Abstract
Hypothalamic kisspeptin neurons serve as the nodal regulatory centre of reproductive function. These neurons are subjected to a plethora of regulatory factors that ultimately affect the release of kisspeptin, which modulates gonadotropin- releasing hormone (GnRH) release from GnRH neurons to control the reproductive axis. The presence of sufficient energy reserves is critical to achieve successful reproduction. Consequently, metabolic factors impose a very tight control over kisspeptin synthesis and release. This Review offers a synoptic overview of the different steps in which kisspeptin neurons are subjected to metabolic regulation, from early developmental stages to adulthood. We cover an ample array of known mechanisms that underlie the metabolic regulation of KISS1 expression and kisspeptin release. Furthermore, the novel role of kisspeptin neurons as active players within the neuronal circuits that govern energy balance is discussed, offering evidence of a bidirectional role of these neurons as a nexus between metabolism and reproduction.
Metabolic state determines a number of behavioural and physiological adaptations to ensure survival. Situations of negative energy balance increase hunger signals, induce food- seeking mechanisms and halt physiological functions that can be spared until energy reserves are restored. Sexual maturation and reproductive success require a minimum threshold of energy reserves1,2 owing to the high energetic cost of reproduction and are therefore subjected to very tight regulation by metabolic factors.
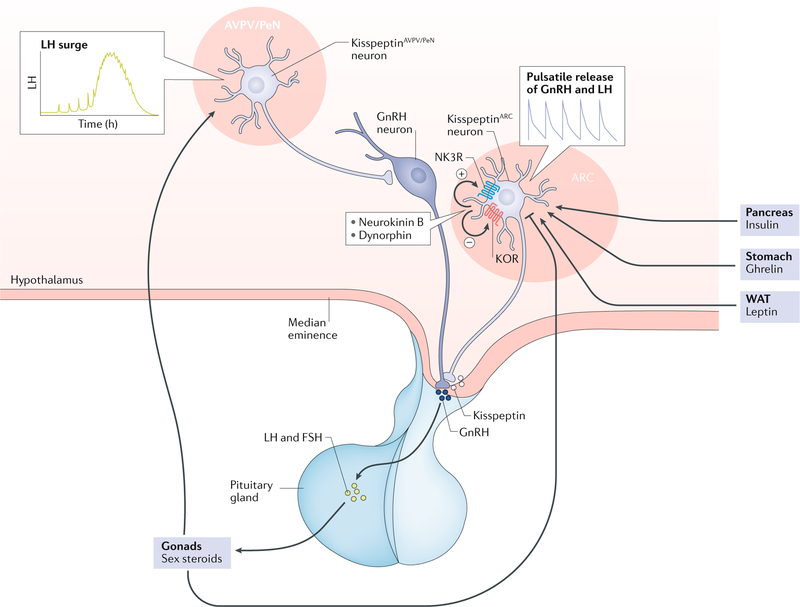
Reproductive function is controlled by the hypothalamic–pituitary–gonadal (HPG) axis, in which gonadotropin- releasing hormone (GnRH) neurons located in the hypothalamus control the production and release of gonadotropins that regulate gonadal function. GnRH secretion requires the stimulatory action of kisspeptin, produced by kisspeptin neurons located primarily in the arcuate nucleus (ARC) and the anteroventral periventricular nucleus/periventricular nucleus continuum (AVPV/PeN) of the hypothalamus. Importantly, kisspeptin neurons in the ARC (kisspeptinARC) co- express the neuropeptides neurokinin B and dynorphin A3,4. In these neurons, the coordinated action of neurokinin B (stimulatory) and dynorphin (inhibitory) facilitates the release of kisspeptin in a pulsatile manner, which is mirrored by GnRH and luteinizing hormone (LH) pulses5 (FIG. 1). By contrast, kisspeptinAVPV/PeN neurons, which are almost exclusive to the female brain, are involved in the positive feedback of sex steroids leading to the pre- ovulatory LH surge6.
Fig. 1 |. The HPG axis with the two main populations of kisspeptin neurons.
Arcuate kisspeptin (kisspeptinARC) neuron together with neurokinin B and dynorphin are involved in the tonic (pulsatile) release of kisspeptin (positive and negative symbols indicate the effect on kisspeptin release) and, therefore, gonadotropin-releasing hormone (GnRH). By contrast, anteroventral periventricular/periventricular nucleus kisspeptin (kisspeptinAVPV/PeN) neurons are involved in the control of the luteinizing hormone (LH) surge and are almost absent in the male brain. Major peripheral metabolic factors are depicted (leptin, insulin and ghrelin) acting at the level of the brain to regulate kisspeptin output. ARC, arcuate nucleus; FSH, follicle-stimulating hormone; HPG axis, hypothalamic–pituitary–gonadal axis; KOR, κ-opioid receptor; NK3R, neurokinin B receptor; WAT, white adipose tissue.
The development of kisspeptin pulsatility (and therefore GnRH pulses) is essential for sexual maturation. Patients with inactivating mutations in the genes encoding kisspeptin and kisspeptin receptor (KISS1 (REF.7) or KISS1R8,9) do not undergo puberty onset, whereas activating mutations of KISS1R10 cause precocious puberty. External factors can accelerate or delay the timing of puberty onset by modifying kisspeptin output onto GnRH neurons. For example, metabolic factors play an essential part by relaying the energetic status of the organism to central networks that ultimately regulate reproductive function11. This regulation is critical for organism survival, given the high energetic burden of reproduction, that is mostly of relevance in female mammals12. In humans, energy excess can also alter reproductive function, leading to the advancement of puberty onset13 and in individuals with severe obesity, to the inhibition of the reproductive axis and hypogonadism14,15 In this context, a strong association is observed between childhood obesity and early activation of the HPG axis due to the early onset of kisspeptin–GnRH pulses. Indeed, obesity during early developmental stages has become a leading cause of precocious puberty16–18 and polycystic ovary syndrome19,20 in adolescent girls, with subsequent fertility impairment.
Attention has mostly been focused on the metabolic regulation of fertility; however, a bidirectional regulation where energy balance is affected by reproductive factors also exists. Changes in levels of sex hormones (oestradiol or testosterone) lead to metabolic disorders. For instance, during pregnancy, the energy demands of the mother rapidly increase, leading to the induction of hunger signals over those inducing satiety21. Moreover, the anorectic effect of oestradiol has been documented in several species22,23. In humans, the decline in levels of sex steroids in women after menopause and low levels of testosterone in men are associated with increased body weight24–26. However, despite this known reproductive axis- dependent regulation of energy balance, the underlying connecting mechanisms remain largely unknown.
This Review focuses on the different mechanisms by which metabolic cues can affect the maturation and function of kisspeptin neurons throughout development to modulate reproductive function. In addition, the latest advances demonstrating these neurons as active players in the regulation of metabolic function are discussed, describing the role of kisspeptin neurons as bidirectional regulatory elements between metabolism and reproduction.
The energetic state and kisspeptin
Effects on kisspeptin neuron development
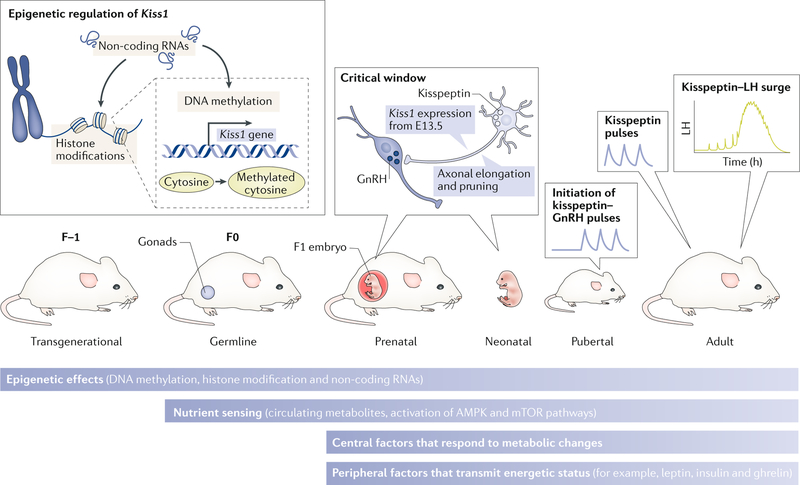
Metabolic factors can influence the development and function of vital systems in an organism. First, the genome can carry adaptations to metabolic conditions that determine the pattern of gene expression, through the inheritance of epigenetic modifications that occurred in previous generations27–29. Second, epigenetic changes occurring in the germline in response to changes in the metabolic state of the progenitor before fertilization can be passed down to the embryo and can have effects into adulthood27,28,30,31, affecting sexual maturation and fertility. Third, once fertilization has occurred, metabolic factors that cross the placenta can fundamentally alter the development of neurons — including those necessary for sexual maturation — during the critical window of differentiation of the brain32–34. This period occurs before birth in precocial animals (for example, during the first trimester in humans) and perinatally in altricial animals (for example, up until postnatal day 10 in mice and rats). During this critical period, axonal and dendritic elongation and pruning take place, to establish the synaptic connections that will form specific neuronal circuits to govern metabolism and reproduction (FIG. 2). Importantly, the location of the hypothalamus in the brain, sitting in the ventral side and exposed to a porous section of the blood–brain barrier35–37, makes this area, and therefore the developing kisspeptin neurons, susceptible to the action of peripheral signals. Such signals can include metabolic cues from the fetus and/or the mother. Indeed, numerous studies have described impairments in axonal elongation in perinatally undernourished rodents that can affect the development of agouti- related peptide (AgRP) and proopiomelanocortin (POMC) neurons38–40, which are essential in the control of energy balance.
Fig. 2 |. Levels of metabolic regulation of kisspeptin neurons throughout development.
Schematic representation of the different developmental stages in which metabolic factors might affect the expression of the Kiss1 gene. First, epigenetic effects (DNA methylation, histone modification and non-coding RNAs) might permanently affect the expression of Kiss1. This effect can occur transgenerationally or at any stage of development. Second, during the perinatal period there is a critical window in which conformational changes in kisspeptin neurons might happen as a consequence of the exposure to metabolic factors. These pre-existing modifications (epigenetic and conformational during development) might determine the timing of the activation of kisspeptin neurons (puberty onset) and their function in adulthood (luteinizing hormone (LH) pulses and surge). Nonetheless, during and after development, kisspeptin neurons can still be regulated by metabolic factors at different levels: epigenetic, nutrient sensing and central and peripheral factors. AMPK, 5'-AMP-activated protein kinase; E13.5, embryonic day 13.5; GnRH, gonadotropin-releasing hormone; mTOR, mammalian target of rapamycin.
Precocial.
Born mature, without the need for parental care for feeding.
Altricial.
Born undeveloped, requiring parental care for feeding.
By embryonic day 13.5 (E13.5) in mice, direct connections are established between kisspeptinARC neurons and GnRH neurons41,42. However, these connections increase progressively during postnatal development to reach a maximum plexus that connects GnRH neuronal somas and terminals at the time of puberty onset (approximately postnatal day 25) in mice43. Of note, in adulthood, kisspeptinARC neurons project largely to GnRH terminals, whereas kisspeptinAVPV/PeN neurons project to the GnRH somas44. Unlike kisspeptinARC neurons, which are detectable prenatally, kisspeptin expression in the AVPV/PeN is not present until postnatal day 10 in rodents45, highlighting the existence of different developmental processes in the formation of each hypothalamic kisspeptin neuron population.
Although no direct studies have addressed the effect of metabolic cues on the differentiation and axonal elongation of kisspeptin neurons, compelling evidence in rodents indicates a long- lasting effect of perinatal metabolic insults on Kiss1 expression throughout an animal’s lifespan. For example, neonatal manipulations in energy resources in mice can be achieved by adjusting the litter size, leading to a model of neonatal energy deprivation (increased litter size) or energy excess (reduced litter size). Energy deprivation or excess leads to a statistically significant delay or advancement, respectively, in the time of puberty onset, driven by permanent changes in the hypothalamic expression of Kiss1 (REF.46). Animals from small litters had increased Kiss1 expression, whereas the animals from large litters showed decreased expression; these changes were maintained despite equal ad libitum access to food after weaning46. This finding strongly suggests that, similar to POMC and AgRP neurons38–40, the kisspeptin system is also sensitive to metabolic alterations from early developmental stages and that these changes can be carried on into adulthood, leading to reproductive disorders.
These data in rodents agree with findings from human studies. Such studies in humans support a strong association between maternal obesity and hyperglycaemia during gestation with higher incidence of precocious puberty in the daughters of mothers with obesity41,42; however, the involvement of kisspeptin signalling in this process in humans remains to be demonstrated. Nonetheless, puberty onset depends on the re- awakening of pulsatile GnRH release, which in turn depends on the activation of kisspeptin neurons. Therefore, we can infer that, as in rodents, perinatal alterations in humans induced by the metabolic state permanently impinge on the expression of KISS1 later in life (directly or through the action on upstream regulators), thus affecting the timing of puberty onset (discussed later).
Effects on kisspeptin neuron activity
Puberty is defined as a complex biological process involving sexual development, accelerated growth and adrenal maturation, heralded by the secretion of GnRH47,48, which initiates the pulsatile release of gonadotropins, gonadal secretion of sex steroids and gametogenesis. A number of central and peripheral factors have been proposed to mediate puberty; however, exactly what triggers puberty onset remains elusive. Accumulating evidence suggests that hypothalamic kisspeptin is a likely candidate to serve as gatekeeper of puberty onset. For example, humans and mice with deficient kisspeptin signalling present with hypogonadotropic hypogonadism, absence of puberty onset and infertility8,9,49. Moreover, compelling evidence shows that Kiss1 expression in the hypothalamus increases at the time of puberty onset in rodents50–53. However, most of these studies refer to the expression of Kiss1 in whole hypothalami, whereas the specific contribution of each kisspeptin population of neurons (that is kisspeptinARC versus kisspeptinAVPV/PeN) to puberty onset, at least in female animals given the vestigial nature of the PeN population in male animals, remains unknown. Of note, the synthesis and release of kisspeptin are critical targets for metabolic regulation induced by energy imbalances, which leads to changes in the timing of puberty onset in rodents. For example, undernutrition delays puberty onset, which is a phenotype that can be rescued by exogenous kisspeptin51, further supporting the role of this neuropeptide as a pubertal gatekeeper sensitive to the metabolic status.
Neurokinin B (encoded by Tac2) is the kisspeptin co-transmitter in the ARC that participates in the auto-synaptic stimulation of kisspeptin release54. Importantly, this factor also acts as a metabolic target to regulate the HPG axis. In mice, the expression of Tac2 is inhibited in conditions of negative energy balance55,56, which further prevents the release of kisspeptin (FIG. 1). In addition to energy deficiency, a prepubertal caloric excess can advance puberty onset in a process that involves the early activation of the kisspeptin system46. Thus, we can consider kisspeptin neurons as the main conveyor of metabolic cues to facilitate sexual maturation.
In adulthood, once sexual maturity has been achieved, successful reproduction depends upon the presence of sufficient energy reserves. Kisspeptin neurons continue to convey metabolic signals to determine the appropriate pattern of GnRH release in adulthood. Indeed, conditions of negative energy balance such as anorexia nervosa or excessive exercise often lead to functional hypothalamic amenorrhoea57. Although the neuronal pathways underlying functional hypothalamic amenorrhoea in humans under energy deficit have not been addressed, studies in rodent models have shown that adult females subjected to energy-depleting conditions (for example, chronic undernutrition or fasting) display disrupted oestrous cycles and low circulating levels of gonadotropin that resemble human hypothalamic amenorrhoea, as a consequence of reduced Kiss1 and Tac2 expression46,51,55. These findings highlight the importance of kisspeptinARC neurons as conveyors of the energy state to the HPG axis.
Functional hypothalamic amenorrhoea.
A condition derived from the insufficient secretion of GnRH from the hypothalamus, leading to anovulation and hypogonadotropic hypogonadism.
The effect of the metabolic state on kisspeptin neurons (described in a previous section) can critically affect sexual maturation and fertility; however, the mechanisms and factors underlying this regulation of kisspeptin neurons, from early developmental stages to adulthood, are not fully understood. This regulation could occur at a multilevel scale, including subcellular mechanisms, central networks and peripheral cues that ultimately directly affect kisspeptin neurons or act through intermediate neuronal effectors, which are described in the following sections.
Kisspeptin synthesis and release
This section summarizes what is known about the mechanisms of metabolic regulation of kisspeptin synthesis and release.
Subcellular mechanisms
Nutrient sensing.
The first mechanism of neuronal adaptation to the nutritional status of the organism is through the direct response of individual cells to metabolites. As mentioned previously, kisspeptin neurons send axonal projections to the hypophysial medial eminence58–61, which is situated in a fenestrated area of the basal hypothalamus35–37, and therefore this neuronal population is directly exposed to circulating nutrients. Circulating levels of glucose and amino acids can induce a direct response on neurons through the 5'-AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) pathways, which integrate cellular metabolism and the metabolic status of the entire organism.
AMPK is a highly conserved 5'-activated protein kinase that comprises a catalytic a-subunit and two regulatory subunits (β and γ)62. The AMPK complex is a highly selective sensor for the AMP to ATP ratio. When glucose levels decrease during energy deficit, the increase in the AMP to ATP ratio induces the activation of AMPK by phosphorylation, which in turn increases the translocation of glucose transporters to the cell membrane, activates catalytic processes and slows down anabolism in an effort to replenish ATP levels62,63. AMPK is present in the hypothalamus at high levels and is involved in numerous pathways that determine the regulation of food intake and energy expenditure64. More importantly, the AMPK pathway is present in both kisspeptinARC and kisspeptinAVPV/PeN neurons65,66, thus serving as the first-order sensor of the nutritional status of the organism to regulate reproductive function.
Studies in rodent models have demonstrated that AMPK phosphorylation is increased in kisspeptinARC neurons under negative energy balance (for example, chronic undernutrition), which leads to the inhibition of Kiss1 expression and the resultant delay in puberty onset65. Additionally, the deletion of AMPKα from kisspeptin neurons in mice protects the HPG axis from the disruption induced by acute fasting66. Of note, although kisspeptin neurons are a nodal conveyor of metabolic and reproductive cues, glucose sensing through AMPK has also been described at different levels of the HPG axis, for example, GnRH neurons67 and gonadotropes68, suggesting that glucose sensing would have a role in the control of reproduction at multiple levels of the HPG axis.
Besides glucose, every cell requires amino acids for protein synthesis. The role of amino acid sensor is played by the mTOR complex, another protein kinase. In the presence of amino acids, mTOR is activated, leading to the phosphorylation of a number of protein complexes, of which S6 kinase 1 (SK61) and 4E-BP1 have a key conserved role69,70. Given their involvement in nutrient sensing, it is not surprising that mTOR and AMPK interact to further adapt cellular metabolism and energy status71–74. As with AMPK, the mTOR pathway not only actively controls food intake and energy expenditure at the hypothalamic level, but it also directly regulates reproductive function. For example, studies in rodents have shown that activation of mTOR with the amino acid leucine activates the HPG axis and advances puberty onset, whereas blockade of mTOR signalling using rapamycin causes the opposite effect through a decrease in Kiss1 expression in the ARC while the expression of Gnrh1 is unaffected75. Nonetheless, a role of mTOR signalling at the level of gonadotropes in the control of reproductive function cannot be excluded76.
Although metabolite sensing enables the neuron to respond in real time to metabolic changes in its environment (such as circulating nutrients), the effect of these changes can reverse or cease as soon as the environment, and hence the neuron, reach a new set point of energy balance. Of note, nutrient sensing pathways also participate in the induction of epigenetic changes. Phosphorylated AMPK can induce changes in DNA methylation and histone acetylation in response to glucose levels77–79 (described later). This process is considered the main mechanism by which disrupted energy balance (that is, deficit or excess of nutrients) during critical developmental periods can lead to the development of metabolic and reproductive conditions during adulthood depending on the neurons and genes affected27,28,30,31, including Kiss1.
Epigenetic regulation.
Numerous studies have provided information on the indirect, transgenerational epigenetic influence of sustained metabolic imbalances (for example, severe obesity or chronic undernutrition) from the parents to the offspring80–85, as well as on the direct metabolic imprinting in an individual from early developmental periods (prenatally and perinatally)86–90, as mentioned earlier (FIG. 2). These epigenetic modifications often contribute to the onset of obesity and reproductive impairments during postnatal development. In the past few decades, the most frequent scenario for epigenetic-induced changes in metabolism and reproduction in Western societies does not come from transgenerational or parental exposure but through childhood obesity in children of parents without obesity. This phenomenon is strikingly associated with permanent developmental alterations in energy balance and reproduction for the lifespan of the individual18,19,28,64,83,91. This effect strongly suggests that obesity in early developmental stages induces epigenetic changes in genes involved in reproduction and energy homeostasis, which are mostly located in the hypothalamus. Indeed, studies have shown that the decrease in the average age of menarche between the mid-nineteenth and the mid- twentieth centuries has been attributed in part to improvements in nutrition92. Epigenetics has been suggested as a molecular mechanism that mediates the interplay between behavioural and/or environmental factors and genetics by adapting the genome to environmental exposures93. In this context, the main epigenetic mechanisms that might ultimately regulate KISS1 expression can be organized in three categories: DNA methylation, histone modification and non-coding RNAs.
DNA methylation occurs predominantly in cytosine–guanine (CpG) sites, referred to as CpG islands, through the action of methyltransferases, which leads to gene silencing or activation depending on the methylated region93. An extensive body of literature documents the association between obesity and diabetes mellitus with changes in DNA methylation91,94. Not surprisingly, the promoter region of the Kiss1 gene is highly methylated95–97 and developmental changes in the methylation pattern occur at the initiation of puberty in kisspeptinAVPV/PeN neurons96,98 (FIG. 2). Importantly, methylation is not a permanent phenomenon. Studies in rodents have documented the role of ten–eleven translocation (TET) enzymes in the oxidative demethylation of DNA sites99–101. Moreover, the TET isoform TET2 is required for the maintenance of GnRH release102 and preliminary studies in vivo (J. Kurian, personal communication) have indicated that they are also necessary for Kiss1 expression. Because TET2 is phosphorylated and activated by AMPK77–79, we can infer a direct relationship between the metabolic state of the organism and the methylation pattern of reproductive genes, that is, Kiss1 and Gnrh1.
Histone modifications consist of chromatin changes that alter the accessibility of the DNA to polymerases to enable transcription, replication or DNA repair103. The most common modification is acetylation, which is a reversible process in which arginine or lysine residues are acetylated on histone tails. This process is highly sensitive to the energetic state of the organism104,105 and is also able to regulate the activity of the Kiss1 promoter, at least in rodents106 (FIG. 2). Indeed, the activation of the H3 histone by H3K9/14 acetylation is associated with an increase in Kiss1 expression in all populations of kisspeptin neurons106 and oestrogen is able to increase acetylation of the Kiss1 promoter in kisspeptinAVPV/PeN neurons and facilitate deacetylation of the promoter in kisspeptinARC neurons106. This effect of oestrogen coincides with the differential regulation of Kiss1 in each hypothalamic area as part of the positive feedback of sex steroids on kisspeptinAVPV/PeN neurons versus negative feedback on kisspeptinARC neurons. Moreover, sirtuin 1 (SIRT1), a fuel- sensing deacetylase107, halts Kiss1 expression in the ARC during infantile periods in rodents, therefore contributing to the proper timing of puberty onset. The activity of SIRT1 is increased by undernutrition, thus repressing Kiss1 expression in conditions of negative energy balance108,109.
Histone modification can also take place through methylation and the regulation of the methylated status of specific sites can substantially affect the expression of a gene. In this context, lysine-specific demethylase 1 (LSD1) has emerged as a critical link in the epigenetic regulation of obesity and ageing110–112. Preliminary studies uncovered an additional role for LSD1 as regulator of the timing of puberty onset. Lsd1-null mice display precocious puberty and elevated levels of gonadotropin (J. Gill and U.B. Kaiser, personal communication). As LSD1 is expressed in kisspeptin neurons113, we can infer that LSD1 contributes to the metabolic regulation of Kiss1 expression and, therefore, the energetic regulation of puberty onset and fertility (FIG. 2).
In addition to DNA methylation and histone modifications, non-coding RNAs have emerged as novel epigenetic mechanisms with critical importance in the regulation of protein synthesis through the regulation of RNA levels. Particularly, microRNAs (miRNAs) consist of RNA sequences that bind complementary mRNAs, silencing the transcript and targeting it for degradation, thereby leading to lower or absent translation of the transcript. A number of these miRNAs serve as mediators of the metabolic state of the organism to further regulate the activity of the HPG axis. Among these, the main miRNAs identified to date with documented activity in the regulation of both metabolism and reproduction are miR-7a2, Let-7a and miR-30b.
The global absence of miR-7a in a mouse model leads to hypogonadotropic hypogonadism114. Although the presence of miR-7a2 in kisspeptin neurons remains to be determined, its co- expression with AgRP in AgRP neurons115 already suggests a reproductive and metabolic role for this miRNA, by modulating the output of the hunger signal AgRP (see later text). The mechanism for this effect involves, at least in part, the action of miR-7a on mTOR within the AgRP neuron116.
Let-7a is a highly evolutionarily conserved miRNA that is upregulated in the hypothalamus of rodents in cone ditions of aberrant energy balance, that is, caloric restriction or diet- induced obesity117. Importantly, Let-7a and its associated protein Lin-28b (which regulates the processing of Let-7a into mature miRNA) are critical components of the neuroendocrine machinery controlling the time of puberty onset118–120. These findings suggest that Let-7a must act through the transmission of the metabolic state onto kisspeptin and/or GnRH neurons.
A 2019 study provided compelling evidence for a role of miR-30b in the control of puberty onset. This miRNA is expressed in kisspeptinARC neurons and regulates the expression of kisspeptin by binding to the 3' untranslated region of the imprinted makorin 3 (Mkrn3) gene to repress its activity121. MKRN3 inhibits pubertal progression, and loss of function mutations in this gene lead to central precocious puberty in humans122. Because miR-30b is significantly upregulated in the hypothalamus by diet- induced obesity in mice fed a high- fat diet117, we can infer that metabolic changes — especially those related to excess energetic state of the organism — might modulate puberty onset through the miR-30b–MKRN3–Kiss1 pathway. Whether this pathway is involved in the advancement of the timing of puberty onset observed in children with obesity remains to be deciphered.
Overall, compelling evidence from a wealth of studies suggests that kisspeptin neurons are able to directly sense the nutritional state of the organism and adapt to it to induce functional changes, for example, transcriptional and translational modifications, in response to the circulating levels of metabolites. However, despite this direct metabolic regulation, an important layer of complexity arises from the effect that regulatory networks of neuroendocrine pathways upstream of kisspeptin neurons, as well as peripheral factors, exert on kisspeptin neuron activity to precisely adapt to the nutritional environment.
Extracellular mechanisms
Neuronal factors.
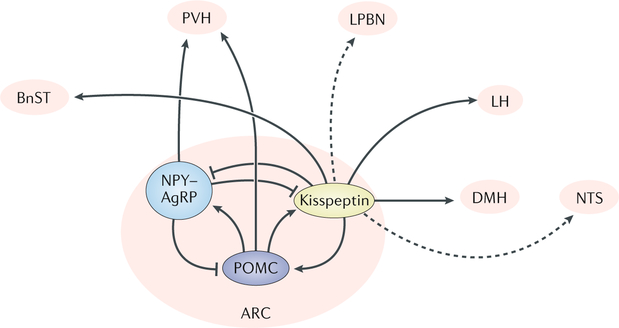
The neuroendocrine regulation of food intake can occur acutely through fast- acting factors that rapidly promote hunger or satiety. Furthermore, on a long-term scale, factors can promote the increase in body weight or leanness, usually as a direct measure of the amount of energy stored in the adipose tissue123,124. In addition to food intake, the control of energy expenditure has a critical role in the maintenance of the overall homeostasis of the organism. Strong evidence exists that two sets of hypothalamic neurons (mostly located in the ARC) with antagonistic functions play a key part in the control of energy balance: first, the hunger-inducing AgRP neurons, which co-express neuropeptide Y and GABA; and second, POMC neurons, which co-express cocaine- and amphetamine-regulated transcript (CART), are predominantly glutamatergic and induce satiety125,126. In the hypothalamus, α-melanocyte-stimulating hormone (α-MSH) is the main product of POMC neurons, which binds the melanocortin 4 receptor (MC4R) to promote satiety127 and increase energy expenditure128, whereas AgRP antagonizes the same receptor to induce the opposite effect129 (FIG. 3).
Fig. 3 |. Neuroendocrine circuits involved in the metabolic role of kisspeptin neurons.
Representation of a sagittal section of the mouse brain depicting documented (solid lines) and predicted (dotted lines) connections from kisspeptinARC neurons to and from known metabolic nuclei. AgRP, agouti-related peptide; ARC, arcuate nucleus; BnST, bed nucleus of the stria terminalis; DMH, dorsomedial hypothalamus; LH, lateral hypothalamus; LPBN, lateral parabrachial nucleus; NPY, neuropeptide Y; NTS, nucleus of the solitary tract; POMC, proopiomelanocortin; PVH, paraventricular hypothalamic nucleus.
Dioestrus.
Phase of the oestrous cycle in female mice, which precedes the ovulation phase, that is proestrus.
Situations of negative energy balance rapidly suppress the reproductive axis as a fail-safe mechanism due to the high energetic cost of reproduction. This effect occurs in part through the direct action of hunger neurons (that is, AgRP neurons through AgRP and GABA release) on both kisspeptinARC and kisspeptinAVPV/PeN neurons130. Conditions of severe obesity, such as leptin deficiency, lead to a perceived permanent state of starvation, which also induces the activation of AgRP neurons and subsequently leads to infertility131. This role of AgRP neurons in reproduction is supported by studies showing that the ablation of AgRP neurons from leptin-deficient mice (ob/ob) is sufficient to restore fertility131. Moreover, in mice, the activation of AgRP neurons directly inhibits kisspeptin neurons, disrupting oestrous cycles and gonadotropin release (pulsatile and surge-like LH release) in a process that is largely mediated by GABA130. Altogether, these studies suggest that the main regulatory pathway by which hunger signals regulate reproductive function is through the direct inhibition of kisspeptin neurons.
Similarly, melanocortin signalling deficiency leads to severe obesity and reduced fertility in rodents132. The findings that the POMCARC neurons express oestrogen receptor-α133–135 and are responsive to kisspeptin136 led to the hypothesis that the melanocortin system might be a critical component in the central regulation of reproduction in a bidirectional POMC–kisspeptin manner. Indeed, α-MSH immunoreactive fibres are found in close apposition to kisspeptin neuron cell bodies of the ARC137, which express MC4R137; however, whether these projections represent synaptic contacts that could modulate the activity of kisspeptin neurons remains unknown. Central stimulation of α-MSH signalling strongly activates the reproductive axis in rats, whereas chronic inhibition of melanocortin receptors delays puberty137. Interestingly, melanocortin receptor agonists are unable to modulate the firing of kisspeptinARC neurons from prepubertal and adult female mice in dioestrus in a series of cell-attached recordings137; however, further investigation is necessary under different sex steroid conditions to rule out a direct action of melanocortin on kisspeptin neurons. Of note, 50% of GnRH neurons of the medial preoptic area express MC4R138, yet GnRH neurons are not the main target of α-MSH in controlling reproduction, as the stimulatory effect of α-MSH on LH secretion depends on the presence of kisspeptin signalling137.
POMC neurons express CART, which contributes to the satiety signals from these neurons to control appetite and energy balance139. Importantly, CART is able to exert a strong direct depolarization of kisspeptinARC neurons in rodents140, demonstrating that multiple stimulatory (and inhibitory, from AgRP neurons) pathways act on kisspeptin neurons to enable fine metabolic regulation of the activity of kisspeptinARC neurons.
In addition to the control of pulsatile LH release, AgRP and POMC neurons also participate in the control of the preovulatory LH surge. First, AgRP neurons contact and inhibit kisspeptinAVPV/PeN neurons130 and the hypothalamic expression of Agrp decreases at the time of proestrus in rodents141. Second, although the existence of direct projections from POMC neurons to AVPV/PeN neurons has not been proven yet, the expression of Pomc142 and Mc4r141 increases at the time of the LH surge, which is blunted by MC4R antagonists, further suggesting the existence of metabolic mechanisms that directly affect ovulation, probably through the regulation of the activity of kisspeptinAVPV/PeN neurons.
Overall, although additional metabolic centres exist in the brain, both within and outside the hypothalamus (FIG. 3), to date, the two major regulators of food intake and energy balance (that is, AgRP and POMC neurons) are also the major neuronal populations that directly transmit metabolic information to kisspeptin neurons. How these neurons sense the overall energetic state of the organism appears to be multifactorial. Although metabolite sensing contributes to the adaptation of the HPG axis to energy balance, the main regulatory process depends on, and is reinforced by, peripheral metabolic cues such as leptin, insulin or ghrelin acting directly on, or upstream of, kisspeptin neurons.
Peripheral factors.
The main peripheral signal informing the hypothalamus of the energy reserves is the adipokine leptin. Leptin is synthesized in adipocytes and secreted at levels proportional to existing adipose deposits124. Sufficient leptin levels are required for puberty onset, reproductive function and fertility (FIG. 1). Delayed or absent puberty, hypogonadotropic hypogonadism and infertility can result from disorders associated with reduced leptin levels143–146. However, despite this critical role of leptin in the maturation and maintenance of reproductive function, its mechanisms and sites of action are not fully understood. Although leptin receptors (LEPR) are expressed at multiple levels of the HPG axis, leptin action at the level of the hypothalamus is sufficient to maintain its metabolic and reproductive effects147,148. Regarding the control of reproduction, GnRH neurons do not express LEPR149,150 and kisspeptin neurons express few LEPR; however, these receptors in kisspeptin neurons are not the direct target involved in the reproductive action of leptin151.
Importantly, GABAergic neurons have been identified as the main interplay in the reproductive action of leptin152,153, resembling the findings for the metabolic role of leptin154. These findings exclude a large number of glutamatergic neurons that could be direct targets of leptin to fine-tune leptin’s action; however, glutamatergic neurons do not have a fundamental role in the effects of leptin on reproduction and body weight. Indeed, the deletion of LEPR specifically from GABAergic neurons largely replicated the reproductive phenotypes of female mice with deficient leptin signalling (ob/ob or db/db mice)155,156, which show delayed or absent puberty onset, hypogonadotropic hypogonadism, disrupted oestrous cycles and infertility152,153. These mice also have substantially lower expression of Kiss1 in the two main populations of hypothalamic kisspeptin neurons (AVPV/PeN and ARC)153. Of note, these studies showed the role of leptin action on all GABAergic neurons. Given the wide distribution of GABAergic neurons in the brain, further studies are required to decipher which specific subpopulation mediates the metabolic role and which the reproductive role of leptin.
Taken together, these findings suggest that the GABAergic action that mediates leptin’s role in reproduction impinges on the kisspeptin system in female mice153; however, the nature of the precise GABAergic neurons that mediate this role remains unknown. Although the GABAergic-mediated action of leptin in reproduction appears to be critical for successful reproduction in female mice, male mice devoid of leptin signalling in these neurons display delayed puberty but normal reproductive function in adulthood152. Similar findings in db/db mice and in humans have also been reported157,158. This sexual dimorphism in the reproductive role of leptin is reasonable, given the larger energetic investment of females in reproduction.
Interestingly, studies have documented a critical contribution of the ventral pre-mammillary nucleus (PMV) to the reproductive action of leptin. First, lesions in this area prevent the activation of the HPG axis by leptin151. Second, re-insertion of LEPR into the PMV of Lepr-deficient mice restores puberty onset and substantially improves reproductive function151. Although these studies offer compelling evidence supporting a role of the PMV in the reproductive role of leptin, it is important to highlight that the PMV is predominantly glutamatergic151,154,159, which generates a puzzling discrepancy with the genetic studies described earlier that show that the reproductive role of leptin is mediated by GABAergic neurons.
One important caveat to the re-insertion studies of LEPR into Lepr-null mice is that the restricted expression of Lepr to a specific area throughout development might force the action of leptin on this area, and might not be a faithful representation of the existing neurocircuitry in wild-type animals. Nonetheless, the PMV receives projections from several brain areas, including the ARC and dorsomedial hypothalamus nuclei, which hold considerable numbers of GABAergic neurons160. These inputs suggest that the PMV is profusely innervated by neurons from GABAergic areas. It is therefore tempting to speculate that GABAergic LEPR-positive neurons that project to the PMV could contribute to the regulation of the reproductive role of leptin (for example, AgRP neurons). Indeed, as ARC neurons project to the PMV160, and the selective re-insertion of LEPR into AgRP neurons of Lepr-null mice faithfully replicates the re-insertion of LEPR into the PMV161, we can further speculate that in the PMV studies, AgRP and/or other GABAergic LEPR-positive neurons that project to the PMV might have contributed to the observed phenotype.
These hypotheses remain to be experimentally tested; however, if confirmed they would reconcile the ABAergic versus PMV-mediated (glutamatergic) pathways of leptin action in reproduction. Of note, this contention would not preclude a role for glutamatergic PMV LEPR-expressing neurons in the control of reproduction, although this effect would be subtle and involved in the fine-tuning of the responses of the reproductive axis to leptin. In this context, in 2018, we reported that pituitary adenylate cyclase-activating peptide neurons from the PMV, which express LEPR, directly contact both populations of kisspeptin neurons and are essential for the proper timing of puberty onset and the acquisition of full reproductive capabilities in females, probably acting as mediators of leptin’s action on kisspeptin neurons162.
In addition to leptin, several other peripheral factors transmit essential information about the energy status of the organism to the hypothalamic centres regulating metabolism and/or reproduction, among which insulin has a critical role. Insulin resistance is often associated with hypogonadotropic hypogonadism163,164, however, the exact mechanism underlying this causative association is unknown. Insulin action in the brain is essential for gonadotropin release, as documented in brain-specific insulin receptor-deficient mice165. KisspeptinARC neurons express insulin receptor113, which suggests a likely direct effect of insulin on these neurons to transmit postprandial information on glucose levels to the HPG axis, an effect that could contribute to the onset of hypogonadotropic hypogonadism in patients with type 2 diabetes mellitus163. However, the specific deletion of insulin receptor from kisspeptin neurons does not affect fertility, nor does the deletion of both insulin receptor and LEPR from kisspeptin neurons166 — as important cross-activation of downstream pathways has been documented167. Nonetheless, kisspeptin neuron-specific insulin receptor-null mice exhibit a slight delay in puberty onset166. This finding suggests that while insulin might be involved in the maturation of the reproductive axis, it is not necessary for maintaining reproductive function in adulthood. Moreover, in studies using a rat model of streptozotocin-induced type 1 diabetes mellitus, in which the rats developed hypogonadotropic hypogonadism due to inhibited hypothalamic Kiss1 expression, exogenous insulin was unable to restore the activity of the HPG axis168. This finding indicates that insulin action on kisspeptin neurons is neither necessary nor sufficient for the activation of the reproductive axis.
The orexigenic gut hormone ghrelin has also been found to be a direct regulator of kisspeptin neurons. Ghrelin levels increase with fasting, acting as an additional hunger signal at the central level. Importantly, kisspeptinARC neurons express ghrelin receptor (GHSR) in direct proportion to circulating levels of oestradiol169 and exogenous ghrelin decreases levels of Kiss1 mRNA in rats170 — as expected for a hunger factor signalling insufficient energy reserves. Paradoxically, in the absence of sex steroids, which is typical of starvation states due to the subsequent hypogonadism, the expression of GHSR in kisspeptin neurons is reduced and limited to ~25% of normal levels. This observation suggests that either this subpopulation of kisspeptin neurons expressing GHSR is sufficient for ghrelin to directly inhibit the HPG axis, or that the inhibitory action of Kiss1 expression is indirect through the documented activation of GHSR in AgRP neurons171–173. Nonetheless, the global deletion of ghrelin in mice does not affect fertility174, suggesting that the role of ghrelin in reproduction is subtle and subject to compensation.
Regulation of energy balance
In this field, most of the attention has been paid to the metabolic regulation of fertility; however, the regulation of the metabolic function by reproductive cues has remained under-studied.
Kisspeptin neurons have emerged as an active player in the control of energy balance, based on several lines of evidence. First, central injections of kisspeptin reduces food intake in mice175, jerboa176 and rats177,178, suggesting that kisspeptin could act as an anorexigenic factor. Second, kisspeptin neurons co-express LEPR and insulin receptor, indicating that they are a target for metabolic signals166,179,180. Third, Kiss1r- null (Kiss1r KO) mice develop substantial obesity compared with controls181. Fourth, kisspeptin neurons receive direct inputs from AgRP and POMC neurons, suggesting that they could serve as intermediate neurons in the hunger and/or satiety pathways130,137. Fifth, kisspeptin neurons modulate the activity of POMC and AgRP neurons through kisspeptin136 and glutamate release in a process that is exacerbated in the presence of oestradiol182,183 (FIG. 3). Finally, silencing of kisspeptinARC neurons in female mice increases body weight184.
Interestingly, despite the anorexigenic action of kisspeptin found in these initial studies, further studies of kisspeptin administration in humans185, and those in Kiss1r KO181 and kisspeptinARC-silenced mice184, do not support a significant role of kisspeptin in food intake. Moreover, the metabolic impairments observed in Kiss1r KO mice are restored in a mouse model of selective reinsertion of Kiss1r only in GnRH neurons186. This manipulation prevented any changes in body weight during adulthood but did not prevent the body weight changes observed in prepubertal and young adult Kiss1r KO mice. This finding indicates that a large component of the metabolic phenotype observed in adult Kiss1r KO mice is sex steroid-dependent186. Furthermore, the optogenetic activation of kisspeptinARC neurons in genetic mouse models is unable to reduce food intake over a short period of time, despite the demonstrated ability to project to and stimulate neurons in the paraventricular hypothalamic (PVH) nucleus187, a key nucleus in the control of food intake.
Overall, these studies suggest that the contribution of kisspeptin neurons to energy balance must be mediated by the regulation of energy expenditure rather than food intake, and that this role must be carried out by kisspeptinARC neurons (but not kisspeptinAVPV/PeN neurons) because, first, the population of kisspeptinAVPV/PeN neurons in the male is vestigial and, second, silencing kisspeptinARC neurons in female mice increases body weight without changing overall food intake184. These studies of kisspeptinARC neuron silencing uncovered a previously unknown role of kisspeptinARC neurons, as mediators of the circadian control of feeding behaviour. Thus, the loss of kisspeptinARC neurons in female mice leads to the loss of the predominantly nocturnal pattern of feeding behaviour, leading to the consumption of similar amounts of food during the dark and light phases184.
A wealth of studies have shown that the circadian rhythm of feeding behaviour is essential to maintaining proper body weight and that the elimination of this cycle induces obesity in the face of equal calories consumed188. Whether the origin of the circadian pattern of feeding behaviour stems from the oscillations in the central circadian clock, that is, the suprachiasmatic nucleus (SCN), from intermediate neurons or from kisspeptin neurons themselves remains to be deciphered. Of note, the circadian genes Clock, Per1 and Per2 have been found to be expressed in kisspeptinARC neurons113, suggesting that an intrinsic oscillator within these neurons is possible, which could be connected to the regulation of a master oscillator upstream, that is SCN. In this context, kisspeptinARC neurons do not appear to exert any regulatory effect on the SCN, although interactions at the level of SCN projections are possible184.
A critical and unresolved question in the understanding of the role of kisspeptinARC neurons in the control of energy balance relates to the nature of the player carrying the main burden of this metabolic action. In this regard, although Kiss1r KO mice develop obesity181, the increase in body weight observed after acute silencing of kisspeptinARC neurons184 is substantially faster and larger than in Kiss1r KO mice. This finding suggests that additional factors, besides kisspeptin, must be at play in this metabolic role. The main candidate to have this role is glutamate, as glutamate from kisspeptinARC neurons directly modifies the activity of POMC and AgRP neurons182,183 (FIG. 3). However, because the overall food intake is largely unaffected after the elimination of kisspeptinARC neurons, we can infer that the role of kisspeptin neurons through glutamate and kisspeptin release is not related to the promotion of satiety signals but to the modulation of the neuroendocrine circuits that control energy expenditure. In this context, projections from kisspeptinARC neurons to hypothalamic centres involved in energy expenditure have been described, including the PVH nucleus, the bed nucleus of the stria terminalis and the lateral hypothalamus61,189. Whether kisspeptinARC neurons also project to extra-hypothalamic areas involved in energy expenditure, such as the lateral parabrachial nucleus (LPBN) and the nucleus of the solitary tract, remains to be addressed (FIG. 3).
An additional factor in the participation of kisspeptinARC neurons in this metabolic process is neurokinin B, through the binding to its putative receptor NK3R in the medial preoptic area190. NK3R-expressing neurons in this area form a so-called thermoregulatory centre. KisspeptinARC neurons directly contact these neurons190 and through the release of neurokinin B they can statistically significantly alter the temperature of the organism, causing vasomotor symptoms characterized by an increase in body temperature followed by adaptive vasodilatation of peripheral capillary veins to dissipate heat190–192. It is tempting to speculate that individuals with affected thermoregulatory capabilities, that is, the inability to activate brown adipose tissue or muscle shivering to induce heat, will burn fewer calories than their unaffected counterparts, further contributing to the increase in body weight observed overtime after kisspeptinARC neuron silencing.
An additional question to improve our understanding of the role of kisspeptin neurons as metabolic players relates to whether they are first order responders to key metabolic cues (such as leptin and insulin) or whether they serve as intermediate effectors within larger neuronal networks that control energy balance. In this vein, studies of selective deletion of LEPR or insulin receptor from kisspeptin neurons have clearly shown the absence of any metabolic phenotype, such as increased body weight or development of insulin resistance166. These findings therefore suggest that kisspeptin neurons are intermediate metabolic players rather than first- order responders. Nonetheless, it is possible that the role of kisspeptin neurons is to serve as a bypass or reinforcing mechanism for the reproductive axis to regulate metabolic function in specific conditions, such as during pregnancy. In this context, prolactin might be an additional regulatory layer on kisspeptin neurons. The majority of kisspeptin neurons express prolactin receptor193. Importantly, prolactin is a well-known orexigenic factor194 during pregnancy, through a mechanism that does not involve AgRP neurons195, suggesting that kisspeptin neurons might contribute to the metabolic actions of prolactin during pregnancy.
Finally, kisspeptin neurons are essential in the photoperiod-dependent activation of the HPG axis in mammalian seasonal breeders196. Most seasonal breeders also display concurrent changes in food intake, which frequently develop in opposite directions, such as a decrease in food intake during the breeding season. For instance, in sheep, short days during autumn and winter stimulate reproductive function196, which coincides with lower voluntary food intake197,198. Whether this correlation is causative of the activation of kisspeptin neurons, leading to the synchronous control of reproductive function and metabolic changes, remains to be deciphered.
Conclusions
Kisspeptin expression, synthesis and release are tightly regulated by metabolic cues at multiple levels. As described in this Review, kisspeptin neurons are the main conveyor of the current metabolic status of the organism, by transmitting real-time information on circulating nutrients and stored energetic reserves to adapt reproductive capabilities. This process ensures that reproduction only happens in situations of energy surplus, mostly in female mammals, as the population that bears the major energetic burden. However, epigenetic modifications induced by metabolic alterations, such as long periods of low food intake or obesity, can have long-lasting effects in the expression of the Kiss1 gene. These changes can be passed on from previous generations, induced during early developmental stages or be derived from late- onset metabolic alterations.
No specific studies demonstrating the ability of metabolic factors to alter the Kiss1 gene have been performed yet; however, the presence of methylation sites in the Kiss1 gene and of enzymes involved in the methylation and acetylation states of DNA and chromatin in kisspeptin neurons, as well as the well-documented epigenetic effect that metabolic alterations have in a large array of studied genetic targets, indicates the existence of such interactions in kisspeptin neurons. Indeed, the expected changes derived from epigenetic alterations of the Kiss1 gene, such as changes in the timing of puberty onset, in mouse models where the metabolic insults are no longer present, serves as a proof of persistent metabolic alterations that affect the Kiss1 gene and/or upstream regulators.
An important system in the metabolic regulation of reproduction is the one formed by the intricate network of neuroendocrine circuits, which ultimately regulates the activity of kisspeptin neurons. Although evidence shows that AgRP and POMC neurons are critical components of this network by acting directly upstream of kisspeptin neurons, we cannot exclude additional hypothalamic centres (for example, the ventromedial nucleus) and extra-hypothalamic areas such as the LPBN or the amygdala. These areas target hypothalamic metabolic centres; however, the direct interaction with kisspeptin neurons has not been demonstrated. However, they might indirectly affect kisspeptin output through the action on other neurons, that is, AgRP and/or POMC neurons.
It is important to note that although peripheral signals transmit fundamental metabolic information to achieve successful reproduction, kisspeptin neurons do not seem to be first-order responders for the main metabolic cues, that is, leptin, insulin and ghrelin. Rather, kisspeptin neurons rely on as-yet-incompletely understood upstream neuroendocrine regulatory networks to respond to peripheral metabolic factors. Deciphering this neurocircuitry is a matter of intense research in the field.
Finally, a subject of increasing interest is the active metabolic role of kisspeptin neurons in energy balance. Some discrepancy exists between the different research models, with some presenting evidence of an anorexigenic role of kisspeptin and others indicating a predominant role in energy expenditure; however, what is clear is that kisspeptin neurons can ultimately affect energy homeostasis. Whether these discrepancies are due to the animal models or depend on specific physiological conditions of the individual remain to be deciphered. For the first time, however, a pivotal player in reproductive function has been described that influences the energetic status. This knowledge is important, given that a number of situations occur where not all the metabolic changes can be attributed to changes in the circulating levels of sex steroids, for example seasonal breeders, pregnant females or postmenopausal women.
Overall, the metabolic regulation of reproductive function is an extremely complex phenomenon composed of multiple regulatory levels, from subcellular to extracellular, as described in this Review. Unfortunately, this complexity prevents the complete understanding of the mechanism (or mechanisms) of action of metabolic cues that ensure successful reproduction. Moreover, it poses a limitation in determining which regulatory level among the ones described here has the most critical role in the control of kisspeptin neurons. Nonetheless, with time, as the neuroscience field evolves and new, more potent and precise techniques are developed, some of these questions will find an answer.
Key points.
Metabolic factors can modulate the development and function of kisspeptin neurons at multiple developmental stages.
These metabolic changes induced on kisspeptin neurons can be transient (for example, depending on the existing energetic reserves) or permanent (for example, epigenetic modifications).
Kisspeptin neuron activity can be regulated by metabolic factors at subcellular, neuroendocrine and endocrine levels, which enables kisspeptin neurons to directly adapt to circulating metabolites and to the overall energetic state.
Kisspeptin neurons serve as the main conveyor of metabolic cues to control the reproductive axis, thus determining the timing of puberty onset and reproductive success.
Controversy exists regarding the role of kisspeptin neurons on food intake; however, mounting data suggest a predominant role of kisspeptin neurons in energy expenditure, potentially mediated by kisspeptin and/or its co- transmitters, such as glutamate.
Acknowledgements
The author acknowledges the support of NIH/NICHD R01HD090151 and R21HD095383.
Footnotes
Competing interests
The author declares no competing interests.
Peer review information
Nature Reviews Endocrinology thanks A. Herbison, M. Lehman and O. Ronnekleiv for their contribution to the peer review of this work.
Publisher’s note
Publisher's Disclaimer: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1. Manfredi-Lozano M, Roa J & Tena-Sempere M Connecting metabolism and gonadal function: novel central neuropeptide pathways involved in the metabolic control of puberty and fertility. Front. Neuroendocrinol. 48, 37–49 (2018). This study is important in demonstrating the interaction between the melanocortin system and the reproductive axis, indicating that the reproductive action of melanocortins is kisspeptin-dependent.
- 2.Navarro VM & Tena-Sempere M Neuroendocrine control by kisspeptins: role in metabolic regulation of fertility. Nat. Rev. Endocrinol. 8, 40–53 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Goodman RL et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 148, 5752–5760 (2007). [DOI] [PubMed] [Google Scholar]
- 4. Navarro VM et al. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J. Neurosci. 29, 11859–11866 (2009). The first study demonstrating the concept of kisspeptinARC neurons as the GnRH pulse generator.
- 5. Clarkson J et al. Definition of the hypothalamic GnRH pulse generator in mice. Proc. Natl Acad. Sci. USA 114, E10216–E10223 (2017). This study demonstrates through a series of optogenetic approaches that kisspeptinARC neurons are indeed the GnRH pulse generator.
- 6.Zhang C, Bosch MA, Qiu J, Ronnekleiv OK & Kelly MJ 17β-Estradiol increases persistent Na(+) current and excitability of AVPV/PeN Kiss1 neurons in female mice. Mol. Endocrinol. 29, 518–527 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silveira LG et al. Mutations of the KISS1 gene in disorders of puberty. J. Clin. Endocrinol. Metab. 95, 2276–2280 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Roux N et al. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc. Natl Acad. Sci. USA 100, 10972–10976 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seminara SB et al. The GPR54 gene as a regulator of puberty. N. Engl. J. Med. 349, 1614–1627 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Teles MG et al. A GPR54-activating mutation in a patient with central precocious puberty. N. Engl. J. Med. 358, 709–715 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navarro VM & Kaiser UB Metabolic influences on neuroendocrine regulation of reproduction. Curr. Opin. Endocrinol. Diabetes Obes. 20, 335–341 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manfredi-Lozano M et al. Defining a novel leptin-melanocortin-kisspeptin pathway involved in the metabolic control of puberty. Mol. Metab. 5, 844–857 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shalitin S & Kiess W Putative effects of obesity on linear growth and puberty. Horm. Res. Paediatr. 88, 101–110 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Calderon B et al. Prevalence of male secondary hypogonadism in moderate to severe obesity and its relationship with insulin resistance and excess body weight. Andrology 4, 62–67 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Lamm S, Chidakel A & Bansal R Obesity and hypogonadism. Urol. Clin. North. Am. 43, 239–245 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Ahmed ML, Ong KK & Dunger DB Childhood obesity and the timing of puberty. Trends Endocrinol. Metab. 20, 237–242 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Li W et al. Association between obesity and puberty timing: a systematic review and meta- analysis. Int. J. Env. Res. Public. Health 14, E1266 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandhu J, Ben-Shlomo Y, Cole TJ, Holly J & Davey Smith G The impact of childhood body mass index on timing of puberty, adult stature and obesity: a follow-up study based on adolescent anthropometry recorded at Christ’s Hospital (1936–1964). Int. J. Obes. 30, 14–22 (2006). [DOI] [PubMed] [Google Scholar]
- 19.He Y, Tian J, Oddy WH, Dwyer T & Venn AJ Association of childhood obesity with female infertility in adulthood: a 25-year follow-up study. Fertil. Steril. 110, 596–604.e1 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Vilmann LS, Thisted E, Baker JL & Holm JC Development of obesity and polycystic ovary syndrome in adolescents. Horm. Res. Paediatr. 78, 269–278 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Brunton PJ & Russell JA Endocrine induced changes in brain function during pregnancy. Brain Res. 1364, 198–215 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Eckel LA The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiol. Behav. 104, 517–524 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butera PC Estradiol and the control of food intake. Physiol. Behav. 99, 175–180 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rivera HM & Stincic TL Estradiol and the control of feeding behavior. Steroids 133, 44–52 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allan CA & McLachlan RI Androgens and obesity. Curr. Opin. Endocrinol. Diabetes Obes. 17, 224–232 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Brand JS, van der Tweel I, Grobbee DE, Emmelot-Vonk MH & van der Schouw YT Testosterone, sex hormone- binding globulin and the metabolic syndrome: a systematic review and meta-analysis of observational studies. Int. J. Epidemiol. 40, 189–207 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Aiken CE & Ozanne SE Transgenerational developmental programming. Hum. Reprod. Update 20, 63–75 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Vickers MH Developmental programming and transgenerational transmission of obesity. Ann. Nutr. Metab. 64 (Suppl. 1), 26–34 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Zambrano E The transgenerational mechanisms in developmental programming of metabolic diseases. Rev. Invest. Clin. 61, 41–52 (2009). [PubMed] [Google Scholar]
- 30.Baptissart M et al. Multigenerational impacts of bile exposure are mediated by TGR5 signaling pathways. Sci. Rep. 8, 16875 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huypens P et al. Epigenetic germline inheritance of diet-induced obesity and insulin resistance. Nat. Genet. 48, 497–499 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Miranda A & Sousa N Maternal hormonal milieu influence on fetal brain development. Brain Behav. 8, e00920 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moog NK et al. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience 342, 68–100 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeltser LM & Leibel RL Roles of the placenta in fetal brain development. Proc. Natl Acad. Sci. USA 108, 15667–15668 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciofi P The arcuate nucleus as a circumventricular organ in the mouse. Neurosci. Lett. 487, 187–190 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Ciofi P et al. Brain-endocrine interactions: a microvascular route in the mediobasal hypothalamus. Endocrinology 150, 5509–5519 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norsted E, Gomuc B & Meister B Protein components of the blood-brain barrier (BBB) in the mediobasal hypothalamus. J. Chem. Neuroanat. 36, 107–121 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Li C, McDonald TJ, Wu G, Nijland MJ & Nathanielsz PW Intrauterine growth restriction alters term fetal baboon hypothalamic appetitive peptide balance. J. Endocrinol. 217, 275–282 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plagemann A et al. Observations on the orexigenic hypothalamic neuropeptide Y-system in neonatally overfed weanling rats. J. Neuroendocrinol. 11, 541–546 (1999). [DOI] [PubMed] [Google Scholar]
- 40.Vogt MC et al. Neonatal insulin action impairs hypothalamic neurocircuit formation in response to maternal high-fat feeding. Cell 156, 495–509 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar D, Periasamy V, Freese M, Voigt A & Boehm U In utero development of kisspeptin/GnRH neural circuitry in male mice. Endocrinology 156, 3084–3090 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Kumar D et al. Murine arcuate nucleus kisspeptin neurons communicate with GnRH neurons in utero. J. Neurosci. 34, 3756–3766 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clarkson J & Herbison AE Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147, 5817–5825 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yip SH, Boehm U, Herbison AE & Campbell RE Conditional viral tract tracing delineates the projections of the distinct kisspeptin neuron populations to gonadotropin- releasing hormone (GnRH) neurons in the mouse. Endocrinology 156, 2582–2594 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Semaan SJ et al. BAX- dependent and BAX-independent regulation of Kiss1 neuron development in mice. Endocrinology 151, 5807–5817 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Castellano JM et al. Early metabolic programming of puberty onset: impact of changes in postnatal feeding and rearing conditions on the timing of puberty and development of the hypothalamic kisspeptin system. Endocrinology 152, 3396–3408 (2011). This study is important in demonstrating the effect of perinatal metabolic alterations in the function of kisspeptin neurons in adulthood, probably due to epigenetic changes.
- 47.Day FR, Perry JR & Ong KK Genetic regulation of puberty timing in humans. Neuroendocrinology 102, 247–255 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ojeda SR, Lomniczi A, Sandau U & Matagne V New concepts on the control of the onset of puberty. Endocr. Dev. 17, 44–51 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Funes S et al. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem. Biophys. Res. Commun. 312, 1357–1363 (2003). [DOI] [PubMed] [Google Scholar]
- 50.Bentsen AH et al. Maturation of kisspeptinergic neurons coincides with puberty onset in male rats. Peptides 31, 275–283 (2010). [DOI] [PubMed] [Google Scholar]
- 51.Castellano JM et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology 146, 3917–3925 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Gill JC et al. Reproductive hormone-dependent and -independent contributions to developmental changes in kisspeptin in GnRH-deficient hypogonadal mice. PLoS One 5, e11911 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Navarro VM et al. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology 145, 4565–4574 (2004). [DOI] [PubMed] [Google Scholar]
- 54.Navarro VM Interactions between kisspeptins and neurokinin B. Adv. Exp. Med. Biol. 784, 325–347 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Navarro VM et al. Role of neurokinin B in the control of female puberty and its modulation by metabolic status. J. Neurosci. 32, 2388–2397 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruiz- Pino F et al. Neurokinin B and the control of the gonadotropic axis in the rat: developmental changes, sexual dimorphism, and regulation by gonadal steroids. Endocrinology 153, 4818–4829 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gordon CM et al. Functional hypothalamic amenorrhea: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 102, 1413–1439 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Abbara A et al. A second dose of kisspeptin-54 improves oocyte maturation in women at high risk of ovarian hyperstimulation syndrome: a phase 2 randomized controlled trial. Hum. Reprod. 32, 1915–1924 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hrabovszky E et al. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur. J. Neurosci. 31, 1984–1998 (2010). [DOI] [PubMed] [Google Scholar]
- 60.Matsuyama S et al. Morphological evidence for direct interaction between kisspeptin and gonadotropin-releasing hormone neurons at the median eminence of the male goat: an immunoelectron microscopic study. Neuroendocrinology 94, 323–332 (2011). [DOI] [PubMed] [Google Scholar]
- 61.Yeo SH et al. Visualisation of Kiss1 neurone distribution using a Kiss1-CRE transgenic mouse. J. Neuroendocrinol. 10.1111/jne.12435 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hardie DG, Ross FA & Hawley SA AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 13, 251–262 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Egan DF et al. Phosphorylation of ULK1 (hATG1) by AMP- activated protein kinase connects energy sensing to mitophagy. Science 331, 456–461 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Obri A & Claret M The role of epigenetics in hypothalamic energy balance control: implications for obesity. Cell Stress 3, 208–220 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Roa J et al. Metabolic regulation of female puberty via hypothalamic AMPK-kisspeptin signaling. Proc. Natl Acad. Sci. USA 115, E10758–E10767 (2018). This study demonstrates the role of AMPK in kisspeptin neurons as an essential regulatory pathway in the release of kisspeptin.
- 66.Torsoni MA et al. AMPKalpha2 in Kiss1 neurons is required for reproductive adaptations to acute metabolic challenges in adult female mice. Endocrinology 157, 4803–4816 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roland AV & Moenter SM Glucosensing by GnRH neurons: inhibition by androgens and involvement of AMP-activated protein kinase. Mol. Endocrinol. 25, 847–858 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andrade J, Quinn J, Becker RZ & Shupnik MA AMP- activated protein kinase is a key intermediary in GnRH- stimulated LHβ gene transcription. Mol. Endocrinol. 27, 828–839 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brown EJ et al. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature 369, 756–758 (1994). [DOI] [PubMed] [Google Scholar]
- 70.Khaleghpour K, Pyronnet S, Gingras AC & Sonenberg N Translational homeostasis: eukaryotic translation initiation factor 4E control of 4E-binding protein 1 and p70 S6 kinase activities. Mol. Cell Biol. 19, 4302–4310 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Inoki K, Kim J & Guan KL AMPK and mTOR in cellular energy homeostasis and drug targets. Annu. Rev. Pharmacol. Toxicol. 52, 381–400 (2012). [DOI] [PubMed] [Google Scholar]
- 72.Jing K et al. Docosahexaenoic acid induces autophagy through p53/AMPK/mTOR signaling and promotes apoptosis in human cancer cells harboring wild- type p53. Autophagy 7, 1348–1358 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Egan D, Kim J, Shaw RJ & Guan KL The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy 7, 643–644 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim J, Kundu M, Viollet B & Guan KL AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13, 132–141 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Roa J et al. The mammalian target of rapamycin as novel central regulator of puberty onset via modulation of hypothalamic Kiss1 system. Endocrinology 150, 5016–5026 (2009). This study demonstrates the role of mTOR in kisspeptin neurons of rodents in the context of reproduction.
- 76.Edwards BS, Isom WJ & Navratil AM Gonadotropin releasing hormone activation of the mTORC2/Rictor complex regulates actin remodeling and ERK activity in LβT2 cells. Mol. Cell. Endocrinol. 439, 346–353 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fiedler EC & Shaw RJ AMPK regulates the epigenome through phosphorylation of TET2. Cell Metab. 28, 534–536 (2018). [DOI] [PubMed] [Google Scholar]
- 78.Wu D et al. Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature 559, 637–641 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang T et al. Phosphorylation of TET2 by AMPK is indispensable in myogenic differentiation. Epigenetics Chromatin 12, 32 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arora P Obesity genetics and epigenetics: dissecting causality. Circ. Cardiovasc. Genet. 7, 395–396 (2014). [DOI] [PubMed] [Google Scholar]
- 81.Herrera BM, Keildson S & Lindgren CM Genetics and epigenetics of obesity. Maturitas 69, 41–49 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Horsthemke B A critical view on transgenerational epigenetic inheritance in humans. Nat. Commun. 9, 2973 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pigeyre M, Yazdi FT, Kaur Y & Meyre D Recent progress in genetics, epigenetics and metagenomics unveils the pathophysiology of human obesity. Clin. Sci. 130, 943–986 (2016). [DOI] [PubMed] [Google Scholar]
- 84.Rohde K et al. Genetics and epigenetics in obesity. Metabolism 92, 37–50 (2019). [DOI] [PubMed] [Google Scholar]
- 85.Sun X et al. From genetics and epigenetics to the future of precision treatment for obesity. Gastroenterol. Rep. 5, 266–270 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu Z, Cao F & Li X Epigenetic programming and fetal metabolic programming. Front. Endocrinol. 10, 764 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nugent BM & Bale TL The omniscient placenta: metabolic and epigenetic regulation of fetal programming. Front. Neuroendocrinol. 39, 28–37 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sookoian S, Gianotti TF, Burgueno AL & Pirola CJ Fetal metabolic programming and epigenetic modifications: a systems biology approach. Pediatr. Res. 73, 531–542 (2013). [DOI] [PubMed] [Google Scholar]
- 89.Heerwagen MJ, Miller MR, Barbour LA & Friedman JE Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299, R711–R722 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li Y Epigenetic mechanisms link maternal diets and gut microbiome to obesity in the offspring. Front. Genet. 9, 342 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Candler T, Kuhnen P, Prentice AM & Silver M Epigenetic regulation of POMC; implications for nutritional programming, obesity and metabolic disease. Front. Neuroendocrinol. 54, 100773 (2019). [DOI] [PubMed] [Google Scholar]
- 92.Abreu AP & Kaiser UB Pubertal development and regulation. Lancet Diabetes Endocrinol. 4, 254–264 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jaenisch R & Bird A Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 33, 245–254 (2003). [DOI] [PubMed] [Google Scholar]
- 94.Loh M, Zhou L, Ng HK & Chambers JC Epigenetic disturbances in obesity and diabetes: epidemiological and functional insights. Mol. Metab. 27S, S33–S41 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Semaan SJ, Dhamija S, Kim J, Ku EC & Kauffman AS Assessment of epigenetic contributions to sexually-dimorphic Kiss1 expression in the anteroventral periventricular nucleus of mice. Endocrinology 153, 1875–1886 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wyatt AK et al. Changes in methylation patterns of kiss1 and kiss1r gene promoters across puberty. Genet. Epigenet 5, 51–62 (2013). This study demonstrates the epigenetic modifications in the kisspeptin system that determine the timing of puberty onset.
- 97.Luo L et al. Identification of differential genomic DNA methylation in the hypothalamus of pubertal rat using reduced representation bisulfite sequencing. Reprod. Biol. Endocrinol. 15, 81 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Uenoyama Y et al. Molecular and epigenetic mechanism regulating hypothalamic Kiss1 gene expression in mammals. Neuroendocrinology 103, 640–649 (2016). [DOI] [PubMed] [Google Scholar]
- 99.He YF et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science 333, 1303–1307 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hu L et al. Structural insight into substrate preference for TET-mediated oxidation. Nature 527, 118–122 (2015). [DOI] [PubMed] [Google Scholar]
- 101.Ito S et al. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science 333, 1300–1303 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kurian JR et al. The methylcytosine dioxygenase ten-eleven translocase-2 (tet2) enables elevated GnRH gene expression and maintenance of male reproductive function. Endocrinology 157, 3588–3603 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jenuwein T & Allis CD Translating the histone code. Science 293, 1074–1080 (2001). [DOI] [PubMed] [Google Scholar]
- 104.Gao T, Diaz- Hirashi Z & Verdeguer F Metabolic signaling into chromatin modifications in the regulation of gene expression. Int. J. Mol. Sci. 19, E4108 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mikula M, Majewska A, Ledwon JK, Dzwonek A & Ostrowski J Obesity increases histone H3 lysine 9 and 18 acetylation at Tnfa and Ccl2 genes in mouse liver. Int. J. Mol. Med. 34, 1647–1654 (2014). [DOI] [PubMed] [Google Scholar]
- 106.Tomikawa J et al. Epigenetic regulation of Kiss1 gene expression mediating estrogen-positive feedback action in the mouse brain. Proc. Natl Acad. Sci. USA 109, E1294–E1301 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rickert E et al. Neuronal SIRT1 regulates metabolic and reproductive function and the response to caloric restriction. J. Endocr. Soc. 3, 427–445 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Vazquez MJ et al. SIRT1 mediates obesity- and nutrient-dependent perturbation of pubertal timing by epigenetically controlling Kiss1 expression. Nat. Commun. 9, 4194 (2018). This study is important in demonstrating the action of SIRT1 in the epigenetic regulation of kisspeptin neurons.
- 109.Choi I, Rickert E, Fernandez M & Webster NJG SIRT1 in astrocytes regulates glucose metabolism and reproductive function. Endocrinology 160, 1547–1560 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Duteil D et al. Lsd1 ablation triggers metabolic reprogramming of brown adipose tissue. Cell Rep. 17, 1008–1021 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Duteil D, Tosic M & Schule R Lsd1, a metabolic sensor of environment requirements that prevents adipose tissue from aging. Adipocyte 6, 298–303 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Duteil D et al. Lsd1 prevents age-programed loss of beige adipocytes. Proc. Natl Acad. Sci. USA 114, 5265–5270 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Campbell JN et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat. Neurosci. 20, 484–496 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ahmed K et al. Loss of microRNA-7a2 induces hypogonadotropic hypogonadism and infertility. J. Clin. Invest. 127, 1061–1074 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Herzer S, Silahtaroglu A & Meister B Locked nucleic acid-based in situ hybridisation reveals miR-7a as a hypothalamus-enriched microRNA with a distinct expression pattern. J. Neuroendocrinol. 24, 1492–1504 (2012). [DOI] [PubMed] [Google Scholar]
- 116.Fang Y, Xue JL, Shen Q, Chen J & Tian L MicroRNA-7 inhibits tumor growth and metastasis by targeting the phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma. Hepatology 55, 1852–1862 (2012). [DOI] [PubMed] [Google Scholar]
- 117. Sangiao-Alvarellos S, Pena-Bello L, Manfredi-Lozano M, Tena-Sempere M & Cordido F Perturbation of hypothalamic microRNA expression patterns in male rats after metabolic distress: impact of obesity and conditions of negative energy balance. Endocrinology 155, 1838–1850 (2014). This study demonstrates the critical role of miRNAs in the control of metabolic function.
- 118.Corre C et al. Sex-specific regulation of weight and puberty by the Lin28/let-7 axis. J. Endocrinol. 228, 179–191 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sangiao-Alvarellos S et al. Changes in hypothalamic expression of the Lin28/let-7 system and related microRNAs during postnatal maturation and after experimental manipulations of puberty. Endocrinology 154, 942–955 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhu H et al. Lin28a transgenic mice manifest size and puberty phenotypes identified in human genetic association studies. Nat. Genet. 42, 626–630 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Heras V et al. Hypothalamic miR-30 regulates puberty onset via repression of the puberty-suppressing factor, Mkrn3. PLoS Biol. 17, e3000532 (2019). This study provides important information on the role of a newly describes miRNA (miR-30) in the control of Kiss1 expression via Mkrn3.
- 122.Abreu AP et al. Central precocious puberty caused by mutations in the imprinted gene MKRN3. N. Engl. J. Med. 368, 2467–2475 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhan C et al. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J. Neurosci. 33, 3624–3632 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lowell BB New neuroscience of homeostasis and drives for food, water, and salt. N. Engl. J. Med. 380, 459–471 (2019). [DOI] [PubMed] [Google Scholar]
- 125.Dicken MS, Tooker RE & Hentges ST Regulation of GABA and glutamate release from proopiomelanocortin neuron terminals in intact hypothalamic networks. J. Neurosci. 32, 4042–4048 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stincic TL, Grachev P, Bosch MA, Ronnekleiv OK & Kelly MJ Estradiol drives the anorexigenic activity of proopiomelanocortin neurons in female mice. eNeuro 5, ENEURO.0103–18.2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xu Y et al. Glutamate mediates the function of melanocortin receptor 4 on Sim1 neurons in body weight regulation. Cell Metab. 18, 860–870 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Millington GW The role of proopiomelanocortin (POMC) neurones in feeding behaviour. Nutr. Metab. 4, 18 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Garfield AS et al. A neural basis for melanocortin-4 receptor-regulated appetite. Nat. Neurosci. 18, 863–871 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Padilla SL et al. AgRP to Kiss1 neuron signaling links nutritional state and fertility. Proc. Natl Acad. Sci. USA 114, 2413–2418 (2017). This study demostrates that AgRP neurons contact both populations of kisspeptin neurons directly and regulate their activity through AgRP and GABA release.
- 131.Wu Q, Whiddon BB & Palmiter RD Ablation of neurons expressing agouti-related protein, but not melanin concentrating hormone, in leptin-deficient mice restores metabolic functions and fertility. Proc. Natl Acad. Sci. USA 109, 3155–3160 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sandrock M et al. Reduction in corpora lutea number in obese melanocortin-4-receptor-deficient mice. Reprod. Biol. Endocrinol. 7, 24 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lehman MN, Ebling FJ, Moenter SM & Karsch FJ Distribution of estrogen receptor-immunoreactive cells in the sheep brain. Endocrinology 133, 876–886 (1993). [DOI] [PubMed] [Google Scholar]
- 134.de Souza FSJ et al. The estrogen receptor a colocalizes with proopiomelanocortin in hypothalamic neurons and binds to a conserved motif present in the neuron-specific enhancer nPE2. Eur. J. Pharmacol. 660, 181–187 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xu Y et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 14, 453–465 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fu LY & van den Pol AN Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J. Neurosci. 30, 10205–10219 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cravo RM et al. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience 173, 37–56 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Israel DD et al. Effects of leptin and melanocortin signaling interactions on pubertal development and reproduction. Endocrinology 153, 2408–2419 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lau J & Herzog H CART in the regulation of appetite and energy homeostasis. Front. Neurosci. 8, 313 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.True C, Verma S, Grove KL & Smith MS Cocaine- and amphetamine-regulated transcript is a potent stimulator of GnRH and kisspeptin cells and may contribute to negative energy balance-induced reproductive inhibition in females. Endocrinology 154, 2821–2832 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zandi MR et al. Hypothalamic expression of melanocortin-4 receptor and agouti-related peptide mRNAs during the estrous cycle of rats. Int. J. Mol. Cell Med. 3, 183–189 (2014). [PMC free article] [PubMed] [Google Scholar]
- 142.Bohler HC Jr., Tracer H, Merriam GR & Petersen SL Changes in proopiomelanocortin messenger ribonucleic acid levels in the rostral periarcuate region of the female rat during the estrous cycle. Endocrinology 128, 1265–1269 (1991). [DOI] [PubMed] [Google Scholar]
- 143.Ahima RS, Dushay J, Flier SN, Prabakaran D & Flier JS Leptin accelerates the onset of puberty in normal female mice. J. Clin. Invest. 99, 391–395 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Farooqi IS et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 341, 879–884 (1999). [DOI] [PubMed] [Google Scholar]
- 145.Mathew H, Castracane VD & Mantzoros C Adipose tissue and reproductive health. Metabolism 86, 18–32 (2018). [DOI] [PubMed] [Google Scholar]
- 146.Welt CK et al. Recombinant human leptin in women with hypothalamic amenorrhea. N. Engl. J. Med. 351, 987–997 (2004). [DOI] [PubMed] [Google Scholar]
- 147.Donato J Jr., Cravo RM, Frazao R & Elias CF Hypothalamic sites of leptin action linking metabolism and reproduction. Neuroendocrinology 93, 9–18 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Leshan RL, Bjornholm M, Munzberg H & Myers MG Jr. Leptin receptor signaling and action in the central nervous system. Obesity 14 (Suppl. 5), 208S–212S (2006). [DOI] [PubMed] [Google Scholar]
- 149.Chan JL & Mantzoros CS Leptin and the hypothalamic- pituitary regulation of the gonadotropin-gonadal axis. Pituitary 4, 87–92 (2001). [DOI] [PubMed] [Google Scholar]
- 150.Quennell JH et al. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology 150, 2805–2812 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Donato J Jr. et al. Leptin’s effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J. Clin. Invest. 121, 355–368 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zuure WA, Roberts AL, Quennell JH & Anderson GM Leptin signaling in GABA neurons, but not glutamate neurons, is required for reproductive function. J. Neurosci. 33, 17874–17883 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Martin C et al. Leptin-responsive GABAergic neurons regulate fertility through pathways that result in reduced kisspeptinergic tone. J. Neurosci. 34, 6047–6056 (2014). This study demonstrates that the action of leptin in regulating reproductive function occurs through GABAergic neurons in the mouse.
- 154.Vong L et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71, 142–154 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen H et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84, 491–495 (1996). [DOI] [PubMed] [Google Scholar]
- 156.Zhang Y et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994). [DOI] [PubMed] [Google Scholar]
- 157.Dehghani MR et al. Potential role of gender specific effect of leptin receptor deficiency in an extended consanguineous family with severe early-onset obesity. Eur. J. Med. Genet. 61, 465–467 (2018). [DOI] [PubMed] [Google Scholar]
- 158.Johnson LM & Sidman RL A reproductive endocrine profile in the diabetes (db) mutant mouse. Biol. Reprod. 20, 552–559 (1979). [DOI] [PubMed] [Google Scholar]
- 159.Xu Y et al. Glutamate release mediates leptin action on energy expenditure. Mol. Metab. 2, 109–115 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cavalcante JC, Bittencourt JC & Elias CF Distribution of the neuronal inputs to the ventral premammillary nucleus of male and female rats. Brain Res. 1582, 77–90 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Egan OK, Inglis MA & Anderson GM Leptin signaling in AgRP neurons modulates puberty onset and adult fertility in mice. J. Neurosci. 37, 3875–3886 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Ross RA et al. PACAP neurons in the ventral premammillary nucleus regulate reproductive function in the female mouse. eLife 7, e35960 (2018). This study demonstrates that neurons from additional hypothalamic nuclei (outside the arcuate and the preoptic area) might contact directly and regulate kisspeptin neurons, transmitting the information on the metabolic state via LEPR to the HPG axis.
- 163.Dandona P & Dhindsa S Update: hypogonadotropic hypogonadism in type 2 diabetes and obesity. J. Clin. Endocrinol. Metab. 96, 2643–2651 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gevi F, Fanelli G & Zolla L Metabolic patterns in insulin-resistant male hypogonadism. Cell Death Dis. 9, 671 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bruning JC et al. Role of brain insulin receptor in control of body weight and reproduction. Science 289, 2122–2125 (2000). [DOI] [PubMed] [Google Scholar]
- 166.Qiu X et al. Insulin and leptin signaling interact in the mouse Kiss1 neuron during the peripubertal period. PLoS One 10, e0121974 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Thon M, Hosoi T & Ozawa K Possible integrative actions of leptin and insulin signaling in the hypothalamus targeting energy homeostasis. Front. Endocrinol. 7, 138 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Castellano JM et al. Expression of hypothalamic KiSS-1 system and rescue of defective gonadotropic responses by kisspeptin in streptozotocin-induced diabetic male rats. Diabetes 55, 2602–2610 (2006). [DOI] [PubMed] [Google Scholar]
- 169.Frazao R et al. Estradiol modulates Kiss1 neuronal response to ghrelin. Am. J. Physiol. Endocrinol. Metab. 306, E606–E614 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Forbes S, Li XF, Kinsey-Jones J & O’Byrne K Effects of ghrelin on Kisspeptin mRNA expression in the hypothalamic medial preoptic area and pulsatile luteinising hormone secretion in the female rat. Neurosci. Lett. 460, 143–147 (2009). [DOI] [PubMed] [Google Scholar]
- 171.Chen SR et al. Ghrelin receptors mediate ghrelin-induced excitation of agouti-related protein/neuropeptide Y but not pro-opiomelanocortin neurons. J. Neurochem. 142, 512–520 (2017). [DOI] [PubMed] [Google Scholar]
- 172.Wang Q et al. Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Mol. Metab. 3, 64–72 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wu CS et al. Suppression of GHS-R in AgRP neurons mitigates diet-induced obesity by activating thermogenesis. Int. J. Mol. Sci. 18, E832 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sun Y, Ahmed S & Smith RG Deletion of ghrelin impairs neither growth nor appetite. Mol. Cell Biol. 23, 7973–7981 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Stengel A, Wang L, Goebel- Stengel M & Tache Y Centrally injected kisspeptin reduces food intake by increasing meal intervals in mice. Neuroreport 22, 253–257 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Talbi R, Laran-Chich MP, Magoul R, El Ouezzani S & Simonneaux V Kisspeptin and RFRP-3 differentially regulate food intake and metabolic neuropeptides in the female desert jerboa. Sci. Rep. 6, 36057 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Saito R et al. Centrally administered kisspeptin suppresses feeding via nesfatin-1 and oxytocin in male rats. Peptides 112, 114–124 (2019). [DOI] [PubMed] [Google Scholar]
- 178.Sahin Z, Canpolat S, Ozcan M, Ozgocer T & Kelestimur H Kisspeptin antagonist prevents RF9-induced reproductive changes in female rats. Reproduction 149, 465–473 (2015). [DOI] [PubMed] [Google Scholar]
- 179.Backholer K et al. Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology 151, 2233–2243 (2010). [DOI] [PubMed] [Google Scholar]
- 180.Smith JT, Acohido BV, Clifton DK & Steiner RA KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J. Neuroendocrinol. 18, 298–303 (2006). [DOI] [PubMed] [Google Scholar]
- 181. Tolson KP et al. Impaired kisspeptin signaling decreases metabolism and promotes glucose intolerance and obesity. J. Clin. Invest. 124, 3075–3079 (2014). This is the first study demonstrating a potentially active role of kisspeptin in the control of energy balance.
- 182. Nestor CC et al. Optogenetic stimulation of arcuate nucleus Kiss1 neurons reveals a steroid- dependent glutamatergic input to POMC and AgRP neurons in male mice. Mol. Endocrinol. 30, 630–644 (2016). This is an important study in demonstrating for the first time that kisspeptin neurons might regulate the activity of POMC and AgRP neurons through glutamate. This finding might have critical implications in the bidirectional regulation of energy balance and reproduction.
- 183.Qiu J et al. Estrogenic-dependent glutamatergic neurotransmission from kisspeptin neurons governs feeding circuits in females. eLife 7, e35656 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Padilla SL et al. Kisspeptin neurons in the arcuate nucleus of the hypothalamus orchestrate circadian rhythms and metabolism. Curr. Biol. 29, 592–604.e4 (2019). This is a seminal study that demonstrates the increase in body weight in mice after ablation of kisspeptinARC neurons and the disruption of the circadian feeding behaviour and activity, uncovering a novel role for kisspeptinARC neurons.
- 185.Izzi-Engbeaya C et al. The effects of kisspeptin on β-cell function, serum metabolites and appetite in humans. Diabetes Obes. Metab. 20, 2800–2810 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Velasco I et al. Gonadal hormone-dependent vs. -independent effects of kisspeptin signaling in the control of body weight and metabolic homeostasis. Metabolism 98, 84–94 (2019). This study is critical to understanding the part of the metabolic phenotype in Kiss1r KO mice that is due to the direct action of kisspeptin versus the absence of sex steroids (hypogonadism) induced by the absence of kisspeptin.
- 187.Fenselau H et al. A rapidly acting glutamatergic ARC→PVH satiety circuit postsynaptically regulated by α-MSH. Nat. Neurosci. 20, 42–51 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chaix A, Lin T, Le HD, Chang MW & Panda S Time-restricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock. Cell Metab. 29, 303–319.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yeo SH & Herbison AE Projections of arcuate nucleus and rostral periventricular kisspeptin neurons in the adult female mouse brain. Endocrinology 152, 2387–2399 (2011). [DOI] [PubMed] [Google Scholar]
- 190. Padilla SL, Johnson CW, Barker FD, Patterson MA & Palmiter RD A neural circuit underlying the generation of hot flushes. Cell Rep. 24, 271–277 (2018). This study demonstrates the role of kisspeptin neurons in thermorregulation through the projections to the medial preoptic area and the release of neurokinin B.
- 191. Krajewski- Hall SJ et al. Glutamatergic neurokinin 3 receptor neurons in the median preoptic nucleus modulate heat-defense pathways in female mice. Endocrinology 160, 803–816 (2019). This is an important study investigating the characteristics of the neurons in the preoptic area that receive direct contact from kisspeptinARC neurons in the context of thermoregulation.
- 192.Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, McMullen NT & Rance NE Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc. Natl Acad. Sci. USA 109, 19846–19851 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kokay IC, Petersen SL & Grattan DR Identification of prolactin-sensitive GABA and kisspeptin neurons in regions of the rat hypothalamus involved in the control of fertility. Endocrinology 152, 526–535 (2011). [DOI] [PubMed] [Google Scholar]
- 194.Gerardo-Gettens T, Moore BJ, Stern JS & Horwitz BA Prolactin stimulates food intake in a dose-dependent manner. Am. J. Physiol. 256, R276–R280 (1989). [DOI] [PubMed] [Google Scholar]
- 195.Ladyman SR et al. Prolactin receptors in Rip- cre cells, but not in AgRP neurones, are involved in energy homeostasis. J. Neuroendocrinol. 29, e12474 (2017). [DOI] [PubMed] [Google Scholar]
- 196.Weems PW, Goodman RL & Lehman MN Neural mechanisms controlling seasonal reproduction: principles derived from the sheep model and its comparison with hamsters. Front. Neuroendocrinol. 37, 43–51 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Clarke IJ Sex and season are major determinants of voluntary food intake in sheep. Reprod. Fertil. Dev. 13, 577–582 (2001). [DOI] [PubMed] [Google Scholar]
- 198.Iason G, Sim D, Foreman E, Fenn P & Elston D Seasonal variation of voluntary food intake and metabolic rate in three contrasting breeds of sheep. Anim. Prod. 58, 381–387 (1994). [Google Scholar]