Abstract
Background:
The objective was to evaluate patterns of pediatric coronavirus disease 2019 testing in a large health system throughout the pandemic, before and after school reopening.
Methods:
This was a cross-sectional time-series study of clinical virology results from children tested for severe acute respiratory syndrome coronavirus 2 in Southern Connecticut and areas of New York and Rhode Island. Data collected include demographics, hospital admission, changes in percent positive tests over time, detection intervals in persistently positive children and cycle threshold values. The setting was the Yale New Haven Health System has 6 hospitals at 4 Connecticut locations, 1 hospital in Rhode Island and ambulatory locations in Connecticut, Rhode Island and New York. Participants included twenty-three–thousand one-hundred thirty-seven children ≤ 18 years of age, tested for coronavirus disease 2019 at an ambulatory testing site, the emergency department or on an inpatient unit within the Yale New Haven Health System.
Results:
Among all tests, 3.2% were positive. Older children consistently made up the larger portion of positive pediatric cases, regardless of community prevalence. Increased pediatric cases later in the pandemic when prevalence in adults was relatively low correlates with a higher number of tests performed in children and not with an increased positivity rate. No significant changes in trends of positivity were detected after the reopening of schools. Symptomatic and asymptomatic children had similar cycle threshold values regardless of age, and a subset of children demonstrated persistent viral detection, some for as long as 6 weeks.
Conclusion:
An increase in pediatric cases documented in the late summer was predominately due to increased access to testing for children. The percent positivity in children did not change in the first 3 weeks after school opened. A subset of children has detectable severe acute respiratory syndrome coronavirus 2 RNA in the upper respiratory tract for weeks after the initial infection.
Keywords: coronavirus disease 2019, severe acute respiratory syndrome coronavirus 2 testing, children
The clinical syndrome (coronavirus disease 2019 [COVID-19]) from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children has been milder compared with adults.1–4 However, serious and potentially fatal postinfectious complications such as multisystem inflammatory syndrome in children have raised concerns about the long-term effects of this virus on previously healthy children.5 School and child care closures are one enacted strategy to interrupt transmission of the virus and protect children. However, these closures negatively affect children, especially those from disadvantaged backgrounds.6
It is not clear how children of various ages contribute to community SARS-CoV-2 spread. Household studies suggest children are both less susceptible to becoming infected and less likely to transmit disease.7,8 Yet children can have high viral loads, prolonged SARS-CoV-2 detection in the respiratory tract and transmit virus to children of similar ages, suggesting a larger role in viral transmission than previously thought.9–11
While school closings may decrease disease spread, other evidence suggests that schools and child care facilities can safely remain open with appropriate risk mitigation.12–14 In late summer, as schools began to reopen in the United States, large numbers of children tested positive for SARS-CoV-2 causing many school districts to choose between in-person, hybrid and remote learning.15 In Connecticut, the 14-day average community prevalence of <10 cases/100,000 people favors in person learning and >25 cases/100,000 people favors remote education.16 The youngest and oldest children have higher rates of infection compared with school-age children such that children might safely attend elementary school while older children remain remote.2,4,15
Repeat testing is no longer recommended to clear healthy COVID-19 patients from isolation.17 Yet many schools and universities require negative COVID-19 tests before in-person education regardless of previous results. Children often shed SARS-CoV-2 beyond 10 days, preventing return to school for some children no longer considered infectious.9,10,18,19
We examined the evolution of pediatric testing for SARS-CoV-2 within the Yale New Haven Health System (YNHH-S) from March to the end of September to better understand how testing patterns contributed to the identification of COVID-19 positive children. We studied the duration of viral shedding to inform policies on repeat testing and return to school and the correlations between age and/or symptoms and the cycle threshold (Ct) value to characterize SARS-CoV-2 levels in the upper respiratory tract.
METHODS
Setting
For this cross-sectional time-series, we captured electronic health record and laboratory data from the YNHH-S, which consists of 4 hospitals across 6 campuses in Southern Connecticut, a hospital in Rhode Island and a network of ambulatory providers with service areas in New York, Connecticut and Rhode Island. Yale New Haven Children’s Hospital is a full-service pediatric hospital, while other community hospitals have dedicated pediatric wards and neonatal intensive care units. Data spanned the first 7 months of the pandemic (March 1, 2020, to September 26, 2020) and included any patient tested for SARS-CoV-2 by YNHH-S laboratories or processed and sent-out by YNHH-S laboratories. Test results from specimens originating from nursing homes, state employee testing and YNHH-S employee screening were excluded from further analysis. Clinical and sociodemographic data were extracted from the electronic medical record system (Epic, Verona, WI) for children and adolescents ≤ 18 years of age (Table 1).
TABLE 1.
Characteristics of Children ≤ 18 Years Tested for SARS-CoV-2
| Characteristic | Test Results | |||
|---|---|---|---|---|
| Positive, n = 734 | Negative, n = 22,403 | % Positive Tests, 3.2% | P | |
| Median age, yr (IQR) | 12.0 (5.0–16.0) | 10.0 (4.0–16.0) | <0.001 | |
| Gender (% male) | 359 (48.9%) | 11,638 (51.9%) | 0.10 | |
| Race/ethnicity | <0.001 | |||
| Hispanic | 312 (42.5%) | 5161 (23.0%) | 5.7% | |
| Non-Hispanic Black | 142 (19.3%) | 2959 (13.2%) | 4.6% | |
| Non-Hispanic White | 191 (26.0%) | 11,304 (50.5%) | 1.7% | |
| Non-Hispanic other | 50 (6.8%) | 1520 (6.8%) | 3.2% | |
| Missing | 39 (5.3%) | 1459 (6.5%) | 2.6% | |
| Testing interval | <0.001 | |||
| March 1 to April 11 | 126 (17.2%) | 797 (3.6%) | 13.7% | |
| April 12 to May 23 | 253 (34.5%) | 2150 (9.6%) | 10.5% | |
| May 24 to July 4 | 78 (10.6%) | 3819 (17.0%) | 2.0% | |
| July 5 to August 15 | 153 (20.8%) | 7238 (32.3%) | 2.1% | |
| August 16 to September 26 | 124 (16.9%) | 8399 (37.5%) | 1.4% | |
| Patient location | <0.001 | |||
| Ambulatory | 688 (93.7%) | 19,714 (88.0%) | 3.4% | |
| Inpatient | 46 (6.3%) | 2689 (12.0%) | 1.7% | |
IQR indicates interquartile range.
Testing for COVID-19
Samples tested were predominantly nasopharyngeal or deep nasal (mid-turbinate) swabs collected in viral transport media or saline, but some deep nasal swab samples were directly collected into proprietary collection devices (Aptima Multitest; Hologic, San Diego, CA). Samples were tested for COVID-19 either (1) locally, with a Food and Drug Administration authorized version of the Centers for Disease Control and Prevention (CDC) assay (implemented March 13, 202020), Xpert Xpress SARS-CoV-2 Test (March 29, 2020; Cepheid, Sunnyvale, CA), BioGx SARS-CoV-2 Reagents for BD Max (April 11, 2020; Becton Dickinson, Franklin Lakes, NJ), TaqPath COVID-19 Combo Kit (May 6, 2020; ThermoFisher, Waltham, MA), Aptima SARS-CoV-2 Assay (May 14, 2020; Hologic) and Simplexa COVID-19 Direct (June 4, 2020; Diasorin, Cypress, CA) or (2) sent out to an affiliated reference laboratory. Ct values were available for samples tested by CDC assay, Xpert Xpress and TaqPath assays. For Food and Drug Administration emergency use authorized assays, all manufacturers’ instructions were followed. Indications for testing are provided for children where Ct values were available (Table, Supplementary Digital Content 1, http://links.lww.com/INF/E263 and Table, Supplementary Digital Content 2, http://links.lww.com/INF/E264). For data analysis, test results were organized by the date and patient age at the time of sample collection.
Descriptive Statistics
Analyses for the pediatric population (≤18 years of age who had at least 1 SARS-CoV-2 test during the study period) were conducted according to the variables of interest (age, race and hospitalization status). Different patient characteristics by test positivity were examined using χ2 or Wilcoxon rank-sum tests for categorical and continuous variables, respectively.
Time-Series Analysis
To calculate weekly rates and trends of SARS-CoV-2, positive tests were de-duplicated for each patient (eg, only the first positive test in a patient with multiple positives was included). Rates for each time period and each age cohort were estimated using the total number of tests performed that week as the denominator. To compare trends, we fit the weekly positivity rate to an ordinary least-squares regression model with a linear trend and used a first-order autoregressive function with Newey-West standard errors. To assess for differences in the trend (slope) of rates between the different cohorts (pediatric or adult) and time periods, we expanded the model to include both indicator variables for various change points (peaks and troughs) and interaction terms for the different cohorts. Most school districts in Southern Connecticut opened in some form after the week of September 8. We also incorporated a dummy variable into the model to capture any potential changes in trajectory following the reopening of schools. Last, we used Spearman’s correlation to assess the relationship between the weekly number of tests performed and positive test results.
Analysis of Persistently Positive Tests
Outcomes of repeat testing were restricted to the patients with a positive test followed by at least one other test result. The first positive test date was considered t = 0, and the difference in days from the first positive test to all subsequent tests was calculated.
Comparison of Ct Values of Symptomatic and Asymptomatic Children
Yale New Haven Children’s Hospital began COVID-19 screening for all admitted patients on April 23 and for all preoperative patients on May 7. We used Ct values as a proxy of SARS-CoV-2 viral load and compared the mean Ct values in symptomatic children with those hospitalized for other reasons such as surgery or psychiatric care.
Analyses were conducted using Stata statistical software 15.0 (StataCorp, College Station, TX). This study complied with Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines for cross-sectional studies. This protocol was approved, and a waiver for informed consent was granted by the Institutional Review Board of Yale University.
RESULTS
Evolution of COVID-19 Testing in Children
Between March 1, 2020, and September 26, 2020, there were 235,415 SARS-CoV-2 tests in the YNHH-S, of which 15,036 (6.3%) tested positive. Overall, 23,137 (9.8%) of the tests were performed in patients ≤ 18 years of age, including 734 (3.2%) positive tests from 688 unique patients (Table 1). The first adult COVID-19 case was identified the week of March 2, 2020, and the first pediatric case was identified the following week. During the first month of the pandemic, local and national guidelines called for testing symptomatic patients, and most (94%) patients tested were adults (Fig. 1A–D and Fig. 2). For adults, the percent positivity increased at a rate of 8.0% per week until the week of March 23, when it peaked at 33.5% and slowly declined by 3.4% per week until it reached a plateau the week of June 8 at 1.8%. After the first positive case, the positivity rate in children and adolescents increased by 3.7% per week, a significantly slower pace than the adults (4.3% slower, 95% CI: 2.2%–6.4%; P < 0.01). The rate of positivity in the pediatric population peaked at 18% the week of March 30 and plateaued the week of June 22 at <1%. After peaking, the rate of decline in children was 1.6% slower than that of adults (95% CI: 0.7%–2.5%; P < 0.01). No significant trend in percent positivity was observed in the adult population after it plateaued; how-ever, a second change point was noted in the positivity trends among children and adolescents (Fig. 1A). After an initial plateau the week of June 22, the rate of positivity in children and adolescents increased by 0.5% per week (95% CI: 0.4%–0.6%; P < 0.01), peaked at 3.1% the week of July 20 and then trended back down to 1.1% 4 weeks later.
FIGURE 1.
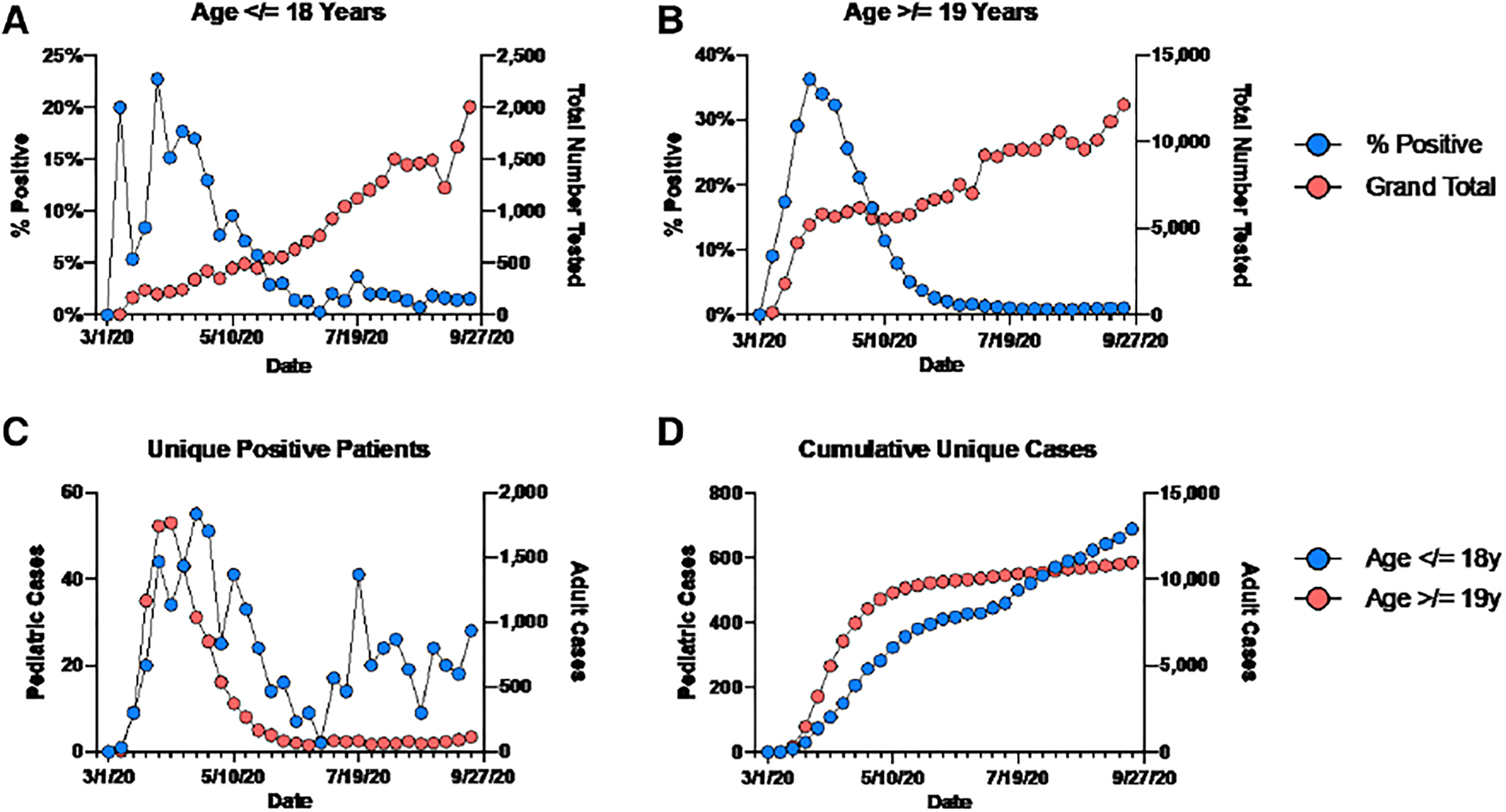
SARS-CoV-2 testing parameters over time. Total tests performed and the %-positivity are shown for children (A) and adults (B) from March to September. C: Unique positive patients and (D) cumulative positive patients by age are shown by week. The date shown is the first day of the week, and tick marks denote individual weeks.
FIGURE 2.
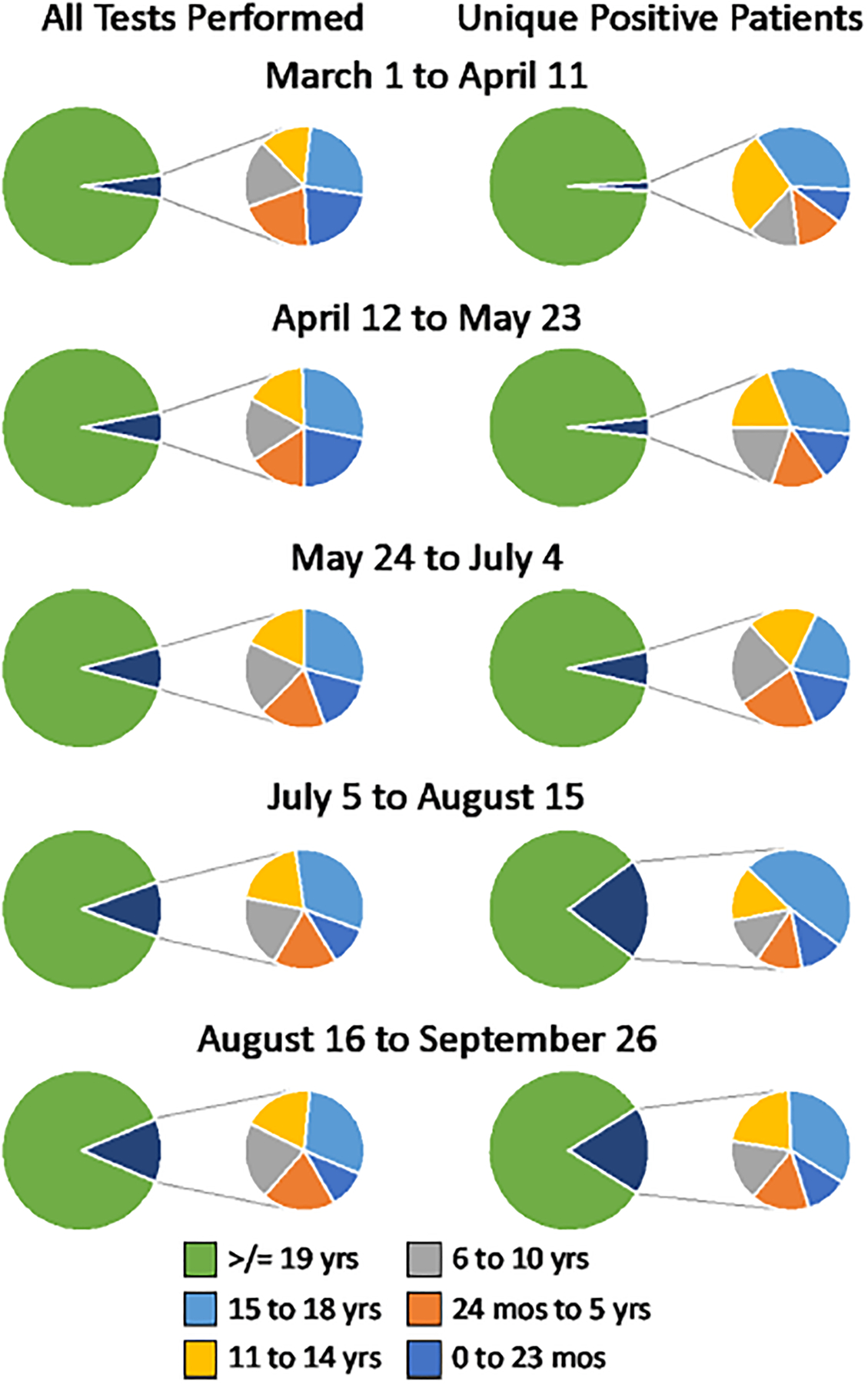
Proportion of all tests and positives by age group over time.
Using Spearman’s correlation, we found a strong and statistically significant positive correlation between number of tests performed in the pediatric population and week of the year with more tests performed over time (rs = 0.97; P < 0.001). On the week of March 30, when the number of pediatric cases first peaked, the YNHH-S performed 217 tests on children and adolescents. In contrast, on the week of July 20, during the second pediatric peak, the health system performed 5.3 times more tests in this age-cohort (n = 1160 tests). After the second peak, SARS-CoV-2 testing in children continued to expand over time, reaching up to 1806 tests during the week of September 14. However, no significant correlation was found between the number of tests performed and the count of positive tests per week after this second peak (rs = 0.51; P = 0.06). Moreover, the positivity rate remained low and did not significantly change even after schools reopened (trend: 0.02% per week; 95% CI: −0.06% to 0.09%; P = 0.65).
COVID-19 Results by Age
Next, we examined COVID-19 testing patterns and results based on age groupings typically associated with school, 0–2 years and 2–5 years (preschool), 6–10 years (school-age), 11–14 years (middle school) and 15–18 years (high school) (Fig. 2 and Figure, Supplementary Digital Content 3, http://links.lww.com/INF/E265). The proportion of unique positive patients in each age group remained similar throughout the pandemic, with older children accounting for most positive cases, especially July through mid-August (Fig. 2 and Figure, Supplementary Digital Content 3, http://links.lww.com/INF/E265). The total number of positive preschool and school-age children was consistently lower than older children, with an increased proportion of positives starting around 12 years of age (Fig. 2 and Figure, Supplementary Digital Content 3, http://links.lww.com/INF/E265).
Ct data were available for 89 children with positive SARS-CoV-2 test results, of which 48 (55.8%) were symptomatic at the time of testing. Fever, cough and congestion were the most commonly reported reason for testing (52%), followed by gastrointestinal symptoms (21%) (Table, Supplementary Digital Content 1, http://links.lww.com/INF/E263 and Table, Supplementary Digital Content 2, http://links.lww.com/INF/E264). Mean viral load, as estimated by the Ct value, did not correlate with symptoms, age, gender or race/ethnicity (Fig. 3A–D and Table, Supplementary Digital Content 4, http://links.lww.com/INF/E266). However, significant differences in viral load were observed between the different tests used to detect the virus. On average, the CDC test reported a Ct value that was 6.9 units lower than the TaqPath assay (95% CI: 0.7–13.1; P = 0.03) (Fig. 3C). These assays were deployed at different points in the pandemic when viral burdens may have differed.
FIGURE 3.
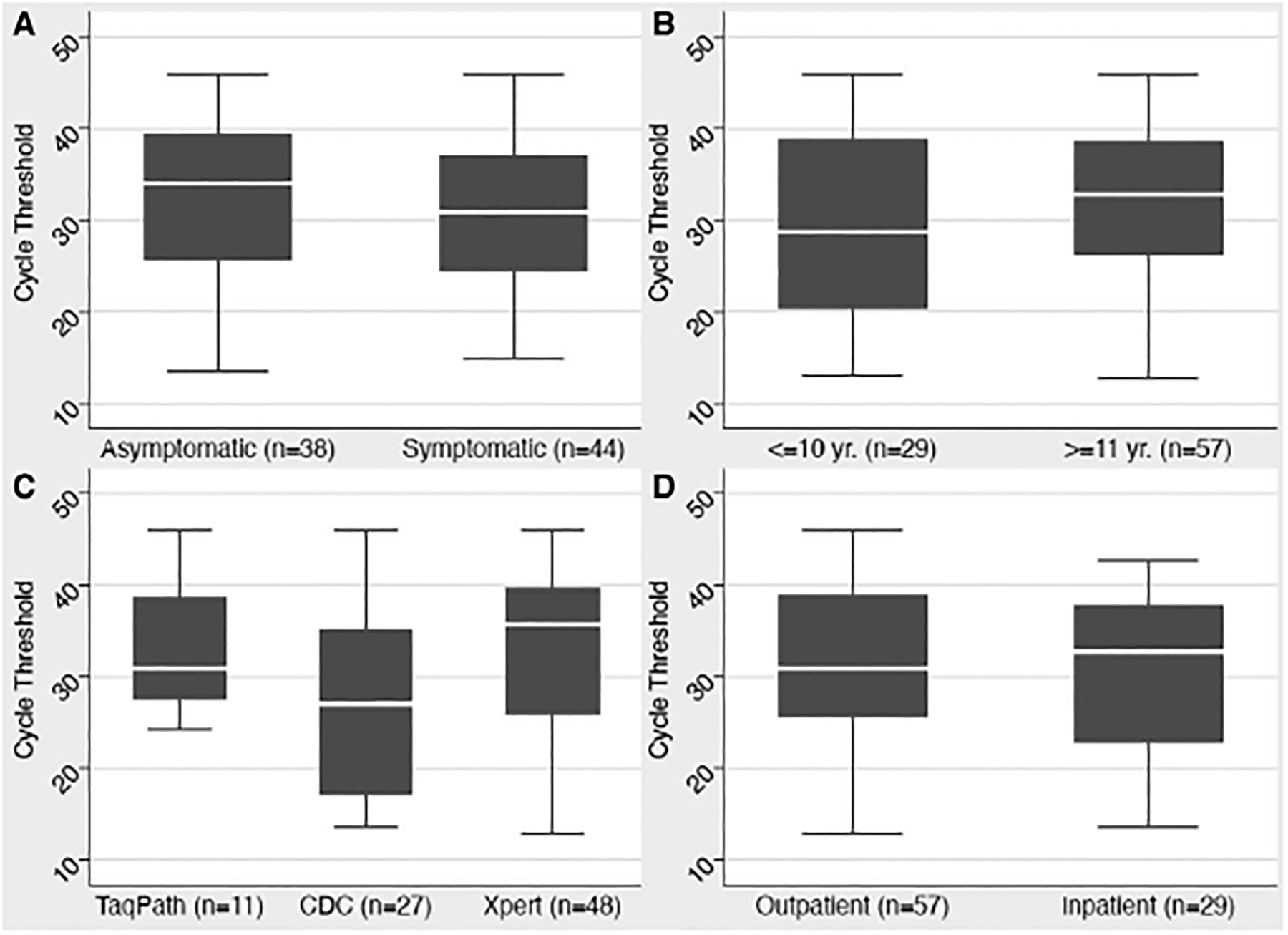
Comparison of Ct values in SARS-CoV-2 positive children. No differences were found based on either the presence of symptoms (A) or age (B). The CDC molecular test was positive at a statistically significant lower Ct value than the other tests (C). There was no difference in Ct values based on patient location as the time of testing (D).
SARS-CoV-2 RNA Detection Over Time
There were 129 pediatric patients who had at least 1 test following a positive test, and 31 had at least 1 second positive test (Fig. 4A). In contrast, 98 pediatric patients had a positive test followed by only a negative test (Fig. 4B). The median number of days from positive test to repeat testing was 22 days (interquartile range: 12–55 days). The median number of days from positive test to negative test was 34 days (interquartile range: 15–69 days). For patients with more than 1 positive test, the longest interval between initial positive test and last positive test was 104 days, with 64.5% having a repeat positive ≥10 days from the initial positive result. Among the 31 with repeat positives, 15 ultimately had a subsequent negative test. One patient had a positive test followed by a negative test on day 5 but was subsequently positive again on days 7 and 11 before testing and remaining negative ≥ day 15. Forty-one patients had testing within 14 days of a positive test, and 19 (46.3%) had a positive result in that time. Interestingly, repeat testing patterns were highly variable and 82.9% of COVID-19 positive children were tested within 90 days, something no longer recommended by the CDC for healthy individuals.17
FIGURE 4.
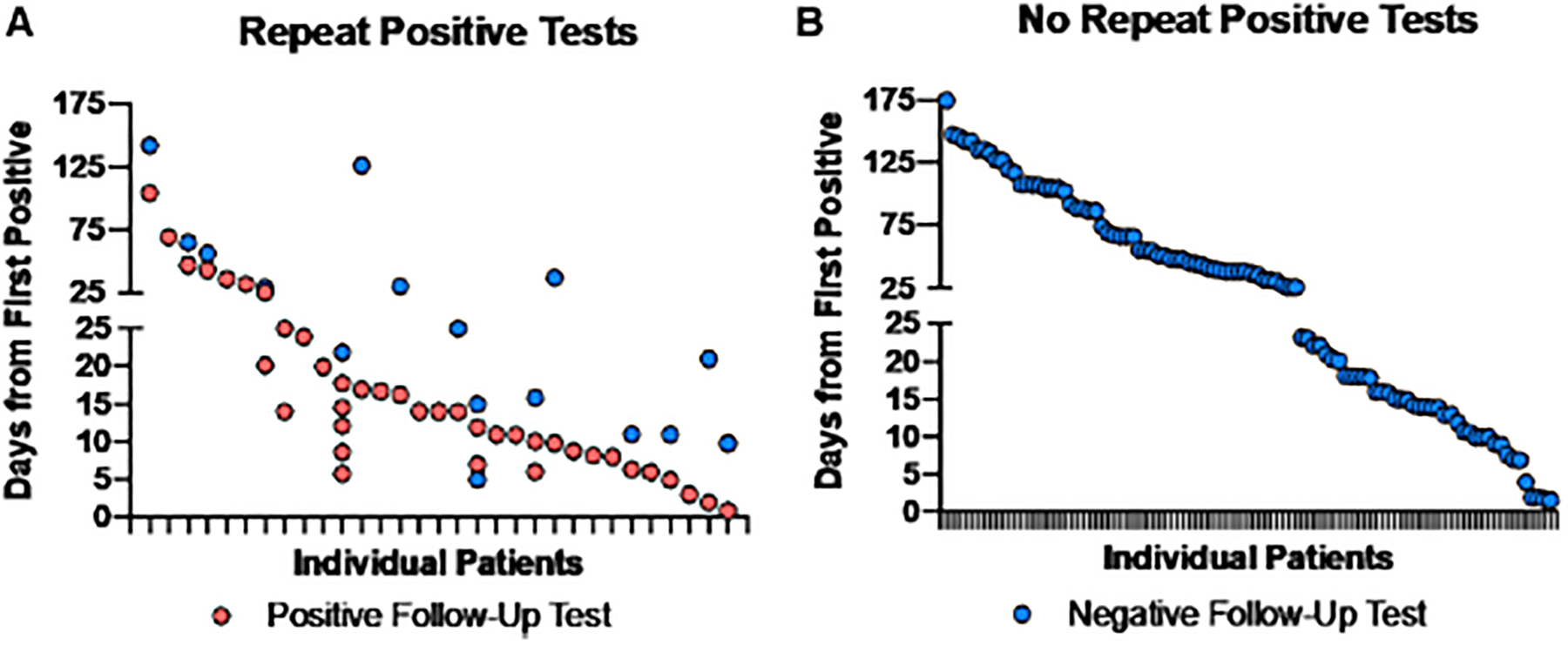
Repeat SARS-COV-2 testing in children. Interval from first positive test to subsequent positive (A) or negative (B) tests among pediatric patients. Patients who tested positive (t = 0) and the interval to subsequent tests were analyzed. With the exception of 1 patient who tested negative and then positive again, the first negative test is shown.
DISCUSSION
This study examines pediatric COVID-19 testing within a large health network based in Southern Connecticut from the beginning of the pandemic through September. Our data support a model where improved access to testing for children led to more complete case detection in late summer. For example, in the first 6 weeks of the pandemic, 126 pediatric cases were identified with 797 tests, while later in the study period, 8398 tests were required to find 124 infected children. Early in the pandemic, when testing strategies prioritized testing individuals with more severe symptoms, fewer children were tested likely underestimating the number of infected children. As the pandemic progressed, local capacity for SARS-CoV-2 testing expanded, along with continuous improvements in the ordering, scheduling and sample collection practices, which ultimately led to increased access to testing for children. Importantly, more testing did not cause more cases of COVID-19.
Adolescents consistently make up a larger proportion of the pediatric population that tests positive for SARS-CoV-2.15 The most pronounced week-to-week increase in unique pediatric cases was seen in weeks 30 to 32, corresponding to July 19 to August 8. While we do not have access to contact tracing information, an adolescent outbreak associated with end-of-school parties was reported for the Fairfield County, Connecticut area during this time.21 Notably, 63% of new positive pediatric cases identified by our laboratories during those weeks were from facilities located in Fairfield County, while these facilities reported only 34% of total unique COVID-19 results overall. Adolescents have higher risk exposure due to unsupervised out of school gatherings and employment and are more likely to be symptomatic when infected compared with younger children. Older children may also require testing to participate in extracurricular activities and thus make up a larger proportion of those tested. Younger children are also less likely to be tested because of their milder symptoms. These data highlight the value of looking at age-specific infection rates when determining the optimal strategy for return to school and extracurricular activities. There may be situations when a high school and the surrounding community are best served by remote learning, while elementary schools continue in-person education with appropriate risk mitigation.
Importantly, schools in the catchment area opened with generally low community rates and with varied combinations of in-person, hybrid and remote only learning. The weather in September in Southern Connecticut was warm, allowing for outdoor activities and open windows and doors. With schools opening for the first time during the pandemic, there was likely increased attention to risk mitigation protocols such as mask wearing. This may wane later in the school year if mitigation fatigue sets in. It will be interesting to watch how the positivity rate in children evolves during the second wave with increasing community prevalence and many schools moving toward remote education.
The inability to attend school in person can have negative effects on the education and health of children, particularly for those in underserved communities. Under-represented minority groups are disproportionately affected by the COVID-19 pandemic.22 To avoid exacerbating inequities, pediatric virologic data should be considered when drafting return to school policies for children who test positive for COVID-19. Consistent with other studies, we found that over half of the children with SARS-CoV-2 who were retested continued to have detectable viral RNA beyond the recommended 10-day isolation period.7,19 This provides further support for a time-based strategy, rather than a testing strategy, in safely returning previously infected children to school.
Higher viral burden in the upper respiratory tract may facilitate community spread. There is contradictory data regarding whether symptomatic children have lower Ct values than asymptomatic children.4,23 A large multicenter study found that symptomatic children have lower Ct values (higher SARS-CoV-2 RNA levels) compared with asymptomatic children.23 However, our results are consistent with previous work that did not find a significant difference in Ct values comparing symptomatic and asymptomatic children.4 The timing of SARS-CoV-2 testing with respect to symptom onset was not known in our study and may explain this discrepancy.
Strengths of this work include the large number of children tested (n = 688 unique positive results). This work also looks at how testing availability impacted the number of reported pediatric cases as the pandemic and testing capacity have evolved. Finally, it captures the highly variable testing patterns for individual children with positive results, reinforcing the need for clear guidance for repeat testing. There were limitations as well: clinical data were only available for a subset of patients. We did not capture pediatric testing at unaffiliated walk-in clinics, and we did not conduct active surveillance of asymptomatic children, potentially biasing the population toward sicker and older children. This study was not designed to evaluate the susceptibility of young children to SARS-CoV-2 infection or the role of younger children in asymptomatic transmission. The epidemiology of COVID-19 in Southern Connecticut during the study period, COVID-19 testing availability and school reopening plans do not necessarily reflect other areas of the United States. Our study was limited to the first 3 to 4 weeks after school reopening and would not detect any increase in COVID-19 from spread within schools beyond this time frame. The length of viral detection is based on testing intervals determined by the provider rather than from symptom onset. Therefore, these results approximate how long children remain positive for SARS-CoV-2.
Despite these limitations, we show that additional testing of children is largely responsible for the late summer increase in pediatric COVID-19 cases in the Southern Connecticut region and that some children have detectable SARS-CoV-2 RNA in the upper respiratory tract for prolonged periods. In the setting of low community prevalence, the percent of positive tests remained stable in the presence of increased access to testing leading to increased identification of cases during the earliest stage of school reopening.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.pidj.com).
The authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145:e20200702. [DOI] [PubMed] [Google Scholar]
- 2.Ladhani SN, Amin-Chowdhury Z, Davies HG, et al. COVID-19 in children: analysis of the first pandemic peak in England. Arch Dis Child. 2020;105:1180–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Størdal K, Bakken IJ, Greve-Isdahl M, et al. SARS-CoV-2 in children and adolescents in Norway: confirmed infection, hospitalisations and underlying conditions. Tidsskr Nor Laegeforen. 2020;140. [DOI] [PubMed] [Google Scholar]
- 4.Maltezou HC, Magaziotou I, Dedoukou X, et al. ; for Greek Study Group on SARS-CoV-2 Infections in Children. Children and adolescents with SARS-CoV-2 infection: epidemiology, clinical course and viral loads. Pediatr Infect Dis J. 2020;39:e388–e392. [DOI] [PubMed] [Google Scholar]
- 5.Feldstein LR, Rose EB, Horwitz SM, et al. ; Overcoming COVID-19 Investigators; CDC COVID-19 Response Team. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Lancet Child Adolescent Health. Pandemic school closures: risks and opportunities. Lancet Child Adolesc Health. 2020;4:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han MS, Choi EH, Chang SH, et al. Clinical characteristics and viral RNA detection in children with coronavirus disease 2019 in the Republic of Korea. JAMA Pediatr. 2020:e203988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Posfay-Barbe KM, Wagner N, Gauthey M, et al. COVID-19 in children and the dynamics of infection in families. Pediatrics. 2020;146:e20201576. [DOI] [PubMed] [Google Scholar]
- 9.DeBiasi RL, Delaney M. Symptomatic and asymptomatic viral shedding in pediatric patients infected with severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2): under the surface [published online ahead of print August 28, 2020]. JAMA Pediatr. doi: 10.1001/jamapediatrics.2020.3996. [DOI] [PubMed] [Google Scholar]
- 10.Hua CZ, Miao ZP, Zheng JS, et al. Epidemiological features and viral shedding in children with SARS-CoV-2 infection [published Epub ahead of print June 15, 2020]. J Med Virol. 2020;92:2804–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laxminarayan R, Wahl B, Dudala SR, et al. Epidemiology and transmission dynamics of COVID-19 in two Indian states. Science. 2020;370:691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Auger KA, Shah SS, Richardson T, et al. Association between statewide school closure and COVID-19 incidence and mortality in the US. JAMA. 2020;324:859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walger P, Heininger U, Knuf M, et al. ; German Society for Hospital Hygiene (DGKH); German Society for Pediatric Infectious Diseases (DGPI); German Academy for Pediatric and Adolescent Medicine (DAKJ); Society of Hygiene, Environmental and Public Health Sciences (GHUP); Professional Association of Pediatricians in Germany (bvkj e.V.). Children and adolescents in the CoVid-19 pandemic: schools and daycare centers are to be opened again without restrictions. The protection of teachers, educators, carers and parents and the general hygiene rules do not conflict with this. GMS Hyg Infect Control. 2020;15:Doc11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilliam WS, Malik AA, Shafiq M, et al. COVID-19 transmission in US child care programs. Pediatrics. 2020:e2020031971. [DOI] [PubMed] [Google Scholar]
- 15.American Academy of Pediatrics and Children’s Hospital Association. Children and COVID-19 State Data Report. 2020. Available at: https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/. Accessed November 3, 2020.
- 16.Connecticut State Department of Education. Addendum 4: Interim Guidance for Decision Making Regarding the Use of In-Person, Hybrid (Blended) or Remote Learning Models in Connecticut Schools during COVID-19. 2020. Available at: https://portal.ct.gov/-/media/SDE/COVID-19/Addendum-4-Interim-Guidance-for-DecisionMaking-Regarding-the-Use-of-InPerson-Hybrid-Blended-or-Remot.pdf. Accessed October 31, 2020.
- 17.Centers for Disease Control and Prevention. Duration of Isolaton and Precautions for Adults With COVID-19. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html. Accessed October 31, 2020.
- 18.Xu CLH, Raval M, Schnall JA, et al. Duration of respiratory and gastrointestinal viral shedding in children with SARS-CoV-2: a systematic review and synthesis of data. Pediatr Infect Dis J. 2020;39:e249–e256. [DOI] [PubMed] [Google Scholar]
- 19.Lu Y, Li Y, Deng W, et al. Symptomatic infection is associated with prolonged duration of viral shedding in mild coronavirus disease 2019: a retrospective study of 110 children in Wuhan. Pediatr Infect Dis J. 2020;39:e95–e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landry ML, Criscuolo J, Peaper DR. Challenges in use of saliva for detection of SARS CoV-2 RNA in symptomatic outpatients. J Clin Virol. 2020;130:104567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaveri M In Ultra-Wealthy Greenwich, Teen Parties Lead to Jump in Virus Cases. New York Times. 2020. Available at: https://www.nytimes.com/2020/07/31/nyregion/greenwich-ct-coronavirus-covid-parties.html. Accessed November 7, 2020.
- 22.Fernandes DM, Oliveira CR, Guerguis S, et al. ; Tri-State Pediatric COVID-19 Research Consortium Authors. SARS-CoV-2 clinical syndromes and predictors of disease severity in hospitalized children and youth [published online ahead of print November 13, 2020]. J Pediatr. doi: 10.1016/j.jpeds.2020.11.016. [DOI] [Google Scholar]
- 23.Kociolek LK, Muller WJ, Yee R, et al. Comparison of upper respiratory viral load distributions in asymptomatic and symptomatic children diagnosed with SARS-CoV-2 infection in pediatric hospital testing programs [published online ahead of print October 22, 2020]. J Clin Microbiol. doi: 10.1128/JCM.02593-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


