Abstract
Background:
Brivanib is a selective inhibitor of vascular endothelial growth factor and fibroblast growth factor (FGF) signalling. We performed a phase II randomised discontinuation trial of brivanib in 7 tumour types (soft-tissue sarcomas [STS], ovarian cancer, breast cancer, pancreatic cancer, non-small-cell lung cancer [NSCLC], gastric/esophageal cancer and transitional cell carcinoma [TCC]).
Patients and methods:
During a 12-week open-label lead-in period, patients received brivanib 800 mg daily and were evaluated for FGF2 status by immunohistochemistry. Patients with stable disease at week 12 were randomised to brivanib or placebo. A study steering committee evaluated week 12 response to determine if enrolment in a tumour type would continue. The primary objective was progression-free survival (PFS) for brivanib versus placebo in patients with FGF2-positive tumours.
Results:
A total of 595 patients were treated, and stable disease was observed at the week 12 randomisation point in all tumour types. Closure decisions were made for breast cancer, pancreatic cancer, NSCLC, gastric cancer and TCC. Criteria for expansion were met for STS and ovarian cancer. In 53 randomised patients with STS and FGF2-positive tumours, the median PFS was 2.8 months for brivanib and 1.4 months for placebo (hazard ratio [HR]: 0.58, p Z 0.08). For all randomised patients with sarcomas, the median PFS was 2.8 months (95% confidence interval [CI]: 1.4–4.0) for those treated with brivanib compared with 1.4 months (95% CI: 1.3–1.6) for placebo (HR Z 0.64, 95% CI: 0.38–1.07; p Z 0.09). In the 36 randomised patients with ovarian cancer and FGF2-positive tumours, the median PFS was 4.0 (95% CI: 2.6–4.2) months for brivanib and 2.0 months (95% CI: 1.2–2.7) for placebo (HR: 0.56, 95% CI: 0.26–1.22). For all randomised patients with ovarian cancer, the median PFS in those randomised to brivanib was 4.0 months (95% CI: 2.6–4.2) and was 2.0 months (95% CI: 1.2–2.7) in those randomised to placebo (HR = 0.54, 95% CI: 0.25–1.17; p = 0.11).
Conclusion:
Brivanib demonstrated activity in STS and ovarian cancer with an acceptable safety profile. FGF2 expression, as defined in the protocol, is not a predictive biomarker of the efficacy of brivanib.
Keywords: Brivanib, Solid tumours, Randomised, discontinuation trial, FGF2 status, Sarcomas, Ovarian cancer
1. Introduction
Brivanib is a small-molecule inhibitor of the vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) family of tyrosine kinase receptors [1,2]. The FGF pathway is involved in cell proliferation, differentiation, survival, angiogenesis and wound healing [3]. A variety of specific abnormalities of the FGF pathway (mutations, translocations, amplifications and overexpression) exist in multiple solid tumours [3]. A retrospective analysis of a phase I trial of brivanib suggested that patients with tumours expressing FGF2 by immunohistochemistry (IHC) were more likely to benefit from therapy [4].
The randomised discontinuation trial (RDT) is an approach to evaluate cytostatic drugs, incorporating a lead-in phase in which all patients are treated with the test drug, and was pivotal in the development of sorafenib for renal cell carcinoma [5,6]. Patients with disease progression after the lead-in phase withdraw from the trial and those with a partial response (PR) continue on test drugs. Patients with stable disease (SD) at the end of the lead-in phase are then randomised to receive the test drug or placebo [7]. This design has a number of advantages as all enrolled patients receive the test drug leading to rapid accrual [7].
We performed a randomised discontinuation phase II trial to evaluate the efficacy and safety of brivanib in multiple tumour types based on their known expression of FGF2 (soft-tissue sarcomas [STS], ovarian cancer, breast cancer, pancreatic cancer, non-small-cell lung cancer [NSCLC], gastric/esophageal cancer and transitional cell carcinoma [TCC]) and hypothesised that FGF2 overexpression would be predictive of efficacy [4].
2. Patients and methods
This trial was approved by the institutional review board or ethics committee at each participating centre (Clinicaltrials.gov identifier: NCT00633789). The trial was conducted according to Good Clinical Practice guidelines, the Declaration of Helsinki and local laws. All patients provided written informed consent.
The study enrolled 7 tumour types: STS, ovarian cancer, breast cancer, pancreatic cancer, NSCLC, gastric/esophageal cancer and TCC. Eligible patients had a histologically or cytologically confirmed diagnosis of one of the eligible tumours (unresectable or metastatic) for which no approved therapy was available. Other key inclusion criteria were as follows: an Eastern Cooperative Oncology Group performance status (PS) of 0–1, at least 3 weeks since the last dose of anticancer therapy, and adequate renal, hepatic and bone marrow function. A tumour sample (archival block) evaluable for FGF2 expression was required for randomisation.
This phase II RDT consisted of a 12-week lead-in period, during which all patients received oral, open-label brivanib 800 mg once daily. At week 12, patients with SD were randomised 1:1 to receive either brivanib or placebo. Unblinding was permitted when disease progression was documented, and patients on placebo could then cross over to brivanib. A maximum of 2 dose reductions was permitted on the trial (to 400 mg daily), and no re-escalation was allowed.
A study steering committee (SSC) reviewed and made changes as needed during the trial. The SSC reviewed accrual, FGF2 expression frequency and tumour response at the end of the lead-in phase (week 12) and determined if enrolment in a given tumour type would continue.
The primary objective of this trial was to compare progression-free survival (PFS) for brivanib versus placebo in randomised patients (in one or more selected tumour types) with FGF2-positive tumours. PFS was also analysed in all randomised patients, regardless of FGF2 status. The secondary end-points included disease stabilisation rate, objective response rate and safety.
Central review of FGF2 status by IHC was performed based on criteria from a previous clinical trial [8]. Tumours were classified as FGF2 positive if the expression score was 1, 2 or 3 and negative if the expression score was 0. Analysis for correlation between grading intensity and efficacy was not performed.
Radiological response was evaluated every 6 weeks. For randomised patients, response was evaluated every 6 weeks up to week 36 and subsequently every 12 weeks. Radiological response was evaluated according to modified World Health Organization criteria using bidimensional measurements [5]. Complete response or PR was confirmed by a second tumour assessment 4 weeks or more after the response was first documented.
Safety assessments were performed on all patients for the entire treatment period. Adverse events (AEs) and laboratory values were graded according to National Cancer Institute Common Terminology Criteria (version 3.0).
2.1. Statistical methods
The primary analysis was the comparison of PFS between brivanib and placebo in the randomised FGF2-positive cohort. This comparison was performed separately for each tumour type (that was selected for expansion) using a 2-sided 10% level log-rank test with 80% power. No adjustment was made for multiple testing. Fifty-two events were required to detect a hazard ratio of 0.5, corresponding to a doubling in the median PFS for brivanib compared with placebo (i.e. 2–4 months). Assuming that 70 patients with FGF2-positive tumours were randomised during a 16-month period, 52 events were expected to be observed after 16 months.
The total number of randomised patients for the primary analysis in the STS and ovarian cohorts was lower than originally planned owing to the relatively low FGF2 positivity rate. As the required number of events in the FGF2-positive STS cohort was not reached, the sample size requirements (52 events required in the randomised period) were applied to the overall population rather than to the FGF2-positive population. Consequently, the statistical power of the primary analysis was lower than 80%. The alternative hypothesis around the effect size was made more stringent, and PFS comparison was conducted on all randomised patients (regardless of FGF2 status) to ensure 80% power.
Forty randomised patients with ovarian cancer (regardless of FGF2 status) were required to reach 28 events when comparing PFS for brivanib and placebo at a HR of 0.33, 2-sided alpha of 5% and power 80%.
The Kaplane–Meier product-limit method was used to estimate median PFS; its corresponding confidence interval (CI) was compared by the method used by Brockmeyer and Crowley [8]. For randomised patients, HR with the corresponding CI was calculated using the Cox proportional hazards model. Because all patients received brivanib at the same initial dose, the safety analysis was performed on the pooled population.
3. Results
Between June 2008 and August 2011, 595 patients with 7 tumour types were treated with brivanib within the phase II trial. The baseline characteristics of these patients are shown in Table 1. Most patients were female (377, 63%), and 290 patients (49%) had a PS of 0. This was a heavily pre-treated population, with 18% of patients having received 2 prior lines of systemic therapy and 55% having received ≥3 lines of therapy. The FGF2 status at the baseline and randomisation for all tumour types are displayed in Table 2. Owing to logistical issues, publication of this manuscript was delayed.
Table 1.
Baseline characteristics by tumour type.
| Characteristic | Tumour type | Overall (n = 595) | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| STS (n = 251) | NSCLC (n = 42) | TCC (n = 31) | Gastric cancer (n = 34) | Pancreatic cancer (n = 38) | Ovarian cancer (n = 126) | Breast cancer (n = 73) | ||
|
| ||||||||
| Mean (SD) age, years | 54 (15) | 64 (11) | 61 (10) | 60 (8) | 58 (10) | 58 (11) | 73 (9) | 57 (13) |
| Male gender, n (%) | 177 (47) | 24 (57) | 25 (81) | 25 (74) | 27 (71) | 0 | 0 | 218 (37) |
| Time from diagnosis ≥2 years, n (%) | 154 (61) | 30 (71) | 13 (42) | 10 (29) | 11 (29) | 101 (80) | 71 (97) | 390 (66) |
| ECOG PS, n (%) | ||||||||
| 0 | 143 (57) | 7 (17) | 12 (39) | 10 (29) | 13 (34) | 59 (47) | 46 (53) | 290 (49) |
| 1 | 107 (43) | 34 (81) | 19 (61) | 23 (68) | 25 (66) | 65 (52) | 27 (37) | 300 (50) |
| Prior treatments, n (%) | ||||||||
| Radiotherapy | 132 (53) | 21 (50) | 13 (42) | 15 (44) | 12 (32) | 14 (18) | 57 (78) | 264 (44) |
| Antiangiogenic therapy | 27 (11) | 16 (38) | 2 (7) | 2 (6) | 0 | 15 (19) | 19 (26) | 81 (14) |
| Other systemic therapy | 199 (79) | 42 (100) | 31 (100) | 34 (100) | 36 (95) | 126 (100) | 73 (100) | 541 (91) |
| No. of prior systemic regimens, n (%) | ||||||||
| 0 | 50 (20) | 0 | 0 | 0 | 2 (5) | 0 | 0 | 52 (9) |
| 1 | 71 (28) | 3 (7) | 15 (48) | 10 (29) | 11 (29) | 11 (9) | 0 | 112 (19) |
| 2 | 47 (19) | 7 (17) | 11 (36) | 11 (32) | 13 (34) | 19 (15) | 1 (1) | 109 (18) |
| ≥ 3 | 83 (33) | 32 (76) | 5 (16) | 13 (38) | 12 (32) | 96 (76) | 72 (99) | 327 (55) |
| FGF2-positive by IHC, % (assessable patients) | 65 (142/218) | 79 (27/34) | 88 (22/25) | 97 (30/31) | 79 (23/29) | 89 (86/97) | 79 (50/63) | 76 (380/497) |
FGF, fibroblast growth factor; IHC, immunohistochemistry; ECOG, Eastern Cooperative Oncology Group; PS, performance status; STS, soft tissue sarcomas; NSCLC, non-small-cell lung cancer; SD, stable disease; TCC, transitional cell carcinoma.
Table 2.
FGF2 status at the baseline and randomisation and secondary efficacy end-points at the end of the lead-in period (week 12).
| Tumour type | Number of FGF2-positive patients/number of assessed patients (%) | |
|---|---|---|
|
|
||
| Baseline | Randomisation | |
|
| ||
| Soft tissue sarcomas | 142/218 (65%) | 53/76 (70%) |
| Ovarian cancer | 97/126 (77%) | 36/39 (92%) |
| Breast cancer | 50/63 (79%) | 9/12 (75%) |
| Pancreatic cancer | 23/29 (79%) | 4/5 (80%) |
| Non-small-cell lung cancer | 27/42 (79%) | 7/11 (64%) |
| Gastric/esophageal carcinoma | 30/31 (97%) | 5/5 (100%) |
| Transitional cell carcinoma | 22/25 (88%) | 4/4 (100%) |
| Overall trial population | 391/510 (77%) | 118/152 (78%) |
| Tumour type | Objective response rate | Disease stabilisation rate | Change in tumour size |
|---|---|---|---|
|
| |||
| Soft tissue sarcomas | 2.8% (95% CI: 0.8–7.1) | 28.9% (95% CI: 21.6–37.1) | 40%a |
| Ovarian cancer | 8.2% (95% CI: 3.6–15.6) | 38.1% (95% CI: 28.5–48.6) | NA |
| Breast cancer | 8.2% (95% CI: 3.1–17.3) | 20.5% (95% CI: 12.0–31.6) | NA |
| Pancreatic cancer | 0 | 13.2% (95% CI: 4.4–28.1) | NA |
| Non-small-cell lung cancer | 0 | 23.8% (95% CI: 12.1–39.5) | NA |
| Gastric/esophageal cancer | 8.8% (95% CI: 1.9–23.7) | 8.8% (95% CI: 1.9–23.7) | 75%b |
| Transitional cell carcinoma | 0 | 16.1% (95% CI: 5.5–33.7) | NA |
FGF, fibroblast growth factor; CI, confidence interval.
FGF2-positive treated patients.
All but one patient was FGF2 positive.
3.1. Efficacy
The randomisation rate (i.e. SD at week 12) for the overall population was 30%. In addition, objective responses were observed in a number of disease cohorts (Table 2), and these patients were continued on open-label brivanib. The SSC regularly reviewed Kaplane–Meier estimates of the conditional probability that a proportion of patients with a tumour type would reach the week 12 randomisation point before making a decision whether to continue to accrue patients with each tumour type. Closure decisions were made for the breast cancer, pancreatic cancer, NSCLC, gastric cancer and TCC tumour types based on evaluation by the SSC (≤42 patients per tumour type). Therefore, the primary end-point was not assessed in these tumours. The SSC determined that the criteria for expansion were met for STS and ovarian cancer.
3.2. Soft-tissue sarcomas
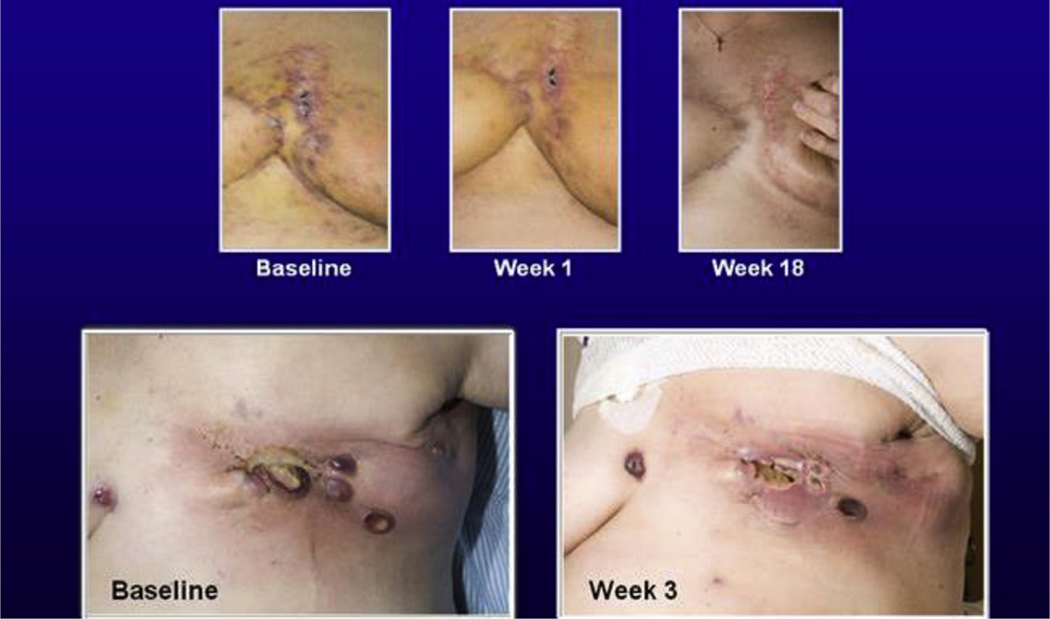
At the week 12 evaluation point, 7 patients with sarcomas (2.8%) had a PR, 4 of these had FGF2-positive tumours. Radiological responses were seen in angiosarcomas (n = 3, Fig. 1), synovial, endometrial stromal, follicular dendritic cell sarcomas and leiomyosarcoma (1 each). Time to response ranged from 1.1 to 2.8 months, and duration of response ranged from 3.2 to 8.4 months.
Fig. 1.
Clinical responses to brivanib in patients with angiosarcoma.
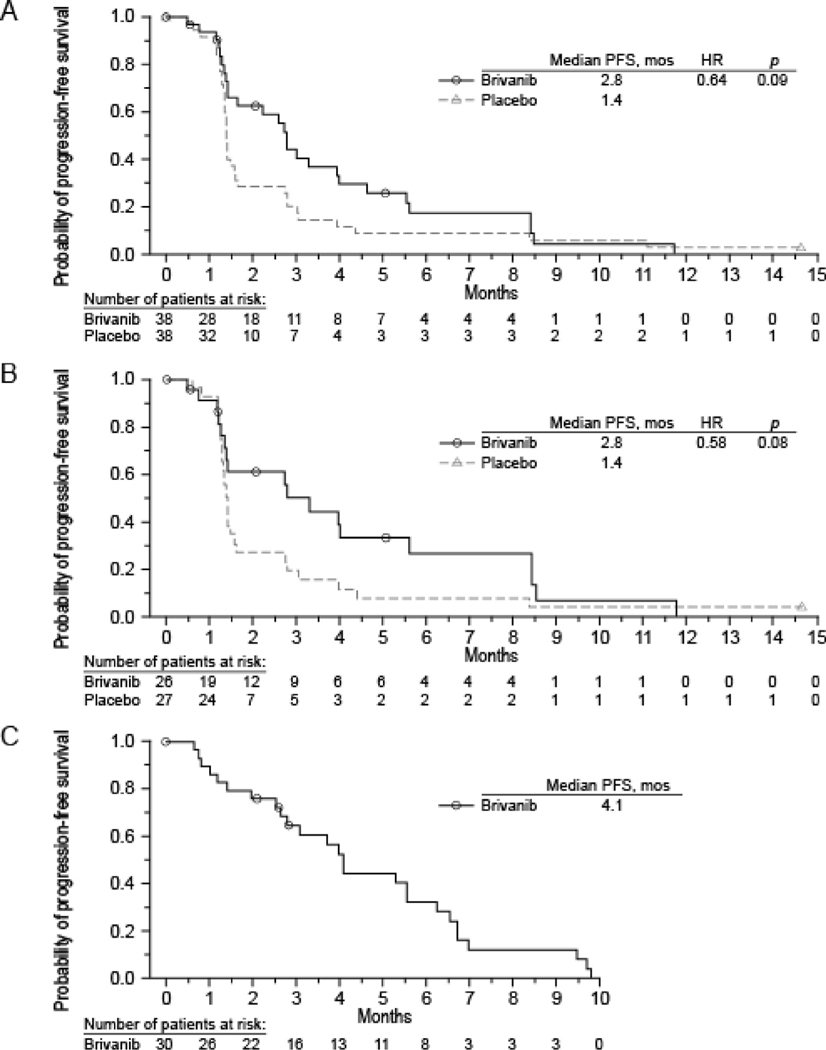
Seventy-six patients (34%) had SD and were randomised to receive brivanib (n = 37) or placebo (n = 36). Three randomised patients were not treated, two had Progressive disease (PD) at week 12 and were randomised in error, and one patient with SD was not treated. In 53 randomised patients with FGF2-positive tumours, the median PFS was 2.8 months for brivanib compared with 1.4 months for placebo (HR: 0.58, p = 0.08), Fig. 2B.
Fig. 2.
Kaplane–Meier curves of progression-free survival in all randomised patients with soft tissue sarcomas (A), patients with FGF2-positive tumours (B) and randomised patients with progression on placebo and treated with brivanib (C). FGF, fibroblast growth factor.
For all randomised patients with sarcomas, the median PFS was 2.8 months (95% CI: 1.4–4.0) for those treated with brivanib compared with 1.4 months (95% CI: 1.3–1.6) for placebo (HR = 0.64, 95% CI: 0.38–1.07; p = 0.09, Fig. 2A).
Seventy-five percent of patients randomised to placebo progressed by their first (after randomisation) scan. Among the 30 randomised patients whose disease progressed while on placebo and then crossed over to openl-abel brivanib, the median PFS was 4.1 months (95% CI: 2.8–6.2) (Fig. 2C). Most patients (87%; 95% CI: 69.3–96.2) had disease restabilisation on retreatment with brivanib. One additional brivanib-treated patient achieved a PR in the randomised period.
3.3. Ovarian cancer
A total of 126 patients with ovarian cancer were treated. At the week 12 randomisation point, 9 patients (8.2%) had a PR, and 43 (34%) had SD. Thirty-nine patients were randomised, 19 to brivanib and 20 to placebo.
In the 36 randomised patients with ovarian cancer and FGF2-positive tumours, the median PFS was 4.0 months (95% CI: 2.6–4.2) for those treated with brivanib and 2.0 months (95% CI: 1.2–2.7) for those given placebo (HR: 0.56, 95% CI: 0.26–1.22).
For all randomised patients with ovarian cancer, the median PFS in those randomised to brivanib was 4.0 months (95% CI: 2.6–4.2) and was 2.0 months (95% CI: 1.2–2.7) in those randomised to placebo (HR = 0.54, 95% CI: 0.25–1.17; p = 0.11).
Three patients achieved a PR to brivanib during the randomised period. The time to response for these patients was 6.7, 3.9 and 1.7 months.
Patients who crossed over from placebo to brivanib had a subsequent median PFS of 1.5 months (95% CI: 1.2–2.8).
3.4. Entire trial population
A post hoc Kaplane–Meier analysis was performed in all randomised patients (n = 152), irrespective of the tumour type. The median PFS for all randomised patients (stratified by tumour type and FGF2 status) was 2.8 months (95% CI: 2.2–3.9) for patients treated with brivanib and was 1.4 months (95% CI: 1.3–1.8) for those on placebo (HR: 0.6, 95% CI: 0.41–0.88). An unstratified analysis of all randomised patients showed similar results.
3.5. Safety
AEs (regardless of causality) that occurred in ≥10% of the overall trial population are shown in Table 3. The most common AEs (>25% of patients) were fatigue, nausea, vomiting, decreased appetite, hypertension and dizziness. The most common grade ≥III AEs (reported for >5% of patients, regardless of causality) were hypertension, fatigue, increased alanine aminotransferase, increased aspartate aminotransferase, dyspnoea, malignant neoplasm and abdominal pain.
Table 3.
Summary of adverse events (N = 595).
| Adverse event, n (%) | All grades | Grade III–V |
|---|---|---|
|
| ||
| Total patients with an event | 591 (99) | 423 (71) |
| Diarrhoea | 301 (51) | 276 (46) |
| Nausea | 280 (47) | 262 (44) |
| Vomiting | 198 (33) | 177 (30) |
| Constipation | 131 (22) | 124 (21) |
| Abdominal pain | 118 (20) | 85 (14) |
| Stomatitis | 64 (11) | 58 (10) |
| Fatigue | 382 (64) | 303 (51) |
| Asthenia | 65 (11) | 49 (8) |
| Alanine aminotransferase increase | 103 (17) | 33 (6) |
| Aspartate aminotransferase increase | 99 (17) | 54 (9) |
| Weight decrease | 91 (15) | 87 (15) |
| Decreased appetite | 269 (45) | 246 (41) |
| Back pain | 85 (14) | 78 (13) |
| Dizziness | 181 (30) | 176 (30) |
| Headache | 148 (25) | 140 (24) |
| Dyspnoea | 101 (17) | 68 (11) |
| Dysphonia | 82 (14) | 82 (14) |
| Cough | 73 (12) | 71 (12) |
| Hypertension | 234 (39) | 156 (26) |
AEs, adverse events.
The list includes AEs (all grades, any relationship) that occurred in ≥10% of the treated patients pooled from 7 cohorts and AEs with onset on or after the first dosing date and on or before the last dosing date, +14 days.
AEs leading to discontinuation were reported for 143 (24%) patients. The most common AEs leading to discontinuation were disease progression (12/143, 8%), vomiting (11/143, 8%) and dyspnoea (11/143, 8%).
Serious AEs (SAEs) were reported for 45% of treated patients. The most common SAEs (≥2%, regardless of causality) were malignant neoplasm, vomiting, dehydration, dyspnoea, hypertension, abdominal pain and nausea.
Sixty-eight patients (11%) died within 30 days of the last dose of brivanib. The primary cause of death was disease progression (51/68, 75%). In 6 patients (2 breast cancer and 1 each of gastric cancer, ovarian cancer, pancreatic cancer and NSCLC), the cause of death was potentially drug toxicity, ascribed to multiorgan failure, cerebral haemorrhage, hypovolemic shock due to dissection of aortic aneurysm, intracranial haemorrhage, bowel perforation and pulmonary haemorrhage.
4. Discussion
This randomised discontinuation phase II trial suggests that brivanib may have activity in multiple solid tumours, especially STS. The key finding supporting this conclusion is the improvement in PFS with brivanib compared with placebo in patients randomised to continuous brivanib. In patients with sarcomas, 75% of those on placebo had disease progression after randomisation, indicating that disease stabilisation during the lead-in period was most likely due to brivanib and reversed rapidly when the treatment ended. Further evidence of activity was provided by patients whose disease progressed while on placebo and received brivanib during the crossover period, with a median PFS of 4.1 months and SD rate of 87%, suggesting that interruption of brivanib did not interfere with responsiveness to subsequent treatment. Temporary withdrawal of brivanib could lead to greater activity than continuous dosing, by allowing re-engagement of the angiogenesis process. Alternatively, a greater percentage of patients randomised to placebo were actually benefitting from the drug (relative to those randomised to brivanib).
In certain subtypes such as angiosarcomas, synovial sarcomas, chondrosarcoma and fibrosarcoma, three-month PFS rates exceeded 50%, indicating the activity of brivanib in these subtypes [9]. To put these results in perspective, the PFS rate in the current trial is similar to that reported in a phase II trial of pazopanib [10,11]. Furthermore, a randomised trial of maintenance pazopanib, following first-line therapy in ovarian cancer, reported an improvement in median PFS for pazopanib compared with 5.6 months for placebo [12]. In our trial, the median PFS for all randomised patients with ovarian cancer treated with brivanib was 4 months.
The results of our trial and the role of the VEGF and FGF pathways in the biology of sarcomas suggest that brivanib should be further evaluated. It is unclear whether the activity of brivanib is due to inhibition of the vascular endothelial growth factor receptor (VEGFR) or FGF receptor (FGFR). As the FGF pathway has a potential role as a mediator of resistance to VEGFR inhibitors, the simultaneous inhibition of the VEGFR/FGFR is rational [13].
In addition, objective responses were observed in ovarian, breast and gastric/esophageal cancer. Activated mutations of FGFR3 occur in 38–66% of non-invasive and in 15–20% of invasive urothelial cancer, with occasional observation of FGFR gene fusions [14,15]. The pan-FGFR inhibitor, erdafitinib, has been approved for FGFR-mutated urothelial carcinoma [16].
FGF2 expression, as defined in the protocol, did not appear to be a biomarker that could be used to select patients with responsive tumours. This was supported by several lines of evidence. First, the median PFS was similar in the FGF2-positive population and all treated patients, regardless of FGF2 status. Second, the proportion of FGF-positive patients at baseline was similar to that at randomisation. A better understanding of the FGF pathway may help to identify other markers of FGF dependence.
5. Conclusion
This randomised discontinuation phase II trial suggests that brivanib may have activity in STS and ovarian cancer. This trial showed that FGF2 expression is not a biomarker for brivanib.
Supplementary Material
Acknowledgments
Funding
This trial was funded by the Bristol-Myers Squibb company.
Footnotes
Conflict of interest statement
Robin L Jones has been a consultant for Adaptimmune, Blueprint, Clinigen, Eisai, Epizyme, Daichii, Deciphera, Immunedesign, Lilly, Merck, Pharmamar, Tracon, Upto Date.
Mark Ratain reports receiving grants from AbbVie, Dicerna and Genentech, reports receiving personal fees from AbbVie, Amgen, Ascentage, Cyclacel, Elion Oncology, multiple generic pharmaceutical companies, Shionogi and Portola Pharmaceuticals, outside the submitted work, is a coinventor on patent US6395481B1 with royalties paid from Mayo Clinic, patent US20160239636A1 pending, patent US8877723B2 issued and patent EP1629111B1 with royalties paid from Mayo Clinic, and serves as a director and treasurer of the Value in Cancer Care Consortium.
Peter J. O’Dwyer reports research support from Pfizer, Genentech, BMS, GSK, Five Prime, Forty Seven, BBI, Novartis, Celgene, Incyte, Lilly / Imclone, Array, H3 Biomedicine and Taiho Pharma, has been consulting in the past 2 years for Genentech, Celgene and Array, provides expert testimony for Bayer and Lilly and is not a stock owner of any companies.
Lillian Siu serves as a consultant for Merck (compensated), Pfizer (compensated), Celgene (compensated), AstraZeneca/MedImmune (compensated), MorphoSys (compensated), Roche (compensated), Geneseeq (compensated), Loxo (compensated), Oncorus (compensated), Symphogen (compensated), Seattle Genetics (compensated) and GSK (compensated), is not a member of the speaker’s bureau for any companies, reports grant/research support (clinical trials for institution) from Novartis, Bristol-Myers Squibb, Pfizer, Boehringer-Ingelheim, GlaxoSmithKline, Roche/Genentech, Karyopharm Therapeutics, Inc., AstraZeneca/MedImmune, Merck, Celgene, Astellas, Bayer, AbbVie, Amgen, Symphogen, Intensity Therapeutics, Mirati and Shattucksm Avid, is a stockholder of Agios (spouse) and is not an employee of any companies.
Jacek Jassem serves as a speaker for AstraZeneca, Roche and Pfizer and reports advisory roles for AstraZeneca, BMS, Pfizer, MSD and Takeda and travel support from Roche.
Jacques Medioni reports honoraria and advisory roles from Pierre Fabre, AstraZeneca, Invectys and Astellas.
Robert Maki reports consulting fees from Arcus, Bayer, Deciphera, Eisai, Immune Design, Janssen R&D, Karyopharm Therapeutics, Lilly, Novartis, Pfizer, Presage, Sarcoma Alliance for Research Through Collaboration (SARC), SpringWorks, American Board of Internal Medicine and UpToDate and reports institutional receipts for clinical trials from Bayer, Karyopharm, Lilly, Pfizer, Regeneron, Presage, Sarcoma Alliance for Research Through Collaboration (SARC), SpringWorks and Tracon.
Ian Walters is an employee/stock owner of BMS at the time of the study and is currently, a CEO/board member of several small companies, including one public company (Portage Biotech).
Joanna Vitfell-Rasmussen, Stan Kaye, Samir Undevia, Ahmad Awada and other authors report no conflict of interest/disclosure.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2019.07.024.
References
- [1].Bhide RS, Lombardo LJ, Hunt JT, et al. The antiangiogenic activity in xenograft models of brivanib, a dual inhibitor of vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1 kinases. Mol Cancer Ther 2010;9(2):369–78. [DOI] [PubMed] [Google Scholar]
- [2].Cai ZW, Zhang Y, Borzilleri RM, et al. Discovery of brivanib alaninate ((S)-((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5methylpyrrolo[2,1-f][1,2,4] triazin-6-yloxy)propan-2-yl)2-aminopropanoate), a novel prodrug of dual vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1 kinase inhibitor (BMS-540215). J Med Chem 2008;51(6):1976–80. [DOI] [PubMed] [Google Scholar]
- [3].Ahmad I, Iwata T, Leung HY. Mechanisms of FGFR-mediated carcinogenesis. Biochim Biophys Acta 2012;1823(4):850–60. [DOI] [PubMed] [Google Scholar]
- [4].Platero S, Mokliatchouk O, Jayson GC, et al. Correlation of FGF2 tumor expression with tumor response, PFS and changes in plasma pharmacodynamics markers following treatment with brivanib alaninate, an oral dual inhibitor of VEGFR FGFR tyrosine kinases. J Clin Oncol 2008;26(15S):3506. [Google Scholar]
- [5].Ratain MJ, Eisen T, Stadler WM, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 2006; 24(16):2505–12. [DOI] [PubMed] [Google Scholar]
- [6].Escudier B, Eisen T, Stadler WM, et al. , Target Study Group. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007;356(2):125–34. [DOI] [PubMed] [Google Scholar]
- [7].Rosner GL, Stadler W, Ratain MJ. Randomized discontinuation design: application to cytostatic antineoplastic agents. J Clin Oncol 2002;20(22):4478–84. [DOI] [PubMed] [Google Scholar]
- [8].Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics 1993;169:1517–23. [Google Scholar]
- [9].Van Glabbeke M, Verweij J, Judson J, Nielsen OS. Progressionfree rate as the principal end point for phase II trials in soft tissue sarcomas. Eur J Cancer 2002;38:543–9. [DOI] [PubMed] [Google Scholar]
- [10].Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European Organisation for Research and Treatment of Cancer—soft Tissue and Bone Sarcoma Group (EORTC Study 62043). J Clin Oncol 2009;27:3126–32. [DOI] [PubMed] [Google Scholar]
- [11].van der Graaf WTA, Blay J-Y, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012;3789: 1879–86. [DOI] [PubMed] [Google Scholar]
- [12].du Bois A, Floquet A, Kim JW, et al. Incorporation of pazopanib in maintenance therapy of ovarian cancer. J Clin Oncol 2014; 32(30):3374–82. [DOI] [PubMed] [Google Scholar]
- [13].Welti JC, Gourlaouen M, Powles T, et al. Fibroblast growth factor 2 regulates endothelial cell sensitivity to sunitinib. Oncogene 2011;30(10):1183–93. [DOI] [PubMed] [Google Scholar]
- [14].Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer 2015;15(1):25–41. [DOI] [PubMed] [Google Scholar]
- [15].Tomlinson DC, Baldo O, Harnden P, Knowles MA. FGFR3 protein expression and its relationship to mutation status and prognostic variables in bladder cancer. J Pathol 2007;213(1): 91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Loriot Y, Necchi A, Park SH, et al. Erdafitinib (ERDA; JNJ-42756493), a pan-fibroblast growth factor receptor (FGFR) inhibitor, in patients (pts) with metastatic or unresectable urothelial carcinoma (mUC) and FGFR alterations (FGFRa): phase 2 continuous versus intermittent dosing. In: Genitourinary Cancers Symposium, 2018; February 8–10, 2018; San Francisco, California; 2018. Abstract 411. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.