Abstract
GABA, glutamate, and glycine release in the locus coeruleus were measured as a function of sleep/wake state in the freely-behaving cat using the microdialysis technique. GABA release was found to increase during rapid-eye-movement sleep as compared to waking values. GABA release during slow-wave sleep was intermediate between that of waking states and rapid-eye-movement sleep. The concentration of glutamate and glycine in microdialysis samples was unchanged across sleep and wake states. Our findings are consistent with the hypothesis that GABAergic inhibition is responsible for the cessation of discharge in locus coeruleus neurons during REM sleep. The data suggest that a population of GABAergic neurons innervating the locus coeruleus are selectively active during rapid-eye-movement sleep.
Keywords: glutamate, glycine, microdialysis, noradrenaline, rapid-eye-movement sleep, slow-wave sleep
The locus coeruleus (LC) and peri-locus coeruleus (p-LC) nuclei in the cat contain noradrenergic neurons that project to widespread areas of the forebrain, brainstem and spinal cord. The activity of these neurons is thought to play an important role in cortical desynchronization, vigilance, response to stressors, regulation of hormone release, and in orienting and alerting behaviours.5,10,18,22
Noradrenergic neurons of the cat LC and p-LC exhibit their highest rates of firing during active waking (1–2 Hz), fire tonically during quiet wake (0.5–1.5 Hz), slow considerably during slow-wave sleep (SWS) (0.3–0.7 Hz), and cease firing during rapid-eye-movement (REM) sleep.35,36,38 This REM-off pattern of activity is found in noradrenergic LC neurons, serotonergic raphe neurons,23 and histaminergic neurons of the tuberomammillary nucleus.47
Several lines of evidence suggest that this pattern of activity is permissive to the induction of the REM sleep state in the cat. Stimulation of the LC promotes wakefulness.8,12,16 Localized cooling of the LC promotes REM sleep.8 Microinjection of beta-adrenoceptor and alpha-2 antagonists into the pontine reticular formation (PRF) increases REM sleep time.7,43 In addition, LC stimulation inhibits the activity of sleep-active neurons and increases the activity of waking-active neurons in the rat preoptic area (POA).31 Microinjection of the beta-adrenoceptor agonist isoproterenol into the POA induces wakefulness.20 Thus, the noradrenergic nuclei of the LC appear to inhibit REM sleep production and promote wakefulness at many levels of the brain.
The REM-off pattern of activity may also be an important function of REM sleep. Data suggest that the sensitivity44 and density25,26 of cortical beta-adrenergic receptors is decreased by REM sleep deprivation lasting 72 or 96 hours. This effect is not seen when sleep deprivation lasts longer than seven days.34,45 According to one hypothesis,42 the constant release of norepinephrine during wake and SWS produces a desensitization of post-synaptic adrenoceptor mechanisms. REM sleep is thought to offer the only period for up-regulation/re-sensitization of adrenoceptors.
The importance of the near cessation of activity of LC noradrenergic neurons for REM sleep initiation is clear; however, the mechanism producing this pattern of activity is unknown. The near cessation of activity could occur through disfacilitation or through inhibition. However, LC neurons exhibit spontaneous activity in the slice preparation.1,50 In addition, the reduced activity of LC neurons during REM sleep is maintained in the presence of local field potentials associated with increased LC unit discharge during waking.3 Thus, it is likely that active inhibition of LC neurons is, at least in part, responsible for their reduced firing in REM sleep.3
Among the compounds that can inhibit LC unit activity are the inhibitory amino acids GABA and glycine.2,4,19,32,35 The GABAergic input from the praepositus hypoglossal (PrH) nucleus is one of two primary inputs to the area of the LC containing noradrenergic cell bodies in the rat.4 Stimulation of this pathway potently inhibits noradrenergic cell firing in vivo.9 In addition, a population of GABAergic neurons has been identified in the dorsal pontine tegmentum, including the LC nucleus.15 Glycine-immunoreactive boutons have been identified within the LC nucleus.11
In this study, we tested the hypothesis that GABA and/or glycine release in the LC is responsible for the cessation of discharge in LC neurons during REM sleep. To this end, we employed the microdialysis technique in freely-behaving cats to measure the release of GABA and glycine across the sleep/wake cycle. This technique has been used successfully to detect changes in neurotransmitter release in several areas of the cat brain as a function of sleep/wake state.17,33,48,49 Glutamate was also measured to assess its possible role in disfacilitation of LC and p-LC noradrenergic neurons during REM sleep.
EXPERIMENTAL PROCEDURES
Experimental procedures were conducted in accordance with NIH guidelines on the use and care of animals.
Surgery was performed on four mongrel cats using sodium pentobarbital anaesthesia (35 mg/kg i.p.) and sterile conditions. Screw electrodes were placed in the posterior orbit and sensorimotor cortex for the recording of eye movements and electroencephalographic (EEG) activity. Tripolar electrodes were implanted into the lateral geniculate nucleus for the recording of ponto-geniculo-occipital spikes. Flexible stainless steel wires were inserted into the neck musculature for the recording of electromyographic (EMG) activity. Stainless steel guide cannulae (21-gauge thin wall) were stereotaxicallv implanted at a 36 degree angle to vertical at sites 1 mm above the LC and p-LC at two brainstem levels known to contain noradrenergic neurons in the cat.15,40 Coordinates for the two sites were6: P 2.1, L 2.2, V 0.5 and P 3.1, L 2.5, V 1.6. All cannulae and electrodes were secured to the skull with dental cement.
At least two weeks following surgery, animals were connected to a recording cable for polygraphic recording and a 50 mm concentric microdialysis probe with a 2 mm semi-permeable membrane 0.27 mm in diameter was inserted to a position 3 mm beyond the tip of the guide. The probe was perfused at a rate of 2 μl/min with artificial cerebrospinal fluid of the following composition: 145 mM Na+, 2.7 mM K+, 1.0 mM Mg2+, 1.2 mM Ca2+, 152.1 mM Cl−, 2.0 mM Na2HPO4, pH 7.4. The interval between probe insertion and sample collection was 17 h. Microdialysis samples were then collected from immediately adjacent 4–10 min periods of SWS, REM sleep, quiet wake and active wake.
SWS and REM sleep were identified by standard criteria.46 During quiet wake, cats were lying down with raised head or in the sphinx posture. Active wake was elicited by engaging cats in play behaviour using a string and consisted of continuous motor activity.
Sample collection was timed precisely by the use of an Eicom fraction collector incorporating a 10 minute delay to allow for the time for perfusate to travel from the tip of the probe to the outlet of the tubing. Samples were immediately frozen on dry ice and stored for subsequent analysis.
Content of the amino acids glutamate, glycine, and GABA in microdialysis samples was analysed by high-performance liquid chromatography with electrochemical detection using a system manufactured by Eicom. The system employed a 4.6 × 150 mm C18 separation column (Rainin) and a glassy-carbon electrode held at +700 mV. Portions of the collected samples were reacted with 5 μl 4 mM o-pthaldialdehyde and 2-mercaptoethanol for 2 min before injection onto the separation column. The volume of samples injected onto the column in this study varied between 8 and 20 μl. The mobile phase flow rate was set at either 1.0 or 1.2ml/min. The mobile phase consisted of 0.1 M sodium phosphate buffer with 35% methanol at a pH between 6.06 and 6.14. The pH of the mobile phase was adjusted to obtain the best separation of the compounds of interest. Separation of the GABA peak was especially dependent on precise maintenance of the pH. The assay was able to detect 10 fmoles of the amino acids glutamate, glycine and GABA with a signal/noise ratio of 3:1. Neurotransmitter concentrations were calculated by comparing peak heights of glutamate, glycine, and GABA in microdialysis samples with peak heights of known concentrations of the same compounds analysed on the same day.
Microdialysis samples were collected during 17 separate sleep/wake cycles. Within each of these cycles, individual samples were collected from periods of SWS, REM sleep, and wake that were immediately adjacent in time. Thus, statistical comparisons were always made between samples collected using the same microdialysis probe over a period of less than or equal to 30 min. This design overcomes potential confounds caused by comparing samples collected at different intervals following implantation of the microdialysis probe, since the recovery rate of microdialysis probes decreases over the course of experiments. In addition, the potentially confounding effects of circadian temperature or hormone-release rhythms on neurotransmitter release is minimized by this design.
Animals were killed using deep sodium pentobarbital anaesthesia (50 mg/kg i.p.) and the brains removed for histological verification of microdialysis collection sites. 50 (μm brainstem slices from the pons were stained with Neutral Red.
In one case, slices were immunohistochemically stained for tyrosine hydroxylase using the following technique: 1) 48 h incubation in 0.1 M Tris–saline with antiserum to tyrosine hydroxylase (1:5000) (Eugene Tech) in 1% normal horse serum and 0.25% Triton X-100; 2) 2.5 h in 1:200 goat anti-rabbit IgG: 3) 2h in 1:100 avidin–biotin complex; 4) visualization by Vector diaminobenzidine kit.
RESULTS
Histological identification
Samples were collected from six microdialysis sites in four cats. Histological analysis indicated that the membranes of all six microdialysis probes were positioned on the borderline of the LC nucleus or within the p-LC. Each of these areas contains concentrations of noradrenergic neurons in the cat15,40 (see Fig. 1A). Figure 1B and C depicts the placement of a microdialysis probe within a population of neurons on the border of the LC staining positively for tyrosine hydroxylase. Except for the area of tissue displacement directly surrounding the probe point, the integrity of the noradrenergic LC neurons was maintained. Thus, the borderline position of this probe permitted collection of extracellular constituents from noradrenergic neurons while minimizing damage to the nucleus.
Fig. 1.
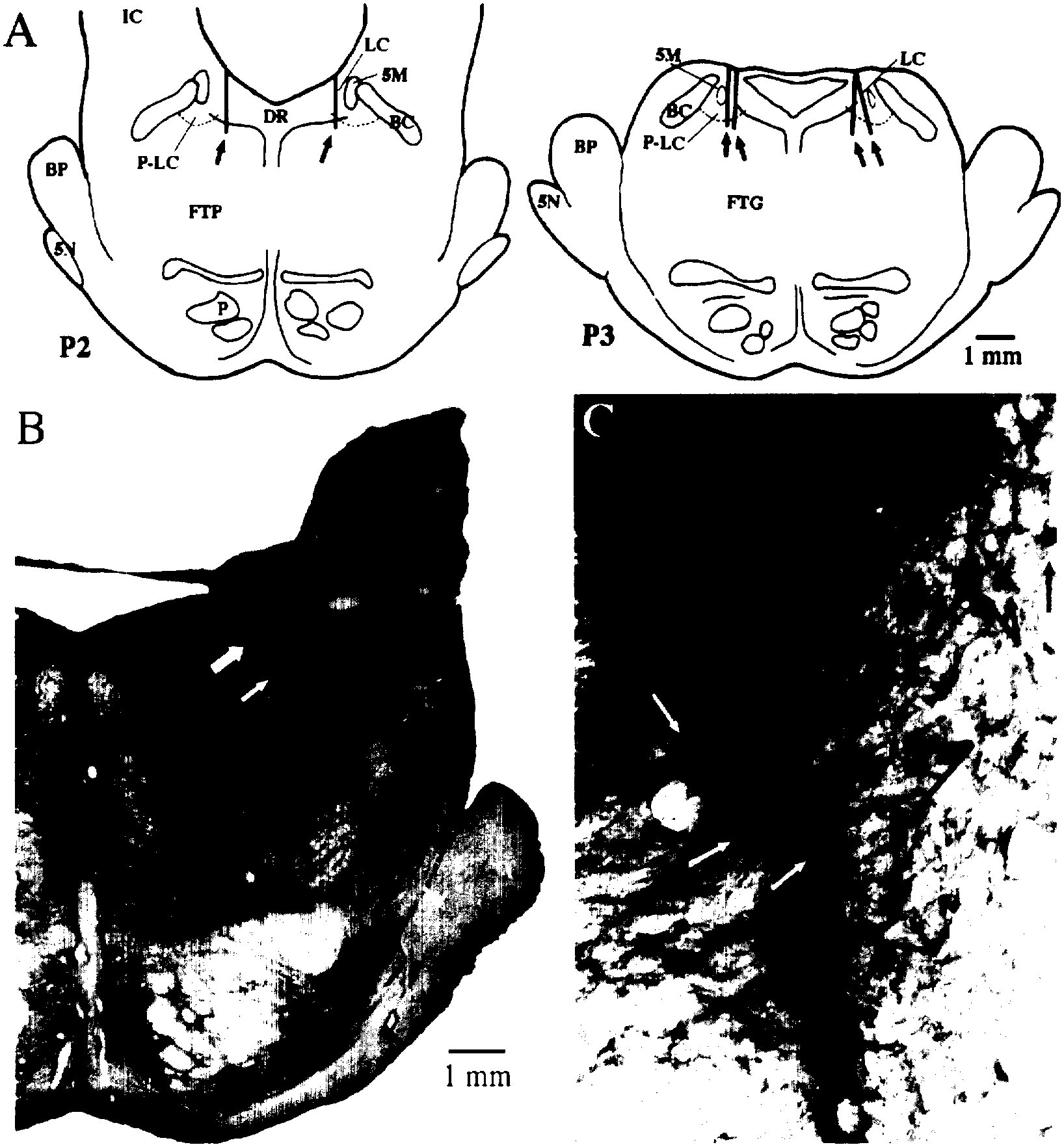
A) Summary of histologically identified placements of microdialysis probes. Black rectangles (indicated by arrows) indicate the placement of microdialysis probe membranes as determined by histology. IC, inferior colliculus; BC, brachium conjunctivum; P, pyramidal tract; DR, dorsal raphe; LC, locus coeruleus; P-LC, peri-locus coeruleus; FTP, paralemniscal tegmental field; FTG, gigantocellular tegmental field; BP, brachium pontis; 5M, tract of the mesencephalic trigeminal nucleus; 5N, 5th cranial nerve. B and C) Identification of tyrosine hydroxylase staining in a microdialysis collection area at the border of the LC and P-LC nuclei. B) Photomicrograph (1 ×) of an individual microdialysis site. Large and small white arrows indicate, respectively, placement of the base and tip of the microdialysis probe membrane. C) Photomicrograph (40 ×) of the area between the white arrows in B. Black and white arrows identify some of the tyrosine hydroxylase immunopositive cells surrounding the microdialysis site.
Dialysate amino acid concentrations
The concentration of GABA across all samples (n=51) was 1.6 ± 0.3 fmoles/μl. Glutamate and glycine concentrations were 0.901 ± 0.20 (n=48) and 0.489 ± 0.14 (n=42) pmoles/μl, respectively. It was possible to compare the concentration of GABA, glutamate (six sample pairs each), and glycine (four sample pairs) in samples collected from adjacent periods of quiet wake and active wake. GABA concentration (in fmoles/μl ± S.E.M.) in these quiet wake and active wake sample pairs was, respectively, 1.26 ± 0.31 and 1.40 ± 0.31. Glutamate concentration (in pmoles/μl ± S.E.M.) in quiet wake versus active wake samples was 0.71 ± 0.29 and 0.73 ± 0.29. Glycine concentration (also in pmoles/μ1 ± S.E.M.) was 0.92 ± 0.43 and 0.85 ± 0.44 in quiet versus active wake. No consistent differences in the release of GABA, glutamate, or glycine were seen and paired t-tests indicated no significant changes in the release of any of the three neurotransmitters as a function of activity level during waking. Therefore, active and quiet waking values were combined for comparison with SWS and REM sleep values.
Release of GABA was analysed across 17 sleep/wake cycles collected from six microdialysis sites. We found a significant increase in GABA release during REM sleep as compared to SWS and waking values (REM vs SWS, P<0.05; REM vs wake, P<0.005. ANOVA with Newman–Keuls post hoc t-tests) (Table 1). GABA levels were, on average, higher during SWS than in wake samples. This difference approached statistical significance (P=0.06). Figure 2 depicts GABA peaks in microdialysis samples collected from SWS, REM sleep, and wake. Table 2 lists GABA content in microdialysis samples collected over the course of two consecutive sleep/wake cycles at a single site.
Table 1.
Extracellular GABA concentration across behavioural state for each microdialysis site
| Site | SWS | REM | WAKE |
|---|---|---|---|
| 1 | 3.03 (1.3) | 3.64 (0.7) | 1.47 (0.2) |
| 2 | 1.55 (0.2) | 2.30 (0.4) | 1.81 (0.6) |
| 3 | 1.28 (0.3) | 1.22 (0.2) | 1.11 (0.2) |
| 4 | 0.46 (0.1) | 0.72 (0.1) | 0.53 (0.1) |
| 5 | 1.85 (0.4) | 1.95 (0.2) | 1.65 (0.2) |
| 6 | 1.30 (0.1) | 1.60 (0.4) | 0.60 (0.3) |
| Mean | 1.58 (0.2) | 1.91 (0.2)a,b | 1.2 (0.3) |
GABA concentration (± S.D.) in fmoles/μl.
REM vs SWS, P<0.05.
REM vs WAKE, P<0.005.
Fig. 2.
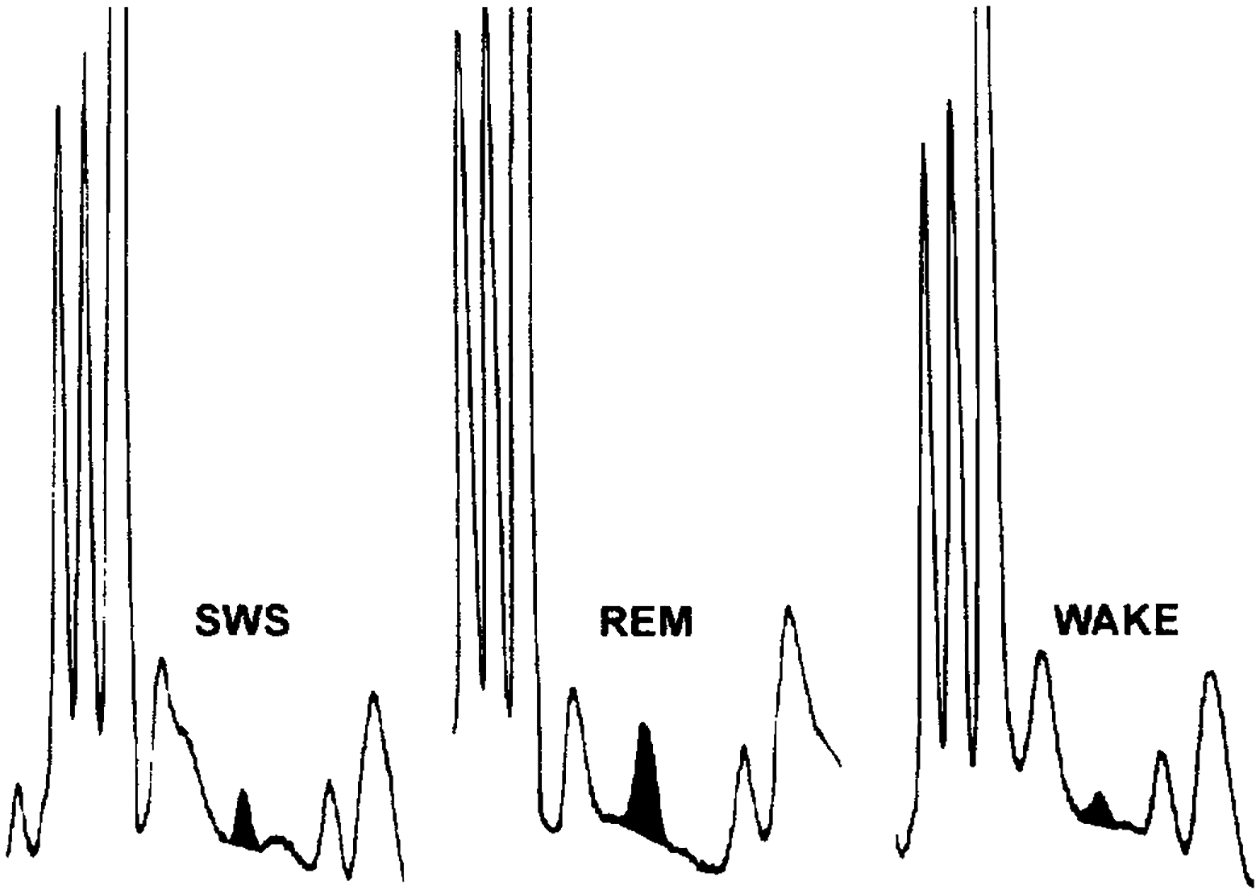
Chromatographic peaks corresponding to GABA from an individual sleep/wake cycle. In each case, the area of the GABA peak has been blackened.
Table 2.
GABA content (in fmoles/μl) in microdialysis samples collected from a single site over the course of two consecutive sleep/wake cycles
| SWS | REM | Wake | SWS | REM | Wake |
|---|---|---|---|---|---|
| 0.75 | 1.05 | 0.6 | 0.8 | 1,0 | 0.7 |
Release of glutamate and glycine was analysed across 16 and 14 sleep/wake cycles respectively. Mean glutamate concentrations (± S.E.M.) in SWS, REM sleep, and wake microdialysis samples were, respectively, 0.90 ± 0.20, 0.95 ± 0.22, and 0.88 ± 0.20 pmoles/μl. Mean glycine concentrations (± S.E.M.) in samples collected during SWS, REM sleep, and wake were, respectively, 0.45 ± 0.14, 0.48 ± 0.13, and 0.54 ± 0.16 pmoles/μl. Glutamate and glycine concentration was unchanged as a function of sleep/wake state as determined by ANOVA.
DISCISSION
Glycine concentration in our samples was unchanged as a function of sleep/wake state. The present data suggest that glycine does not mediate changes in the state-dependent firing of noradrenergic LC neurons.
We found no significant reduction in glutamate concentration in samples collected during REM sleep. In fact, mean levels were higher in REM sleep than in waking. Thus, the cessation of firing of LC neurons during REM sleep is unlikely to be mediated by disfacilitation by an excitatory glutamatergic input.
Glutamate projections from the medullary nucleus paragigantocellularis (PG) are known to mediate the phasic excitation of LC unit activity in response to some environmental stimuli.5 Furthermore. the behaviours elicited during active wake in this study (arousal to the opening of the cage door, orienting responses associated with high motor activity) are associated with increased LC unit activity in the cat.35 Therefore, we expected to find increases in glutamate release during active wake as compared to quiet wake, SWS, or REM sleep. One explanation for our not finding significantly increased release of glutamate during active wake is that the behaviours elicited in this study during periods of active wake were insufficient to increase the activity of glutamatergic neurons projecting to the LC. On the other hand, the microdialysis technique may be incapable of resolving small changes in naturally-occurring glutamate and/or glycine release. In the absence of chemical, electrical, or mechanical stimulation of the brain, the percentage of synaptically-released glutamate (and possibly glycine) in microdialysis samples may be small relative to the percentage of these compounds used in metabolic processes. This may also explain the high concentrations of glutamate and glycine relative to GABA (300 ×) in our samples.
The increased release of GABA during REM sleep suggests the existence of GABAergic neurons that increase firing during REM sleep as compared to waking. Anatomical and electrophysiological evidence has demonstrated that the primary GABAergic input to the rat LC derives from the PrH nucleus of the medulla.4 Neurons firing selectively during REM sleep have not been found in this area; however, very few neurons have been recorded in this region across sleep/wake states. Moreover, neurons exhibiting selective firing during REM sleep make up only a small percentage of neuronal types in the brainstem areas (p-LC-alpha and ventromedial medulla) where they have been located.41 Neurons in the PrH nucleus were found to be immunoreactive for the immediate early gene c-fos following the induction of REM sleep by microinjections of the muscarinic receptor agonist carbachol into the PRF of the cat.51 C-fos immunohistochemistry has been used extensively as a marker of neuronal activity.13,24,27,37 Thus, some neurons in this area may indeed fire maximally during REM sleep.
Alternatively, an increase in GABA release could be effected by changes in the release of other neurotransmitters or neuropeptides at GABAergic axon terminals in the LC and p-LC. Removal of a tonic inhibition at GABAergic axon terminals in the LC and p-LC could account for increased GABA release in the LC and p-LC even without change in the firing rate of GABAergic units.
Although not great in number, GABAergic neurons are found within the p-LC region of the cat pons and in the PG of the rat which projects heavily to the LC.14,15,28 REM-on neurons have been identified in each of these areas.39 It is possible that REM-on neurons in either of these areas are GABAergic and account for the increased GABA release in the LC found in this study. The possibility that some REM-on neurons are GABAergic is especially significant in light of the observed firing rate reductions and increases in, respectively, LC REM-off and REM-on neurons during short-term REM sleep deprivation.21 GABA neurons could potentially mediate compensatory responses of noradrenergic LC neurons to REM sleep disruption.
The important role of GABA release in mediating state-related changes in unit activity in the brain is becoming increasingly apparent. GABA release in the dorsal raphe (DR) is increased during REM sleep when serotonergic DR neurons exhibit a near-cessation of activity while GABA release in the posterior hypothalamus (PH) is greatest during SWS when PH units exhibit reduced discharge relative to wake and REM sleep.29,30 As in the present study, then, the release of GABA across the sleep/wake cycle is opposite that of the predominant state-related activity characteristic of neurons in the DR and PH. Together, these findings indicate that state-dependent fluctuations in GABA release are regionally specific.
CONCLUSIONS
The present findings are consistent with our hypothesis that the cessation of activity in LC neurons during REM sleep is mediated by GABAergic inhibition. The source of this GABAergic input remains to be determined.
Acknowledgements—
This research was supported by the Medical Research Service of the Veterans Administration, PHS grants NS14610 and HL41370, and PHS pre-doctoral fellowship no. 5F31MH10451-02.
Abbreviations:
- DR
dorsal raphe
- EEG
electroencephalographic
- EMG
electromyographic
- LC
locus coeruleus
- PG
paragigantocellularis
- PGO
ponto-geniculo-occipital
- PH
posterior hypothalamus
- p-LC
peri-LC
- POA
preoptic area
- PRF
pontine reticular formation
- PrH
praepositus hypoglossal
- REM
rapid-eye-movement
- SWS
slow-wave sleep
REFERENCES
- 1.Aghajanian GK, Vandermaelen CP and Andrade R (1983) Intracellular studies on the role of calcium in regulating the activity and reactivity of locus coeruleus neurons in vivo. Brain Res 273, 237–243. [DOI] [PubMed] [Google Scholar]
- 2.Aghajanian G and Wang Y (1987) Common alpha-2 and opiate effector mechanisms in the locus coeruleus: intracellular studies in the slice. Neuropharmacology 267B, 793–799. [DOI] [PubMed] [Google Scholar]
- 3.Aston-Jones G and Bloom F (1981) Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J. Neurosci 18, 876–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aston-Jones G, Shipley MT, Ennis M, Williams JT, Pieribone VA (1990) Restricted afferent control of locus coeruleus neurones revealed by anatomical, physiological, and pharmacological studies. In: The Pharmacology of Noradrenaline in the Central Nervous System (eds Marsden CA and Heal DJ), pp 187–247. Oxford University Press, Oxford. [Google Scholar]
- 5.Aston-Jones G, Chiang C and Alexisnky T (1991) Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog. Brain Res 88, 501–520. [DOI] [PubMed] [Google Scholar]
- 6.Berman AT (1968) The Brainstem of the Cat University of Wisconsin Press, Madison, WI. [Google Scholar]
- 7.Bier MJ and McCarley RW (1994) REM-enhancing effects of the adrenergic antagonist idazoxan infused into the medial pontine reticular formation of the freely moving cat. Brain Res 634, 333–338. [DOI] [PubMed] [Google Scholar]
- 8.Cespuglio R, Gomez ME, Faradji H and Jouvet M (1982) Alterations in the sleep-waking cycle induced by cooling of the locus coeruleus area. Electroenccph. clin. Neurophysiol 54, 570–578. [DOI] [PubMed] [Google Scholar]
- 9.Ennis M and Aston-Jones G (1989) GABA-mediated inhibition of locus coeruleus from the dorsomedial rostral medulla. J. Neurosci 98, 2973–2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foote SL, Berridge CW, Adams LM and Pineda JA (1991) Electrophysiological evidence for the involvement of the locus coeruleus in alerting, orienting, and attending. Prog. Brain Res 88, 521–532. [DOI] [PubMed] [Google Scholar]
- 11.Fort P, Luppi P and Jouvet M (1993) Glycine-immunoreactive neurons in the cat brain stem reticular formation. NeuroReport 49, 1123–1126. [PubMed] [Google Scholar]
- 12.Fredrickson CJ and Hobson JA (1970) Electrical stimulation of the brain stem and subsequent sleep. Archs ital. Biol 108, 564–576. [PubMed] [Google Scholar]
- 13.Hunt S, Pini A and Evan G. (1987) Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature 328, 632–634. [DOI] [PubMed] [Google Scholar]
- 14.Jones BE (1988) The role of noradrenergic locus coeruleus neurons and neighboring cholinergic neurons of the pontomesencephalic tegmentum in sleep-wake states. Prog. Brain Res 88, 533–543. [DOI] [PubMed] [Google Scholar]
- 15.Jones BE (1991) Noradrenergic locus coeruleus neurons: their distant connections and their relationship to neighboring (including cholinergic and GABAersic) neurons of the central gray and reticular formation. Prog. Brain Res 88, 15–30. [DOI] [PubMed] [Google Scholar]
- 16.Kaitin K, Bliwise D, Gleason C, Nino-Murcia G, Dement W and Libet B (1986) Sleep disturbance produced by electrical stimulation of the locus coeruleus in a human subject. Biol. Psychiat 218–9, 710–716. [DOI] [PubMed] [Google Scholar]
- 17.Kodama T, Takahashi Y and Honda Y (1990) Enhancement of acetylcholine release during REM sleep in the dorsal tegmental field of the cat brainstem. Neurosci. Lett 114, 277–282. [DOI] [PubMed] [Google Scholar]
- 18.Levine E, Litto W and Jacobs B (1990) Activity of cat locus coeruleus noradrenergic neurons during the defense reaction. Brain Res 5311–2, 189–195. [DOI] [PubMed] [Google Scholar]
- 19.Luppi PH, Charlety PJ, Fort P, Akaoka H, Chouvet G and Jouvet M (1991) Anatomical and electrophysiological evidence for a glycinergic inhibitory innervation of the rat locus coeruleus. Neurosci. Lett 128, 33–36. [DOI] [PubMed] [Google Scholar]
- 20.Mallick B and Alam MN (1992) Different types of norepinephrmergic receptors are involved in preoptic area mediated independent modulation of sleep-wakefulness and body temperature. Brain Res 591, 8–19. [DOI] [PubMed] [Google Scholar]
- 21.Mallick B, Siegel J and Fahringer H (1989) Changes in pontine unit activity with REM sleep deprivation. Brain Res 515, 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCormick DA, Pape HC and Williamson A (1991) Actions of norepinephrine in the cerebral cortex and thalamus: implications for function of the central noradrenergic system. Prog. Brain Res 88, 293–305. [DOI] [PubMed] [Google Scholar]
- 23.McGinty DJ and Harper RM (1976) Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res 101, 569–575. [DOI] [PubMed] [Google Scholar]
- 24.Menetrey D, Gannon A, Levine J and Basbaum A (1989) Expression of c-fos protein in interneurons and projection neurons of the rat spinal cord in response to noxious somatic, articular, and visceral stimulation. J. comp. Neurol 2852, 177–195. [DOI] [PubMed] [Google Scholar]
- 25.Mogilnicka E, Arbilla S, Depoortere H and Langer S (1980) Rapid-eye-movement sleep deprivation decreases the density of 3H-imipramine binding sites in the rat cerebral cortex. Eur. J. Pharmac 65, 289–292. [DOI] [PubMed] [Google Scholar]
- 26.Mogilnicka E, Przewlocka B, Van Luijtelaar ELJM, Klimek V and Coenen ML (1986) Effects of REM sleep deprivation on central alpha-1 and beta-adrenoceptors in rat brain. Pharmac. Biochem. Behav 25, 329–332. [DOI] [PubMed] [Google Scholar]
- 27.Morgan JI and Curran T (1991) Stimulus-transcnption coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. A. Rev. Neurosci 14, 421–451. [DOI] [PubMed] [Google Scholar]
- 28.Mugnaini E and Oertel WH (1985) An atlas of the distribution of GABAergic neurons and terminals in the rat CNS as revealed by GAD immunohistochemistry. In: Handbook of Chemical Neuroanatomy (eds Bjorklund A and Hokfelt T.). Elsevier, Amsterdam. [Google Scholar]
- 29.Nitz D and Siegel J (1993) Inhibitory amino acid neurotransmission in the dorsal raphe nucleus during sleep-wake states. Soc. Neurosci. Abstr 19, 742. [Google Scholar]
- 30.Nitz D and Siegel J (1996) GABA, glutamate, and glycine release in the posterior hypothalamus across the sleep/wake cycle. Am. J. Physiol (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osaka T and Matsumura H (1993) Noradrenergic inputs to sleep-related neurons in the preoptic area from the locus coeruleus and ventrolateral medulla in the rat. Neurosci. Res 191, 39–50. [DOI] [PubMed] [Google Scholar]
- 32.Osmanovic SS and Shefner SA (1990) Gamma-aminobutyric acid responses in rat locus coeruleus neurones in vitro: a current-clamp and voltage-clamp study. J. Physiol 421, 151–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Portas CM and McCarley RW (1994) Behavioral state-related changes of extracellular serotonin concentration in the dorsal raphe nucleus: a microdialysis study in the freely moving cat. Brain Res 6482, 306–312. [DOI] [PubMed] [Google Scholar]
- 34.Radulovacki M and Micovic N (1982) Effects of REM sleep deprivation and desipramine on beta-adrenergic binding sites in rat brain. Brain Res 235, 393–396. [DOI] [PubMed] [Google Scholar]
- 35.Rasmussen K, Morilak DA and Jacobs BL (1986) Single-unit activity of locus coeruleus neurons in the freely moving cat I. During naturalistic behaviors and in response to simple and complex stimuli. Brain Res 371, 324–334. [DOI] [PubMed] [Google Scholar]
- 36.Reiner P (1986) Correlational analysis of central noradrenergic activity and sympathetic tone in behaving cats. Brain Res 378, 86–96. [DOI] [PubMed] [Google Scholar]
- 37.Sagar SM, Sharp FR and Curran T (1988) Expression c-fos protein in brain: metabolic mapping at the cellular level. Science 240, 1328–1331. [DOI] [PubMed] [Google Scholar]
- 38.Saito H, Sakai K and Jouvet M (1977) Discharge patterns of the nucleus parabrachialis lateralis neurons of the cat during sleep and waking. Brain Res 134, 59–72. [DOI] [PubMed] [Google Scholar]
- 39.Sakai K (1988) Executive mechanisms of paradoxical sleep. Archs ital Biol 1264, 239–257. [PubMed] [Google Scholar]
- 40.Shiromani PJ, Armstrong DM, Berkowitz A, Jeste DV and Gillin JC (1988) Distribution of choline acetyltransferase immunoreactive somata in the feline brainstem: implications for REM sleep generation. Sleep 111, 1–16. [DOI] [PubMed] [Google Scholar]
- 41.Siegel J (1994) Brainstem mechanisms generating REM sleep. In: Principles and Practice of Sleep Medicine, 2nd edn, (eds Kryger M, Roth T and Dement W), pp. 125–144. New York, WB Saunders. [Google Scholar]
- 42.Siegel JM and Rogawski MA (1988) A function for REM sleep: regulation of noradrenergic receptor sensitivity. Brain Res. Rev 13, 213–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tononi G, Pompeiano M and Cirelli C (1991) Effects of local pontine injection of noradrenergic agents on desynchronized sleep of the cat. Prog. Brain Res 88, 545–553. [DOI] [PubMed] [Google Scholar]
- 44.Troncone L, Braz S, Benedito M and Tufik S (1986) REM sleep deprivation induces a decrease in norepinephrine-stimulated 3H-cyclic AMP accumulation in slices from rat brain. Pharmac. Biochem. Behav 251, 223–225. [DOI] [PubMed] [Google Scholar]
- 45.Tsai L, Bergmann B, Perry B and Rechtschaffen A (1993) Effects of chronic total sleep deprivation on central noradrenergic receptors in rat brain. Brain Res 6022, 221–227. [DOI] [PubMed] [Google Scholar]
- 46.Ursin R and Sterman B (1981) A Manual for Standardized Scoring of Sleep and Waking States in the Adult Cat Brain Information Service, Brain Research Institute. UCLA. [Google Scholar]
- 47.Vanni-Mercier G, Sakai K and Jouvet M (1984) Neurons specifique de l’eveil dans l’hypothalamus posterieur du chat. C. r. hebd. Séane. Acad. Sci., Paris 298, 195–200. [PubMed] [Google Scholar]
- 48.Wilkinson L, Auerbach S and Jacobs B (1991) Extracellular serotonin levels change with behavioral state but not with pyrogen-induced hyperthermia. J. Neurosci 119, 2732–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams J, Comisarow J, Day J, Fibiger H and Reiner P (1994) State-dependent release of acetylcholine in rat thalamus measured by in vivo microdialysis. J. Neurosci 149, 5235–5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams JT, North RA, Shefner SA, Nishi S and Egan TM (1984) Membrane properties of rat locus coeruleus neurones. Neuroscience 13, 137–156. [DOI] [PubMed] [Google Scholar]
- 51.Yamuy J, Mancillas JR, Morales FR and Chase MH (1993) C-fos expression in the pons and medulla of the cat during carbachol-induced active sleep. J. Neurol 136, 2703–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]


