69[0.64–0.75]).9 Finally, a Spanish study found 64% more men with strokes (including ischemic, ICH, undetermined and transient ischemic attacks [TIA]) among age 35 to 44 years (IRR 0.61[0.39–0.97]).16 The two former studies were retrospective and relied on administrative ascertainment of strokes. The latter study was prospective and performed clinical case ascertainment.
Four studies found a sex difference with higher incidence amongst women, which was most evident among the younger age groups. One study in Dijon, France found 89% more women with strokes (including ischemic, ICH, subarachnoid hemorrhages [SAH], and undetermined) among adults age 18 to 35 years (IRR 1.89[1.27–2.8]).17 Another study in Ontario, Canada found 26% more women with strokes (including ischemic, ICH, SAH, and TIA) in adults younger than age 30 years (IRR 1.26[1.1–1.45]).22 This difference increased to 33% more women when only ischemic strokes were considered in those younger than age 30 years (IRR 1.47[1.19–1.83]). Finally, two studies in the Netherlands and United States both found more women than men with ischemic stroke in adults younger than age 45 years.5, 6 This difference was largest among the younger age groups. In the Dutch cohort, twice as many women developed ischemic strokes among adults younger than age 30 years (IRR 2.19[1.72–2.81]), which attenuated to 20% more women among adults age 40 to 44 years (1.20[1.11–1.31]).5 Among Americans, 42% more women had ischemic strokes in adults age 24 to 34 years (IRR 1.42[1.16–1.75]) compared to 14% more women in adults age 35 to 44 years (IRR 1.14[1.02–1.28]).6 The French study was prospective and relied on clinical stroke ascertainment. Meanwhile, the Canadian, Dutch, and American studies were all retrospective and relied on administrative data.
Among the studies which reported on ischemic strokes, there was an overall effect of higher incidence in women younger than age 35 years (IRR 1.44[1.18–1.76], I2=82%, p<0.01, Figure 1), no statistical sex difference in among adults age 35 to 45 years (IRR 1.10[0.91–1.33], I2=95%, p=0.53, Figure 2) and an overall effect of more women younger than age 45 years (IRR 1.24[1.08–1.43], I2=91%, p<0.01, Figure 3). This effect became attenuated when all stroke types were considered with higher incidence in women younger than age 35 years (IRR 1.26[1.07–1.48], I2=86%, p<0.01, Supplemental Figure F2), no statistical difference among adults age 35 to 45 years (IRR 0.89[0.75–1.05], I2=94%, p=0.16, Supplemental Figure F3) or younger than age 45 years (IRR 1.07[0.96–1.18], I2=90%, p=0.21, Supplemental Figure F4). After the inclusion of data prior to 2008 from Appelros et al., there was no change in the direction or significance of our findings (≤35 IRR 1.23[1.05–1.44] I2=86%, 35–45 IRR 0.86[0.73–1.02] I2=93%, ≤ 45 IRR 1.07[0.94–1.15] I2=90%).
Figure 1.
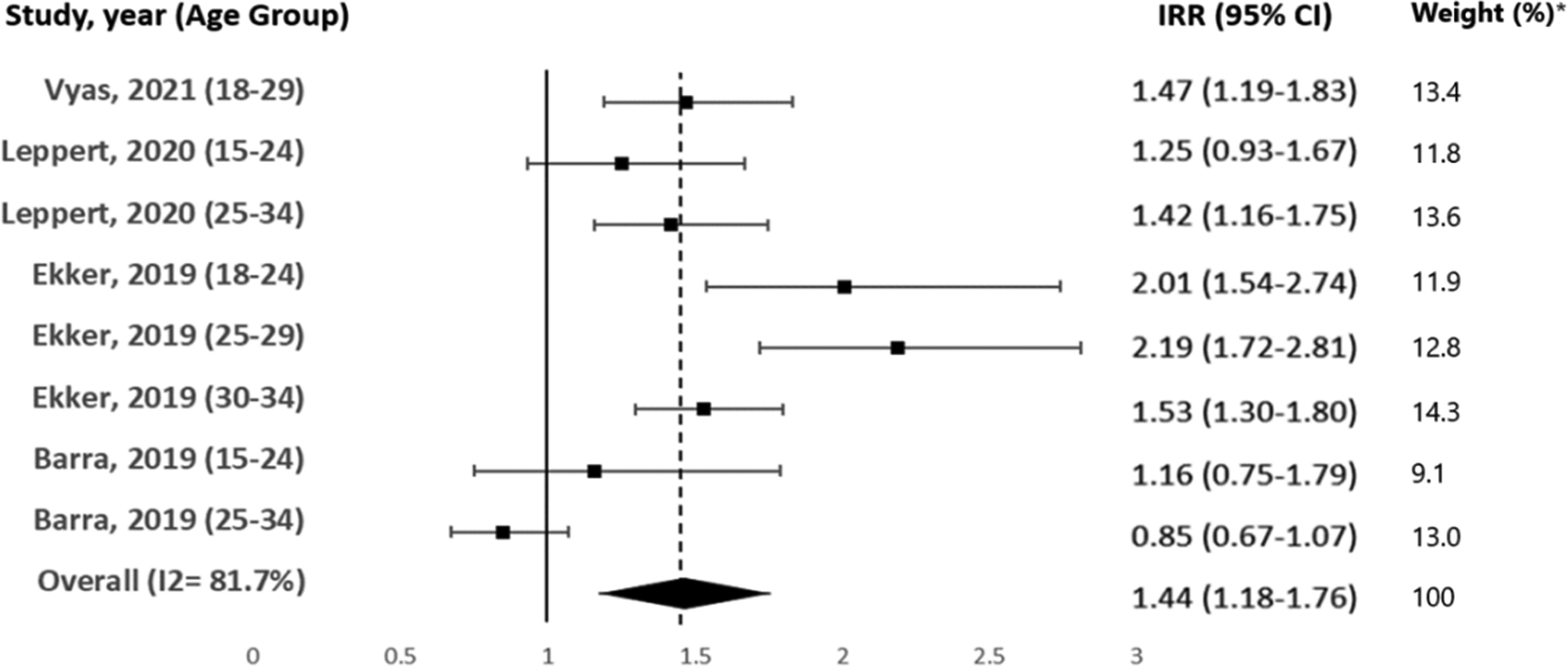
Age <35 years, Incidence rate ratio(women/men) of ischemic strokes with 95%CI for different populations and overall effect. Analyses of overall effect were performed with random effects models on the log of the ratio estimates. The combined ratios were estimated, along with 95%CI testing the null hypothesis of the IRR equaling one. *Study weights were calculated based on variability of the IRR.
Figure 2.

Age 35–45 years, Incidence rate ratios(women/men) of ischemic strokes with 95%CI for different populations and overall effect.
Figure 3.

Age ≤ 45 years. Incidence rate ratio(women/men) of ischemic strokes with 95%CI for different populations and overall effect.
Discussion:
In this review, studies that identified a sex difference generally found that there were higher incidences of women with ischemic strokes in the younger age groups (<30–35 years). This finding was demonstrated in multiple developed countries, including France, Canada, the Netherlands, and the United States.5, 6, 17, 22 The sex difference in the incidence of ischemic strokes was the greatest and most evident among adults younger than age 35 years, with an estimated 44% more women, though there is notable heterogeneity in this effect. This sex difference narrows among adults ages 35 to 45 years, and there is contradictory evidence whether young men may be more at risk of ischemic strokes in this age group.
Some variability exists in sex differences across studies among the older age group (>30–35 years). Two retrospective administrative studies from the Netherlands and United States found more women between age 35 to 45 years with ischemic strokes while three studies (two retrospective administrative from Norway and the United Kingdom and one prospective from Spain) found more men between age 30 to 45 years with strokes. Methods of stroke ascertainment may play a role in this difference. Both the Dutch and American studies favoring more women used previously validated administrative algorithms of identifying ischemic strokes25, which was then validated in young adults during the Dutch study.5 The two administrative studies favoring men did not. The Norwegian study reported on the unique individuals who suffered ischemic strokes with episodes each year, which did not guarantee that it was their first-ever ischemic stroke.10, 26 Meanwhile, the British study adapted the code list from CALIBER, an open access phenotyping algorithm for United Kingdom’s electronic health record’s data (www.caliberresearch.org), to define strokes that included administrative codes for sequelae of cerebral infarction.27 Hence, both studies favoring men likely included recurrent strokes and non-acute strokes. Sex differences among stroke recurrence and non-acute strokes are unknown and could favor men.
The uncertainty surrounding sex differences in the incidence of ischemic strokes among young adults is likely related to three main factors (1) small sample sizes, (2) failure to differentiate ischemic strokes from other stroke types, and (3) broad age group categorizations. The incidence of ischemic stroke increases exponentially with age and only 15% of all ischemic strokes occur in adults younger than age 50 years.28 While it is relatively easy to study epidemiological sex differences among older adults, the same studies in younger adults necessitate a much larger population. Prospective studies in this review had smaller stroke case numbers and more difficulty detecting a statistically significant sex difference. Six of the nine studies that did not find a sex difference identified fewer than 100 cases of stroke (4 identified less than 50 cases) among adults younger than age 45 years. The three studies that identified more than 100 cases were the Greater Cincinnati Northern Kentucky Stroke Study with 587 strokes (age <45),18 a Chinese cross-sectional survey with 152 strokes (age <50),24 and a community-based cohort in Joinville, Brazil with 200 strokes (age 20–49).29 With the exception of the Chinese study, all were prospective and relied on stroke ascertainment based on clinical data. In contrast, retrospective studies were more successful at capturing a large number of cases (all identified more than 1000 strokes in age ≤ 45) but relied on administrative case ascertainment.
To compensate for small sample sizes, there has been a tendency to group stroke types together (i.e., ischemic, ICH, SAH, undetermined, and occasionally TIA). Unfortunately, this can make results harder to interpret, as different prevalent etiologies inform specific stroke types, i.e., ruptured aneurysms in SAH or uncontrolled hypertension in ICH.30 In addition, epidemiological differences between these subtypes are already known to exist. For example, while the incidence of ischemic stroke increased over time among young adults, this trend has not been observed in hemorrhagic strokes.31 Notably, the sex differences observed in ischemic stroke among young adults was not seen in ICH.5 Underscoring this point is that sex differences observed by both the Canadian and Dutch cohorts in this review were larger when only ischemic strokes were considered compared to the addition of other stroke types.5, 22 Attenuation of sex differences by including other stroke types may also explain why nine studies failed to discern a sex difference. Not only did they start with smaller sample sizes, but all except one included other stroke types (9 included ICH, 8 included SAH, 2 included undetermined, 1 included TIA). Chief among them is ICH, which has the second highest incidence behind ischemic stroke and has not shown sex differences among young adults.1 The IBERICTUS study from Spain was the only prospective study to find more men (age 35–44) with strokes (including ischemic, ICH, SAH, TIA, undetermined) and only one of two studies to include TIA.16 The inclusion of so many different stroke types could significantly distort the sex difference in ischemic stroke incidence, especially when only a 79 cases were captured in this age group.
Since ischemic stroke incidence increases exponentially with age, age-group breakdowns become especially important. For example, among the 8,444 ischemic strokes in adults age 18 to 49 years in the Dutch cohort, the mean age was 46 years, with almost half of all cases (n= 3,997) occurring in the oldest age group (age 45–49).5 Hence, larger age groups among ischemic strokes are most representative of the oldest rather than the average of the age group. In one analysis of the Dijon Stroke Registry, Bejot et al. noted a higher incidence of strokes among men in adults younger than age 55 years.32 However, these results were most representative of the cohort closest to 55 years old, as a subsequent analysis of the same data by Giroud et al. found more strokes in women younger than age 35 years, and concurrently more strokes in men older than age 45 years.17 Therefore, consistency of age break downs and avoiding wide age ranges are vitally important to ensuring comparability between studies and teasing out sex differences by age.
An assertion that young women may be disproportionately at risk of ischemic stroke represents a significant departure from our current scientific understanding and may have important implications about the etiology of ischemic strokes in young adults. Endogenous estrogen is known to increase vasodilation and inhibit the response of blood vessels to the development of atherosclerosis.33 Hence, the prevalence of atherosclerotic disease is lower in premenopausal women and rises in postmenopausal women.34 This phenomenon is thought to result in larger sex differences in myocardial infarction incidence in adults younger than age 45 years, with more than double the incidence in young men compared to young women.35 We previously found that the rate of index acute myocardial infarction was 1.6 times higher among men age 25 to 34 years (Figure 4) whereas the rate of index ischemic stroke was 1.4 times higher among women in this age group.6 Ischemic strokes are often a sequela of atherosclerotic disease and are subject to the same cardiovascular risk factors as ischemic heart disease. The observation of a higher incidence of ischemic stroke in premenopausal women compared with men of the same age is therefore a departure from our previous understanding. This finding suggests that the role of non-atherosclerotic risk factors (ie. maternal strokes, hormonal contraceptives,36 migraine headaches37) in young women may be more important, yet poorly understood risk factors for ischemic stroke.
Figure 4.

Forest plot of incidence rate ratio (IRR-men/women) by age group using a national US claims sample. IRR for Myocardial Infarctions(MI) in 15–24 is 3.38(2.05–5.57). Index ischemic stroke(IS) or MI is the first admission for IS or MI by ICD-9 code.6
We still do not know how much of this sex difference, especially in younger women, is attributable to pregnancy associated strokes. One study found that maternal strokes accounted for as much as 18% of all strokes in women age 12 to 35 years and 1.4% of strokes in women 35 to 55 years. However, in addition to ischemic strokes, this study included TIA, ICH, SAH, and cerebral venous thrombosis.38 Overall, ischemic stroke and TIA accounted for less than a quarter of these combined stroke events during pregnancy. In the Helsinki Young Stroke Registry, which found proportionally more ischemic strokes in women than men age 15 to 35 years, only 2.6% of women with ischemic strokes were pregnant or postpartum.39 More work is required to better understand how pregnancy and sex differences among other thromboembolic conditions (e.g., cryptogenic strokes with concurrent patent foramen ovale) contribute to the development of different stroke types in young adults.
This study has several limitations. There is significant heterogeneity between studies, as evidenced by the high I2 values in our analyses. Some of this heterogeneity can be attributed to study populations spanning different continents, including 15 different countries with varying levels of development, race make up, ethnicities and cultures. Notably, the incidence of ischemic strokes in young adults is higher among Black and Hispanic individuals.40 However, most studies do not report the incidence of strokes by race and ethnicity, so we were unable to include this breakdown in our analysis. Heterogeneity may also be due to methodologically differing study designs, with the two main groups being prospective with clinical stroke ascertainment or retrospective with stroke ascertainment based on administrative algorithms. Even among these two subgroups, however, methods of clinical and administrative case ascertainment varied. There were many more prospective than retrospective studies, but retrospective studies collected more stroke cases overall. The combined effect would favor the results from these larger studies, even though retrospective studies did not always agree on the direction of the effect. Publication bias, a failure to publish smaller studies, may affect our results. We did not exclude studies based on sample size. However, larger epidemiological studies may not have published results in the younger age groups due to the relatively small number of cases captured. This publication bias may further reduce the sample size of prospective studies and skew the results towards retrospective studies. Finally, heterogeneity in studies of young adult strokes may be related to the heterogeneity of the disease itself, which may have more widely distributed etiologies in the young adult population compared with stroke in older adults. Despite the limitations of this data, this analysis included nearly 70,000 young adult stroke cases, many fold more than previously evaluated in this age group. Caution is needed in interpreting our results given the heterogeneous studies, but they do suggest the possibility of a different distribution of stroke across sex in young adults.
Experts in young adult stroke have highlighted the increasing contribution of traditional vascular risk factors and the diminishing role of ‘rare’ risk factors including migraines, oral contraceptives and pregnancy or postpartum.41 There is no doubt that traditional atherosclerotic risk factors are a major contributor to ischemic strokes in both young men and women, and become increasingly important with age. However, these risk factors are less prevalent in younger women1, 34 and may not account for the observed higher incidence of ischemic strokes in women younger than age 35. Young women who are survivors of ischemic stroke also have worse outcomes with 2 to 3 times higher risk of poorer functional outcomes compared to their male counterparts.42 Sex differences among young adults with ischemic stroke is a problem that demands attention. More research is needed to better define the etiological sex differences of ischemic stroke in young adults and the contributions that non-traditional risk factors such as pregnancy, postpartum, and hormonal contraceptives play in the overall burden of ischemic strokes in young women.
Supplementary Material
Sources of Funding:
Dr. Daugherty is funded by NIH (R01HL133343).
Disclosures:
Dr. Leppert is supported by NIH/NCATS Colorado CTSA KL2 (TR002534). Dr. Ho is supported by NHLBI, VA HSR&D, and University of Colorado School of Medicine. He has a research agreement with Bristol-Myers Squibb through the University of Colorado. He serves as the Deputy Editor for Circulation: Cardiovascular Quality and Outcomes. Dr. Madsen is supported by NHLBI (K23HL140081).
Non-Standard Abbreviations and Acronyms:
- IS
Ischemic Strokes
- ICH
Intracranial Hemorrhage
- SAH
Subarachnoid Hemorrhage
- TIA
Transient Ischemic Attack
- IRR
Incidence Rate Ratio
Footnotes
Supplemental Materials:
PRISMA_2020 Checklist
PRISMA_2020 Abstract Checklist
References:
- 1.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart disease and stroke statistics-2021 update: A report from the american heart association. Circulation. 2021;143:e254–e743 [DOI] [PubMed] [Google Scholar]
- 2.Bushnell CD, Chaturvedi S, Gage KR, Herson PS, Hurn PD, Jimenez MC, Kittner SJ, Madsen TE, McCullough LD, McDermott M, et al. Sex differences in stroke: Challenges and opportunities. J Cereb Blood Flow Metab. 2018;38:2179–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carcel C, Woodward M, Wang X, Bushnell C, Sandset EC. Sex matters in stroke: A review of recent evidence on the differences between women and men. Front Neuroendocrinol. 2020;59:100870. [DOI] [PubMed] [Google Scholar]
- 4.Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology: A systematic review. Stroke. 2009;40:1082–1090 [DOI] [PubMed] [Google Scholar]
- 5.Ekker MS, Verhoeven JI, Vaartjes I, van Nieuwenhuizen KM, Klijn CJM, de Leeuw FE. Stroke incidence in young adults according to age, subtype, sex, and time trends. Neurology. 2019;92:e2444–e2454 [DOI] [PubMed] [Google Scholar]
- 6.Leppert MH, Ho PM, Burke J, Madsen TE, Kleindorfer D, Sillau S, Daugherty S, Bradley CJ, Poisson SN. Young women had more strokes than young men in a large, united states claims sample. Stroke. 2020;51:3352–3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alhazzani AA, Mahfouz AA, Abolyazid AY, Awadalla NJ, Aftab R, Faraheen A, Khalil SN. Study of stroke incidence in the aseer region, southwestern saudi arabia. Int J Environ Res Public Health. 2018;15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aked J, Delavaran H, Norrving B, Lindgren A. Temporal trends of stroke epidemiology in southern sweden: A population-based study on stroke incidence and early case-fatality. Neuroepidemiology. 2018;50:174–182 [DOI] [PubMed] [Google Scholar]
- 9.Akyea RK, Vinogradova Y, Qureshi N, Patel RS, Kontopantelis E, Ntaios G, Asselbergs FW, Kai J, Weng SF. Sex, age, and socioeconomic differences in nonfatal stroke incidence and subsequent major adverse outcomes. Stroke. 2021;52:396–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barra M, Labberton AS, Faiz KW, Lindstrøm JC, Rønning OM, Viana J, Dahl FA, Rand K. Stroke incidence in the young: Evidence from a norwegian register study. Journal of neurology. 2019;266:68–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Béjot Y, Duloquin G, Thomas Q, Mohr S, Garnier L, Graber M, Giroud M. Temporal trends in the incidence of ischemic stroke in young adults: Dijon stroke registry. Neuroepidemiology. 2021;55:239–244 [DOI] [PubMed] [Google Scholar]
- 12.Cabral NL, Cougo-Pinto PT, Magalhaes PS, Longo AL, Moro CH, Amaral CH, Costa G, Reis FI, Gonçalves AR, Nagel V, et al. Trends of stroke incidence from 1995 to 2013 in joinville, brazil. Neuroepidemiology. 2016;46:273–281 [DOI] [PubMed] [Google Scholar]
- 13.Cabral NL, Goncalves AR, Longo AL, Moro CH, Costa G, Amaral CH, Souza MV, Eluf-Neto J, Fonseca LA. Trends in stroke incidence, mortality and case fatality rates in joinville, brazil: 1995–2006. J Neurol Neurosurg Psychiatry. 2009;80:749–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Correia M, Magalhães R, Felgueiras R, Quintas C, Guimarães L, Silva MC. Changes in stroke incidence, outcome, and associated factors in porto between 1998 and 2011. International journal of stroke : official journal of the International Stroke Society. 2017;12:169–179 [DOI] [PubMed] [Google Scholar]
- 15.Corso G, Bottacchi E, Giardini G, Di Giovanni M, Meloni T, Pesenti Campagnoni M, Veronese Morosini M. Epidemiology of stroke in northern italy: The cerebrovascular aosta registry, 2004–2008. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2013;34:1071–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Díaz-Guzmán J, Egido JA, Gabriel-Sánchez R, Barberá-Comes G, Fuentes-Gimeno B, Fernández-Pérez C. Stroke and transient ischemic attack incidence rate in spain: The iberictus study. Cerebrovascular diseases (Basel, Switzerland). 2012;34:272–281 [DOI] [PubMed] [Google Scholar]
- 17.Giroud M, Delpont B, Daubail B, Blanc C, Durier J, Giroud M, Bejot Y. Temporal trends in sex differences with regard to stroke incidence: The dijon stroke registry (1987–2012). Stroke. 2017;48:846–849 [DOI] [PubMed] [Google Scholar]
- 18.Madsen TE, Khoury JC, Leppert M, Alwell K, Moomaw CJ, Sucharew H, Woo D, Ferioli S, Martini S, Adeoye O, et al. Temporal trends in stroke incidence over time by sex and age in the gcnkss. Stroke. 2020:STROKEAHA120028910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newbury J, Kleinig T, Leyden J, Arima H, Castle S, Cranefield J, Paterson T, Jannes J, Crotty M, Anderson CS. Stroke epidemiology in an australian rural cohort (search). International journal of stroke : official journal of the International Stroke Society. 2017;12:161–168 [DOI] [PubMed] [Google Scholar]
- 20.Palm F, Urbanek C, Rose S, Buggle F, Bode B, Hennerici MG, Schmieder K, Inselmann G, Reiter R, Fleischer R, et al. Stroke incidence and survival in ludwigshafen am rhein, germany: The ludwigshafen stroke study (lusst). Stroke. 2010;41:1865–1870 [DOI] [PubMed] [Google Scholar]
- 21.Vaartjes I, Reitsma JB, de Bruin A, Berger-van Sijl M, Bos MJ, Breteler MM, Grobbee DE, Bots ML. Nationwide incidence of first stroke and tia in the netherlands. European journal of neurology. 2008;15:1315–1323 [DOI] [PubMed] [Google Scholar]
- 22.Vyas MV, Silver FL, Austin PC, Yu AYX, Pequeno P, Fang J, Laupacis A, Kapral MK. Stroke incidence by sex across the lifespan. Stroke. 2021;52:447–451 [DOI] [PubMed] [Google Scholar]
- 23.Walker R, Whiting D, Unwin N, Mugusi F, Swai M, Aris E, Jusabani A, Kabadi G, Gray WK, Lewanga M, et al. Stroke incidence in rural and urban tanzania: A prospective, community-based study. Lancet Neurol. 2010;9:786–792 [DOI] [PubMed] [Google Scholar]
- 24.Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, Wang L, Jiang Y, Li Y, Wang Y, et al. Prevalence, incidence, and mortality of stroke in china: Results from a nationwide population-based survey of 480 687 adults. Circulation. 2017;135:759–771 [DOI] [PubMed] [Google Scholar]
- 25.Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke. 2005;36:1776–1781 [DOI] [PubMed] [Google Scholar]
- 26.Rand K, Dahl FA, Viana J, Rønning OM, Faiz KW, Barra M. Fewer ischemic strokes, despite an ageing population: Stroke models from observed incidence in norway 2010–2015. BMC Health Serv Res. 2019;19:705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuan V, Denaxas S, Gonzalez-Izquierdo A, Direk K, Bhatti O, Husain S, Sutaria S, Hingorani M, Nitsch D, Parisinos CA, et al. A chronological map of 308 physical and mental health conditions from 4 million individuals in the english national health service. The Lancet. Digital health 2019;1:e63–e77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boot E, Ekker MS, Putaala J, Kittner S, De Leeuw FE, Tuladhar AM. Ischaemic stroke in young adults: A global perspective. J Neurol Neurosurg Psychiatry. 2020;91:411–417 [DOI] [PubMed] [Google Scholar]
- 29.Cabral NL, Freire AT, Conforto AB, Dos Santos N, Reis FI, Nagel V, Guesser VV, Safanelli J, Longo AL. Increase of stroke incidence in young adults in a middle-income country: A 10-year population-based study. Stroke. 2017;48:2925–2930 [DOI] [PubMed] [Google Scholar]
- 30.Hathidara MY, Saini V, Malik AM. Stroke in the young: A global update. Current neurology and neuroscience reports. 2019;19:91. [DOI] [PubMed] [Google Scholar]
- 31.George MG, Tong X, Kuklina EV, Labarthe DR. Trends in stroke hospitalizations and associated risk factors among children and young adults, 1995–2008. Ann Neurol. 2011;70:713–721 [DOI] [PubMed] [Google Scholar]
- 32.Bejot Y, Daubail B, Jacquin A, Durier J, Osseby GV, Rouaud O, Giroud M. Trends in the incidence of ischaemic stroke in young adults between 1985 and 2011: The dijon stroke registry. J Neurol Neurosurg Psychiatry. 2014;85:509–513 [DOI] [PubMed] [Google Scholar]
- 33.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. New England Journal of Medicine. 1999;340:1801–1811 [DOI] [PubMed] [Google Scholar]
- 34.Man JJ, Beckman JA, Jaffe IZ. Sex as a biological variable in atherosclerosis. Circ Res. 2020;126:1297–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alkhouli M, Alqahtani F, Jneid H, Al Hajji M, Boubas W, Lerman A. Age-stratified sex-related differences in the incidence, management, and outcomes of acute myocardial infarction. Mayo Clin Proc. 2021;96:332–341 [DOI] [PubMed] [Google Scholar]
- 36.Gillum LA, Mamidipudi SK, Johnston SC. Ischemic stroke risk with oral contraceptives: A meta-analysis. JAMA. 2000;284:72–78 [DOI] [PubMed] [Google Scholar]
- 37.Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: Case-control study. The world health organisation collaborative study of cardiovascular disease and steroid hormone contraception. BMJ. 1999;318:13–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller EC, Gatollari HJ, Too G, Boehme AK, Leffert L, Elkind MS, Willey JZ. Risk of pregnancy-associated stroke across age groups in new york state. JAMA Neurol. 2016;73:1461–1467 [DOI] [PubMed] [Google Scholar]
- 39.Putaala J, Metso AJ, Metso TM, Konkola N, Kraemer Y, Haapaniemi E, Kaste M, Tatlisumak T. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: The helsinki young stroke registry. Stroke. 2009;40:1195–1203 [DOI] [PubMed] [Google Scholar]
- 40.Jacobs BS, Boden-Albala B, Lin IF, Sacco RL. Stroke in the young in the northern manhattan stroke study. Stroke. 2002;33:2789–2793 [DOI] [PubMed] [Google Scholar]
- 41.Maaijwee NA, Rutten-Jacobs LC, Schaapsmeerders P, van Dijk EJ, de Leeuw FE. Ischaemic stroke in young adults: Risk factors and long-term consequences. Nat Rev Neurol. 2014;10:315–325 [DOI] [PubMed] [Google Scholar]
- 42.Synhaeve NE, Arntz RM, van Alebeek ME, van Pamelen J, Maaijwee NA, Rutten-Jacobs LC, Schoonderwaldt HC, de Kort PL, van Dijk EJ, de Leeuw FE. Women have a poorer very long-term functional outcome after stroke among adults aged 18–50 years: The future study. Journal of neurology. 2016;263:1099–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


