Abstract
This study was designed to investigate the effect of hypercholesterolemia on the reproductive performance of premature male rats and to evaluate the influence of L-Carnitine (CAR) in maintaining their fertility. Sixty rats were divided randomly into three groups. Control group (CG n=20 rats), cholesterol feeding group 1 (CFG1 n=20 rats) fed 1.5% cholesterol with diet for one month, and cholesterol feeding group 2 (CFG2 n=20 rats) fed 1.5% cholesterol with diet + CAR 150 mg/kg body weight (B.W.) given by water for one month. Results showed a significant increase in body weight of CFG1 compared with CG and CFG2. The lipid profile of CFG1 after one month of feeding cholesterol showed a significant increase in serum cholesterol and triglyceride compared with CG and with the group that watered by CAR and CFG2. Results of sperms parameters in CGF2 showed a significant increase in sperms count with sperms live percentage and a significant decrease in sperms abnormalities percentage compared with CGF1 and CG. The hormonal profile showed a significant decrease in serum testosterone levels in rats from CFG1 compared with CFG2 and CG. In conclusion, CAR is a powerful antioxidant that can maintain the parameters of sperms of hypercholesterolemic premature rats, which may enhance the fertilizing ability of subfertile rats that may occur due to hyperlipidemia.
Keywords: premature rats, cholesterol, hyperlipidemia, L-Carnitine, sperm parameters, lipid profile
Keywords: CAR-L – Carnitine, CG – control group, CFG1– cholesterol-fed group1, CFG2 – cholesterol-fed group 2, LH – Luteinizing Hormone, FSH – Follicle Stimulating Hormone, hCG – Human chorionic gonadotropin, ROS – reactive oxygen species, NFKB – Nuclear Factor kappa-light-chain-enhancer of activated B cells
Introduction
A high-fat diet can affect reproductive efficiency due to the accumulation of free radicals in testicular tissue that causes damages in Sertoli and Leydig cells, which results in functional disorders of the hypothalamic-pituitary-gonadal axis responsible for the clear spermatogenesis process [1, 2]. Using antioxidants as protective agents against the formation of free radicals was known as a strong tool in decreasing oxidative stress and maintaining the biological activities of cells. Using antioxidants has a confirmed positive action on enhancing the fertile properties of semen because they scavenge free radicals that form during the metabolic activities of sperms and leukocytes, decrease the development of premature sperms, and prevent DNA fragmentation of sperms [3].
L-Carnitine (CAR) is a potent antioxidant used as a co-factor for several important mitochondrial enzymes [4]. The therapeutic activity of CAR depends on its action as a scavenger of reactive oxygen species (ROS), metal chelating abilities, capability to repair oxidative damage, and its capacity to regenerate endogenous antioxidants activity [5]. Hyperlipidemia decreases male fertility due to its harmful effect on spermatogenesis [6–9].
The main objective of this study was to evaluate the underlying mechanism of the ameliorative effects of L-carnitine, enhancing the reproductive performance of mature hyperlipidemic premature male rats.
Material and Methods
The present study was performed on 60 premature albino male rats; their ages were around 4 weeks, with a body weight ranging between 75–100 grams. Rats were obtained from the College of Veterinary Medicine/University of Al-Qadisiyah and were fed and housed under standard nutrition and environment during the whole experimentation days.
Rats were divided randomly into two main groups: control group CG (20 rats) and treatment group (40 rats). The treatment group was subdivided into two groups (20 rats for each). Group 1 (CFG1) cholesterol-fed group were fed on a diet supplemented with 1.5% cholesterol alone while rats from treatment group 2 (CFG2) were fed on a diet supplemented with 1.5% cholesterol (BDH, England) + CAR (AMS, USA) 150 mg/kg body weight given by water, from day 30 to day 60 of age. Rats in the control group were fed on a diet that did not contain cholesterol or CAR from day 30 to day 60 of age. One day after ceasing the cholesterol supplement (on day 61 of age), the weights of rats in all groups were checked, and blood samples were collected to measure serum total cholesterol and triglyceride. The rats were sacrificed, and specimens from the liver and testis of all groups were fixed in 10% formalin for histology [10]. Semen samples were taken from the tail of the epididymis and checked for semen analysis [11].
Tissue specimens, including liver, testes, and epididymis, are extracted by scissors and collected at the end of the study to perform a histological examination of the collected specimens. A sequence of successive histological processing steps was made, as shown in this study [12].
The statistical package for social sciences (SPSS) was used to calculate the statistical analysis. The statistical analysis of physiological and histological parameters was done using Chi-square [13].
Results
Weights of rats of all groups at day 30 were 92.87±7.21 g, 94.87±6.33 g, and 95.87±5.73 g for CFG1, CFG2, and CG, respectively. After 30 days of cholesterol feeding (at day 61 of age), the weights for groups were 182.4±12.32 g, 147.4±12.32 g, and 133.96±10.43 g for CGF1, CGF2, and CG, respectively. The weight of CGF1 was significantly higher compared with CGF2 and CG, as shown in Table 1.
Table 1.
Bodyweight (B.W.) of all groups before and after feeding cholesterol (M±SD).
| Groups | CFG1 | CFG2 | CG |
|---|---|---|---|
| B.W at 1 month of age (grams) | 92.87±7.21 | 94.87±6.33 | 95.87±5.73 |
| B.W after 2 months of age (grams) | 182.4±12.32a | 147.4±12.32c | 133.96±10.43c |
*ac – significant difference in rows (P<0.01).
Table 2 represents the measures of the lipid profile of CFG1 and CFG2 after one month of cholesterol feeding (at day 61 of age) and the lipid profile of CG. The serum cholesterol for CFG1 was 126.15±5.91, and serum triglyceride was 176.06±3.56. For CFG2, serum cholesterol was 107.34±4.12, and serum triglyceride was 111.77±6.63. Serum cholesterol for the control group was 95.32±3.85, and serum triglyceride was 98.51±3.76, with significant differences between all groups.
Table 2.
Lipid profile (cholesterol and triglyceride mg/dl) for all groups (M±SD).
| GROUPS | CFG1 | CFG2 | CG |
|---|---|---|---|
| Cholesterol | 126.15±5.91a | 107.34±4.12b | 95.32±3.85c |
| Triglyceride | 176.06±3.56a | 111.77±6.63b | 98.51±3.76c |
*ab – significant difference in rows (P<0.05); *bc – significant difference in rows (P<0.05); *ac – significant difference in rows (P<0.01).
Sperm parameters after 30 days of cholesterol feeding: sperms count in 1ml of semen were 99.55±7.62×106, 121.05±8.25×106, and 115.27±4.02×106 for CFG1, CFG2, and CG, respectively, with significant differences between groups. Live sperm percentages for CFG1, CFG2, and CG were 74.32±4.38, 80.25±4.51, and 81.13±5.31 percent, respectively, with a significant difference between CGF1 compared with CFG2 and CG. Abnormal sperms percentages were 6.25±1.32, 4.73±1.37, and 3.37±1.92 percent for CFG1, CFG2, and CG, respectively, with significant differences between groups, as shown in Table 3.
Table 3.
Sperm parameters of all groups at day 61 of age.
| Groups | Sperm count ×106 | Live % | Abnormal sperms % |
|---|---|---|---|
| CFG1 | 99.55±7.62c | 74.32±4.38a | 6.25±1.32a |
| CFG2 | 121.05±8.25a | 80.25±4.51b | 4.73±1.37b |
| CG | 115.27±4.02b | 81.13±5.31b | 3.37±1.92c |
*ab – significant difference in columns (P<0.05); *bc – significant difference in columns (P<0.05); *ac – significant difference in columns (P<0.01).
The hormonal profile of all groups was recorded at day 61 of age. Serum LH levels were 15.31±1.20, 16.95±1.18, and 6.34±1.17 mIU/ml in CFG1, CFG2, and CG, respectively, with a significant difference between CFG1 and CFG2, compared with CG. Serum FSH levels were 11.77±1.82, 13.29±2.78, and 8.23±2.77 mIU/ml for CFG1, CFG2, and CG, respectively, with a significant difference between CFG1 and CFG2, compared with CG. Serum testosterone levels were 4.24±0.42, 5.66±0.53, and 7.81±1.89 ng/ml for CFG1, CFG2, and CG, respectively, with a significant difference between CGF1 and CFG2, and CG as in Table 4.
Table 4.
Hormonal profile of all groups at day 61 of age (M±SD)
| Parameters | |||
|---|---|---|---|
| Groups | LH (mIU/ml) | FSH (mIU/ml) | Testosterone (ng/ml) |
| CFG1 | 15.31±1.20a | 11.77±1.82a | 4.24±0.42c |
| CFG2 | 16.95±1.18a | 13.29±2.78a | 5.66±0.53b |
| CG | 6.34±1.17c | 8.23±2.77c | 7.81±1.89a |
*ab – significant difference in columns (P<0.05); *bc – significant difference in columns (P<0.05); *ac – significant difference in columns (P<0.01).
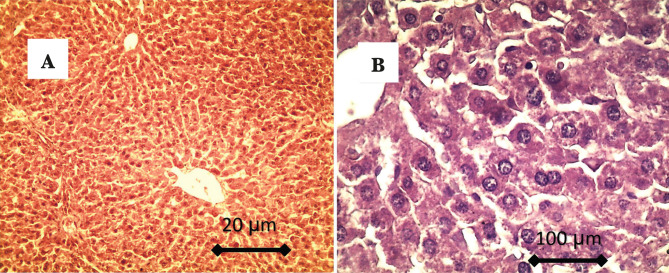
Histological findings of the liver in CG showed normal hepatic tissue, characterized by radially arranged hepatocytes around the normal central vein. The hepatocytes showed hexagonal and normal shape in higher magnification, as shown in Figure 1 AB.
Figure 1.
A – showed normal hepatic tissue, characterized by the presence of radially arrangement of hepatocytes around the normal central vein; B – In higher magnification, the hepatocytes showed hexagonal and normal shape.
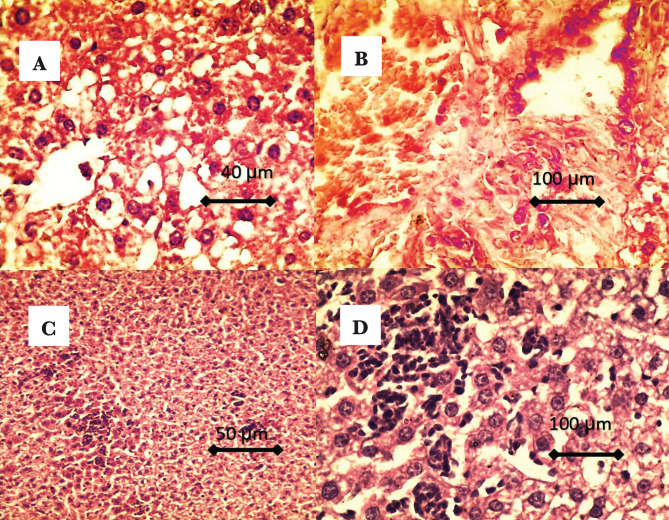
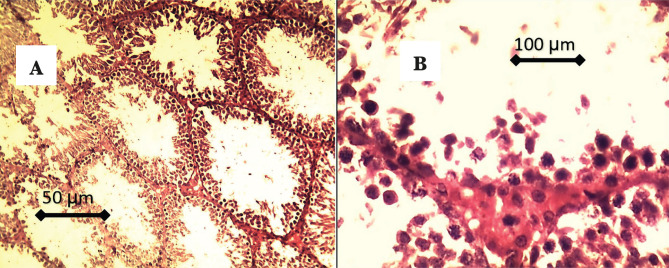
After one month of cholesterol administration, histological findings of CFG1 livers showed congestion of the central vein and loss of hepatic architecture; the bile duct showed congestion with hyperplasia (Figure 2 AB). Marked vacuolation of the hepatocyte, presence of fatty change within hepatocyte, hepatocyte showed as binucleated. Also, there is infiltration (aggregation) of the inflammatory cells (mainly macrophage) within hepatic tissue, hepatocyte shown as a signet (Figure 2 CD).
Figure 2.
A – Showed loss of hepatic architecture, marked vacuolation of the hepatocyte, presence of fatty change within hepatocyte (hepatocyte shown as signet-like shape); B – The bile duct showed congestion with hyperplasia; C and D – There is infiltration (aggregation) of the inflammatory cells (mainly macrophage) within hepatic tissue, and the hepatocytes showed as binucleated.
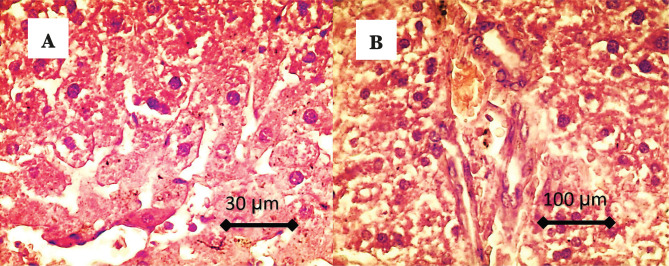
While in the CFG2 (CAR) group, histological findings of the liver show the presence of hepatic architecture (radial arrangement of hepatocyte around normal central vein), presence of dilation of the sinusoid, mild proliferation of the Kupffer cells, the hepatocyte showed normal hexagonal shape, congestion and mild hyperplasia of the bile duct (Figure 3 AB).
Figure 3.
A – The histological findings of the liver showed the presence of hepatic architecture (radial arrangement of hepatocyte around normal central vein), presence of dilation of the sinusoid, mild proliferation of the Kupffer cells, and the hepatocyte showed normal hexagonal shape; B – There is congestion and mild hyperplasia of the bile duct.
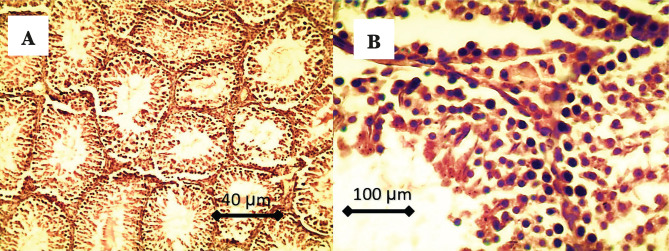
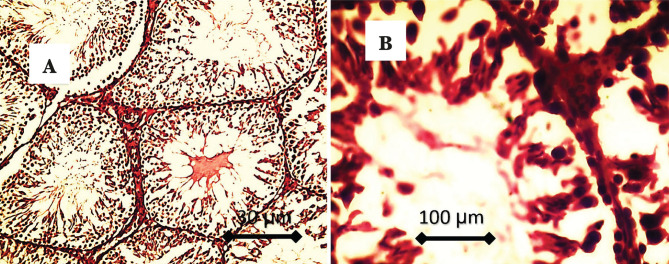
Histological findings of testicular tissue in CG are characterized by complete spermatogenesis, and the seminiferous tubules showed compact, circular, and normal shapes. A higher number of spermatogonia, primary and secondary spermatocyte, and spermatids can be seen in the lumen of seminiferous tubules, as represented in Figure 4 AB,
Figure 4.
A – There is complete spermatogenesis, and the seminiferous tubules showed compact, circular, and normal shapes; B – Higher number of spermatogonia, primary and secondary spermatocytes, and spermatids can be seen in the lumen of seminiferous tubules. Also, there are high numbers of Leydig cells in the interstitial tissue.
Histological findings of testes in cholesterol feeding group (CFG1) showed suppression of spermatogenesis characterized by vacuolation of spermatogonia, with few numbers of primary and secondary spermatocytes, absence of sperms in the lumen of the seminiferous tubules which showed very wide, few numbers of Leydig cells in the interstitial tissue with presence of adipose tissue, as shown in Figure 5 AB.
Figure 5.
A – There is suppression of spermatogenesis characterized by the absence of sperms in the lumen of the seminiferous tubules, which was very wide; B – Vacuolation of spermatogonia, few numbers of primary and secondary spermatocytes, few numbers, and vacuolation of Leydig cells in the interstitial tissue.
In CFG2, there is complete spermatogenesis characterized by the presence of spermatogonia, high numbers of spermatocytes, and spermatids in the lumen of seminiferous tubules. There is mild vacuolation of spermatogonia in a few seminiferous tubules and proliferation of Leydig cells in the interstitial tissue, as seen in Figure 6 AB.
Figure 6.
A – There is complete spermatogenesis characterized by the presence of spermatogonia, high numbers of spermatocytes, spermatids, and spermatozoa in the lumen of seminiferous tubules; B – Higher magnification showed mild vacuolation of spermatogonia in a few seminiferous tubules and proliferation of Leydig cells in the interstitial tissue.
Discussion
Body weights of CFG1 rats increased significantly compared with CFG2 and CG after one month of cholesterol feeding due to high lipid intake. This causes the accumulation of lipids in the bodies and internal organs of rats, as represented by the increase of body weight, and induces fatty changes of hepatocytes such as vacuolation and loss of architecture. Moreover, blood levels of total cholesterol and triglyceride increased significantly in CFG1 and CFG2 compared with CG after one month of high lipid intake, as shown in Table 2. Those results resemble other studies [14–20] that fed rats and mice with 0.5–1% cholesterol for several weeks and gained body weight and hypercholesterolemia with hypertriglyceridemia.
This study was performed to induce partial infertility by feeding a high lipid content diet that causes hyperlipidemia that affects the spermatogenesis process. Our results meet the goal of reducing rats’ fertility, as shown in Table 3. The results are similar to many previous data obtained from feeding high cholesterol diet to lab animals (mice, rats, rabbits) which caused detrimental effects on testicular tissue and functions such as spermatogenesis, steroidogenesis, epididymal sperm maturation process, sperm quality parameters, sperm fertilizing capacity and fertility index [20–26]. Other research [9, 27, 28] suggested that feeding a high cholesterol diet for several weeks caused an adverse effect on the secretory functions of Leydig and Sertoli cells reducing sperm concentration and motility percentages and increasing abnormal sperm morphology. Ouvrier et al. [25] suggested that high cholesterol diet intake can alter the epididymal epithelium structure due to the accumulation of cholesterol droplets in the smooth muscles that lines the epididymal epithelium weakening the peristaltic movement of the epididymis delaying sperms maturation and progression. Table 4 showed that high lipid diet intake decreased serum levels of testosterone. Similar results were recorded in another study [29], which mentioned that hypercholesterolemia inhibited the steroidogenesis process in testis by modulating the bioactive peptides of the renin-angiotensin system that occur in testicles which leads to decreased testosterone production. Tanaka et al. [21] mentioned that hypercholesterolemia reduces rats’ serum testosterone levels due to the reduction in testicular LH\HCG binding. Many types of research on humans [30–33] reported a negative correlation between serum triglyceride levels and serum testosterone levels. They mentioned that hypercholesterolemia leads to elevated serum triglyceride levels, which have deleterious effects on spermatogenesis, decrease sperms motility, and decrease serum testosterone levels in infertile men. Our results showed the same data in a rat model. Hypercholesterolemia also causes damage to testicular tissue due to the excessive formation of free radicals that have a cytotoxic effect on spermatozoa [22, 34–36]. Previous studies indicated that a diet supplemented with (antioxidants and/or agents that enhances lipid metabolism) could maintain the reproductive functions of the testis in hypercholesteremic rats [21, 33, 34]. L-Carnitine is known to have antioxidant [37] and anti-inflammatory [38] properties. L-Carnitine can easily cross the biological membranes because of its small size and high lipophilicity [39]. L-Carnitine can improve mitochondrial functions given its ability to stimulate Sirtuin 1 and 3 [40–42], quench free radicals and inhibit ROS generators.
Furthermore, it down-regulates the CAR-dependent pro-inflammatory NF-kB pathway [38]. Our study showed significant testicular histological protection in the hyperlipidemic group of rats treated with CAR compared with the not-treated hyperlipidemic group. This may be attributed to CAR’s ability to scavenge many free radicals such as singlet oxygen, H2O2, and hydroxyl radicals which formed excessively during lipid peroxidation and metabolic processes [43]. This study showed good histological protection for spermatogenic layers in the CAR treated group compared with the non-CAR treated group, reflecting the better concentration of sperms and the lesser percentage of sperms abnormalities in the treated group.
Conclusions
According to our study’s results and discussions, oral administration of CAR (150 mg/kg.B.W) with diet established lowering ROS has a considerable favorable influence on male reproductive function by increasing sperm parameters. In addition to being a hepato-protective agent and having an anti-hyperlipidemic effect by lowering blood cholesterol and triglyceride levels, dealing of CAR to premature male rats resulted in the greatest reproductive profile.
Acknowledgments
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The approval for this study was obtained from the Ethics Committee of the College of Veterinary Medicine, University of Al-Qadisiyah, under Ref. number 543/2018.
Personal thanks
The authors would like to thank the institute of lab animals at the University of Al-Qadisiyah for their cooperation in achieving this study.
Authorship
KMK and ASA contributed to conceptualizing, KMK and DHA contributed to the methodology, DHA contributed to writing the original draft, KMK contributed to editing the manuscript, ASA and KGA contributed to data collection, data analysis, and histopathological configurations.
References
- 1.Awoniyi DO, Aboua YG, Marnewick J, Brooks N. The Effects of Rooibos (Aspalathus linearis), Green Tea (Camellia sinensis) and Commercial Rooibos and Green Tea Supplements on Epididymal Sperm in Oxidative Stress-induced Rats. Phytotherapy Research. 2012;26(8):1231–1239. doi: 10.1002/ptr.3717. [DOI] [PubMed] [Google Scholar]
- 2.Nemzer B, Chang T, Xie Z, Pietrzkowski Z, et al. Decrease of free radical concentrations in humans following consumption of a high antioxidant capacity natural product. Food Science & Nutrition. 2014;2(6):647–654. doi: 10.1002/fsn3.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nikolovski M, Mickov L, Dovenska M, Petkov V, et al. Influence of glutathione on kinetic parameters of frozen-thawed spermatozoa from ovchepolian pramenka rams. Mac Vet Rev. 2014;37(2):121–128. doi: 10.14432/j.macvetrev.2014.05.014. [DOI] [Google Scholar]
- 4.Agarwal A, Nallella KP, Allamaneni SS, Said TM. Role of antioxidants in treatment of male infertility: an overview of the literature. Reprod Biomed Online. 2004;8:616–27. doi: 10.1016/s1472-6483(10)61641-0. [DOI] [PubMed] [Google Scholar]
- 5.Gorąca A, Huk-Kolega H, Piechota A, Kleniewska P, et al. Lipoic acid - biological activity and therapeutic potential. Pharmacol Rep. 2011;63(4):849–58. doi: 10.1016/s1734-1140(11)70600-4. [DOI] [PubMed] [Google Scholar]
- 6.Gupta RS, Dixit VP. Effect of dietary cholesterol on spermatogenesis. Z Ernahrungswiss. 1988 Dec;27(4):236–43. doi: 10.1007/BF02019512. [DOI] [PubMed] [Google Scholar]
- 7.Saez Lancellotti TE, Boarelli PV, Monclus MA, Cabrillana ME, et al. Hypercholesterolemia impaired sperm functionality in rabbits. PLoS One. 2010 Oct 18;5(10):e13457. doi: 10.1371/journal.pone.0013457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Purohit A, Daradka HM. Effect of mild hyperlipidaemia on testicular cell population dynamics in albino rats. Indian J Exp Biol. 1999 Apr;37(4):396–8. [PubMed] [Google Scholar]
- 9.Yamamoto Y, Shimamoto K, Sofikitis N, Miyagawa I. Effects of hypercholesterolaemia on Leydig and Sertoli cell secretory function and the overall sperm fertilizing capacity in the rabbit. Hum Reprod. 1999 Jun;14(6):1516–21. doi: 10.1093/humrep/14.6.1516. [DOI] [PubMed] [Google Scholar]
- 10.Verstegen J, Iguer-Ouada M, Onclin K. Computer assisted semen analyzers in andrology research and veterinary practice. Theriogenology. 2002 Jan 1;57(1):149–79. doi: 10.1016/s0093-691x(01)00664-1. [DOI] [PubMed] [Google Scholar]
- 11.Ombelet W, Deblaere K, Bosmans E, Cox A, et al. Semen quality and intrauterine insemination. Reprod Biomed Online. 2003 Oct-Nov;7(4):485–92. doi: 10.1016/s1472-6483(10)61894-9. [DOI] [PubMed] [Google Scholar]
- 12.Bolon B, Duryea D, Foley JF. Pathology of the Developing Mouse. CRC Press; 2015. Histotechnological Processing of Developing Mice. pp. 195–209. [Google Scholar]
- 13.Machin D, Campbell MJ, Walters SJ. 4th ed. England: John Wiley & Sons; 2007. Medical statistics: a textbook for the health sciences. pp. 99–116. [Google Scholar]
- 14.Ismail MF, Gad MZ, Hamdy MA. Study of the hypolipidemic properties of pectin, garlic and ginseng in hypercholesterolemic rabbits. Pharmacol Res. 1999 Feb;39(2):157–66. doi: 10.1006/phrs.1998.0421. [DOI] [PubMed] [Google Scholar]
- 15.Yu-Ming W, Bei Z, Yong X, Zhao J, et al. The mechanism of dietary cholesterol effects on lipids metabolism in rats. Lipids in Health and Disease. 2010;9(4):1–6. doi: 10.1186/1476-511X-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okazaki M, Morio Y, Iwai S, Miyamoto K, et al. Age-related changes in blood coagulation and fibrinolysis in mice fed on a high-cholesterol diet. Exp Anim. 1998 Oct;47(4):237–46. doi: 10.1538/expanim.47.237. [DOI] [PubMed] [Google Scholar]
- 17.Ali M, Al-Qattan K, Al-Enezi F, Khanafer R, Mustafa T. Effect of allicin from garlic powder on serum lipids and blood pressure in rats fed with a high cholesterol diet Prostaglandins Leukot. Essent. Fatty Acids. 2000;62(4):253–9. doi: 10.1054/PLEF.2000.0152. [DOI] [PubMed] [Google Scholar]
- 18.Bennani-Kabchi N, Kehel L, El Bouayadi F, Fdhil H, et al. New model of atherosclerosis in insulin resistant sand rats: hypercholesterolemia combined with D2 vitamin. Atherosclerosis. 2000;150(1):55–61. doi: 10.1016/s0021-9150(99)00365-2. [DOI] [PubMed] [Google Scholar]
- 19.Vlad M, Bordas E, Caseanu E, Uza G, et al. Effect of cuprofilin on experimental atherosclerosis.Biol. Trace Elem.Res. 1995;48(1):99–109. doi: 10.1007/BF02789082. [DOI] [PubMed] [Google Scholar]
- 20.Miao J, Zang X, Cui X, Zhang J. Autophagy, Hyperlipidemia, and Atherosclerosis. Adv Exp Med Biol. 2020;1207:237–264. doi: 10.1007/978-981-15-4272-5_18. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka M, Nakaya S, Kumai T, Watanabe M, et al. Impaired testicular function in rats with diet-induced hypercholesterolemia and/or streptozotocin diabetes mellitus. Endocr. Res. 2001;27:109–117. doi: 10.1081/erc-100107174. [DOI] [PubMed] [Google Scholar]
- 22.Shalaby MA, el-Zorba HY, Kamel GM. Effect of alpha-tocopherol and simvastatin on male fertility in hypercholesterolemic rats. Pharmacol Res. 2004 Aug;50(2):137–42. doi: 10.1016/j.phrs.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Liu C, Chou Y, Lin S, Wu S, et al. Serum lipid profiles are associated with semen quality. Asian J Androl. 2017 Nov-Dec;19(6):633–638. doi: 10.4103/1008-682X.195240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saes lancillotti T, Boarelli P, Monclus M, Cabrillana M, et al. Hypercholesterolemia impaired sperm functionality in rabbits. PLoS one. 2010;5:e13457. doi: 10.1371/journal.pone.0013457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ouvrier A, Alves G, Damon-Soubeyrand C, Marceao G, et al. Diertary cholesterol induced post testicular infertility. PLoS one. 2011;6:e26966. doi: 10.1371/journal.pone.0026966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashrafi H, Ghabili K, Alihemmati A, Jouyban A, et al. The effect of quince leaf (Cydonia Oblonga Miller) decoction on testes in hypercholesterolemic rabbits: a pilot study. Afr J Tradit Complement Altern Med. 2013;10:277–282. doi: 10.4314/ajtcam.v10i2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diaz-Fontdevilla M, Bustos-Obregon E, Fornes M. Distribution of filipin-sterol complexes in sperm membranes from hypercholesterolemic rabbits. Andrologia. 1992;24:279–283. doi: 10.1111/j.1439-0272.1992.tb02653.x. [DOI] [PubMed] [Google Scholar]
- 28.Diaz-Fontdevila M, Bustos-Obregón E. Cholesterol and polyunsaturated acid enriched diet: effect on kinetics of the acrosome reaction in rabbit spermatozoa. Mol Reprod Dev. 1993 Jun;35(2):176–80. doi: 10.1002/mrd.1080350211. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Martos J, Arrazola M, Mayas M, Carrera-Gonzalez M, et al. Diet-induced hypercholesterolemia impaired testicular steroidogenesis in mice through the renin angiotensin system. Gen Comp Indocrinol. 2011;173:15–19. doi: 10.1016/j.ygcen.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Zmuda J, Cauley J, Kriska A. Longitudinal relation between endogenous testosterone and cardiovascular disease risk factors in middle-aged men. A 13-year follow-up of former Multiple Risk Factor Intervention Trial participants. Am J Epidemiol. 1997;146:609–617. doi: 10.1093/oxfordjournals.aje.a009326. [DOI] [PubMed] [Google Scholar]
- 31.Pushpendra A, Jain G. Hyper-Lipidemia and Male Fertility: A Critical Review of Literature. Andrology. 2015;(Los Angel) 4:141. doi: 10.4172/2167-0250.1000141. [DOI] [Google Scholar]
- 32.Vignon F, Koll-Back MH, Clavert A, Cranz C. Lipid composition of human seminal plasma. Arch Androl. 1989;22:49–53. doi: 10.3109/01485018908986750. [DOI] [PubMed] [Google Scholar]
- 33.Ergün A, Köse SK, Aydos K, Ata A, Avci A. Correlation of seminal parameters with serum lipid profile and sex hormones. Arch Androl. 2007;53:21–23. doi: 10.1080/01485010600888961. [DOI] [PubMed] [Google Scholar]
- 34.Bashandy A. Effect of fixed oil of nigella sativa on male fertility in normal and hyperlipidemic rats. Int J Pharmacol. 2007;3:27–33. doi: 10.3923/ijp.2007.27.33. [DOI] [Google Scholar]
- 35.Liu CY, Chang TC, Lin SH, Wu ST, et al. Metformin Ameliorates Testicular Function and Spermatogenesis in Male Mice with High-Fat and High-Cholesterol Diet-Induced Obesity. Nutrients. 2020 Jun 29;12(7):1932. doi: 10.3390/nu12071932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang K, Lv Z, Jia X, Huang D. Melatonin prevents testicular damage in hyperlipidaemic mice. Andrologia. 2012 Aug;44(4):230–6. doi: 10.1111/j.1439-0272.2012.01272.x. [DOI] [PubMed] [Google Scholar]
- 37.Boyacioglu M, Turgut H, Akgullu C, Eryilmaz U, et al. The effect of L-carnitine on oxidative stress responses of experimental contrast-induced nephropathy in rats. J Vet Med Sci. 2014 Jan;76(1):1–8. doi: 10.1292/jvms.13-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sawicka AK, Renzi G, Olek RA. The bright and the dark sides of L-carnitine supplementation: a systematic review. Journal of the International Society of Sports Nutrition. 2020;17(1):1932. doi: 10.1186/s12970-020-00377-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki Y, Tsuchiya M, Packer L. Thioctic acid and dihydrolipoic acid are novel antioxidants which interact with reactive oxygen species. Free Radical Res. Communications. 1991;15(5):255–263. doi: 10.3109/10715769109105221. [DOI] [PubMed] [Google Scholar]
- 40.Valdecantos MP, Pérez-Matute P, González-Muniesa P, Prieto-Hontoria PL, et al. Lipoic acid improves mitochondrial function in nonalcoholic steatosis through the stimulation of sirtuin 1 and sirtuin 3. Obesity (Silver Spring). 2012 Oct;20(10):1974–83. doi: 10.1038/oby.2012.32. [DOI] [PubMed] [Google Scholar]
- 41.Elnaga N. Effect of cholesterol and/or Methionine on the testis of rats. The Egyptian Journal of Hospital Medicine. 2012;49:857–878. doi: 10.21608/EJHM.2012.16220. [DOI] [Google Scholar]
- 42.Vicari E, Calogero AE. Effects of treatment with carnitines in infertile patients with prostato-vesiculo-epididymitis. Hum Reprod. 2001 Nov;16(11):2338–42. doi: 10.1093/humrep/16.11.2338. [DOI] [PubMed] [Google Scholar]
- 43.Moini H, Packer L, Saris NE. Antioxidant and prooxidant activities of alpha-lipoic acid and dihydrolipoic acid. Toxicol Appl Pharmacol. 2002 Jul 1;182(1):84–90. doi: 10.1006/taap.2002.9437. [DOI] [PubMed] [Google Scholar]