Abstract
With the aging of the world’s population, a large proportion of patients seen in cardiovascular practice are older adults, but many patients also exhibit signs of physical frailty. Cardiovascular disease and frailty are interdependent and have the same physiologic underpinning that predispose to the progression of both disease processes. Frailty can be defined as a phenomenon of increased vulnerability to stressors due to decreased physiological reserves in older patients, and thus leads to poor clinical outcomes after cardiovascular insults. There are various pathophysiologic mechanisms for the development of frailty: cognitive decline, physical inactivity, poor nutrition, and lack of social supports; these risk factors provide opportunity for various types of interventions that aim to prevent, improve, or reverse the development of frailty syndrome in the context of cardiovascular disease. There is no compelling study demonstrating a successful intervention to improve a global measure of frailty. Emerging data from patients admitted with heart failure indicate that interventions associated with positive outcomes on frailty and physical function are multidimensional and include tailored cardiac rehabilitation. Contemporary cardiovascular practice should actively identify patients with physical frailty who could benefit from frailty interventions and aim to deliver these therapies in a patient-centered model to optimize quality of life particularly after cardiovascular interventions.
Keywords: Aging, Older Adult, Frailty, Cardiovascular Disease, Interventions
CONDENSED ABSTRACT
Frailty can be defined as a phenomenon of increased vulnerability to stressors due to decreased physiological reserves, which leads to worse clinical outcomes after cardiovascular insults. The various pathophysiological mechanisms for the development of frailty include cognitive decline, physical inactivity, poor nutrition, and lack of social supports. Multiple interventions for frailty have been studied that target these mechanisms and the ones that have demonstrated maximal benefit are multidimensional. Contemporary cardiovascular practice should actively identify patients with physical frailty who could benefit from frailty interventions and aim to deliver these therapies in a patient-centered care model to optimize quality of life particularly after cardiovascular interventions.
INTRODUCTION
In the United States, like the rest of the developed world, there is a rapidly growing older adult populations with adults 65 years or older composing 16.5% of the population in 2019.(1) This number is projected to increase to 20.3% by 2030 when all baby boomers reach 65 years of age. By 2034, older adults will outnumber children and nearly one in four Americans will be older than 65 by 2060.(2) This has many implications for the practice of cardiovascular medicine because older adults are disproportionately affected by cardiovascular disease (CVD). The prevalence of CVD increases with age and outcomes are more detrimental for those above 75 years with coexisting Geriatric Syndromes.(3) Between 2015 and 2018, the prevalence of CVD, was 75 to 77% in those age 60-79 and 89 to 90% in those 80 and older.(4) The very old patients have a higher mortality rate(5) and greater risk of disability after hospitalizations(6). They are also more likely to have longer hospital length of stay and less likely to be discharged back to their original place of residence.(4) This phenomenon of increased vulnerability to stressors due to decreased physiological reserves in the older adults is termed frailty, which has recently gained a great interest from cardiologists because of the changing demographics of the U.S. population.(7)
Frailty syndrome has been described over a spectrum ranging from the absence of frailty, termed robust, to pre-frail, and then physically frail.(8) The pre-frail state increases the risk of progression to frailty and frailty increases the risk of disability, a state that is distinct from frailty.(9) Depending on the instrument used to assess for frailty, the prevalence of frailty among community-dwelling older adults ranges from 4.0% to 59.1% and the prevalence of pre-frailty ranges from 18.7% to 53.1%,(10) but the highest estimates are observed among older patients with CVD. There is a strong bidirectional association between CVD and frailty with a dose-dependent response seen from robust to frail. Pre-frailty and frailty are independently associated with a higher risk for developing CVD.(11)
The hospital environment, with immobilization, fasting, sleep deprivation, and disorientation, can dramatically worsen physical frailty with rapid, severe loss of muscle mass and function. The result is the “post-hospital syndrome,” with high rates of rehospitalization, mortality, and nursing home admissions; prolonged physical disability; poor quality of life; and high healthcare costs. (12) Thus, interventions aimed at preventing, delaying, or reversing frailty may influence cardiovascular health in older patients. In this state-of-the-art review, we discuss the various definitions of frailty, the instruments used to measure frailty in practice, and proposed interventions to prevent, reverse, or slow the progression of frailty in patients with CVD.
DOMAINS AND DEFINITIONS
There are various proposed definitions and conceptual frameworks of frailty. Since frailty involves dysregulation across many physiological systems(13), with multifactorial etiologies, there is a wide range of clinical phenotypes. Therefore, frailty has been categorized into different functional domains: physical, cognitive, psychosocial, and nutritional (Table 1).
Table 1.
Conceptual Definitions of Frailty Syndromes among Patients with Cardiovascular Disease.
| Frailty Type | Definition |
|---|---|
| Physical Frailty | |
| - Fried Physical Frailty Phenotype | Clinical syndrome of increased vulnerability resulting from age associated decline in reserve and function across multiple physiologic systems such that the ability to cope with everyday acute stress is compromised. |
| - Consensus Definition (JAMDA) | A medical syndrome with multiple causes and contributors that is characterized by diminished strength, endurance, and reduced physiological function that increases an individual’s vulnerability for developing increased dependency and/or death. |
| - WHO Definition | A clinically recognizable state in which the ability of older people to cope with everyday or acute stressors is compromised by an increased vulnerability brought by age-associated declines in physiological reserve and function across multiple organ systems. |
| Cognitive Frailty | |
| - Cognitive Frailty/Predementia Syndrome | State of cognitive vulnerability exposed to vascular risk factors with an increased likelihood of progression to overt dementia. |
| - I.A.N.A./I.A.G.G. Definition | A heterogenous clinical manifestation characterized by the simultaneous presence of both physical frailty and cognitive impairment. |
| - Ruan Definition | A heterogenous clinical syndrome of cognitive impairment (CDR ≤0.5) that develops in older patients and caused by physical factors (e.g., physical frailty and pre-physical frailty) and is excluded from dementia resulting from AD or other conditions. The two subtypes are:
|
| Psychosocial Frailty | |
| - Integral Conceptual Definition of Frailty | A dynamic state affecting an individual who experiences losses in one or more domains of human functioning (physical, psychological, social), which is caused by the influence of a range of variables and which increases the risk of adverse outcomes.
|
| - Social Frailty | A continuum of being at risk of losing, or having lost, social and general resources, activities, or abilities that are important for fulfilling one or more basic social needs during the life span. |
| Nutritional Frailty | |
| - Nutritional Frailty | A state commonly seen in vulnerable older adults, characterized by sudden, significant weight loss and loss of muscle mass and strength (sarcopenia), or an essential loss of physiologic reserves, making the individual susceptible to disability. |
Abbreviations: CDR= Clinical Dementia Rating; I.A.G.G.= International Association of Gerontology and Geriatrics; I.A.N.A.= International Academy on Nutrition and Aging; JAMDA= Journal of the American Medical Directors Association; MCI= Mild Cognitive Impairment
Physical frailty is defined by Fried et al. as, “a clinical syndrome of increased vulnerability resulting from age associated decline in reserve and function across multiple physiologic systems such that the ability to cope with everyday acute stress is compromised”.(8) It uses the presence of the following components to establish the diagnosis of frailty: shrinking, weakness, poor endurance and energy, slowness, and low physical activity level. Shrinking is defined by unintentional weight loss and alludes to the presence of sarcopenia contributing to the development of frailty.
Cognitive dysfunction leads to increased vulnerability; therefore, many investigators have proposed adding cognition into the definition of frailty. Cognitive frailty was first defined by Panza, et al. as, “a particular state of cognitive vulnerability in mild cognitive impairment and other similar clinical entities exposed to vascular risk factors and with a subsequent increased progression to dementia, particularly vascular dementia”.(14) A workshop on cognitive frailty was conducted by an international consensus group that defined cognitive frailty to be “a heterogenous clinical manifestation characterized by the simultaneous presence of both physical frailty and cognitive impairment”.(15) Ruan, et al. refined the definition by proposing subtypes of potentially reversible and irreversible cognitive frailty so that interventions could be accurately divided into primary prevention and secondary prevention.(16)
In an aim to develop a conceptual framework of frailty, two additional expert meetings took place that resulted in an integral conceptual model of frailty that included psychological frailty and social frailty. The consensus document defined frailty as, “A dynamic state affecting an individual who experiences losses in one or more domains of human functioning (physical, psychological, social), which is caused by the influence of a range of variables and increases the risk of adverse outcomes.”(17) Psychological frailty was defined as a decline in cognition, mood and coping, and social frailty was defined as a decline in social relations and social support. Recognizing that social frailty is the most unexplored of all frailty domains, Bunt, et al. defined it as “a continuum of being at risk of losing, or having lost, resources that are important for fulfilling one or more basic social needs during the life span” and suggesting that not only the absence of resources, but also the absence of social behaviors, social activities as well as self-management abilities be included in the concept of social frailty.(18)
Nutritional frailty was defined by Bales and Ritchie as “rapid, unintentional loss of body weight and accompanying disability that often signals the beginning of a terminal decline in an older individual.”(19) Using this conceptual framework, numerous nutritional interventions to reverse frailty have been proposed.
Frailty can be a result of physical, cognitive, nutritional, and/or psychosocial vulnerabilities and there is a lack of a comprehensive definition that incorporates all components of every domain, but several of these domains cannot be practically addressed in cardiovascular practice. Since physical frailty can be measured objectively, investigators argue that physical factors should be identified by clinicians as they are more likely to be medically treatable.(6) Similar to other academic consortiums in cardiovascular medicine, a universal definition of frailty through the “frailty academic research consortium” is needed to improve the quality of research in the field and particularly to accurately interpret the results of interventions aimed to prevent and reverse frailty as part of the comprehensive cardiovascular evaluation and management in older adults
FRAILTY AND CARDIOVASCULAR DISEASE: A BIDIRECTIONAL ASSOCIATION
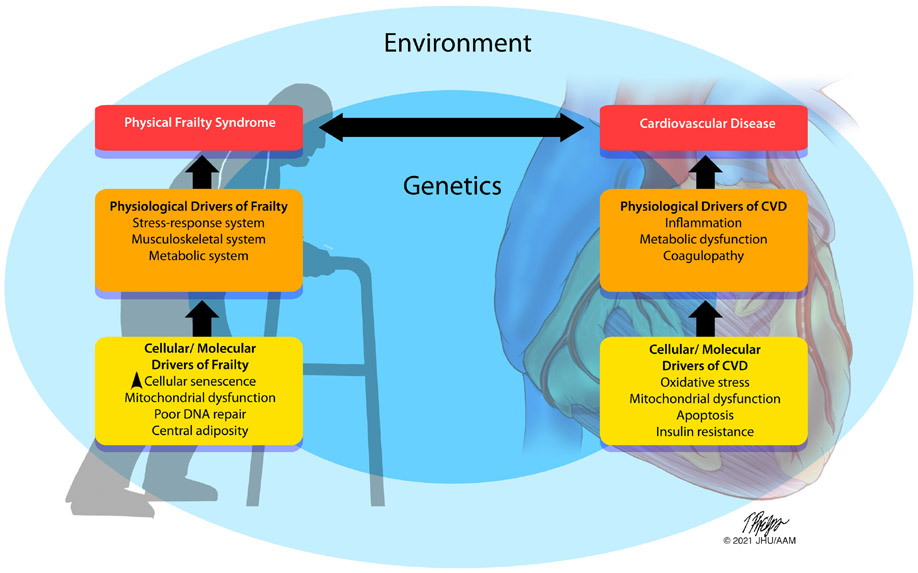
Whereas CVD can lead to worsening frailty due to hospitalization, debilitation, and immobility, underlying systemic, metabolic, and hormonal dysregulation such as chronic low-grade inflammation, central adiposity and insulin resistance can explain the molecular and physiologic underpinning for both physical frailty and CVD (Figure 1).(21,22) There is a strong association between frailty and adverse cardiovascular outcomes, which was explored in depth elsewhere.(23) Among patients who underwent percutaneous coronary intervention (PCI), frailty status was independently associated with increased mortality and adverse cardiovascular events.(24) In patients with non-ST elevation myocardial infarction (NSTEMI), an invasive strategy is associated with improved outcomes in non-frail patients, but it was associated with a higher incidence of procedural complications in frail patients.(25) In patients presenting with ST-elevation myocardial infarction (STEMI) undergoing PCI, frailty was associated with higher in-hospital mortality even with prompt revascularization strategies.(26) In patients with acute coronary syndrome (ACS), frailty was associated with lower adherence to quality metrics including longer admission to balloon time, longer hospital stay, and higher 30-day and 1-year mortality.(27) Frailty is highly prevalent in patients with heart failure (HF), ranging from 36.2-52.8%,(28) and is a strong predictor of outcomes. The FRAILTY-AVR study showed that the prevalence of frailty was 26-68% in patients undergoing aortic valve replacement, which increased the risk of midterm mortality, functional decline, and disability.(29) A study on patients undergoing percutaneous mitral valve repair showed that 45.5% of patients were frail and frailty was associated with higher risk of mortality and heart failure decompensation during follow-up.(30)
Figure 1. Theoretical model of the drivers of frailty syndrome and CVD.
A combination of genetic and environmental factors cause dysfunction at a cellular/molecular scale (yellow) driving physiological responses in different organ systems (orange) resulting in clinical manifestations of CVD and/or the physical frailty syndrome (red). Both are intertwined in a bidirectional relationship as the presence of one entity drives the progression of the other. *Revised with author’s permission. Nat Aging 1, 36–46 (2021).
The same biological underpinnings that cause cardiovascular disease may also lead to the development of other chronic cardiovascular and non-cardiovascular conditions in the older patient populations that need to be carefully managed. These multiple chronic conditions, also known as multimorbidity, result in the introduction of several concurrent medications, or polypharmacy, which has long been associated with an increased risk of frailty, falls and worsening cognitive impairment.(7) In addition to more data on optimal pharmacotherapy in older adults, individualized care informed by geriatric principles may reduce frailty risk in this patient population. Due to recognition that various pharmacotherapies in frail patients with CVD can result to adverse effects, e.g., antiplatelet therapy increasing the risk of bleeding in frail patients or polypharmacy secondary to guideline directed medical therapy increases risk of adverse events,(32) frailty is becoming a forerunner of undertreatment or overtreatment among older geriatric populations. Hence, frailty can worsen cardiovascular outcomes and efforts to address and prevent frailty syndrome in the setting of CVD are critical.
Taken together, cumulative evidence has shown that the high prevalence of frailty in patients with different forms of CVD is strongly associated with adverse clinical outcomes, but the association between frailty and cardiovascular disease is bidirectional. Recent data from the National Health and Aging Trends Study (NHATS) has shown that among patients with no known coronary artery disease, frailty was strongly associated with high incidence of CVD outcomes including mortality, myocardial infarction, stroke, and vascular disease during five-year follow-up. Frailty and CVD are interconnected and therefore integration of frailty in cardiovascular practice is necessary.
INSTRUMENTS TO MEASURE FRAILTY DURING CARDIOVASCULAR ILLNESS
Measuring frailty is important for prognostication and planning of an appropriate treatment plan in patients with CVD. However, there are numerous tools that have been developed to measure frailty, some focusing on physical frailty as a construct, but others incorporate cognitive and psychosocial domains of frailty. The most widely cited definition of frailty is the Fried Physical Frailty Phenotype. The Fried Frailty Phenotype was developed from two large epidemiological studies: The Cardiovascular Health Study and the Women’s Health and Aging Study. It measured frailty as ≥3 abnormal domains of the following: shrinking or weight loss, weakness, poor endurance and energy, slowness, and low physical activity level (Figure 2).(8) However, other instruments detect physical frailty by measuring one or more components of the Fried phenotype combined with other metrics of functional decline. For example, the Green score, is composed of gait speed and hand grip strength in addition to independence in activities of daily living and a biomarker: serum albumin.(33) There are other scales that include components to evaluate not only physical but also cognitive, psychosocial, and nutritional frailty.
Figure 2. Selected instruments for frailty screening in patients with CVD.
In the Physical Frailty Phenotype model, frailty is defined by meeting three or more of the five criteria of unintentional weight loss, exhaustion, low activity, slowness, and weakness. The Essential Frailty Toolset is scored from 0 (least frail) to 5 (most frail), based on the following components chair stands, cognitive impairment, hemoglobin, and serum albumin. The Deficit Accumulation Index is a deficit-accumulation approach based off the theory that vulnerability stems from the accumulation of health deficits.
Cognitive Frailty can be measured by the Fried+ Scale which is composed of the criteria in the Fried Frailty Phenotype in addition to a cognitive assessment measured by the mini mental status exam and mood assessed by the short-form geriatric depression scale.(29) Similarly, the Edmonton scale includes an assessment of cognition, social support, and nutrition in addition to function.(34) Some tools use a deficit accumulation approach to formulate an index of frailty, one of which is the Canadian Study on Health and Aging (CSHA) Frailty Index. It is based on the accumulation of deficits, a 70-item list that encompasses physical, cognitive, and psychological domains (Figure 2).(35)
In addition to the large number of tools used to measure frailty, there is variability due to the subjective nature of some of these instruments. The CSHA Clinical Frailty Scale (CFS) is solely based on a clinician’s judgement of a patient’s functional status and independence.(36) On the other hand, there are many questionnaires, including the Fatigue, Resistance, Ambulation, Illness, Loss of Weight (FRAIL) scale(37) which are solely based on a subject’s answers on a questionnaire. To address limitations related to subjective tools or complex instruments, the Essential Frailty Toolset (EFT) was developed by Afilalo and colleagues.(29) The EFT is an objective and parsimonious instrument used to measure frailty in older patients with CVD. The components of the instrument include biomarkers like serum albumin and hemoglobin combined with an evaluation of (1) cognitive function using the Mini-Mental State Examination (MMSE) or Mini-Cog Scale and (2) physical function utilizing the chair rise test, i.e., the time it takes to perform five sit-to-stand repetitions without using arms (Figure 2). The EFT was compared to the Fried scale, Fried+ scale, CFS, Short Physical Performance Battery (SPPB), Bern scale, and Columbia scale and demonstrated superior ability in predicting worsening disability at one year post-operatively and death at 30 days.(29) It has also been applied to patients undergoing CABG and proved to be highly prognostic for short- and intermediate-term outcomes.(38) Due to ease of use and the objective nature of this tool, the essential frailty toolset has been incorporated in research and practice.
Implementation of frailty scales can be challenging due to the time and resources required for measurement. Thereby, many single-item measures of frailty are used, e.g., 5-meter gait speed and hand grip strength.(39-41) However, these measures can be a surrogate for pre-disability frailty or a single domain of frailty and do not represent physical frailty as a construct (Table 2).
Table 2.
Clinical and Administrative Instruments used to Measure Frailty in Patients with Cardiovascular Disease.
| Type | Instrument | Criteria |
|---|---|---|
| Physical Frailty | Fried Frailty Phenotype | Three or more of the following components of the hypothesized cycle of frailty:
|
| Study of Osteoporotic Fractures Frailty Criteria | A frailty index using three components: weight loss, one’s inability to rise from a chair 5 times without using her arms, and reduced energy level. | |
| The Simplified Frailty Scale | A scale consisting of five components: slowness as measured by gait speed, weakness as measured by handgrip strength, exhaustion, low activity level, and weight loss. | |
| FIFA Score | A frailty score based on data collected from a wearable health-monitoring device including heart rate, preprocedural stress, and walking, found to demonstrate great predictive power for hospital mortality after transcatheter aortic valve implantation. | |
| Deficit accumulation Frailty | Frailty Screening Questionnaire | A self-report frailty measurement tool based on the modified Fried frailty components used to identify older adults with higher risk of adverse health outcomes. |
| Brief Risk Identification of Geriatric Health Tool | An 11-item questionnaire composed of questions on health status, independence, falls risk, depression, and cognitive limitations. A score of 3 or more identifies those with disability-related needs. | |
| PRISMA-7 | A 7-item questionnaire on age, gender, health problems, limitations, dependence, social support, and mobility. A score of 4 or more indicates frailty. | |
| Geriatric 8 frailty questionnaire | An 8-question screening tool to identify patients who could benefit from a comprehensive geriatric assessment. Evaluates appetite, weight loss, mobility, neuropsychological problems, BMI, medications, relative health status, and age. | |
| Hospital Frailty Risk Score | A risk score based on administrative hospital data (ICD-10 codes) used to identify frailty risk. | |
| Electronic Frailty Index | A risk score derived from automatically populated routinely collected data contained within the primary care electronic health record in the UK. | |
| The Johns Hopkins Claims-Based Frailty Indicator | A frailty indicator derived from Medicare claims that uses administrative data to classify patients as frail. | |
| Claims-based Frailty Index | An index calculated from Medicare data using the deficit accumulation approach. | |
| The CSHA Frailty Index | Comprised of assessments in 10 standard domains: cognitive status, mood and motivation, communication, mobility, balance, bowel function, bladder function, IADLs and ADLs, nutrition, and social resources. A count of 70 deficits including the presence and severity of current diseases, ability in ADLs and physical signs from clinical and neurologic exams. | |
| Frailty Index of Accumulated Deficits | The proportion of deficits present in the individuals at the time of their health appraisal. | |
| Schoenenberger Frailty Index | A summary score of performance on various components including MMSE, TUG Test, Mini Nutritional Assessment, Basic Activities of Daily Living, and Instrumental Activities of Daily Living. Also includes an assessment of pre-clinical mobility disability. | |
| Pre-Disability Frailty | CSHA Function Scale | Based on the Older American Resources Survey, with 12 IADL and ADL items. Scores patients on each of 12 ADLs as 0 (the patient is independent in carrying out this ADL), 1 (needs assistance) or 2 (is incapable). |
| Frail Non-Disabled (FiND) instrument | A 5-item questionnaire assessing mobility, disability, weight loss, exhaustion and sedentary behavior categorizes patients into disabled, frail, or robust. | |
| Edmonton Frailty Scale | Screening questionnaire assessing nine domains of frailty: cognition, functional performance, general health status, functional independence, social support, pharmacological condition, nutritional aspect, mental condition and continence. Patients are classified into the following categories: no frailty, apparently vulnerable, and severe frailty. | |
| Bern Scale | An 8-element frailty score, including the domains of cognition, instrumental activity of living, nutrition, energy level, weight loss, limb strength, comorbidities, and psychological factors, scoring patients on a scale of 0 to 9 (least frail to most frail). | |
| The 11-Item Simplified Frailty Index | An index composed of 11 overlapping items from the CSHA-Frailty Index and the ACS NSQIP: DM, lung problems, CHF, MI, cardiac problems, HTN, cognitive impairment, cerebrovascular problems, history of stroke, and PVD. | |
| Groningen Frailty Indicator | A screening instrument consisting of 15 self-report items in multiple frailty domains. Score of 4 of more indicates frailty. | |
| Health Deficits Index | Derived from a self-administered questionnaire of health deficits, this tool has been found to be associated with adverse health outcomes. | |
| Evaluative Frailty Index for Physical Activity | A 50-item multi-domain questionnaire assessing physical functioning, psychological functioning, social functioning and general health. | |
| Frailty Risk Score | A risk score based on 16 factors derived from the electronic health record including symptoms, syndromes, conditions and serum biomarkers. | |
| Vulnerable Elders Survey | A function-based self-assessment tool consisting of age, self-rated health, independence, and physical performance for screening community-dwelling adults to identify the vulnerable, defined as a score of greater than 3. | |
| Katz Index | A simple scale composed of six items that evaluate basic daily activities to provide a measure of independence. | |
| Barthel Index | A scale assessing mobility and performance in ADLs, with higher number indicating greater independence and a lower number indicating a greater degree of assistance is required. | |
| Gait speed | Gait speed of <0.8 m/s is found to have a high sensitivity (0.99) and moderate specificity (0.64) for identifying frailty. | |
| Hand grip strength | Reduction in hand grip strength is associated with functional decline, mortality, disability, and medical complications. | |
| Rising time from bed | Rising time from bed, measured within 2 days of admission, is an independent predictor of frailty at hospital discharge in elderly patients hospitalized for heart failure. | |
| Short Physical Performance Battery | A measure of lower limb function which can predict future risk of nursing home admission and mortality. It consists of three individual sub-tests: standing balance, 4-meter gait speed, and 5-repetition sit-to-stand. | |
| 6-Minute Walk Test | A 300-m or less walk distance is a significant predictor of frailty in patients with CHF. | |
| 5-m Gait Speed Test | Gait speed of ≥6 s over a five-meter distance has proven to be a predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. | |
| Tinetti Performance Oriented Mobility Assessment | A task-oriented test composed of various maneuvers that measure gait and balance abilities. | |
| Knee Extension Strength Test | A measure of lower extremity strength which is measured in seated position with hips and knees in 90 degrees, by a force transducer mounted in a chair. | |
| Appendicular Lean Mass | Quantified using bioimpedance analysis of body measurements, a measurement found to correlate with mortality in pre-frail and frail adults. | |
| Mixed Assessments | Fried+ Scale | Consists of the Fried Frailty Phenotype components in addition to a cognition assessment as evaluated by the MMSE and mood as assessed by the short-form geriatric depression scale. |
| The CSHA Clinical Frailty Scale | Utilizes information from a clinical encounter to summarize a person’s health on a scale from 1 (very fit) to 7 (severely frail). | |
| CSHA Rules-Based Definition of Frailty | Categorizes subjects as 0 (having no cognitive or functional impairment), 1 (isolated urinary incontinence), 2 (dependent in 1 ADL or having a diagnosis of CIND) or 3 (dependent in at least 2 ADLs, having mobility impairment or having a diagnosis of dementia). | |
| Frailty Staging System | Evaluates the functional status of patients using carefully selected tests of vision, hearing, arm and leg function, urinary incontinence, mental status, instrumental and basic activities of daily living, environmental hazards, and social support system which are conducted by a brief questionnaire and easily observed tasks. | |
| Columbia Scale | A frailty scale consisting of 4 items: gait speed, grip strength, serum albumin, and ADL disability. | |
| Green Score | A frailty score based on serum albumin, independence in ADLs, gait speed, and hand grip strength. | |
| Essential Frailty Toolset | A brief, 4-item frailty scale based on evaluation of chair rise, cognition, hemoglobin and serum albumin. | |
| Comprehensive Assessment of Frailty | A score composed of different items to quantify the physical performance and coordinative abilities of the patient in addition to biomarkers and scores that are already used to define frailty, such as the Fried criteria. | |
| Gérontopôle Frailty Screening Tool | An 8-item questionnaire evaluating a patient’s status (living alone, involuntary weight loss, fatigue, mobility difficulties, memory problems and gait speed) in addition to the general practitioner’s personal view about the frailty status of the individual. | |
| The Frailty Trait Scale | A 12-item multi-dimensional assessment on energy balance-nutrition, physical activity, nervous system, vascular system, strength, endurance, and gait speed. | |
| The Dutch Tilburg Frailty Indicator | Consists of two subscales, one comprises of sociodemographic data and data about life-events and chronic diseases. Another consists of physical, social and psychological factors. A score of 5 or greater is associated with frailty. | |
| SHARE Frailty Instrument | An assessment tool composed of grip strength and four self-reported items (fatigue, loss of appetite and/or eating less than usual, difficulties climbing stairs and/or walking 100 meters, and low level physical activity) utilized to screen community-dwelling adults for frailty. It is based on the first wave of the Survey of Health, Ageing and Retirement in Europe. | |
| The Comprehensive Geriatric Assessment | An interdisciplinary, multidimensional assessment of various domains of health including medical conditions, mental health, functioning, social circumstances, and environment. | |
| FRAIL Scale | A questionnaire composed of a self-assessment of five components: fatigue, resistance, ambulation, illness, and loss of weight which categorizes patients into robust, pre-frail or frail. | |
| SARC-F | A 5-component questionnaire consisting of self-assessment of strength, assistance walking, rise from a chair, climb stairs, and falls. | |
| Sherbrooke Postal Questionnaire | A simple mailed questionnaire consisting of six items in multiple frailty domains. Those who score two or higher, or those who do not respond to the questionnaire, are assumed to be frail. | |
| Kihon Checklist | A 25-item questionnaire including seven categories: daily life, physical ability, nutrition, oral condition, the extent to which one is housebound, cognitive status, and depression risk. |
ACS NSQIP= American College of Surgeons National Surgical Quality Improvement Program; ADL= Activities of Daily Living; BMI= Body Mass Index; CHF= Congestive Heart Failure; CIND= Cognitive Impairment No Dementia; CSHA= Canadian Study of Health and Ageing; DM= Diabetes Mellitus; HTN= Hypertension; FIFA= Fitness-Tracker Assisted Frailty-Assessment; FRAIL= Fatigue, Resistance, Ambulation, Illnesses, & Loss of Weight; IADL= Instrumental Activities of Daily Living; ICD= International Classification of Diseases; MI= Myocardial Infarction; MMSE= Mini Mental Status Exam; PVD= Peripheral Vascular Disease; TUG= Timed-up-and-go
To conclude, numerous tools are used to measure frailty, some are based on a single domain of physical frailty phenotype, others are more comprehensive and aim to capture frailty as a construct. These comprehensive tools may be resource intensive, time consuming, and require additional training. To overcome this heterogeneity and balance that with accuracy in measuring frailty syndrome as a construct, the EFT can be used to measure frailty in older patients with CVD, which allows for standardized frailty assessment in research and practice (Figure 2).
CHALLENGES IN MEASURING FRAILTY DURING ACUTE CVD ILLNESS
Despite the availability of numerous instruments (Table 2), measurement of frailty during acute cardiovascular illness is challenging. For example, measuring physical domains like gait speed and grip strength can be impaired in acute cardiovascular illness (42) and can even be impractical in critically ill patients in the intensive care unit.(43) To overcome this challenge, some questionnaire-based or simplified clinical instruments were proposed. Prior work showed that the CFS required only 3 to 5 minutes to complete, and the FRAIL scale required 1 to 3 minutes to complete in acute settings.(42) Whereas the FRAIL scale is a questionnaire that can be administered by any staff member, the CFS requires clinical expertise. While these questionnaires can be helpful, other challenges during acute cardiovascular illness among older adults include delirium, poor recall and memory, and inaccuracy in physical testing due to their bedbound status. Since most frailty-assessment tools were developed in the outpatient settings, some investigators argue that new instruments to detect frailty during acute illness are needed utilizing objective measures routinely performed in the inpatient settings including laboratory and imaging techniques, which can best characterize older populations vulnerable to stressors as they relate to CVD.
REVERSIBILITY OF FRAILTY
A large longitudinal study on transitions of frailty among community-dwelling adults showed that frailty is in fact a dynamic process. In a 54-month follow-up period, more than half of the participants had a transition between the frailty states and although the majority of those transitions was to a state of greater frailty, there were some who transitioned to a state of less frailty, indicating that frailty is a reversible process.(44) Xue, et al.(45) conducted a study using the NHATS cohort that measured the Fried’s physical frailty phenotype at different intervals over time and evaluated its association with mortality. The investigators showed that frailty is a dynamic process and ~ 60% of participants had either an increase or a decrease in frailty scores over time. The results also showed that a score of 5 was associated with a mortality rate more than three times higher than that of scores of 3 and 4. High scores, 4 and 5, were also associated with a decreased likelihood of complete reversibility of frailty to a score of 0. While these results suggest that there is a point of irreversibility, it also indicates that implementing measures to intervene on frailty syndrome early rather than late may be beneficial.
FRAILTY AS OUTCOME
Because frailty is potentially a reversable state, it’s necessary to study frailty as an outcome and to examine the underlying risk factors of frailty so that at-risk patients can be identified early and clinical interventions including risk-mitigation can be implemented to prevent or delay the onset of frailty in the context of CVD prevention. Studies have shown that obesity, tobacco use, heavy alcohol use, low socioeconomic status, female gender, minimum physical activity, lower level of education attained, polypharmacy, falls, multimorbidity, social isolation, impaired cognitive function, depression, and spouse’s depression are associated with an increased risk of frailty development.(46-48) Treatment of underlying risk factors for frailty can potentially delay or prevent frailty, which in turn, is a precursor of CVD outcomes.
OVERVIEW OF FRAILTY INTERVENTIONS
Various interventions have been proposed to influence frailty status (Table 3; Central Illustration-Figure 3). Some interventions, like cardiac rehabilitation, are already a part of CVD management, but other interventions, like resistance and balance training, are not routinely prescribed. Although most of the attention has focused on physical frailty, interventions targeting other domains of frailty exist including cognitive, nutritional, and psychosocial. Some pharmacologic interventions targeting systemic inflammation have been proposed. Numerous trials are ongoing, and we reviewed the results from these data, which we present below.
Table 3.
Frailty Interventions in Patients with Cardiovascular Disease
| Type of Intervention |
Type of Study |
Intervention | Subjects | Frailty Instrument Used |
outcomes |
|---|---|---|---|---|---|
| Physical | RCT | Multicomponent Cardiac Rehabilitation(55) | 136 patients with elective transcatheter aortic valve implantation and subsequent inpatient cardiac rehabilitation | Schoenenberger Frailty Index 6MWD Maximum workload in bicycle ergometry | Improved functional capacity, quality of life, and reduction in frailty |
| RCT | Resistance and Balance Training in Exercise-Based Cardiac Rehabilitation(58) | 252 patients admitted to cardiac rehab early after valve surgery/intervention | 6MWD SPPB 5-minute walk test Strength (one repetition maximum test for leg press) | Improved functional and exercise capacity, physical performance, muscular strength, and reduced physical frailty levels | |
| RCT | Cardiac rehabilitation(68) | 89 cardiovascular disease patients with age ≥65 years old who participated in the outpatient cardiac rehabilitation program for 3 months | Japanese Version of the Cardiovascular Health Study Standard Walking speed Maximal grip strength Lower extremity strength | Reduction in frailty and improved physical function | |
| RCT | Structured physical activity intervention after cardiac rehabilitation(67) | 140 frail elderly patients who completed cardiac rehabilitation after elective cardiac surgery | SPPB | Improved physical function | |
| Observational Study | Cardiac Rehabilitation(56) | 60 patients who underwent TAVI and were thereafter referred to cardiac rehabilitation | 6MWD Cumulative Illness Rating Scale | Improvement in function, autonomy and quality of life | |
| Cross-sectional Study | Exercise-based Cardiac Rehabilitation(102) | 78 patients who underwent TAVI compared to 80 patients who underwent sAVR | 6MWD | Enhanced independence, mobility and functional capacity | |
| Retrospective Observational Study | Cardiac Rehabilitation(103) | 3277 patients hospitalized for acute HF | The CSHA Frailty Index | Improved physical functioning and exercise capacity with favorable long-term outcomes in frail patients with HF | |
| Observational Study | 4-week Inpatient Cardiac Rehabilitation(104) | 160 patients aged 75 years and older referred to an outpatient cardiac rehabilitation unit after an acute coronary event or cardiac surgery | 6MWD Peak torque (strength) using an isokinetic dynamometer | Improvement in all domains of physical performance and particularly in those with poorer baseline performance | |
| Prospective Pilot Study | 8-week Combined Endurance and Resistance Exercise Training(105) | 30 patients who underwent TAVI | Muscular strength 6MWD | Improved exercise capacity, muscular strength, and quality of life | |
| Retrospective Study | 3-week cardiac rehabilitation(62) | 442 patients after TAVI or sAVR who were referred to cardiac rehabilitation | 6MWD Bicycle exercise test | Improved functional status and exercise capacity | |
| Retrospective Cohort Study | Cardiac rehabilitation program enhanced with psychological support(63) | 523 elderly inpatients aged ≥75 years admitted to a cardiac rehabilitation ward due to heart disease | Barthel Index | Improvement in psycho-physical health of elderly subjects and significant delay in re-hospitalization | |
| Observational Study | Home-based preoperative rehabilitation (prehab)(49) | 22 patients planned to undergo CABG or valve surgery | Clinical Frailty Score 6MWD SPPB | Improved clinical frailty score, functional ability, and reduced hospital length of stay | |
| Retrospective Analysis | Cardiac rehabilitation(64) | 243 patients with cardiovascular disease who completed phase II cardiac rehabilitation program | Fried Criteria | Improvement in multiple domains of physical function among frail patients, similar to or greater than those achieved by intermediate-frail and nonfrail patients | |
| Observational Study | Patient-centered cardiac rehabilitation(106) | 160 patients over 70 years old admitted in the cardiac rehabilitation unit soon after cardiac surgery | Not available | Improved objective and subjective functional status | |
| Retrospective Study | Geriatric rehabilitation-cardio program(69) | 58 patients hospitalized because of cardiovascular disease | Functional status | Improved functional status and health-related quality of life | |
| Retrospective Study | Comprehensive cardiac rehabilitation, including nutrition, physical exercise and medication(52) | 322 inpatients with cardiovascular disease | Muscle mass (skeletal muscle index) Muscle strength (grip strength) Physical performance (gait speed) | Improved handgrip strength, gait speed, leg weight bearing index, and nutritional intake after exercise training in patients both with and without sarcopenia | |
| RCT | Prehabilitation (PREQUEL Study)(51) | 164 patients who are pre-frail and frail, awaiting CABG with or without valvular repair/replacement | The Clinical Frailty Scale 5m Gait Speed The Essential Frailty Toolset | Unpublished | |
| RCT | Personalized physiotherapy program in-hospital(53) | 224 patients aged 70-87 years who underwent cardiac surgery | Tinetti Performance Oriented Mobility Assessment Get-Up-and-Go Test Mobility Balance Muscle strength | Improved independence and mobility and shorter hospital length of stay | |
| RCT | Pre-operative Rehabilitation (PREHAB Study)(50) | 244 patients age 65 and older who underwent elective cardiac surgery and had a clinical frailty score of 4-7 | Clinical Frailty Score | Unpublished | |
| RCT | Physical activity intervention (HULK Trial)(107) | Elderly (≥70 years) patients with ACS who had an uneventful first month and showed reduced physical performance | SPPB | Unpublished | |
| Retrospective Cohort Study | Early mobilization in the CICU(54) | 264 patients ≥60 years of age admitted to the CICU | Level of Function 1-4 (bedbound to walk >50 ft) Rockwood’s Clinical Frailty Scale | Improvement in functional status in both frail and non-frail older adults | |
| Retrospective Cohort Study | Cardiac rehabilitation(108) | 114 cardiac surgery patients who underwent cardiac rehabilitation | Clinical Frailty Scale Modified Fried Criteria SPPB Functional Frailty Index | No change in frailty scores from baseline to 1-year post-operation, however improvement in cognitive impairment and functional domains of the frailty criteria | |
| Pilot Trial | 6-month cardiac rehabilitation (RECOVER-TAVI Pilot)(57) | 27 patients who underwent TAVI | 6MWD Fried and Edmonton Frailty Scores | Improvement in outcome scores | |
| RCT | Cardiac rehabilitation with resistance training and special balance training(59) | 173 patients aged 75 and older who underwent CABG | 6MWD TUG Test Maximal isometric strength test | Improvements in all measured variables | |
| RCT | 12-week multi-domain physical rehabilitation (REHAB-HF Trial)( 109) | 360 patients age 60 years and older hospitalized with ADHF | SPPB | In process | |
| Pilot Study | 12-week multi-domain physical rehabilitation (REHAB-HF)(60) | 27 patients with ADHF age 60 years and older hospitalized with ADHF | SPPB | Improved SPPB score and reduced all-cause rehospitalization rate | |
| RCT | Acute Phase Intensive Electrical Muscle Stimulation (ACTIVE-EMS Trial)(71) | Frail patients age 75 years and older with AHF | Quadriceps isometric strength Handgrip strength SPPB Gait speed 6MWD Digit Symbol Substitution Test Mini-Cog MOS 36-Item Short-Form Health Survey physical functioning scale Frailty score SARC-F | In process | |
| RCT | Structured physical activity vs health education program (LIFE study)(110) | 1635 sedentary mean and women aged 70-89 years who had physical limitations, defined as a score on SPPB of 9 or below, but able to walk 400 m | Mobility disability defined by loss of ability to walk 400 m | Reduced major mobility disability in the structured, moderate-intensity physical activity program compared with a health education program | |
| Pharmacotherapy | RCT | Rapamycin, an mTOR inhibitor(84) | 13 elderly patients undergoing cardiac rehabilitation | Physical performance Frailty | Some correlation between some senescence markers and physical performance, but no improvement in frailty with rapamycin |
| RCT | Testosterone (intramuscular)(76) | Men 70 years and older, undergoing elective cardiovascular revascularization with extracorporeal circulation | Clinical and functional outcomes | In process | |
| RCT | Vitamin D3(72) | 64 patients with HF | 6MWD TUG test Knee isokinetic muscle strength | No improvement in physical performance for patients with HF despite a robust increase in serum 25OHD | |
| RCT | Vitamin D and Quadriceps Resistance Exercise (FITNESS Trial)(74) | 243 frail older people discharged from the hospital | Physical performance | Neither vitamin D supplementation nor a home-based program of high-intensity quadriceps resistance exercise improved outcomes in frail older people after hospitalization | |
| RCT | High Omega-3 Fatty Acid Multinutrient Supplement (Efalex Active 50+) for 6 months(77) | 27 non acutely ill postmenopausal women (age 60-84 years) | Mobility (habitual walking speed and fast walking speed) Cognitive performance | Improved cognition and mobility | |
| Cross-sectional Study | Exposure to Metformin(78) | 763 community-dwelling veterans age 65 years and older with type 2 diabetes | Frailty Index | Exposure to metformin was associated with lower risk of frailty | |
| RCT | Metformin(79) | Adults more than 65 years who are prediabetic and not frail at baseline | Fried criteria SPPB | In process | |
| RCT | Antihypertensive medication reduction (OPTIMISE Trial)(85) | 540 adults age 80 years and older with hypertension, prescribed 2 or more antihypertensive treatments | The CSHA Frailty Index Electronic Frailty Index FRAIL Scale | No significant differences in frailty | |
| RCT | Allogeneic human mesenchymal stem cells via intravenous delivery (CRATUS study)(81) | Age 60-95 showing signs of frailty | Activity (CHAMPS questionnaire) Mobility (4-m gait speed test and 6MWD, handgrip strength, SPPB) Exhaustion (multidimensional fatigue inventory questionnaire) | In process | |
| RCT | Testosterone supplementation with and without progressive resistance training(75) | 167 community-dwelling older men with low-normal baseline total testosterone levels | Continuous-scale physical functional performance Bilateral grip strength Leg extensor power Nottingham leg extensor power rig | No effect on functional performance, but improved upper body strength | |
| Meta-analysis | β-Hydroxy-β-Methylbutyrate (HMB) Supplementation(83) | 10 RCTs including 384 participants 50 years or older | Muscle Strength (isokinetic knee flexion, extension, isometric knee extension, handgrip strength, bench press, leg press Physical performance (6MWD, SPPB, gait speed, get-up-and-go) | No or fairly low impact on improving muscle strength or physical performance | |
| Nutrition | RCT | Nutritional Supplement vs Resistance Training(86) | 100 elderly nursing home residents | Muscle strength and size Gait velocity Stair-climbing power | High-intensity resistance exercise training improves muscle strength, however multinutrient supplementation has neither an independent nor an additive effect on these outcomes |
| RCT | Diet, exercise, cognitive training and vascular risk monitoring (FINGER Trial)(96) | 1260 individuals aged 60-77 years with a CAIDE Dementia Risk Score of at least 6 points and cognition at mean level or slightly lower than expected for age | Change in cognition measured through comprehensive neuropsychological test battery (NTB) Z score | Greater improvement in NTB score in the intervention group | |
| Prospective Cohort Study | Mediterranean-style diet(93) | 690 community-living persons (≥65 years of age) | Frailty defined as at least 2 of the following criteria: poor muscle strength, feeling of exhaustion, low walking speed, and low physical activity | Higher adherence to a Mediterranean-style diet was associated with lower odds of developing frailty compared with those with lower adherence. | |
| Meta-analysis | Mediterranean diet(94) | Analysis of 4 studies including a total 5789 community-dwelling older adults with a mean age of greater than 60 | Frailty | Greater adherence to a Mediterranean diet is associated with significantly lower risk of incident frailty in community-dwelling older people | |
| RCT | Protein-energy supplementation for 12 weeks(90) | 87 frail older adults | Change of physical functioning SPPB Gait speed TUG test Hand grip strength One-legged stance | Physical functioning increased and SPPB remained stable with the intervention although it decreased in the control group. | |
| RCT | Co-supplementation with creatine and protein supplementation combined with resistance training (from the Pro-Elderly study) (87) | 18 subjects | Handgrip strength TUG test Timed-stands test | Whey protein plus creatine and whey protein alone were similarly effective in improving muscle function | |
| RCT | Whey protein supplementation(88) | 47 frail, hospitalized elderly | Grip strength Knee extensor force | Improvements in grip strength and knee extensor force | |
| RCT | Vitamin D and leucine-enriched whey protein nutritional supplement for 13 weeks (PROVIDE study)(89) | 380 sarcopenic primarily independent-living older adults with SPPB scores between 4 and 9 and a low skeletal muscle mass index | Handgrip strength SPPB score Chair-stand test Gait speed Balance score Appendicular muscle mass | Improvement in muscle mass and lower-extremity function | |
| Prospective Cohort Study | ‘Prudent’ dietary pattern characterized by high intake of olive oil and vegetables compared to a ‘Westernized’ pattern with high intake of refined bread, whole dairy products, and red and processed meat(111) | 1872 non-institutionalized individuals aged ≥60 | Fried Criteria | A prudent dietary pattern showed an inverse dose-response relationship with the risk of frailty while a Westernized pattern had a direct relationship with slow walking speed and weight loss. | |
| Meta-analysis | Alcohol consumption(112) | 4 studies on 44,051 subjects age 55 and older | Frailty | Heavier alcohol consumption is associated with lower incident frailty compared with no alcohol consumption among community-dwelling middle-aged and older people | |
| Prospective Cohort Study | Dairy products(95) | 1871 community-dwelling aduls age 60 years and older | Modified version of the Fried criteria | Increased use of low-fat milk or yogurt was associated with a lower risk of frailty, however consumption of whole-milk dairy or cheese did not affect frailty status. | |
| Cognitive | |||||
| RCT | Cognitive stimulation and physical exercise (MIND&GAIT Project)(99) | Older adults age 65 years or more who are supported by the consortium end-user organizations who are frail or at risk of developing frailty | Barthel Index | In process | |
| RCT | Nutritional supplementation vs cognitive training vs physical training vs combination treatment(97) | 246 community-dwelling prefrail and frail old adults with a mean age of 70 years | Fried Criteria | Combination training resulted in the greatest frailty reduction, followed by physical, and then cognitive and nutritional interventions | |
| RCT | Multi-component physical exercise, cognitive training, dietary counseling, and promotion of psychosocial support (WE-RISE trial)(98) | Community-dwelling older adults aged 60 years and above with cognitive frailty | Cognitive frailty status as proposed by I.A.N.A./I.A.G.G. | In process | |
| Social | RCT | Physical training and nutritional intervention program vs social support intervention that included cognitive training(100) | 80 community-dwelling pre-frail and frail adults age 65 years or older | Frailty Status (SHARE-FI) | Decreased frailty with both interventions. Social support alone also resulted in improvement in frailty. |
Abbreviations: 6MWD= 6-Minute Walk Distance; CABG= Coronary Artery Bypass Graft; CHS= Cardiovascular Health Study; HF= Heart Failure; MMSE= Mini-Mental State Exam; RCT= Randomized Control Trial; SPPB= Short Physical Performance Battery; SARC-F= strength, assistance walking, rise from a chair, climb stairs, and falls; SAVR= Surgical Aortic Valve Repair; TAVI= Transcatheter Aortic Valve Intervention; TUG= Timed-up-and-go
Central Illustration. Interventions aimed to prevent or reverse frailty in patients with CVD.
Physical, pharmacological, cognitive, nutritional, and psychosocial interventions or a combination thereof have the potential to prevent the onset of frailty (primary prevention), reverse frailty (secondary prevention), or improve the quality of life in older patients with preexisting frailty (tertiary prevention).
Physical Interventions
Since frailty leads to poor post-operative outcomes, there is amounting interest in improving frailty prior to surgery by pre-operative rehabilitation, or pre-habilitation. Waite, et al. conducted a small prospective pilot study with 22 frail patients who had been listed for CABG or valve surgery.(49) The intervention was a home program that consisted of balance and strength-training exercises and resulted in reduction of frailty in 18% of patients, along with improvement in 6MWT distance, walking speed, and SPPB score. The Pre-operative Rehabilitation for reduction of Hospitalization After coronary Bypass and valvular surgery (PREHAB) study is enrolling older patients with a CFS of ≥4 and <7 in Canada.(50) The intervention consists of an 8-week comprehensive exercise therapy and education program targeting physical, psychological, social, and cognitive aspects of cardiac disease and frailty. The intervention is personalized based off an intake of health status assessment and requires a minimum of two sessions of supervised structured exercise sessions per week and four educational sessions on topics including risk factor reduction, medication use, cardiovascular physiology, smoking cessation, and healthy eating. The frailty outcomes are measured using the Modified Fried Criteria and 6MWT. The PREhabilitation for improving Quality of recovery after Elective cardiac surgery (PREQUEL) study is currently enrolling adults undergoing elective cardiac surgery with a CFS of 4-6.(51) The intervention is composed of a structured 6–10-week exercise training. The frailty outcomes studied include CFS score, gait speed, and EFT scores.
A large retrospective study of a physical intervention during hospitalization was conducted by Harada, et al. which studied the impact of a comprehensive cardiac rehabilitation program on inpatients with CVD and/or those who had undergone cardiovascular surgery.(52) The intervention consisted of exercise training starting the day of their admission. The patients who underwent in-hospital cardiac rehabilitation had decreased weight and skeletal muscle index, however significant improvement in gait speed and muscle strength assessed by handgrip and leg weight bearing index. The Barthel index of activities of daily living score was enhanced indicating an improvement in overall physical function. Opasich, et al. studied the impact of in-hospital personalized physical therapy program based on each individual’s frailty level as compared to traditional physiotherapy program for older patients post-cardiac surgery.(53) Frailty measures, assessed by get-up-and-go test, chair stand, arm curl, and 6MWT improved in the intervention group and no participant in the intervention group went home severely frail. Among cardiac intensive care unit patients, Goldfarb et al. showed that physical intervention with early mobilization resulted in improved level of function in both frail and non-frail patients.(54)
Multiple studies on cardiac rehabilitation post-procedure and post-hospitalization have shown improvement in frailty measures. Eichler et al. evaluated a three-week multicomponent inpatient cardiac rehabilitation program consisting of patient education, dietary counseling, psychological support, risk factor management and individualized physical training after elective TAVR.(55) The intervention resulted in improved distance in 6MWT, a reduction in the proportion of frail patients, and an average decrease in the Frailty Index score. Another study that evaluated the effects of multidimensional cardiac rehabilitation after TAVR found that there was improvement in frailty (measured by 6MWT and Barthel Index) at the end of cardiac rehabilitation, which persisted in the majority of patients at mid-term follow-up.(56) A pilot study for the RECOVER-TAVI Trial showed that cardiac rehabilitation after TAVR resulted in improvement in frailty, measured by multiple frailty scores including Fried and Edmonton.(57) In one study, patients were either randomized to the intervention group consisting of three sessions per week of specially tailored resistance/balance training incorporated in three-weeks of standard inpatient cardiac rehabilitation or the control group who participated in standard cardiac rehabilitation alone.(58) There was no statistically significant difference in frailty improvement as measured by 6MWT and SPPB between the groups at the end of the intervention, although in mid-term follow-up, the patients who had undergone the resistance/balance training had significantly lower frailty levels. Daily resistance and balance training in cardiac rehabilitation has also been studied in patients who underwent CABG and results showed that the resistance/balance training led to greater improvement in physical frailty compared to conventional cardiac rehabilitation as measured by the 6MWT and the timed up and go (TUG) time.(59)
Cardiac Rehabilitation has also proven to improve frailty in patients with HF. The REHAB-HF trial studied a progressive physical rehabilitation intervention that begins during admission for acute decompensated HF and continues for 3 months post-discharge would improve frailty outcomes.(60) Among the 349 patients who were randomized, a greater improvement in physical function, frailty, 6MWT, quality of life and depression were all associated with the early, transitional, tailored progressive rehabilitation group when compared to “usual care”.(61)
There is additional evidence that showed improvements in assessments of cognitive and psychosocial domains of frailty are related to cardiac rehabilitation. One retrospective study showed that patients who underwent surgical aortic valve replacement had improvement in both anxiety and depression scores after cardiac rehabilitation.(62) Another study showed an improvement in depression and cognitive functioning in all patients who underwent comprehensive cardiac rehabilitation consisting of an individualized multi-disciplinary program.(63) The pilot study for the RECOVER-TAVI trial showed an improvement in anxiety and depression scores after cardiac rehabilitation.(57) These studies show that cardiac rehabilitation not only improve physical frailty, but benefits extend to include cognitive and psychosocial frailty.
Benefits of cardiac rehabilitation extend to patients with preexisting frailty. Lutz et al. studied 243 patients referred to 2-6 weeks of phase II cardiac rehabilitation and results showed an improvement in frailty measured by gait speed, TUG, hand grip strength, and 6MWD in intermediately-frail and severely-frail patients.(64) In fact, the frail group experienced a greater percentage improvement from baseline in gait speed, TUG, and 6MWD. The long-term effects of cardiac rehabilitation may persist after cardiac illness. Machi, et al. found that 65% of patients that adhered to at least light physical activity post-cardiac rehabilitation, and the degree of physical activity had a direct correlation with the distance walked at 6MWT one-year after rehabilitation.(65) This supports the notion that there may be some lifestyle changes associated with cardiac rehabilitation that have long-term effects on preventing or reversing frailty (Figure 3 - Central Illustration).
Because of the positive outcomes from the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) Study,(66) which evaluated outpatient physical activity intervention among the general frail older adult population, a similar intervention was studied in the CVD population, who remained frail post-cardiac rehabilitation. The study showed that the 1-year outpatient physical activity intervention improved frailty as measured by the SPPB.(67) Ushijima et al. examined CVD patients aged ≥65 years of age and showed that a 3-month outpatient cardiac rehabilitation program resulted in improvement in frailty status in 87% of patients to either pre-frail (57%) or robust (30%) and all the non-frail patients remained non-frail during follow-up.(68)
Van Dam van Isselt, et al. studied geriatric rehabilitation,(69) a term coined by the Boston Working Group as “evaluative, diagnostic, and therapeutic interventions whose purpose is to restore functional ability or enhance residual functional capability in older people with disabling impairments.”(70) The intervention addressed body structure and function, functional status, and self-management and was studied in patients with CVD who were discharged to a skilled nursing facility. Results showed an improvement in physical frailty as measured by performance on the 6MWT and Barthel index. Subgroup analysis in patients with HF and showed improvement in the same outcomes. The data support early initiation of physical function program, whether through geriatric rehabilitation or cardiac rehabilitation, may reverse frailty in patients with CVD. Other physical interventions that are under investigation include electrical muscle stimulation as an adjunct to cardiac rehabilitation while the patient is resting to stimulate and potentially strengthen the skeletal muscles. This can potentially turn periods of immobility associated with functional decline into a productive time and may ultimately influence frailty status.(71)
Pharmacological Interventions
Several pharmacological interventions to influence frailty have been studied targeting different underlying physiological mechanisms of multisystem dysregulation. Since changes in muscular structure and sarcopenia is associated with frailty, the effect of hormones on frailty is an area of active investigation. Among older patients with HF, the effect of vitamin D3 supplementation on physical frailty as measured by the 6MWT and knee isokinetic muscle strength test was evaluated, but no significant change in frailty status was associated with vitamin D supplementation during follow-up.(72-74) Treatment with testosterone replacement therapy in older men results in an increase in lean mass and decrease in fat mass, but testosterone supplementation did not result in any significant improvement in physical function, measured by the physical function performance test. However, there was an improvement in measurements of upper body strength, including grip strength, bench press, incline press, and average upper body strength as compared to placebo.(75) A clinical trial designed to evaluate the effect of perioperative testosterone injections on frailty outcomes after rehabilitation among older patients undergoing CABG surgery is under way.(76)
Since cognitive impairment has been described as a risk factor for development of frailty, there has been interest in pharmacotherapy that may slow cognitive decline in preventing or improving frailty. There is a weak evidence that omega-3 polyunsaturated fatty acids docosahexanoic acid (DHA) and eicosapentaenoic acid (EPA) may be neuroprotective and reduce the risk of cognitive impairment. A pilot study was conducted to evaluate the impact of Efalex Active 50+ supplement which contains 1g of DHA and 160mg of EPA in addition to Ginkgo biloba, phosphatidylserine, alpha-tocopherol, folic acid and vitamin B12 among older patients over the age of 60 years.(77) While these are weak associations, the patients in the intervention group had mild improvement in some measures of mobility and cognition (motor screening task latency and verbal recognition memory recall). The protective role of these supplementation is yet to be studied in a larger trial.
Since frailty and CVD have bidirectional association, pharmacotherapy that target systemic inflammation can potentially influence both disease processes. A large proportion of patients with CVD are diabetic and metformin is widely utilized as a treatment of diabetes which also has anti-inflammatory and antioxidant effects. Few observation studies have reported an association between metformin therapy and improvement in frailty status, but these results are obtained from non-randomized retrospective experiments.(78) A randomized controlled trial is currently underway to examine the effects of metformin on frailty status in older patients with prediabetes.(79) Finally, the association between impaired endogenous stem cell repair and frailty status is being preliminary examined by Golpanian et al. utilizing allogeneic human mesenchymal stem cells (hMSCs). Phase-1 study showed that allo-hMSCs are safe and well-tolerated in older participants.(80) While the results remain exploratory because the small sample (n=15), improvement in frailty status, 6MWT, and cognitive frailty as measured by the MMSE were reported and Phase II is underway.(81)
The mechanistic Target of Rapamycin (mTOR) signaling pathway has been proposed to influence frailty. The mTOR is an enzyme that is activated by nutrients and is involved in protein synthesis and cell growth. Insulin and IGF-1 activate mTOR, and low levels of both factors is associated with longevity, indicating inhibition of mTOR can result in a longer lifespan.(82) On the other hand, stimulation of the pathway can lead to muscle anabolism and skeletal muscle growth. Therefore, pharmacotherapy on both stimulation and inhibition of the pathway has been studied. Beta-hydroxy-beta-methylbutyrate (HMB) is a molecule that exerts downstream effects activating mTOR, therefore several studies evaluated the impact of HMB supplementation on frailty. A meta-analysis of seven of those studies showed that HMB supplementation in addition to physical exercise had no or low impact on muscle strength and physical performance in older subjects compared to exercise alone.(83) Rapamycin is an inhibitor of mTOR and was studied by Singh, et al. in an observational study of 13 patients 60 years and older with coronary artery disease who were eligible for cardiac rehabilitation.(84) The intervention consisted solely of rapamycin administration and frailty was assessed by gait speed. Although there was no significant association between rapamycin and frailty status, there remains the question of whether rapamycin may improve physical function if studied in a population with a higher burden of frailty over a longer follow-up period.
Relevant to the pharmacologic interventions, polypharmacy has been associated with frailty. A recent large trial studied the effects of anti-hypertensive medication reduction on frailty as secondary outcome.(85) Medication reduction was noninferior to usual care in regards to blood pressure control and contrary to expectation, there was no statistically significant difference between the groups in frailty status measured by the Frailty Index, the Morley FRAIL scale, and Electronic Frailty Index. Further study on the association between polypharmacy and physical frailty is needed in the context of guideline directed medical therapy for CVD.
Dietary Interventions
The nutritional domain of frailty has also been widely studied, although most nutritional interventions have been in combination with physical interventions. The Boston FICSIT (Frailty and Injuries: Cooperative Studies of Intervention Techniques) study was a randomized, placebo-controlled, 10-week clinical trial comparing lower-extremity resistance training, nutritional supplement, both treatments, or a placebo activity and supplement.(86) Results showed that the resistance training improved muscle strength and mobility, however nutritional supplementation, consisting of a 360 kcal combination of carbohydrate, fat, soy-based protein, vitamins and minerals to augment caloric intake by 20%, did not have any effect on outcomes. However, few studies that evaluated protein supplementation have shown that whey protein supplementation with (87) and without(88) resistance training may improve frailty domains by measures of muscle strength.
Several studies investigated the effect of protein supplementation on frailty status, as reduced dietary protein intake is one factor that leads to sarcopenia. The PROVIDE study enrolled 380 older adults with mild to moderate limitations in physical function, and low skeletal muscle mass index and radomized to a protein-rich supplement with vitamins or an iso-caloric control product without protein or micronutrients (i.e., only carbohydrates and fat).(89) Frailty was measured by handgrip strength, SPPB, gait speed, chair stand test, and balance. Results showed that after 13 weeks, there was no significant difference in handgrip strength or SPPB, howevere there was a significant gain in muscle mass as measured by appendicular muscle mass and improvement in chair stand ability. Similarly, multiple nutritional interventions had not resulted in a significant improvement in frailty status. Kim et al. designed a trial to study whether protein supplementation may be more beneficial in frail individuals of low socioeconomic status.(90) Frailty was measured by functional performance, SPPB, TUG test, one-legged stance, and maximal hand grip strength. The study found improvement in multiple areas, suggesting that a nutritonal intervention in “at-risk older patient” may reduce the progression of frailty.
The association between certain dietary patterns and frailty were investigated, but significant confounding and selection bias exist. The Mediterranean diet is of great interest as it has been associated with a decreased likelihood of cognitive decline(91) and CVD(92) possibly because of a higher composition of antioxidants like beta-carotene equivalents, vitamin C and vitamin E which through their anti-inflammatory effects may also prevent frailty. In the Invechiare in Chianti study of aging, Talegawkar et al. conducted a longitudinal analysis in which they found that a greater adherence to a Mediterranean diet was associated with a lower odds of frailty as defined by Fried’s criteria along with a lower odds of having low physical activity and walking speed during follow-up.(93) A systematic review and meta-analysis of four large prospective cohort studies showed that greater adherence to a mediterranean diet was associated with a significantly lower incident frailty, as assessed by modified versions of the Fried frailty criteria or the FRAIL scale.(94)
Studying dairy consumption, Lana, et al. conducted a prospective cohort study based off data from the Study on Nutrition and Cardiovascular Risk in Spain (Seniors-ENRICA) showed that over a follow-up period of 3.5 years, those who had a greater consumption of low-fat dairy products had a lower risk of frailty development.(95) It is difficult to ascertain any causal associaitons between nutritional interventions and frailty status because significant counfonding and selection bias, but certain nutritional interventions, e.g. mediterranean diet, protein supplementation combined with physical intervention may have impact on frailty in at risk patients with CVD.
Cognitive Interventions
Cognitive interventions have generally been studied in combination with physical interventions to prevent or delay frailty. One of the largest randomized controlled trials studying the effects of a multicomponent intervention on cognitive frailty was reported by Ngandu et al.(96) The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) Trial enrolled a total of 1,260 older patients aged 60-77 years at risk for dementia and followed them over two-year period. The intervention group consisted of four components including nutritional therapy consisting of dietary education, physical exercise training program, cognitive training and social activities. The intervention resulted in significant improvement in cognitive frailty, the neuropsychiatric test battery, along with secondary outcomes of executive functioning and processing speed. Additionally, the intervention resulted in favorable effects on cardiovascular risk factors such as BMI, dietary habits and physical activity.
The Singapore Frailty Intervention Trial compared different types of interventions that target underlying domains of frailty.(97) The study was a randomized controlled trial in which 246 participants were randomly assigned to 12 weeks of nutritional supplementation, cognitive training, physical training, combination treatment, and usual care control. The cognitive training consisted of 2-hour weekly sessions of cognitive-enhancing activities. Frailty was measured by Fried’s operational definition. Results showed that the combination intervention was associated with the highest odds of frailty reduction, followed by the physical intervention, and then cognitive and nutritional interventions. The WE-RISE trial is an ongoing single blinded, randomized controlled trial aimed to examine a multi-domain intervention to reverse cognitive frailty.(98) It consisted of a multi-component exercise program, an in-person cognitive intervention consisting of “pen-to-paper” tasks such as “spot-the-difference,” mazes, matrix reasoning, and jigsaw puzzles. The nutritional intervention consisted of dietary counseling and psychosocial intervention consisting of group-based activity. The primary outcome studied in this trial is cognitive frailty using the Fried’s criteria and clinical dementia rating scale. The MIND&GAIT project aimes to promote independent living for frail older adults in Europe through a combined intervention using technology and traditional resources to develop and test a cognitive stimulation program, physical exercise program, and fall reduction plan.(99) More data on cognitive interventions and reversal of cognitive frailty is expected to result in the upcoming years.
Psychosocial Interventions
Acknowledging the importance of social supports in maintaining healthy habits, Luger et al. conducted a study on non-professional volunteer-administered interventions, comparing physical training and nutrition intervention to a social support group in which participants were visited twice a week by acquaintances over the 12-week duration of the study.(100) Results showed that frailty, as measured by the Frailty Instrument of the Survey of Health Ageing and Retirement in Europe, was reduced in both groups. Social support, such as a buddy system, may potentially play a role in addressing social determinants of frailty.
GAPS IN KNOWLEDGE
There is a lack of consensus on the definition of frailty which presents many challenges for research. Additionally, the abundance of tools used to measure frailty have resulted in heterogeneity in research output that limits consistency and reproducibility. The ideal frailty assessment tool should be a simple, quantitative, objective, and universally accepted method, capable of providing a consistent, valid, and reproducible definition that can then be used in real time by clinicians to determine a well-defined either presence or absence of the phenotype, much like hypertension or diabetes. The Essential Frailty Toolset is a promising instrument that is simple, quantitative, objective, and easy to administer, however its use to date is limited to cardiovascular medicine and its applicability to other fields is not known.
Most studies on frailty interventions use tools that measure one component of physical frailty, e.g., 6MWT, due to ease of administration. Although the 6MWT is correlated with the frailty syndrome in cardiac patients,(1) there are more comprehensive, validated tools and instruments available to measure the frailty syndrome (Figure 2). Future trials should study whether interventions show improvements in the global measure termed “Frailty Syndrome”.
Although there are data to suggest that frailty status can be reversed, whether reversal in frailty influences the development and progression of CVD remains unknown. Further studies are needed on interventions that reverse frailty and the impacts thereof on long-term cardiovascular outcomes. Future prospective clinical trial data in the older adult populations are needed to better outline the exact interactions between frailty status, CVD risk factors, and the potential downstream consequences, using prespecified and robust frailty assessment methods. Although there is a strong association between CVD and frailty(8,9), the direct “causal” association between frailty and CVD is not yet elucidated as frailty can theoretically be an epiphenomenon of the aging process itself, which can result in higher oxidative stress levels and contribute to CVD.(10) However, basic research on the biologic underpinning of frailty has shown that there is underlying inflammation, metabolic dysregulation, and coagulopathy, which are significantly associated with the development of cardiovascular disease.(11) If there is a “causal association” between frailty and CVD, then we need therapies that actively target the frailty syndrome as a standalone entity.
CONCLUSION
Frailty is a syndrome that is relevant to the practice of cardiovascular medicine because each disease process can predispose to physical impairments that ultimately cause worsening of the other. Because of its multi-factorial etiology, multiple underlying frailty domains exist including physical, cognitive, nutritional, and psychosocial, that constitute the frailty construct. Multi-dimensional interventions targeting these domains have been proposed to prevent or reverse frailty. There is no compelling study demonstrating a successful intervention to improve a global measure of frailty. Emerging data from patients admitted with heart failure indicate that interventions associated with positive outcomes on frailty and physical function are multidimensional and include tailored cardiac rehabilitation. Systematic efforts to integrate frailty assessment in cardiovascular practice is needed to improve the overall health and wellbeing of older patients, but clinical trials to evaluate frailty interventions in specific cardiac populations should further help optimize cardiovascular care in older adults.
Table 4.
Clinical pearls and take-home points for clinical practice and future research.
| Clinical Pearls |
|---|
| - Identification of the frailty syndrome in older adults with cardiovascular disease is critical and integrating interventions to reduce frailty should be part of the comprehensive cardiac management for the expanding older adult population. |
| - Frailty syndrome in older patients with cardiovascular disease can be measured objectively using the Essential Frailty Toolset. |
| - Frail and pre-frail patients should be referred for interventions such as tailored cardiac rehabilitation program to improve frailty status and influence cardiovascular outcomes. |
| - Randomized experiments in cardiovascular aging are needed using consistent frailty tools to evaluate the efficacy and safety of frailty interventions. |
HIGHLIGHTS.
Physical frailty syndrome is associated with poor outcomes after cardiovascular events.
The etiology of frailty in older adults is multifactorial and patient-specific.
Multimodal interventions, including cardiac rehabilitation, can reduce frailty.
ACKNOWLEDEMENT:
The authors would like to acknowledge Tim Phelps, MS, FAMI for his assistance with medical illustration.
FUNDING AND FINANCIAL DISCLOSURE:
Drs. Damluji, Xue, Bandeen-Roche, Walston, and Gerstenblith receive research funding from the Pepper Scholars Program of the Johns Hopkins University Claude D. Pepper Older Americans Independence Center funded by the National Institute on Aging P30-AG021334 and mentored patient-oriented research career development award from the National Heart, Lung, and Blood Institute K23-HL153771-01.
ABBREVIATIONS
- 6MWT
6-Minute Walk Test
- CSHA
Canadian Study of Healthy Aging
- CVD
Cardiovascular Disease
- CFS
Clinical Frailty Scale
- HF
Heart Failure
- MMSE
Mini-Mental State Examination
- mTOR
Mechanistic Target of Rapamycin
- NSTEMI
Non-ST Elevation Myocardial Infarction
- PCI
Percutaneous Coronary Intervention
- SPPB
Short Physical Performance Battery
- STEMI
ST-Elevation Myocardial Infarction
- TAVR
Transcatheter Aortic Valve Replacement
- TUG
Timed Up and Go
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST: The authors declare that they have no relevant interests.
REFERENCES
- 1.U.S. Census Bureau. Current Population Survey, Annual Social and Economic; Table 1: Population by Age and Sex: 2019. Available at: http://census.gov/data/tables/2019/demo/age-and-sex/2019-older-population.html, 2020.
- 2.Medina L, Sabo S, Vespa J. Living Longer: Historical and Projected Life Expectancy in the United States, 1960 to 2060. Current Population Reports. Washington, DC: U.S. Census Bureau, 2020. [Google Scholar]
- 3.Rodgers JL, Jones J, Bolleddu SI et al. Cardiovascular Risks Associated with Gender and Aging. J Cardiovasc Dev Dis 2019;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Virani SS AA, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elking MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, Liu J, Loop MS, Lutsey PL, Ma J, Mackey J, Martin SS, Matchar DB, Mussolino ME, Navaneethan SD, Perak AM, Roth GA, Samad Z, Satou GM, Schroeder EB, Shah SH, Shay CM, Stokes A, VanWagner LB, Wang N-Y, and Tsao CW on behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2021 update: a report from the American Heart Association. Circulation 2021. [Google Scholar]
- 5.Kulmala J, Nykanen I, Hartikainen S. Frailty as a predictor of all-cause mortality in older men and women. Geriatr Gerontol Int 2014;14:899–905. [DOI] [PubMed] [Google Scholar]
- 6.Buurman BM, Hoogerduijn JG, de Haan RJ et al. Geriatric conditions in acutely hospitalized older patients: prevalence and one-year survival and functional decline. PLoS One 2011;6:e26951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clin Interv Aging 2014;9:433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fried LP, Tangen CM, Walston J et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–56. [DOI] [PubMed] [Google Scholar]
- 9.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 2004;59:255–63. [DOI] [PubMed] [Google Scholar]
- 10.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012;60:1487–92. [DOI] [PubMed] [Google Scholar]
- 11.Damluji AA, Chung SE, Xue QL et al. Frailty and cardiovascular outcomes in the National Health and Aging Trends Study. Eur Heart J 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krumholz HM. Post-hospital syndrome--an acquired, transient condition of generalized risk. N Engl J Med 2013;368:100–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013;381:752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panza F, D'Introno A, Colacicco AM et al. Cognitive frailty: Predementia syndrome and vascular risk factors. Neurobiol Aging 2006;27:933–40. [DOI] [PubMed] [Google Scholar]
- 15.Kelaiditi E, Cesari M, Canevelli M et al. Cognitive frailty: rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J Nutr Health Aging 2013;17:726–34. [DOI] [PubMed] [Google Scholar]
- 16.Ruan Q, Yu Z, Chen M, Bao Z, Li J, He W. Cognitive frailty, a novel target for the prevention of elderly dependency. Ageing Res Rev 2015;20:1–10. [DOI] [PubMed] [Google Scholar]
- 17.Gobbens RJ, Luijkx KG, Wijnen-Sponselee MT, Schols JM. Towards an integral conceptual model of frailty. J Nutr Health Aging 2010;14:175–81. [DOI] [PubMed] [Google Scholar]
- 18.Bunt S, Steverink N, Olthof J, van der Schans CP, Hobbelen JSM. Social frailty in older adults: a scoping review. Eur J Ageing 2017;14:323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bales CW, Ritchie CS. Sarcopenia, weight loss, and nutritional frailty in the elderly. Annu Rev Nutr 2002;22:309–23. [DOI] [PubMed] [Google Scholar]
- 20.Lally F, Crome P. Understanding frailty. Postgrad Med J 2007;83:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fontana L, Addante F, Copetti M et al. Identification of a metabolic signature for multidimensional impairment and mortality risk in hospitalized older patients. Aging Cell 2013;12:459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sergi G, Veronese N, Fontana L et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. study. J Am Coll Cardiol 2015;65:976–83. [DOI] [PubMed] [Google Scholar]
- 23.Afilalo J, Alexander KP, Mack MJ et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol 2014;63:747–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel A, Goodman SG, Yan AT et al. Frailty and Outcomes After Myocardial Infarction: Insights From the CONCORDANCE Registry. J Am Heart Assoc 2018;7:e009859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Llao I, Ariza-Sole A, Sanchis J et al. Invasive strategy and frailty in very elderly patients with acute coronary syndromes. EuroIntervention 2018;14:e336–e342. [DOI] [PubMed] [Google Scholar]
- 26.Yoshioka N, Takagi K, Morita Y et al. Impact of the clinical frailty scale on mid-term mortality in patients with ST-elevated myocardial infarction. Int J Cardiol Heart Vasc 2019;22:192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murali-Krishnan R, Iqbal J, Rowe R et al. Impact of frailty on outcomes after percutaneous coronary intervention: a prospective cohort study. Open Heart 2015;2:e000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denfeld QE, Winters-Stone K, Mudd JO, Gelow JM, Kurdi S, Lee CS. The prevalence of frailty in heart failure: A systematic review and meta-analysis. Int J Cardiol 2017;236:283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Afilalo J, Lauck S, Kim DH et al. Frailty in Older Adults Undergoing Aortic Valve Replacement: The FRAILTY-AVR Study. J Am Coll Cardiol 2017;70:689–700. [DOI] [PubMed] [Google Scholar]
- 30.Metze C, Matzik AS, Scherner M et al. Impact of Frailty on Outcomes in Patients Undergoing Percutaneous Mitral Valve Repair. JACC Cardiovasc Interv 2017;10:1920–1929. [DOI] [PubMed] [Google Scholar]
- 31.O'Neill DE, Forman DE. Cardiovascular care of older adults. BMJ 2021;374:n1593. [DOI] [PubMed] [Google Scholar]
- 32.Pavasini R, Maietti E, Tonet E et al. Bleeding Risk Scores and Scales of Frailty for the Prediction of Haemorrhagic Events in Older Adults with Acute Coronary Syndrome: Insights from the FRASER study. Cardiovasc Drugs Ther 2019;33:523–532. [DOI] [PubMed] [Google Scholar]
- 33.Diez-Villanueva P, Ariza-Sole A, Vidan MT et al. Recommendations of the Geriatric Cardiology Section of the Spanish Society of Cardiology for the Assessment of Frailty in Elderly Patients With Heart Disease. Rev Esp Cardiol (Engl Ed) 2019;72:63–71. [DOI] [PubMed] [Google Scholar]
- 34.Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006;35:526–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rockwood K, Rockwood MR, Mitnitski A. Physiological redundancy in older adults in relation to the change with age in the slope of a frailty index. J Am Geriatr Soc 2010;58:318–23. [DOI] [PubMed] [Google Scholar]
- 36.Rockwood K, Song X, MacKnight C et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 2012;16:601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solomon J, Afilalo J, Morin J et al. THE ESSENTIAL FRAILTY TOOLSET IN OLDER ADULTS UNDERGOING CABG. Canadian Journal of Cardiology 2019;35:S25. [Google Scholar]
- 39.Green P, Woglom AE, Genereux P et al. Gait speed and dependence in activities of daily living in older adults with severe aortic stenosis. Clin Cardiol 2012;35:307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chainani V, Shaharyar S, Dave K et al. Objective measures of the frailty syndrome (hand grip strength and gait speed) and cardiovascular mortality: A systematic review. Int J Cardiol 2016;215:487–93. [DOI] [PubMed] [Google Scholar]
- 41.Boxer RS, Wang Z, Walsh SJ, Hager D, Kenny AM. The utility of the 6-minute walk test as a measure of frailty in older adults with heart failure. Am J Geriatr Cardiol 2008;17:7–12. [DOI] [PubMed] [Google Scholar]
- 42.Chong E, Ho E, Baldevarona-Llego J et al. Frailty in Hospitalized Older Adults: Comparing Different Frailty Measures in Predicting Short- and Long-term Patient Outcomes. J Am Med Dir Assoc 2018;19:450–457 e3. [DOI] [PubMed] [Google Scholar]
- 43.Falvey JR, Ferrante LE. Frailty assessment in the ICU: translation to 'real-world' clinical practice. Anaesthesia 2019;74:700–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med 2006;166:418–23. [DOI] [PubMed] [Google Scholar]
- 45.Xue QL, Bandeen-Roche K, Tian J, Kasper JD, Fried LP. Progression of Physical Frailty and the Risk of All-Cause Mortality: Is There a Point of No Return? J Am Geriatr Soc 2021;69:908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strawbridge WJ, Shema SJ, Balfour JL, Higby HR, Kaplan GA. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci 1998;53:S9–16. [DOI] [PubMed] [Google Scholar]
- 47.Ntanasi E, Yannakoulia M, Mourtzi N et al. Prevalence and Risk Factors of Frailty in a Community-Dwelling Population: The HELIAD Study. J Aging Health 2020;32:14–24. [DOI] [PubMed] [Google Scholar]
- 48.Cheong CY, Nyunt MSZ, Gao Q et al. Risk Factors of Progression to Frailty: Findings from the Singapore Longitudinal Ageing Study. J Nutr Health Aging 2020;24:98–106. [DOI] [PubMed] [Google Scholar]
- 49.Waite I, Deshpande R, Baghai M, Massey T, Wendler O, Greenwood S. Home-based preoperative rehabilitation (prehab) to improve physical function and reduce hospital length of stay for frail patients undergoing coronary artery bypass graft and valve surgery. J Cardiothorac Surg 2017;12:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stammers AN, Kehler DS, Afilalo J et al. Protocol for the PREHAB study-Pre-operative Rehabilitation for reduction of Hospitalization After coronary Bypass and valvular surgery: a randomised controlled trial. BMJ Open 2015;5:e007250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yau DKW, Wong MKH, Wong WT et al. PREhabilitation for improving QUality of recovery after ELective cardiac surgery (PREQUEL) study: protocol of a randomised controlled trial. BMJ Open 2019;9:e027974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harada H, Kai H, Niiyama H et al. Effectiveness of Cardiac Rehabilitation for Prevention and Treatment of Sarcopenia in Patients with Cardiovascular Disease - A Retrospective Cross-Sectional Analysis. J Nutr Health Aging 2017;21:449–456. [DOI] [PubMed] [Google Scholar]
- 53.Opasich C, Patrignani A, Mazza A, Gualco A, Cobelli F, Pinna GD. An elderly-centered, personalized, physiotherapy program early after cardiac surgery. Eur J Cardiovasc Prev Rehabil 2010;17:582–7. [DOI] [PubMed] [Google Scholar]
- 54.Goldfarb M, Afilalo J, Chan A, Herscovici R, Cercek B. Early mobility in frail and non-frail older adults admitted to the cardiovascular intensive care unit. J Crit Care 2018;47:9–14. [DOI] [PubMed] [Google Scholar]
- 55.Eichler S, Salzwedel A, Reibis R et al. Multicomponent cardiac rehabilitation in patients after transcatheter aortic valve implantation: Predictors of functional and psychocognitive recovery. Eur J Prev Cardiol 2017;24:257–264. [DOI] [PubMed] [Google Scholar]
- 56.Zanettini R, Gatto G, Mori I et al. Cardiac rehabilitation and mid-term follow-up after transcatheter aortic valve implantation. J Geriatr Cardiol 2014;11:279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rogers P, Al-Aidrous S, Banya W et al. Cardiac rehabilitation to improve health-related quality of life following trans-catheter aortic valve implantation: a randomised controlled feasibility study: RECOVER-TAVI Pilot, ORCA 4, For the Optimal Restoration of Cardiac Activity Group. Pilot Feasibility Stud 2018;4:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tamuleviciute-Prasciene E, Beigiene A, Thompson MJ, Balne K, Kubilius R, Bjarnason-Wehrens B. The impact of additional resistance and balance training in exercise-based cardiac rehabilitation in older patients after valve surgery or intervention: randomized control trial. BMC Geriatr 2021;21:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Busch JC, Lillou D, Wittig G et al. Resistance and balance training improves functional capacity in very old participants attending cardiac rehabilitation after coronary bypass surgery. J Am Geriatr Soc 2012;60:2270–6. [DOI] [PubMed] [Google Scholar]
- 60.Reeves GR, Whellan DJ, O'Connor CM et al. A Novel Rehabilitation Intervention for Older Patients With Acute Decompensated Heart Failure: The REHAB-HF Pilot Study. JACC Heart Fail 2017;5:359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kitzman DW, Whellan DJ, Duncan P et al. Physical Rehabilitation for Older Patients Hospitalized for Heart Failure. N Engl J Med 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Voller H, Salzwedel A, Nitardy A, Buhlert H, Treszl A, Wegscheider K. Effect of cardiac rehabilitation on functional and emotional status in patients after transcatheter aortic-valve implantation. Eur J Prev Cardiol 2015;22:568–74. [DOI] [PubMed] [Google Scholar]
- 63.Balestroni G, Panzeri A, Omarini P et al. Psychophysical health of elderly inpatients in cardiac rehabilitation: a retrospective cohort study. Eur J Phys Rehabil Med 2020;56:197–205. [DOI] [PubMed] [Google Scholar]
- 64.Lutz AH, Delligatti A, Allsup K, Afilalo J, Forman DE. Cardiac Rehabilitation Is Associated With Improved Physical Function in Frail Older Adults With Cardiovascular Disease. J Cardiopulm Rehabil Prev 2020;40:310–318. [DOI] [PubMed] [Google Scholar]
- 65.Macchi C, Polcaro P, Cecchi F et al. One-year adherence to exercise in elderly patients receiving postacute inpatient rehabilitation after cardiac surgery. Am J Phys Med Rehabil 2009;88:727–34. [DOI] [PubMed] [Google Scholar]
- 66.Investigators LS, Pahor M, Blair SN et al. Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci 2006;61:1157–65. [DOI] [PubMed] [Google Scholar]
- 67.Molino-Lova R, Pasquini G, Vannetti F et al. Effects of a structured physical activity intervention on measures of physical performance in frail elderly patients after cardiac rehabilitation: a pilot study with 1-year follow-up. Intern Emerg Med 2013;8:581–9. [DOI] [PubMed] [Google Scholar]
- 68.Ushijima A, Morita N, Hama T et al. Effects of cardiac rehabilitation on physical function and exercise capacity in elderly cardiovascular patients with frailty. J Cardiol 2020. [DOI] [PubMed] [Google Scholar]
- 69.van Dam van Isselt EF, van Wijngaarden J, Lok DJA, Achterberg WP. Geriatric rehabilitation in older patients with cardiovascular disease: a feasibility study. Eur Geriatr Med 2018;9:853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boston Working Group on Improving Health Care Outcomes Through Geriatric Rehabilitation. Proceedings from the conference. May 16-18, 1996. Med Care 1997;35:JS1–133. [PubMed] [Google Scholar]
- 71.Tanaka S, Kamiya K, Matsue Y et al. Effects of Acute Phase Intensive Electrical Muscle Stimulation in Frail Elderly Patients With Acute Heart Failure (ACTIVE-EMS): Rationale and protocol for a multicenter randomized controlled trial. Clin Cardiol 2017;40:1189–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boxer RS, Kenny AM, Schmotzer BJ, Vest M, Fiutem JJ, Pina IL. A randomized controlled trial of high dose vitamin D3 in patients with heart failure. JACC Heart Fail 2013;1:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boxer RS, Dauser DA, Walsh SJ, Hager WD, Kenny AM. The association between vitamin D and inflammation with the 6-minute walk and frailty in patients with heart failure. J Am Geriatr Soc 2008;56:454–61. [DOI] [PubMed] [Google Scholar]
- 74.Latham NK, Anderson CS, Lee A et al. A randomized, controlled trial of quadriceps resistance exercise and vitamin D in frail older people: the Frailty Interventions Trial in Elderly Subjects (FITNESS). J Am Geriatr Soc 2003;51:291–9. [DOI] [PubMed] [Google Scholar]
- 75.Hildreth KL, Barry DW, Moreau KL et al. Effects of testosterone and progressive resistance exercise in healthy, highly functioning older men with low-normal testosterone levels. J Clin Endocrinol Metab 2013;98:1891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maggio M, Nicolini F, Cattabiani C et al. Effects of testosterone supplementation on clinical and rehabilitative outcomes in older men undergoing on-pump CABG. Contemp Clin Trials 2012;33:730–8. [DOI] [PubMed] [Google Scholar]
- 77.Strike SC, Carlisle A, Gibson EL, Dyall SC. A High Omega-3 Fatty Acid Multinutrient Supplement Benefits Cognition and Mobility in Older Women: A Randomized, Double-blind, Placebo-controlled Pilot Study. J Gerontol A Biol Sci Med Sci 2016;71:236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baskaran D, Aparicio-Ugarriza R, Ferri-Guerra J, Milyani R, Florez H, Ruiz JG. Is There an Association Between Metformin Exposure and Frailty? Gerontol Geriatr Med 2020;6:2333721420924956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Espinoza SE, Musi N, Wang CP et al. Rationale and Study Design of a Randomized Clinical Trial of Metformin to Prevent Frailty in Older Adults With Prediabetes. J Gerontol A Biol Sci Med Sci 2020;75:102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Golpanian S, DiFede DL, Khan A et al. Allogeneic Human Mesenchymal Stem Cell Infusions for Aging Frailty. J Gerontol A Biol Sci Med Sci 2017;72:1505–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Golpanian S, DiFede DL, Pujol MV et al. Rationale and design of the allogeneiC human mesenchymal stem cells (hMSC) in patients with aging fRAilTy via intravenoUS delivery (CRATUS) study: A phase I/II, randomized, blinded and placebo controlled trial to evaluate the safety and potential efficacy of allogeneic human mesenchymal stem cell infusion in patients with aging frailty. Oncotarget 2016;7:11899–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stallone G, Infante B, Prisciandaro C, Grandaliano G. mTOR and Aging: An Old Fashioned Dress. Int J Mol Sci 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Courel-Ibanez J, Vetrovsky T, Dadova K, Pallares JG, Steffl M. Health Benefits of beta-Hydroxy-beta-Methylbutyrate (HMB) Supplementation in Addition to Physical Exercise in Older Adults: A Systematic Review with Meta-Analysis. Nutrients 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singh M, Jensen MD, Lerman A et al. Effect of Low-Dose Rapamycin on Senescence Markers and Physical Functioning in Older Adults with Coronary Artery Disease: Results of a Pilot Study. J Frailty Aging 2016;5:204–207. [DOI] [PubMed] [Google Scholar]
- 85.Sheppard JP, Burt J, Lown M et al. Effect of Antihypertensive Medication Reduction vs Usual Care on Short-term Blood Pressure Control in Patients With Hypertension Aged 80 Years and Older: The OPTIMISE Randomized Clinical Trial. JAMA 2020;323:2039–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fiatarone MA, O'Neill EF, Ryan ND et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 1994;330:1769–75. [DOI] [PubMed] [Google Scholar]
- 87.Collins J, Longhurst G, Roschel H, Gualano B. Resistance Training and Co-supplementation with Creatine and Protein in Older Subjects with Frailty. J Frailty Aging 2016;5:126–34. [DOI] [PubMed] [Google Scholar]
- 88.Niccoli S, Kolobov A, Bon T et al. Whey Protein Supplementation Improves Rehabilitation Outcomes in Hospitalized Geriatric Patients: A Double Blinded, Randomized Controlled Trial. J Nutr Gerontol Geriatr 2017;36:149–165. [DOI] [PubMed] [Google Scholar]
- 89.Bauer JM, Verlaan S, Bautmans I et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 2015;16:740–7. [DOI] [PubMed] [Google Scholar]
- 90.Kim CO, Lee KR. Preventive effect of protein-energy supplementation on the functional decline of frail older adults with low socioeconomic status: a community-based randomized controlled study. J Gerontol A Biol Sci Med Sci 2013;68:309–16. [DOI] [PubMed] [Google Scholar]
- 91.Feart C, Samieri C, Rondeau V et al. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA 2009;302:638–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martinez-Gonzalez MA, Salas-Salvado J, Estruch R et al. Benefits of the Mediterranean Diet: Insights From the PREDIMED Study. Prog Cardiovasc Dis 2015;58:50–60. [DOI] [PubMed] [Google Scholar]
- 93.Talegawkar SA, Bandinelli S, Bandeen-Roche K et al. A higher adherence to a Mediterranean-style diet is inversely associated with the development of frailty in community-dwelling elderly men and women. J Nutr 2012;142:2161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kojima G, Avgerinou C, Iliffe S, Walters K. Adherence to Mediterranean Diet Reduces Incident Frailty Risk: Systematic Review and Meta-Analysis. J Am Geriatr Soc 2018;66:783–788. [DOI] [PubMed] [Google Scholar]
- 95.Lana A, Rodriguez-Artalejo F, Lopez-Garcia E. Dairy Consumption and Risk of Frailty in Older Adults: A Prospective Cohort Study. J Am Geriatr Soc 2015;63:1852–60. [DOI] [PubMed] [Google Scholar]
- 96.Ngandu T, Lehtisalo J, Solomon A et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 2015;385:2255–63. [DOI] [PubMed] [Google Scholar]
- 97.Ng TP, Feng L, Nyunt MS et al. Nutritional, Physical, Cognitive, and Combination Interventions and Frailty Reversal Among Older Adults: A Randomized Controlled Trial. Am J Med 2015;128:1225–1236 e1. [DOI] [PubMed] [Google Scholar]
- 98.Murukesu RR, Singh DKA, Shahar S, Subramaniam P. A Multi-Domain Intervention Protocol for the Potential Reversal of Cognitive Frailty: "WE-RISE" Randomized Controlled Trial. Front Public Health 2020;8:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Apostolo J, Couto F, Bobrowicz-Campos E et al. An Interregional, Transdisciplinary and Good Practice-Based Approach for Frailty: the Mind&Gait Project. Transl Med UniSa 2019;19:11–16. [PMC free article] [PubMed] [Google Scholar]
- 100.Luger E, Dorner TE, Haider S, Kapan A, Lackinger C, Schindler K. Effects of a Home-Based and Volunteer-Administered Physical Training, Nutritional, and Social Support Program on Malnutrition and Frailty in Older Persons: A Randomized Controlled Trial. J Am Med Dir Assoc 2016;17:671 e9–671 e16. [DOI] [PubMed] [Google Scholar]
- 101.Boxer R, Kleppinger A, Ahmad A, Annis K, Hager D, Kenny A. The 6-minute walk is associated with frailty and predicts mortality in older adults with heart failure. Congest Heart Fail 2010;16:208–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Russo N, Compostella L, Tarantini G et al. Cardiac rehabilitation after transcatheter versus surgical prosthetic valve implantation for aortic stenosis in the elderly. Eur J Prev Cardiol 2014;21:1341–8. [DOI] [PubMed] [Google Scholar]
- 103.Kamiya K, Sato Y, Takahashi T et al. Multidisciplinary Cardiac Rehabilitation and Long-Term Prognosis in Patients With Heart Failure. Circ Heart Fail 2020;13:e006798. [DOI] [PubMed] [Google Scholar]
- 104.Baldasseroni S, Pratesi A, Francini S et al. Cardiac Rehabilitation in Very Old Adults: Effect of Baseline Functional Capacity on Treatment Effectiveness. J Am Geriatr Soc 2016;64:1640–5. [DOI] [PubMed] [Google Scholar]
- 105.Pressler A, Christle JW, Lechner B et al. Exercise training improves exercise capacity and quality of life after transcatheter aortic valve implantation: A randomized pilot trial. Am Heart J 2016;182:44–53. [DOI] [PubMed] [Google Scholar]
- 106.Mazza A, Camera F, Maestri A et al. [Elderly patient-centered rehabilitation after cardiac surgery]. Monaldi Arch Chest Dis 2007;68:36–43. [DOI] [PubMed] [Google Scholar]
- 107.Tonet E, Maietti E, Chiaranda G et al. Physical activity intervention for elderly patients with reduced physical performance after acute coronary syndrome (HULK study): rationale and design of a randomized clinical trial. BMC Cardiovasc Disord 2018;18:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kimber DE, Kehler DS, Lytwyn J et al. Pre-Operative Frailty Status Is Associated with Cardiac Rehabilitation Completion: A Retrospective Cohort Study. J Clin Med 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reeves GR, Whellan DJ, Duncan P et al. Rehabilitation Therapy in Older Acute Heart Failure Patients (REHAB-HF) trial: Design and rationale. Am Heart J 2017;185:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pahor M, Guralnik JM, Ambrosius WT et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA 2014;311:2387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Leon-Munoz LM, Garcia-Esquinas E, Lopez-Garcia E, Banegas JR, Rodriguez-Artalejo F. Major dietary patterns and risk of frailty in older adults: a prospective cohort study. BMC Med 2015;13:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kojima G, Liljas A, Iliffe S, Jivraj S, Walters K. A systematic review and meta-analysis of prospective associations between alcohol consumption and incident frailty. Age Ageing 2018;47:26–34. [DOI] [PubMed] [Google Scholar]