Abstract
Aims:
Intensive lifestyle intervention (ILS) targeting health behaviors is efficacious for diabetes mellitus prevention, but there is heterogeneity in the effect of ILS. We tested whether diabetes genetic risk modifies the association of successful lifestyle changes with incident diabetes.
Materials and Methods:
We studied 823 individuals randomized to the ILS arm of the Diabetes Prevention Program who were diabetes-free one year after enrollment. We tested additive and multiplicative interactions of a 67-variant diabetes genetic risk score (GRS) with achievement of three ILS goals at one year (≥7% weight loss, ≥150 minutes/week of moderate leisure-time physical activity, and/or a goal for self-reported total fat intake) on the primary outcome of incident diabetes over 3 years of follow-up.
Results:
A lower GRS and achieving each or all three ILS goals were each associated with lower incidence of diabetes (all p<0.05). Additive interactions were significant between the GRS and achievement of the weight loss goal (p<0.001), physical activity goal (p=0.02), and all three ILS goals (p<0.001) for diabetes risk. Achievement of all three ILS goals was associated with 1.8 [0.3, 3.4], 3.1 [1.5, 4.7], and 3.9 [1.6, 6.2] fewer diabetes cases/100-person-years in the 1st, 2nd, and 3rd GRS tertiles (p<0.001 for trend). Multiplicative interactions between the GRS and ILS goal achievement were significant for the diet goal (p<0.001) but not for weight loss (p=0.18) or physical activity (p=0.62) goals.
Conclusions:
Genetic risk may identify high-risk subgroups for whom successful lifestyle modification is associated with greater absolute reduction in the risk of incident diabetes.
Introduction
Diabetes mellitus is a highly morbid condition with increasing incidence and prevalence.1 Lifestyle modifications targeting weight loss, increased physical activity, and dietary change have been clearly shown to reduce the risk of incident diabetes in randomized trials enrolling high-risk individuals2–4 and in modeling studies of hypothetical mid-life behavioral interventions using observational data.5 In the Diabetes Prevention Program (DPP) randomized trial, weight loss through intensive counseling on dietary modification and increased exercise reduced the risk of transition from prediabetes to overt diabetes compared to control subjects.2 While the intensive lifestyle intervention (ILS) reduced diabetes incidence compared to placebo in the DPP, a subset of participants randomized to ILS progressed to diabetes despite achieving each or a combination of the ILS goals (7% weight loss, 150 minutes/week of leisure-time physical activity, and <25% of weekly dietary calories from fat).6 If participant characteristics can predict how well individuals will respond to lifestyle modification, then alternative diabetes prevention modalities (e.g., metformin) can be targeted rationally.
Genetics is an attractive tool for such precision prevention, and recent studies have identified common variation associated with type 2 diabetes and related traits.7,8 However, gene-lifestyle interaction studies have failed to demonstrate significant interactions between most cross-sectional lifestyle risk factors and diabetes genetic risk in observational cohort studies.9,10 Similarly, secondary analysis of the DPP failed to find a statistical interaction between a 34-variant type 2 diabetes genetic risk score (GRS) and intervention arm for diabetes incidence.11 The prior studies did not examine the effects of interactions between successful change in lifestyle risk factors and genetic risk on incident diabetes. In addition, most prior assessments of gene-lifestyle interactions have focused on multiplicative interactions (interaction on the risk ratio or relative scale) that elucidate how risk factors jointly contribute to diabetes pathogenesis. In contrast, additive interactions (interactions on the risk difference or absolute scale) may have greater relevance to public health as they describe absolute effects of risk factors on disease risk in subgroups of the population.
A recent analysis of the DPP examined interactions with randomization to metformin treatment and found that baseline glycemia and a history of gestational diabetes mellitus modify the effect of metformin on incident diabetes on both the additive and multiplicative scales.12 An analogous evaluation of additive and multiplicative interactions between diabetes genetic risk and successful lifestyle modification has not been reported. Accordingly, we examined whether the association between achievement of ILS goals and incident diabetes was modified by underlying diabetes genetic risk by evaluating both additive and multiplicative interaction models using data from the DPP.
Materials and Methods
Study participants
The details of the Diabetes Prevention Program randomized clinical trial have been described in detail previously.2,13 Briefly, the DPP enrolled individuals ≥25 years old (maximum age 84 years) at elevated diabetes risk based on body mass index (BMI) ≥24 kg/m2 (≥22 kg/m2 in Asian Americans), fasting plasma glucose of 5.3 to 6.9 mmol/L (95 to 125 mg/dL), and plasma glucose 7.8 to 11.0 mmol/L (140 to 199 mg/dL) after a 75-gram oral glucose load.2,13 Exclusion criteria from DPP enrollment included taking medicines that could alter glucose tolerance, life-limiting illness, and illnesses that could limit trial participation. Enrolled participants were randomized to standard lifestyle counseling (written instructions and an annual in-person visit encouraging health lifestyle) plus placebo, standard lifestyle counseling plus metformin (850 mg twice daily), or an intensive lifestyle intervention (ILS) plus placebo. A fourth arm studying troglitazone was terminated early due to reports of liver toxicity. The ILS included a 16-lesson curriculum administered individually in person to study participants by case managers over 24 weeks with follow up in person sessions at least every other month, covering diet, exercise, and behavior modification. Study participants were followed for a mean of 3.2 years during the masked treatment phase of DPP before results were unmasked and the protocol modified.
In this secondary analysis, we included all participants randomized to the intensive lifestyle intervention arm, who provided written informed consent for genetic analysis, who had not developed diabetes within the first year of study enrollment, who had physical activity, diet, or weight assessed at one year after enrollment, and who had fewer than 3 out of 67 missing single nucleotide polymorphisms (SNPs) for the construction of the diabetes genetic risk score (see below). Of the 955 participants who were randomized to the ILS arm and consented to genetic studies, 823 individuals met the inclusion criteria. 813, 790, 820, and 785 individuals had non-missing assessments of physical activity, diet, weight, and all three ILS goals at one year after enrollment and were included in analyses of the respective ILS goals. Each clinical center and the coordinating center obtained institutional review board approval; the DPP study is registered on ClinicalTrials.gov (Identifier: NCT00004992).
Predictors of interest
The primary predictor explored was the achievement of each of three ILS goals at one year from study enrollment. We focused on ILS goal achievement at one year after study enrollment since the ILS curriculum was delivered over 24 weeks with a goal of achieving 7% weight loss by one year. In addition, prior work has shown that participants achieved greater weight loss and increases in physical activity in the first year after enrollment than in the subsequent years of follow-up.6 The weight loss goal was 7% weight loss from baseline body weight. The physical activity goal was 150 minutes of self-reported moderate-to-strenuous physical activity per week. The dietary goal in the DPP focused on reducing fat intake as a means of achieving the weight loss goal; the goal was a fat gram intake of fewer than 25% of calories from fat each week based on calorie targets that would promote weight loss of 1–2 pounds per week. A calorie goal was added to the fat intake goal for participants who were not on track to achieve 7% weight loss during the 24-week ILS curriculum, but dietary goal achievement for this study was based on the fat intake goal irrespective of achievement of the weight loss goal. Weight was assessed at semiannual and annual study visits. Self-reported physical activity was assessed annually using the Modifiable Activity Questionnaire14 and calculated as the weighted sum across all activities of the product of the duration and frequency of each activity, weighted by the estimated metabolic equivalents (MET) for each activity. The result was the average MET-hours/week of leisure-time physical activity in the previous year. Dietary intake during the first year of enrollment was assessed using a modified Block food-frequency questionnaire,15 including the usual daily caloric intake from fat, carbohydrate, protein, and other nutrients. Finally, we examined the achievement of all three ILS goals as a fourth predictor. Achievement of each of the ILS goals was not mutually exclusive, and Table S2 shows the pairwise correlation in achievement of each of the goals.
Diabetes genetic risk
Genetic risk for diabetes was estimated as an additive GRS using 67 SNPs previously found to be associated with type 2 diabetes at genome-wide significance in genome-wide association studies (GWAS).7 Genotyping in the DPP has been described previously, and details are provided in the Online Supplementary Material.11,16,17 If the index SNP reported in GWAS was not available, a proxy in strong linkage disequilibrium was selected (r2 >0.8). Of 72 candidate SNPs for the GRS,7 67 were present or had suitable proxies that passed genotyping quality control on the Illumina MetaboChip or the Illumina Human Core Exome Array (Online Supplementary Material, Table S1). Each participant could carry 0, 1, or 2 diabetes risk alleles at each of 67 loci, meaning that the diabetes GRS could take values from 0 to 134. SNPs were not weighted as their effects on diabetes risk are largely comparable, and we found similar associations of an unweighted GRS and one weighting each allele proportionally to its effect size in the source GWAS with incident diabetes (Table S3). previous analyses using previously published weights have not changed results substantially. We examined the GRS as a continuous variable, such that associations with incident diabetes reflected the average effect per risk allele, and categorized into tertiles with the 1st tertile representing the lowest genetic risk of diabetes and the 3rd tertile representing the highest genetic risk of diabetes.
Outcomes
The primary outcome of this study was incident diabetes at 3 years diagnosed during follow-up based on an oral glucose-tolerance test or fasting plasma glucose. Oral glucose tolerance tests were performed annually for all study participants, and diabetes was diagnosed if plasma glucose was 11.1 mmol/L (200 mg/dL) or higher two hours after a 75-gram oral glucose load. Fasting plasma glucose tests were performed semiannually, and diabetes was diagnosed if fasting glucose was 7.0 mmol/L (126 mg/dL) or higher. The diagnosis of diabetes was confirmed by a second test applying the same criteria, typically within six weeks of the first positive test.
Statistical analysis
We compared participant demographics, baseline adiposity, baseline glycemia, and diabetes genetic risk across levels of ILS goal achievement using chi-square tests for categorical data and two-sample t-tests for continuous or ordinal data. We performed the primary analyses in three steps. First, we estimated associations of achievement of ILS goals and of the diabetes GRS with incident diabetes in separate models using multivariable Cox proportional hazards regression. Second, we included achievement of ILS goals and the diabetes GRS together in multivariable Cox proportional hazards regression models with incident diabetes as the outcome. Third, we evaluated additive (difference scale) and multiplicative (ratio scale) interactions between achievement of ILS goals and the diabetes GRS using multivariable Poisson regression. We opted for this modeling framework for the interaction analysis as it permits estimating incidence rate differences and incidence rate ratios, allowing straightforward comparison of associations and interactions on both the additive and multiplicative scales. We assessed interaction on the risk difference scale by estimating diabetes incidence rate differences between those who did and did not achieve ILS goals stratified by GRS tertile in generalized linear models specifying a Poisson distribution and log link function. We tested heterogeneity in the tertile-specific diabetes incidence rate differences using a test for a directional trend across subgroups. We assessed interaction on the ratio scale using an ILS goal * GRS tertile product term in generalized linear models specifying a Poisson distribution and log link function. Finally, we confirmed multiplicative interactions in Cox proportional hazards regression models using an ILS goal * GRS term with the continuous GRS rather than stratifying into tertiles. All regression models were adjusted for age at randomization, sex, baseline waist circumference (in centimeters), and the first ten genetic principal components to account for ancestry. We employed a significance threshold of p<0.05 for all association tests, with the tests of interaction representing the primary hypothesis test for this study. All analyses were conducted in SAS 9.3 (SAS Inc., Cary, NC) or R (version 3.3, R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 823 individuals were randomized to the ILS arm of DPP, were diabetes-free at one year after enrollment, had at least one ILS goal assessed at that time, and had genetic data available. Of these participants, 414 (50.5%), 605 (74.4%), 363 (46.0%), and 168 (21.4%) individuals achieved the weight loss goal, physical activity goal, diet goal, and all three goals at one year after study enrollment, respectively. Of the 823 individuals included in this study, the mean diabetes GRS was 65.7 (standard deviation [SD] 4.4, Figure S1) and did not differ substantially between individuals who did and did not achieve each or all three ILS goals (Table 1). For all three goals examined, white participants were more likely to achieve ILS goals, but other baseline characteristics did not differ consistently on the basis of ILS goal achievement (Table 1). Baseline glycemia, measured as hemoglobin A1c or fasting glucose, was similar between those who did and did not achieve each or all three ILS goals (Table 1).
Table 1.
Study participant characteristics.
| All n=823 |
Lifestyle Goals | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight Loss † | Physical Activity † | Diet † | All three † | |||||||||
| p-value | p-value | p-value | p-value | |||||||||
| Female sex, n (%) | 557 (67.7) | 286 (70.4) | 0.09 | 172 (82.7) | <0.001 | 277 (64.9) | 0.08 | 0.9 | ||||
| Race/Ethnicity, n (%) | ||||||||||||
| White | 449 (54.6) | 188 (46.3) | ref | 103 (49.5) | ref | 220 (51.5) | ref | ref | ||||
| Black | 154 (18.7) | 102 (25.1) | <0.001 | 49 (23.6) | 0.03 | 68 (15.9) | 0.4 | 0.004 | ||||
| Latino (non-white) | 148 (18.0) | 76 (18.7) | 0.04 | 39 (18.8) | 0.4 | 90 (21.1) | 0.03 | 0.09 | ||||
| Other | 72 (8.8) | 40 (9.9) | 0.02 | 17 (8.2) | 0.8 | 49 (11.5) | 0.001 | 0.002 | ||||
| Family history of diabetes, n (%) | 573 (69.7) | 298 (73.6) | 0.01 | 156 (75.0) | 0.06 | 293 (68.6) | 0.5 | 0.1 | ||||
| Age, year (SD) | 50.8 (11.3) | 49.3 (10.7) | <0.001 | 48.8 (10.9) | 0.004 | 50.0 (11.3) | 0.06 | 0.02 | ||||
| BMI, kg/m2 (SD) | 33.9 (6.5) | 34.1 (6.7) | 0.2 | 36.0 (7.3) | <0.001 | 33.2 (6.1) | 0.003 | 0.6 | ||||
| Waist circ, cm (SD) | 105.2 (14.6) | 105.7 (15.1) | 0.3 | 108.6 (15.8) | <0.001 | 104.2 (14.2) | 0.07 | 0.8 | ||||
|
Fasting glucose, mmol/mol (SD)
[mg/dL (SD)] |
5.9 (0.4) [106.4 (7.8)] |
5.9 (0.4) [106.5 (8.0)] |
0.6 | 5.9 (0.4) [105.6 (7.1)] |
0.1 | 5.9 (0.4) [106.3 (7.9)] |
0.8 | 0.7 | ||||
|
HbA1c, mmol/mol (SD)
[% (SD)] |
41 (3.5) [5.9 (0.5)] |
41 (3.5) [5.9 (0.5)] |
0.3 | 41 (3.5) [5.9 (0.5)] |
0.2 | 41 (3.5) [5.9 (0.5)] |
0.9 | 1.0 | ||||
| Diabetes genetic risk score, count (SD) | 65.7 (4.4) | 65.6 (4.4) | 0.2 | 65.6 (4.5) | 0.4 | 65.9 (4.5) | 0.8 | 0.3 | ||||
Of 823 total participants, 820, 813, 790, and 785 had non-missing assessments of weight loss, physical activity, diet, and all three ILS goals at 1-year after enrollment.
Achievement of each and achievement of all three ILS goals were associated with incident diabetes in multivariable Cox proportional hazards models adjusted for age, sex, baseline waist circumference, and genetic principal components for ancestry (Table 2). Similarly, the diabetes GRS was associated with incident diabetes in an adjusted model with each diabetes risk-raising allele associated with a 5% increased hazard of incident diabetes, corresponding to a 23% increased hazard of incident diabetes per standard deviation increase in the GRS (Table 2). When categorized, tertiles of the diabetes GRS were also associated with incident diabetes (Table 2). When a term for ILS goal achievement was included in adjusted models with the diabetes GRS, both ILS goal achievement and the GRS remained associated with incident diabetes (Table 3). Similar results were observed when the diabetes GRS was categorized into tertiles, with the exception that the confidence intervals for achieving the diet goal widened to include 1.00 (Table S4).
Table 2.
Association of achievement of lifestyle modification goals or diabetes genetic risk with incident diabetes mellitus in the DPP.
| HR (95% CI) | p-value | ||
|---|---|---|---|
| ILS goals | Weight loss | 0.19 (0.12, 0.32) | <0.0001 |
| Physical activity | 0.50 (0.33, 0.76) | 0.001 | |
| Fat intake | 0.67 (0.45, 1.00) | 0.05 | |
| All three ILS goals | 0.26 (0.13, 0.54) | 0.0003 | |
| Genetic risk | |||
| Per SD increase | 1.23 (1.01, 1.49) | 0.04 | |
| 0.009 | |||
| 3rd vs 1st | 2.05 (1.27, 3.30) |
Table 3.
Association of achievement of lifestyle modification goals and diabetes genetic risk with incident diabetes mellitus examined together in the DPP.
| HR (95% CI) | p-value | |
|---|---|---|
| Genetic risk score | 1.06 (1.01, 1.10) | 0.02 |
| Genetic risk score | 1.05 (1.01, 1.10) | 0.02 |
| Genetic risk score | 1.05 (1.00, 1.10) | 0.05 |
| Genetic risk score | 1.05 (1.00, 1.10) | 0.03 |
Next we used multivariable Poisson regression models to evaluate incidence rate differences and incidence rate ratios in the same modeling framework. Those who failed to achieve had a higher risk of incident diabetes than those who successfully achieved the weight loss, physical activity, or all three ILS goals on both the risk difference and risk ratio scales (Table 4, Figure 1). For example, those who did not achieve all three ILS goals had 2.9 (95% CI 1.8–4.1) more incident diabetes cases/100-person-years and 3.7-fold (95% CI 1.8–7.6) higher risk of incident diabetes than those who successfully achieved all three ILS goals (Table 4, Figure 1). In contrast to the Cox proportional hazards models, achievement of the dietary fat intake goal was not associated with incident diabetes on either the risk difference scale (1.1 [-0.2, 2.3] more incident cases/100-person-years) or risk ratio scale (1.4 [0.9, 2.0]) in multivariable Poisson models owing to wider confidence intervals.
Table 4.
Diabetes incidence rates associated with lifestyle goal achievement and interactions with diabetes genetic risk.
| Genetic Risk Tertiles | |||||
|---|---|---|---|---|---|
| All Participants | Low | Intermediate | High | Heterogeneity P-value† | |
| Weight loss | |||||
| Incidence Rate (95% CI) ‡ | |||||
| Not Achieved | 5.9 (4.7, 7.5) | 3.9 (2.5, 6.1) | 5.4 (3.7, 7.8) | 8.2 (5.9, 11.4) | |
| Achieved | 1.3 (0.8, 2.0) | 0.8 (0.3, 1.9) | 0.6 (0.2, 1.9) | 2.1 (1.3, 3.7) | |
| Risk Difference ‡ | −4.7 (−3.2, −6.1) | −3.1 (−1.2, −5.0) | −4.7 (−2.6, −6.8) | −6.0 (−3.2, −8.9) | <0.001 |
| Rate Ratio | 0.22 (0.14, 0.34) | 0.20 (0.07, 0.56) | 0.12 (0.05, 0.38) | 0.26 (0.48, 0.14) | 0.18 |
| Physical Activity | |||||
| Incidence Rate (95% CI) ‡ | |||||
| Not Achieved | 5.3 (3.8, 7.5) | 3.2 (1.7, 6.1) | 6.0 (3.5, 10.1) | 7.0 (4.3, 11.3) | |
| Achieved | 2.9 (2.2, 3.7) | 1.8 (1.1, 3.0) | 2.3 (1.5, 3.7) | 4.2 (2.9, 5.9) | |
| Risk Difference ‡ | −2.5 (−0.6, −4.3) | −1.4 (0.8, −3.6) | −3.6 (−0.3, −7.0) | −2.8 (0.8, −6.4) | 0.02 |
| Rate Ratio | 0.56 (0.36, 0.83) | 0.56 (0.25. 1.25) | 0.4 (0.19, 0.83) | 0.59 (0.33, 1.11) | 0.62 |
| Diet | |||||
| Incidence Rate (95% CI) ‡ | |||||
| Not Achieved | 3.9 (3.0, 5.1) | 3.2 (2.0,5.1) | 2.9 (1.8,4.9) | 5.1 (3.6,7.4) | |
| Achieved | 2.9 (2.1, 3.9) | 1.4 (0.7,3.0) | 2.8 (1.6, 4.6) | 4.2 (2.7,6.5) | |
| Risk Difference ‡ | −1.1 (0.2, −2.3) | −1.7 (−0.0, −3.5) | −0.2 (1.9, −2.2) | −0.9 (1.6, −3.4) | 0.07 |
| Rate Ratio | 0.71 (0.50, 1.11) | 0.45 (0.20, 1.11) | 0.91 (0.45, 2.0) | 0.83 (0.48, 1.43) | <0.001 |
| All three goals | |||||
| Incidence Rate (95% CI) ‡ | |||||
| Not Achieved | 4.0 (3.2, 5.0) | 2.6 (1.7, 4.1) | 3.6 (2.5, 5.1) | 5.6 (4.1, 7.7) | |
| Achieved | 1.1 (0.5, 2.2) | 0.8 (0.2, 3.5) | 0.5 (0.1, 3.4) | 1.7 (0.7, 4.1) | |
| Risk Difference ‡ | −2.9 (−1.8, −4.1) | −1.8 (−0.3, −3.4) | −3.1 (−1.5, −4.7) | −3.9 (−1.6, −6.2) | <0.001 |
| Rate Ratio | 0.27 (0.13, 0.56) | 0.30 (0.07, 1.43) | 0.13 (0.05, 1.0) | 0.31 (0.13, 0.77) | 0.92 |
Test for heterogeneity reflects: test for directional trend across genetic risk tertiles for risk difference models; and lifestyle*genetic risk interaction term for rate ratio models
events/100 person-years
Figure 1. Association of achievement of lifestyle modification goals with incident diabetes mellitus on the risk difference and risk ratio scales, stratified by diabetes genetic risk tertiles.
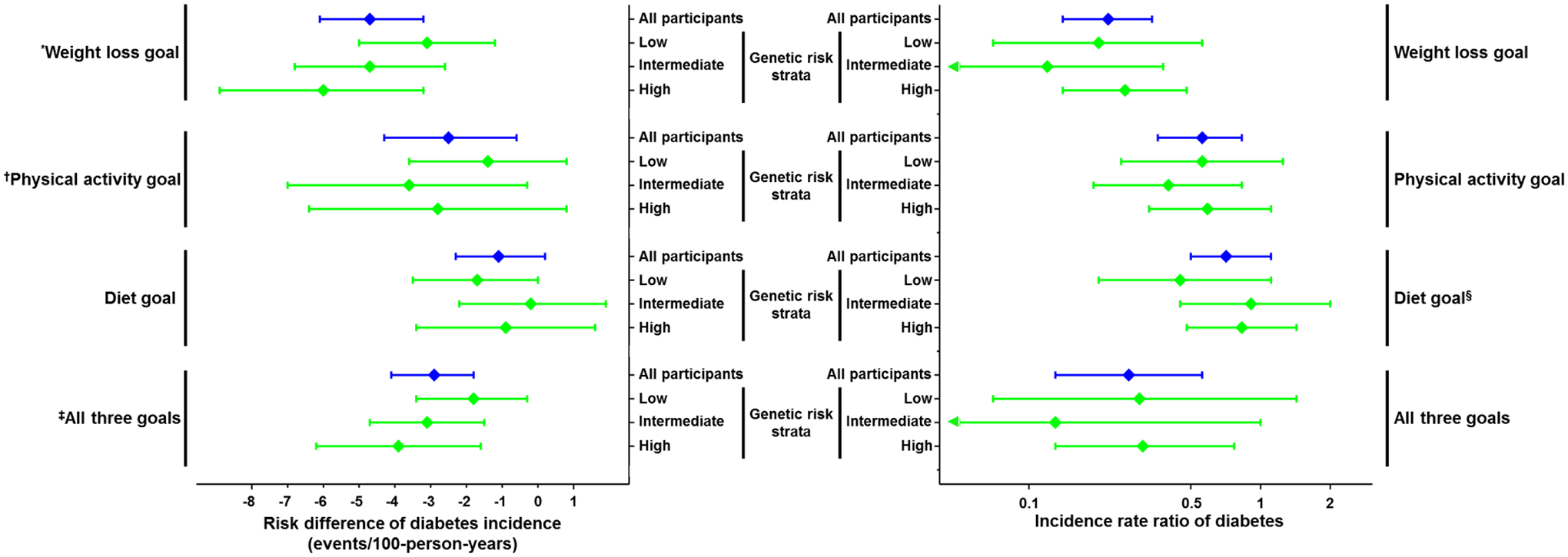
Forest plot of diabetes incidence rate differences (left) and incidence rate ratios (right) for individuals who did relative to those who did not achieve intensive lifestyle intervention goals for weight loss, physical activity, diet, or all three at one year after study enrollment across subgroups defined by genetic risk tertiles. Statistically significant heterogeneity across subgroups (P<0.05) noted for: *weight loss on risk difference scale (P<0.001 for trend across genetic risk tertiles); †physical activity on risk difference scale (P=0.02 for trend across genetic risk tertiles); ‡all three goals on risk difference scale (P<0.001 for trend across genetic risk tertiles); §diet on the risk ratio scale (P<0.001 for diet*genetic risk interaction term).
Additive but not multiplicative interactions, that is interaction on the risk difference but not the risk ratio scale, were significant between GRS tertiles and achievement of the weight loss, physical activity, and all three ILS goals (Table 4, Figure 1). The risk reduction associated with achievement of all three ILS goals increased across GRS tertiles from 1.8 [0.3, 3.4] to 3.1 [1.5, 4.7] to 3.9 [1.6, 6.2] fewer incident diabetes cases/100-person-years in the first, second, and third genetic risk tertile, respectively (p<0.001 for trend). In contrast, multiplicative but not additive interaction was significant between GRS tertiles and achievement of the dietary goal with a larger relative risk reduction of diabetes incidence observed in those in the lowest GRS tertile than in those with a higher diabetes GRS (Table 4, Figure 1). Similarly, tests for multiplicative interaction in Cox proportional hazards models using the diabetes GRS as a continuous variable were non-significant for each combination of the diabetes GRS and ILS goals (Table S5).
Discussion
In this study, we have shown that a common variant diabetes genetic risk score and ILS goal achievement were independently associated with incident diabetes among participants randomized to the ILS intervention in the DPP, and that the absolute reduction in diabetes risk – that is the number of cases prevented per person-year of follow-up – associated with achievement of specific ILS goals (weight loss, physical activity, or all three goals) was greatest in individuals with the highest diabetes genetic risk. In contrast, the relative reduction in the risk of incident diabetes – that is the proportional reduction in incident cases – associated with achieving weight loss, physical activity, or all three lifestyle modification goals did not vary depending on the underlying genetic risk of diabetes. Our findings suggest that the number of incident diabetes cases prevented from successful lifestyle modification is greater in individuals at high genetic risk than in those at lower genetic risk.
Whether genetic information can personalize lifestyle modification approaches to mitigate diabetes risk has motivated a number of gene-lifestyle interaction studies.9,10,18–27 While some studies have identified statistically significant interactions between genetic risk and lifestyle factors on either risk of type 2 diabetes or intermediate phenotypes such as insulin resistance,18–24 whether those findings can be effectively incorporated into clinical care has been unclear. Of note, the majority of gene-lifestyle interaction evaluations have focused on lifestyle measurements at a single “baseline” timepoint. Examination of interactions between genetics and lifestyle modification or change in risk factors have been less common except secondary analyses of randomized trials of specific diets.18–25,28
We extend prior work by focusing specifically on interactions between genetic risk and lifestyle risk factor change. The evaluation of additive and multiplicative interactions provides insight two different approaches for examining the combinatorial effect of diabetes genetic risk and lifestyle modification on diabetes incidence. The additive interaction quantifies differences across populations (in this case defined by GRS tertiles) in the absolute reduction of diabetes cases associated with successful lifestyle modification. In contrast, the multiplicative interaction quantifies whether genetic risk and lifestyle modification combine to yield a proportional or relative effect on diabetes incidence that differs from what would be expected from their individual or independent contributions to diabetes risk. The interpretation of the additive interaction, therefore, largely pertains to the impact of lifestyle risk factor modification in populations of at-risk individuals, whereas the multiplicative interaction may have a biological or pathophysiological interpretation pertaining to diabetes risk in individuals who achieve lifestyle modification goals in different strata of diabetes genetic risk. Importantly, we observed a significant trend for greater incident diabetes absolute risk reduction from weight loss, physical activity, and achievement of all three ILS goals across increasing GRS tertiles. Variation in absolute risk reduction from successful lifestyle modification on diabetes incidence would be expected to be observed across a gradient of an independent diabetes risk factor (in this case genetic risk).29,30 However, the direction of that gradient in absolute risk reduction across genetic risk strata is difficult to anticipate a priori. Taken together, this study and prior work provide further motivation for additional prospective investigation of the population health impacts of intensive lifestyle interventions across strata of diabetes genetic risk, acknowledging that improved lifestyle should be recommended for all individuals irrespective of genetic risk.
Our observation that the relative benefit of achieving weight change, physical activity change, or all three lifestyle modification goals is similar in genetically low- and high-risk individuals complements prior work. This finding suggests that in a population similar to that enrolled in the DPP, a diabetes GRS is unlikely to provide predictive insight into who will or will not respond to an intensive lifestyle intervention, with the possible exception of dietary change for which we did observe interaction on the ratio scale. An earlier study found that a 34-SNP diabetes GRS predicted incident diabetes but did not have a significant interaction with treatment arm in the full DPP study population.11 Notably, the number of SNPs included in the GRS in this study expanded to 67, and the HR per allele increase in the GRS was larger in the current study than previously. Another prior study examined whether a GRS associated with insulin resistance modified improvements in insulin sensitivity associated with the ILS and metformin treatment arms of the DPP.17 While improvement of insulin sensitivity and insulin secretion from ILS is a predictor of diabetes risk reduction in the DPP,31 changes in insulin sensitivity from ILS did not vary across levels of insulin resistance genetic risk.17
Although the DPP demonstrated the efficacy of ILS on reducing diabetes risk in a high-risk population, the effects of real-world programs based on the DPP ILS have been heterogeneous in terms of reach, retention, and effectiveness.32–37 Lower engagement, retention, and weight loss than seen in the original DPP trial may be due to the resource intensiveness of the programs for participants and the communities and/or health systems in which they are deployed.38–41 This difficulty of effectively executing DPP-style programs highlights that selecting the highest risk patients or those who are likely to derive the greatest diabetes risk reduction is critical to lifestyle intervention success. Indeed, an earlier study found that the DPP interventions – ILS or metformin – were more effective in the highest risk enrolled participants based on a diabetes risk prediction model.42 In addition, analyses of the DPP including up to 15-years of follow-up found that the absolute risk reduction of diabetes incidence from metformin treatment was greater in individuals with higher baseline glycemia (by fasting glucose or HbA1c) or with a history of gestational diabetes mellitus.12,43 Thus, this study and prior work suggest that concentrating ILS efforts in real-world settings on genetically or clinically high-risk individuals may result in more diabetes cases prevented from a resource-intensive intervention.
The strengths of our study include the use of DPP randomized trial data, including a standardized lifestyle counseling intervention and standardized measurement of lifestyle modification at one year after randomization. In addition, we used a more comprehensive set of diabetes genetic risk loci than previous DPP studies.11 There are also several important limitations worth noting. First, by restricting our study to the ILS treatment arm of the DPP, we limited sample size and thus the power to detect interactions. Second, recent genome-wide association studies of diabetes have expanded the number of associated loci beyond those used in our study. However, expanding the GRS using common variants with small effect sizes is unlikely to substantially impact the overall predictive utility of a diabetes GRS44,45 or reclassify individuals’ genetic risk sufficiently to impact the interaction tests conducted in this study. Third, we have treated the ILS arm of the DPP as an observational cohort for the purposes of this study. That is, the primary predictors in this study, achievement of ILS goals, were not randomized; therefore, residual confounding is a threat to the validity of the associations estimated in our study. To address this limitation, we included age, sex, and baseline waist circumference as covariates in all multivariable models, but we cannot exclude potential residual confounding. Fourth, the study population was limited to individuals with BMI ≥24 kg/m2, elevated fasting glucose, and impaired glucose tolerance, which were major inclusion criteria for the DPP. Thus, we cannot necessarily extrapolate our results to the general population of individuals who may be at lower risk of type 2 diabetes but could benefit from lifestyle modification to attenuate that risk further. Fifth, by design we excluded participants randomized to the ILS intervention who developed diabetes within the first year after study enrollment. This may have resulted in the exclusion of the highest risk individuals and consequently led to misestimation of the association of successful lifestyle modification or of the GRS with diabetes incidence. However, the number of individuals excluded based on this criterion was small (7 participants), reducing the likelihood that this study design choice substantially influenced our results. Sixth, the dietary goal in the DPP focused on fat intake and secondarily on caloric intake; thus, our study does not provide insight on the interactions of other dietary components with diabetes genetic risk. Finally, the genetic loci used to build the GRS used in this study were initially discovered in individuals of European ancestry,7 and trans-ethnic studies have demonstrated differential effect sizes for many diabetes-associated loci across different ancestry groups.46,47
While lifestyle modification remains a mainstay of diabetes prevention for all at-risk individuals, we conclude that diabetes genetic risk may help identify individuals who derive greater absolute benefit from intensive lifestyle modification programs than the average person at high-risk of diabetes. On the other hand, diabetes genetic risk does not fully explain the heterogeneous effect of ILS goal achievement on incident diabetes in the DPP study. Additional observational studies utilizing larger cohorts and genetically-stratified randomized trials of lifestyle intervention are needed to confirm whether the absolute risk reduction from lifestyle modification on diabetes incidence varies across levels of underlying genetic risk and whether genetic data can inform referrals to intensive lifestyle modification programs in real-world clinical practice.
Supplementary Material
Acknowledgments
The Research Group gratefully acknowledges the commitment and dedication of the participants of the DPP and DPPOS.
Funding
SR is supported by American Heart Association Award 17MCPRP33670728 and US Veterans Affairs award IK2-CX001907-01. PWF is supported by European Research Council award ERC-2015-CoG - 681742_NASCENT. During the DPP and DPPOS, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health provided funding to the clinical centers and the Coordinating Center for the design and conduct of the study, and collection, management, analysis, and interpretation of the data (U01 DK048489). The Southwestern American Indian Centers were supported directly by the NIDDK, including its Intramural Research Program, and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources, and the Department of Veterans Affairs supported data collection at many of the clinical centers. Funding was also provided by the National Institute of Child Health and Human Development, the National Institute on Aging, the National Eye Institute, the National Heart Lung and Blood Institute, the National Cancer Institute, the Office of Research on Women’s Health, the National Institute on Minority Health and Health Disparities, the Centers for Disease Control and Prevention, and the American Diabetes Association. Bristol-Myers Squibb and Parke-Davis provided additional funding and material support during the DPP, Lipha (Merck-Sante) provided medication and LifeScan Inc. donated materials during the DPP and DPPOS. This research was also supported, in part, by the intramural research program of the NIDDK. LifeScan Inc., Health O Meter, Hoechst Marion Roussel, Inc., Merck-Medco Managed Care, Inc., Merck and Co., Nike Sports Marketing, Slim Fast Foods Co., and Quaker Oats Co. donated materials, equipment, or medicines for concomitant conditions. McKesson BioServices Corp., Matthews Media Group, Inc., and the Henry M. Jackson Foundation provided support services under subcontract with the Coordinating Center. The sponsor of this study was represented on the Steering Committee and played a part in study design, how the study was done, and publication. The funding agency was not represented on the writing group, although all members of the Steering Committee had input into the report’s contents. The opinions expressed are those of the investigators and do not necessarily reflect the views of the funding agencies. A complete list of Centers, investigators, and staff can be found an online Supplement. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Duality of interest
The authors have no conflicts of interest to report pertaining to this manuscript.
References
- 1.NCD Risk Factor Collaboration. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):1513–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537–44. [DOI] [PubMed] [Google Scholar]
- 4.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–50. [DOI] [PubMed] [Google Scholar]
- 5.Danaei G, Pan A, Hu FB, Hernan MA. Hypothetical midlife interventions in women and risk of type 2 diabetes. Epidemiology. 2013;24(1):122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29(9):2102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris AP, Voight BF, Teslovich TM, Ferreira T, Segre AV, Steinthorsdottir V, et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44(9):981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott RA, Lagou V, Welch RP, Wheeler E, Montasser ME, Luan J, et al. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet. 2012;44(9):991–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langenberg C, Sharp SJ, Franks PW, Scott RA, Deloukas P, Forouhi NG, et al. Gene-lifestyle interaction and type 2 diabetes: the EPIC interact case-cohort study. PLoS Med. 2014;11(5):e1001647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Said MA, Verweij N, van der Harst P. Associations of Combined Genetic and Lifestyle Risks With Incident Cardiovascular Disease and Diabetes in the UK Biobank Study. JAMA Cardiol. 2018;3(8):693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hivert MF, Jablonski KA, Perreault L, Saxena R, McAteer JB, Franks PW, et al. Updated genetic score based on 34 confirmed type 2 diabetes Loci is associated with diabetes incidence and regression to normoglycemia in the diabetes prevention program. Diabetes. 2011;60(4):1340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diabetes Prevention Program Research Group. Long-term Effects of Metformin on Diabetes Prevention: Identification of Subgroups That Benefited Most in the Diabetes Prevention Program and Diabetes Prevention Program Outcomes Study. Diabetes Care. 2019;42(4):601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22(4):623–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kriska AM, Caspersen CJ Introduction to a collection of physical activity questionnaires. Med Sci Sports Exerc. 1997;29(Suppl):S5–S9. [PubMed] [Google Scholar]
- 15.Mayer-Davis EJ, Vitolins MZ, Carmichael SL, Hemphill S, Tsaroucha G, Rushing J, et al. Validity and reproducibility of a food frequency interview in a Multi-Cultural Epidemiology Study. Ann Epidemiol. 1999;9(5):314–24. [DOI] [PubMed] [Google Scholar]
- 16.Varga TV, Winters AH, Jablonski KA, Horton ES, Khare-Ranade P, Knowler WC, et al. Comprehensive Analysis of Established Dyslipidemia-Associated Loci in the Diabetes Prevention Program. Circ Cardiovasc Genet. 2016;9(6):495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hivert MF, Christophi CA, Franks PW, Jablonski KA, Ehrmann DA, Kahn SE, et al. Lifestyle and metformin ameliorate insulin sensitivity independently of the genetic burden of established insulin resistance variants in Diabetes Prevention Program participants. Diabetes. 2016;65(2):520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardner CD, Trepanowski JF, Del Gobbo LC, Hauser ME, Rigdon J, Ioannidis JPA, et al. Effect of Low-Fat vs Low-Carbohydrate Diet on 12-Month Weight Loss in Overweight Adults and the Association With Genotype Pattern or Insulin Secretion: The DIETFITS Randomized Clinical Trial. JAMA. 2018;319(7):667–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang T, Huang J, Qi Q, Li Y, Bray GA, Rood J, et al. PCSK7 genotype modifies effect of a weight-loss diet on 2-year changes of insulin resistance: the POUNDS LOST trial. Diabetes Care. 2015;38(3):439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattei J, Qi Q, Hu FB, Sacks FM, Qi L. TCF7L2 genetic variants modulate the effect of dietary fat intake on changes in body composition during a weight-loss intervention. Am J Clin Nutr. 2012;96(5):1129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi Q, Bray GA, Smith SR, Hu FB, Sacks FM, Qi L. Insulin receptor substrate 1 gene variation modifies insulin resistance response to weight-loss diets in a 2-year randomized trial: the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Circulation. 2011;124(5):563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi Q, Xu M, Wu H, Liang L, Champagne CM, Bray GA, et al. IRS1 genotype modulates metabolic syndrome reversion in response to 2-year weight-loss diet intervention: the POUNDS LOST trial. Diabetes Care. 2013;36(11):3442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang T, Huang T, Zheng Y, Rood J, Bray GA, Sacks FM, et al. Genetic variation of fasting glucose and changes in glycemia in response to 2-year weight-loss diet intervention: the POUNDS LOST trial. Int J Obes (Lond). 2016;40(7):1164–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Qi Q, Zhang C, Smith SR, Hu FB, Sacks FM, et al. FTO genotype and 2-year change in body composition and fat distribution in response to weight-loss diets: the POUNDS LOST Trial. Diabetes. 2012;61(11):3005–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang T, Ley SH, Zheng Y, Wang T, Bray GA, Sacks FM, et al. Genetic susceptibility to diabetes and long-term improvement of insulin resistance and beta cell function during weight loss: the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Am J Clin Nutr. 2016;104(1):198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klimentidis YC, Chen Z, Arora A, Hsu CH. Association of physical activity with lower type 2 diabetes incidence is weaker among individuals at high genetic risk. Diabetologia. 2014;57(12):2530–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott RA, Chu AY, Grarup N, Manning AK, Hivert MF, Shungin D, et al. No interactions between previously associated 2-hour glucose gene variants and physical activity or BMI on 2-hour glucose levels. Diabetes. 2012;61(5):1291–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qi Q, Zheng Y, Huang T, Rood J, Bray GA, Sacks FM, et al. Vitamin D metabolism-related genetic variants, dietary protein intake and improvement of insulin resistance in a 2 year weight-loss trial: POUNDS Lost. Diabetologia. 2015;58(12):2791–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology 3rd Edition. Lippincott Williams Wilkins; 2012. [Google Scholar]
- 30.Kent DM, Paulus JK, van Klaveren D, et al. The Predictive Approaches to Treatment effect Heterogeneity (PATH) Statement. Ann Intern Med. 2019. [DOI] [PubMed] [Google Scholar]
- 31.Kitabchi AE, Temprosa M, Knowler WC, Kahn SE, Fowler SE, Haffner SE, et al. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes. 2005;54(8):2404–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ali MK, Echouffo-Tcheugui J, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff (Millwood). 2012;31(1):67–75. [DOI] [PubMed] [Google Scholar]
- 33.Aziz Z, Absetz P, Oldroyd J, Pronk NP, Oldenburg B. A systematic review of real-world diabetes prevention programs: learnings from the last 15 years. Implement Sci. 2015;10:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ely EK, Gruss SM, Luman ET, Gregg EW, Ali MK, Nhim K, et al. A National Effort to Prevent Type 2 Diabetes: Participant-Level Evaluation of CDC’s National Diabetes Prevention Program. Diabetes Care. 2017;40(10):1331–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nhim K, Gruss SM, Porterfield DS, Jacobs S, Elkins W, Luman ET, et al. Using a RE-AIM framework to identify promising practices in National Diabetes Prevention Program implementation. Implement Sci. 2019;14(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Venkataramani M, Pollack CE, Yeh HC, Maruthur NM. Prevalence and Correlates of Diabetes Prevention Program Referral and Participation. Am J Prev Med. 2019;56(3):452–7. [DOI] [PubMed] [Google Scholar]
- 37.Whittemore R A systematic review of the translational research on the Diabetes Prevention Program. Transl Behav Med. 2011;1(3):480–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson M, Jones R, Freeman C, Woods HB, Gillett M, Goyder E, et al. Can diabetes prevention programmes be translated effectively into real-world settings and still deliver improved outcomes? A synthesis of evidence. Diabet Med. 2013;30(1):3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayer-Davis EJ, D’Antonio AM, Smith SM, Kirkner G, Levin Martin S, Parra-Medina D, et al. Pounds off with empowerment (POWER): a clinical trial of weight management strategies for black and white adults with diabetes who live in medically underserved rural communities. Am J Public Health. 2004;94(10):1736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Damschroder LJ, Moin T, Datta SK, Reardon CM, Steinle N, Weinreb J, et al. Implementation and evaluation of the VA DPP clinical demonstration: protocol for a multi-site non-randomized hybrid effectiveness-implementation type III trial. Implement Sci. 2015;10:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Damschroder LJ, Reardon CM, AuYoung M, Moin T, Datta SK, Sparks JB, et al. Implementation findings from a hybrid III implementation-effectiveness trial of the Diabetes Prevention Program (DPP) in the Veterans Health Administration (VHA). Implement Sci. 2017;12(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sussman JB, Kent DM, Nelson JP, Hayward RA. Improving diabetes prevention with benefit based tailored treatment: risk based reanalysis of Diabetes Prevention Program. BMJ. 2015;350:h454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herman WH, Pan Q, Edelstein SL, Mather KJ, Perreault L, Barrett-Connor E, et al. Impact of Lifestyle and Metformin Interventions on the Risk of Progression to Diabetes and Regression to Normal Glucose Regulation in Overweight or Obese People With Impaired Glucose Regulation. Diabetes Care. 2017;40(12):1668–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meigs JB, Shrader P, Sullivan LM, McAteer JB, Fox CS, Dupuis J, et al. Genotype score in addition to common risk factors for prediction of type 2 diabetes. N Engl J Med. 2008;359(21):2208–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vassy JL, Hivert MF, Porneala B, Dauriz M, Florez JC, Dupuis J, et al. Polygenic type 2 diabetes prediction at the limit of common variant detection. Diabetes. 2014;63(6):2172–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu CT, Raghavan S, Maruthur N, Kabagambe EK, Hong J, Ng MC, et al. Trans-ethnic Meta-analysis and Functional Annotation Illuminates the Genetic Architecture of Fasting Glucose and Insulin. Am J Hum Genet. 2016;99(1):56–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ng MC, Shriner D, Chen BH, Li J, Chen WM, Guo X, et al. Meta-analysis of genome-wide association studies in African Americans provides insights into the genetic architecture of type 2 diabetes. PLoS Genet. 2014;10(8):e1004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


