Abstract
Background & Aims
Scale-up of highly effective direct-acting antivirals (DAAs) for HCV among people who inject drugs (PWID) in Scotland has led to a reduction in the prevalence of viraemia in this population. However, the extent of reinfection among those treated with DAAs remains uncertain. We estimated HCV reinfection rates among PWID in Scotland by treatment setting, pre- and post-introduction of DAAs, and the potential number of undiagnosed reinfections resulting from incomplete follow-up testing.
Methods
Through linkage of national clinical and laboratory HCV data, a retrospective cohort of PWID who commenced treatment between 2000-2018 and achieved a sustained virological response (SVR) were followed up for reinfection to December 2019. Reinfection was defined as a positive HCV antigen or RNA test.
Results
Of 5,686 SVRs among 5,592 PWID, 4,126 (73%) had an HCV RNA or antigen test post-SVR. Of those retested, we identified 361 reinfections (3.9/100 person-years [PY]). The reinfection rate increased from 1.5/100 PY among PWID treated in 2000-2009 to 8.8/100 PY in 2017-2018. The highest reinfection rates were observed among those treated in prison (14.3/100 PY) and community settings (9.5/100 PY). Among those treated in the DAA era (2015-2018), 68% were tested within the first year post-SVR but only 30% in the second year; while 169 reinfections were diagnosed in follow-up, an estimated 200 reinfections (54% of the estimated total) had gone undetected.
Conclusions
HCV reinfection rates among PWID in Scotland have risen alongside the scale-up of DAAs and broadened access to treatment for those at highest risk, through delivery in community drug services. Promotion of HCV testing post-SVR among PWID is essential to ensure those reinfected are identified and retreated promptly.
Lay summary
Increased rates of hepatitis C reinfection in Scotland were observed following the rapid scale-up of highly effective direct-acting antiviral (DAA) treatments among people who inject drugs. This demonstrates that community-based treatment pathways are reaching high-risk groups, regarded vital in efforts to eliminate the virus. However, we estimate that less than half of reinfections have been detected in the DAA era because of inadequate levels of retesting beyond the first year following successful treatment. Sustained efforts that involve high coverage of harm reduction measures and high uptake of annual testing are required to ensure prompt diagnosis and treatment of those reinfected if the goals of elimination are to be met.
Keywords: Reinfection, HCV, PWID, Sustained virological response, SVR, Injecting, Incidence
Graphical abstract
Highlights
-
•
HCV reinfection rates increased in the early phase of treatment scale-up with direct-acting antivirals among PWID in Scotland.
-
•
Community-based treatment pathways are reaching groups at high risk of reinfection, regarded vital for elimination.
-
•
Low levels of retesting for HCV were observed beyond the first year following successful treatment.
-
•
Considerable numbers of HCV reinfections may have gone undetected in the early phase of treatment scale-up.
-
•
Concerted effort to ensure prompt diagnosis of reinfection needs to be integral to national strategies for PWID.
Introduction
The World Health Organisation (WHO) has set a target of reducing HCV incidence by 80% by 2030 in their global viral hepatitis elimination strategy.[1], [2], [3] People who inject drugs (PWID) are one of the priority groups in elimination efforts.4 Approximately 40% of PWID are infected, accounting for over 6 million cases of the virus and at least 10% of the global population living with the virus.5,6 Prior to the introduction of highly effective direct-acting antivirals (DAAs) for HCV, treatment uptake among PWID was low.7,8 This was partly a result of clinician concerns around adherence to less well tolerated interferon-based treatments and the perceived risk of reinfection.9 Evidence has since emerged that PWID adhere well to DAAs with sustained virological response (SVR) rates above 90%,10,11 but concerns remain that reinfection may hinder efforts to eliminate HCV.12,13
Scaling up HCV DAA treatment to PWID is viewed as a key strategy, with modelling studies illustrating its potential to reduce both HCV prevalence and incidence.3,14,15 In Scotland, treatment with DAAs has been scaled up considerably, especially in the Tayside and Greater Glasgow and Clyde (GGC) areas, through expanded care pathways for HCV testing and treatment in the community, including pharmacies, drug treatment centres, needle and syringe programmes (NSPs), nurse-led outreach clinics and prisons.16,17 In Tayside, all community pathways were in environments accessible by PWID, with testing and treatment available in each setting delivered by multidisciplinary groups of staff.18 This has led to substantial reductions in chronic HCV prevalence among PWID19 but there remains uncertainty around the extent of reinfection among those treated with DAAs. To detect and monitor reinfection, guidelines recommend that PWID be tested annually for HCV RNA post-SVR.20 Most reinfection studies have been relatively small scale within drug treatment services or community clinics.21 One large study from British Columbia confined to those treated with DAAs suggested that showed that reinfection rates were higher among people who had injected recently – though still comparatively modest at 3 per 100 person-years (PY).22
Using record-linkage of established HCV clinical and laboratory surveillance systems in Scotland,23 we aim to estimate the incidence of HCV reinfection among PWID following SVR according to treatment settings and time periods since 2000. More specifically, we aim to (i) provide the first population-level estimates of HCV reinfection since the introduction of DAAs in Scotland; (ii) compare reinfection rates pre and post DAAs and by setting of treatment; and (iii) estimate the extent of undiagnosed reinfection resulting from incomplete follow-up testing for HCV post-SVR among PWID.
Patients and methods
Study population and data sources
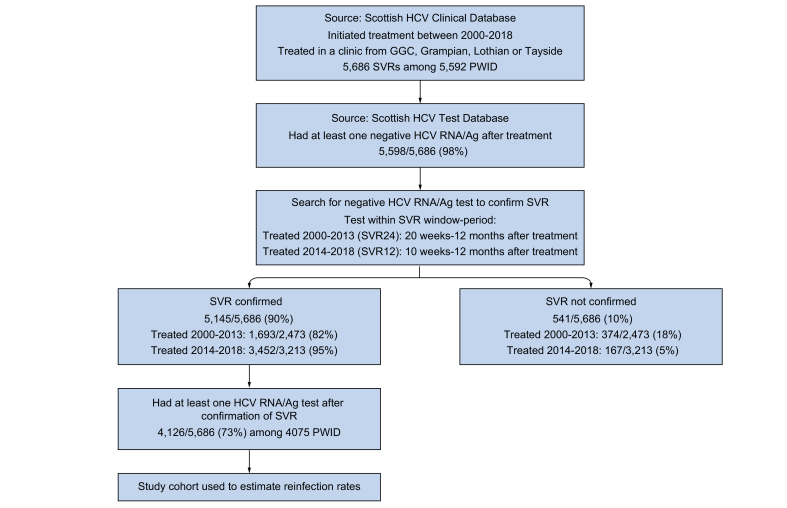
The Scottish HCV clinical database contains data collected during clinical follow-up of patients treated for HCV (either in hospital, prisons or community settings) in Scotland.24 We identified individuals on the database who reported injecting drug use as a potential risk factor for their HCV infection, commenced HCV therapy between 1st January 2000 and 31st December 2018, and achieved a SVR according to the clinical database. The cohort was confined to those treated in 1 of the 4 largest National Health Service (NHS) board areas in Scotland (GGC, Grampian, Lothian or Tayside) and with an available Community Health Index patient identifier (a unique number that identifies individuals across healthcare systems in Scotland), as comprehensive HCV testing data is available for these areas. These areas relate to over 70% of all PWID treated in Scotland and 68% of the anti-HCV diagnosed population.25 For analysis, Lothian and Grampian were combined to provide sufficient numbers of PWID for statistical modelling.
The cohort was then linked to the Scottish HCV test database, which contains data on tests performed in Scottish virus laboratories for HCV antibody, antigen (Ag), RNA and genotype from 1st January 1999 to 31st December 2019. The test database was used, first, to corroborate SVR by identifying the first negative HCV RNA/Ag test within the following periods post completion of therapy: 20 weeks to 12 months for those who initiated treatment between 2000-2013, and 10 weeks to 12 months for those who initiated treatment from 2014 onwards. We refer to this test as the confirmatory negative SVR test and these window periods were based on changes to testing for SVR pre and post the introduction of DAAs in Scotland. The test database was then used to follow up the cohort for HCV reinfection by searching for subsequent HCV RNA/Ag tests (which we refer to as follow-up tests performed post-SVR); those with both a confirmatory negative SVR test and at least 1 follow up RNA/Ag test (positive or negative) were used to estimate reinfection rates (Fig. 1). Information on opioid or injection-related hospital admissions (identified using ICD-10 codes described previously26 and used as an indicator of recent or ongoing injection risk), death and migration were ascertained through linkage to Scottish Morbidity Record data, National Records of Scotland mortality data and the Community Health Index database, respectively.
Fig. 1.
Flowchart illustrating the selection process for the PWID cohort followed up for HCV reinfection.
GGC, Greater Glasgow and Clyde; PWID, people who inject drugs; SVR, sustained virological response.
Statistical analysis
Diagnosed reinfection rates
Diagnosed HCV reinfection was defined as a positive HCV RNA/Ag test during follow-up. The date of reinfection was taken as the midpoint between the date of the first positive HCV RNA/Ag test post-SVR and their most recent preceding negative HCV RNA/Ag test. PY were calculated from the time of their confirmatory negative SVR test up to the date of reinfection or otherwise latest negative HCV RNA/Ag test. PWID that were reinfected and retreated, achieving SVR more than once were followed up again in the same way. Incidence of diagnosed reinfection was calculated as the rate per 100 PY, with 95% CIs calculated using a compound Poisson process model to account for patients achieving SVR more than once.27 We used Cox proportional hazards regression to determine risk factors for reinfection. Recurrent reinfections were accounted for by using the Prentice, Williams and Peterson-gap time version of the Cox model.28 Period of treatment initiation was a key risk factor, categorised into 2 pre-DAA periods (2000-2009 and 2010-2014; the latter relates to a period of modest scale-up in treatment associated with Scotland’s HCV Action Plan29,30) and 2 DAA periods (2015-2016 and 2017-2018; the former relates to an initial period of prioritisation of DAA therapy for those with advanced liver fibrosis31).
Estimates of undiagnosed reinfection
An issue that affects the estimation of reinfection rates is that PWID have variable amounts of follow-up due to differences in retesting rates post-SVR. If patients are not regularly retested post-SVR, failure to detect reinfections may occur and potentially bias estimates of reinfection. We thus performed simulations on the unobserved time periods (i.e. the period between a patient’s last HCV RNA/Ag negative test and the end of the study period) for the whole cohort of PWID that initiated treatment in 2015-2018 and achieved SVR (with corroboration), regardless of whether they had follow-up RNA/Ag tests post-SVR, to estimate the number of undiagnosed reinfections during follow-up to the end of 2019. For sensitivity analyses, we halved reinfection rates used in simulations on the basis that those that sought a test soon after SVR may have higher reinfection risk and thus, reinfection risk may reduce as patients are followed up for longer.21 We also conducted a sensitivity analysis where reinfection risk was increased by 50% to consider the alternative scenario where those not retested are at a higher risk of reinfection, possibly because of unwillingness or difficulty in accessing health services or a perception that health-related matters are low priority. Patients were followed up to the earliest of the end of 2019, death or migration.
Rates of reinfection for the unobserved period were estimated based on PWID that commenced treatment after 2014 (relating to the DAA era) and had been retested, accounting for variation by NHS board, treatment setting and age. The cumulative probability of reinfection over each patient’s whole unobserved time period was estimated as where is the reinfection rate for NHS board , treatment setting and age group , and is the unobserved PY for patient . Time of reinfection was chosen as any day during a patient’s unobserved period with equal probability.
Results
Characteristics of the PWID cohort achieving SVR
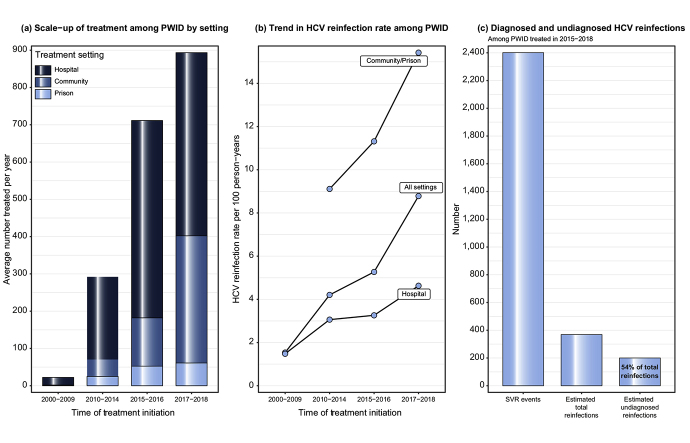
The numbers of PWID achieving SVR increased from 886 in 2000-2009 (average of 89 per year) to 1,587 in 2010-2014 (317 per year), 1,426 in 2015-2016 (713 per year), and 1,787 in 2017-2018 (894 per year) (Table 1). This equates to a 2.5-fold increase in the average annual number of PWID achieving SVR in the DAA period (2015-2018) compared to the period immediately prior to DAAs (2010-2014), equivalent to a 2.8-fold increase in GGC, 2.4-fold in Tayside and 2-fold in Grampian/Lothian. A greater proportion of the PWID SVR cohort were treated in community and prison settings during 2017-2018 (45%) compared to 2010-2014 (22%) and 2015-2016 (26%). This change was particularly apparent in Tayside where community and prison settings accounted for 85% of PWID treated during 2017-2018, compared to 35% in other areas (Table S1). For inclusion in the cohort to estimate reinfection: 5,145/5,686 (90%) SVR events recorded on the Clinical Database had a negative HCV RNA/Ag test located in the test database to corroborate SVR and 4,126 (73%) had a least 1 follow-up HCV RNA/Ag test (Fig. 1). The lowest cohort inclusion rate was among those treated in Tayside (512/1,018, 50%) (Table S2).
Table 1.
Characteristics of the PWID cohort achieving SVR in Scotland by time of initiating treatment for HCV.
| 2000-2009 (col %) | 2010-2014 (col %) | 2015-2016 (col %) | 2017-2018 (col %) | |
|---|---|---|---|---|
| Total PWID achieving SVR∗ | 886 | 1,587 | 1,426 | 1,787 |
| NHS board of treatment | ||||
| Greater Glasgow and Clyde | 410 (46.3) | 863 (54.4) | 854 (59.9) | 1,102 (61.7) |
| Grampian/Lothian | 331 (37.4) | 421 (26.5) | 346 (24.3) | 341 (19.1) |
| Tayside | 145 (16.4) | 303 (19.1) | 226 (15.8) | 344 (19.3) |
| Treatment setting | ||||
| Hospital | 206 (23.3) | 1,109 (69.9) | 1,059 (74.3) | 983 (55.0) |
| Community | 7 (0.8) | 227 (14.3) | 259 (18.2) | 682 (38.2) |
| Prison | 11 (1.2) | 125 (7.9) | 105 (7.4) | 122 (6.8) |
| Other/not known∗∗ | 662 (74.7) | 126 (7.9) | 3 (0.2) | 0 (0.0) |
| Sex | ||||
| Female | 235 (26.5) | 349 (22.0) | 319 (22.4) | 452 (25.3) |
| Male | 651 (73.5) | 1,238 (78.0) | 1,107 (77.6) | 1,335 (74.7) |
| Age (years) | ||||
| 50+ | 86 (9.7) | 227 (14.3) | 438 (30.7) | 467 (26.1) |
| 35-49 | 498 (56.2) | 884 (55.7) | 806 (56.5) | 1,098 (61.4) |
| <35 | 302 (34.1) | 476 (30.0) | 182 (12.8) | 222 (12.4) |
| Treatment regimen | ||||
| Interferon-based | 882 (99.5) | 1,522 (95.9) | 444 (31.1) | 28 (1.6) |
| Interferon-free | 0 (0.0) | 57 (3.6) | 982 (68.9) | 1,759 (98.4) |
| Other/not known | 4 (0.5) | 8 (0.5) | 0 (0.0) | 0 (0.0) |
| Cirrhosis at time of treatment | ||||
| No | 820 (92.6) | 1,291 (81.3) | 971 (68.1) | 1,462 (81.8) |
| Yes | 66 (7.4) | 296 (18.7) | 455 (31.9) | 325 (18.2) |
| Opioid/injection hospital admission | ||||
| >3 years pre-treatment/never pre-Treatment | 771 (87.0) | 1,285 (81.0) | 1,124 (78.8) | 1,272 (71.2) |
| During treatment/in the 3 years pre-treatment | 115 (13.0) | 302 (19.0) | 302 (21.2) | 515 (28.8) |
NHS, National Health Service; PWID, people who inject drugs; SVR, sustained virological response.
Achieved SVR according to the Scottish HCV clinical database. Patients can achieve SVR more than once if they were reinfected.
Whilst treatment setting was not recorded on the clinical database during 2000-2009, the vast majority would have been hospital.
Diagnosed reinfection
There were 361 reinfections detected up to the end of 2019 among 4,075 PWID and relating to 4,126 SVR events (9%) between 2000-2018; with 9,196 PY of follow-up yielding an overall reinfection rate of 3.9 per 100 PY (95% CI 3.5-4.3) (Table 2). Almost half of these reinfections were among those treated in 2015-2016 (n = 95, 26%) or 2017-2018 (n = 74, 20%). Two consecutive positive HCV RNA/Ag results were available for 96% (346/361) of these reinfections. HCV genotype was known before and after reinfection for 184 reinfections, with 93 reinfections showing evidence of genotype change. Ninety-four PWID were confirmed to have achieved SVR twice (26% of reinfections); 51 of their second SVRs were followed up for 68 PY and 10 were reinfected twice, giving a reinfection rate of 14.7 per 100 PY among second SVRs. Almost two-thirds of reinfections detected between 2015-2017 were retreated, with retreatment occurring, on average, just over a year following diagnosis of reinfection (median 1.2 years, IQR 0.6-1.8) (Table S3).
Table 2.
HCV reinfection rates and risk factors for reinfection.
| Reinfections (col %)∗ | PY | Reinfection rate per 100 PY (95% CI) | HR (95% CI) | aHR (95% CI) | |
|---|---|---|---|---|---|
| Total | 361 | 9,196 | 3.9 (3.5–4.4) | ||
| Time of treatment initiation | |||||
| 2000-2009 | 48 (13.3) | 3,127 | 1.5 (1.1–2.1) | 1.00 | 1.00 |
| 2010-2014 | 144 (39.9) | 3,424 | 4.2 (3.5–5.0) | 2.22 (1.59–3.09) | 1.64 (1.15–2.34) |
| 2015-2016 | 95 (26.3) | 1,803 | 5.3 (4.3–6.5) | 2.57 (1.80–3.69) | 2.11 (1.42–3.12) |
| 2017-2018 | 74 (20.5) | 843 | 8.8 (6.9–11.1) | 4.47 (3.06–6.54) | 3.36 (2.20–5.13) |
| NHS board of treatment | |||||
| Greater Glasgow and Clyde | 204 (56.5) | 5,940 | 3.4 (3.0–4.0) | 1.00 | 1.00 |
| Grampian/Lothian | 54 (15.0) | 2,095 | 2.6 (2.0–3.4) | 0.74 (0.55–1.00) | 0.77 (0.57–1.04) |
| Tayside | 103 (28.5) | 1,161 | 8.9 (7.2–10.9) | 2.46 (1.94–3.12) | 1.69 (1.28–2.23) |
| Treatment setting | |||||
| Hospital∗∗ | 197 (54.6) | 7,695 | 2.6 (2.2–3.0) | 1.00 | 1.00 |
| Community | 101 (28.0) | 1,061 | 9.5 (7.8–11.7) | 3.13 (2.44–4.02) | 1.81 (1.35–2.43) |
| Prison | 63 (17.5) | 440 | 14.3 (11.1–18.5) | 4.79 (3.60–6.38) | 3.20 (2.28–4.47) |
| Sex | |||||
| Female | 72 (19.9) | 1,934 | 3.7 (2.9–4.7) | 1.00 | 1.00 |
| Male | 289 (80.1) | 7,262 | 4.0 (3.5–4.5) | 1.06 (0.82–1.37) | 1.01 (0.77–1.32) |
| Age (years) | |||||
| 50+ | 38 (10.5) | 1,230 | 3.1 (2.2–4.3) | 1.00 | 1.00 |
| 35-49 | 203 (56.2) | 5,398 | 3.8 (3.3–4.3) | 1.38 (0.97–1.96) | 1.24 (0.86–1.78) |
| <35 | 120 (33.2) | 2,569 | 4.7 (3.9–5.6) | 1.91 (1.32–2.77) | 1.53 (1.01–2.32) |
| Cirrhosis at time of treatment | |||||
| No | 301 (83.4) | 7,367 | 4.1 (3.6–4.6) | 1.00 | 1.00 |
| Yes | 60 (16.6) | 1,830 | 3.3 (2.5–4.3) | 0.72 (0.55–0.95) | 0.87 (0.64–1.18) |
| Opioid/injection hospital admission | |||||
| >3 years pre-treatment/never pre-treatment | 248 (68.7) | 7,337 | 3.4 (3.0–3.9) | 1.00 | 1.00 |
| During treatment/in the 3 years pre-treatment | 113 (31.3) | 1,859 | 6.1 (5.0–7.4) | 1.69 (1.35–2.11) | 1.37 (1.09–1.73) |
Unadjusted and adjusted hazard ratios for HCV reinfection were estimated from Cox proportional hazards models.
aHR, adjusted hazard ratio; HR, hazard ratio; NHS, National Health Service; PY, person-years; SVR, sustained virological response.
346/361 reinfections had 2 consecutive positive HCV RNA/Ag tests; 93/184 patients with HCV genotype data before and after treatment had evidence of genotype change; 10/94 that achieved SVR twice were reinfected twice.
Includes 46 reinfections and 2,766 person-years where treatment setting was other/not known.
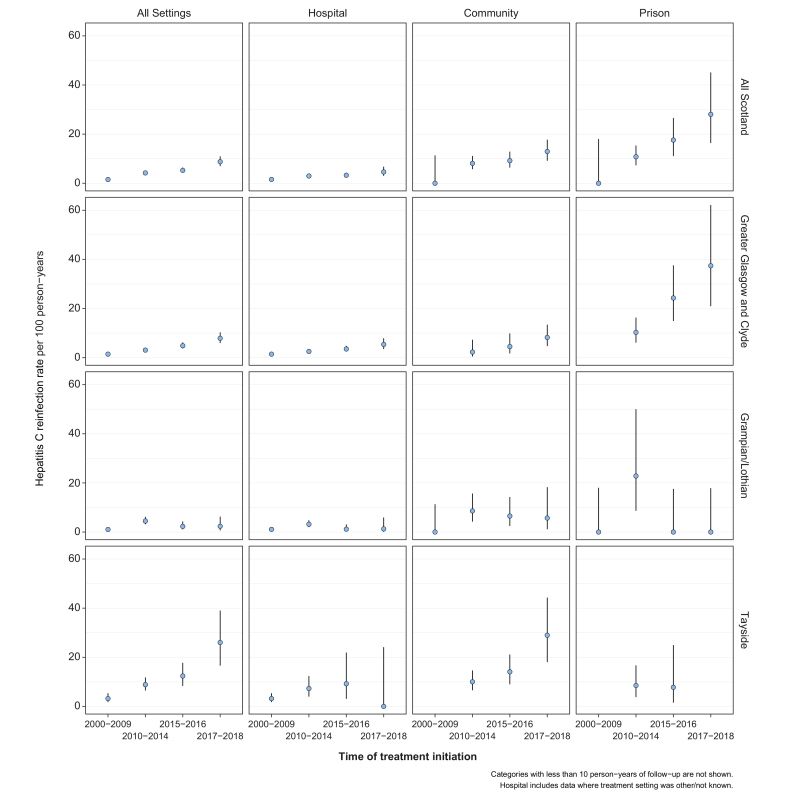
Reinfection rates were greater among those treated since the introduction of DAAs: 5.3 per 100 PY (4.3-6.5) and 8.8 per 100 PY (6.9-11.1) among those treated in 2015-2016 and 2017-2018, respectively, compared to 4.2 (3.5-5.0) for 2010-2014. For those treated in 2015-2016, reinfection rates were lower in the first year following SVR (4.0, 2.8-5.4) compared to the second year following SVR (8.7, 6.4-11.5); however, the same pattern was not observed for those treated in 2017-2018 (9.4, 7.3-11.9 in year one vs. 6.3, 3.1-11.4 in year two) (Table S4). High reinfection rates were observed among PWID treated in prisons (14.3, 11.1-18.5), community settings (9.5, 7.8-11.7) and those treated in Tayside (8.9, 7.2-10.9). Reinfection rates increased over time across all treatment settings, rising to over 20 per 100 PY among PWID treated in prisons during 2017-2018 (Fig. 2).
Fig. 2.
HCV reinfection rates per 100 person-years for all of Scotland and within NHS boards by treatment settings and time of treatment initiation.
Note that treatment settings were not categorised in Tayside for those that initiated treatment between 2000-2009 and these have been assumed as hospital.
Factors associated with reinfection
PWID that initiated treatment in 2017-2018 had a >3-fold increased risk of reinfection compared with those treated in 2000-2009 (adjusted hazard ratio [aHR] 3.4, 2.2-5.1) (Table 2). Compared with PWID treated at GGC, those treated in Tayside had significantly increased risk of reinfection (aHR 1.7, 1.3-2.2). There was insufficient evidence for any differential in reinfection rates by calendar period and NHS board (test for interaction; p = 0.09). Other factors associated with an increased risk of reinfection included being treated in prison (aHR 3.2, 2.3-4.5), being treated in community settings (aHR 1.8, 1.3-2.4), and having an opiate- or injection-related hospital admission during treatment or in the 3 years preceding treatment (aHR 1.4, 1.1-1.7). PWID aged under 35 years at treatment initiation had an increased risk of reinfection (aHR 1.5, 1.0-2.3).
Estimates of undiagnosed reinfection among PWID treated in the DAA era (2015-2018)
Among PWID that initiated treatment in 2015-2018, 78% were tested at least once post-SVR, with 68% tested within the first year post-SVR but only around 30% tested in years beyond this (Table S5). More person-time was unobserved (3,997 PY) than observed (2,646 PY) (Table S6) – unobserved person-time includes 678 SVR events with a confirmatory RNA/Ag test but no follow-up test thereafter. We estimate that there were an additional 200 (174-225) undetected reinfections by the end of 2019 (Table 3), which is more than the 169 reinfections observed during that period. The simulations estimated on average more undiagnosed reinfections than those diagnosed for those treated in hospital (82 vs. 68), community settings (82 vs. 66), Tayside (76 vs. 48) and Grampian/Lothian (25 vs. 11). A conservative sensitivity analysis that assumed reinfection rates for the unobserved period were half those of the observed period yielded an overall estimate of 104 (87-125) undiagnosed reinfections (Table S7), while a sensitivity analysis that assumed a 50% increased reinfection risk produced an overall estimate of 285 (257-315) undiagnosed reinfections (Table S8).
Table 3.
Diagnosed and estimated undiagnosed HCV reinfection rates per 100 person-years based on observed data and estimated from simulation of complete follow-up for PWID who achieved SVR following treatment initiation during 2015-2018.
| Observed data |
Estimated from simulation |
|||||||
|---|---|---|---|---|---|---|---|---|
| SVR events | Diagnosed reinfections | PY | Reinfection rate per 100 PY (95% CI)∗ | Undiagnosed reinfections (Median, 95% CI)∗ | Total reinfections (Median, 95% CI)∗ | PY (Median)∗ | Reinfection rate per 100 PY (Median, 95% CI) | |
| Total | 2,401 | 169 | 2,646 | 6.4 (5.5-7.5) | 200 (174-225) | 369 (343-394) | 6,416 | 5.8 (4.8-6.8) |
| Time of treatment initiation∗∗ | ||||||||
| 2015-2016 | 1,185 | 95 | 1,803 | 5.3 (4.3-6.5) | 98 (80-114) | 193 (175-209) | 4,057 | 4.8 (3.7-5.9) |
| 2017-2018 | 1,216 | 74 | 843 | 8.8 (7.0-11.1) | 102 (84-120) | 176 (158-194) | 2,359 | 7.5 (5.7-9.5) |
| Treatment setting | ||||||||
| Hospital | 1,633 | 68 | 1,871 | 3.6 (2.8-4.6) | 82 (66-99) | 150 (134-167) | 4,533 | 3.3 (2.5-4.3) |
| Community | 610 | 66 | 608 | 10.9 (8.5-13.9) | 82 (66-98) | 148 (132-164) | 1,507 | 9.8 (7.3-12.7) |
| Prison | 158 | 35 | 167 | 20.9 (14.8-29.5) | 35 (26-45) | 70 (61-80) | 376 | 18.7 (12.2-26.7) |
| NHS board of treatment | ||||||||
| Greater Glasgow and Clyde | 1,649 | 110 | 1,870 | 5.9 (4.9-7.1) | 99 (82-116) | 209 (192-226) | 3,929 | 5.3 (4.2-6.6) |
| Grampian/Lothian | 485 | 11 | 477 | 2.3 (1.3-4.3) | 25 (16-35) | 36 (27-46) | 1,540 | 2.3 (1.2-4.0) |
| Tayside | 267 | 48 | 299 | 16.1 (12.0-21.6) | 76 (62-90) | 124 (110-138) | 947 | 13.1 (9.5-17.4) |
| Age (years) | ||||||||
| 50+ | 730 | 25 | 724 | 3.5 (2.3-5.1) | 37 (26-48) | 62 (51-73) | 1,946 | 3.2 (2.0-4.7) |
| 35-49 | 1,414 | 110 | 1,612 | 6.8 (5.6-8.3) | 116 (98-136) | 226 (208-246) | 3,734 | 6.1 (4.8-7.5) |
| <35 | 257 | 34 | 310 | 11.0 (7.8-15.5) | 46 (35-58) | 80 (69-92) | 736 | 10.9 (7.3-15.5) |
An additional 678 patients with a confirmatory SVR test identified on the HCV test database but no follow-up test post-SVR were included in simulations.
NHS, National Health Service; PY, person-years; SVR, sustained virological response.
Median and 95% CIs estimated from 1,000 simulations.
Estimates of undiagnosed reinfection were based on observed data on diagnosed reinfection; estimates for those initiating treatment in 2015-16 were based on observed data from those treated during 2014-16, and estimates for those initiating treatment in 2017-18 were based on observed data from those treated during 2014-18.
Discussion
In the first 4 years of DAAs (2015-2018), the average annual number of people with an injecting history achieving HCV SVR increased almost 5-fold in Scotland compared to the 15 year pre-DAA period (2000-2014). HCV reinfection rates in PWID who have successfully been treated for HCV were 2- to 3-fold higher in the DAA era compared with the pre-DAA period in Scotland. Reinfection rates were substantially higher in PWID treated in the community and prison settings as well as in those geographical areas that were the first to majorly scale up community treatment. Overall, the majority (78%) of those treated in the DAA era had been tested at least once post-SVR, but only 30% were tested in years beyond the first year post-SVR. We estimate that less than half of reinfections have been identified in the DAA era.
There are a number of possible explanations for the higher reinfection rates observed in the DAA era. The environmental and individual factors that influence reinfection risk could account for changes in rates over time but the national surveillance of PWID – reporting high coverage of NSP and moderate coverage of opioid agonist therapy (OAT) among PWID,32 with relatively stable trends in uptake of harm reduction services and in injecting risk behaviours (with the exception of a recent rise in cocaine injection) across Scotland – would not indicate this as the primary reason.33 As recognised in the Scottish Government HCV treatment strategy, the defining attributes of DAAs – i.e. easy to administer, highly-effective, short duration and safe – make it practical to deliver therapy in the community and overcome the barrier of accessing treatment in hospital settings.30,31,34 Thus, with the availability of DAAs and strategic change to service delivery in Scotland, scale-up of treatment in the community has likely reached individuals with greater reinfection risk who would not otherwise have accessed tertiary care for interferon-based therapies.
It has been acknowledged previously that elevated reinfection rates may occur in the early scale-up of HCV treatment among high-risk populations,21 as successful treatment leads to a larger pool of individuals who are susceptible to reinfection. In this regard, uptake of HCV treatment (in the last year among those therapy-eligible) has doubled between 2013-2014 (9%) and 2017-2018 (21%) among PWID in Scotland, relating to a period of transition when treatment shifted from hospital to community settings. Whilst we observed elevated reinfection risk during the early scale-up of DAAs, the rate of 10.9 per 100 PY (8.5-13.9) among those treated in community settings is consistent with HCV incidence rates captured in population-based surveys of PWID in Scotland (10-15 per 100 PY).33 The highest rates of reinfection (16.1 per 100 PY, 12-21.6) in the DAA era were observed in the Tayside region, which has achieved the greatest scale-up in the proportion of the PWID population treated for HCV across Scotland (with 43% and 65% of those therapy-eligible reporting treatment in the last year and ever, respectively, among PWID from Tayside participating in a national survey in 2017-2018).19 Through a coordinated programme of HCV treatment across multiple community services (including drug treatment, pharmacies, needle exchanges and prisons), the rapid scale-up of DAA therapy in Tayside between 2015 and 2019 has contributed to a reduction in HCV viraemia prevalence in the PWID population from 32% in 2015-2016 to 24% in 2017-2018 and is on track to reduce to below 10%, a level that is expected to considerably limit the potential for onward spread of the virus.16,19 As therapy is scaled-up among PWID, increased attention should be placed on strengthening harm reduction services (increasing coverage of and minimising lapses in NSP and OAT) and prompt retreatment of reinfection20 – observed here in almost two-thirds of Scottish cases detected in the DAA era – to help reach and maintain low prevalence of HCV in high-risk populations.
The reinfection rates we observed are higher than those found in many other studies involving treatment with DAAs. A systematic review on HCV reinfection found an overall pooled reinfection estimate of 6.2 per 100 PY among people with recent injecting drug use across 31 studies.21 A lower reinfection rate of 3.9 per 100 PY was found among those that were treated with DAA therapy across 19 studies, but this included estimates from studies that involved those with non-injecting drug use as well as recent injecting drug use. The largest study within the systematic review found a reinfection rate of 3.1 per 100 PY among recent PWID (defined as having a major drug or injection-related diagnosis in the last 3 years prior to SVR) in British Columbia, Canada.22 However, this estimate was based on a study period where DAA treatment was restricted to patients with at least moderate fibrosis. Hence, the treated population is likely to be representative of one with more advanced liver disease and a relatively lower risk of HCV reinfection. The high reinfection rate of 40.6 per 100 PY (95% CI 26.0-61.2) reported from a recent small study of 111 individuals treated with DAAs in prisons in North East England35 corroborates our findings that prisoners represent a group with potentially elevated reinfection risk. Reinfection rates for Tayside are also aligned with an earlier analysis in this region, following a pilot of interferon-based therapy in a community NSP, that found a rate of 21.5 per 100 PY (95% CI 13.0-35.7).17 The higher reinfection rates observed here are unlikely to be limited to Scotland, but it is important to note that reinfection rates may vary considerably across settings, likely reflecting the population-level incidence of (primary) infection. Previous modelling predicted that rapid scale-up of treatment may result in higher numbers of reinfection initially, but this is expected to decline thereafter in line with reductions in the prevalence of viraemia in the population.12
This is one of few population-level studies on HCV reinfection and the first to (i) directly compare rates between the periods pre and post the introduction of DAAs, in the context of major scale-up of these new interferon-free therapies among PWID facilitated through delivery in community-based settings, and (ii) estimate the overall number of reinfections – both detected and undetected – in PWID who had achieved an SVR. Our estimates of undetected reinfection are based on our observed data for those who have been retested post-SVR, taking account of variation by age, treatment setting and NHS Board, which is close to assuming Missing Completely at Random.
It is possible that those tested for HCV post-SVR have done so out of concern for reinfection and therefore those not retested may in comparison be less at risk. Simulations of undetected reinfections did not account for the possibility of decreased risk of reinfection with increasing years of follow-up and we do not know if those that were not followed up differ in terms of their reinfection risk in ways that we were unable to measure. A meta-regression analysis found that reinfection risk decreased by 23% for each additional year of mean/median post-treatment follow-up21 and a small study among 150 PWID on OAT in New York found that 6/10 reinfections occurred 12-24 weeks post-treatment, with only 4 reinfections in the subsequent 18 months.36 Our results signal a slightly lower reinfection rate in the second vs. first year following SVR for those treated in 2017-2018, and so may reflect that risk of reinfection is reducing over time among this group but further follow-up is required to verify this. Thus, the number of undiagnosed reinfections could have been overestimated in the main analysis. However, simulations that assumed half the risk of reinfection for the unobserved compared to observed time still estimated that a substantial proportion (38%, n = 104) of all reinfections had gone undetected. To account for the possibility that patients not retested are actually at greater risk of reinfection (e.g. because of underprioritizing their personal health or obstacles to healthcare access), we also conducted simulations that assumed a 50% increased risk. This estimated that 63% (n = 285) of reinfections had gone undetected and provides a reasonable upper bound to the scale of undetected reinfections. Further follow-up and increased HCV testing of this SVR cohort is ultimately needed to more accurately measure reinfection, including across the range of community treatment settings (i.e. drug treatment, pharmacy and NSP) which were necessarily grouped together here for the purpose of statistical analysis – future analyses of reinfection will have more power to investigate reinfection within specific community settings.
Our analysis focussed on PWID based on information held on risk factors for HCV acquisition in the Scottish clinical database. However, this lacked information on a number of factors useful for assessing reinfection risk, such as if patients recently injected drugs at the point of SVR, injection frequency and sharing of injection equipment. By linking to hospital admission data, we were able to identify those with an opioid- or injection-related hospital admission within 3 years prior to or during HCV treatment. This group of individuals had an increased risk of reinfection, which suggests that it serves as a reasonable indicator of (albeit will likely under-estimate) recent injecting risk; based on this indicator, a higher proportion of patients treated in the DAA era had recent injecting risk compared to the pre-DAA era.
Reinfection analysis was based on testing data only and centred around those with a confirmatory negative SVR test. Therefore, reinfections that occurred immediately after the end of treatment will not be detected and we were unable to distinguish between reinfection and late relapse of viraemia which occurs rarely but usually soon after stopping treatment.37 The exact timing of reinfection was not known precisely and hence, we used the midpoint between the date of first positive HCV RNA/Ag test post-SVR and their most recent preceding negative test as an approximation. This method has also been used by a number of larger studies to date.22,23 As person-time accumulated by those reinfected was a relatively small proportion of the entire cohort person-time, using the midpoint has very limited impact on reinfection rates (using the date of the first positive HCV RNA/Ag yielded a rate of 8.6 per 100 PY among those treated in 2017-2018 compared to 8.8 when using the midpoint). Reinfection rates within some treatment settings and NHS boards among those treated in 2017-2018 were based on few PY and thus, these rates are uncertain and could change as more follow-up testing data becomes available.
Our evidence from Scotland highlights that major scale-up of HCV therapy (and associated SVR) can be achieved among PWID through delivery of treatment in community settings alongside harm reduction services. The elevated rates of HCV reinfection in the early phase of DAA treatment scale-up confirms that these community-based treatment pathways are reaching high-risk groups regarded vital for elimination. However, our study also revealed a significant shortfall in follow-up HCV RNA testing of PWID post-SVR, with potential for many as yet undiagnosed reinfections. These findings highlight that concerted and sustained efforts post-SVR are required among populations of PWID – involving high coverage of harm reduction measures to minimise reinfection and high uptake of annual RNA testing to ensure prompt diagnosis and treatment of those reinfected – if the goals of HCV elimination are to be met.
Abbreviations
Ag, antigen; aHR, adjusted hazard ratio; DAA, direct-acting antiviral; GGC, Greater Glasgow and Clyde; HR, hazard ratio; NHS, National Health Service; NSP, needle and syringe programme; OAT, opioid agonist therapy; PWID, people who inject drugs; PY, person-years; SVR, sustained virological response.
Financial support
This study is funded by the National Institute for Health Research (NIHR) Programme Grants for Applied Research programme (Grant Reference Number RP-PG-0616-20008). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Authors’ contributions
SJH, MH and NEP conceived and designed the study. AY led on the data analysis and drafting of the manuscript; SJH also contributed substantially to the drafting of the manuscript. NEP, JFD, SM, SB, PCH, DJG, MH and SJH assisted with data analysis and contributed to manuscript revisions. AY, NEP, JFD, SM, SS, SB, PCH, RNG, KT, DJG and SJH contributed to the implementation of national surveillance of HCV infection in Scotland. All authors interpreted the findings and all authors approved the submitted manuscript.
Data availability statement
Data may be obtained from a third party and are not publicly available. Access to the individual level data can be sought through approval of the Public Benefit and Privacy Panel for Health and Social Care (www.informationgovernance.scot.nhs.uk/pbpphsc/home/for-applicants/).
Conflict of interest
SJH has received honoraria from Gilead, outside the submitted work. JFD reports research grants and honoraria from Gilead, Abbvie and MSD, all outside the submitted work. SB reports grants, personal fees and other from Gilead; grants, personal fees and other from AbbVie; all outside the submitted work. PCH recently acted in an advisory capacity, has been paid to speak at meetings with or had travel support from AbbVie, BMS, Eisai Ltd, Falk, Ferring, Gilead, Gore, Janssen, Lundbeck, MSD, Norgine, Novartis, ONO Pharmaceuticals, Pfizer and Roche. KT reports grants from GenMark, grants from Cepheid, grants from Qiagen, other from SpeedX, all outside the submitted work. All other authors declare no competing interests.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found online at https://doi/org/10.1016/j.jhep.2021.09.038.
Supplementary data
The following are the supplementary data to this article:
References
- 1.World Health Organization . 2016. Global health sector strategy on viral hepatitis 2016-2021. Towards ending viral hepatitis.https://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en [Google Scholar]
- 2.Wiktor S. How feasible is the global elimination of HCV infection? Lancet. 2019;393:1265–1267. doi: 10.1016/S0140-6736(18)32750-8. [DOI] [PubMed] [Google Scholar]
- 3.Heffernan A., Cooke G.S., Nayagam S., Thursz M., Hallett T.B. Scaling up prevention and treatment towards the elimination of hepatitis C: a global mathematical model. Lancet. 2019;393:1319–1329. doi: 10.1016/S0140-6736(18)32277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Health Protection Scotland . 2017. Blood borne viruses and sexually transmitted infections: Scotland 2017.https://www.hps.scot.nhs.uk/web-resources-container/blood-borne-viruses-and-sexually-transmitted-infections-scotland-2017 [Google Scholar]
- 5.Grebely J., Larney S., Peacock A., Colledge S., Leung J., Hickman M., et al. Global, regional, and country-level estimates of hepatitis C infection among people who have recently injected drugs. Addiction. 2019;114:150–166. doi: 10.1111/add.14393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Degenhardt L., Peacock A., Colledge S., Leung J., Grebely J., Vickerman P., et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health. 2017;5:e1192–e1207. doi: 10.1016/S2214-109X(17)30375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iversen J., Grebely J., Topp L., Wand H., Dore G., Maher L. Uptake of hepatitis C treatment among people who inject drugs attending Needle and Syringe Programs in Australia, 1999–2011. J Viral Hepat. 2014;21:198–207. doi: 10.1111/jvh.12129. [DOI] [PubMed] [Google Scholar]
- 8.Wiessing L., Ferri M., Grady B., Kantzanou M., Sperle I., Cullen K.J., et al. Hepatitis C virus infection epidemiology among people who inject drugs in Europe: a systematic review of data for scaling up treatment and prevention. PLoS One. 2014;9 doi: 10.1371/journal.pone.0103345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalgard O. Follow-up studies of treatment for hepatitis C virus infection among injection drug users. Clin Infect Dis. 2005;40:S336–S338. doi: 10.1086/427449. [DOI] [PubMed] [Google Scholar]
- 10.Grebely J., Dalgard O., Conway B., Cunningham E.B., Bruggmann P., Hajarizadeh B., et al. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol. 2018;3:153–161. doi: 10.1016/S2468-1253(17)30404-1. [DOI] [PubMed] [Google Scholar]
- 11.Aspinall E.J., Corson S., Doyle J.S., Grebely J., Hutchinson S.J., Dore G.J., et al. Treatment of hepatitis C virus infection among people who are actively injecting drugs: a systematic review and meta-analysis. Clin Infect Dis. 2013;57:S80–S89. doi: 10.1093/cid/cit306. [DOI] [PubMed] [Google Scholar]
- 12.Grebely J., Hajarizadeh B., Dore G.J. Direct-acting antiviral agents for HCV infection affecting people who inject drugs. Nat Rev Gastro Hepat. 2017;14:641. doi: 10.1038/nrgastro.2017.106. [DOI] [PubMed] [Google Scholar]
- 13.Ingiliz P., Rockstroh J.K. Hepatitis C virus reinfection—more to come? Lancet Gastroenterol Hepatol. 2017;2:150–151. doi: 10.1016/S2468-1253(16)30223-0. [DOI] [PubMed] [Google Scholar]
- 14.Martin N., Foster G., Vilar J., Ryder S.E., Cramp M., Gordon F., et al. HCV treatment rates and sustained viral response among people who inject drugs in seven UK sites: real world results and modelling of treatment impact. J Viral Hepat. 2015;22:399–408. doi: 10.1111/jvh.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Innes H., Goldberg D., Dillon J., Hutchinson S.J. Strategies for the treatment of Hepatitis C in an era of interferon-free therapies: what public health outcomes do we value most? Gut. 2015;64:1800–1809. doi: 10.1136/gutjnl-2014-308166. [DOI] [PubMed] [Google Scholar]
- 16.Hickman M., Dillon J.F., Elliott L., De Angelis D., Vickerman P., Foster G., et al. Evaluating the population impact of hepatitis C direct acting antiviral treatment as prevention for people who inject drugs (EPIToPe)–a natural experiment (protocol) BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-029538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulkind J., Stephens B., Ahmad F., Johnston L., Hutchinson S., Thain D., et al. High response and re-infection rates among people who inject drugs treated for hepatitis C in a community needle and syringe programme. J Viral Hepat. 2019;26:519–528. doi: 10.1111/jvh.13035. [DOI] [PubMed] [Google Scholar]
- 18.Byrne C., Beer L., Inglis S.K., Robinson E., Radley A., Goldberg D., et al. Real-world outcomes of rapid regional hepatitis C virus treatment scale-up among people who inject drugs in Tayside, Scotland. Aliment Pharmacol Ther. 2021 doi: 10.1111/apt.16728. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmateer N.E., McAuley A., Dillon J.F., McDonald S., Yeung A., Smith S., et al. Reduction in the population prevalence of HCV viraemia among people who inject drugs associated with scale-up of direct-acting antiviral therapy in community drug services: real world data. Addiction. 2021 doi: 10.1111/add.15459. [DOI] [PubMed] [Google Scholar]
- 20.Pawlotsky J.-M., Negro F., Aghemo A., Berenguer M., Dalgard O., Dusheiko G., et al. EASL recommendations on treatment of hepatitis C: final update of the series. J Hepatol. 2020;73:1170–1218. doi: 10.1016/j.jhep.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 21.Hajarizadeh B., Cunningham E.B., Valerio H., Martinello M., Law M., Janjua N.Z., et al. Hepatitis C reinfection after successful antiviral treatment among people who inject drugs: a meta-analysis. J Hepatol. 2020;72:643–657. doi: 10.1016/j.jhep.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Rossi C., Butt Z.A., Wong S., Buxton J.A., Islam N., Yu A., et al. Hepatitis C virus reinfection after successful treatment with direct-acting antiviral therapy in a large population-based cohort. J Hepatol. 2018;69:1007–1014. doi: 10.1016/j.jhep.2018.07.025. [DOI] [PubMed] [Google Scholar]
- 23.Weir A., McLeod A., Innes H., Valerio H., Aspinall E.J., Goldberg D.J., et al. Hepatitis C reinfection following treatment induced viral clearance among people who have injected drugs. Drug Alcohol Depen. 2016;165:53–60. doi: 10.1016/j.drugalcdep.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 24.McDonald S., Hutchinson S., Innes H., Allen S., Bramley P., Bhattacharyya D., et al. Attendance at specialist hepatitis clinics and initiation of antiviral treatment among persons chronically infected with hepatitis C: examining the early impact of Scotland’s Hepatitis C Action Plan. J Viral Hepat. 2014;21:366–376. doi: 10.1111/jvh.12153. [DOI] [PubMed] [Google Scholar]
- 25.McLeod A., Glancy M., Went A., Smith S., Weir A., McAuley A., et al. 2019. Surveillance of hepatitis C testing, diagnosis and treatment in Scotland, 2019 update.https://www.hps.scot.nhs.uk/web-resources-container/surveillance-of-hepatitis-c-testing-diagnosis-and-treatment-in-scotland-2019-update [Google Scholar]
- 26.Valerio Heather, Goldberg David J., Lewsey James, Weir Amanda, Allen Samuel, Aspinall Esther J., et al. Evidence of continued injecting drug use after attaining sustained treatment-induced clearance of the hepatitis C virus: implications for reinfection. Drug Alcohol Depen. 2015;154:125–131. doi: 10.1016/j.drugalcdep.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 27.Kegler S.R. Applying the compound poisson process model to the reporting of injury-related mortality rates. Epidemiologic Perspect Innov. 2007;4:1–9. doi: 10.1186/1742-5573-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amorim L.D., Cai J. Modelling recurrent events: a tutorial for analysis in epidemiology. Int J Epidemiol. 2015;44:324–333. doi: 10.1093/ije/dyu222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldberg D., Hutchinson S., Innes H., Dillon J. 2019. Scotland’s hepatitis C action plan: achievements of the first decade and proposals for a Scottish Government strategy (2019) for the elimination of both infection and disease.https://www.hps.scot.nhs.uk/web-resources-container/blood-borne-viruses-and-sexually-transmitted-infections-scotland-2017 [Google Scholar]
- 30.Hutchinson S.J., Dillon J.F., Fox R., McDonald S.A., Innes H.A., Weir A., et al. Expansion of HCV treatment access to people who have injected drugs through effective translation of research into public health policy: Scotland’s experience. Int J Drug Pol. 2015;26:1041–1049. doi: 10.1016/j.drugpo.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 31.The Scottish Government . 2015. Hepatitis C treatment & therapies group report.http://www.hepatitisscotland.org.uk/files/2814/4431/5598/treatment_and_therapies_group.pdf [Google Scholar]
- 32.Larney S., Peacock A., Leung J., Colledge S., Hickman M., Vickerman P., et al. Global, regional, and country-level coverage of interventions to prevent and manage HIV and hepatitis C among people who inject drugs: a systematic review. Lancet Glob Health. 2017;5:e1208–e1220. doi: 10.1016/S2214-109X(17)30373-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Health Protection Scotland . 2019. Glasgow Caledonian University and the West of Scotland Specialist Virology Centre. The Needle Exchange Surveillance Initiative (NESI): prevalence of blood-borne viruses and injecting risk behaviours among people who inject drugs (PWID) attending injecting equipment provision (IEP) services in Scotland, 2008-09 to 2017-18.https://www.hps.scot.nhs.uk/web-resources-container/needle-exchange-surveillance-initiative-nesi-2008-09-to-2017-18 [Google Scholar]
- 34.Bruggmann P., Grebely J. Prevention, treatment and care of hepatitis C virus infection among people who inject drugs. Int J Drug Pol. 2015;26:S22–S26. doi: 10.1016/j.drugpo.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Bhandari R., Morey S., Hamoodi A., Thompson C., Jones D., Hewett M., et al. High rate of hepatitis C reinfection following antiviral treatment in the North East England Prisons. J Viral Hepat. 2020;27:449–452. doi: 10.1111/jvh.13240. [DOI] [PubMed] [Google Scholar]
- 36.Akiyama M.J., Lipsey D., Heo M., Agyemang L., Norton B.L., Hidalgo J., et al. Low hepatitis C reinfection following direct-acting antiviral therapy among people who inject drugs on opioid agonist therapy. Clin Infect Dis. 2020;70:2695–2702. doi: 10.1093/cid/ciz693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarrazin C., Isakov V., Svarovskaia E., Hedskog C., Martin R., Chodavarapu K., et al. Late relapse versus HCV reinfection in patients with sustained virologic response after sofosbuvir-based therapies. Clin Infect Dis. 2016:ciw676. doi: 10.1093/cid/ciw676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data may be obtained from a third party and are not publicly available. Access to the individual level data can be sought through approval of the Public Benefit and Privacy Panel for Health and Social Care (www.informationgovernance.scot.nhs.uk/pbpphsc/home/for-applicants/).